Applications of Polymeric Composites in Bone Tissue Engineering and Jawbone Regeneration
Abstract
1. Introduction
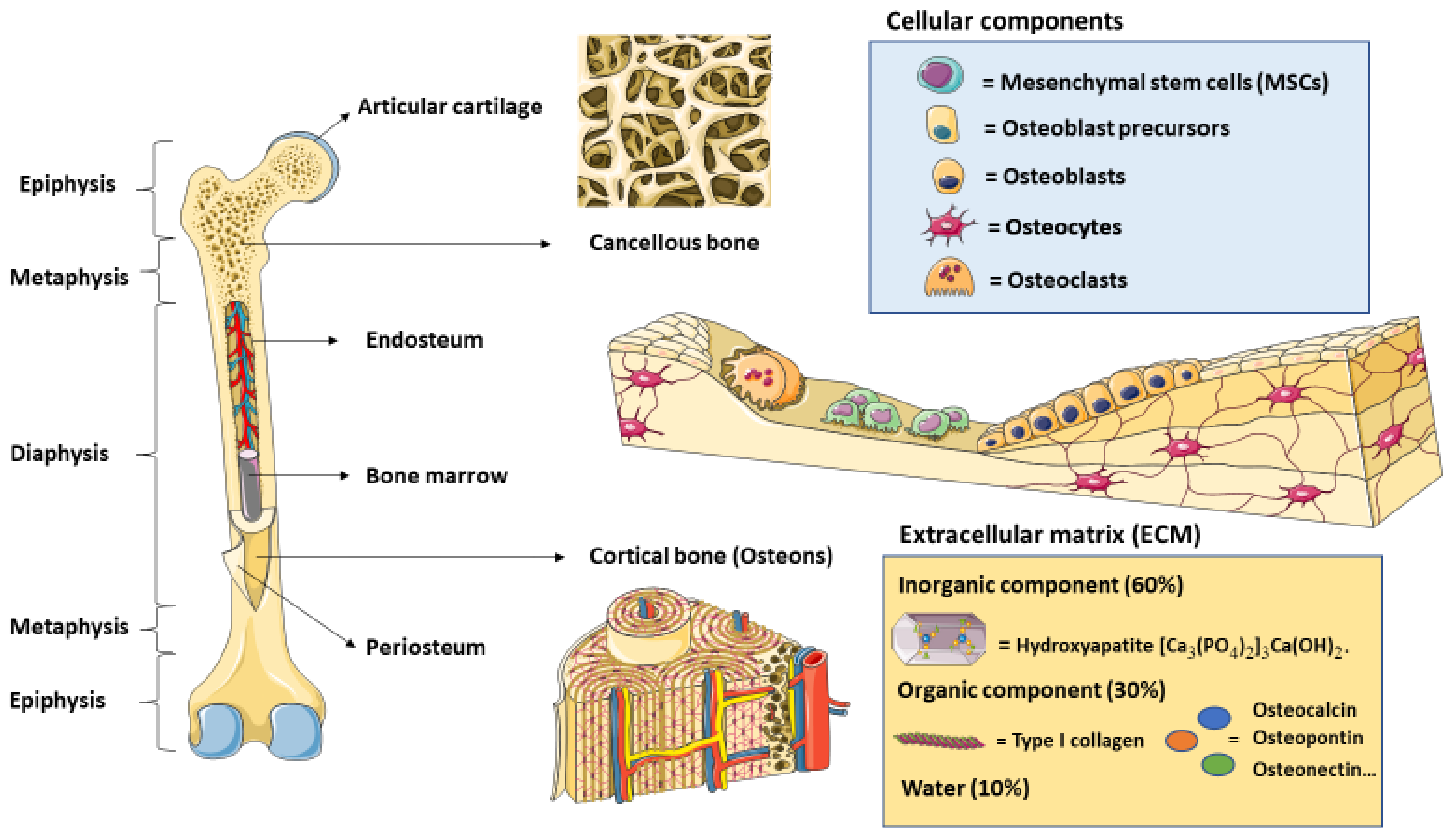
2. Bone Cytoarchitecture and Remodeling
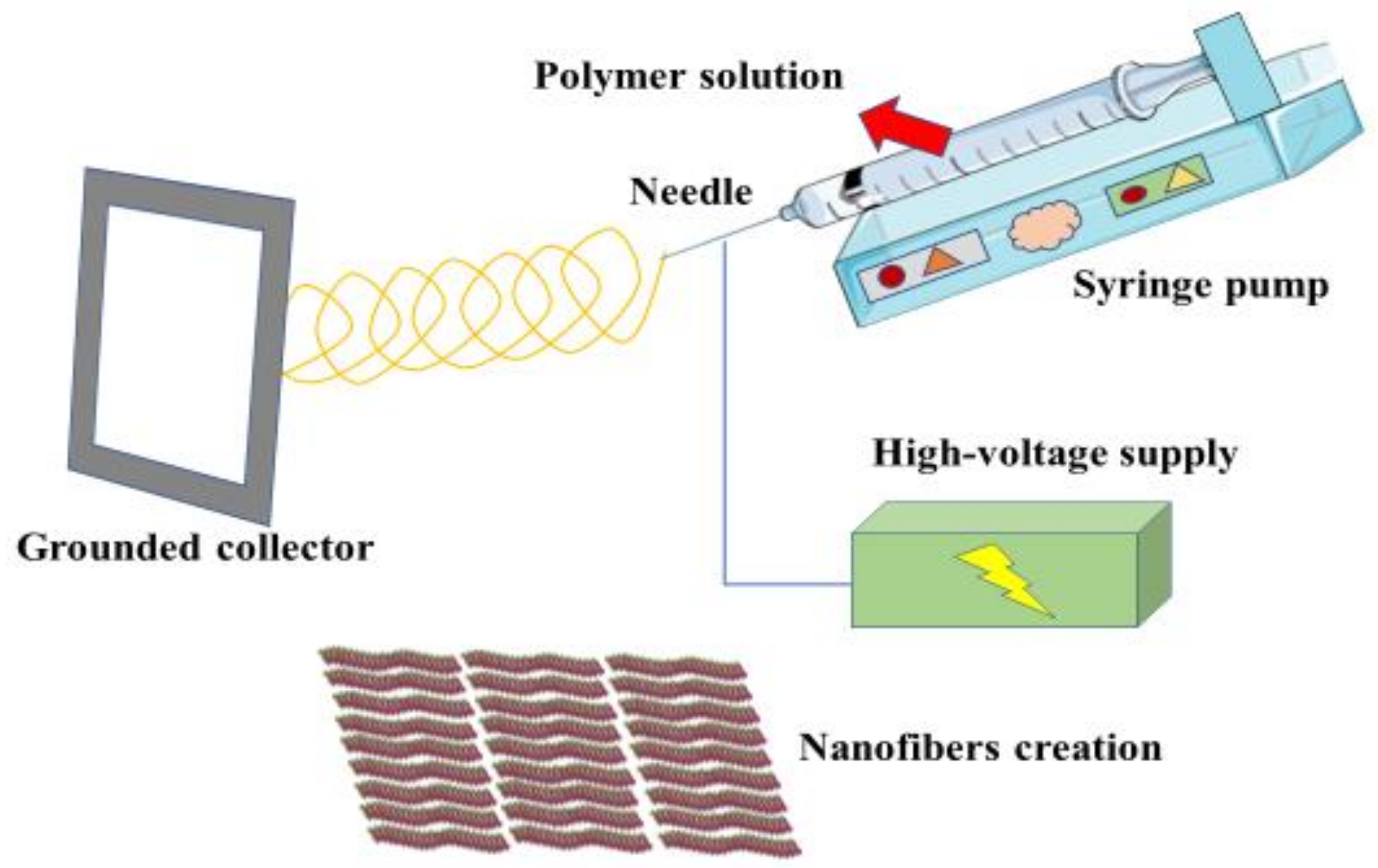
3. Polymeric Composites: Concept, Technology and Biomedical Applications
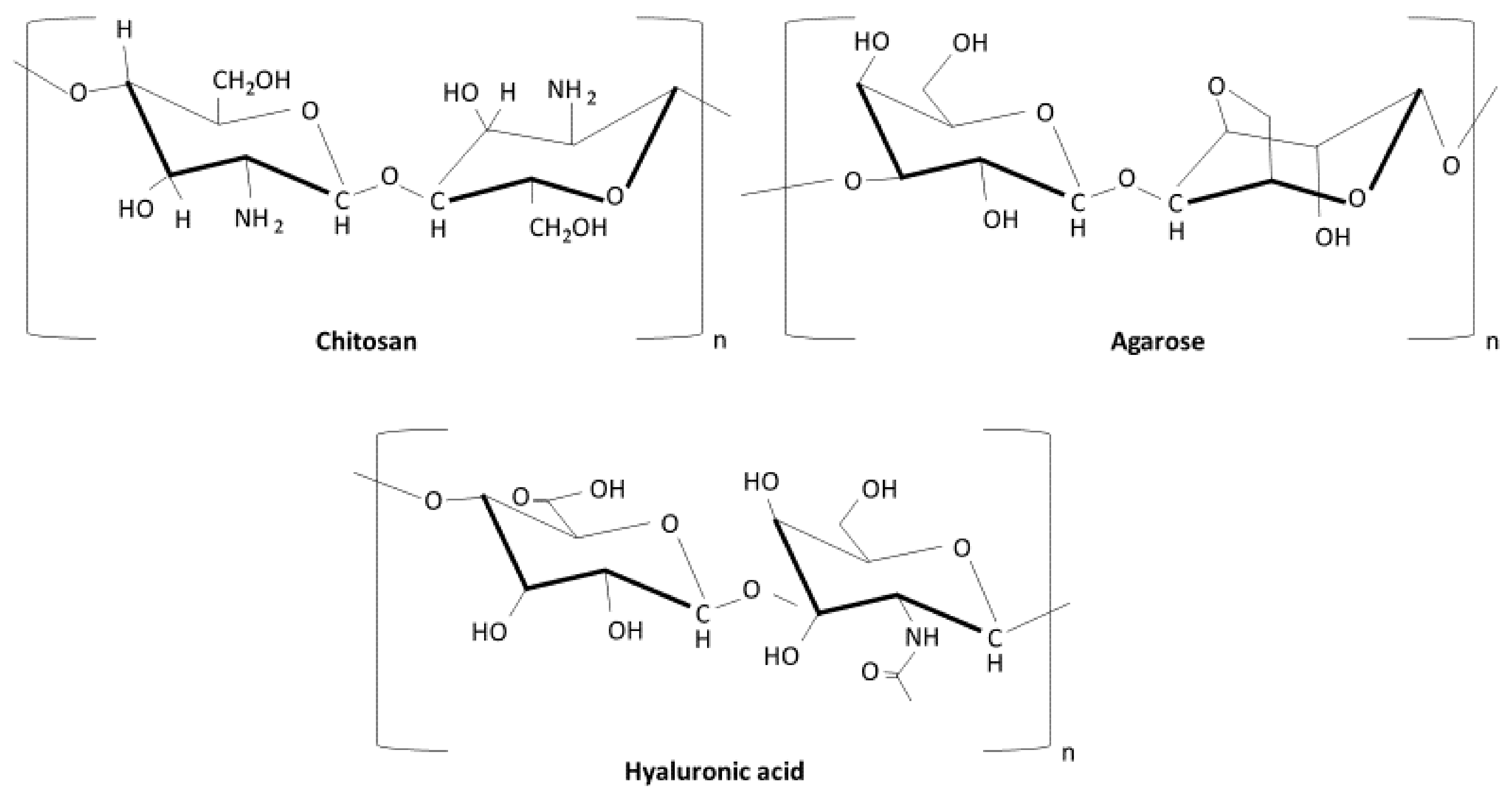
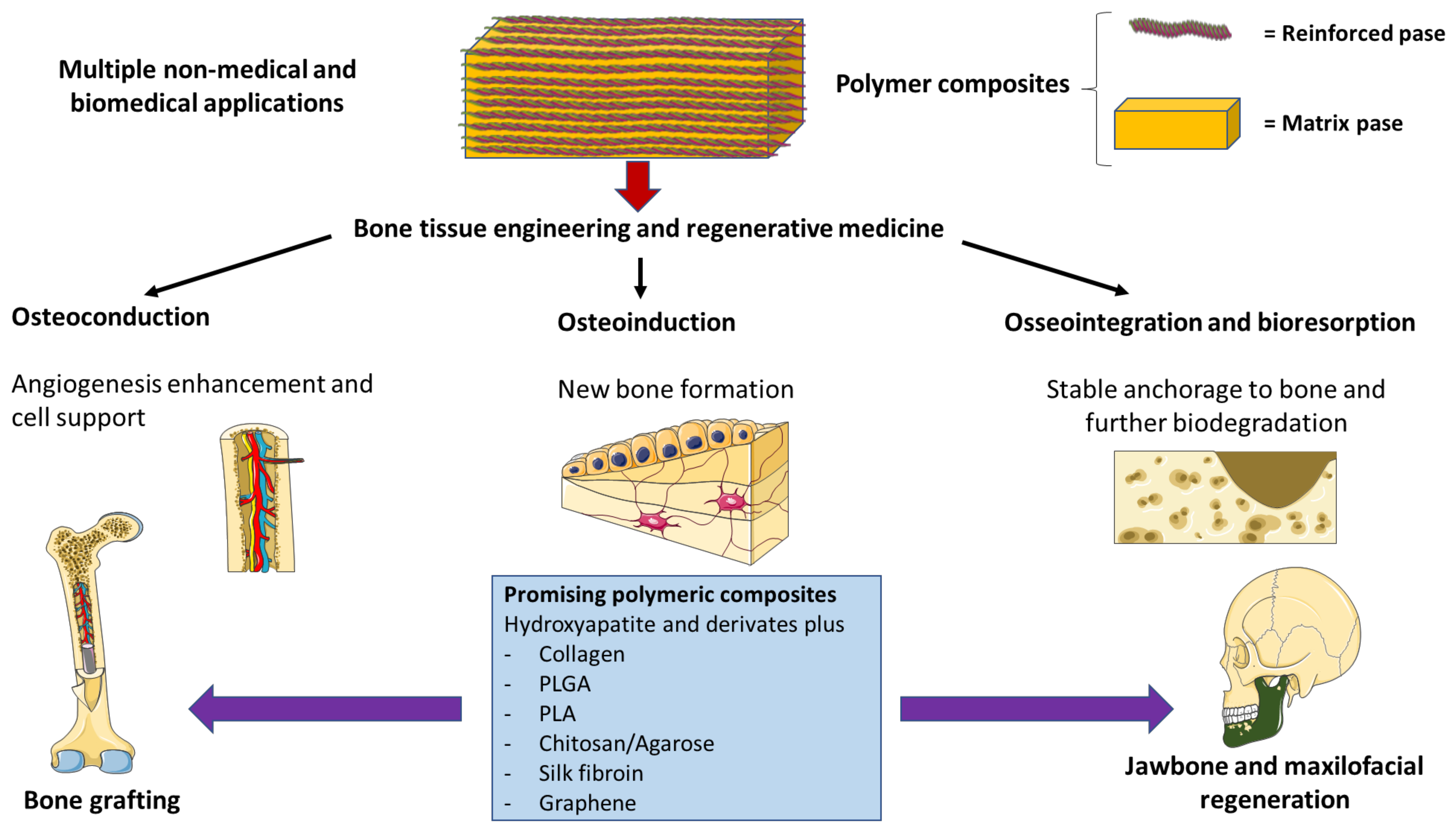
4. Most Relevant Polymeric Composites in Bone Regeneration
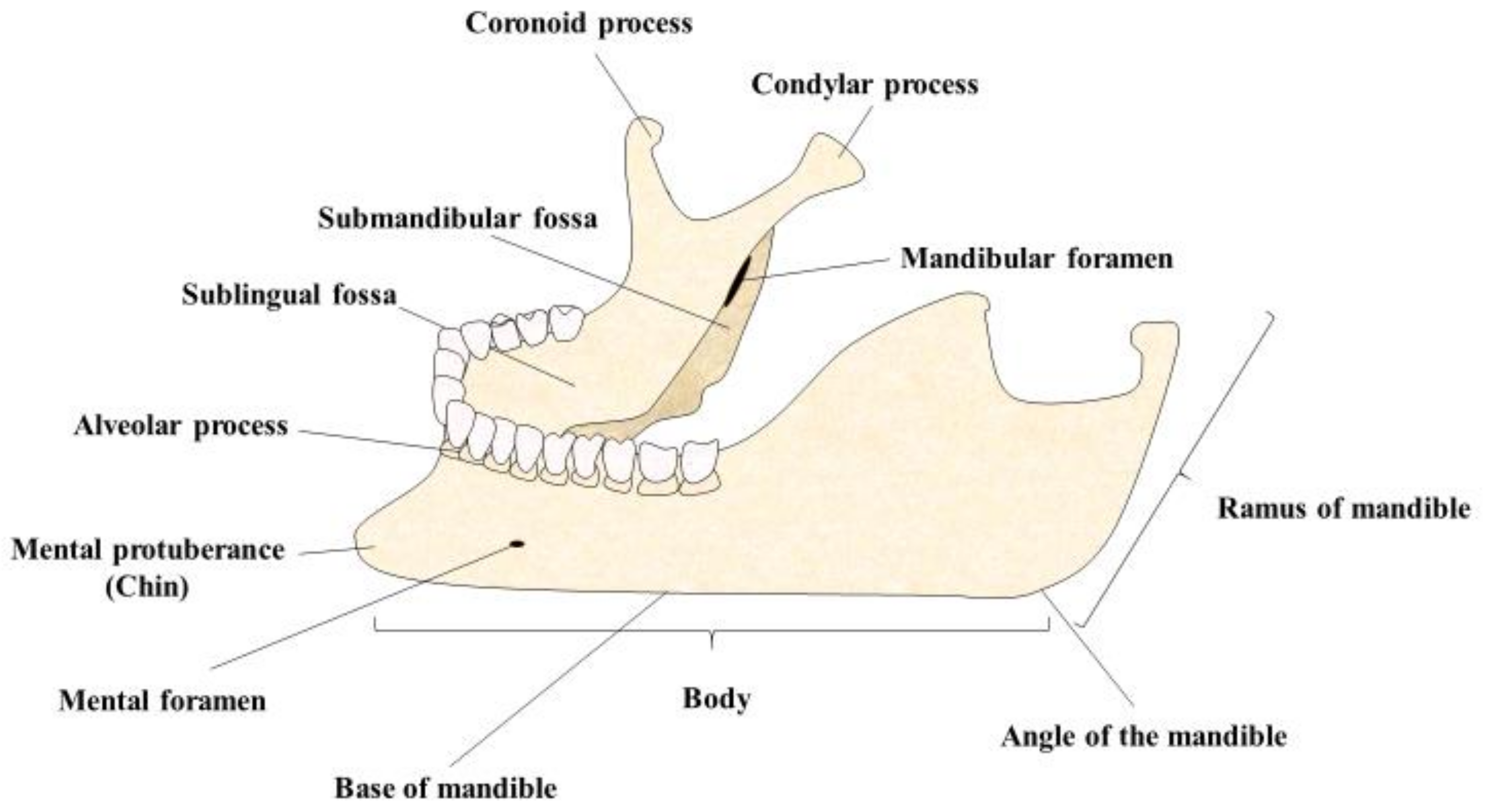
5. Polymer Composites and Jawbone Regeneration
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dittmer, K.E.; Firth, E.C. Mechanisms of bone response to injury. J. Vet. Diagn. Investig. 2017, 29, 385–395. [Google Scholar] [CrossRef]
- Schindeler, A.; McDonald, M.M.; Bokko, P.; Little, D.G. Bone remodeling during fracture repair: The cellular picture. Semin. Cell Dev. Biol. 2008, 19, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A. The cell and molecular biology of fracture healing. Clin. Orthop. Relat. Res. 1998, 355, S7–S21. [Google Scholar] [CrossRef]
- Ansari, M. Bone tissue regeneration: Biology, strategies and interface studies. Prog. Biomater. 2019, 8, 223. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, allograft, and bone graft substitutes: Clinical evidence and indications for use in the setting of orthopaedic trauma surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Bauer, T.W.; Muschler, G.F. Bone graft materials. An overview of the basic science. Clin. Orthop. Relat. Res. 2000, 371, 10–27. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96. [Google Scholar] [CrossRef] [PubMed]
- Moraschini, V.; de Almeida, D.C.F.; Calasans-Maia, M.D.; Kischinhevsky, I.C.C.; Louro, R.S.; Granjeiro, J.M. Immunological response of allogeneic bone grafting: A systematic review of prospective studies. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2020, 49, 395–403. [Google Scholar] [CrossRef]
- Shibuya, N.; Jupiter, D.C. Bone graft substitute: Allograft and Xenograft. Clin. Podiatr. Med. Surg. 2015, 32, 21–34. [Google Scholar] [CrossRef]
- Nandi, S.; Roy, S.; Mukherjee, P.; Kundu, B.; De, D.; Basu, D. Orthopaedic applications of bone graft & graft substitutes: A review. Indian J. Med. Res. 2010, 132, 15–30. [Google Scholar]
- Zafar, M.; Najeeb, S.; Khurshid, Z.; Vazirzadeh, M.; Zohaib, S.; Najeeb, B.; Sefat, F. Potential of electrospun nanofibers for biomedical and dental applications. Materials 2016, 9, 73. [Google Scholar] [CrossRef]
- Zagho, M.M.; Hussein, E.A.; Elzatahry, Á.A. Recent overviews in functional polymer composites for biomedical applications. Polymers 2018, 10, 739. [Google Scholar] [CrossRef]
- Ramesh, N.; Moratti, S.C.; Dias, G.J. Hydroxyapatite-polymer biocomposites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2046–2057. [Google Scholar] [CrossRef]
- El Sayed, S.A.; Nezwek, T.A.; Varacallo, M. Physiology, Bone; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Caetano-Lopes, J.; Canhão, H.; Fonseca, J.E. Osteoblasts and bone formation. Acta Reumatol. Port. 2007, 32, 103–110. [Google Scholar] [PubMed]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baghaban Eslaminejad, M. Role of Mesenchymal stem cells in bone regenerative medicine: What is the evidence? Cells Tissues Organs 2017, 204, 59–83. [Google Scholar] [CrossRef]
