A Comparative Study on Hexavalent Chromium Adsorption onto Chitosan and Chitosan-Based Composites
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Reagents
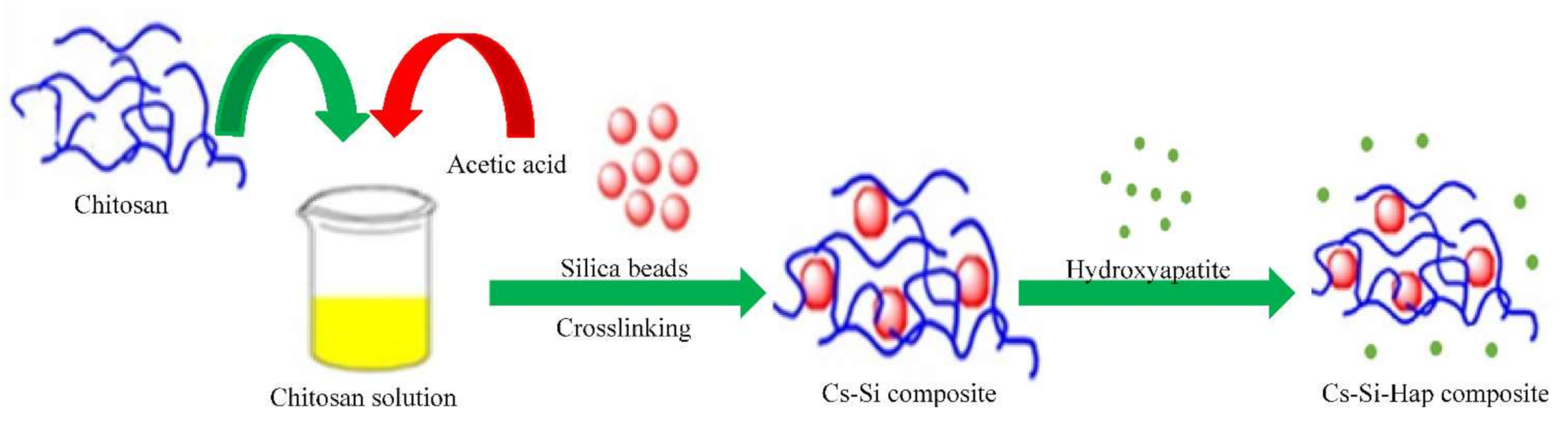
2.2. Preparation of Cs–Si–Hap Composite
2.2.1. Preparation of Chitosan (Cs)
2.2.2. Preparation of Chitosan-Silica (Cs–Si) Composite
2.2.3. Preparation of Chitosan–Silica–Hydroxyapatite (Cs–Si–Hap) Composite
2.3. Characterization
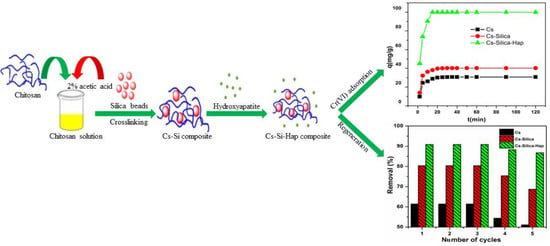
2.4. Adsorption, Coexisting Anions, and Regeneration Studies
3. Results and Discussion
3.1. Characterization
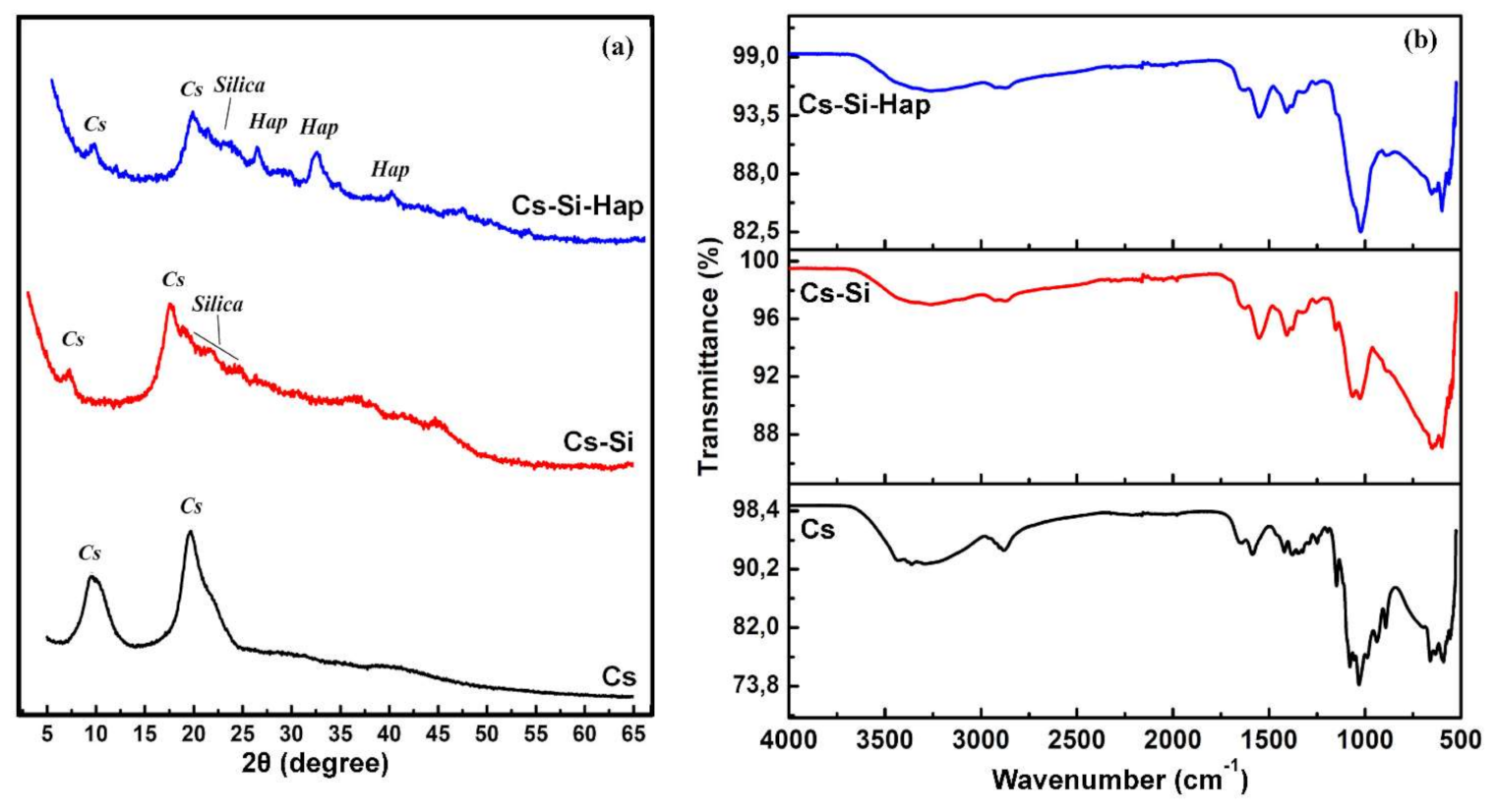
3.1.1. X-ray Diffraction (XRD) Analysis
3.1.2. Fourier Transform Infrared (FT-IR) Analysis
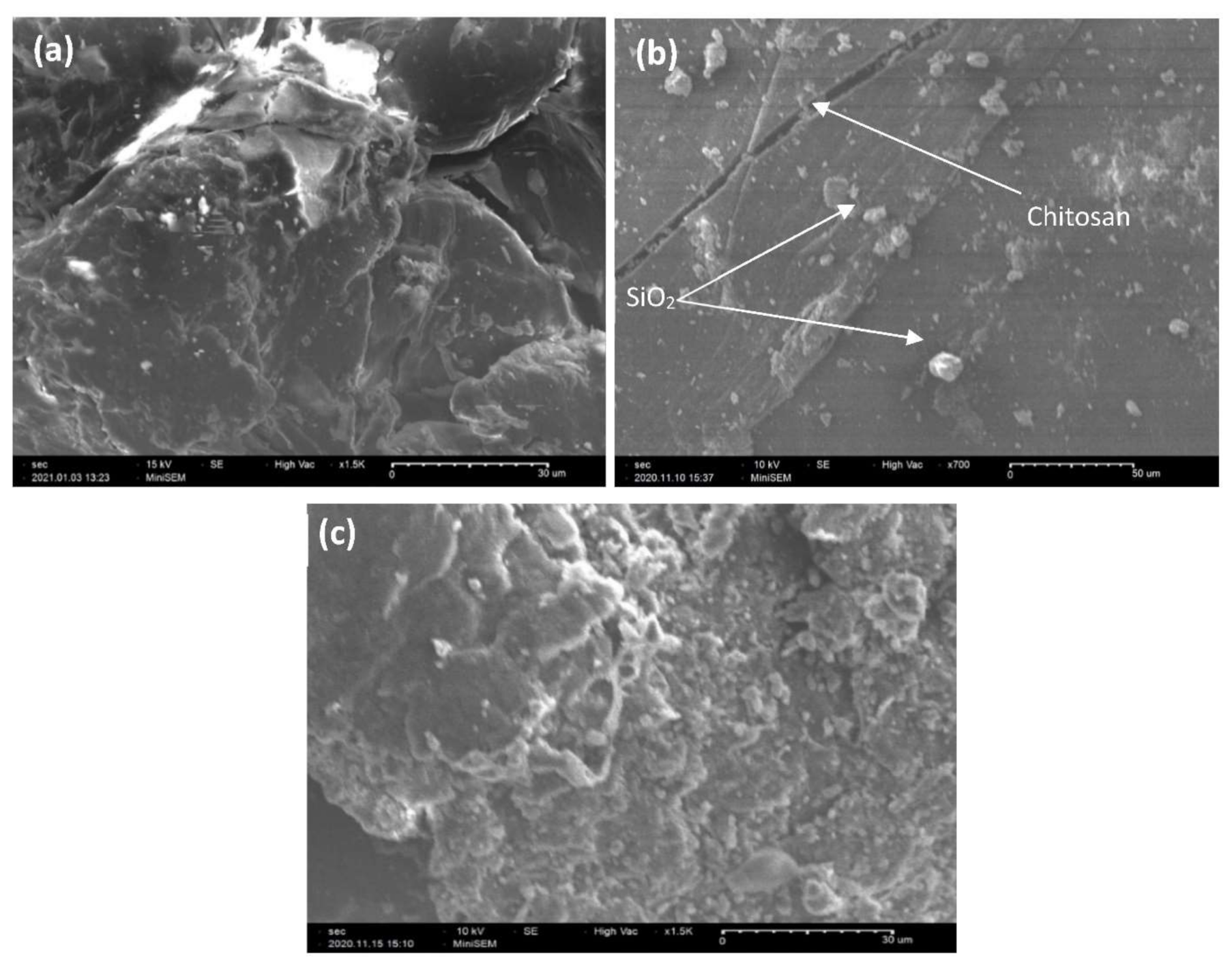
3.1.3. Scanning Electron Microscopy (SEM) Analysis
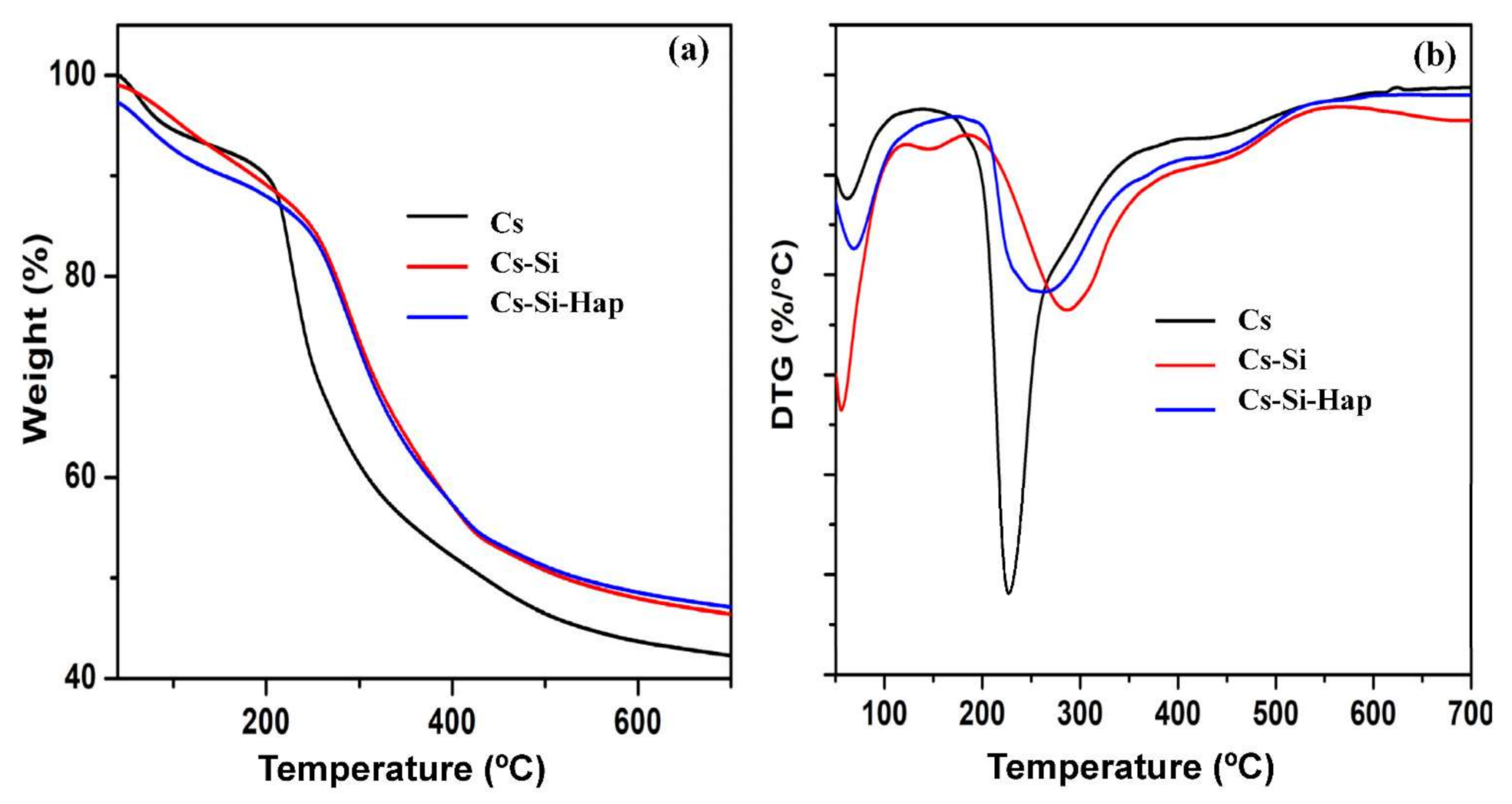
3.1.4. Thermal Analysis
3.2. Parameters Affecting Cr(VI) Adsorption
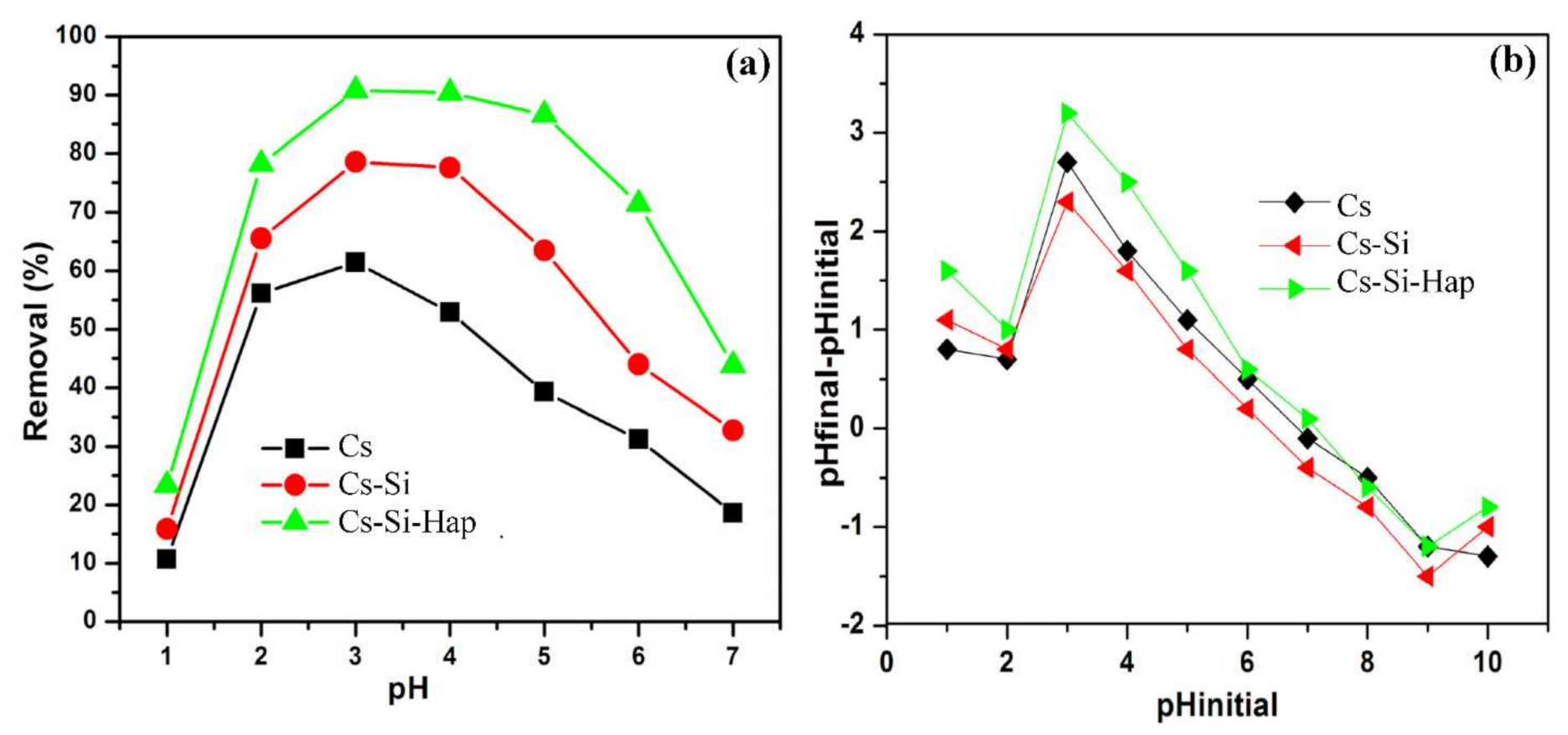
3.2.1. Effect of pH
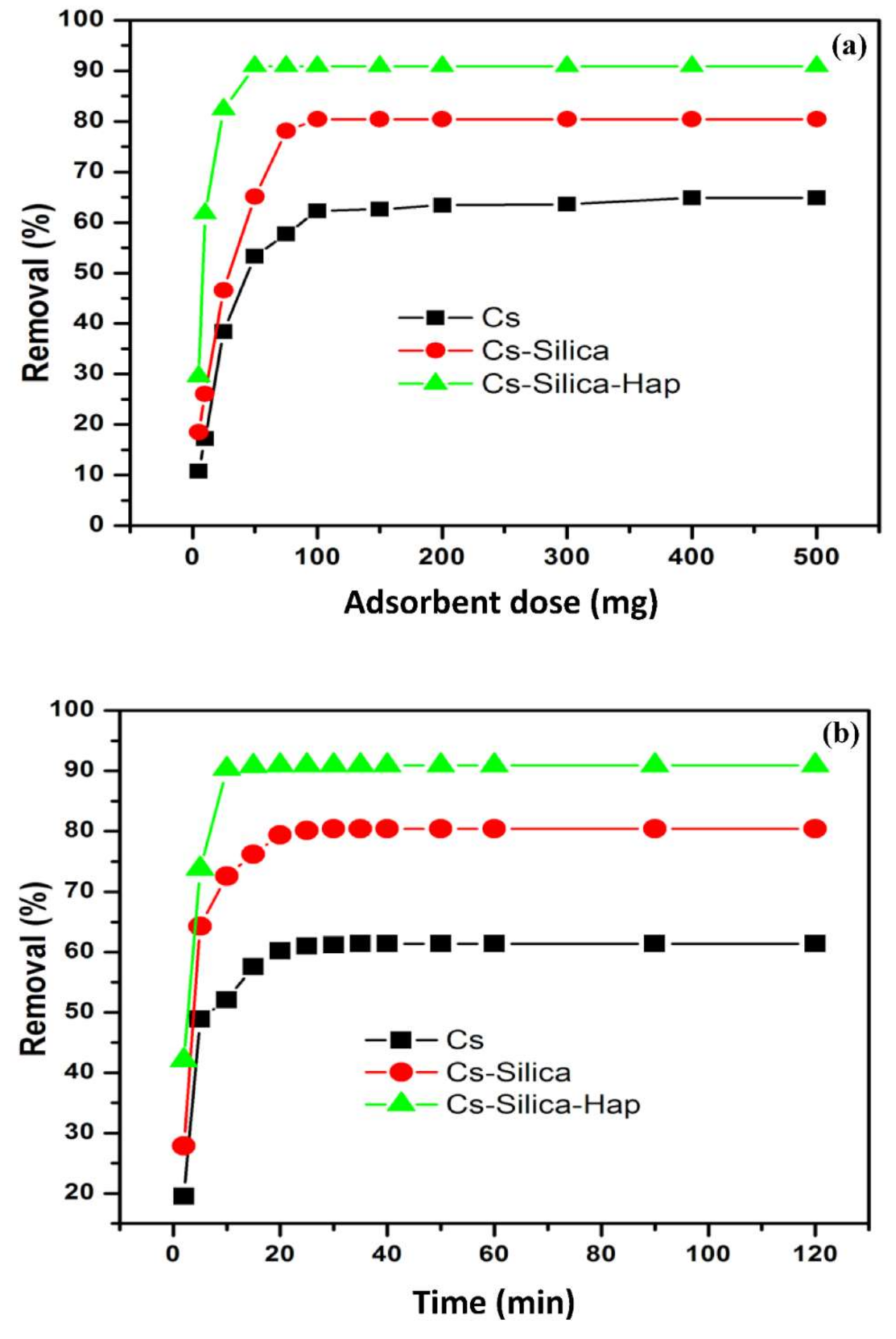
3.2.2. Effect of Adsorbent Dose and Contact Time
3.3. Adsorption Modeling
3.3.1. Kinetic Modeling
3.3.2. Isotherm Modeling
3.3.3. Thermodynamic Parameters
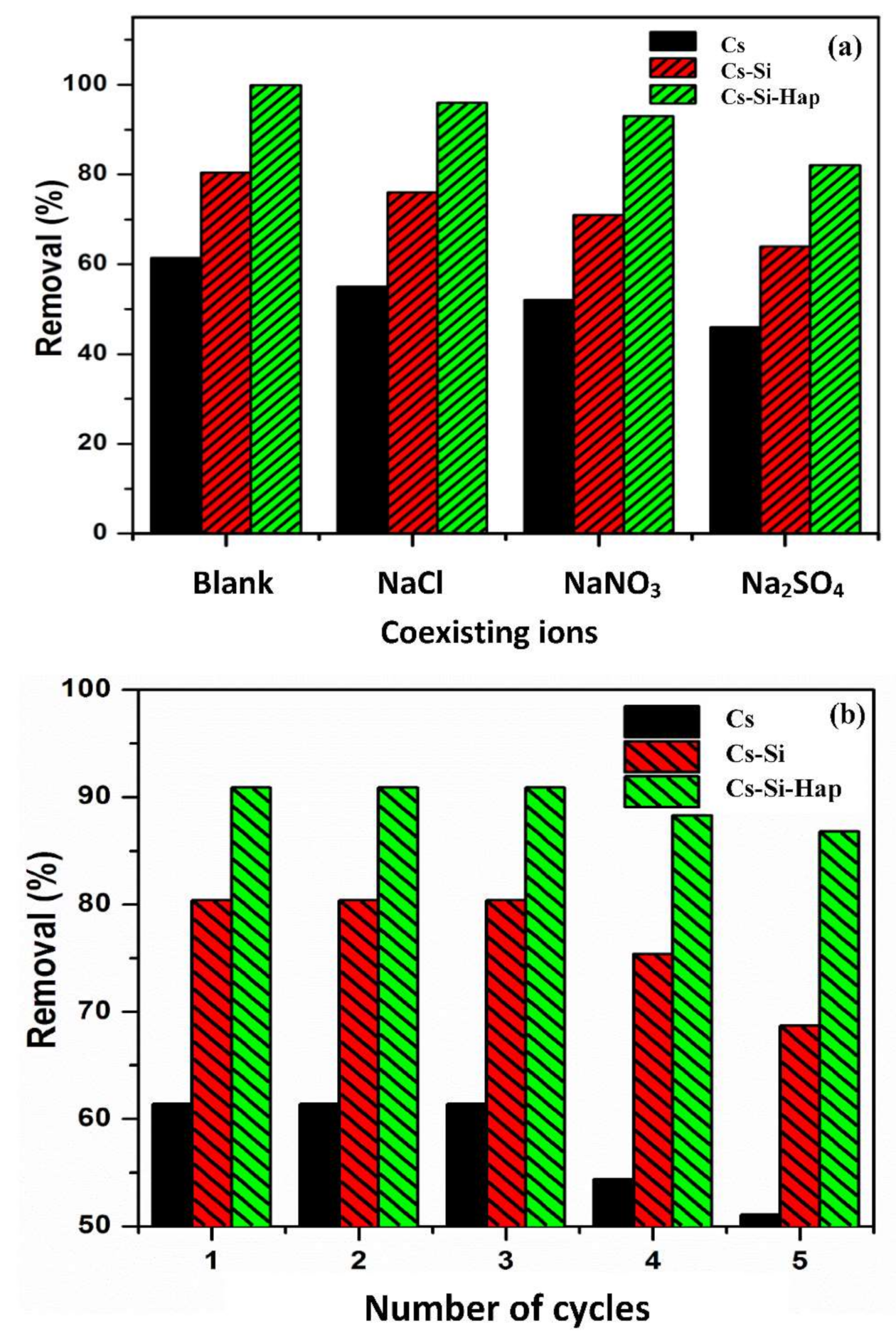
3.4. Effect of Coexisting Anions on Cr(VI) Adsorption
3.5. Regeneration Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahbazi, A.; Younesi, H.; Badiei, A. Functionalized SBA-15 Mesoporous Silica by Melamine-Based Dendrimer Amines for Adsorptive Characteristics of Pb(II), Cu(II) and Cd(II) Heavy Metal Ions in Batch and Fixed Bed Column. Chem. Eng. J. 2011, 168, 505–518. [Google Scholar] [CrossRef]
- Zare, E.N.; Mudhoo, A.; Khan, M.A.; Otero, M.; Bundhoo, Z.M.A.; Patel, M.; Srivastava, A.; Navarathna, C.; Mlsna, T.; Mohan, D.; et al. Smart Adsorbents for Aquatic Environmental Remediation. Small 2021, 17, 2007840. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, Y.; Wang, J.; Zhou, C.; Tang, Q.; Rao, X. Calcined Graphene/MgAl-Layered Double Hydroxides for Enhanced Cr(VI) Removal. Chem. Eng. J. 2013, 221, 204–213. