Influence of Formate Concentration on the Rheology and Thermal Degradation of Xanthan Gum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Rheological Measurements
2.3. Thermal Aging and Degradation
3. Results and Discussion
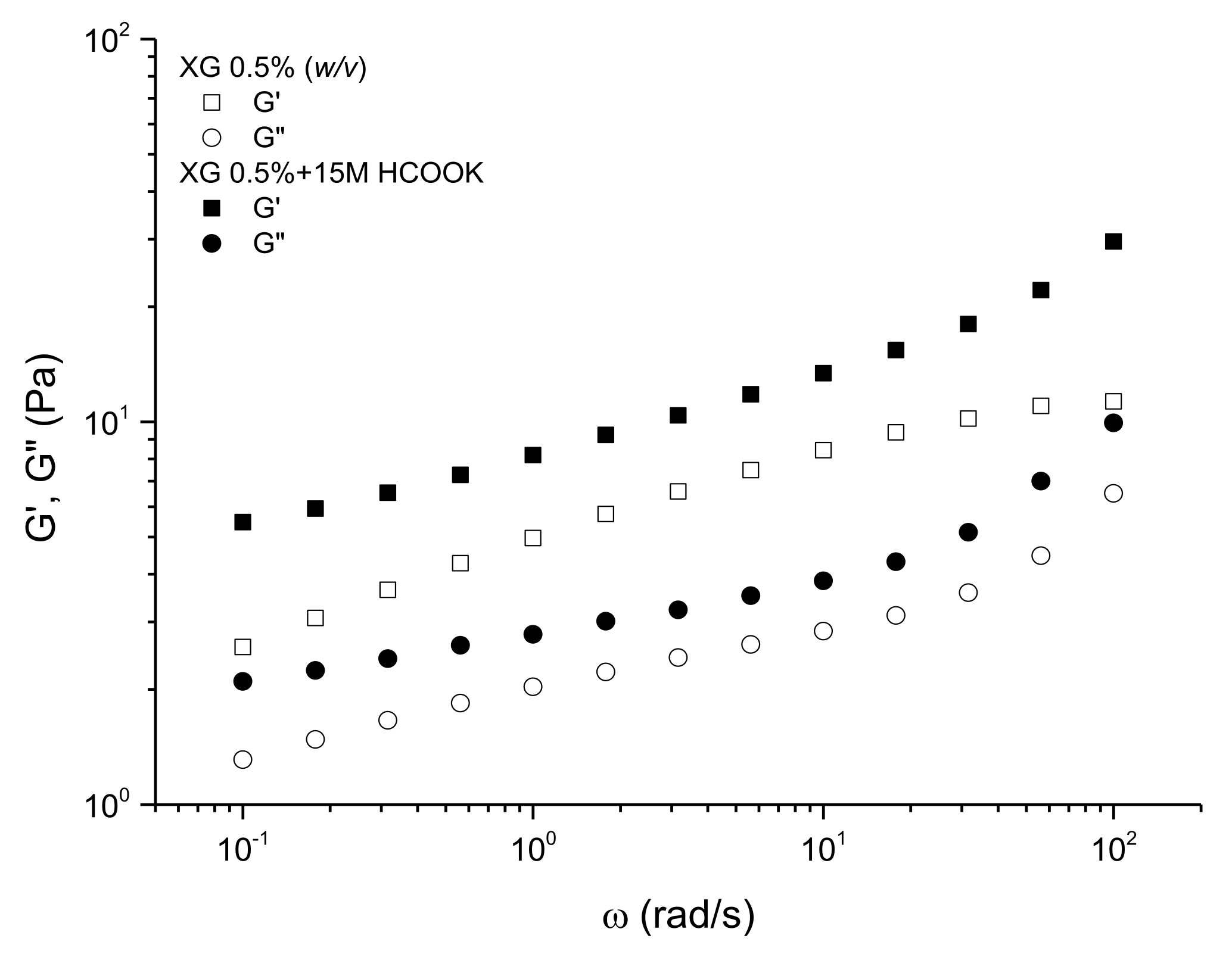
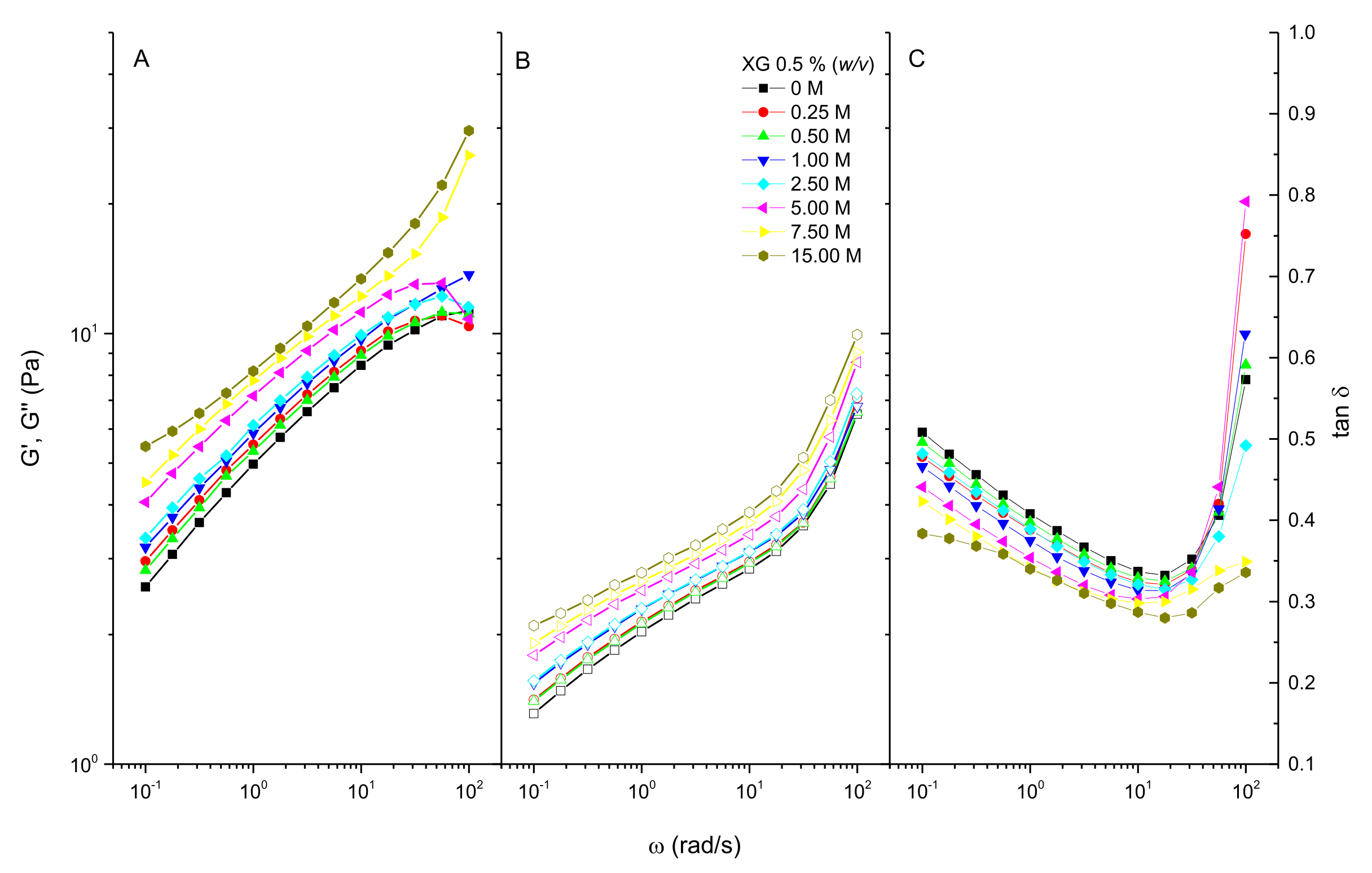
3.1. Viscoelastic Behavior
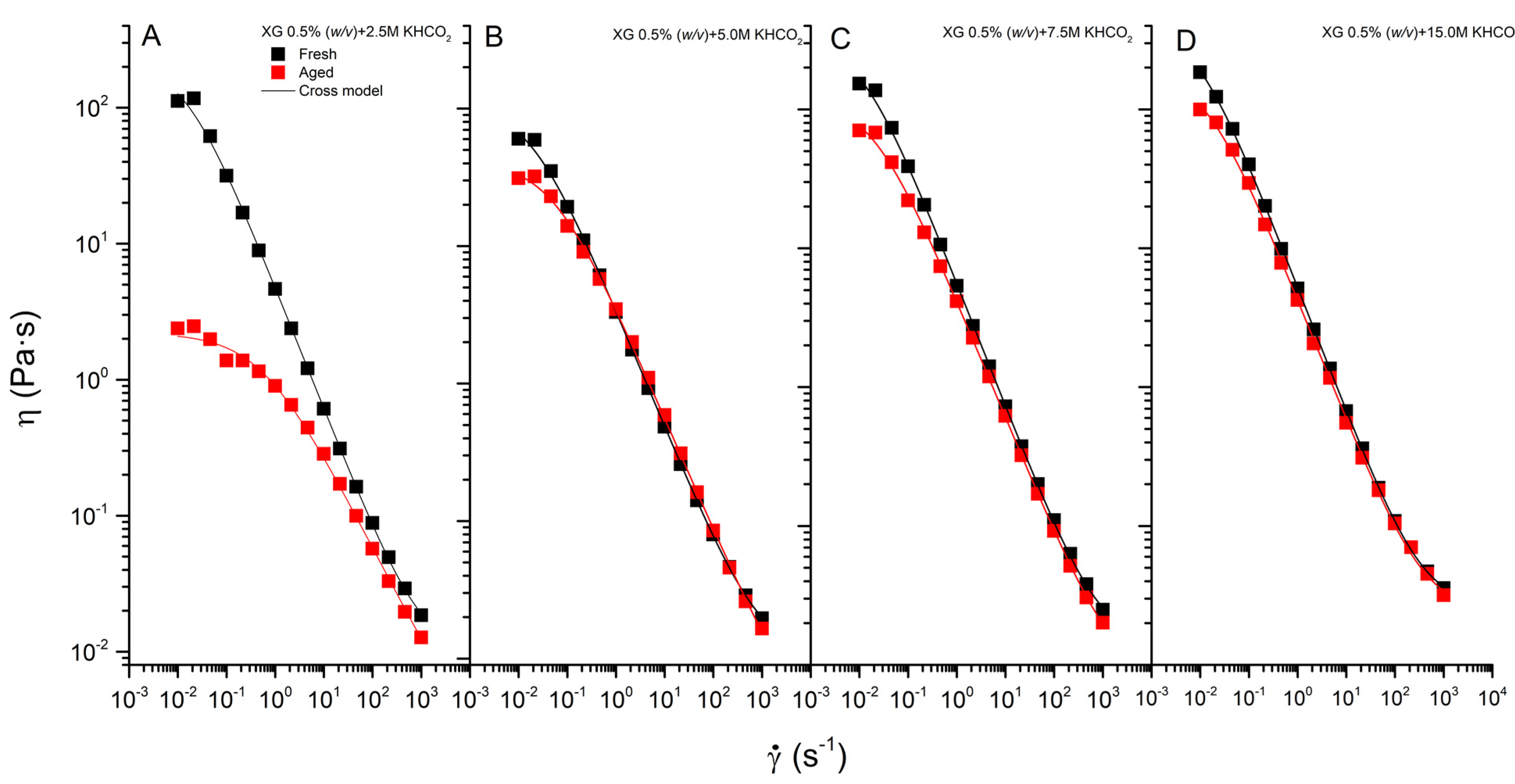
3.2. Flow Behavior
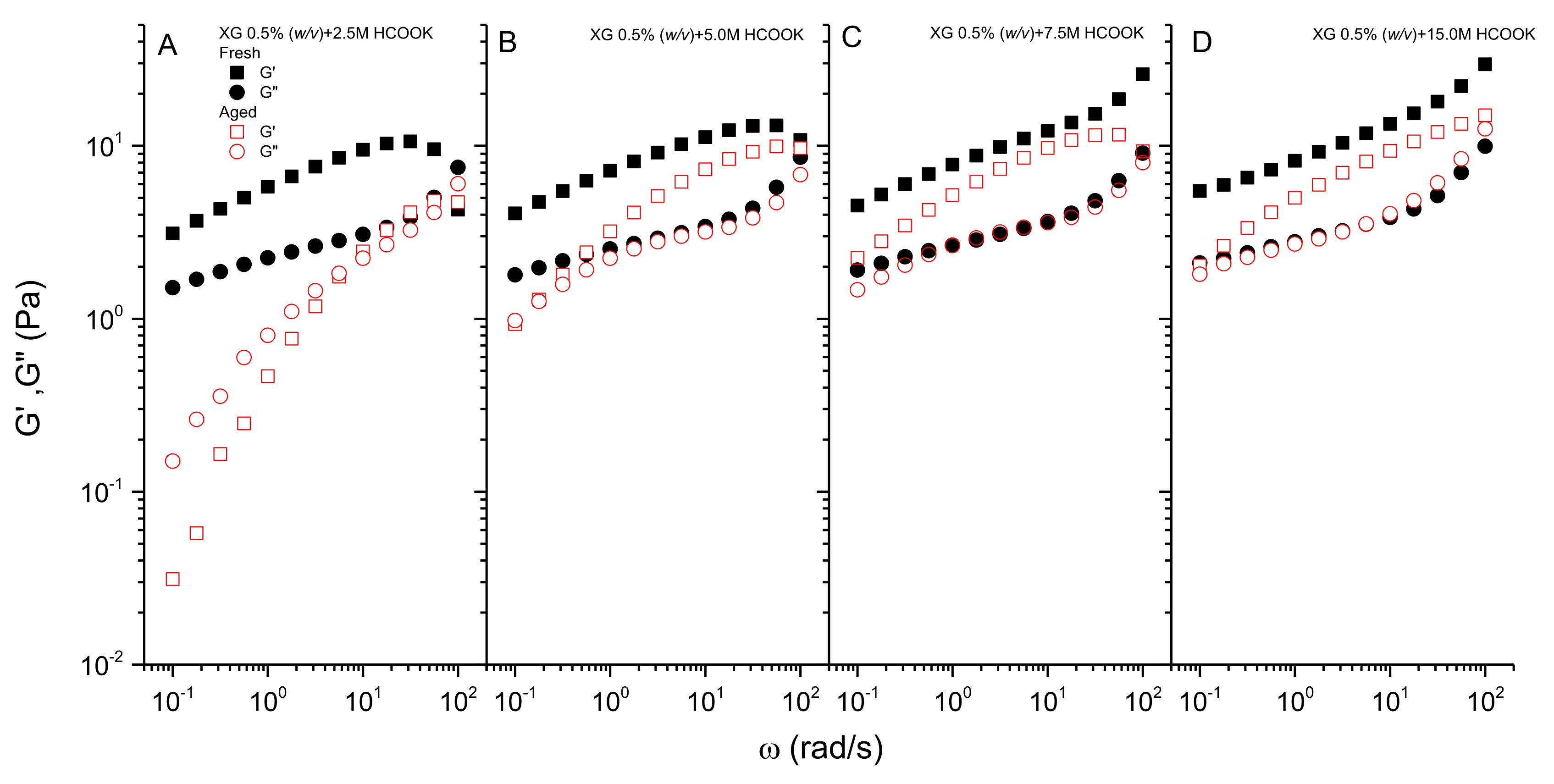
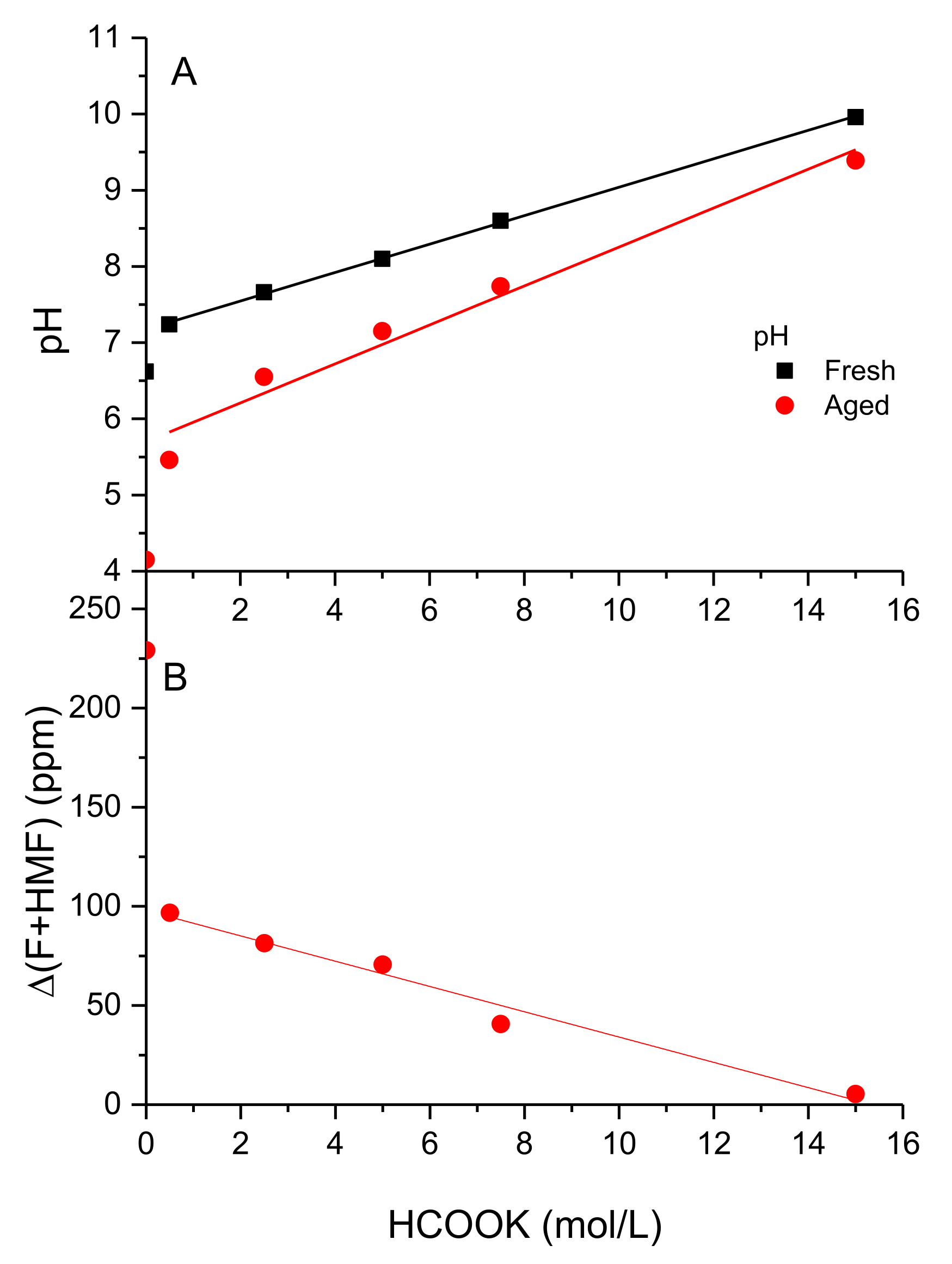
3.3. Effect of Aging on the Rheological Behavior
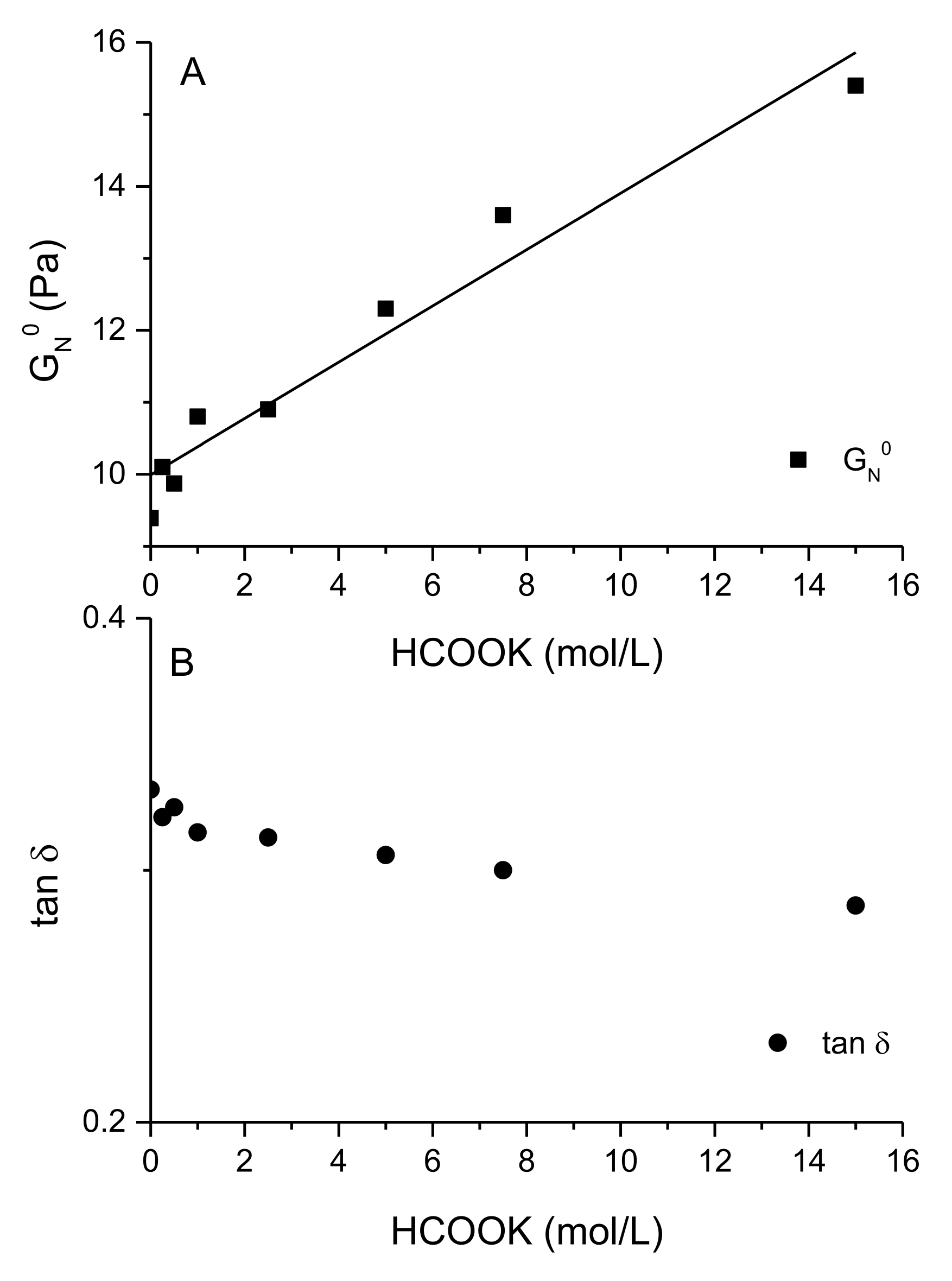
3.4. Structure-Properties Relationships
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, B.; Romero-Zerón, L.; Rodrigue, D. Mechanical properties and flow behavior of polymers for enhanced oil recovery. J. Macromol. Sci. Part B Phys. 2014, 53, 625–644. [Google Scholar] [CrossRef]
- Jang, H.Y.; Zhang, K.; Chon, B.H.; Choi, H.J. Enhanced oil recovery performance and viscosity characteristics of polysaccharide xanthan gum solution. J. Ind. Eng. Chem. 2015, 21, 741–745. [Google Scholar] [CrossRef]
- Asafa, K.A.; Shah, S.N. Rheology and flow characteristics of xanthan in calcium chloride brine. In Proceedings of the SPE/ICoTA Coiled Tubing and Well Intervention Conference and Exhibition, The Woodlands, TX, USA, March 2014. [Google Scholar] [CrossRef]
