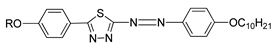2. Structure–Property Relationship
Despite being lyotropic or thermotropic, most liquid crystal can exhibit at least one liquid crystal phase. According to Safinya et al. (1986), a thermotropic liquid crystal can exhibit one to several liquid crystal phases between the crystal and isotropic states [
18]. However, there is a time where a liquid crystal does not exhibit any mesophase at all. This study will explain the possibilities of liquid crystal disclosing different types of liquid crystal phase, why a structure with the same central unit gives out different liquid crystal phases, and how any substitution can affect the mesomorphic.
In accordance with a study by Collings and Hird (1997), the structure–properties relationship is crucial to understand for the synthesizing or altering of a molecule in a certain way or arrangement to get a certain mesomorphic phase [
14]. This understanding is important, especially when the liquid crystal is synthesized for a particular application that requires a particular liquid crystal phase.
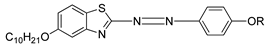
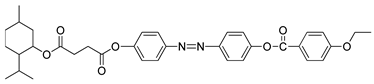
A study was conducted in 1997 by Parra et al. on the structure–properties relationship of azo-based compounds [
19]. In this study, Parra and her co-worker synthesized two azo compounds,
1(a–f) and
2(a–f). All homologues of compound
1a were claimed to display a nematic phase. In addition, homologues
n = 9 and 10, compounds
1e and
1f, respectively, show a monotropic smectic C phase. Similar to compound
1, all homologues of compound
2 also exhibit an enantiotropic nematic phase. However, compounds
2(a–c) displays a monotropic smectic C while compound
2(d–f) exhibit an enantiotropic smectic C phase. Compound
2 appears to have more extensive mesomorphic range compared to compound
1. Parra and her co-worker stated that the low mesophase stability of compound
1 is caused by the presence of a thiophene ring which produces an additional deviation that hinders the formation of a stable mesophase.
![Polymers 13 03462 i001]() |
| Comp | 1a | 1b | 1c | 1d | 1e | 1f |
| R | C5H11 | C6H13 | C7H15 | C8H17 | C9H19 | C10H21 |
![Polymers 13 03462 i002]() |
| Comp | 2a | 2b | 2c | 2d | 2e | 2f |
| R | C5H11 | C6H13 | C7H15 | C8H17 | C9H19 | C10H21 |
Two years later, Belmar et al. (1999) synthesized quite a similar azo compound, namely compounds
3(a–h) [
20]. All homologues of compound
3 give out a nematic phase showing that corresponding molecular interactions occur that give the same outcome in terms of molecular arrangement and thermal stability. However, not all homologues exhibit smectic C phase, such as compounds
3(a–d) as a layered smectic order is not ideal due to the difference in the volume occupied by the opposite chain. Nevertheless, a tilted smectic C order is formed as the alkoxy chain lengthens starting of homologues 7 to 10, and almost the same to the opposite chain.
![Polymers 13 03462 i003]() |
| Comp | 3a | 3b | 3c | 3d | 3e | 3f | 3g | 3h |
| R | C3H7 | C4H9 | C5H11 | C6H13 | C7H15 | C8H17 | C9H19 | C10H21 |
In 2001, Lee et al. synthesized two crystalline dyes containing a non-activated pyranylazo group, compounds
4(a–f) and
5(a–f) [
21]. At the first heating phase, none of the homologues of compounds
4 and
5 display any mesomorphic property due to the thermo- and photochromic properties of the dye. This property causes a small ring-opened merocyanine species, which is not favored at high temperatures. Only after the first heating-cooling phase does the compound starting to exhibit a mesophase. Compounds
4a and
4c show an enantiotropic nematic phase, compounds
4b and
4d display a monotropic nematic phase, while compounds
4e and
4f do not form any liquid crystal phase. In contrast, all homologues of compound
5 form a nematic phase, and some even display a smectic A phase, except compounds
5d and
5e.
![Polymers 13 03462 i004]() |
| Comp | 4a | 4b | 4c | 4d | 4e | 4f |
| R | (CH2)4CH3 | (CH2)5CH3 | (CH2)6CH3 | (CH2)7CH3 | (CH2)8CH3 | (CH2)9CH3 |
![Polymers 13 03462 i005]() |
| Comp | 5a | 5b | 5c | 5d | 5e | 5f |
| R | (CH2)4CH3 | (CH2)5CH3 | (CH2)6CH3 | (CH2)7CH3 | (CH2)8CH3 | (CH2)9CH3 |
In the same year, two azo compounds were synthesized containing a similar structural unit, comprised of pyridine in compounds
6(a–f) and 1,3,4-thiadiazole rings in both compounds
6(a–f) and
7(a–f) [
22]. All homologues of both compounds exhibit a liquid crystal phase. As for compound
6, all homologues display a crystal to isotropic transition, and only two of the highest homologues, compounds
6e and
6f exhibit a monotropic nematic phase. Compound
7 shows a dimorphism of a nematic phase and a smectic C phase. However, compounds
7(a–c) exhibit a monotropic smectic C phase. Nevertheless, the thermal stability of compound
7 is higher compared to the thermal stability of compound
6. The most prominent difference between compounds
6 and
7 can be seen from the structure. Compound
6 comprises a pyridine unit at the end of the central core and only one lateral chain, while compound
7 has a benzene ring instead of a pyridine, two lateral units, and a greater molecular length. To briefly summarize, compound
6 contains a pyridine unit, and is not long enough to be polarized enough to exhibit a stable liquid crystal phase compared to compound
7.
