Microwave Enabled Physically Cross Linked Sodium Alginate and Pectin Film and Their Application in Combination with Modified Chitosan-Curcumin Nanoparticles. A Novel Strategy for 2nd Degree Burns Wound Healing in Animals
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Film Formulation
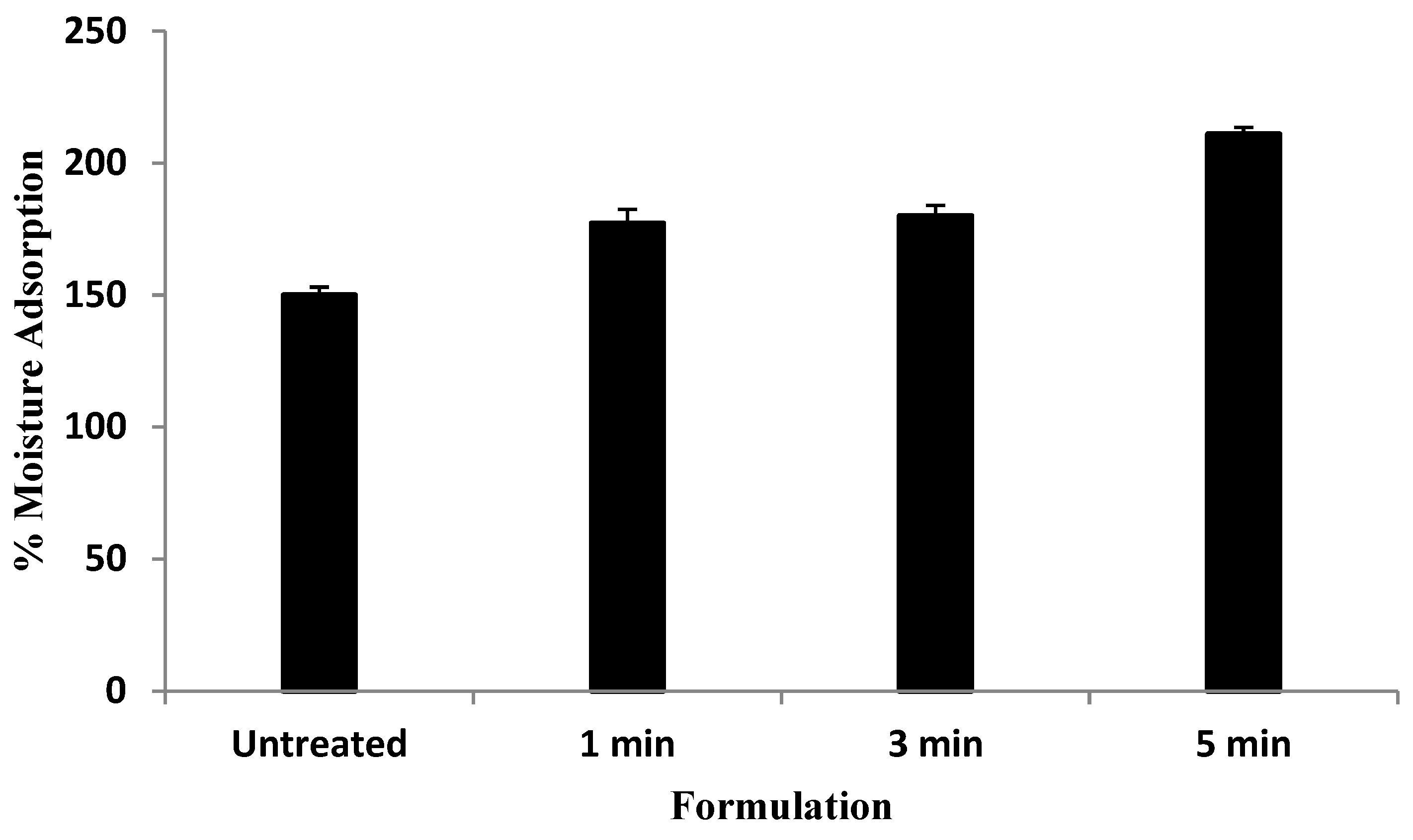
2.3. Moisture Adsorption
2.4. Water Vapor Transmission (WVTR) and Vapor Permeability Rate (WVP)
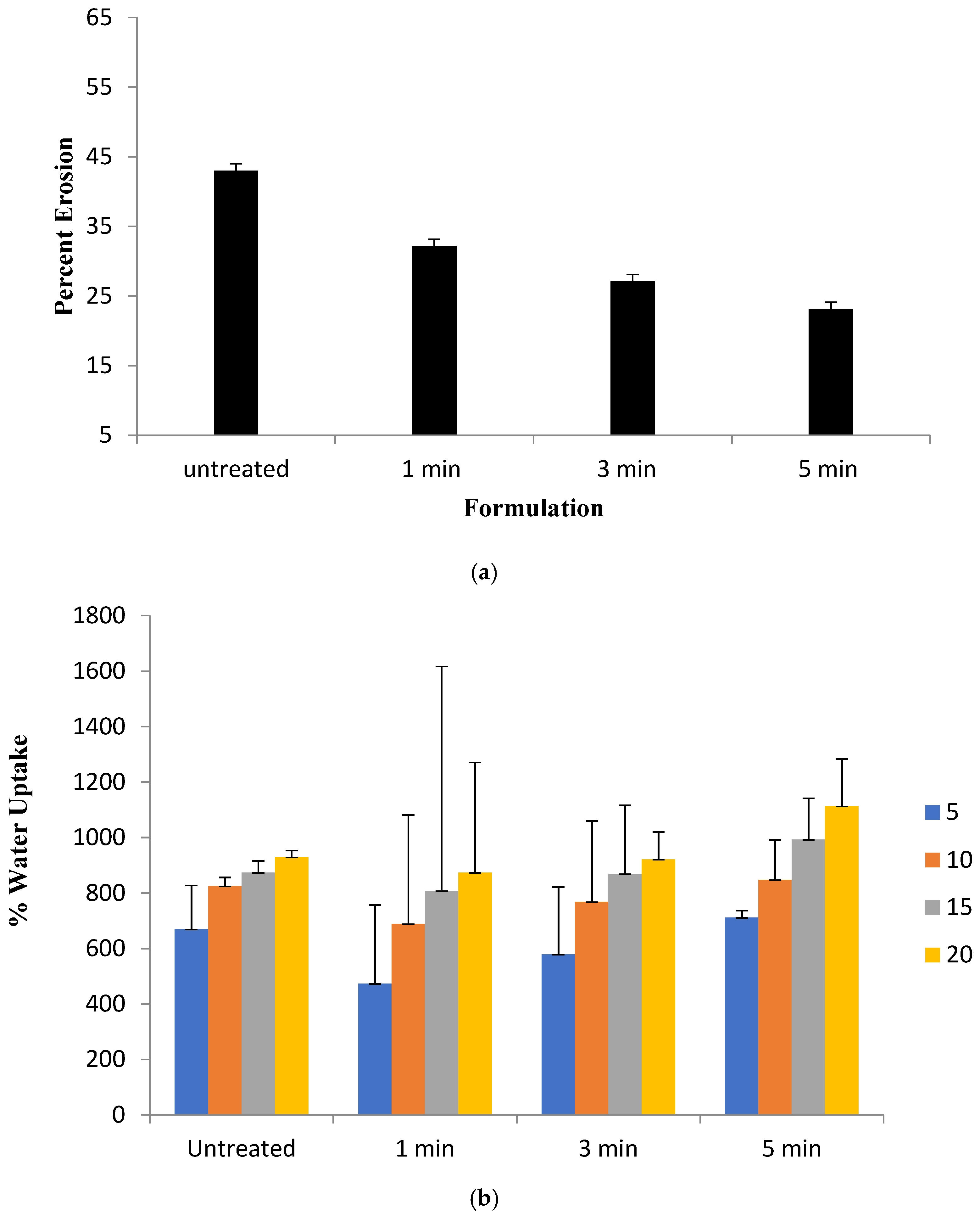
2.5. Erosion and Water Uptake
2.6. Tensile Strength
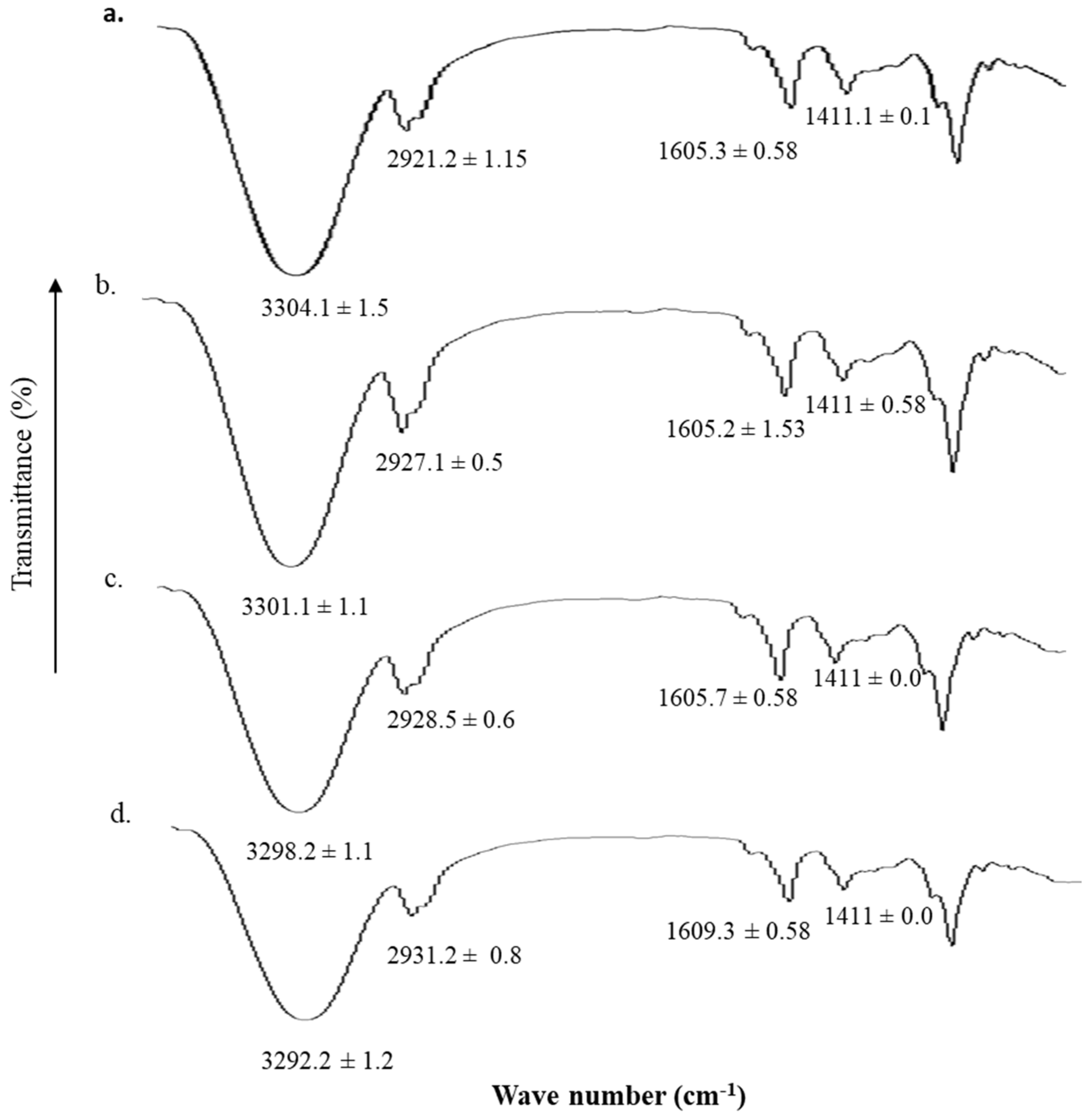
2.7. Vibrational Spectroscopic Analysis
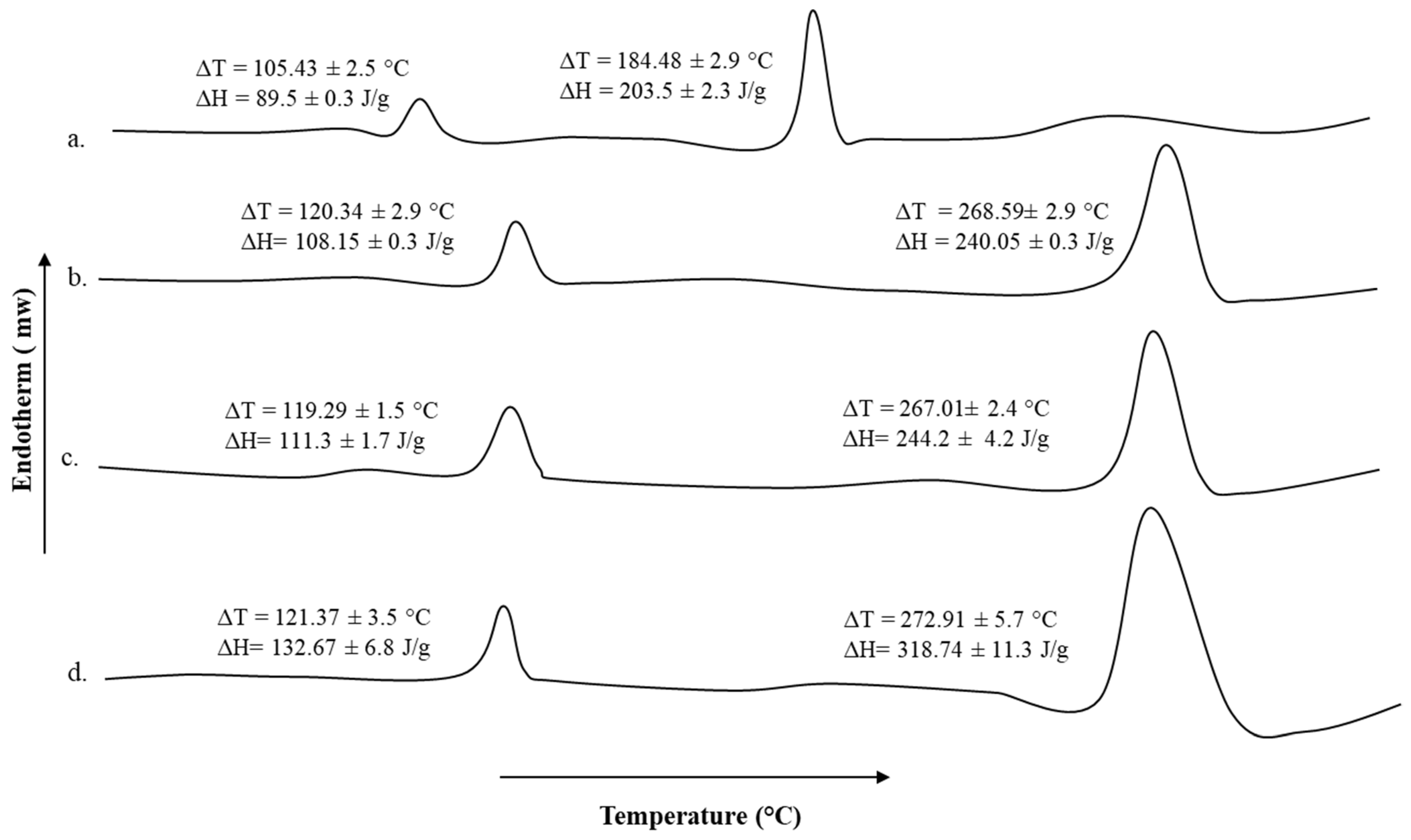
2.8. Thermal Analysis
2.9. Morphology
2.10. In Vivo Wound Healing
2.11. Wound Morphology
2.12. Skin Histology
2.13. Vibrational and Thermal Analysis of Skin
2.14. Tensile Strength of Skin Samples
2.15. Statistical Analysis
3. Results
3.1. Moisture Adsorption
3.2. Water Vapor Transmission Rate (WVTR) and Water Vapor Permeability (WVP)
3.3. Erosion and Water Uptake
3.4. Tensile Strength
3.5. Vibrational Analysis
3.6. Thermal Analysis
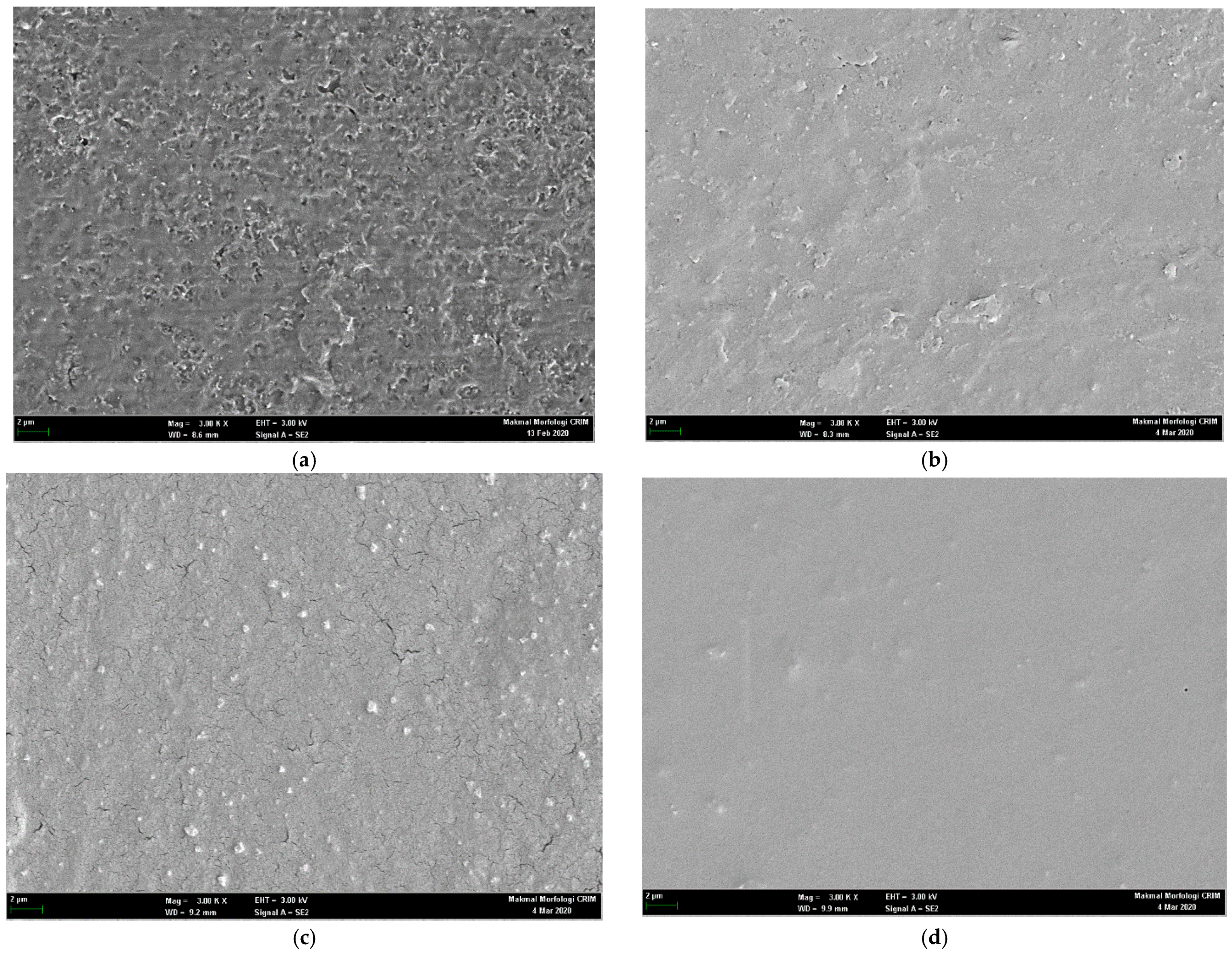
3.7. Scanning Electron Microscopy
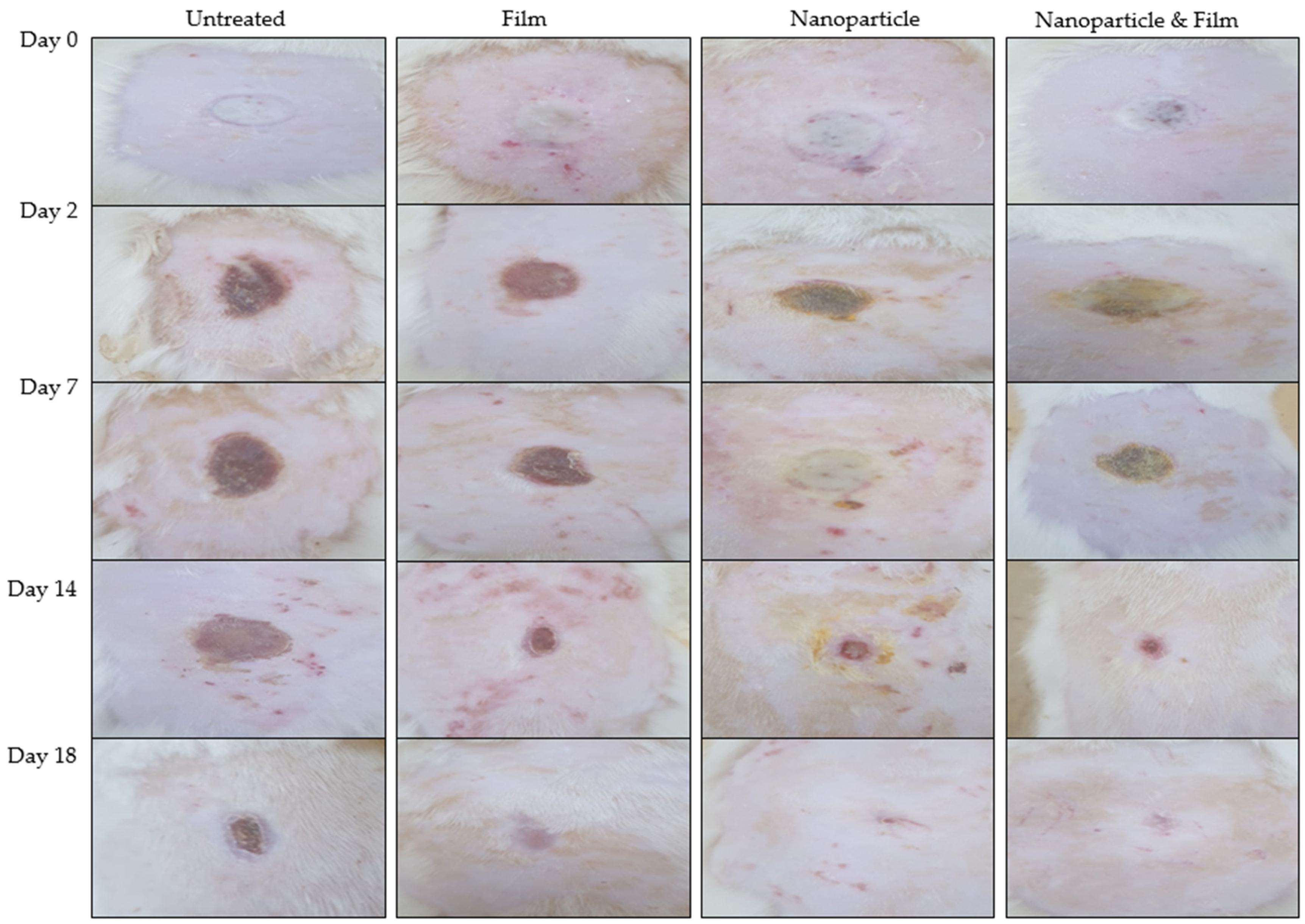
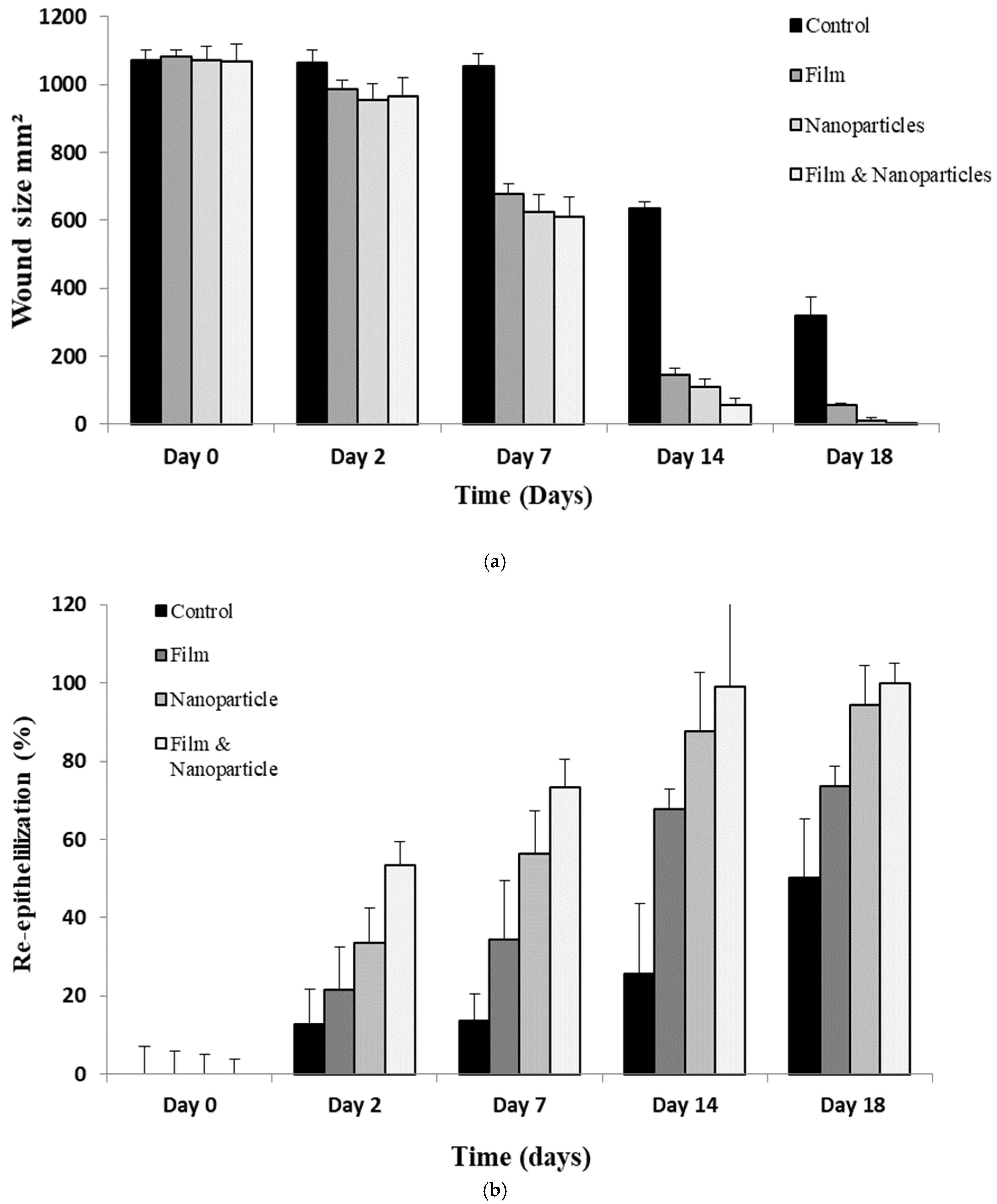
3.8. Wound Morphology
3.9. Vibrational Spectroscopic Analysis (ATR-FTIR) of Skin Samples
3.10. Thermal and Mechanical Strength Analysis of Skin Samples
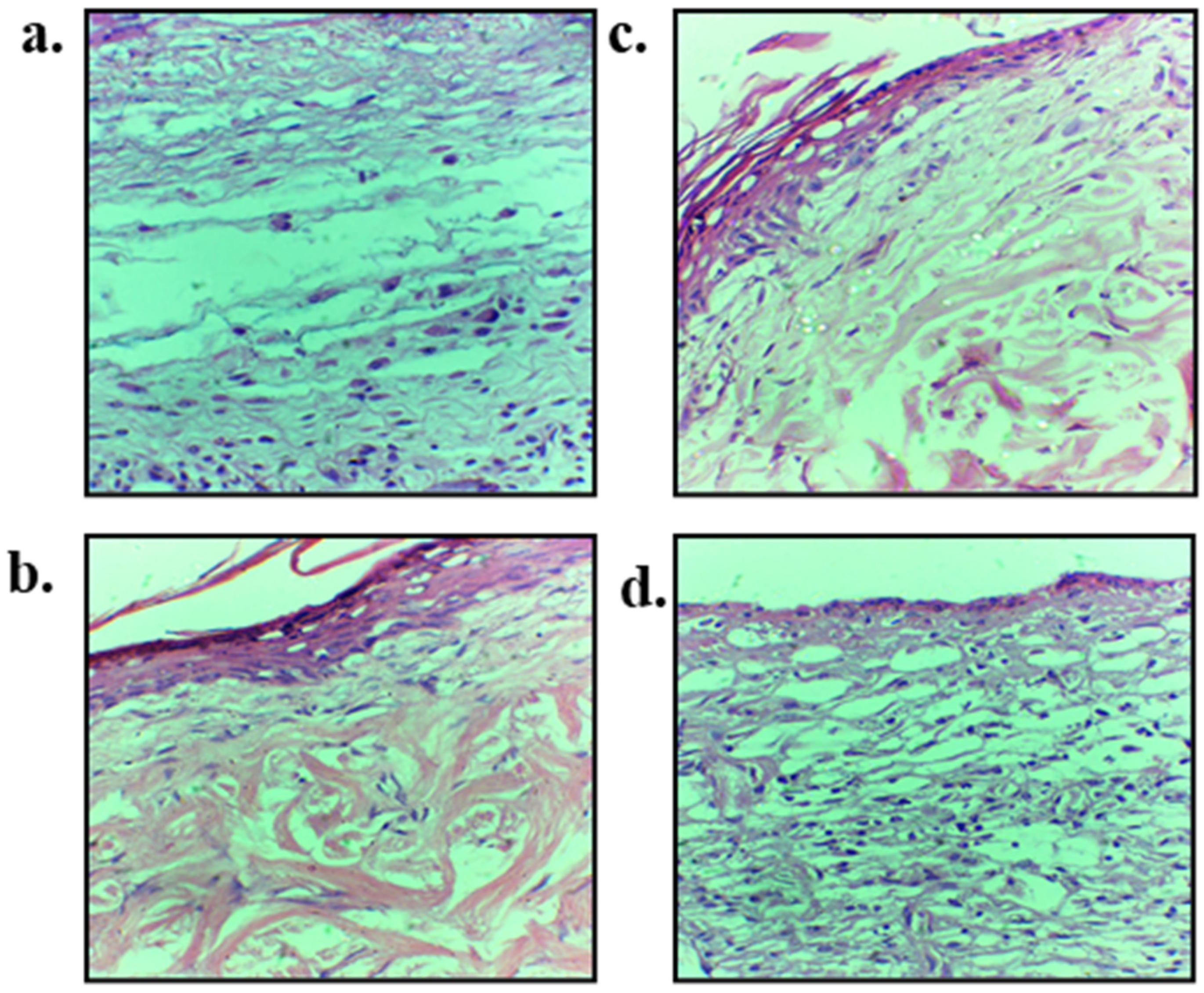
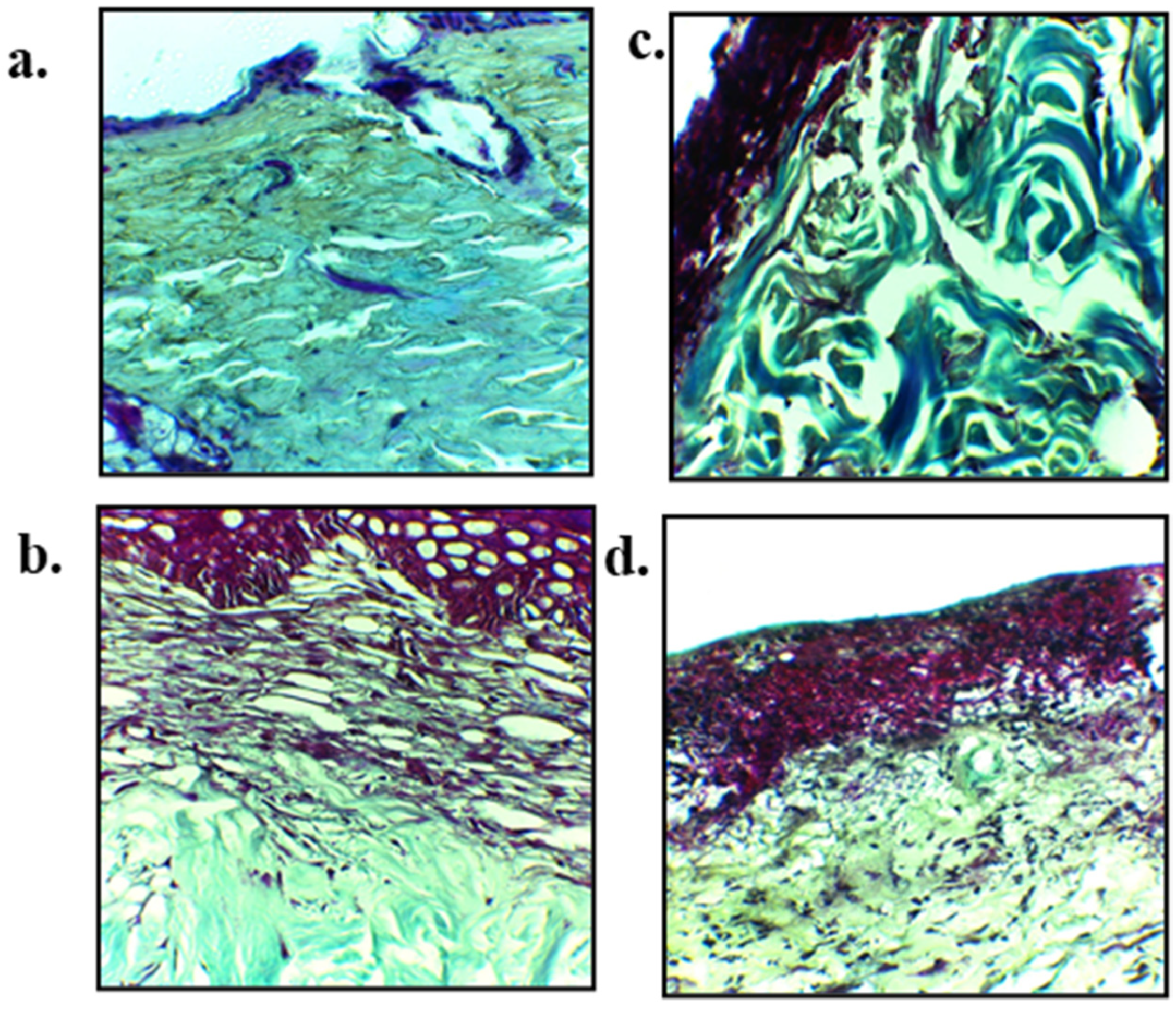
3.11. Skin Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 1–25. [Google Scholar] [CrossRef]
- World Health Organization. BASIC EMERGENCY CARE: Approach to the Acutely Ill and Injured; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Boyce, S.T.; Lalley, A.L. Tissue engineering of skin and regenerative medicine for wound care. Burn. Trauma 2018, 6. [Google Scholar] [CrossRef]
- Chen, F.-M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Ahadian, S.; Civitarese, R.; Bannerman, D.; Mohammadi, M.H.; Lu, R.; Wang, E.; Davenport-Huyer, L.; Lai, B.; Zhang, B.; Zhao, Y. Organ-on-a-chip platforms: A convergence of advanced materials, cells, and microscale technologies. Adv. Healthc. Mater. 2018, 7, 1700506. [Google Scholar] [CrossRef] [PubMed]
- Castano, O.; Pérez-Amodio, S.; Navarro-Requena, C.; Mateos-Timoneda, M.Á.; Engel, E. Instructive microenvironments in skin wound healing: Biomaterials as signal releasing platforms. Adv. Drug Deliv. Rev. 2018, 129, 95–117. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Douroumis, D.; Boateng, J. 3D printed scaffolds for wound healing and tissue regeneration. Ther. Dress. Wound Health Appl. 2020, 1, 385–398. [Google Scholar]
- Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A.; Marycz, K. Study on alginate–chitosan complex formed with different polymers ratio. Polymers 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Valle, K.Z.M.; Saucedo Acuña, R.A.; Ríos Arana, J.V.; Lobo, N.; Rodriguez, C.; Cuevas-Gonzalez, J.C.; Tovar-Carrillo, K.L. Natural Film Based on Pectin and Allantoin for Wound Healing: Obtaining, Characterization, and Rat Model. BioMed Res. Int. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Torres, F.G.; Commeaux, S.; Troncoso, O.P. Starch-based biomaterials for wound-dressing applications. Starch-Stärke 2013, 65, 543–551. [Google Scholar] [CrossRef]
- Escárcega-Galaz, A.A.; Sánchez-Machado, D.I.; López-Cervantes, J.; Sanches-Silva, A.; Madera-Santana, T.J.; Paseiro-Losada, P. Mechanical, structural and physical aspects of chitosan-based films as antimicrobial dressings. Int. J. Biol. Macromol. 2018, 116, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Khan, N.R.; Basit, H.M.; Mahmood, S. Physico-chemical based mechanistic insight into surfactant modulated sodium Carboxymethylcellulose film for skin tissue regeneration applications. J. Polym. Res. 2020, 27, 1–11. [Google Scholar] [CrossRef]
