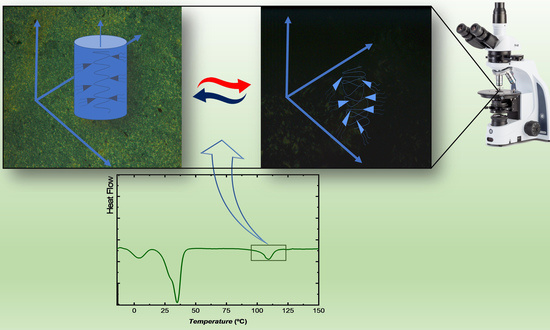
Membranes for Cation Transport Based on Dendronized Poly(epichlorohydrin-co-ethylene oxide). Part 1: The Effect of Dendron Amount and Column Orientation on Copolymer Mobility
Abstract
:1. Introduction
2. Materials and Methods
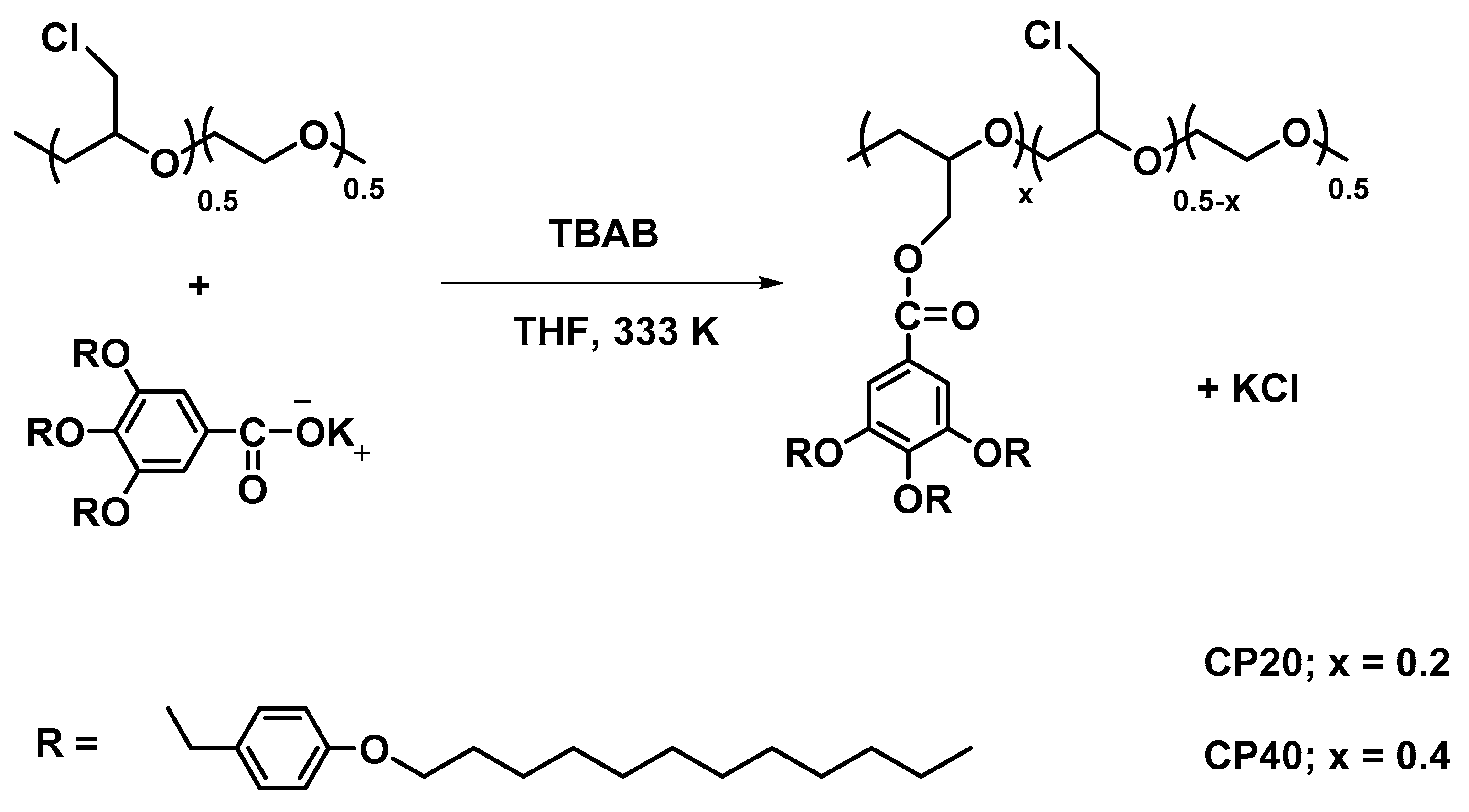
2.1. Materials
2.2. Membrane Preparation
2.3. Characterization Techniques
3. Results
3.1. Thermal, Microscopic and X-ray Diffraction Characterization
3.2. Optimization of the Orientation Procedure
- -
- For sample CP40 (a), the membrane was heated up (10 K/min) to 398 K, then it was cooled slowly (1 K/min) to 363 K where it was kept for 50 h and subsequently cooled to room temperature at a rate of 10 K/min.
