Thermal Degradation Behavior of Epoxy Resin Containing Modified Carbon Nanotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
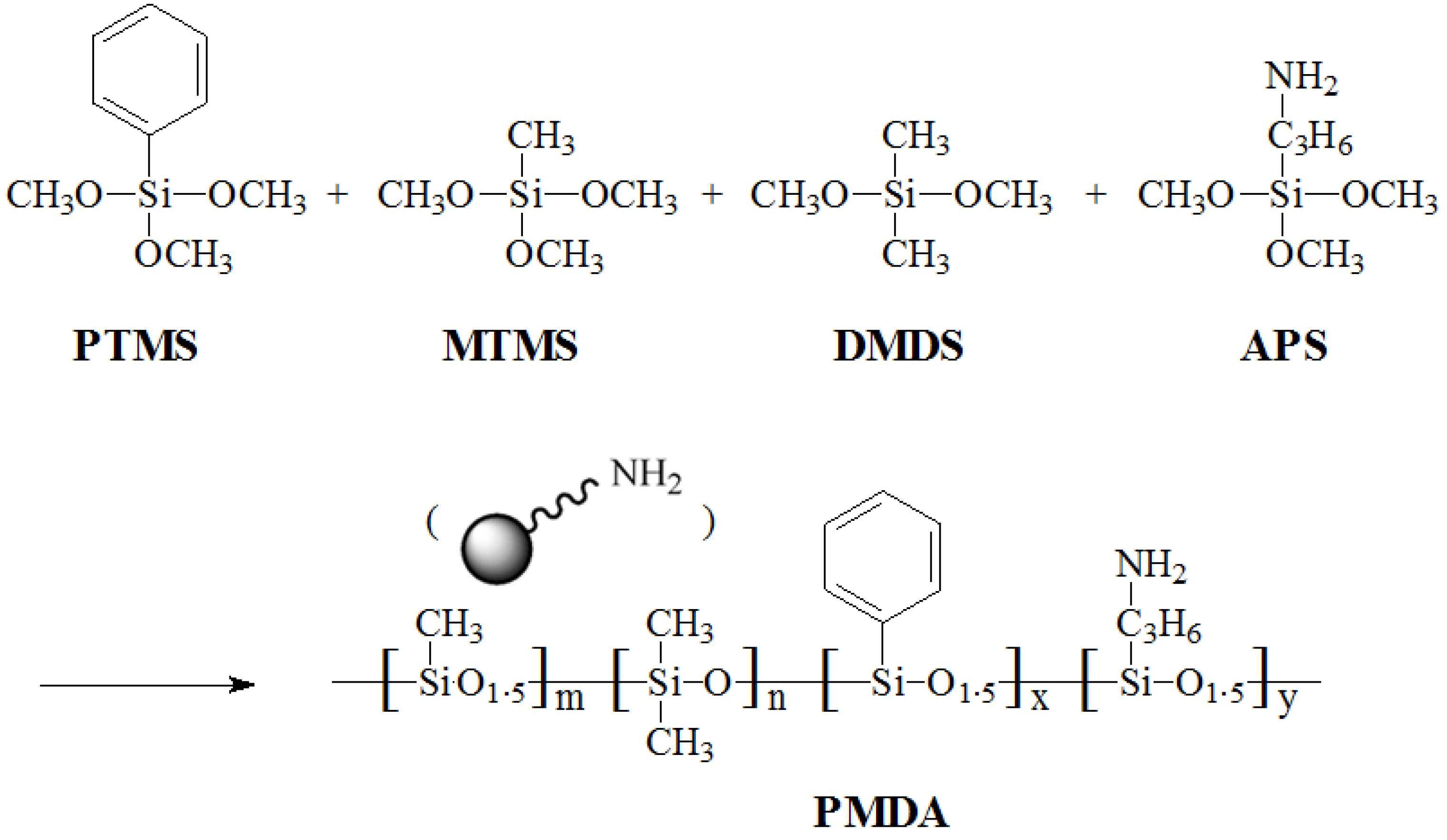
2.2. Synthesis of Polysilicone (PMDA)
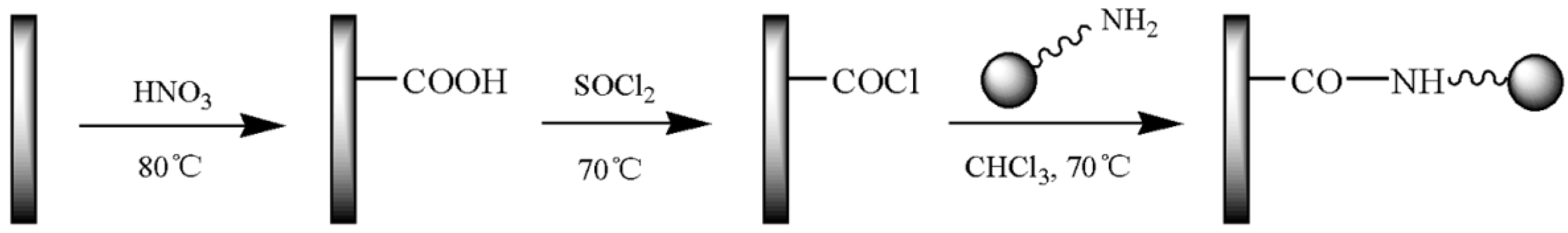
2.3. Functionalization of CNTs
2.4. Preparation of Epoxy Composites
2.5. Characterization and Measurement
2.6. Thermal Degradation Theory
2.6.1. Kissinger Method
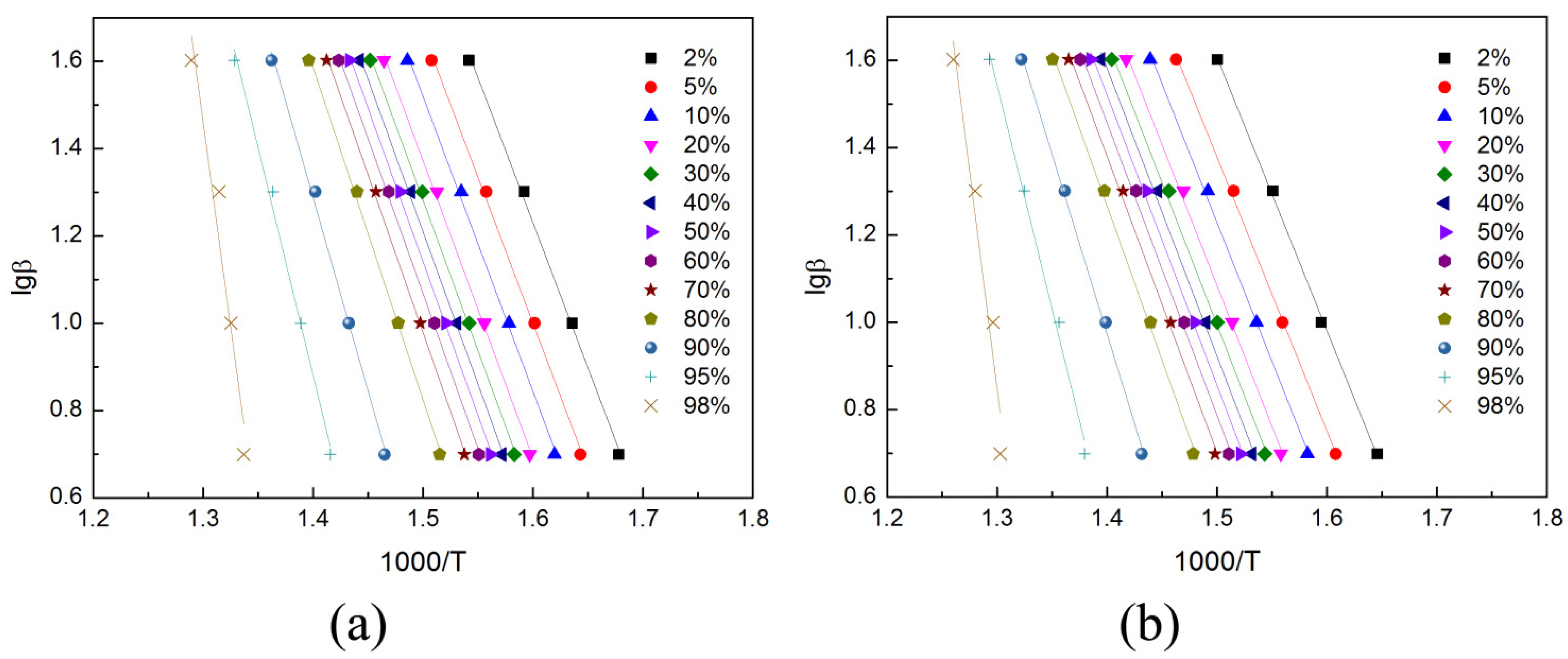
2.6.2. Flynn-Wall-Ozawa Method
3. Results
3.1. Structural Characterization
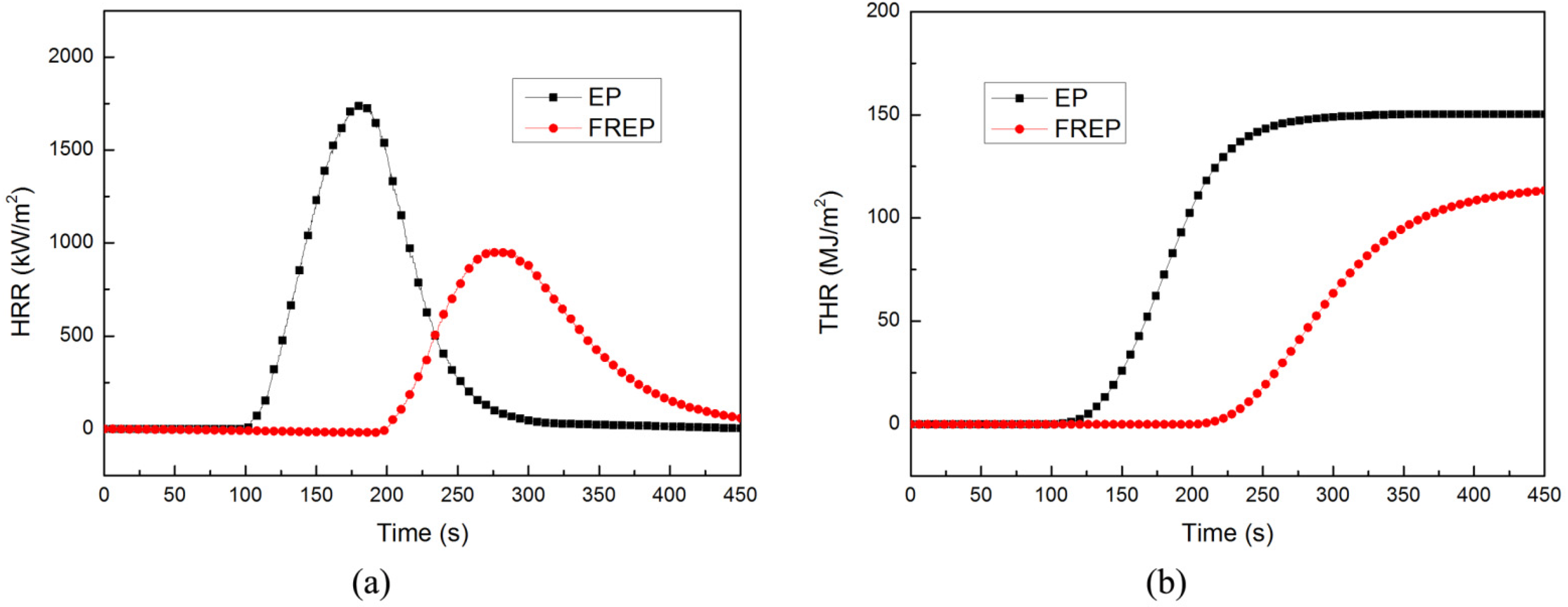
3.2. Flame Retardancy
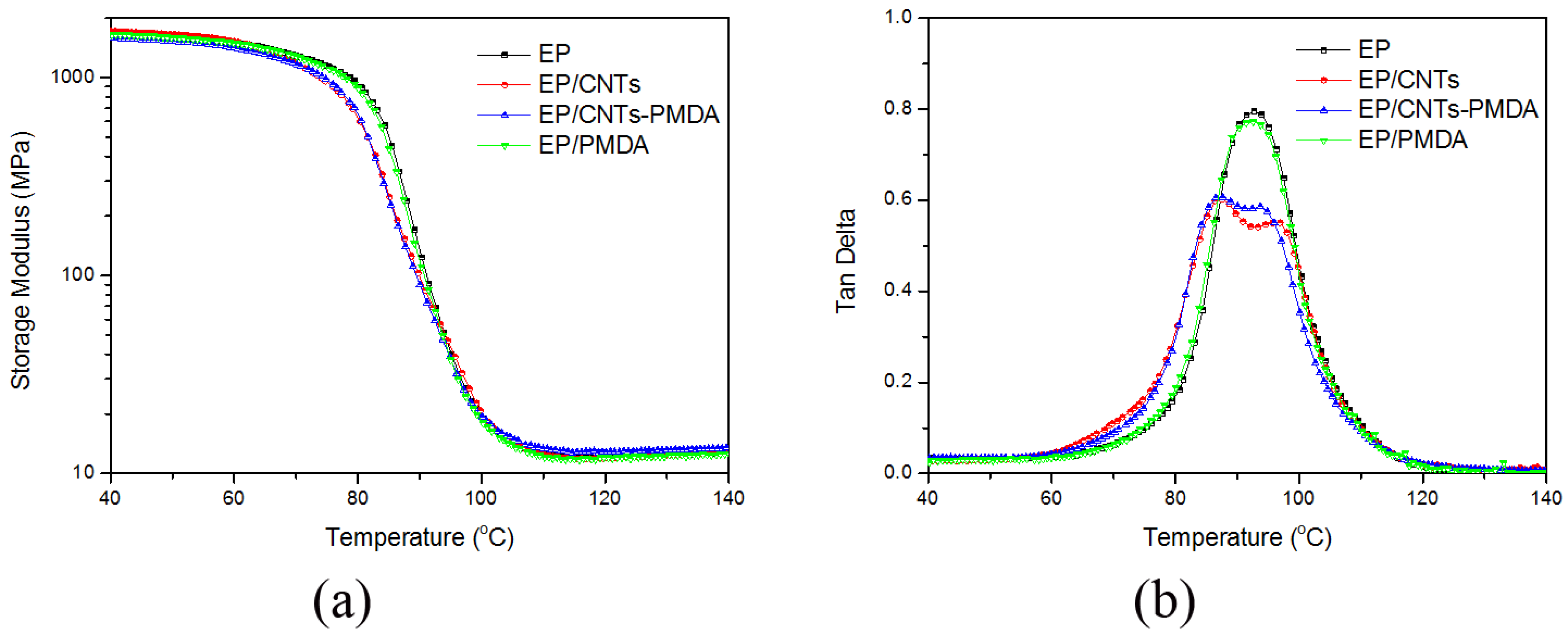
3.3. Thermal Stability
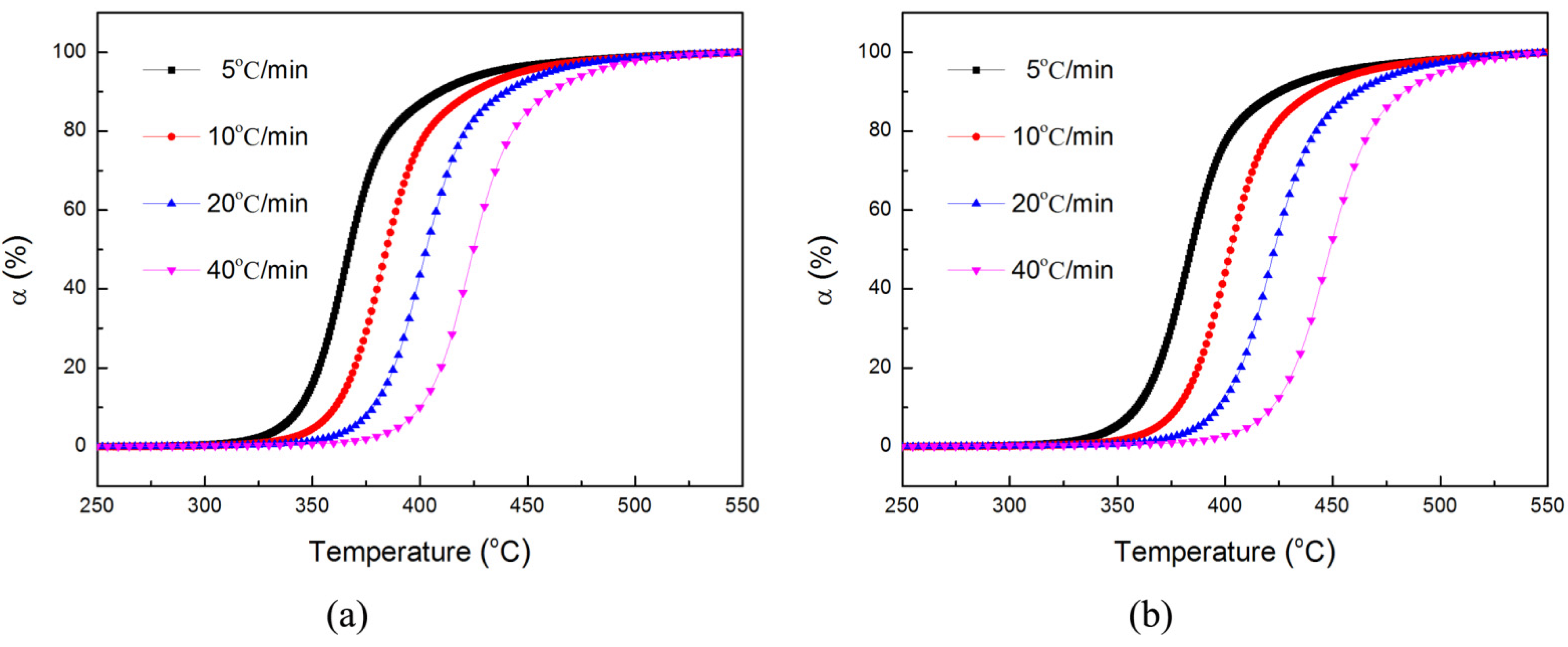
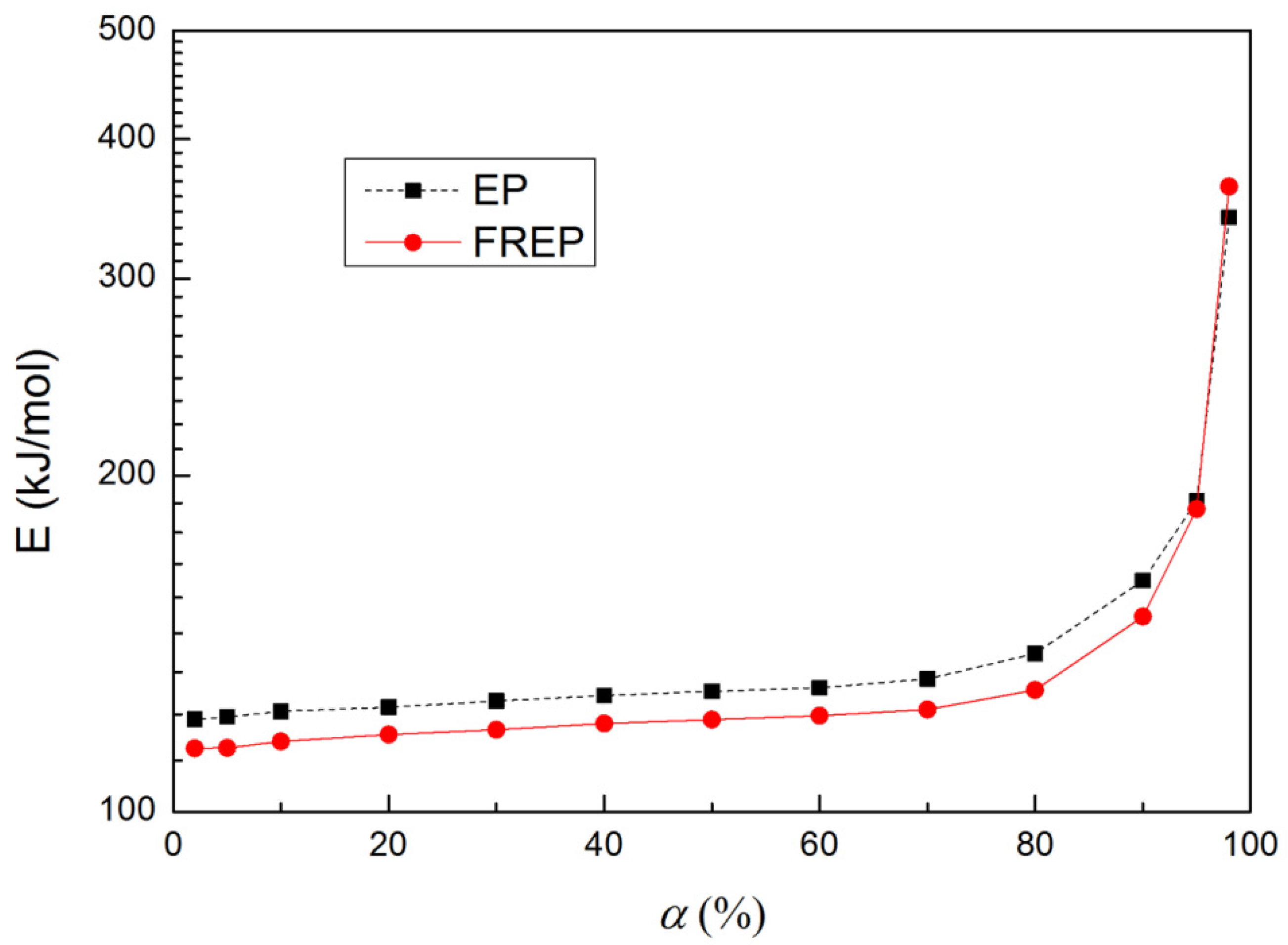
3.4. Thermal Degradation Kinetics
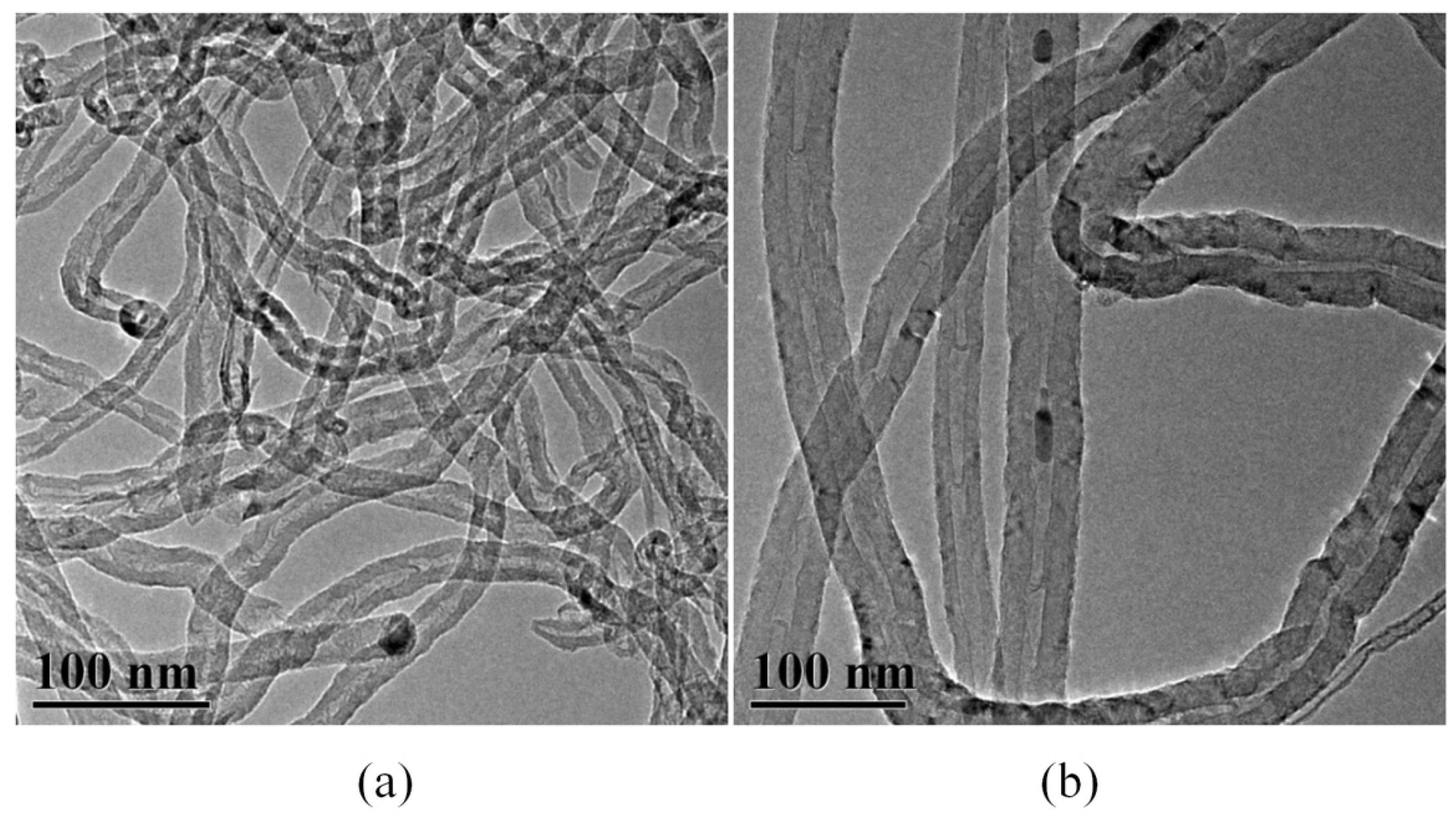
3.5. Dispersion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, H.; Ma, C.; Gu, J.; Guo, J.; Yan, X.; Huang, J.; Zhang, Q.; Guo, Z. An overview of multifunctional epoxy nanocomposites. J. Mater. Chem. C 2016, 4, 5890–5906. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, K.; Jiang, J.; Li, J.; Liu, Y. Highly dispersed melamine cyanurate flame-retardant epoxy resin composites. Polym. Int. 2017, 66, 85–91. [Google Scholar] [CrossRef]