- Maver, T.; Maver, U.; Mostegel, F.; Griesser, T.; Spirk, S.; Smrke, D.M.; Stana-Kleinschek, K. Cellulose based thin films as a platform for drug release studies to mimick wound dressing materials. Cellulose 2015, 22, 749–761. [Google Scholar] [CrossRef]
- Hubner, P.; Donati, N.; de Menezes Quines, L.K.; Tessaro, I.C.; Marcilio, N.R. Gelatin-based films containing clinoptilolite-Ag for application as wound dressing. Mater. Sci. Eng. C 2020, 107, 110215. [Google Scholar] [CrossRef] [PubMed]
- Michalska-Sionkowska, M.; Walczak, M.; Sionkowska, A. Antimicrobial activity of collagen material with thymol addition for potential application as wound dressing. Polym. Test. 2017, 63, 360–366. [Google Scholar] [CrossRef]
- Akkaya, N.E.; Ergun, C.; Saygun, A.; Yesilcubuk, N.; Akel-Sadoglu, N.; Kavakli, I.H.; Turkmen, H.S.; Catalgil-Giz, H. New biocompatible antibacterial wound dressing candidates; agar-locust bean gum and agar-salep films. Int. J. Biol. Macromol. 2020, 155, 430–438. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef]
- Soni, M.L.; Kumar, M.; Namdeo, K. Sodium alginate microspheres for extending drug release: Formulation and in vitro evaluation. Int. J. Drug Deliv. 2010, 2, 64–68. [Google Scholar] [CrossRef]
- Sachan, N.K.; Pushkar, S.; Jha, A.; Bhattcharya, A. Sodium alginate: The wonder polymer for controlled drug delivery. J. Pharm. Res. 2009, 2, 1191–1199. [Google Scholar]
- Luna, S.; Gomes, M.E.; Mano, J.; Reis, R. Development of a novel cell encapsulation system based on natural origin polymers for tissue engineering applications. J. Bioact. Compat. Polym. 2010, 25, 341–359. [Google Scholar] [CrossRef]
- Dettmar, P.W.; Strugala, V.; Richardson, J.C. The key role alginates play in health. Food Hydrocoll. 2011, 25, 263–266. [Google Scholar] [CrossRef]
- Shalaka, D.; Naik, S.; Amruta, A.; Parimal, K. Vitamin E loaded pectin alginate microspheres for cosmetic application. J. Pharm. Res. 2009, 2, 1098–1102. [Google Scholar]
- Xia, Y.; Mei, F.; Duan, Y.; Gao, Y.; Xiong, Z.; Zhang, T.; Zhang, H. Bone tissue engineering using bone marrow stromal cells and an injectable sodium alginate/gelatin scaffold. J. Biomed. Mater. Res. Part A 2012, 100, 1044–1050. [Google Scholar] [CrossRef]
- Devi, M.P.; Sekar, M.; Chamundeswari, M.; Moorthy, A.; Krithiga, G.; Murugan, N.S.; Sastry, T. A novel wound dressing material—fibrin–chitosan–sodium alginate composite sheet. Bull. Mater. Sci. 2012, 35, 1157–1163. [Google Scholar] [CrossRef]
- Mohandas, A.; Sudheesh Kumar, P.; Raja, B.; Lakshmanan, V.-K.; Jayakumar, R. Exploration of alginate hydrogel/nano zinc oxide composite bandages for infected wounds. Int. J. Nanomed. 2015, 10 (Suppl. S1), 53–66. [Google Scholar] [CrossRef] [PubMed]
- Badgujar, B.; Mundada, A. The technologies used for developing orally disintegrating tablets: A review. Acta Pharm. 2011, 61, 117–139. [Google Scholar] [CrossRef]
- Hamrun, N.; Rachman, S.A. Measuring sodium alginate content of brown algae species padina sp. as the basic matter for making dental impression material (irreversible hydrocolloid impression material). J. Dentomaxillofac. Sci. 2016, 1, 129–133. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Wang, X.; Shi, J.; Zhu, R.; Zhang, J.; Zhang, Z.; Ma, D.; Hou, Y.; Lin, F.; Yang, J. A biomimetic silk fibroin/sodium alginate composite scaffold for soft tissue engineering. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Khan, A.; Afzal, Z.; Umer, M.F.; Khan, J.; Khan, G.M. Formulation development and characterization of cefazolin nanoparticles-loaded cross-linked films of sodium alginate and pectin as wound dressings. Int. J. Biol. Macromol. 2019, 124, 255–269. [Google Scholar] [CrossRef]
- Rezvanian, M.; Amin, M.C.I.M.; Ng, S.-F. Development and physicochemical characterization of alginate composite film loaded with simvastatin as a potential wound dressing. Carbohydr. Polym. 2016, 137, 295–304. [Google Scholar] [CrossRef]