- -
- For sample CP40 (b), the membrane was heated up (10 K/min) to 413 K, then it was cooled slowly (1 K/min) to 378 K where it was kept for 50 h. In the end, the membrane was allowed to cool (10 K/min) to room temperature.
- -
- In the case of sample CP40 (c), the membrane was heated up (10 K/min) to 413 K, then it was cooled slowly (0.1 K/min) to 378 K where it was kept for 69 h. Finally, the membrane was allowed to cool (10 K/min) to room temperature.
- -
- For sample CP40 (d), the membrane was heated up (10 K/min) to 413 K. It was kept at the same temperature for 30 min. Then it was cooled slowly (0.1 K/min) to 380 K where it was kept for 120 h. Finally, the membrane was allowed to cool to room temperature at 10 K/min.
- -
- The cooling rate from the isotropic phase to the annealing temperature seems to play a crucial role: the lower the rate, the better the orientation.
- -
- The annealing temperature should be as close as possible to the clearing point determined by DSC. In this regard, given the higher mobility of the copolymer at higher temperatures, one has to increase the annealing time accordingly, i.e., we passed from 69 h in the case of Tclearing − Tannealing = 5 K (CP40 (c)), to 120 h for Tclearing − Tannealing = 3 K (CP40 (d)). It must be also pointed out that when CP40 was annealed at 378 K (Tclearing − Tannealing = 5 K) for longer times, no improvement in column orientation was achieved.
3.3. Dynamic-Mechanical Thermal Analysis (DMTA)
3.4. Dielectric Thermal Analysis (DETA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vorländer, D. Research on molecular shape with the aid of crysalline fluids. Z. Fur Phys. Chem.-Stochiom. Und Verwandtschaftslehre 1923, 105, 211–254. [Google Scholar] [CrossRef]
- Lyu, X.; Xiao, A.; Shi, D.; Li, Y.; Shen, Z.; Chen, E.Q.; Zheng, S.; Fan, X.H.; Zhou, Q.F. Liquid crystalline polymers: Discovery, development, and the future. Polymer 2020, 202, 122740. [Google Scholar] [CrossRef]
- Kato, T.; Uchida, J.; Ichikawa, T.; Soberats, B. Functional liquid-crystalline polymers and supramolecular liquid crystals. Polym. J. 2018, 50, 149–166. [Google Scholar] [CrossRef] [Green Version]
- Shibaev, V. Liquid Crystalline Polymers. Basic Concepts Polym. Prop. 2012, 1, 259–285. [Google Scholar]
- Kato, T.; Gupta, M.; Yamaguchi, D.; Gan, K.P.; Nakayama, M. Supramolecular association and nanostructure formation of liquid crystals and polymers for new functional materials. Bull. Chem. Soc. Jpn. 2021, 94, 357–376. [Google Scholar] [CrossRef]
- Vicari, L. Optical Applications of Liquid Crystals; Taylor & Francis: Bristol, UK, 2010. [Google Scholar]
- Bustamante, E.A.S.; Haase, W. Synthesis and characterization of new liquid crystalline monomers for non-linear optics. X-ray study of re-entrant nematic behaviour with smectic-like fluctuations of C-type. Liq. Cryst. 1997, 23, 603–612. [Google Scholar] [CrossRef]
- Shih, H.F.; Wu, D.Y.; Tien, C.L.; Dai, C.L. Optical compensator with switchable modes using polymer stabilized liquid crystals. Opt. Rev. 2013, 20, 218–223. [Google Scholar] [CrossRef]
- Funahashi, M. Development of liquid-crystalline semiconductors with high carrier mobilities and their application to thin-film transistors. Polym. J. 2009, 41, 459–469. [Google Scholar] [CrossRef]