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Maschio, L.J.; da Silva, R.E.; da Silva, M.L.C.P. Adsorption of Cr(VI) from Aqueous Solution by Hydrous Zirconium Oxide. J. Hazard. Mater. 2010, 173, 630–636. [Google Scholar] [CrossRef]
- Langård, S. Chromium Carcinogenicity; a Review of Experimental Animal Data. Sci. Total Environ. 1988, 71, 341–350. [Google Scholar] [CrossRef]
- Alemu, A.; Lemma, B.; Gabbiye, N.; Alula, M.T.; Desta, M.T. Removal of Chromium (VI) from Aqueous Solution Using Vesicular Basalt: A Potential Low Cost Wastewater Treatment System. Heliyon 2018, 4, e00682. [Google Scholar] [CrossRef]
- Saf, A.Ö.; Alpaydin, S.; Coskun, A.; Ersoz, M. Selective Transport and Removal of Cr(VI) through Polymer Inclusion Membrane Containing 5-(4-Phenoxyphenyl)-6H-1,3,4-Thiadiazin-2-Amine as a Carrier. J. Membrane Sci. 2011, 377, 241–248. [Google Scholar] [CrossRef]
- Barrera-Díaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. A Review of Chemical, Electrochemical and Biological Methods for Aqueous Cr(VI) Reduction. J. Hazard. Mater. 2012, 223–224, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Galán, B.; Castañeda, D.; Ortiz, I. Removal and Recovery of Cr(VI) from Polluted Ground Waters: A Comparative Study of Ion-Exchange Technologies. Water Res. 2005, 39, 4317–4324. [Google Scholar] [CrossRef]
- Uysal, M.; Ar, I. Removal of Cr(VI) from Industrial Wastewaters by Adsorption Part I: Determination of Optimum Conditions. J. Hazard. Mater. 2007, 149, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-Y.; Zhang, X.-L.; Yang, C.-Y.; Chen, X.; Zheng, X.-C. Alkali-Hydrothermal Synthesis and Characterization of W-MCM-41 Mesoporous Materials with Various Si/W Molar Ratios. Appl. Surf. Sci. 2013, 270, 590–595. [Google Scholar] [CrossRef]