- Zhao, X.; Babu, H.; Llorca, J.; Wang, D. Impact of halogen-free flame retardant with varied phosphorus chemical surrounding on the properties of diglycidyl ether of bisphenol-A type epoxy resin: Synthesis, fire behaviour, flame-retardant mechanism and mechanical properties. RSC Adv. 2016, 6, 59226–59236. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, W.; Duan, W.; Liu, Y.; Wang, Q. In situ synthesized and dispersed melamine polyphosphate flame retardant epoxy resin composites. J. Appl. Polym. Sci. 2019, 136, 47194. [Google Scholar] [CrossRef]
- Yan, W.; Yu, J.; Zhang, M.; Qin, S.; Wang, T.; Huang, W.; Long, L. Flame-retardant effect of a phenethyl-bridged DOPO derivative and layered double hydroxides for epoxy resin. RSC Adv. 2017, 7, 46236–46245. [Google Scholar] [CrossRef]
- Liu, S.; Yan, H.; Fang, Z.; Wang, H. Effect of graphene nanosheets on morphology, thermal stability and flame retardancy of epoxy resin. Compos. Sci. Technol. 2014, 90, 40–47. [Google Scholar] [CrossRef]
- Jian, R.; Wang, P.; Duan, W.; Wang, J.; Zheng, X.; Weng, J. Synthesis of a novel P/N/S-containing flame retardant and its application in epoxy resin: Thermal property, flame retardance, and pyrolysis behavior. Ind. Eng. Chem. Res. 2016, 55, 11520–11527. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Yin, S.; Lu, W.; Wu, R.; Fan, W.; Guo, C.; Chen, G. Poly(3,4-ethylenedioxythiophene)/Te/single-walled carbon nanotube composites with high thermoelectric performance promoted by electropolymerization. ACS Appl. Mater. Interfaces 2020, 12, 3547–3553. [Google Scholar] [CrossRef]
- Wu, Q.; Bao, J.; Zhang, C.; Liang, R.; Wang, B. The effect of thermal stability of carbon nanotubes on the flame retardancy of epoxy and bismaleimide/carbon fiber/buckypaper composites. J. Therm. Anal. Calorim. 2011, 103, 237–242. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, J.; Kwong, F.; Ng, D.; Tjong, S. Synthesis of multiwalled carbon nanotubes from bamboo charcoal and the roles of minerals on their growth. Biomass Bioenergy 2012, 36, 12–19. [Google Scholar] [CrossRef]
- Fathy, N. Carbon nanotubes synthesis using carbonization of pretreated rice straw through chemical vapor deposition of camphor. RSC Adv. 2017, 7, 28535–28541. [Google Scholar] [CrossRef]