- Wei, C.C.; Lin, A.B.; Hung, S.C. Mesenchymal stem cells in regenerative medicine for musculoskeletal diseases: Bench, bedside, and industry. Cell Transplant. 2014, 23, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Lerner, U.H. Osteoclasts in health and disease. Pediatr. Endocrinol. Rev. PER 2019, 17, 84–99. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-osteoclast interactions. Connect. Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; da Silva Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. BioMed Res. Int. 2015, 2015, 17. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.P.; Bordoni, B. Histology, Osteoblasts; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Feng, X. Chemical and biochemical basis of cell-bone matrix interaction in health and disease. Curr. Chem. Biol. 2009, 3, 189. [Google Scholar] [CrossRef]
- Dwek, J.R. The periosteum: What Is it, where is it, and what mimics it in its absence? Skelet. Radiol. 2010, 39, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, M.G.; Roe, A.K. The osteon: The micromechanical unit of compact bone. Front. Biosci. 2012, 17, 1551–1581. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.R. The mechanobiology of cancellous bone structural adaptation. J. Rehabil. Res. Dev. 2000, 37, 209–216. [Google Scholar] [PubMed]
- Baig, M.A.; Bacha, D. Histology, Bone; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. CJASN 2008, 3, S131. [Google Scholar] [CrossRef]
- Nahian, A.; Chauhan, P.R. Histology, Periosteum and Endosteum; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Gianakos, A.; Ni, A.; Zambrana, L.; Kennedy, J.G.; Lane, J.M. Bone marrow aspirate concentrate in animal long bone healing: An analysis of basic science evidence. J. Orthop. Trauma 2016, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Le, B.Q.; Nurcombe, V.; Cool, S.M.; van Blitterswijk, C.A.; de Boer, J.; LaPointe, V.L.S. The Components of bone and what they can teach us about regeneration. Materials 2018, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Joseph, K.; Malhotra, S.; Goda, K. Advances in polymer composites: Macro- and microcomposites—State of the art, new challenges, and opportunities. In Polymer Composites; Thomas, S., Joseph, K., Malhotra, S.K., Goda, K., Sreekala, M.S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; Volume 1: Macro and Micro, pp. 1–16. [Google Scholar]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Lee, J.K.Y.; Chen, N.; Peng, S.; Li, L.; Tian, L.; Thakor, N.; Ramakrishna, S. Polymer-based composites by electrospinning: Preparation & functionalization with nanocarbons. Prog. Polym. Sci. 2018, 86, 40–84. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Breitenbach, J. Melt extrusion: From process to drug delivery technology. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. EV 2002, 54, 107–117. [Google Scholar] [CrossRef]
- Sarabu, S.; Bandari, S.; Kallakunta, V.R.; Tiwari, R.; Patil, H.; Repka, M.A. An update on the contribution of hot-melt extrusion technology to advance drug delivery in the 21st century: Part II. Expert Opin. Drug Deliv. 2019, 16, 567. [Google Scholar] [CrossRef] [PubMed]
- Hyvärinen, M.; Jabeen, R.; Kärki, T. The modelling of extrusion processes for polymers—A review. Polymers 2020, 12, 1306. [Google Scholar] [CrossRef]
- Grossiord, N.; Hermant, M.C. Latex technology for conductive polymer nanocomposites. Encycl. Polym. Sci. Technol. 2016, 1–25. [Google Scholar] [CrossRef]
