Comparison of Degradation Behavior of Newly Developed Encapsulation Materials for Photovoltaic Applications under Different Artificial Ageing Tests
Abstract
1. Introduction
2. Materials and Methods
2.1. Thermal Desorption Gas Chromatography Coupled to Mass Spectrometry (TD-GC/MS)
- held for two minutes at 50 °C,
- from 50 C to 290 °C, held at 290 °C for six minutes, heating rate of 10 C min−1,
- ion source temperature and interface temperature were set at 300 °C,
- splitless mode.
2.2. UV-Visible-Near Infrared Spectroscopy (UV-Vis-NIR)
2.3. Fourier Transform Infrared Spectroscopy (FT-IR)
2.4. Differential Scanning Calorimetry (DSC)
2.5. Thermogravimetric Analysis (TGA)
3. Results and Discussion
3.1. Thermal Desorption Gas Chromatography Coupled to Mass Spectrometry (TD-GC/MS)
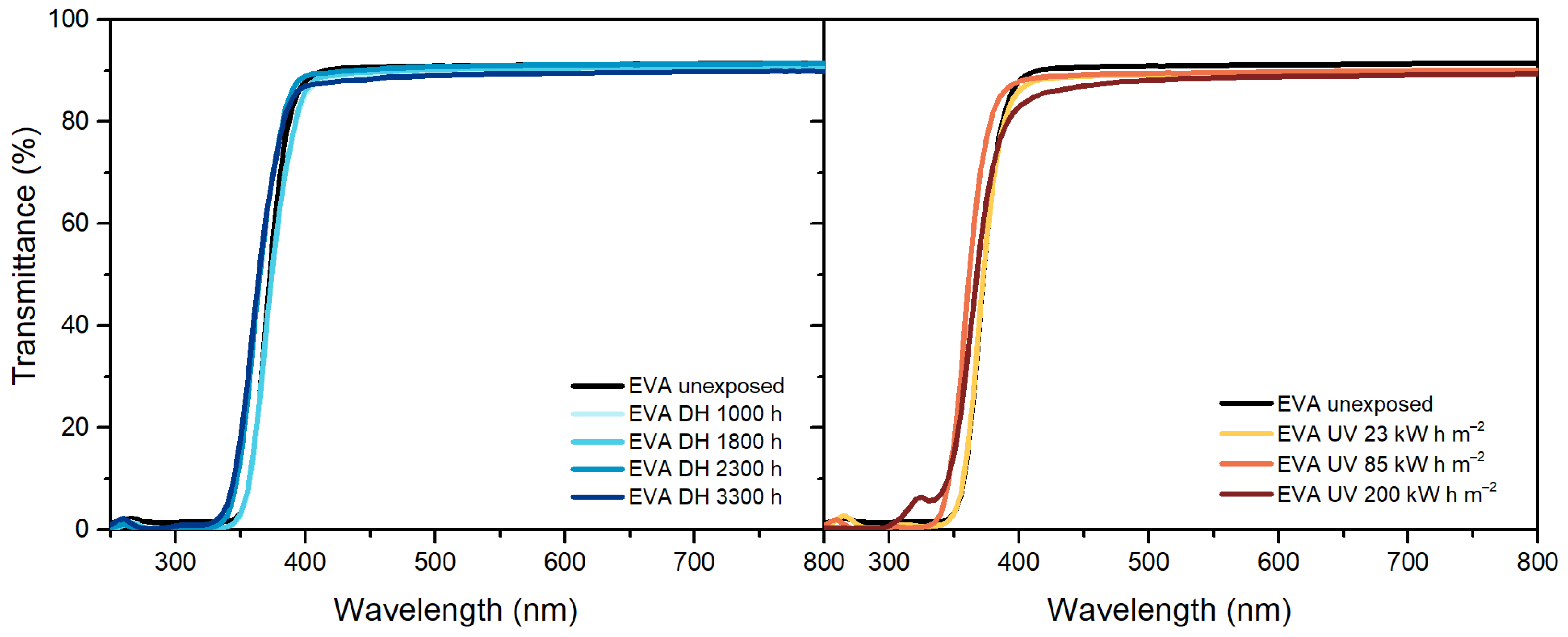
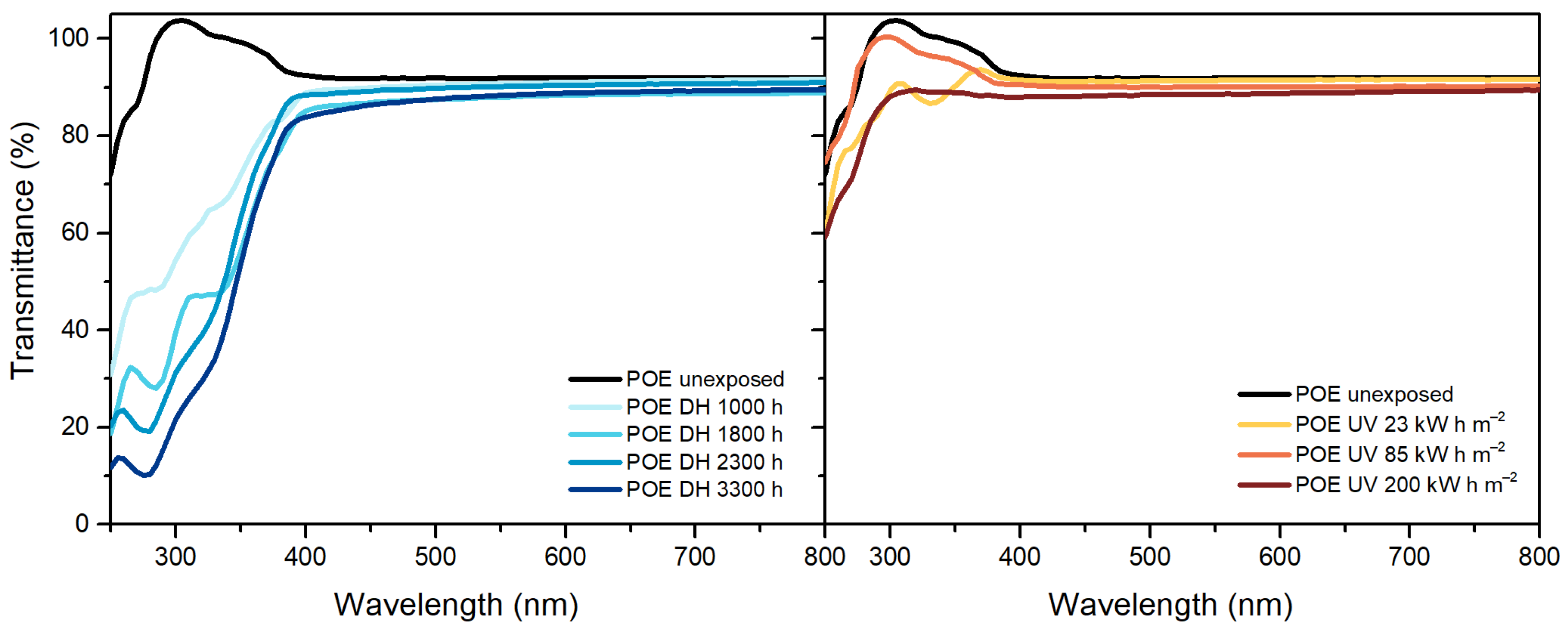
3.2. UV-Visible-Near Infrared Spectroscopy (UV-Vis-NIR)
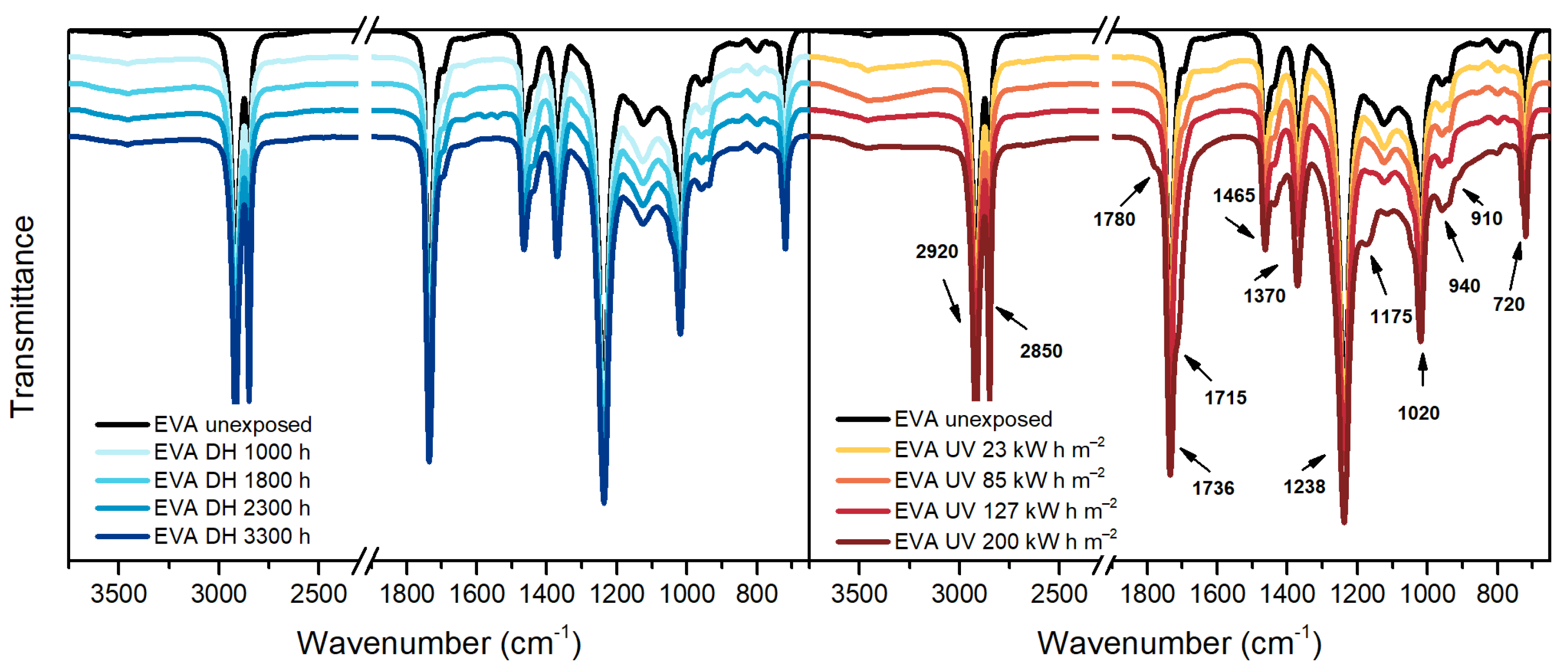
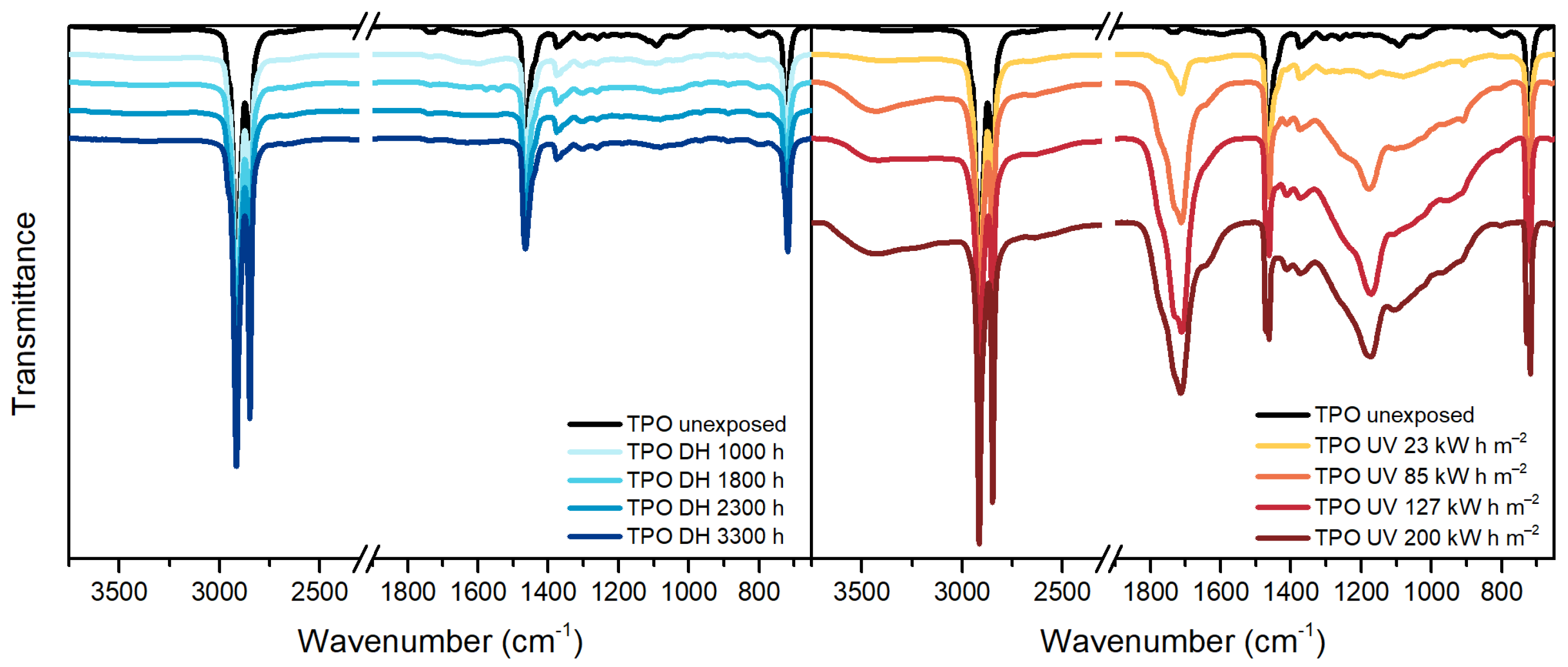
3.3. Fourier Transform Infrared Spectroscopy (FT-IR)
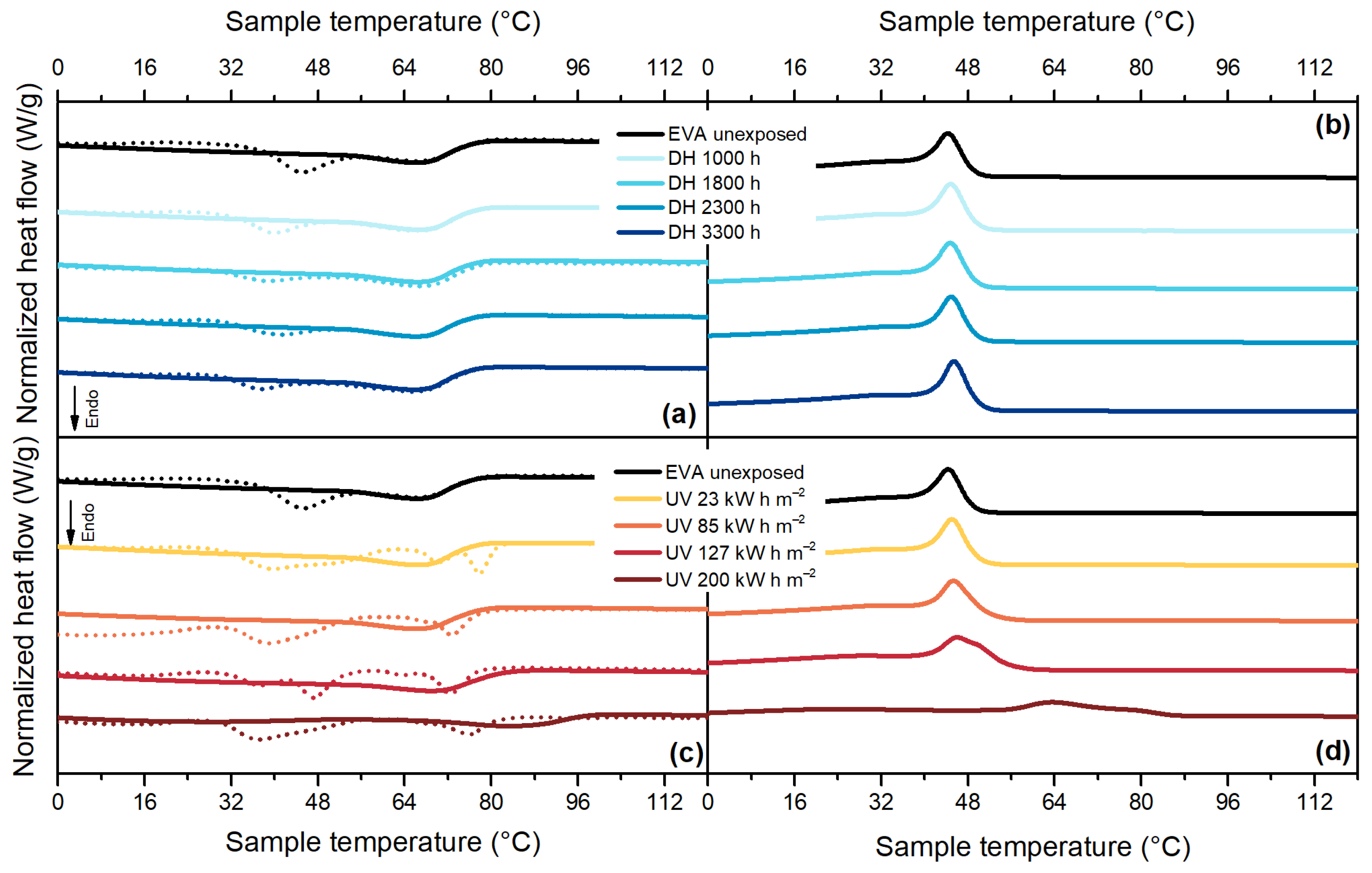
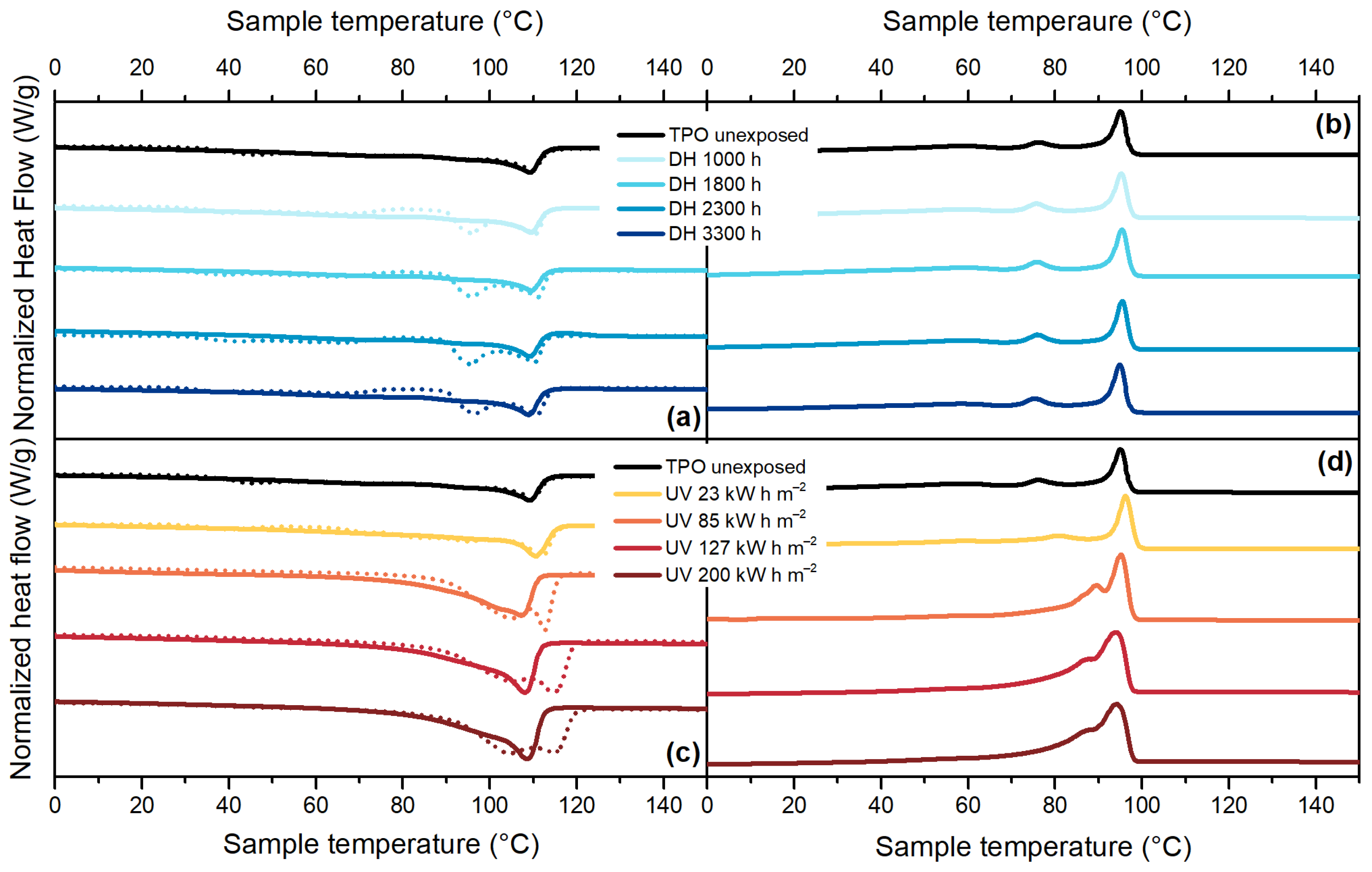
3.4. Differential Scanning Calorimetry (DSC)
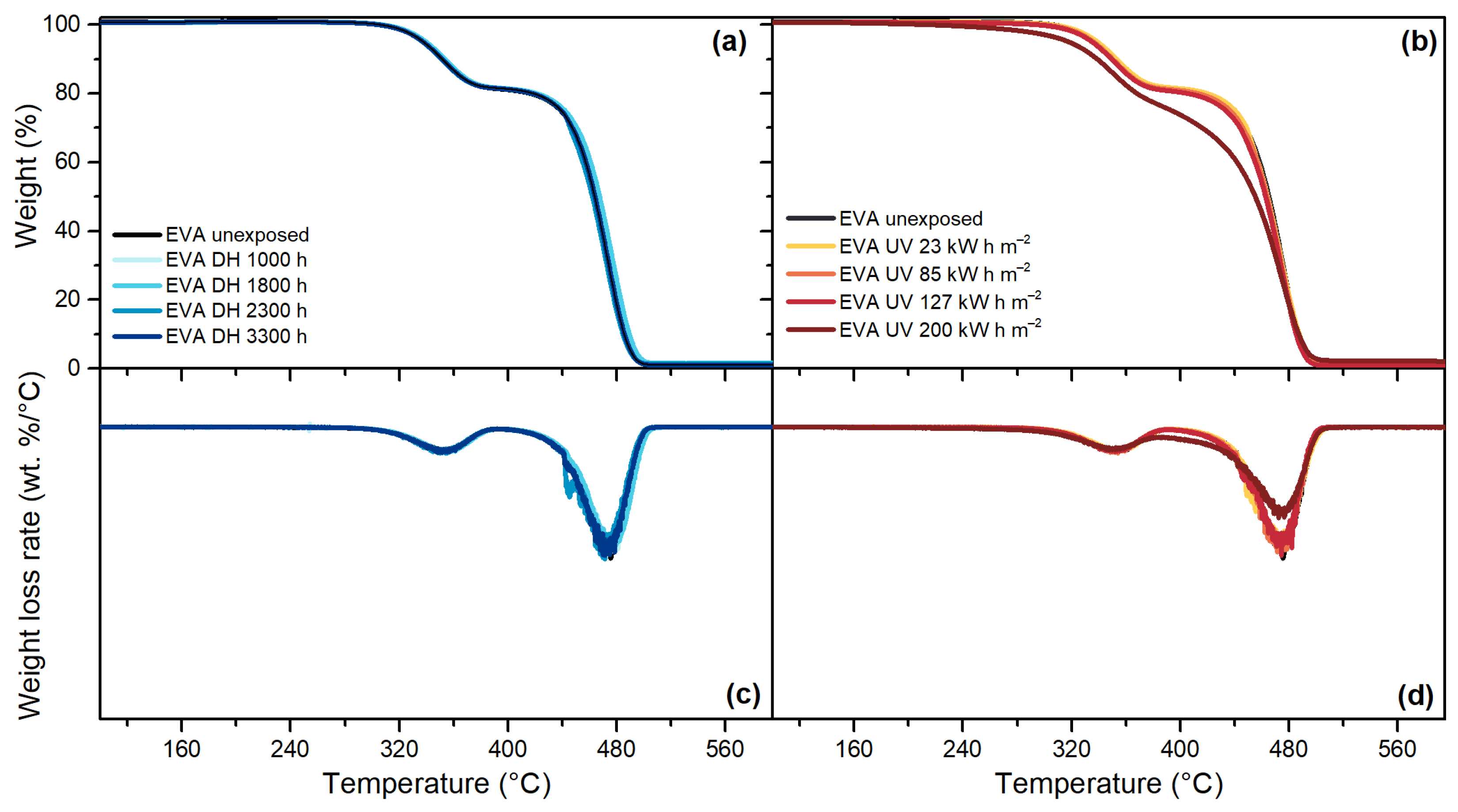
3.5. Thermogravimetric Analysis (TGA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Czanderna, A.W.; Pern, F.J. Encapsulation of PV modules using ethylene vinyl acetate copolymer as a pottant: A critical review. Sol. Energy Mater. Solar Cells 1996, 43, 101–181. [Google Scholar] [CrossRef]
- Ndiaye, A.; Charki, A.; Kobi, A.; Kébé, C.M.; Ndiaye, P.A.; Sambou, V. Degradations of silicon photovoltaic modules: A literature review. Sol. Energy 2013, 96, 140–151. [Google Scholar] [CrossRef]
- Kempe, M. Encapsulant Materials for PV Modules. In Photovoltaic Solar Energy: From Fundamentals to Applications; Reinders, A., Verlinden, P., van Sark, W., Freundlich, A., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 478–490. [Google Scholar] [CrossRef]