- Adewumi, G.; Inambao, F.; Eloka-Eboka, A.; Revaprasadu, N. Synthesis of carbon nanotubes and nanospheres from coconut fibre and the role of synthesis temperature on their growth. J. Electron. Mater. 2018, 47, 3788–3794. [Google Scholar] [CrossRef]
- Ganesan, Y.; Peng, C.; Lu, Y.; Loya, P.; Moloney, P.; Barrera, E.; Yakobson, B.; Tour, J.; Ballarini, R.; Lou, J. Interface toughness of carbon nanotube reinforced epoxy composites. ACS Appl. Mater. Interfaces 2011, 3, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Flame retardancy and dispersion of functionalized carbon nanotubes in thiol-ene nanocomposites. Polymers 2021, 13, 3308. [Google Scholar] [CrossRef]
- Peeterbroeck, S.; Laoutid, F.; Taulemesse, J.; Monteverde, F.; Lopez-Cuesta, J.; Nagy, J.; Alexandre, M.; Dubois, P. Mechanical properties and flame-retardant behavior of ethylene vinyl acetate/high-density polyethylene coated carbon nanotube nanocomposites. Adv. Funct. Mater. 2007, 17, 2787–2791. [Google Scholar] [CrossRef]
- Ma, H.; Tong, L.; Xu, Z.; Fang, Z. Functionalizing carbon nanotubes by grafting on intumescent flame retardant: Nanocomposite synthesis, morphology, rheology, and flammability. Adv. Funct. Mater. 2008, 18, 414–421. [Google Scholar] [CrossRef]