- Shen, J.; Yin, X.; Karpuzov, D.; Semagina, N. PVP-Stabilized Mono- and Bimetallic Ru Nanoparticles for Selective Ring Opening. Catal. Sci. Technol. 2012, 3, 208–221. [Google Scholar] [CrossRef]
- Fechete, I.; Donnio, B.; Ersen, O.; Dintzer, T.; Djeddi, A.; Garin, F. Single Crystals of Mesoporous Tungstenosilicate W-MCM-48 Molecular Sieves for the Conversion of Methylcyclopentane (MCP). Appl. Surf. Sci. 2011, 257, 2791–2800. [Google Scholar] [CrossRef]
- Meski, S.; Tazibt, N.; Khireddine, H.; Ziani, S.; Biba, W.; Yala, S.; Sidane, D.; Boudjouan, F.; Moussaoui, N. Synthesis of Hydroxyapatite from Mussel Shells for Effective Adsorption of Aqueous Cd(II). Water Sci. Technol. 2019, 80, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Kousalya, G.N.; Gandhi, M.R.; Meenakshi, S. Removal of Toxic Cr(VI) Ions from Aqueous Solution Using Nano-Hydroxyapatite-Based Chitin and Chitosan Hybrid Composites. Adsorp. Sci. Technol. 2010, 28, 49–64. [Google Scholar] [CrossRef]
- El Kaim Billah, R.; Khan, M.A.; Wabaidur, S.M.; Jeon, B.-H.; Am, A.; Majdoubi, H.; Haddaji, Y.; Agunaou, M.; Soufiane, A. Chitosan/Phosphate Rock-Derived Natural Polymeric Composite to Sequester Divalent Copper Ions from Water. Nanomaterials 2021, 11, 2028. [Google Scholar] [CrossRef] [PubMed]
- El Kaim Billah, R.; Islam, M.; Agunaou, M.; Soufiane, A. A Promising Chitosan/Fluorapatite Composite for Efficient Removal of Lead (II) from Aqueous Solution. Arabian J. Geosci. 2021, 14, 1134. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Shen, Y.; Zhou, Y.; Wang, D.; Lei, Z.; Feng, W.; Min, Z. Silicone-Based Alumina Composites Synthesized through in Situ Polymerization for High Thermal Conductivity and Thermal Stability. Mater. Lett. 2020, 261, 127002. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Mahadik, D.B.; Parale, V.G.; Park, H.-H. Composites of Silica Aerogels with Organics: A Review of Synthesis and Mechanical Properties. J. Korean Ceram. Soc. 2020, 57, 1–23. [Google Scholar] [CrossRef]