- Gohil, R.M. Synergistic blends of natural polymers, pectin and sodium alginate. J. Appl. Polym. Sci. 2011, 120, 2324–2336. [Google Scholar] [CrossRef]
- Sun, X.; Cameron, R.G.; Bai, J. Effect of spray-drying temperature on physicochemical, antioxidant and antimicrobial properties of pectin/sodium alginate microencapsulated carvacrol. Food Hydrocoll. 2020, 100, 105420. [Google Scholar] [CrossRef]
- Sonker, A.K.; Verma, V. Influence of crosslinking methods toward poly (vinyl alcohol) properties: Microwave irradiation and conventional heating. J. Appl. Polym. Sci. 2018, 135, 46125. [Google Scholar] [CrossRef]
- Hofmann, M.; Fischer, G.; Weigel, R.; Kissinger, D. Microwave-based noninvasive concentration measurements for biomedical applications. IEEE Trans. Microw. Theory Tech. 2013, 61, 2195–2204. [Google Scholar] [CrossRef]
- Chih-Ho Hong, H.; Lupin, M.; O’Shaughnessy, K.F. Clinical evaluation of a microwave device for treating axillary hyperhidrosis. Dermatol. Surg. 2012, 38, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, S.; Azimzadeh, A.; Bahar, M.; Naraghi, Z.S.; Javadi, A.; Khamesipour, A.; Mohamadi, A.M. Efficacy of microwave and infrared radiation in the treatment of the skin lesions caused by leishmania major in an animal model. Iran. J. Public Health 2012, 41, 80. [Google Scholar] [PubMed]
- Vogl, T.J.; Naguib, N.N.; Lehnert, T.; Nour-Eldin, N.-E.A. Radiofrequency, microwave and laser ablation of pulmonary neoplasms: Clinical studies and technical considerations. Eur. J. Radiol. 2011, 77, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Hetta, O.M.; Shebrya, N.H.; Amin, S.K. Ultrasound-guided microwave ablation of hepatocellular carcinoma: Initial institutional experience. Egypt. J. Radiol. Nucl. Med. 2011, 42, 343–349. [Google Scholar] [CrossRef][Green Version]
- Castle, S.M.; Salas, N.; Leveillee, R.J. Initial experience using microwave ablation therapy for renal tumor treatment: 18-month follow-up. Urology 2011, 77, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.-A.; Liang, P.; Yu, X.-L.; Cheng, Z.-G.; Han, Z.-Y.; Liu, F.-Y.; Yu, J. Sonography-guided percutaneous microwave ablation of intrahepatic primary cholangiocarcinoma. Eur. J. Radiol. 2011, 80, 548–552. [Google Scholar] [CrossRef]
- Wong, T. Use of microwave in processing of drug delivery systems. Curr. Drug Deliv. 2008, 5, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Goksu, E.I.; Sumnu, G.; Esin, A. Effect of microwave on fluidized bed drying of macaroni beads. J. Food Eng. 2005, 66, 463–468. [Google Scholar] [CrossRef]
- Romano, V.R.; Marra, F.; Tammaro, U. Modelling of microwave heating of foodstuff: Study on the influence of sample dimensions with a FEM approach. J. Food Eng. 2005, 71, 233–241. [Google Scholar] [CrossRef]
- Verboven, P.; Datta, A.K.; Anh, N.T.; Scheerlinck, N.; Nicolaï, B.M. Computation of airflow effects on heat and mass transfer in a microwave oven. J. Food Eng. 2003, 59, 181–190. [Google Scholar] [CrossRef]
- Meyer, D.E.; Shin, B.; Kong, G.; Dewhirst, M.; Chilkoti, A. Drug targeting using thermally responsive polymers and local hyperthermia. J. Control. Release 2001, 74, 213–224. [Google Scholar] [CrossRef]
- Wong, T.; Wahab, S.; Anthony, Y. Drug release responses of zinc ion crosslinked poly (methyl vinyl ether-co-maleic acid) matrix towards microwave. Int. J. Pharm. 2008, 357, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Anuar, N.K.; Wong, T.W.; Taib, M.N. Microwave modified non-crosslinked pectin films with modulated drug release. Pharm. Dev. Technol. 2012, 17, 110–117. [Google Scholar] [CrossRef]
- Vandelli, M.A.; Romagnoli, M.; Monti, A.; Gozzi, M.; Guerra, P.; Rivasi, F.; Forni, F. Microwave-treated gelatin microspheres as drug delivery system. J. Control. Release 2004, 96, 67–84. [Google Scholar] [CrossRef]