![Polymers 13 03462 i006]() |
| Comp | 6a | 6b | 6c | 6d | 6e | 6f |
| R | C5H11 | C6H13 | C7H15 | C8H17 | C9H19 | C10H21 |
![Polymers 13 03462 i007]() |
| Comp | 7a | 7b | 7c | 7d | 7e | 7f |
| R | C5H11 | C6H13 | C7H15 | C8H17 | C9H19 | C10H21 |
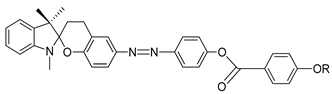
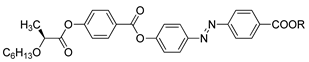
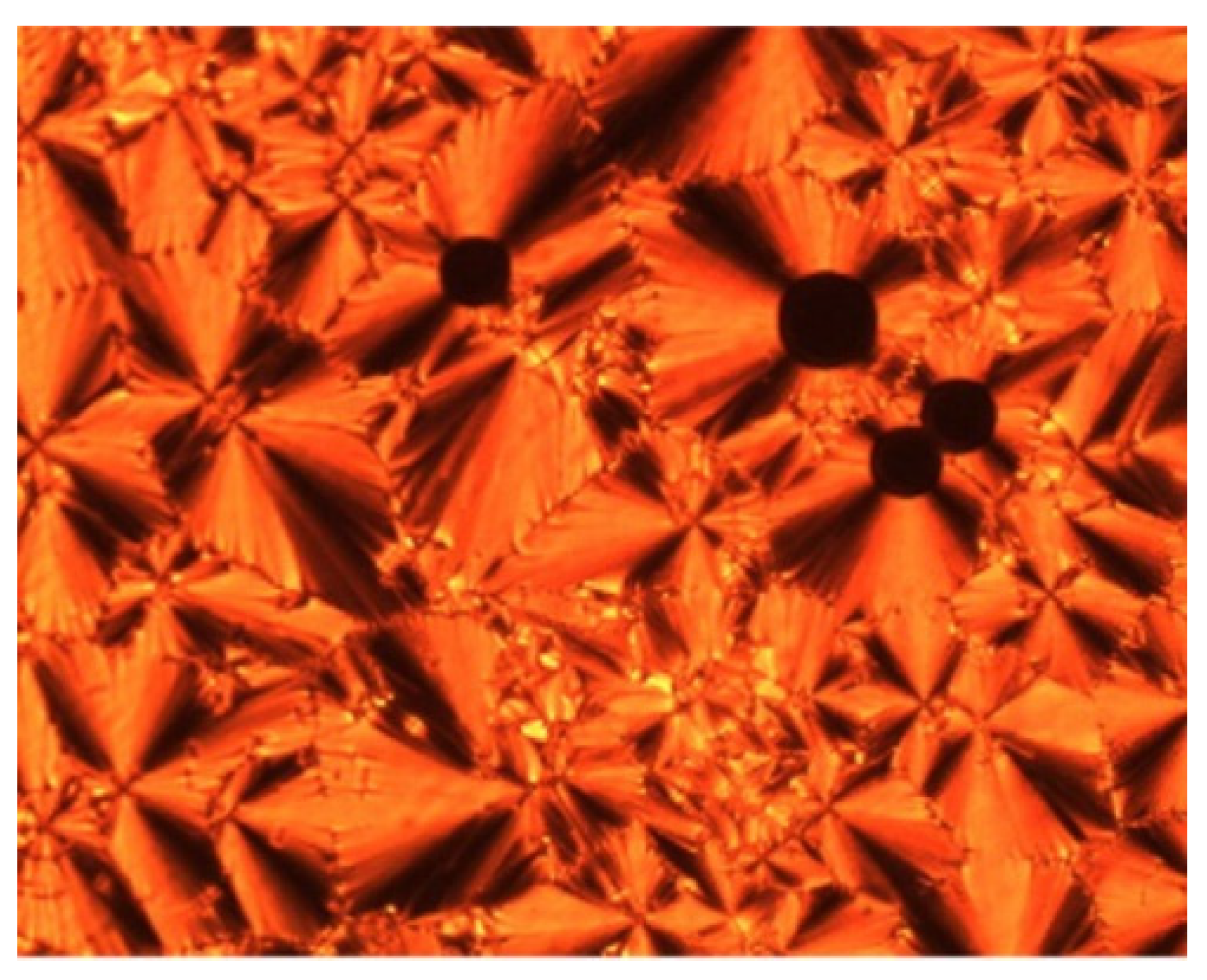
So et al. (2003) reported in his study about compounds
8(a–d), where this compound has two two-ring mesogenic units that are connected by a spacer, namely 2-hydroxy-1,3-dioxypropylene [
23]. All homologues of compound
8 have display an enantiotropic schlieren and/or a broken fan textured smectic C phase except for compound
8a.
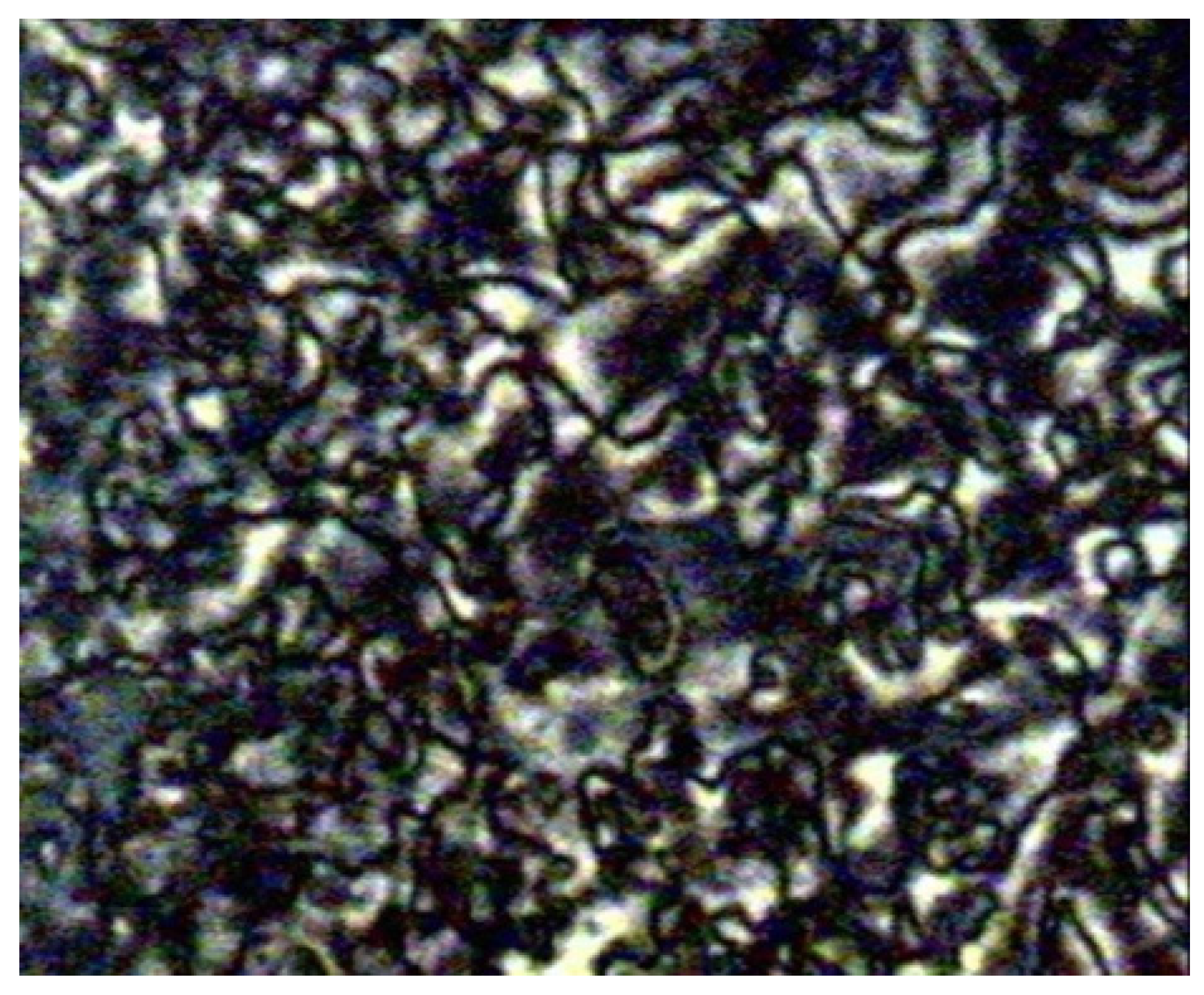
Figure 1 shows a micrograph of compound
8c. This phenomenon happens due to the temperature range of mesophase increase with the increasing length of the terminal alkyl chain.