- Cai, D.; Song, M. Latex technology as a simple route to improve the thermal conductivity of a carbon nanotube/polymer composite. Carbon 2008, 46, 2107–2112. [Google Scholar] [CrossRef]
- Beecroft, L.L.; Ober, C.K. Nanocomposite materials for optical applications. Chem. Mater. 1997, 9, 1302–1317. [Google Scholar] [CrossRef]
- Sun, H.; Bai, Y. Science in China series E: Technological sciences in situ preparation of nanoparticles/polymer composites. Sci. China Ser. E—Tech. Sci. 2007, 51, 1886–1901. [Google Scholar] [CrossRef]
- Shuai, C.; Zan, J.; Deng, F.; Yang, Y.; Peng, S.; Zhao, Z. Core–shell-Structured ZIF-8@PDA-HA with Controllable Zinc Ion Release and Superior Bioactivity for Improving a Poly-l-lactic Acid Scaffold. ACS Sustain. Chem. Eng. 2021, 9, 1814–1825. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, Y.; Deng, F.; Shen, L.; Zhao, Z.; Peng, S.; Shuai, C. A bifunctional bone scaffold combines osteogenesis and antibacterial activity via in situ grown hydroxyapatite and silver nanoparticles. Bio-Des. Manuf. 2021, 4, 452–468. [Google Scholar] [CrossRef]
- Huang, H.-M. Medical application of polymer-based composites. Polymers 2020, 12, 2560. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Poot, Á.A.; Grijpma, D.W. Advanced polymer-based composites and structures for biomedical applications. Eur. Polym. J. 2021, 149, 110388. [Google Scholar] [CrossRef]
- Hench, L.L.; Thompson, I. Twenty-first century challenges for biomaterials. J. R. Soc. Interface 2010, 7, S379. [Google Scholar] [CrossRef] [PubMed]
- Yunus Basha, R.; Sampath, S.K.; Doble, M. Design of biocomposite materials for bone tissue regeneration. Mater. Sci. Eng. C 2015, 57, 452–463. [Google Scholar] [CrossRef]
- Holzapfel, B.M.; Reichert, J.C.; Schantz, J.T.; Gbureck, U.; Rackwitz, L.; Nöth, U.; Jakob, F.; Rudert, M.; Groll, J.; Hutmacher, D.W. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 2013, 65, 581–603. [Google Scholar] [CrossRef]
- Ning, C.; Zhou, L.; Tan, G. Fourth-generation biomedical materials. Mater. Today 2016, 19, 2–3. [Google Scholar] [CrossRef]
- Kołodziejska, B.; Kaflak, A.; Kolmas, J. Biologically inspired collagen/apatite composite biomaterials for potential use in bone tissue regeneration—A review. Materials 2020, 13, 1748. [Google Scholar] [CrossRef]
- Rico-Llanos, G.A.; Borrego-González, S.; Moncayo-Donoso, M.; Becerra, J.; Visser, R. Collagen type I biomaterials as scaffolds for bone tissue engineering. Polymers 2021, 13, 599. [Google Scholar] [CrossRef]
- Noro, T.; Itoh, K. Biomechanical behavior of hydroxyapatite as bone substitute material in a loaded implant model. On the surface strain measurement and the maximum compression strength determination of material crash. Bio-Med Mater. Eng. 1999, 9, 319–324. [Google Scholar]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.J.; Morgan, A.W.; Eurell, J.A.C.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Wagoner Johnson, A.J. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite—Past, present, and future in bone regeneration. Bone Tissue Regen. Insights 2016, 7, BTRI.S36138. [Google Scholar] [CrossRef]
- Teotia, A.K.; Raina, D.B.; Singh, C.; Sinha, N.; Isaksson, H.; Tägil, M.; Lidgren, L.; Kumar, A. Nano-hydroxyapatite bone substitute functionalized with bone active molecules for enhanced cranial bone regeneration. ACS Appl. Mater. Interfaces 2017, 9, 6816–6828. [Google Scholar] [CrossRef]