- Song, P.; Shen, Y.; Du, B.; Guo, Z.; Fang, Z. Fabrication of fullerene-decorated carbon nanotubes and their application in flame-retarding polypropylene. Nanoscale 2009, 1, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Xu, L.; Guo, Z.; Zhang, Y.; Fang, Z. Flame-retardant-wrapped carbon nanotubes for simultaneously improving the flame retardancy and mechanical properties of polypropylene. J. Mater. Chem. 2008, 18, 5083–5091. [Google Scholar] [CrossRef]
- Ma, P.; Siddiqui, N.; Marom, G.; Kim, J. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Mathan, N.; Ponraju, D.; Vijayakumar, C. Kinetics of thermal degradation of intumescent flame-retardant spirophosphates. Bull. Mater. Sci. 2021, 44, 15. [Google Scholar] [CrossRef]
- Lv, X.; Fang, J.; Xie, J.; Yang, X.; Wang, J. Thermal stability of phosphorus-containing epoxy resins by thermogravimetric analysis. Polym. Polym. Compos. 2018, 26, 400–407. [Google Scholar] [CrossRef]
- Cruz, P.; Silva, L.; Fiuzamgr, R.; Polli, H. Thermal dehydrochlorination of pure PVC polymer: Part I—thermal degradation kinetics by thermogravimetric analysis. J. Appl. Polym. Sci. 2021, 138, 50598. [Google Scholar] [CrossRef]