- Shaheen, M.S.; El-Massry, K.F.; El-Ghorab, A.H.; Anjum, F.M. Microwave applications in thermal food processing. Dev. Appl. Microw. Heat. 2012, 3–16. [Google Scholar] [CrossRef][Green Version]
- Basit, H.M.; Mohd Amin, M.C.I.; Ng, S.-F.; Katas, H.; Shah, S.U.; Khan, N.R. Formulation and Evaluation of Microwave-Modified Chitosan-Curcumin Nanoparticles—A Promising Nanomaterials Platform for Skin Tissue Regeneration Applications Following Burn Wounds. Polymers 2020, 12, 2608. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarzadeh, B.; Almasi, H. Physical properties of edible emulsified films based on carboxymethyl cellulose and oleic acid. Int. J. Biol. Macromol. 2011, 48, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Ramli, N.A.; Wong, T.W. Sodium carboxymethylcellulose scaffolds and their physicochemical effects on partial thickness wound healing. Int. J. Pharm. 2011, 403, 73–82. [Google Scholar] [CrossRef]
- Kim, H.S.; Sun, X.; Lee, J.-H.; Kim, H.-W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef]
- Junker, J.P.; Kamel, R.A.; Caterson, E.; Eriksson, E. Clinical impact upon wound healing and inflammation in moist, wet, and dry environments. Adv. Wound Care 2013, 2, 348–356. [Google Scholar] [CrossRef]
- Junker, J.P.; Caterson, E.; Eriksson, E. The microenvironment of wound healing. J. Craniofac. Surg. 2013, 24, 12–16. [Google Scholar] [CrossRef]
- Altiok, D.; Altiok, E.; Tihminlioglu, F. Physical, antibacterial and antioxidant properties of chitosan films incorporated with thyme oil for potential wound healing applications. J. Mater. Sci. Mater. Med. 2010, 21, 2227–2236. [Google Scholar] [CrossRef]
- Peles, Z.; Zilberman, M. Novel soy protein wound dressings with controlled antibiotic release: Mechanical and physical properties. Acta Biomater. 2012, 8, 209–217. [Google Scholar] [CrossRef]
- Belbachir, K.; Noreen, R.; Gouspillou, G.; Petibois, C. Collagen types analysis and differentiation by FTIR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 829–837. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Z.; Khan, N.R.; Hua, Z.; Huo, J.; Li, Y. Microwave assisted chitosan-polyethylene glycol hydrogel membrane synthesis of curcumin for open incision wound healing. Die Pharm.-An Int. J. Pharm. Sci. 2020, 75, 118–123. [Google Scholar]
- Gonçalves, V.S.; Gurikov, P.; Poejo, J.; Matias, A.A.; Heinrich, S.; Duarte, C.M.; Smirnova, I. Alginate-based hybrid aerogel microparticles for mucosal drug delivery. Eur. J. Pharm. Biopharm. 2016, 107, 160–170. [Google Scholar] [CrossRef]
- Monroe, J.; Barry, M.; DeStefano, A.; Aydogan Gokturk, P.; Jiao, S.; Robinson-Brown, D.; Webber, T.; Crumlin, E.J.; Han, S.; Shell, M.S. Water Structure and Properties at Hydrophilic and Hydrophobic Surfaces. Annu. Rev. Chem. Biomol. Eng. 2020, 11, 523–557. [Google Scholar] [CrossRef] [PubMed]
- Pinto, G.D.; Rodrigues, J.; Bernardo, L. A Review on Thermoplastic or Thermosetting Polymeric Matrices Used in Polymeric Composites Manufactured with Banana Fibers from the Pseudostem. Appl. Sci. 2020, 10, 3023. [Google Scholar] [CrossRef]
- Namviriyachote, N.; Lipipun, V.; Akkhawattanangkul, Y.; Charoonrut, P.; Ritthidej, G.C. Development of polyurethane foam dressing containing silver and asiaticoside for healing of dermal wound. Asian J. Pharm. Sci. 2019, 14, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Levina, E.M.; Kharitonova, M.A.; Rovensky, Y.A.; Vasiliev, J.M. Cytoskeletal control of fibroblast length: Experiments with linear strips of substrate. J. Cell Sci. 2001, 114, 4335–4341. [Google Scholar] [CrossRef]
- Hiro, M.E.; Pierpont, Y.N.; Ko, F.; Wright, T.E.; Robson, M.C.; Payne, W.G. Comparative evaluation of silver-containing antimicrobial dressings on in vitro and in vivo processes of wound healing. Eplasty 2012, 12, 409–419. [Google Scholar]