- Hermoso, J.; Martínez-Boza, F.; Gallegos, C. Influence of viscosity modifier nature and concentration on the viscous flow behavior of oil-based drilling fluids at high pressure. Appl. Clay Sci. 2014, 87, 14–21. [Google Scholar] [CrossRef]
- Hermoso, J.; Martínez-Boza, F.; Gallegos, C. Combined effect of pressure and temperature on the viscous behaviour of all-oil drilling fluids. Oil Gas Sci. Technol. 2014, 69, 1283–1296. [Google Scholar] [CrossRef] [Green Version]
- Hermoso, J.; Martínez-Boza, F.; Gallegos, C. Influence of aqueous phase volume fraction, organoclay concentration and pressure on invert-emulsion oil muds rheology. J. Ind. and Eng. Chem. 2015, 22, 341–349. [Google Scholar] [CrossRef]
- Boul, P.J.; Abdulquddos, S.; Thaemlitz, C.J. High performance brine viscosifiers for high temperatures. In Proceedings of the SPE Middle East Oil & Gas Show and Conference, Manama, Kingdom of Bahrain, March 2017. [Google Scholar] [CrossRef]
- Howard, S.; Kaminski, L.; Downs, J. Xanthan stability in formate brines-Formulating non-damaging fluids for high temperature applications. In Proceedings of the SPE European Formation Damage Conference and Exhibition, Budapest, Hungary, June 2015. [Google Scholar] [CrossRef]