- Wang, J. Novel polysilicone flame-retardant functionalized carbon nanotubes: Synthesis, characterization and flame retardancy as used in epoxy based composites. J. Macromol. Sci. B 2021, 60, 88–98. [Google Scholar] [CrossRef]
- Wang, J. Synthesis and characterization of flame retardant-wrapped carbon nanotubes and its flame retardancy in epoxy nanocomposites. Polym. Polym. Compos. 2021. [Google Scholar] [CrossRef]
- Wang, J. Mechanistic study of the flame retardancy of epoxy resin with a novel phosphorus and silicon-containing flame retardant. J. Macromol. Sci. B 2020, 59, 479–489. [Google Scholar] [CrossRef]
- Kissinger, H. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Wang, J.; Xin, Z. Non-isothermal degradation kinetics for polycarbonate/polymethylphenylsilsesquioxane composite. e-Polymers 2010, 10, 1–10. [Google Scholar] [CrossRef][Green Version]
- Flynn, J. A quick, direct method for the determination of activation energy from thermogravimetric data. J. Polym. Sci. Pol. Lett. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Flynn, J. Initial kinetic parameters from thermogravimetric rate and conversion data. J. Polym. Sci. Pol. Lett. 1967, 5, 191–196. [Google Scholar] [CrossRef]