- Omazic, A.; Oreski, G.; Halwachs, M.; Eder, G.C.; Hirschl, C.; Neumaier, L.; Pinter, G.; Erceg, M. Relation between degradation of polymeric components in crystalline silicon PV module and climatic conditions: A literature review. Sol. Energy Mater. Solar Cells 2019, 192, 123–133. [Google Scholar] [CrossRef]
- Badiee, A.; Ashcroft, I.A.; Wildman, R.D. The thermo-mechanical degradation of ethylene vinyl acetate used as a solar panel adhesive and encapsulant. Int. J. Adhes Adhes 2016, 68, 212–218. [Google Scholar] [CrossRef]
- Pern, F.J. Factors that affect the EVA encapsulant discoloration rate upon accelerated exposure. Sol. Energy Mater. Solar Cells 1996, 587–615. [Google Scholar] [CrossRef]
- Pern, F.J.; Czanderna, A.W. Characterization of ethylene vinyl acetate (EVA) encapsulant: Effects of thermal processing and weathering degradation on its discoloration. Sol. Energy Mater. Solar Cells 1992, 25, 3–23. [Google Scholar] [CrossRef]
- Oreski, G.; Ottersböck, B.; Omazic, A. 6-Degradation Processes and Mechanisms of Encapsulants. In Durability and Reliability of Polymers and Other Materials in Photovoltaic Modules; Yang, H.E., French, R.H., Bruckman, L.S., Eds.; William Andrew Publishing: New York, NY, USA, 2019; pp. 135–152. ISBN 978-0-12-811545-9. [Google Scholar]
- Weber, U.; Eiden, R.; Strubel, C.; Soegding, T.; Heiss, M.; Zachmann, P.; Nattermann, K.; Engelmann, H.; Dethlefsen, A.; Lenck, N. Acetic Acid Production, Migration and Corrosion Effects in Ethylene-Vinyl-Acetate- (EVA-) Based PV Modules. Presented at 27th European Photovoltaic Solar Energy Conference and Exhibition, Frankfurt, Germany, 24–28 September 2012; pp. 2992–2995. [Google Scholar]
- Matsuda, K.; Watanabe, T.; Sakaguchi, K.; Yoshikawa, M.; Doi, T.; Masuda, A. Microscopic Degradation Mechanisms in Silicon Photovoltaic Module under Long-Term Environmental Exposure. Jpn. J. Appl. Phys. 2012, 51, 10NF07. [Google Scholar] [CrossRef]
- Jonai, S.; Hara, K.; Tsutsui, Y.; Nakahama, H.; Masuda, A. Relationship between cross-linking conditions of ethylene vinyl acetate and potential induced degradation for crystalline silicon photovoltaic modules. Jpn. J. Appl. Phys. 2015, 54, 08KG01. [Google Scholar] [CrossRef]