![Polymers 13 03462 i008]() |
| Comp | 8a | 8b | 8c | 8d |
| R | C6H13 | C8H17 | C10H21 | C12H25 |
Compounds
9(a–c) was synthesized by Abbasi et al. (2006) [
24]. Compounds
9(a–b) display a monotropic liquid crystal phase during cooling from the isotropic liquid. However, compound
9c formed an enantiotropic mesophase behavior and showed a liquid crystalline characteristic on heating and cooling from the isotropic liquid. Abbasi et al. declared that the stability of mesophase is influenced by the alkyl length (R). Compound
9c with the longest alkyl chain length exhibit enantiotropic mesophase which is thermodynamically stable compound, and compound with a short chain length (
9a and
9b) exhibit unstable mesomorphic behavior.
![Polymers 13 03462 i009]() |
| Comp | 9a | 9b | 9c |
| R | C7H15 | C11H23 | C15H31 |
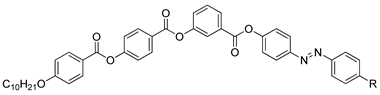
In the same year, compounds
10(a–c) and
11(a–c) were synthesized by Rezvani et al. (2006) [
25]. Compounds
10(a–c) did not show any liquid crystal phase as this compound directly melted into an isotropic liquid. It was claimed that the melting point of the ligands decreases with increasing alkyl chain length value. Compound
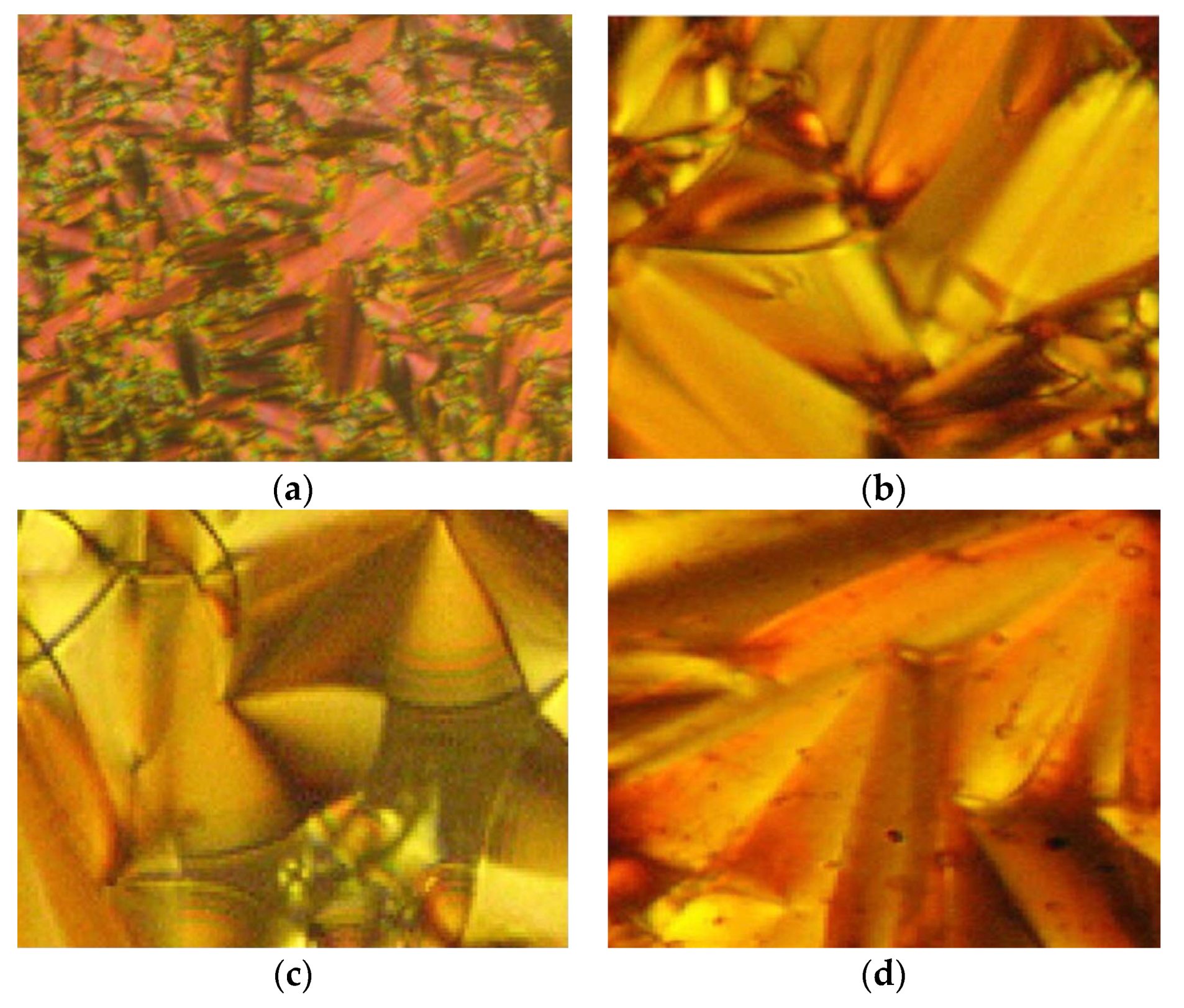
11a exhibit four endothermic transitions, where the first two transitions are equivalent to the crystal-to-crystal transition. The third transition peak at 204.3 °C with enthalpy values of 532.43 kJ mol
−1 is corresponding to a crystal phase to a mesophase. A Schlieren texture typically for the smectic C phase was detected, as reported in
Figure 2. The fourth transition peak at 240.3 °C with a low enthalpy value of 5.25 kJ mol
−1 is responsible for the transition from mesophase to the isotropic liquid. The high clearing enthalpy corresponding to the mesophase to isotropic transition indicates that the mesophase structure is highly in order.