- Ning, L.; Malmström, H.; Ren, Y.-F. Porous collagen-hydroxyapatite scaffolds with mesenchymal stem cells for bone regeneration. J. Oral Implantol. 2015, 41, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Pepla, E.; Besharat, L.K.; Palaia, G.; Tenore, G.; Migliau, G. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: A review of literature. Ann. Stomatol. 2014, 5, 108. [Google Scholar] [CrossRef]
- Coelho, C.C.; Grenho, L.; Gomes, P.S.; Quadros, P.A.; Fernandes, M.H. Nano-hydroxyapatite in oral care cosmetics: Characterization and cytotoxicity assessment. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Asady, S.; Toledano-Osorio, M.; García-Godoy, F.; Serrera-Figallo, M.-A.; Benítez-García, J.A.; Osorio, R. Differential biodegradation kinetics of collagen membranes for bone regeneration. Polymers 2020, 12, 1290. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-W.; Huang, S.-S.; Yu, W.-X.; Hsu, Y.-W.; Hsu, F.-Y. Collagen scaffolds containing hydroxyapatite-CaO fiber fragments for bone tissue engineering. Polymers 2020, 12, 1174. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, S.; Coradin, T.; Worch, H.; Wiesmann, H.P.; Hanke, T. Possibilities and Limitations of preparing silica/collagen/hydroxyapatite composite xerogels as load-bearing biomaterials. Compos. Sci. Technol. 2011, 71, 1873–1880. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, J.; Fan, D. Fabrication of high-strength and porous hybrid scaffolds based on nano-hydroxyapatite and human-like collagen for bone tissue regeneration. Polymers 2020, 12, 61. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Cabral, J.M.S.; Silva CL, d.a.; Vashishth, D. Bone matrix non-collagenous proteins in tissue engineering: Creating new bone by mimicking the extracellular matrix. Polymers 2021, 13, 1095. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Kuo, C.-Y.; Wong, C.-B.; Chien, Y.-M.; Chen, H.-A.; Chen, J.-P. Gelatin/nanohyroxyapatite cryogel embedded poly(lactic-co-glycolic acid)/nanohydroxyapatite microsphere hybrid scaffolds for simultaneous bone regeneration and load-bearing. Polymers 2018, 10, 620. [Google Scholar] [CrossRef]
- Bi, M.; Han, H.; Dong, S.; Zhang, Y.; Xu, W.; Zhu, B.; Wang, J.; Zhou, Y.; Ding, J. Collagen-coated poly(lactide-co-glycolide)/hydroxyapatite scaffold incorporated with dgea peptide for synergistic repair of skull defect. Polymers 2018, 10, 109. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, G.; Qi, F.; Cheng, Y.; Lu, X.; Wang, L.; Zhao, J.; Zhao, B. Enhanced bone regeneration using an insulin-loaded nano-hydroxyapatite/collagen/plga composite scaffold. Int. J. Nanomed. 2017, 13, 117–127. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; Nieto, A.; Vallet-Regí, M. Hydroxyapatite/β-Tricalcium phosphate/agarose macroporous scaffolds for bone tissue engineering. Chem. Eng. J. 2008, 137, 62–71. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156. [Google Scholar] [CrossRef]
- Levengood, S.L.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B Mater. Biol. Med. 2014, 2, 3161. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, P.; Benko, A.; Nocun, M.; Przekora, A. Novel chitosan/agarose/hydroxyapatite nanocomposite scaffold for bone tissue engineering applications: Comprehensive evaluation of biocompatibility and osteoinductivity with the use of osteoblasts and mesenchymal stem cells. Int. J. Nanomed. 2019, 14, 6615. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Palka, K.; Przekora, A. Development and optimization of the novel fabrication method of highly macroporous chitosan/agarose/nanohydroxyapatite bone scaffold for potential regenerative medicine applications. Biomolecules 2019, 9, 434. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic acid: Molecular mechanisms and therapeutic trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef]