- Peike, C.; Purschke, L.; Weiß, K.-A.; Köhl, M.; Kempe, M. Towards the origin of photochemical EVA discoloration. Presented at 39th Photovoltaic Specialists Conference IEEE, Tampa, FL, USA, 16–21 June 2013. [Google Scholar]
- Chang, J.; Yang, H.; Wang, H.; Cao, D. The investigation of snail trails in photovoltaic modules. Presented at IEEE 42nd Photovoltaic Specialist Conference, New Orleans, LA, USA, 12–19 June 2015. [Google Scholar]
- Jentsch, A.; Eichhorn, K.-J.; Voit, B. Influence of typical stabilizers on the aging behavior of EVA foils for photovoltaic applications during artificial UV-weathering. Polym. Test. 2015, 44, 242–247. [Google Scholar] [CrossRef]
- López-Escalante, M.; Caballero, L.J.; Martín, F.; Gabás, M.; Cuevas, A.; Ramos-Barrado, J. Polyolefin as PID-resistant encapsulant material in 5PV6 modules. Sol. Energy Mater. Sol. Cells 2016, 144, 691–699. [Google Scholar] [CrossRef]
- Adothu, B.; Bhatt, P.; Chattopadhyay, S.; Zele, S.; Oderkerk, J.; Sagar, H.P.; Costa, F.R.; Mallick, S. Newly developed thermoplastic polyolefin encapsulant–A potential candidate for crystalline silicon photovoltaic modules encapsulation. Sol. Energy 2019, 194, 581–588. [Google Scholar] [CrossRef]
- Adothu, B.; Bhatt, P.; Zele, S.; Oderkerk, J.; Costa, F.R.; Mallick, S. Investigation of newly developed thermoplastic polyolefin encapsulant principle properties for the c-Si PV module application. J. Mater. Chem. Phys. 2020, 243, 122660. [Google Scholar] [CrossRef]
- Ottersböck, B.; Oreski, G.; Pinter, G. Comparison of different microclimate effects on the aging behavior of encapsulation materials used in photovoltaic modules. Polym. Degrad. Stab. 2017, 182–191. [Google Scholar] [CrossRef]
- Oreski, G.; Omazic, A.; Eder, G.; Voronko, Y.; Neumaier, L.; Mühleisen, W.; Hirschl, C.; Ujvari, G.; Eber, R.; Edler, M. Properties and degradation behaviour of polyolefin encapsulants for PV modules. Prog. Photovolt. 2020, 28, 1277–1288. [Google Scholar] [CrossRef]