- Habash, M.; Reid, G. Microbial biofilms: Their development and significance for medical device—Related infections. J. Clin. Pharmacol. 1999, 39, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.W.; Ramli, N.A. Carboxymethylcellulose film for bacterial wound infection control and healing. Carbohydr. Polym. 2014, 112, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, G.P.; Debone, H.S.; Severino, P.; Souto, E.B.; da Silva, C.F. Design and characterization of chitosan/zeolite composite films—Effect of zeolite type and zeolite dose on the film properties. Mater. Sci. Eng. C 2016, 60, 246–254. [Google Scholar] [CrossRef]
- Bishop, A. Role of oxygen in wound healing. J. Wound Care 2008, 17, 399–402. [Google Scholar] [CrossRef]
- Ichioka, S.; Ando, T.; Shibata, M.; Sekiya, N.; Nakatsuka, T. Oxygen consumption of keloids and hypertrophic scars. Ann. Plast. Surg. 2008, 60, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.A.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, J.C.; Boland, A.; Messiaen, J.; Cambier, P.; Van Cutsem, P. Egg box conformation of oligogalacturonides: The time-dependent stabilization of the elicitor-active conformation increases its biological activity. Glycobiology 2008, 18, 473–482. [Google Scholar] [CrossRef]
- Mashingaidze, F. Design of an Intravaginal Composite Polymeric System for the Reduction and Prevention of STI and HIV Transmission. Ph.D. Thesis, Department of Pharmacy and Pharmacology, University of the Witwatersrand, Johannesburg, South Africa, 2014. [Google Scholar]
- Farzanian, K.; Ghahremaninezhad, A. On the effect of chemical composition on the desorption of superabsorbent hydrogels in contact with a porous cementitious material. Gels 2018, 4, 70. [Google Scholar] [CrossRef]
- Rezvanian, M.; Ahmad, N.; Amin, M.C.I.M.; Ng, S.-F. Optimization, characterization, and in vitro assessment of alginate-pectin ionic cross-linked hydrogel film for wound dressing applications. Int. J. Biol. Macromol. 2017, 97, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Banthia, A.; Majeed, A. Pectin based formulations for biomedical applications: A review. Asian J. Pharm. Clin. Res. 2012, 5, 1–7. [Google Scholar]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Zaman, H.U.; Islam, J.; Khan, M.A.; Khan, R.A. Physico-mechanical properties of wound dressing material and its biomedical application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1369–1375. [Google Scholar] [CrossRef]
- Zahouani, H.; Pailler-Mattei, C.; Sohm, B.; Vargiolu, R.; Cenizo, V.; Debret, R. Characterization of the mechanical properties of a dermal equivalent compared with human skin in vivo by indentation and static friction tests. Ski. Res. Technol. 2009, 15, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Lancerotto, L.; Orgill, D.P. Mechanoregulation of angiogenesis in wound healing. Adv. Wound Care 2014, 3, 626–634. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Qiu, D.; Li, Y.; Yao, L.; Duan, J. Ultrasonic assisted microwave synthesis of poly (Chitosan-co-gelatin)/polyvinyl pyrrolidone IPN hydrogel. Ultrason. Sonochem. 2018, 40, 714–719. [Google Scholar] [CrossRef]
- Ravishankar, K.; Dhamodharan, R. Advances in chitosan-based hydrogels: Evolution from covalently crosslinked systems to ionotropically crosslinked superabsorbents. React. Funct. Polym. 2020, 149, 104517. [Google Scholar] [CrossRef]
- Miao, C.; Hamad, W.Y. Cellulose reinforced polymer composites and nanocomposites: A critical review. Cellulose 2013, 20, 2221–2262. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Chen, X.; Eldin, M.S.M.; Kenawy, E.-R.S. Crosslinked poly (vinyl alcohol) hydrogels for wound dressing applications: A review of remarkably blended polymers. Arab. J. Chem. 2015, 8, 1–14. [Google Scholar] [CrossRef]