- Bakos, D.; Soldán, M.; Hernández-Fuentes, I. Hydroxyapatite-collagen-hyaluronic acid composite. Biomaterials 1999, 20, 191–195. [Google Scholar] [CrossRef]
- Chang, Y.L.; Lo, Y.J.; Feng, S.W.; Huang, Y.C.; Tsai, H.Y.; Lin, C.T.; Huang, H.M.; Fan, K.H. Bone healing improvements using hyaluronic acid and hydroxyapatite/beta-tricalcium phosphate in combination: An animal study. Biomed. Res. Int. 2016, 2016, 8301624. [Google Scholar] [CrossRef] [PubMed]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef]
- Gurumurthy, B.; Griggs, J.A.; Janorkar, A.V. Optimization of collagen-elastin-like polypeptide composite tissue engineering scaffolds using response surface methodology. J. Mech. Behav. Biomed. Mater. 2018, 84, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Tejeda-Montes, E.; Klymov, A.; Nejadnik, M.R.; Alonso, M.; Rodriguez-Cabello, J.C.; Walboomers, X.F.; Mata, A. Mineralization and bone regeneration using a bioactive elastin-like recombinamer membrane. Biomaterials 2014, 35, 8339–8347. [Google Scholar] [CrossRef]
- McCarthy, B.; Yuan, Y.; Koria, P. Elastin-like-polypeptide based fusion proteins for osteogenic factor delivery in bone healing. Biotechnol. Prog. 2016, 32, 1029–1037. [Google Scholar] [CrossRef]
- Gurumurthy, B.; Tucci, M.A.; Fan, L.W.; Benghuzzi, H.A.; Pal, P.; Bidwell, G.L.; Salazar Marocho, S.M.; Cason, Z.; Gordy, D.; Janorkar, A.V. Collagen-elastin-like polypeptide-bioglass scaffolds for guided bone regeneration. Adv. Healthc. Mater. 2020, 9, e1901385. [Google Scholar] [CrossRef]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Farokhi, M.; Mottaghitalab, F.; Samani, S.; Shokrgozar, M.A.; Kundu, S.C.; Reis, R.L.; Fatahi, Y.; Kaplan, D.L. Silk fibroin/hydroxyapatite composites for bone tissue engineering. Biotechnol. Adv. 2018, 36, 68–91. [Google Scholar] [CrossRef] [PubMed]
- Luetchford, K.A.; Chaudhuri, J.B.; de Bank, P.A. Silk fibroin/gelatin microcarriers as scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2020, 106, 110116. [Google Scholar] [CrossRef]
- Kumari, D.; Sheikh, L.; Bhattacharya, S.; Webster, T.J.; Nayar, S. Two-dimensional collagen-graphene as colloidal templates for biocompatible inorganic nanomaterial synthesis. Int. J. Nanomed. 2017, 12, 3605. [Google Scholar] [CrossRef]
- Du, Z.; Wang, C.; Zhang, R.; Wang, X.; Li, X. Applications of graphene and its derivatives in bone repair: Advantages for promoting bone formation and providing real-time detection, challenges and future prospects. Int. J. Nanomed. 2020, 15, 7523. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wong, H.M.; Yeung, K.W.K.; Tjong, S.C. Novel Electrospun polylactic acid nanocomposite fiber mats with hybrid graphene oxide and nanohydroxyapatite reinforcements having enhanced biocompatibility. Polymers 2016, 8, 287. [Google Scholar] [CrossRef]