- Gang, D.; Banerji, S.K.; Clevenger, T.E. Chromium(VI) Removal by Modified PVP-Coated Silica Gel. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2000, 4, 105–110. [Google Scholar] [CrossRef]
- Zeb, S.; Ali, N.; Ali, Z.; Bilal, M.; Adalat, B.; Hussain, S.; Gul, S.; Ali, F.; Ahmad, R.; khan, S.; et al. Silica-Based Nanomaterials as Designer Adsorbents to Mitigate Emerging Organic Contaminants from Water Matrices. J. Water Proc. Eng. 2020, 38, 101675. [Google Scholar] [CrossRef]
- Rasheed, A.; Carvalho, A.A.C.; de Carvalho, G.G.A.; Ghous, T.; Nomura, C.S.; Esposito, B.P. Chromium Removal from Aqueous Solutions Using New Silica Gel Conjugates of Desferrioxamine or Diethylenetriaminepentaacetic Acid. Environ. Sci. Pollut. Res. 2020, 27, 15635–15644. [Google Scholar] [CrossRef] [PubMed]
- Bilgiç, A.; Çimen, A. Removal of Chromium(VI) from Polluted Wastewater by Chemical Modification of Silica Gel with 4-Acetyl-3-Hydroxyaniline. RSC Adv. 2019, 9, 37403–37414. [Google Scholar] [CrossRef]
- Hu, C.; Zheng, H.; Zhao, R.; Zhang, S.; Sun, Q.; Jiang, J.; Sun, Y. Structural Design of a Floating-Magnetically Responsive Silica Adsorbent and Efficient Removal of Dyes. J. Clean. Prod. 2021, 302, 126985. [Google Scholar] [CrossRef]
- Puchol, V.; Haskouri, J.E.; Latorre, J.; Guillem, C.; Beltrán, A.; Beltrán, D.; Amorós, P. Biomimetic Chitosan-Mediated Synthesis in Heterogeneous Phase of Bulk and Mesoporous Silica Nanoparticles. Chem. Commun. 2009, 19, 2694–2696. [Google Scholar] [CrossRef][Green Version]
- Zou, H.; Wu, S.; Shen, J. Polymer/Silica Nanocomposites: Preparation, Characterization, Properties, and Applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef]
- Spirk, S.; Findenig, G.; Doliska, A.; Reichel, V.E.; Swanson, N.L.; Kargl, R.; Ribitsch, V.; Stana-Kleinschek, K. Chitosan–Silane Sol–Gel Hybrid Thin Films with Controllable Layer Thickness and Morphology. Carbohydr. Polym. 2013, 93, 285–290. [Google Scholar] [CrossRef]