- Salvalaggio, M.; Bagatin, R.; Fornaroli, M.; Fanutti, S.; Palmery, S.; Battistel, E. Multi-component analysis of low-density polyethylene oxidative degradation. Polym. Degrad. Stab. 2006, 91, 2775–2785. [Google Scholar] [CrossRef]
- Yang, R.; Christensen, P.A.; Egerton, T.A.; White, J.R. Degradation products formed during UV exposure of polyethylene–ZnO nano-composites. Polym. Degrad. Stab. 2010, 95, 1533–1541. [Google Scholar] [CrossRef]
- Abdelhafidi, A.; Chabira, S.; Yagoubi, W.; Mistretta, M.; Lamantia, F.; Sebaa, M.; Benchatti, A. Sun radiation and temperature impact at different periods of the year on the photooxidation of polyethylene films. IJHT 2017, 35, 255–261. [Google Scholar] [CrossRef]
- Eder, G.C.; Voronko, Y.; Oreski, G.; Mühleisen, W.; Knausz, M.; Omazic, A.; Rainer, A.; Hirschl, C.; Sonnleitner, H. Error analysis of aged modules with cracked polyamide backsheets. Sol. Energy Mater. Solar Cells 2019, 203, 110194. [Google Scholar] [CrossRef]
- Annigoni, E.; Virtuani, A.; Caccivio, M.; Friesen, G.; Chianese, D.; Ballif, C. 35 years of photovoltaics: Analysis of the TISO-10-kW solar plant, lessons learnt in safety and performance—Part 2. Prog. Photovolt. 2019, 27, 760–778. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Liauw, C.M.; Allen, N.S.; Edge, M.; Fontan, E. Degradation and stabilization of poly(ethylene-stat-vinyl acetate): 1—Spectroscopic and rheological examination of thermal and thermo-oxidative degradation mechanisms. Polym. Degrad. Stab. 2006, 91, 154–164. [Google Scholar] [CrossRef]
- Yagoubi, W.; Abdelhafidi, A.; Sebaa, M.; Chabira, S.F. Identification of carbonyl species of weathered LDPE films by curve fitting and derivative analysis of IR spectra. Polym. Test. 2015, 44, 37–48. [Google Scholar] [CrossRef]
- Ehrenstein, G.W.; Riedel, G.; Trawiel, P. Thermal Analysis of Plastics. Theory and Practice; Carl Hanser Verlag: Munich, Germany, 2004; ISBN 978-1569903629. [Google Scholar]