- Aidun, A.; Safaei Firoozabady, A.; Moharrami, M.; Ahmadi, A.; Haghighipour, N.; Bonakdar, S.; Faghihi, S. Graphene oxide incorporated polycaprolactone/chitosan/collagen electrospun scaffold: Enhanced osteogenic properties for bone tissue engineering. Artif. Organs 2019, 43, E264–E281. [Google Scholar] [CrossRef]
- Sharifi, F.; Atyabi, S.M.; Norouzian, D.; Zandi, M.; Irani, S.; Bakhshi, H. Polycaprolactone/carboxymethyl chitosan nanofibrous scaffolds for bone tissue engineering application. Int. J. Biol. Macromol. 2018, 115, 243–248. [Google Scholar] [CrossRef]
- Baylan, N.; Bhat, S.; Ditto, M.; Lawrence, J.G.; Lecka-Czernik, B.; Yildirim-Ayan, E. Polycaprolactone nanofiber interspersed collagen type-i scaffold for bone regeneration: A unique injectable osteogenic scaffold. Biomed. Mater. 2013, 8, 125011. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, V. When 1 + 1 > 2: Nanostructured composites for hard tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 57, 434–451. [Google Scholar] [CrossRef]
- Ahola, N.; Veiranto, M.; Männistö, N.; Karp, M.; Rich, J.; Efimov, A.; Seppälä, J.; Kellomäki, M. Processing and sustained in vitro release of rifampicin containing composites to enhance the treatment of osteomyelitis. Biomatteria 2012, 2, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.; Pereira, M.; Bettencourt, A.F. Osteomyelitis: An overview of antimicrobial therapy. Braz. J. Pharm. Sci. 2013, 49, 13–27. [Google Scholar] [CrossRef]
- Sharma, S.; Sudhakara, P.; Singh, J.; Ilyas, R.A.; Asyraf, M.R.M.; Razman, M.R. Critical review of biodegradable and bioactive polymer composites for bone tissue engineering and drug delivery applications. Polymers 2021, 13, 2623. [Google Scholar] [CrossRef]
- Pal, P.; Nguyen, Q.C.; Benton, A.H.; Marquart, M.E.; Janorkar, A.V. Drug-loaded elastin-like polypeptide–collagen hydrogels with high modulus for bone tissue engineering. Macromol. Biosci. 2019, 19, 1900142. [Google Scholar] [CrossRef]
- Wagoner Johnson, A.J.; Herschler, B.A. A review of the mechanical behavior of CaP and CaP/polymer composites for applications in bone replacement and repair. Acta Biomater. 2011, 7, 16–30. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, X.; Duan, K.; Guo, L.Y.; Zhou, S.B.; Weng, J. Improving mechanical and biological properties of macroporous HA scaffolds through composite coatings. Colloids Surf. B Biointerfaces 2009, 74, 159–166. [Google Scholar] [CrossRef]
- Shahi, S.; Karbasi, S.; Ahmadi, T.; Naeimi, F.; Goodarzi, V.; Ebrahimi-Barough, S. Evaluation of physical, mechanical and biological properties of β-tri-calcium phosphate/Poly-3-hydroxybutyrate nano composite scaffold for bone tissue engineering application. Mater. Technol. 2021, 36, 237–249. [Google Scholar] [CrossRef]
- Dumas, J.E.; Prieto, E.M.; Zienkiewicz, K.J.; Guda, T.; Wenke, J.C.; Bible, J.; Holt, G.E.; Guelcher, S.A. Balancing the rates of new bone formation and polymer degradation enhances healing of weight-bearing allograft/polyurethane composites in rabbit femoral defects. Tissue Eng. Part A 2014, 20, 115–129. [Google Scholar] [CrossRef]
- Barbieri, D.; de Bruijn, J.D.; Luo, X.; Farè, S.; Grijpma, D.W.; Yuan, H. Controlling dynamic mechanical properties and degradation of composites for bone regeneration by means of filler content. J. Mech. Behav. Biomed. Mater. 2013, 20, 162–172. [Google Scholar] [CrossRef]