- Muleja, A.; Mbianda, X.; Krause, R.; Pillay, K. Synthesis, characterization and thermal decomposition behaviour of triphenylphosphine-linked multiwalled carbon nanotubes. Carbon 2012, 50, 2741–2751. [Google Scholar] [CrossRef]
- Wang, S.; Xin, F.; Chen, Y.; Qian, L.; Chen, Y. Phosphorus-nitrogen containing polymer wrapped carbon nanotubes and their flame-retardant effect on epoxy resin. Polym. Degrad. Stab. 2016, 129, 133–141. [Google Scholar] [CrossRef]
- Tang, Y.; Gou, J.; Hu, Y. Covalent functionalization of carbon nanotubes with polyhedral oligomeric silsequioxane for superhydrophobicity and flame retardancy. Polym. Eng. Sci. 2013, 53, 1021–1030. [Google Scholar] [CrossRef]
- Singh, N.; Gupta, V.; Singh, A. Graphene and carbon nanotube reinforced epoxy nanocomposites: A review. Polymer 2019, 180, 121724. [Google Scholar] [CrossRef]

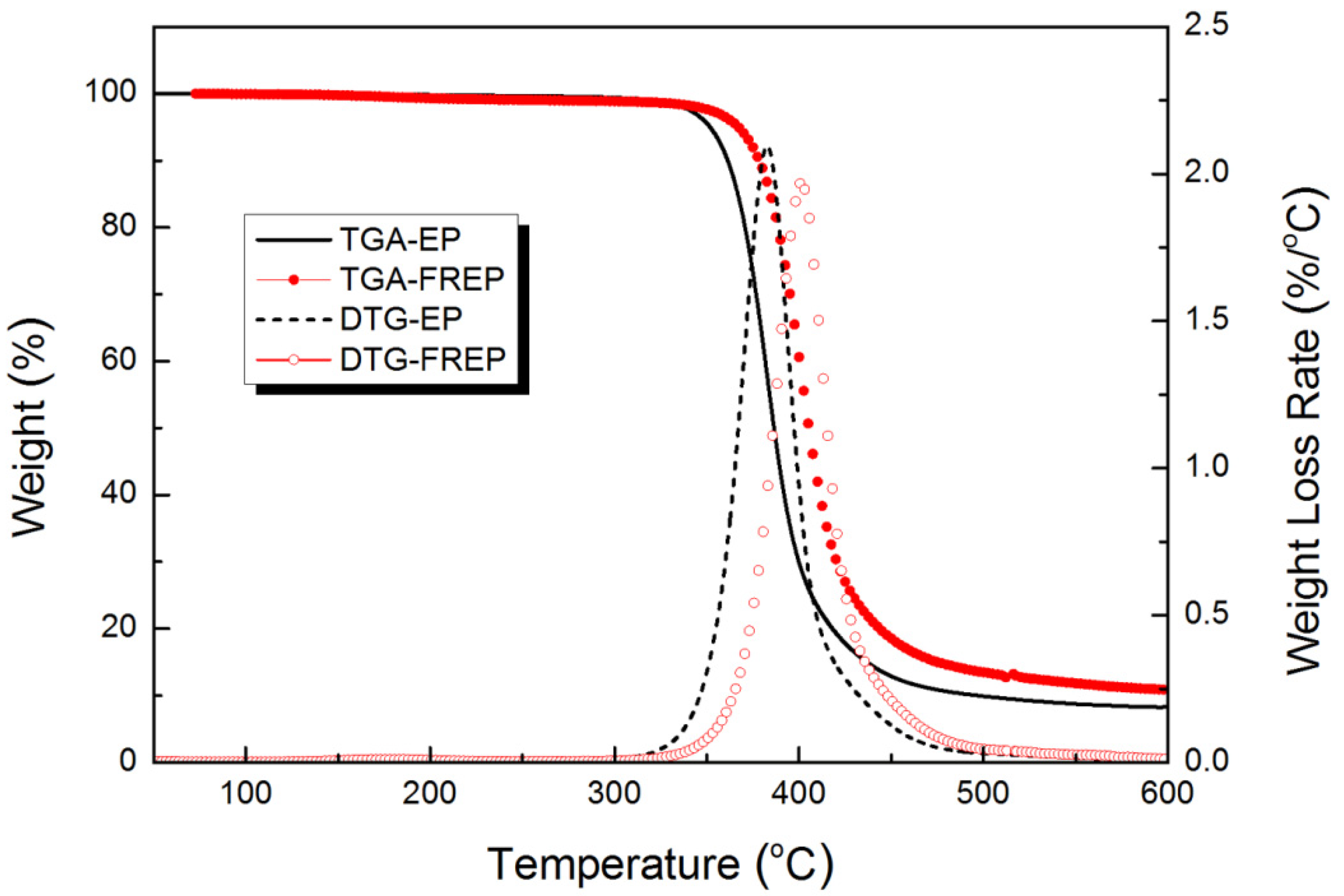



| Temperature (°C) | Peak Rate (wt%/°C) | Residual Char (wt%) | ||
|---|---|---|---|---|
| T5wt% | Tmax | |||
| EP | 351.7 | 382.6 | 2.10 | 8.27 |