- Hirschl, C.; Biebl–Rydlo, M.; DeBiasio, M.; Mühleisen, W.; Neumaier, L.; Scherf, W.; Oreski, G.; Eder, G.; Chernev, B.; Schwab, W.; et al. Determining the degree of crosslinking of ethylene vinyl acetate photovoltaic module encapsulants—A comparative study. Sol. Energy Mater. Solar Cells 2013, 116, 203–218. [Google Scholar] [CrossRef]
- Klemchuk, P.; Ezrin, E.; Lavigne, G.; Holley, W.; Galica, J.; Agro, S. Investigation of the degradation and stabilization of EVA-based encapsulant in field-aged solar energy modules. Polym. Degrad. Stab. 1997, 347–365. [Google Scholar] [CrossRef]
- Hintersteiner, I.; Sternbauer, L.; Beissmann, S.; Buchberger, W.W.; Wallner, G.M. Determination of stabilisers in polymeric materials used as encapsulants in photovoltaic modules. Polym. Test. 2014, 33, 172–178. [Google Scholar] [CrossRef]
- Gugumus, F. Possibilities and limits of synergism with light stabilizers in polyolefins 2. UV absorbers in polyolefins. Polym. Degrad. Stab. 2002, 75, 309–320. [Google Scholar] [CrossRef]
- Nieva-Echevarría, B.; Manzanos, M.J.; Goicoechea, E.; Guillén, M.D. 2,6-Di-Tert-Butyl-Hydroxytoluene and Its Metabolites in Foods. Compr. Rev. Food Sci. Food Saf. 2015, 14, 67–80. [Google Scholar] [CrossRef]
- Miller, D.C.; Bokria, J.G.; Burns, D.M.; Fowler, S.; Gu, X.; Hacke, P.L.; Honeker, C.C.; Kempe, M.D.; Köhl, M.; Phillips, N.H.; et al. Degradation in photovoltaic encapsulant transmittance: Results of the first PVQAT TG5 artificial weathering study. Prog. Photovolt. 2019, 27, 391–409. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies. Tables and Charts, 3rd ed.; John Wiley & Sons, Ltd.: Chichester, West Sussex, UK, 2001. [Google Scholar]
- Allen, N.S.; Edge, M.; Rodriguez, M.; Liauw, C.M.; Fontan, E. Aspects of the thermal oxidation, yellowing and stabilisation of ethylene vinyl acetate copolymer. Polym. Degrad. Stab. 2001, 71, 1–14. [Google Scholar] [CrossRef]
- Gulmine, J.V.; Janissek, P.; Heise, H.; Akcelrud, L. Degradation profile of polyethylene after artificial accelerated weathering. Polym. Degrad. Stab. 2003, 79, 385–397. [Google Scholar] [CrossRef]