![Polymers 13 03462 i010]() |
| Comp | 10a | 10b | 10c |
| R | C9H19 | C11H23 | C13H27 |
![Polymers 13 03462 i011]() |
| Comp | 11a | 11b | 11c |
| R | C9H19 | C11H23 | C13H27 |
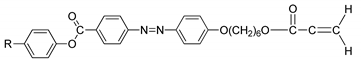
Compounds
12(a–c) were first synthesized by So et al. in 2001. In 2006, he conducted another study using the same compound but with a different alkyl chain [
26]. During the heating phase, compound with the highest homologues, compound
12c, exhibits a crystal to smectic C phase at 163.0 °C, and smectic C to isotropic liquid at 168.0 °C and compounds
12a and
12b does not show any mesomorphic phase. However, during the cooling scan, compounds
12b and
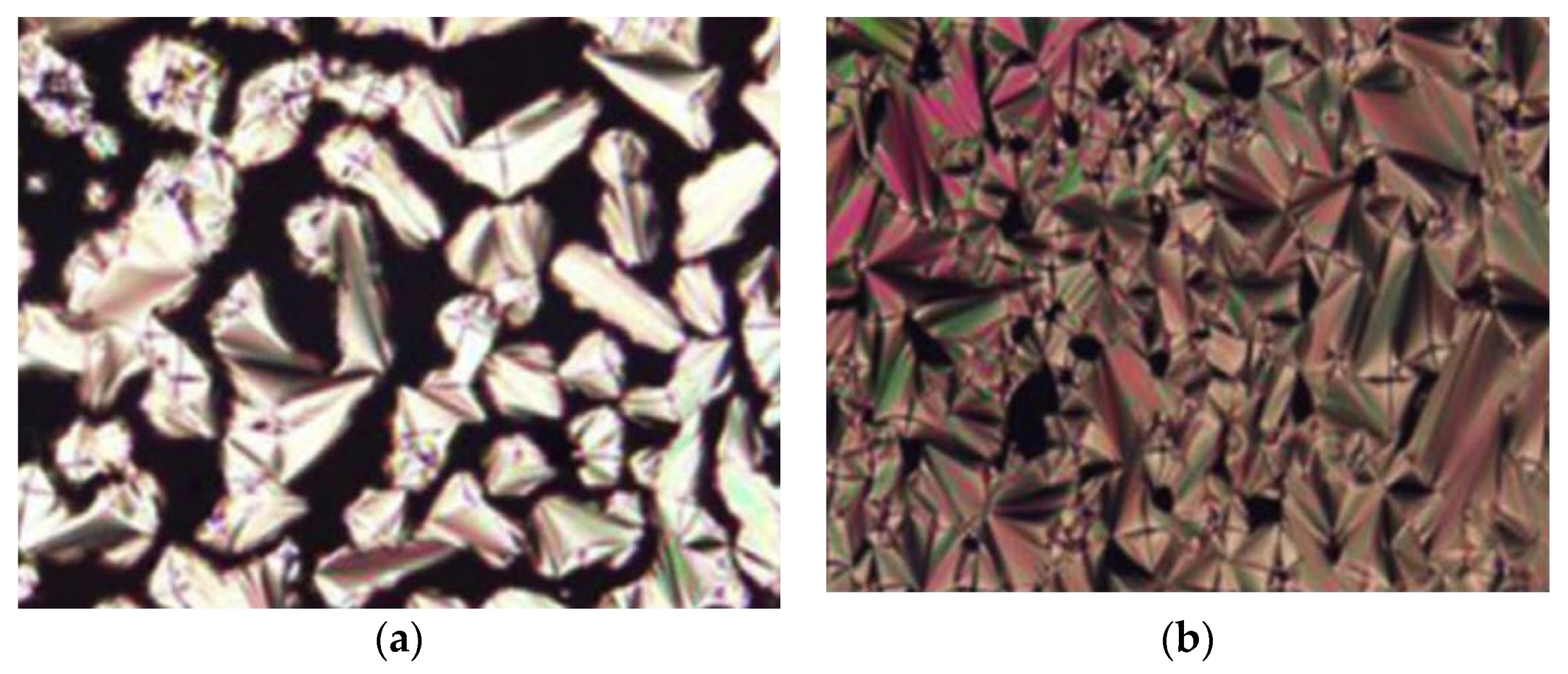
12c show a smectic C mesophase (
Figure 3) but not compound
12a. Compound
12b form a monotropic smectic C liquid crystal phase with a schlieren or broken-fan texture, whereas compound
12c exhibit an enantiotropic smectic C liquid crystal phase with a schlieren texture and compound
12a shows no mesomorphic behavior. So et al. (2006) reported that increasing the alkyl chain is expected to increase the length-to-breadth, resulting in a liquid crystal phase, especially the smectic phase [
27]. He also claimed that as the length of the terminal chain increase, the temperature range of the smectic phase also increases. It was also discussed in the study that the entropy change keeps increasing as the terminal chain increase, hence, a smectic mesophase is prone to show, and as the terminal chain increases, the thermal stability of a tilted smectic C also increases.
![Polymers 13 03462 i012]() |
| Comp | 12a | 12b | 12c |
| R | C5H11 | C7H15 | C9H19 |
Lutfor et al. (2009) have synthesized compounds
13(a–f) with six different homologues [
28]. As for raw compound
13a, two transition peaks were observed during cooling of compound at 175.2 and 123.1 °C, which are assigned for the isotropic to smectic and smectic to crystal transition, respectively. Chloro-substituted compound
13b also shows two peaks on cooling, 144.3 and 126.0 °C, which are corresponded to isotropic to smectic and smectic to crystal transition, respectively. Compound
13c and
13f do not display any liquid crystal phase, and it melts at 168.0 and 165.0 °C and crystallized at 162.0 °C and 160.0 °C, respectively. Compound
13d is a fluoro-substituted compound that displays two peaks on cooling at exactly 150.5 and 127.1°C, corresponding to isotropic to smectic and smectic to crystal transition, respectively. Compound
13e is a fluoro, and chloro-substituted compound that shows two peaks on cooling at 142.9 and 126.3 °C, correspond to isotropic to smectic phase and smectic to crystal phase. In summary, the substitution of F atom to the aromatic core in compound
13d and chloro-substituted of compound
13b decreases the mesophase-isotropic transition temperature compared to compound
13a. Compound
13e possessing both F and Cl atoms have the lowest transition temperature compared to the other compounds.