- Reinoso, D.; Martin-Alfonso, M.J.; Luckham, P.F.; Martinez-Boza, F.J. Rheological characterisation of xanthan gum in brine solutions at high temperature. Carbohydr. Polym. 2019, 203, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ochoa, F.; Santos, V.E.; Casas, J.A.; Gómez, E. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef]
- Fitzpatrick, P.; Meadows, J.; Ratcliffe, I.; Williams, P.A. Control of the properties of xanthan/glucomannan mixed gels by varying xanthan fine structure. Carbohydr. Polym. 2013, 92, 1018–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochefort, W.E.; Middleman, S. Rheology of xanthan gum: Salt, temperature, and strain effects in oscillatory and steady shear experiments. J. Rheol. 1987, 31, 337–369. [Google Scholar] [CrossRef]
- Whitcomb, P.J.; Macosko, C.W. Rheology of xanthan gum. J. Rheol. 1978, 22, 493–505. [Google Scholar] [CrossRef]
- Lee, H.C.; Brant, D.A. Rheology of concentrated isotropic and anisotropic xanthan solutions. 1. A rodlike low molecular weight sample. Macromolecules 2002, 35, 2212–2222. [Google Scholar] [CrossRef]
- Choppe, E.; Puaud, F.; Nicolai, T.; Benyahia, L. Rheology of xanthan solutions as a function of temperature, concentration and ionic strength. Carbohydr. Polym. 2010, 82, 1228–1235. [Google Scholar] [CrossRef]