- Liu, H.; Gong, C.; Wang, J.; Liu, X.; Liu, H.; Cheng, F.; Wang, G.; Zheng, G.; Qin, C.; Wen, S. Chitosan/Silica Coated Carbon Nanotubes Composite Proton Exchange Membranes for Fuel Cell Applications. Carbohydr. Polym. 2016, 136, 1379–1385. [Google Scholar] [CrossRef]
- Osaki, S.; Osaki, T.; Takashima, Y. Determination of chromium(vi) in natural waters by the sorption of chromium-diphenylcarbazone with xad-2 resin. Talanta 1983, 30, 683–686. [Google Scholar] [CrossRef]
- Pflaum, R.T.; Howick, L.C. The chromium-diphenylcarbazide reaction. J. Am. Chem. Soc. 1956, 78, 4862–4866. [Google Scholar] [CrossRef]
- Jastrzębski, W.; Sitarz, M.; Rokita, M.; Bułat, K. Infrared spectroscopy of different phosphates structures. Spectrochim. Acta Part A 2011, 79, 722–727. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, viavaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Carrodeguas, R.G.; Peniche, C.; Solís, Y.; Cameron, R.E. Chitosan/Apatite Composite Beads Prepared by in Situ Generation of Apatite or Si-Apatite Nanocrystals. Acta Biomater. 2010, 6, 466–476. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, R.; Li, R.; Ali, A.; Chen, A.; Zhang, Z. Enhanced Aqueous Cr(VI) Removal Using Chitosan-Modified Magnetic Biochars Derived from Bamboo Residues. Chemosphere 2020, 261, 127694. [Google Scholar] [CrossRef] [PubMed]
- Kaim Billah, R.E.; Elyamani, Y.; Rakhila, Y.; Agunaou, M.; Soufiane, A. Removal of Cr (VI) from aqueous solution by adsorption on the natural and activated fluorapatite. Rasayan J. Chem. 2019, 12, 347–354. [Google Scholar] [CrossRef]
- Mei, J.; Zhang, H.; Li, Z.; Ou, H. A Novel Tetraethylenepentamine Crosslinked Chitosan Oligosaccharide Hydrogel for Total Adsorption of Cr(VI). Carbohydr. Polym. 2019, 224, 115154. [Google Scholar] [CrossRef]