- Hesse, B.B.; Langer, M.; Varga, P.; Pacureanu, A.; Dong, P.; Schrof, S.; Männicke, N.; Suhonen, H.; Olivier, C.; Maurer, P.; et al. Alterations of mass density and 3d osteocyte lacunar properties in bisphosphonate-related osteonecrotic human jaw bone, a synchrotron μct study. PLoS ONE 2014, 9, e88481. [Google Scholar] [CrossRef]
- Iezzi, G.; Mangano, C.; Barone, A.; Tirone, F.; Baggi, L.; Tromba, G.; Piattelli, A.; Giuliani, A. Jawbone remodeling: A conceptual study based on synchrotron high-resolution tomography. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Matsubara, T.; Suardita, K.; Ishii, M.; Sugiyama, M.; Igarashi, A.; Oda, R.; Nishimura, M.; Saito, M.; Nakagawa, K.; Yamanaka, K.; et al. Alveolar bone marrow as a cell source for regenerative medicine: Differences between alveolar and iliac bone marrow stromal cells. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2005, 20, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Akintoye, S.O.; Lam, T.; Shi, S.; Brahim, J.; Collins, M.T.; Robey, P.G. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone 2006, 38, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Aghaloo, T.L.; Chaichanasakul, T.; Bezouglaia, O.; Kang, B.; Franco, R.; Dry, S.M.; Atti, E.; Tetradis, S. Osteogenic potential of mandibular vs. long-bone marrow stromal cells. J. Dent. Res. 2010, 89, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Obradović, O.; Todorovic, L.; Vitanovic, V. Anatomical considerations relevant to implant procedures in the mandible. Bull. Group Int. Rech. Sci. Stomatol. Odontol. 1995, 38, 39–44. [Google Scholar] [PubMed]
- Kumar, B.P.; Venkatesh, V.; Kumar, K.A.; Yadav, B.Y.; Mohan, S.R. Mandibular reconstruction: Overview. J. Maxillofac. Oral. Surg. 2016, 15, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Basyuni, S.; Ferro, A.; Santhanam, V.; Birch, M.; McCaskie, A. Systematic scoping review of mandibular bone tissue engineering. Br. J. Oral Maxillofac. Surg. 2020, 58, 632–642. [Google Scholar] [CrossRef]
- Nelms, L.; Palmer, W.J. Tissue engineering in mandibular reconstruction: Osteogenesis-inducing scaffolds. Plast. Aesthet. Res. 2019, 6, 21. [Google Scholar] [CrossRef]
- Wong, R.C.; Tideman, H.; Kin, L.; Merkx, M.A. Biomechanics of mandibular reconstruction: A review. Int. J. Oral. Maxillofac. Surg. 2010, 39, 313–319. [Google Scholar] [CrossRef]
- Mehrotra, D. TMJ bioengineering: A review. J. Oral Biol. Craniofacial Res. 2013, 3, 140. [Google Scholar] [CrossRef]
- Kumar, P.; Vinitha, B.; Fathima, G. Bone grafts in dentistry. J. Pharm. Bioallied Sci. 2013, 5, S125. [Google Scholar] [CrossRef]
- Nanci, A.; Bosshardt, D.D. Structure of periodontal tissues in health and disease. Periodontol. 2000 2006, 40, 11–28. [Google Scholar] [CrossRef]
- Ohba, S.; Sumita, Y.; Nakatani, Y.; Noda, S.; Asahina, I. Alveolar bone preservation by a hydroxyapatite/collagen composite material after tooth extraction. Clin. Oral Investig. 2019, 23, 2413–2419. [Google Scholar] [CrossRef]
- Jeong, H.J.; Gwak, S.J.; Seo, K.D.; Lee, S.; Yun, J.H.; Cho, Y.S.; Lee, S.J. Fabrication of Three-dimensional composite scaffold for simultaneous alveolar bone regeneration in dental implant installation. Int. J. Mol. Sci. 2020, 21, 1863. [Google Scholar] [CrossRef]