- Schlothauer, J.C.; Grabmayer, K.; Hintersteiner, I.; Wallner, G.M.; Röder, B. Non-destructive 2D-luminescence detection of EVA in aged PV modules: Correlation to calorimetric properties, additive distribution and a clue to aging parameters. Sol. Energy Mater. Sol. Cells 2017, 159, 307–317. [Google Scholar] [CrossRef]
- Ehrenstein, G.W.; Pongratz, S. Resistance and Stability of Polymers; Hanser Publishers: Munich, Germany, 2013. [Google Scholar]
- Sharma, B.K.; Desai, U.; Singh, A.; Singh, A. Effect of vinyl acetate content on the photovoltaic-encapsulation performance of ethylene vinyl acetate under accelerated ultra-violet aging. J. Appl. Polym. Sci. 2020, 137, 48268. [Google Scholar] [CrossRef]
- Brogly, M.; Nardin, M.; Schultz, J. Effect of Vinylacetate Content on Crystallinity and Second-Order Transitions in Ethylene–Vinylacetate Copolymers. J. Appl. Polym. Sci. 1997, 64, 1903–1912. [Google Scholar] [CrossRef]
- White, J.R. Polymer ageing: Physics, chemistry or engineering? Time to reflect. Comptes Rendus Chim. 2006, 9, 1396–1408. [Google Scholar] [CrossRef]
- Fairbrother, A.; Hsueh, H.-C.; Kim, J.H.; Jacobs, D.; Perry, L.; Goodwin, D.; White, C.; Watson, S.; Sung, L.-P. Temperature and light intensity effects on photodegradation of high-density polyethylene. Polym. Degrad. Stab. 2019, 165, 153–160. [Google Scholar] [CrossRef]
- Fayolle, B.; Colin, X.; Audouin, L.; Verdu, J. Mechanism of degradation induced embrittlement in polyethylene. Polym. Degrad. Stab. 2007, 92, 231–238. [Google Scholar] [CrossRef]





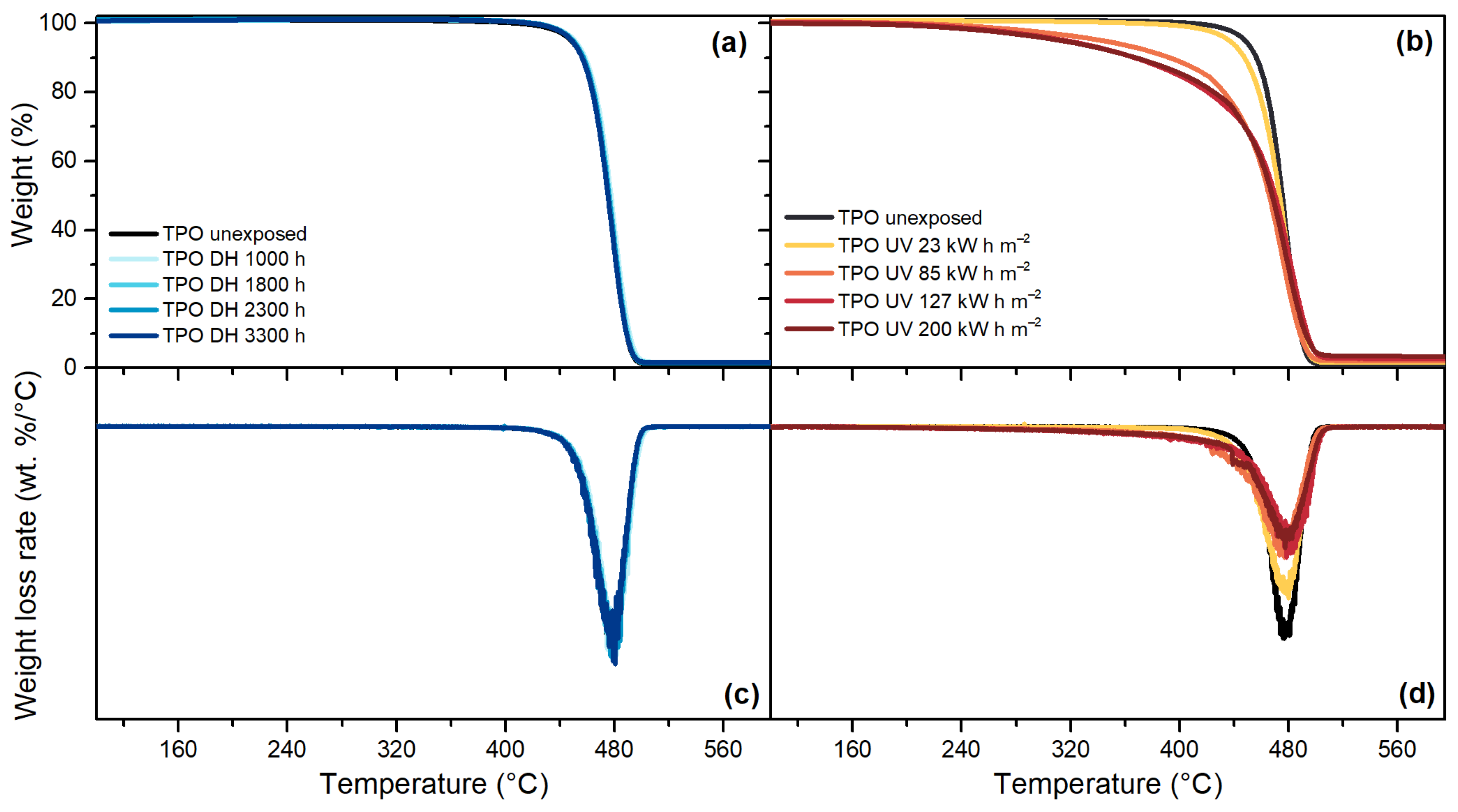

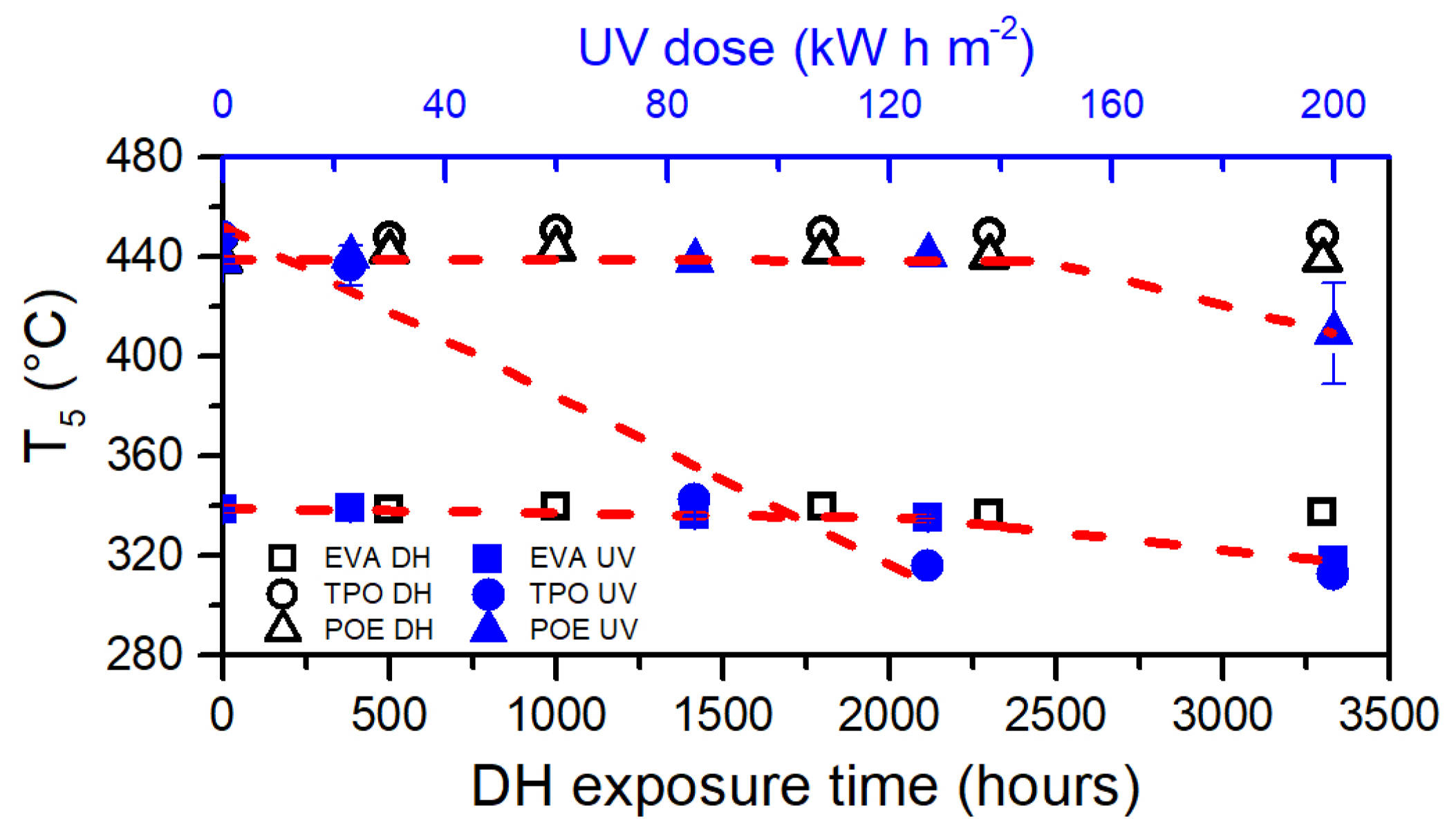
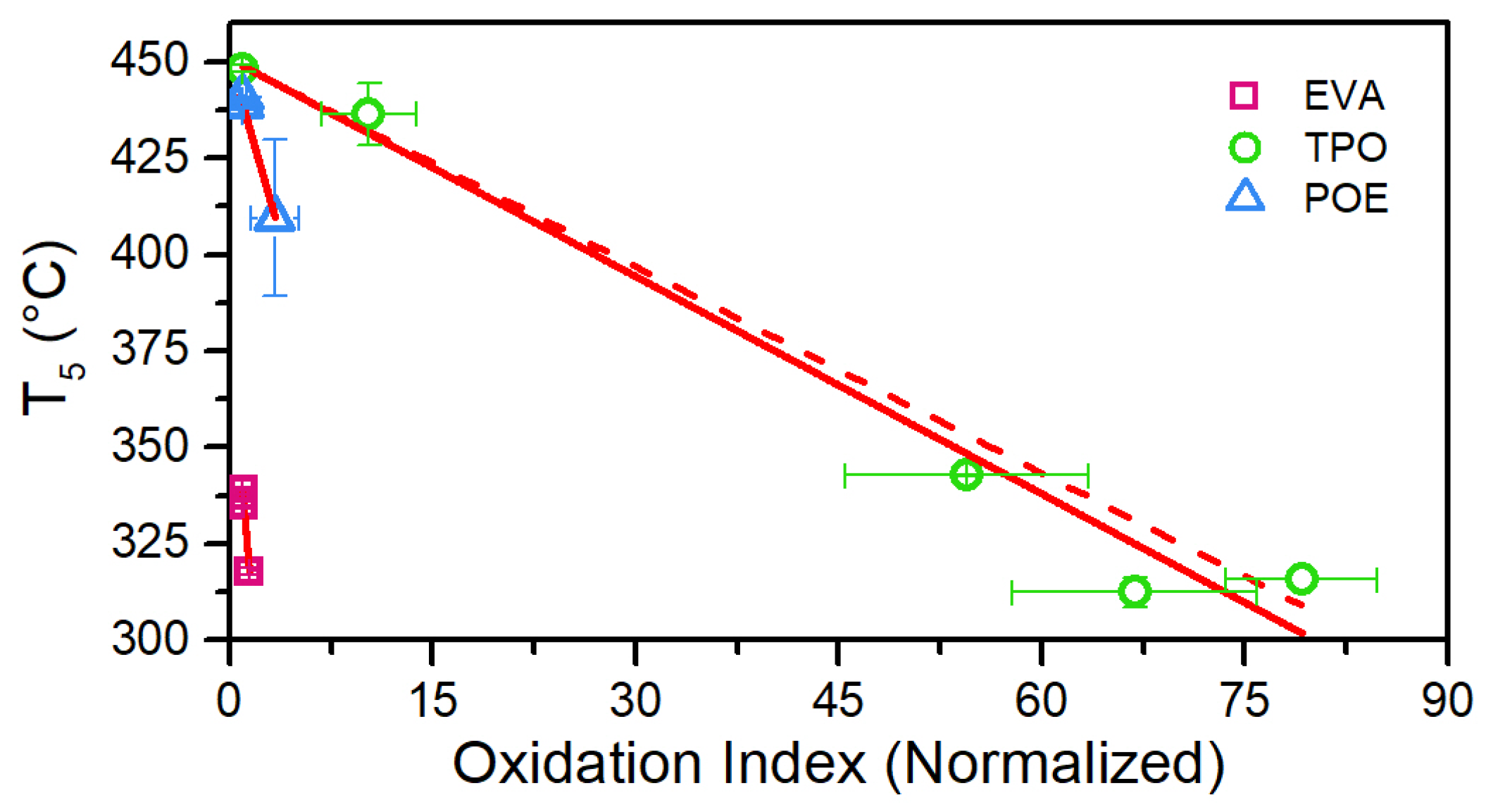
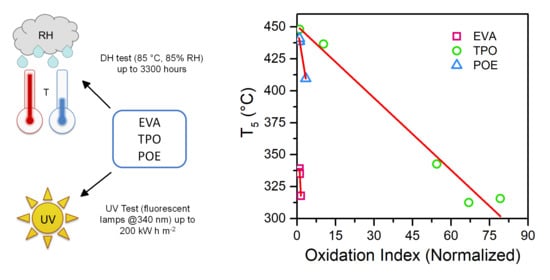
| Encapsulant | Thickness (µm) | Chemical Crosslinking | Acetic Acid |
|---|---|---|---|
| EVA | 450 | Yes, with peroxides | Yes |
| TPO | 500 | No | No |
| POE | 550 | Yes, with peroxides | No |
| Function | Irradiation (W m−2 nm−1) | Black Panel Temperature (°C) | Time (Hours:Minutes) |