- Rodríguez, M.; Osés, J.; Ziani, K.; Mate, J.I. Combined effect of plasticizers and surfactants on the physical properties of starch based edible films. Food Res. Int. 2006, 39, 840–846. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Cerqueira, M.A.; Teixeira, J.A.; Mussatto, S.I. Production and physicochemical properties of carboxymethyl cellulose films enriched with spent coffee grounds polysaccharides. Int. J. Biol. Macromol. 2018, 106, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Sin, L.T.; Bee, S.T.; Wah, T.Y.; Chee, T.M.; Kadhum, A.A.H.; Rahmat, A.R. Microwave effects on montmorillonite reinforced polyvinyl alcohol-starch nanocomposit. J. Vinyl Addit. Technol. 2016, 23, E142–E151. [Google Scholar] [CrossRef]
- Swamy, T.M.; Ramaraj, B.; Lee, J.H. Sodium alginate and its blends with starch: Thermal and morphological properties. J. Appl. Polym. Sci. 2008, 109, 4075–4081. [Google Scholar]
- Sundaram, J.; Durance, T.D.; Wang, R. Porous scaffold of gelatin–starch with nanohydroxyapatite composite processed via novel microwave vacuum drying. Acta Biomater. 2008, 4, 932–942. [Google Scholar] [CrossRef]
- Wang, Y.; Ying, T.; Li, J.; Xu, Y.; Wang, R.; Ke, Q.; Shen, S.G.; Xu, H.; Lin, K. Hierarchical micro/nanofibrous scaffolds incorporated with curcumin and zinc ion eutectic metal organic frameworks for enhanced diabetic wound healing via anti-oxidant and anti-inflammatory activities. Chem. Eng. J. 2020, 402, 126273. [Google Scholar] [CrossRef]
- Wu, N.-C.; Wang, J.-J. Curcumin Attenuates Liver Warm Ischemia and Reperfusion–Induced Combined Restrictive and Obstructive Lung Disease by Reducing Matrix Metalloprotease 9 Activity. In Transplantation Proceedings; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1135–1138. [Google Scholar]
- Song, Z.; Revelo, X.; Shao, W.; Tian, L.; Zeng, K.; Lei, H.; Sun, H.S.; Woo, M.; Winer, D.; Jin, T. Dietary curcumin intervention targets mouse white adipose tissue inflammation and brown adipose tissue UCP1 expression. Obesity 2018, 26, 547–558. [Google Scholar] [CrossRef]
- Merrell, J.G.; McLaughlin, S.W.; Tie, L.; Laurencin, C.T.; Chen, A.F.; Nair, L.S. Curcumin loaded poly (ε-caprolactone) nanofibers: Diabetic wound dressing with antioxidant and anti-inflammatory properties. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1149. [Google Scholar] [CrossRef]
- Dai, X.; Liu, J.; Zheng, H.; Wichmann, J.; Hopfner, U.; Sudhop, S.; Prein, C.; Shen, Y.; Machens, H.-G.; Schilling, A.F. Nano-formulated curcumin accelerates acute wound healing through Dkk-1-mediated fibroblast mobilization and MCP-1-mediated anti-inflammation. NPG Asia Mater. 2017, 9, e368. [Google Scholar] [CrossRef]
- Panchatcharam, M.; Miriyala, S.; Gayathri, V.S.; Suguna, L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol. Cell. Biochem. 2006, 290, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Tapia, E.; Sánchez-Lozada, L.; García-Niño, W.; García, E.; Cerecedo, A.; García-Arroyo, F.; Osorio, H.; Arellano, A.; Cristóbal-García, M.; Loredo, M. Curcumin prevents maleate-induced nephrotoxicity: Relation to hemodynamic alterations, oxidative stress, mitochondrial oxygen consumption and activity of respiratory complex I. Free. Radic. Res. 2014, 48, 1342–1354. [Google Scholar] [CrossRef]
- Laskar, K.; Faisal, S.M.; Rauf, A.; Ahmed, A.; Owais, M. Undec-10-enoic acid functionalized chitosan based novel nano-conjugate: An enhanced anti-bacterial/biofilm and anti-cancer potential. Carbohydr. Polym. 2017, 166, 14–23. [Google Scholar] [CrossRef]
- Adnan, S.; Ranjha, N.M.; Hanif, M.; Asghar, S. O-Carboxymethylated chitosan; A promising tool with in-vivo anti-inflammatory and analgesic properties in albino rats. Int. J. Biol. Macromol. 2020, 156, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Lu, S.; Cheng, Y.; Kong, S.; Li, S.; Li, C.; Yang, L. Investigation of the effects of molecular parameters on the hemostatic properties of chitosan. Molecules 2018, 23, 3147. [Google Scholar] [CrossRef]