- Xiao, F.; Cheng, J.; Cao, W.; Yang, C.; Chen, J.; Luo, Z. Removal of Heavy Metals from Aqueous Solution Using Chitosan-Combined Magnetic Biochars. J. Colloid. Interface Sci. 2019, 540, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, B.; Zhang, H.; Ma, J.; Mu, B.; Zhang, W. A Novel Biochar Modified by Chitosan-Fe/S for Tetracycline Adsorption and Studies on Site Energy Distribution. Biores. Technol. 2019, 294, 122152. [Google Scholar] [CrossRef]
- Rao, R.A.K.; Khan, M.A.; Jeon, B.-H. Utilization of carbon derived from mustard oil cake (CMOC) for the removal of bivalent metal ions: Effect of anionic surfactant on the removal and recovery. J. Hazard. Mater. 2010, 173, 273–282. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. K. Sven. Vetenskapsakad. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surface of glass, mica and platinum. J. Am. Chem. Soc. 1916, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Sethy, T.R.; Sahoo, P.K. Highly toxic Cr (VI) adsorption by (chitosan-g-PMMA)/silica bionanocomposite prepared via emulsifier-free emulsion polymerisation. Int. J. Biol. Macromol. 2019, 122, 1184–1190. [Google Scholar] [CrossRef]
- Zhang, L.; Mu, C.; Zhong, L.; Xue, J.; Zhou, Y.; Han, X. Recycling of Cr (VI) from weak alkaline aqueous media using a chitosan/triethanolamine/Cu (II) composite adsorbent. Carbohydr. Polym. 2019, 205, 151–158. [Google Scholar] [CrossRef]
- Yu, P.; Wang, H.-Q.; Bao, R.-Y.; Liu, Z.; Yang, W.; Xie, B.-H.; Yang, M.-B. Self-Assembled Sponge-like Chitosan/Reduced Graphene Oxide/Montmorillonite Composite Hydrogels without Cross-Linking of Chitosan for Effective Cr(VI) Sorption. ACS Sustain. Chem. Eng. 2017, 5, 1557–1566. [Google Scholar] [CrossRef]
- Chen, D.; Li, W.; Wu, Y.; Zhu, Q.; Lu, Z.; Du, G. Preparation and Characterization of Chitosan/Montmorillonite Magnetic Microspheres and Its Application for the Removal of Cr (VI). Chem. Eng. J. 2013, 221, 8–15. [Google Scholar] [CrossRef]