When cooling from the isotropic liquid, compounds
13d and
13e exhibit a fan-like texture which is typical for a smectic phase. Both compounds form a smectic phase at a lower temperature due to the addition of F atoms in compound
13d and F and Cl atoms in compound
13e. Compound
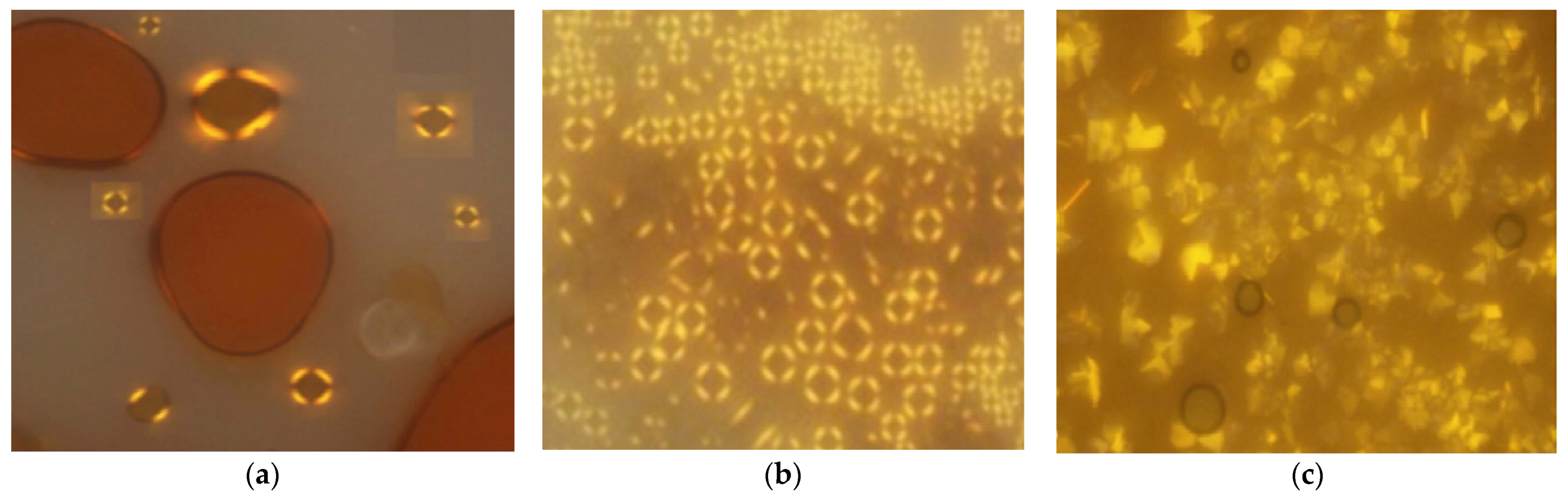
13(a, b, d, and e) were found to be monotropic in nature (
Figure 4), and only compound
13a was studied to be thermodynamically more stable compared to the other compound. However, it was expected that compound
13a has the highest mesomorphic stability as it contains no lateral substitution that may disturb the aromatic core packing.
![Polymers 13 03462 i013]() |
| Comp | 13a | 13b | 13c | 13d | 13e | 13f |
| R | H | H | H | F | F | F |
| R’ | H | Cl | COOH | H | Cl | COOH |
There are several possible reasons for the absence of a smectic mesophase. Firstly, the dipole of the substituent is partly canceled by the dipole of the ester group. Next, no other dipole across the long axis of molecules. Lastly, the strength of intermolecular lateral attractions is reduced as a result of a long narrow to be pushed further apart due to an increase in molecular breadth [
29]. Compounds
14(a–b) consists of CH
3 and F substituted compounds, respectively. Compound
14a is prone to show a nematic phase while compound
14b tend to exhibit both nematic and smectic phase. Briefly summarize, the ratio of lateral to the terminal attraction of compound
14b is higher than compound
14a. This is because the F atom does not increase the molecule width due to the small atomic size compared to the adjacent substituent. Although the C–F bond has a high dipole moment, the bond itself cannot be entirely canceled by the dipole moment because of the ester group that presents in the compound. The dipole moment of the long axis will approach the dipole moment of the lateral axis, causing the attractive terminal forces is almost the same as the attractive lateral forces. Hence, two phases were formed at low temperatures.
![Polymers 13 03462 i014]() |
| Comp | 14a | 14b |
| R | CH3 | F |
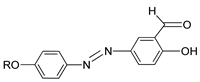
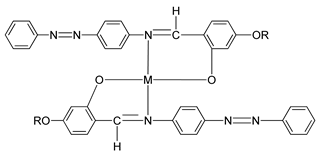
In 2011, two ligands containing Cu and Ni atoms were synthesized by Yeap et al. (2011), namely compounds
15(a–e) and
16(a–e) [
30]. All compound
15 first exhibits a schlieren textured nematic phase followed by a focal conic fan shape textured typically for smectic A. However, all compound
16 does not exhibit a mesomorphic behavior due to direct isotropization during the heating and cooling phase without exhibiting any liquid crystal phase. The layer spacing of compound
15(a–e) is 1.04 Å. This layer spacing corresponds to the molecular length, and the layer thickness of compound
15 is slightly lower than its molecular length. This explains the presence of interdigitation of the alkoxy chain and its neighboring layer.
![Polymers 13 03462 i015]() |
| Comp | 15a | 15b | 15c | 15d | 15e |
| M | Cu | Cu | Cu | Cu | Cu |
| R | C8H17 | C10H21 | C12H25 | C14H29 | C16H33 |
| Comp | 16a | 16b | 16c | 16d | 16e |
| M | Ni | Ni | Ni | Ni | Ni |
| R | C8H17 | C10H21 | C12H25 | C14H29 | C16H33 |
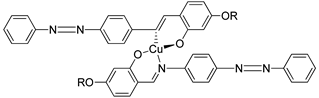
Yeap et al. (2011) also focused on the study of a structure–property relationship of compound
17, which was synthesized by Reddy et al. (1991) [
30,
31]. Compound
17 exhibit a smectic A phase and is thermodynamically enantiotropic up to the clearing temperature of 33.0–261.0 °C. Such a thing happens due to the introduction of azobenzene cored that may induce mesophase formation in compound
17 and lower the temperature of complexes.