|---|---|---|---|
| UV light | 0.76 | 60 | 8:00 |
| Condensation | n/a | 50 | 4:00 |
| EVA | |||||
| Stabilizer | Unexposed | DH Ageing Time 3300 h | UV Dose 85 kW h m−2 | UV Dose 127 kW h m−2 | UV Dose 200 kW h m−2 |
| Antioxidant butylated hydroxytoluene (BHT) | ✓ | ✓ | ✓ | ✓ | n. d. |
| UV absorber (benzophenone) | ✓ | ✓ | ✓ | ✓ | ✓ |
| UV absorber (benzotriazole) | n. d. | ✓ | n. d. | n. d. | n. d. |
| TPO | |||||
| Stabilizer | Unexposed | DH ageing time 3300 h | UV dose 85 kW h m−2 | UV dose 127 kW h m−2 | UV dose 200 kW h m−2 |
| Antioxidant (Antioxidant 1076) | ✓ | fragment | n. d. | n. d. | n. d. |
| UV absorber (benzotriazole) | n. d. | ✓ | n. d. | n. d. | n. d. |
| POE | |||||
| Stabilizer | Unexposed | DH ageing time 3300 h | UV dose 85 kW h m−2 | UV dose 127 kW h m−2 | UV dose 200 kW h m−2 |
| Antioxidant (BHT) | ✓ | fragment | n. d. | n. d. | n. d. |
| UV absorber (benzotriazole) | n. d. | ✓ | n. d. | n. d. | n. d. |
| Antioxidant (Antioxidant 1076) | n. d. | n. d. | traces | traces | traces |
| Wavenumber [cm−1] | Assignment |
|---|---|
| 2920 | Asymmetric stretching vibration of CH2 |
| 2850 | Symmetric deformation vibration of CH2 |
| 1780 | C=O stretching vibration of γ-lactones |
| 1715/1175 | C=O stretching vibration of ketones |
| 1736 | C=O stretching vibration |
| 1465 | Asymmetric deformation vibration of CH2 |
| 1370 | Symmetric deformation of CH3 |
| 1238 | C-O-C stretching vibration |
| 1020 | C-O-C stretching vibration |
| 960–940 | CH out-of-plane deformation vibration of vinyl ether |
| 910 | CH out-of-plane deformation vibration of vinyl |
| 720 | CH2 skeleton rocking vibration |
| Wavenumber [cm−1] | Assignment |
|---|---|
| 2920 | Asymmetric stretching vibration of CH2 |
| 2850 | Symmetric stretching vibration of CH2 |
| 1800–1680 | C=O stretching vibration |
| 1715/1175 | C=O stretching vibration of ketones |
| 1465 | Asymmetric deformation vibration of CH2 |
| 1370 | Symmetric deformation of CH3 |