- Sakuda, J.; Hosono, E.; Yoshio, M.; Ichikawa, T.; Matsumoto, T.; Ohno, H.; Zhou, H.; Kato, T. Liquid-crystalline electrolytes for lithium-ion batteries: Ordered assemblies of a mesogen-containing carbonate and a lithium salt. Adv. Funct. Mater. 2015, 25, 1206–1212. [Google Scholar] [CrossRef]
- Gin, D.L.; Noble, R.D. Designing the next generation of chemical separation membranes. Science 2011, 332, 674–676. [Google Scholar] [CrossRef]
- Rahman, S.N.A.; Masdar, M.S.; Rosli, M.I.; Majlan, E.H.; Husaini, T.; Kamarudin, S.K.; Daud, W.R.W. Overview biohydrogen technologies and application in fuel cell technology. Renew. Sustain. Energy Rev. 2016, 66, 137–162. [Google Scholar] [CrossRef]
- Sazali, N.; Salleh, W.N.W.; Jamaludin, A.S.; Razali, M.N.M. New perspectives on fuel cell technology: A brief review. Membranes 2020, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Yoshio, M.; Kato, T. Handbook of Liquid Crystals; Wiley-VCH: Weinheim, Germany, 2012; pp. 727–749. [Google Scholar]
- Boden, N. Micelles, Membranes, Microemulsions, and Monolayers; Springer New York: New York, NY, USA, 1994; pp. 153–217. [Google Scholar]
- Strzelecka, T.E.; Davidson, M.W.; Rill, R.L. Multiple liquid crystal phases of DNA at high concentrations. Nature 1988, 331, 457–460. [Google Scholar] [CrossRef] [Green Version]
- Percec, V.; Heck, J. Liquid crystalline polymers containing mesogenic units based on half-disc and rod-like moieties—4. Side chain liquid crystalline polymethylsiloxanes containing hemiphasmidic mesogens based on 4-[3,4,5-tri-(alkan-1-yloxy)benzoate] biphenyl groups. J. Polym. Sci. Part A Polym. Chem. 1991, 29, 591–597. [Google Scholar] [CrossRef]
- Sun, H.J.; Zhang, S.; Percec, V. From structure to function via complex supramolecular dendrimer systems. Chem. Soc. Rev. 2015, 44, 3900–3923. [Google Scholar] [CrossRef]
- Bhosale, S.V.; Rasool, M.A.; Reina, J.A.; Giamberini, M. New Liquid crystallne Columnar Poly(epichlorohydrin-co-ethylene oxide) Derivatives Leading to Biomimetic Ion Channels. Polym. Eng. Sci. 2013, 53, 159–167. [Google Scholar] [CrossRef]
- Šakalytė, A.; Reina, J.A.; Giamberini, M. Liquid Crystalline Polyamines Containing Side Dendrons: To-ward the Building of Ion Channels Based on Polyamines. Polymer 2013, 54, 5133–5140. [Google Scholar] [CrossRef]
- Montané, X.; Bhosale, S.V.; Reina, J.A.; Giamberini, M. Columnar Liquid Crystalline Polyglycidol De-rivatives: A Novel Alternative for Proton-Conducting Membranes. Polymer 2015, 66, 100–109. [Google Scholar] [CrossRef]
- Montané, X.; Bogdanowicz, K.A.; Colace, G.; Reina, J.A.; Cerruti, P.; Lederer, A.; Giamberini, M. Advances in the Design of Self-Supported Ion-Conducting Membranes-New Family of Columnar Liquid Crystalline Polyamines. Part 1: Copolymer Synthesis and Membrane Preparation. Polymer 2016, 105, 298–309. [Google Scholar] [CrossRef]
- Bogdanowicz, K.A.; Bhosale, S.V.; Li, Y.; Vankelecom, I.F.J.; Garcia-Valls, R.; Reina, J.A.; Giamberini, M. Mimicking Nature: Biomimetic Ionic Channels. J. Membr. Sci. 2016, 509, 10–18. [Google Scholar] [CrossRef]
- Bogdanowicz, K.A.; Sistat, P.; Reina, J.A.; Giamberini, M. Liquid Crystalline Polymeric Wires for Selec-tive Proton Transport, Part 2: Ion Transport in Solid-State. Polymer 2016, 92, 58–65. [Google Scholar] [CrossRef]
- Montané, X.; Bogdanowicz, K.A.; Prats-Reig, J.; Colace, G.; Reina, J.A.; Giamberini, M. Advances in the Design of Self-Supported Ion-Conducting Membranes—New Family of Columnar Liquid Crystalline Pol-yamines. Part 2: Ion Transport Characterisation and Comparison to Hybrid Membranes. Polymer 2016, 105, 234–242. [Google Scholar] [CrossRef]