- Marcotte, M.; Hoshahili, A.R.T.; Ramaswamy, H.S. Rheological properties of selected hydrocolloids as a function of concentration and temperature. Food Res. Int. 2001, 34, 695–703. [Google Scholar] [CrossRef]
- Wyatt, N.B.; Liberatore, M.W. Rheology and viscosity scaling of the polyelectrolyte xanthan gum. J. Appl. Polym. Sci. 2009, 114, 4076–4084. [Google Scholar] [CrossRef]
- Lambert, F.; Rinaudo, M. On the thermal stability of xanthan gum. Polymer 1985, 26, 1549–1553. [Google Scholar] [CrossRef]
- Xie, W.; Lecourtier, J. Xanthan behaviour in water-based drilling fluids. Polym. Degrad. Stab. 1992, 38, 155–164. [Google Scholar] [CrossRef]
- Reinoso, D.; Martín-Alfonso, M.J.; Luckham, P.F.; Martinez-Boza, F.J. Flow behavior and thermal resistance of xanthan gum in formate brine. J. Petrol. Sci. Eng. 2020, 188, 106881. [Google Scholar] [CrossRef]
- Wu, M.; Shi, Z.; Ming, Y.; Wang, C.; Qiu, X.; Li, G.; Ma, T. Thermostable and rheological properties of natural and genetically engineered xanthan gums in different solutions at high temperature. Int. J. Biol. Macromol. 2021, 182, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Hermoso, J.; Jofore, B.D.; Martínez-Boza, F.J.; Gallegos, C. High pressure mixing rheology of drilling fluids. Ind. Eng. Chem. Res. 2012, 51, 14399–14407. [Google Scholar] [CrossRef]