![Polymers 13 03462 i016]() |
| Comp | 17 |
| R | C5H11 |
A year later, compounds
18(a–d) were synthesized by Yang et al. (2012) [
32]. In this study, all compounds
18 exhibit mesomorphic phases. This is due to the six or eleven segments (
n = 6 or 11) present in the compound that acts as a spacer. In his study, Yang et al. (2012) argued that in order for a compound to exhibit a mesomorphic phase, a liquid crystal must contain a suitable spacer length between the center and the terminal chain [
32]. This flexible spacer is also crucial as it affects the dipole and molecular interaction between the compound, which will play an important role in mesophase formation. During the heating scan, compound
18a melted, and a cholesteric liquid crystal was exhibited between 138.7 and 189.1 °C. On the other hand, compound
18c was observed to show an enantiotropic mesophase at between 160.0–185.4 °C and 135.6–183.1 °C, upon heating and cooling scan, respectively. Compound
18a displayed a broader phase transition temperature range compared to
18c, which shows that the electron-withdrawing nitro moiety intensifies the head-to-tail molecular interactions. However, in compound
18b, the longer methylene spacer decreases the head-to-tail interactions, resulting in a phase transition temperature range. In contrast, compounds
18c and
18d that contain a methoxy terminal chain exhibit different behavior. Compound
18d has a broader phase transition temperature change compared to compound
18c. Based on this observation, the rigidity of the mesomorphic core, length of the flexible spacer, and the type of terminal chain play an important role in the dipole–dipole interaction that leads to the variability of the phase transition temperature. Hence, it will later influence the formation of different types of liquid crystal properties and textures.
![Polymers 13 03462 i017]() |
| Comp | 18a | 18b | 18c | 18d |
| n | 6 | 11 | 6 | 11 |
| R | NO2 | NO2 | OCH3 | OCH3 |
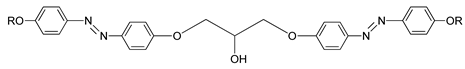
Two azo compounds bearing two different moieties used at the end terminal were synthesized: compounds
19(a–i) and
20(a–i). Lower homologues of both compounds only exhibit a nematic phase, while compounds with higher homologues, which are compounds
19h,
19i,
20f,
20g,
20h, and
20i, possess a longer terminal chain exhibit an additional phase transition directing to a layered mesophase. During the cooling scan, all homologues of both compounds are enantiotropic in nature and exhibit a schlieren and marbled texture typically for a nematic mesophase. As observed, the nematic phase range decreases with an increasing chain length in both compounds [
33].
![Polymers 13 03462 i018]() |
| Comp | 19a | 19b | 19c | 19d | 19e | 19f | 19g | 19h | 19i |
| n | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| X | CN | CN | CN | CN | CN | CN | CN | CN | CN |
| Comp | 20a | 20b | 20c | 20d | 20e | 20f | 20g | 20h | 20i |
| n | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| X | NO2 | NO2 | NO2 | NO2 | NO2 | NO2 | NO2 | NO2 | NO2 |
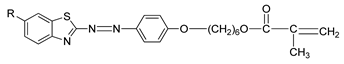
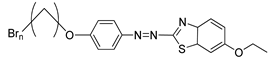
Compounds
21(a–d) have a similar molecular structure except for the R group that was substituted on the benzothiazole ring. Hence, different types of mesophase were displayed by each compound during the heating and cooling cycles. Generally, the terminal methacrylate unit and the substituent on the benzothiazole ring are crucial to forming a liquid crystal. Compound
21a shows only a smectic mesophase, while compounds
21b,
21c, and
21d display both nematic and smectic phases. The terminal methacrylate unit is responsible for forming the smectic phase as this unit elevates the polarizability anisotropy, which is ideal for the lateral attraction of molecules to form a strong smectic phase. As mentioned earlier, compounds
21b,
21c, and
21d exhibited both smectic and nematic liquid crystal phases. Nematic liquid crystal is favored as the replacement of hydrogen atom on compound
21a by a methyl group on compound
21b, methoxy group on compound
21c, and ethoxy group on compound
21d, to the sixth position of the benzothiazole ring was facilitated by conjugation of the high core polarizability with the short terminal chain [
34]. The polarization of benzothiazole moiety is directly affected by the electron distribution in the electron-donating substituent. The substituent size also influences the mesophase temperature transition in the sixth position of the benzothiazole ring. The ethoxy substituent has the greatest mesophase stability compared to the methoxy or methyl substituent. To conclude, the terminal methacrylate unit and the sixth position substitution play a vital role in confirming the liquid crystal phase formation.
![Polymers 13 03462 i019]() |
| Comp | 21a | 21b | 21c | 21d |
| R | H | CH3 | OCH3 | OC2H5 |
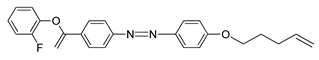
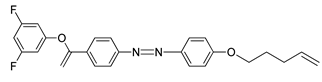
Compounds
22 and
23 are azobenzene chromophores with a fluoro substituent. Both compounds are similar compared to the number of fluorine atom each compound possess, compound
22 contain only one fluorine atom while compound
23 contain two fluorine atoms. Compound
22 shows a nematic to isotropic transition at 158.9 °C, whereas compound
23 exhibit a smectic A to isotropic transition at exactly 157.6 °C. The number of fluorine substituent present in the compound influence the transition temperature by increasing the number of fluorine atom, the transition temperature will decrease [
35].
![Polymers 13 03462 i020]() |
| Compound 22 |
![Polymers 13 03462 i021]() |
| Compound 23 |
All compounds
24(a–f) exhibited a schlieren texture in an enantiotropic liquid crystal phase which is typical for a nematic phase. The thermal transition of the nematic phase of methoxy homologues (
24d,
24e, and
24f) is higher than the methyl homologues compounds (
24a,
24b, and
24c). The presence of an oxygen atom on the methoxy group is vital domination in the mesophase thermal stability and the thermal phase range. Upon heating, the enthalpy values of the crystal to the nematic phase transition of compounds
24a,
24b, and
24c are 38.8, 46.6, and 50.3 °C, respectively. As for compounds
24d,
24e, and
24f are 99.0, 77.8, and 72.4 °C, respectively. These large enthalpy values suggest a strong Van der Waals interaction between the end unit of an adjacent molecule. However, upon cooling, the enthalpy values recorded for the crystal to the nematic phase transition are 26.2, 27.1, 25.8, 46.6, 43.7, and 47.0 °C for each compound, respectively. These lower enthalpy values upon cooling are lower compared to the enthalpy values upon heating. This is due to the fact that the strong Van der Waals interactions between the end of the chain of a neighboring molecule keep them static in the crystalline state before the gradation to liquid crystal starts in the heating cycle [
36].