| 909 | CH out-of-plane deformation vibration of vinyl |
| 720 (doublet) | CH2 skeleton rocking vibration |
| EVA | POE | TPO | |
|---|---|---|---|
| Chemical crosslinking | Yes | Yes | No |
| Acetic acid | Yes | No | No |
| General DH stability after 3300 h | Very good | Very good, transmittance decreases in UV range | Very good, transmittance decreases in UV range |
| Presence of stabilizers upon UV exposure | Yes | Partial | No |
| Optical properties upon UV exposure | Slight transmittance decrease | Slight transmittance decrease | Not measurable |
| Chemical oxidation upon UV exposure | Initial stage | Initial stage | Severe |
| Crystallinity changes upon UV exposure | Not relevant | Not relevant | Yes |
| Thermal stability upon UV exposure | Decreased | Decreased | Very much decreased |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barretta, C.; Oreski, G.; Feldbacher, S.; Resch-Fauster, K.; Pantani, R. Comparison of Degradation Behavior of Newly Developed Encapsulation Materials for Photovoltaic Applications under Different Artificial Ageing Tests. Polymers 2021, 13, 271. https://doi.org/10.3390/polym13020271
Barretta C, Oreski G, Feldbacher S, Resch-Fauster K, Pantani R. Comparison of Degradation Behavior of Newly Developed Encapsulation Materials for Photovoltaic Applications under Different Artificial Ageing Tests. Polymers. 2021; 13(2):271. https://doi.org/10.3390/polym13020271
Chicago/Turabian StyleBarretta, Chiara, Gernot Oreski, Sonja Feldbacher, Katharina Resch-Fauster, and Roberto Pantani. 2021. "Comparison of Degradation Behavior of Newly Developed Encapsulation Materials for Photovoltaic Applications under Different Artificial Ageing Tests" Polymers 13, no. 2: 271. https://doi.org/10.3390/polym13020271
APA StyleBarretta, C., Oreski, G., Feldbacher, S., Resch-Fauster, K., & Pantani, R. (2021). Comparison of Degradation Behavior of Newly Developed Encapsulation Materials for Photovoltaic Applications under Different Artificial Ageing Tests. Polymers, 13(2), 271. https://doi.org/10.3390/polym13020271