- Hermoso, J.; Martínez-Boza, F.J.; Gallegos, C. Modeling Pressure-Viscosity Behavior of Oil-Based Drilling Fluids. Oil Gas Sci. Technol. 2017, 72, 18. [Google Scholar] [CrossRef] [Green Version]
- Bradshaw, R.J.; Hodge, R.M.; Wolf, N.O.; Knox, D.A.; Hudson, C.E.; Evans, E. Formate-based reservoir drilling fluid resolves high-temperature challenges in the Natuna Sea. In Proceedings of the International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, February 2006. [Google Scholar] [CrossRef]
- Hermoso, J.; Martínez-Boza, F.J.; Gallegos, C. Organoclay influence on high pressure-high temperature volumetric properties of oil-based drilling fluids. J. Pet. Sci. Eng. 2017, 151, 13–23. [Google Scholar] [CrossRef]
- Heggset, E.B.; Chinga-Carrasco, G.; Syverud, K. Temperature stability of nanocellulose dispersions. Carbohydr. Polym. 2017, 157, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, N.B.; Gunther, C.M.; Liberatore, M.W. Increasing viscosity in entangled polyelectrolyte solutions by the addition of salt. Polymer 2011, 52, 2437–2444. [Google Scholar] [CrossRef]
- Pelletier, E.; Viebke, C.; Meadows, J.; Williams, P.A. A rheological study of the order–disorder conformational transition of xanthan gum. Biopolymers 2001, 59, 339–346. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Rubinstein, M. Theory of polyelectrolytes in solutions and at surfaces. Prog. Polym. Sci. 2005, 30, 1049–1118. [Google Scholar] [CrossRef]