- Xiao, Y.; Liang, H.; Chen, W.; Wang, Z. Synthesis and Adsorption Behavior of Chitosan-Coated MnFe2O4 Nanoparticles for Trace Heavy Metal Ions Removal. Appl. Surf. Sci. 2013, 285, 498–504. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, H.; Liu, P.; Fang, W.; Geng, J. A novel modified graphene oxide/chitosan composite used as anadsorbent for Cr(VI) in aqueous solutions. Int. J. Biol. Macromol. 2016, 87, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, B.; Zhang, L.; Huang, R. Adsorptive Removal of Cr(VI) from Aqueous Solutions by Cross-Linked Chitosan/Bentonite Composite. Korean J. Chem. Eng. 2015, 32, 1314–1322. [Google Scholar] [CrossRef]
- Alshareef, S.A.; Otero, M.; Alanazi, H.S.; Siddiqui, M.R.; Khan, M.A.; Alothman, Z.A. Upcycling olive oil cake through wet torrefaction to produce hydrochar for water decontamination. Chem. Eng. Res. Des. 2021, 170, 13–22. [Google Scholar] [CrossRef]
| Adsorbent | qe,exp. (mg/g) | Kinetic Models | |||||
|---|---|---|---|---|---|---|---|
| Pseudo-First-Order | Pseudo-Second-Order | ||||||
| qe,1 (mg/g) | k1 (1/min) | R2 | qe,2 (mg/g) | k2 (g/mg-min) | R2 | ||
| Cs | 31.35 | 24.89 | 0.070 | 0.974 | 30.70 | 0.020 | 0.998 |
| Cs–Si | 40.82 | 34.02 | 0.080 | 0.981 | 40.20 | 0.030 | 0.999 |
| Cs–Si–Hap | 91.74 | 104.45 | 0.212 | 0.983 | 90.90 | 0.040 | 0.999 |
| Adsorbent | Isotherm Models | |||||
|---|---|---|---|---|---|---|
| Langmuir | Freundlich | |||||
| qm (mg/g) | KL (L/mg) | R2 | KF (mg/g)(L/mg)1/n | n | R2 | |
| Cs | 55.51 | 0.080 | 0.991 | 7.91 | 2.88 | 0.971 |
| Cs–Si | 64.42 | 0.011 | 0.998 | 29.44 | 2.74 | 0.985 |
| Cs–Si–Hap | 212.76 | 0.230 | 0.999 | 31.86 | 3.71 | 0.989 |
| Adsorbent | Experimental Conditions | qm, mg/g | Reference |
|---|---|---|---|
| Cs-g-PMMA/ Silica BNC | Co: 10–500 mg/L; pH: 4; T: 25 °C; t: 24 h; m: 0.1 g; V: 20 mL; Agitation speed: 100 rpm. | 92.50 | [44] |
| Cs/triethanolamine/ Cu (II) composite | Co: 50–300 mg/L; pH: 8; T: 40 °C; m: 0.1 g; V: 50 mL. | 44.64 | [45] |
| Cs/GO/montmorillonite composite | Co: 25–250 mg/L; pH: 2; t: 3 h; m: 0.05 g; V: 50 mL | 87.03 | [46] |