![Polymers 13 03462 i022]() |
| Comp | 24a | 24b | 24c | 24d | 24e | 24f |
| n | 4 | 5 | 6 | 4 | 5 | 6 |
| R | CH3 | CH3 | CH3 | OCH3 | OCH3 | OCH3 |
Compounds
25(a,b) are novel benzoates that contain three phenyl rings and a trimethylsilyl group in the terminal position. Both compounds possess a similar structural formula having a central azo compound with an eminently polar nitro group and a pentyl chain except the R group at the end of the chain. Despite having a similar structural formula, only one compound exhibits a liquid crystal property, which is compound
25a. The optical texture of compound
25a is illustrated in
Figure 5. The polar nitro terminal favored a smectic A phase, while compound
25b possessing an alkyl chain does not display any liquid crystalline phase property. To conclude, the property of a liquid crystal is reduced as the chain length lengthen as per the disruption of the packing layers. Hence, the only molecule with a short-chain compound can display any liquid crystalline property [
37].
![Polymers 13 03462 i023]() |
| Comp | 25a | 25b |
| R | NO2 | C5H11 |
Compounds
26(a–e) were synthesized by Selvarasu and Kannan, in 2015 [
38]. Generally, the transition temperature of compounds
26a and
26b is larger compared to the transition temperature range of compounds
26d and
26e, as the electron-withdrawing substituent in compounds
26a and
26b have a strong Π to Π* interaction that increase the head to tail molecular structure. The nematic to the isotropic thermal stability of compounds
26a and
26b are higher compared to compounds
26d and
26e. The reason is that the –CN and –Cl groups as well as the benzene ring located in the terminal position will cause both compounds to have high polarity and thermal attraction that later will causes the thermal stability to increase. The only difference that compounds
26a and
26e show is on the substitution unit at the terminal end. Both of these compounds share the same central unit, a cinnamoyloxy group (–C
6H
5–CH=CH–COO–) which is directly connected to the alkyloxyphenylester group. The presence of the double bond in the cinnamoyloxy group lengthen the length of polarizability and elevate the thermal stability.
![Polymers 13 03462 i024]() |
| Comp | 26a | 26b | 26c | 26d | 26e |
| R | CN | Cl | H | CH3 | OCH3 |
A year later, Selvarasu and Kannan (2016) synthesized a new azobenzene compound, compound
27 [
39]. Compounds
27a,
27b, and
27c exhibit a nematic phase with the lowest homologues of n: 6 forming small droplets that affiliate into a classic schlieren nematic and compound of homologues n: 8 and 10 display a focal-conic textured nematic phase while the compound with the highest homologues exhibits a nematic phase and an addition of a focal conic textured smectic C phase upon cooling from the isotropic liquid. The formation of a nematic phase is a result of the alkoxy chain terminal being more noticeable. Compound
27 contains a cinnamoyloxy group connected directly to the azo compound and the ester group that acts as the central unit of the molecule, and a double bond that increase the polarizability. The core of compound
27 is directly bonded to an ester group, causing the molecule to lose stability. This phenomenon is due to the ability of the oxygen atom of the carbonyl group to bump into the non-bonded sides of the adjacent hydrogen in the aromatic ring, which attributed to the strain in the molecules. An ester linkage contains a ketonic bond, where the electron is being pulled by the ester group which causes the transition temperature to decline. To sum everything up, the attributes responsible for mesophase formation are the effect of terminal alkoxy containing azobenzene moiety and the central linkage with or without the spacer. The presence of an olefinic unit reinforces the length of polarizability, and an additional olefinic unit would proliferate the thermal stability with a high polarizability.
![Polymers 13 03462 i025]() |
| Comp | 27a | 27b | 27c | 27d |
| R | C6H13 | C8H17 | C10H21 | C12H25 |
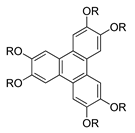
Compounds
28(a–f) are azo-bridged compounds with triphenylene cores. The only difference between the compounds
28(
a–
f) is the length of the methylene units. The length of the spacer has a vital role in the fluctuation of the transition temperature. Upon cooling, the melting point of compound
28a is higher compared to compound
28b. However, it gradually elevates from compounds
28b to
28e. According to Yeap et al. (2016), it has something to do with the spacer length [
40]. As the length of spacer increase, the melting point continue to decrease from compounds
28e to
28f. Nonetheless, the clearing temperature of compound increase from compounds
28b to
28c and keep on descending as the length of alkyl chain increase. By reason, it may be due to the dilution of the mesogenic core, which causes the spacer to be flexible.
Compound
28f exhibits a smectic C phase, where it has been claimed that the formation of a smectic phase results from a side-by-side organization of azobenzene base peripheral units that are adjacent in parallel to each other. Many flexible spacers link all homologues of compound
28. This flexible spacer enables a conformational change so that a rod-shaped unit could align in a parallel arrangement resulting in a layered structure of a smectic phase [
40].
![Polymers 13 03462 i026]() |
| Comp | 28a | 28b | 28c | 28d | 28e | 28f |
| n | 5 | 6 | 7 | 8 | 9 | 10 |
| R | ![Polymers 13 03462 i027]() |
Compounds
29(a–c) were synthesized by Sen et al. (2016) [
41]. All of compounds
29(a–c) exhibit only one mesophase transition (
Figure 6). Uncommonly for a rod-shaped molecule, the enthalpy values for the liquid crystalline phase of the isotropic liquid are higher than the enthalpy values of a solid–liquid crystalline phase. This unusual phenomenon may be due to the molecule’s arrangement within each layer, which the minimized steric forces have driven.
![Polymers 13 03462 i028]() |
| Comp | 29a | 29b | 29c |
| n | 10 | 12 | 18 |
| R | H | H | H |
Jaworska et al. (2017) synthesized compounds
30(a–n) [
42]. Out of 14 compounds with different homologues, he showed that two of them do not exhibit any liquid crystalline property. Compounds
30a and
30b do not possess any liquid crystal phase; by reason both compounds have high melting points compared to other homologues. Other compounds studied exhibit at least one enantiotropic mesophase, which was identified as a focal-conic textured smectic A. Compound
30c is the first homologue displaying smectic A and the width is 30.0 °C, as it can be seen in
Figure 7. Starting from compound
30d until compound
30k, the width of the smectic A phase is broader, ranging from 40.0 to 75.0 °C. The smectic range keeps narrowing corresponding to increasing the alkoxy chain to n: 14 and above.