- Ross-Murphy, S.B. Structure-property relationships in food biopolymer gels and solutions. J. Rheol. 1995, 39, 1451–1463. [Google Scholar] [CrossRef]
- Song, K.W.; Kim, Y.S.; Chang, G.S. Rheology of concentrated xanthan gum solutions: Steady shear flow behavior. Fibers Polym. 2006, 7, 129–138. [Google Scholar] [CrossRef]
- Cross, M.M. Rheology of non-Newtonian fluids: A new flow equation for pseudoplastic systems. J. Colloid Sci. 1965, 20, 417–434. [Google Scholar] [CrossRef]
- Clarke-Sturman, A.J.; Pedley, J.B.; Sturla, P.L. Influence of anions on the properties of microbial polysaccharides in solution. Int. J. Biol. Macromol. 1986, 8, 355–360. [Google Scholar] [CrossRef]
- Ash, S.G.; Clarke-Sturman, A.J.; Calvert, R.; Nisbet, T.M. Chemical stability of biopolymer solutions. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Francisco, CA, USA, October 1983. [Google Scholar] [CrossRef]
- Wellington, S.L. Biopolymer solution viscosity stabilization-Polymer degradation and antioxidant use. SPE J. 1983, 23, 901–912. [Google Scholar] [CrossRef]
- Seright, R.S.; Henrici, B.J. Xanthan stability at elevated temperatures. SPE Reservoir Eng. 1990, 5, 52–60. [Google Scholar] [CrossRef]