- Paz, J.L.C.; Soares, C.J.; Rodrigues, J.F.; de Araújo Almeida, G.; Soares, P.B.F. Fractured alveolar process displacement evaluation-effect of the rigidity of wire-composite splints. Dent. Traumatol. Off. Publ. Int. Assoc. Dent. Traumatol. 2021, 37, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Tumedei, M.; Mourão, C.F.; D’Agostino, S.; Dolci, M.; di Cosola, M.; Piattelli, A.; Lucchese, A. Histological and histomorphometric effectiveness of the barrier membranes for jawbone regeneration: An overview of more than 30 years’ experience of research results of the italian implant retrieval center (1988–2020). Appl. Sci. 2021, 11, 2438. [Google Scholar] [CrossRef]
- Simion, M.; Misitano, U.; Gionso, L.; Salvato, A. Treatment of dehiscences and fenestrations around dental implants using resorbable and nonresorbable membranes associated with bone autografts: A comparative clinical study. Int. J. Oral. Maxillofac. Implant. 1997, 12, 159–167. [Google Scholar]
- Cerrai, P.; Guerra, G.D.; Tricoli, M.; Krajewski, A.; Ravaglioli, A.; Martinetti, R.; Dolcini, L.; Fini, M.; Scarano, A.; Piattelli, A. Periodontal membranes from composites of hydroxyapatite and bioresorbable block copolymers. J. Mater. Sci. Mater. Med. 1999, 10, 677–682. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, B.; Man, C.; Ma, Y.; Hu, J. NEL-like molecule-1-modified bone marrow mesenchymal stem cells/poly lactic-co-glycolic acid composite improves repair of large osteochondral defects in mandibular condyle. Osteoarthr. Cartil. 2011, 19, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Suzuki, O.; Matsui, K.; Tanuma, Y.; Takahashi, T.; Kamakura, S. Octacalcium phosphate collagen composite facilitates bone regeneration of large mandibular bone defect in humans. J. Tissue Eng. Regen. Med. 2017, 11, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Yukna, R.A. Clinical evaluation of HTR polymer bone replacement grafts in human mandibular class II molar furcations. J. Periodontol. 1994, 65, 342–349. [Google Scholar] [CrossRef]
- Tahmasebi, E.; Alam, M.; Yazdanian, M.; Tebyanian, H.; Yazdanian, A.; Seifalian, A.; Mosaddad, S.A. Current biocompatible materials in oral regeneration: A comprehensive overview of composite materials. J. Mater. Res. Technol. 2020, 9, 11731–11755. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraile-Martínez, O.; García-Montero, C.; Coca, A.; Álvarez-Mon, M.A.; Monserrat, J.; Gómez-Lahoz, A.M.; Coca, S.; Álvarez-Mon, M.; Acero, J.; Bujan, J.; et al. Applications of Polymeric Composites in Bone Tissue Engineering and Jawbone Regeneration. Polymers 2021, 13, 3429. https://doi.org/10.3390/polym13193429
Fraile-Martínez O, García-Montero C, Coca A, Álvarez-Mon MA, Monserrat J, Gómez-Lahoz AM, Coca S, Álvarez-Mon M, Acero J, Bujan J, et al. Applications of Polymeric Composites in Bone Tissue Engineering and Jawbone Regeneration. Polymers. 2021; 13(19):3429. https://doi.org/10.3390/polym13193429
Chicago/Turabian StyleFraile-Martínez, Oscar, Cielo García-Montero, Alejandro Coca, Miguel Angel Álvarez-Mon, Jorge Monserrat, Ana M. Gómez-Lahoz, Santiago Coca, Melchor Álvarez-Mon, Julio Acero, Julia Bujan, and et al. 2021. "Applications of Polymeric Composites in Bone Tissue Engineering and Jawbone Regeneration" Polymers 13, no. 19: 3429. https://doi.org/10.3390/polym13193429
APA StyleFraile-Martínez, O., García-Montero, C., Coca, A., Álvarez-Mon, M. A., Monserrat, J., Gómez-Lahoz, A. M., Coca, S., Álvarez-Mon, M., Acero, J., Bujan, J., García-Honduvilla, N., Asúnsolo, Á., & Ortega, M. A. (2021). Applications of Polymeric Composites in Bone Tissue Engineering and Jawbone Regeneration. Polymers, 13(19), 3429. https://doi.org/10.3390/polym13193429