| FREP | 367.1 | 401.3 | 1.97 | 10.86 |
| Tmax (°C) | Fitting Equation | R2 Values | E (kJ/mol) | lnA (1/min) | ||||
|---|---|---|---|---|---|---|---|---|
| 5 °C/min | 10 °C/min | 20 °C/min | 40 °C/min | |||||
| EP | 365.4 | 382.6 | 400.1 | 423.5 | Y = 11.72 − 14.69x | 0.9948 | 122.1 | 14.4 |
| FREP | 383.1 | 401.3 | 420.2 | 447.1 | Y = 10.11 − 14.01x | 0.9906 | 117.0 | 12.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, X.; Wu, F.; Wang, J. Thermal Degradation Behavior of Epoxy Resin Containing Modified Carbon Nanotubes. Polymers 2021, 13, 3332. https://doi.org/10.3390/polym13193332
Bao X, Wu F, Wang J. Thermal Degradation Behavior of Epoxy Resin Containing Modified Carbon Nanotubes. Polymers. 2021; 13(19):3332. https://doi.org/10.3390/polym13193332
Chicago/Turabian StyleBao, Xiaohui, Fangyi Wu, and Jiangbo Wang. 2021. "Thermal Degradation Behavior of Epoxy Resin Containing Modified Carbon Nanotubes" Polymers 13, no. 19: 3332. https://doi.org/10.3390/polym13193332
APA StyleBao, X., Wu, F., & Wang, J. (2021). Thermal Degradation Behavior of Epoxy Resin Containing Modified Carbon Nanotubes. Polymers, 13(19), 3332. https://doi.org/10.3390/polym13193332