- Downs, J.D. High-temperature stabilization of xanthan in drilling fluids by the use of formate salts. In Physical Chemistry of Colloids and Interfaces in Oil Production; Toulhoat, H., Lecourtier, J., Eds.; Editions TECHNIP: Paris, France, 1992; pp. 197–202. ISBN 2-7108-0618-5. [Google Scholar]
- Callet, F.; Milas, M.; Rinaudo, M. On the role of thermal treatments on the properties of xanthan solutions. Carbohydr. Polym. 1989, 11, 127–137. [Google Scholar] [CrossRef]
- Howard, S. Formate Manual, Section B5, Compatibility with Additives, 2nd ed.; Cabot Corporation: Boston, MA, USA, 2009. [Google Scholar]
| Fresh Xanthan 0.5% (w/v) | ||||
|---|---|---|---|---|
| KHCO2 (mol/L) | η0 (Pa·s) | η∞ (Pa·s) | k (s) | m |
| 0 | 145.98 | 6.02 × 10−3 | 52.3 | 0.881 |
| 0.25 | 186.06 | 7.39 × 10−3 | 61.5 | 0.894 |
| 0.5 | 221.43 | 7.47 × 10−3 | 61.9 | 0.890 |
| 1 | 191.68 | 7.82 × 10−3 | 63.1 | 0.891 |
| 2.5 | 216.60 | 9.34 × 10−3 | 68.9 | 0.899 |
| 5 | 266.08 | 1.14 × 10−2 | 73.7 | 0.907 |
| 7.5 | 328.67 | 1.40 × 10−2 | 94.6 | 0.893 |
| 15 | 428.43 | 2.63 × 10−2 | 129.7 | 0.904 |
| HCOOK (mol/L) | η0 (Pa·s) | η∞ (Pa·s) | k (s) | m | η0Ag/η0Fr (%) | kAg/kFr (%) | mAg/mFr (%) |
|---|---|---|---|---|---|---|---|
| 0 | 6.5 × 10−3 | - | - | ||||
| 0.25 | 6.4 × 10−3 | - | - | ||||
| 0.50 | 6.7 × 10−3 | - | - | ||||
| 1.00 | 7.8 × 10−3 | - | - | ||||