- Xing, K.; Zhu, X.; Peng, X.; Qin, S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agron. Sustain. Dev. 2015, 35, 569–588. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Chen, X.G.; Liu, N.; Wang, S.X.; Liu, C.S.; Meng, X.H.; Liu, C.G. Protonation constants of chitosan with different molecular weight and degree of deacetylation. Carbohydr. Polym. 2006, 65, 194–201. [Google Scholar] [CrossRef]
- Howling, G.I.; Dettmar, P.W.; Goddard, P.A.; Hampson, F.C.; Dornish, M.; Wood, E.J. The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro. Biomaterials 2001, 22, 2959–2966. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; ur Rehman, D.I. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Palombo, F.; Cremers, S.G.; Weinberg, P.D.; Kazarian, S.G. Application of Fourier transform infrared spectroscopic imaging to the study of effects of age and dietary L-arginine on aortic lesion composition in cholesterol-fed rabbits. J. R. Soc. Interface 2009, 6, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Cheheltani, R.; McGoverin, C.M.; Rao, J.; Vorp, D.A.; Kiani, M.F.; Pleshko, N. Fourier transform infrared spectroscopy to quantify collagen and elastin in an in vitro model of extracellular matrix degradation in aorta. Analyst 2014, 139, 3039–3047. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, T.; Okamura, Y.; Shigemasa, Y.; Minami, S.; Okamoto, Y. Effects of molecular weight and deacetylation degree of chitin/chitosan on wound healing. Carbohydr. Polym. 2007, 67, 640–644. [Google Scholar] [CrossRef]
- Sahariah, P.; Masson, M. Antimicrobial chitosan and chitosan derivatives: A review of the structure–activity relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Divya, K.; Smitha, V.; Jisha, M. Antifungal, antioxidant and cytotoxic activities of chitosan nanoparticles and its use as an edible coating on vegetables. Int. J. Biol. Macromol. 2018, 114, 572–577. [Google Scholar] [CrossRef]
- Fong, D.; Hoemann, C.D. Chitosan Immunomodulatory Properties: Perspectives on the Impact of Structural Properties and Dosage. Future Sci. 2017. [Google Scholar] [CrossRef]
- Bessa, L.J.; Fazii, P.; Di Giulio, M.; Cellini, L. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: Some remarks about wound infection. Int. Wound J. 2015, 12, 47–52. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Samar, M.M.; El-Kalyoubi, M.; Khalaf, M.; Abd El-Razik, M. Physicochemical, functional, antioxidant and antibacterial properties of chitosan extracted from shrimp wastes by microwave technique. Ann. Agric. Sci. 2013, 58, 33–41. [Google Scholar] [CrossRef]
- Ueno, H.; Mori, T.; Fujinaga, T. Topical formulations and wound healing applications of chitosan. Adv. Drug Deliv. Rev. 2001, 52, 105–115. [Google Scholar] [CrossRef]
- Friedman, A.J.; Phan, J.; Schairer, D.O.; Champer, J.; Qin, M.; Pirouz, A.; Blecher-Paz, K.; Oren, A.; Liu, P.T.; Modlin, R.L. Antimicrobial and anti-inflammatory activity of chitosan–alginate nanoparticles: A targeted therapy for cutaneous pathogens. J. Investig. Dermatol. 2013, 133, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Thu, H.E.; Amjad, M.W.; Hussain, F.; Ahmed, T.A.; Khan, S. Exploring recent developments to improve antioxidant, anti-inflammatory and antimicrobial efficacy of curcumin: A review of new trends and future perspectives. Mater. Sci. Eng. C 2017, 77, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.M.; Cui, X.; Wu, R.; Dong, W.; Zhou, M.; Hu, M.; Simms, H.H.; Wang, P. The anti-inflammatory effect of curcumin in an experimental model of sepsis is mediated by up-regulation of peroxisome proliferator-activated receptor-γ. Crit. Care Med. 2006, 34, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C.; Aggarwal, B.B. “Spicing up” of the immune system by curcumin. J. Clin. Immunol. 2007, 27, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhu, Y.; Ran, X.; Wang, M.; Su, Y.; Cheng, T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J. Surg. Res. 2006, 133, 185–192. [Google Scholar] [CrossRef]