![Polymers 13 03462 i029]() |
| Comp | 30a | 30b | 30c | 30d | 30e | 30f | 30g |
| n | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Comp | 30h | 30i | 30j | 30k | 30l | 30m | 30n |
| n | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
All compounds
31(a–e) possess at least one liquid crystal phase except compound
31e, where it exhibits an extra liquid crystalline phase familiar as a smectic phase. This is a result of the length of the alkoxy chain in compound
31e is long enough to stabilize the lamellar molecular arrangement ideal for the molecule to display a smectic phase. The variation of the terminal group by the methylene spacer located in 1,4-disubstituted triazole derivatives, the molecular alignment of mesophase is stabilized [
43]. Imine as a central unit causes the polarity to increase through retaining of linear patter. Minor changes in the alkyl chain may cause major changes in the transition temperature and types of mesophase formed.
![Polymers 13 03462 i030]() |
| Comp | 31a | 31b | 31c | 31d | 31e |
| R | C6H13 | C7H15 | C8H17 | C9H19 | C10H21 |
In the same year, two identical compounds,
32 and
33, were synthesized. The only difference between these two compounds is the central unit, where it exchanges place with the adjacent group. The melting point and the clearing point of compounds
32 and
33 are 104.8, 130.2, and 166.5, 162.2 °C, respectively. These temperature changes may be due to the length to diameter ratio, which eventually increases the transition temperature [
44].
![Polymers 13 03462 i031]() |
| Compound 32 |
![Polymers 13 03462 i032]() |
| Compound 33 |
Each homologue of compounds
34(a–c) exhibit a different liquid crystal phase despite having similar compound. The formation of different mesophase within this compound is influenced by the substitution terminal (–R). Compound
33a contains an end non-polar methyl group, and it shows a nematic phase at 31.7 °C upon cooling. Compound
33b comprises a relatively polar chloro moiety, revealing a nematic phase about 3.0 °C above the isotropic liquid and a smectic A phase at 47.0 °C upon cooling. A polar nitro moiety is present in compound
33c, a long-range smectic A phase is observed at 106.3 °C in the cooling scan. The polarity of substituent not only affects the formation of different mesophase, but also caused the clearing temperature to change. The mesophase range increase as the polarity of substituent increase from
33a to
33c [
45].
![Polymers 13 03462 i033]() |
| Comp | 34a | 34b | 34c |
| R | CH3 | Cl | NO2 |
Compounds
35(a–e) were synthesized in 2019 by Madiahlagan et al., and are a series of azo-coumarin compounds with different aliphatic chain lengths [
46]. Lower homologues of compound
35 displayed a nematic phase at 120.0 °C for compound
35b, while compound
35e exhibited a broken fan-shaped smectic A at 170.0 °C upon cooling from the isotropic liquid. The difference in mesomorphic formation and the transition temperature is greatly influenced by the increasing carbon atom of the aliphatic substituent.
![Polymers 13 03462 i034]() |
| Comp | 35a | 35b | 35c | 35d | 35e |
| R | C6H13 | C8H17 | C10H21 | C12H25 | C14H29 |
The shortest terminal chain, compounds 36a and 36e, exhibited three liquid crystal phases which are nematic phase, smectic A phase, and smectic C phase, while compound 36c containing a butyl terminal chain exhibit a twist grain boundary smectic A (TGBA) rather than a smectic A itself and a smectic C phase. However, the broadest smectic S phase was observed to be of compound 36b. The TGBA phase does not present in 36b, and 36d is due to the odd-even effect. Compound 36i with the longest aliphatic group displayed a smectic A to smectic C mesophase transition with the temperature narrowed. It was affected by the elongation of the non-chiral alkyl chain, which eventually decreases the mesophase range.
As for compounds
37(a–i), the TGBA phase appeared in compound
37a and disappeared from compounds
37b to
37d and re-appear in compound
37e implying that a change has happened for compounds with the middle length alkyl chain. Further studies were done to identify the cause, however, further increase of the carboxylate group leads to a reduction of mesophase property [
47].
![Polymers 13 03462 i035]() |
| Comp | 36a | 36b | 36c | 36d | 36e | 36f | 36g | 36h | 36i |
| R | C2H5 | C3H7 | C4H9 | C5H11 | C6H13 | C7H15 | C8H17 | C10H21 | C12H25 |
![Polymers 13 03462 i036]() |
| Comp | 37a | 37b | 37c | 37d | 37e | 37f | 37g | 37h | 37i |
| R | C2H5 | C3H7 | C4H9 | C5H11 | C6H13 | C7H15 | C8H17 | C10H21 | C12H25 |
In the following year, compounds
38(a–e) and
39(a–e) were synthesized, where all compounds studied exhibit a liquid crystalline property. Within compound
38 and all higher homologues were observed to be enantiotropic except compounds
38a, and
38b, while compounds
39a and
39d are enantiotropic in nature. Compounds
38b and
38c display an enantiotropic smectic B1 phase, while compound
38d exhibits an enantiotropic smectic B1 phase and an addition of a monotropic smectic B2 phase. Theoretically, a change in the alkyl chain will cause the mesophase property to be varied, and the formation of smectic B proves the point [
48].
![Polymers 13 03462 i037]() |
| Comp | 38a | 38b | 38c | 38d | 38e |
| R | C4H9 | C6H13 | C8H17 | C10H21 | C12H25 |
| Comp | 39a | 39b | 39c | 39d | 39e |
| R | OC4H9 | OC6H13 | OC8H17 | OC10H21 | OC12H25 |
Sardon et al. (2021) synthesized compounds
40(a–d) and compared them with a compound studied back in 2016 by Karim et al., labeled as compounds
41(b–c) [
49,
50]. The result of this observation is illustrated in
Table 1.
Based on
Table 1, it can be observed that the mesophase stability is inconsistent. This inconsistency is caused by the substituent (–H, –Cl, –Br, –CN). However, the nematic mesophase range of compounds
40(b–d) are wider. The substitution of hydrogen atom on the para-position of benzene ring affects the polarizability of mesogens that aid the formation of the liquid crystal phase. Compound
40d presented the highest nematic to isotropic clearing transition temperature because of higher polarization effects caused by the cyano substitution that gives high temperature to break the association within the molecule.
Compounds
40b and
40c only reveal the formation of a nematic phase, while compounds
41b and
41c displayed both nematic and smectic A phases. The methyl side chain substituent in compounds
40b and
40c truncate the melting and clearing temperature compared to compounds
41b and
41c that possess no methyl side chain. Generally, any lateral group attached to the core unit of a compound may cause some changes in the mesomorphic behavior. The presence of methyl side-chain group widens the core unit where the intermolecular separation is eventually increased and leads to lower mesophase stability.
![Polymers 13 03462 i038]() |
| Comp | 40a | 40b | 40c | 40d |
| R | H | Cl | Br | CN |
![Polymers 13 03462 i039]() |
| Comp | 41a | 41b |
| R | Cl | Br |