- Wang, T.; Li, X.; Dong, Z.; Huang, S.; Yu, H. Vertical Orientation of Nanocylinders in Liquid-Crystalline Block Copolymers Directed by Light. ACS Appl. Mater. Interfaces 2017, 9, 24864–24872. [Google Scholar] [CrossRef]
- Bogdanowicz, K.A.; Rapsilber, G.A.; Reina, J.A.; Giamberini, M. Liquid Crystalline Polymeric Wires for Selective Proton Transport, Part 1: Wires Preparation. Polymer 2016, 92, 50–57. [Google Scholar] [CrossRef]
- Teruel-Juanes, R.; Bogdanowicz, K.A.; Badia, J.D.; de Sáenz Juano-Arbona, V.; Graf, R.; Reina, J.A.; Giamberini, M.; Ribes-Greus, A. Molecular Mobility in Oriented and Unoriented Membranes Based on Poly[2-(Aziridin-1-yl)ethanol]. Polymers 2021, 13, 1060. [Google Scholar] [CrossRef]
- Teruel-Juanes, R.; Pascual-Jose, B.; Graf, R.; Reina, J.A.; Giamberini, M.; Ribes-Greus, A. Effect of Dendritic Side Groups on the Mobility of Modified Poly(epichlorohydrin) Copolymers. Polymers 2021, 13, 1961. [Google Scholar] [CrossRef]
- Percec, V.; Glodde, M.; Bera, T.K.; Miura, Y.; Shiyanovskaya, I.; Singer, K.D.; Balagurusamy, V.S.K.; Heiney, P.A.; Schnell, I.; Rapp, A.; et al. Self-organization of supramolecular helical dendrimers into complex electronic materials. Nature 2002, 417, 384–387. [Google Scholar] [CrossRef]
- Charlesworth, J.M. Deconvolution of overlapping relaxations in dynamic mechanical spectra. J. Mater. Sci. 1993, 28, 399–404. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S. A complex plane analysis of α-dispersions in some polymer systems. J. Polym. Sci. Part C Polym. Symp. 1966, 14, 99–117. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S. A complex plane representation of dielectric and mechanical relaxation processes in some polymers. Polymer 1967, 8, 161–210. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, S.; Runt, J. Broadband Dielectric Investigation of Amorphous Poly (methyl methacrylate)/Poly (ethylene oxide) Blends. Macromolecules 2004, 37, 8110–8115. [Google Scholar] [CrossRef]
- Tong, Y.; Lin, Y.; Wang, S.; Song, M. A study of crystallisation of poly (ethylene oxide) and polypropylene on graphene surface. Polymer 2015, 73, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Buckley, C.P.; Kovacs, A.J. Melting behaviour of low molecular weight poly (ethylene-oxide) fractions. Prog. Colloid. Polym. Sci. 1975, 58, 44–52. [Google Scholar]
- Reina, J.A.; Serra, A.; Mantecón, A.; Cádiz, V. Thermosets obtained by chemical modification of epichlorohydrin-ethylene oxide copolymer and their crosslinking through double bonds. J. Polym. Sci. Part A Polym. Chem. 1995, 33, 941–948. [Google Scholar] [CrossRef]
- Ronda, J.C.; Reina, J.A.; Cádiz, V.; Giamberini, M.; Nicolais, L. Self-organized Liquid-crystalline Poly-ethers Obtained by Grafting Tapered Mesogenic Groups onto Poly (Epichlorohydrin): Toward Biomimetic Ion Channels. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2918–2929. [Google Scholar] [CrossRef]
- Ronda, J.C.; Reina, J.A.; Giamberini, M. Self-organized Liquid-crystalline Polyethers Obtained by Grafting Tapered Mesogenic Groups onto Poly (Epichlorohydrin): Toward Biomimetic Ion Channels 2. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 326–340. [Google Scholar] [CrossRef]