| 2.50 | 2.200 | 1.00 × 10−3 | 1.70 | 0.707 | 1.00 | 2.40 | 78.6 |
| 5.00 | 41.41 | 2.64 × 10−3 | 19.3 | 0.817 | 15.2 | 26.1 | 90.1 |
| 7.50 | 120.60 | 7.50 × 10−3 | 52.3 | 0.847 | 36.7 | 55.3 | 94.8 |
| 15.00 | 192.00 | 2.39 × 10−2 | 77.1 | 0.874 | 44.8 | 59.4 | 96.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Alfonso, M.J.; Loaiza, J.M.; Delgado-Sánchez, C.; Martínez-Boza, F.J. Influence of Formate Concentration on the Rheology and Thermal Degradation of Xanthan Gum. Polymers 2021, 13, 3378. https://doi.org/10.3390/polym13193378
Martín-Alfonso MJ, Loaiza JM, Delgado-Sánchez C, Martínez-Boza FJ. Influence of Formate Concentration on the Rheology and Thermal Degradation of Xanthan Gum. Polymers. 2021; 13(19):3378. https://doi.org/10.3390/polym13193378
Chicago/Turabian StyleMartín-Alfonso, María José, Javier Mauricio Loaiza, Clara Delgado-Sánchez, and Francisco José Martínez-Boza. 2021. "Influence of Formate Concentration on the Rheology and Thermal Degradation of Xanthan Gum" Polymers 13, no. 19: 3378. https://doi.org/10.3390/polym13193378
APA StyleMartín-Alfonso, M. J., Loaiza, J. M., Delgado-Sánchez, C., & Martínez-Boza, F. J. (2021). Influence of Formate Concentration on the Rheology and Thermal Degradation of Xanthan Gum. Polymers, 13(19), 3378. https://doi.org/10.3390/polym13193378