| Cs/montmorillonite | Co: 6–24 mg/L; pH: 5; T: 30 °C; t: 3 h; m: 0.015 g; V:25 mL | 35.71 | [47] |
| Cs/MnFe2O4 | Co: 0.1–1 mg/L; pH: 3; T: 20 °C; t: 12 h; m: 0.008 g; V: 300 mL. | 31.32 | [48] |
| Cs/GO/EDTA composite | Co: 20–100 mg/L; pH: 2; T: 25°C; t: 1.5 h; m: 0.02 g; V: 25 mL. | 86.17 | [49] |
| Cross-linked Cs-bentonite composite | pH: 2; t: 4 h; T: 20 °C; Co: 100–300 mg/L; m: 0.1 g; V: 20 mL. | 37.73 | [50] |
| Cs–Si–Hap | pH: 3; t: 1 h; T: 25 °C; Co: 20–140 mg/L; Agitation speed: 100 rpm. | 222 | This study |
| Adsorbent | Thermodynamic Parameters | ||||
|---|---|---|---|---|---|
| ∆H° (kJ/mol) | ∆S° (J/mol-K) | ∆G° (kJ/mol) | |||
| 298 K | 308 K | 333 K | |||
| Cs | 57.37 | 190.85 | −0.75 | −1.56 | −3.24 |
| Cs-S | 58.79 | 202.35 | −1.47 | −3.78 | −5.65 |
| Cs-S-Hap | 33.15 | 135.30 | −5.72 | −7.15 | −8.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Billah, R.E.K.; Khan, M.A.; Park, Y.-K.; AM, A.; Majdoubi, H.; Haddaji, Y.; Jeon, B.-H. A Comparative Study on Hexavalent Chromium Adsorption onto Chitosan and Chitosan-Based Composites. Polymers 2021, 13, 3427. https://doi.org/10.3390/polym13193427
Billah REK, Khan MA, Park Y-K, AM A, Majdoubi H, Haddaji Y, Jeon B-H. A Comparative Study on Hexavalent Chromium Adsorption onto Chitosan and Chitosan-Based Composites. Polymers. 2021; 13(19):3427. https://doi.org/10.3390/polym13193427
Chicago/Turabian StyleBillah, Rachid El Kaim, Moonis Ali Khan, Young-Kwon Park, Amira AM, Hicham Majdoubi, Younesse Haddaji, and Byong-Hun Jeon. 2021. "A Comparative Study on Hexavalent Chromium Adsorption onto Chitosan and Chitosan-Based Composites" Polymers 13, no. 19: 3427. https://doi.org/10.3390/polym13193427
APA StyleBillah, R. E. K., Khan, M. A., Park, Y.-K., AM, A., Majdoubi, H., Haddaji, Y., & Jeon, B.-H. (2021). A Comparative Study on Hexavalent Chromium Adsorption onto Chitosan and Chitosan-Based Composites. Polymers, 13(19), 3427. https://doi.org/10.3390/polym13193427