- Mehrabani, D.; Farjam, M.; Geramizadeh, B.; Tanideh, N.; Amini, M.; Panjehshahin, M.R. The healing effect of curcumin on burn wounds in rat. World J. Plast. Surg. 2015, 4, 29. [Google Scholar] [PubMed]
- Thangapazham, R.L.; Sharma, A.; Maheshwari, R.K. Beneficial role of curcumin in skin diseases. Mol. Targets Ther. Uses Curcumin Health Dis. 2007, 343–357. [Google Scholar]


| Formulation | Sodium Alginate (w/w) | Pectin (w/w) | Glycerol (2%, w/w) | Tween-80 (0.1%w/w) | Distilled Water (w/w) | Microwave Treatment Time (Min) |
|---|---|---|---|---|---|---|
| Untreated | 2 g | 2 g | 2 g | 0.1 g | 93.9 g | 0 |
| 1 min | 2 g | 2 g | 2 g | 0.1 g | 93.9 g | 1 |
| 3 min | 2 g | 2 g | 2 g | 0.1 g | 93.9 g | 3 |
| 5 min | 2 g | 2 g | 2 g | 0.1 g | 93.9 g | 5 |
| Formulation | Film Thickness (mm) | WVTR (g/m2h) | WVP (g mm/h·m2) |
|---|---|---|---|
| Untreated blend | 0.22 ± 0.03 | 180 ± 1.8 | 0.152 ± 0.06 |
| 1 min treated blend | 0.18 ± 0.02 | 240 ± 0.6 | 0.164 ± 0.02 |
| 3 min treated blend | 0.19 ± 0.03 | 256 ± 0.6 | 0.177 ± 0.03 |
| 5 min treated blend | 0.20 ± 0.03 | 276 ± 0.5 | 0.191 ± 0.02 |
| Formulation | Tensile Strength (Mpa) | Elongation Break (%) | Elastic Modulus (Mpa) |
|---|---|---|---|
| Untreated blend | 2.34 ± 0.24 | 40.02 ± 5.52 | 9.39 ± 12.50 |
| 1 min treated blend | 5.21 ± 0.41 | 43.34 ± 2.98 | 60.28 ± 15.55 |
| 3 min treated blend | 7.88 ± 0.04 | 50.01 ± 4.71 | 79.99 ± 25.98 |
| 5 min treated blend | 9.94 ± 0.08 | 66.66 ± 3.29 | 86.78 ± 22.98 |
| Samples | Absorbance Ratios of OH/NH of Test to Untreated Dermal Moieties | Absorbance Ratios of Amide-I (C=O Stretching) to Amide-II (C-N Bending) of Various Test Dermal Moieties |
|---|---|---|
| Film | 0.91 ± 0.005 | 1.01 ± 0.05 |
| Nanoparticle | 1.05 ± 0.005 | 1.8 ± 0.01 |
| Nanoparticle-film combined | 1.12 ± 0.015 | 2.15 ± 0.001 |
| Animal Groups | Tensile Strength (MPa) | Elongation Break (%) | Elastic Modulus (MPa) |
|---|---|---|---|
| Untreated | 8.61 ± 0.7 | 12.09 ± 0.7 | 1.82 ± 2.1 |
| Film | 10.98 ± 0.6 | 14.89 ± 0.5 | 3.32 ± 2.9 |
| Nanoparticles | 13.78 ± 0.9 | 17.14 ± 1.5 | 4.10 ± 1.9 |
| Nanoparticles-film combined | 14.65 ± 0.8 | 17.99 ± 0.7 | 5.78 ± 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basit, H.M.; Ali, M.; Shah, M.M.; Shah, S.U.; Wahab, A.; Albarqi, H.A.; Alqahtani, A.A.; Walbi, I.A.; Khan, N.R. Microwave Enabled Physically Cross Linked Sodium Alginate and Pectin Film and Their Application in Combination with Modified Chitosan-Curcumin Nanoparticles. A Novel Strategy for 2nd Degree Burns Wound Healing in Animals. Polymers 2021, 13, 2716. https://doi.org/10.3390/polym13162716
Basit HM, Ali M, Shah MM, Shah SU, Wahab A, Albarqi HA, Alqahtani AA, Walbi IA, Khan NR. Microwave Enabled Physically Cross Linked Sodium Alginate and Pectin Film and Their Application in Combination with Modified Chitosan-Curcumin Nanoparticles. A Novel Strategy for 2nd Degree Burns Wound Healing in Animals. Polymers. 2021; 13(16):2716. https://doi.org/10.3390/polym13162716
Chicago/Turabian StyleBasit, Hafiz Muhammad, Muhammad Ali, Mian Mufarih Shah, Shefaat Ullah Shah, Abdul Wahab, Hassan A. Albarqi, Abdulsalam A. Alqahtani, Ismail A. Walbi, and Nauman Rahim Khan. 2021. "Microwave Enabled Physically Cross Linked Sodium Alginate and Pectin Film and Their Application in Combination with Modified Chitosan-Curcumin Nanoparticles. A Novel Strategy for 2nd Degree Burns Wound Healing in Animals" Polymers 13, no. 16: 2716. https://doi.org/10.3390/polym13162716
APA StyleBasit, H. M., Ali, M., Shah, M. M., Shah, S. U., Wahab, A., Albarqi, H. A., Alqahtani, A. A., Walbi, I. A., & Khan, N. R. (2021). Microwave Enabled Physically Cross Linked Sodium Alginate and Pectin Film and Their Application in Combination with Modified Chitosan-Curcumin Nanoparticles. A Novel Strategy for 2nd Degree Burns Wound Healing in Animals. Polymers, 13(16), 2716. https://doi.org/10.3390/polym13162716