- Komura, M.; Yoshitake, A.; Komiyama, H.; Iyoda, T. Control of Air-Interface-Induced Perpendicular Nanocylinder Orientation in Liquid Crystal Block Copolymer Films by a Surface-Covering Method. Macromolecules 2015, 48, 672–678. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, S.; Runt, J. Observation of a fast dielectric relaxation in semi-crystalline poly(ethylene oxide). Polymer 2002, 43, 6247–6254. [Google Scholar] [CrossRef]
- Alegría, A.; Elizetxea, C.; Cendoya, I.; Colmenero, J. Anomalous Dynamical Homogeneity of the Dielectric α-Relaxation in Miscible Polymer Blends of Poly(epichlorohydrin) and Poly(vinyl methyl ether). Macromolecules 1995, 28, 8819–8823. [Google Scholar] [CrossRef]
- Silva, M.A.; De Paoli, M.-A.; Felisberti, M.I. Flory-Huggins interaction parameter of poly (ethylene oxide)/poly (epichlorohydrin) and poly (ethylene oxide)/poly (epichlorohydrin-co-ethylene oxide) blends. Polymer 1998, 39, 2551–2556. [Google Scholar] [CrossRef]








| Sample | Chlorine/Tap (mol/mol) | PECH-co-EO (mol·10−3) | Tap (mol·10−3) | Time (days) | T(K) | Modification Degree of PECH-co-EO (%) | Yield (%) |
|---|---|---|---|---|---|---|---|
| CP20 | 1:0.5 | 7.4 | 3.7 | 8 | 333 | 20 | 75 |
| CP35 | 1:1 | 3.7 | 3.7 | 8 | 333 | 35 | 55 |
| CP40 | 1:1 | 3.7 | 3.7 | 10 | 333 | 40 | 65 |
| Sample | Tg (K) | Tm (K) | ΔHm (kJ/mol) | Xc (%) | Tcl (K) | ΔHcl (kJ/mol) |
|---|---|---|---|---|---|---|
| CP0 | 241 | - | - | - | - | - |
| CP20 | 262 | 308 | 2.79 | 32 | 378 | 0.061 |
| CP20 oriented | n.d. | 312 | 3.27 | 38 | 377 * | n.d. |
| CP40 | 262 | 308 | 2.56 | 30 | 383 | 0.17 |
| CP40 oriented | 258 | 314 | 4.78 | 52 | 383 | 0.14 |
| Sample | Annealing Temperature (K) | Cooling Rate (K/min) | Annealing Time (hours) | WHH (°) | Angle of Orientation (°) |
|---|---|---|---|---|---|
| CP40 (a) | 363 | 1 | 50 | No orientation | - |
| CP40 (b) | 378 | 1 | 50 | No orientation | - |
| CP40 (c) | 378 | 0.1 | 69 | 92 | 90 |
| CP40 (d) | 380 | 0.1 | 120 | 8.3 | 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zare, A.; Pascual-Jose, B.; De la Flor, S.; Ribes-Greus, A.; Montané, X.; Reina, J.A.; Giamberini, M. Membranes for Cation Transport Based on Dendronized Poly(epichlorohydrin-co-ethylene oxide). Part 1: The Effect of Dendron Amount and Column Orientation on Copolymer Mobility. Polymers 2021, 13, 3532. https://doi.org/10.3390/polym13203532
Zare A, Pascual-Jose B, De la Flor S, Ribes-Greus A, Montané X, Reina JA, Giamberini M. Membranes for Cation Transport Based on Dendronized Poly(epichlorohydrin-co-ethylene oxide). Part 1: The Effect of Dendron Amount and Column Orientation on Copolymer Mobility. Polymers. 2021; 13(20):3532. https://doi.org/10.3390/polym13203532
Chicago/Turabian StyleZare, Alireza, Borja Pascual-Jose, Silvia De la Flor, Amparo Ribes-Greus, Xavier Montané, José Antonio Reina, and Marta Giamberini. 2021. "Membranes for Cation Transport Based on Dendronized Poly(epichlorohydrin-co-ethylene oxide). Part 1: The Effect of Dendron Amount and Column Orientation on Copolymer Mobility" Polymers 13, no. 20: 3532. https://doi.org/10.3390/polym13203532
APA StyleZare, A., Pascual-Jose, B., De la Flor, S., Ribes-Greus, A., Montané, X., Reina, J. A., & Giamberini, M. (2021). Membranes for Cation Transport Based on Dendronized Poly(epichlorohydrin-co-ethylene oxide). Part 1: The Effect of Dendron Amount and Column Orientation on Copolymer Mobility. Polymers, 13(20), 3532. https://doi.org/10.3390/polym13203532