Flame Retardancy and Dispersion of Functionalized Carbon Nanotubes in Thiol-Ene Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
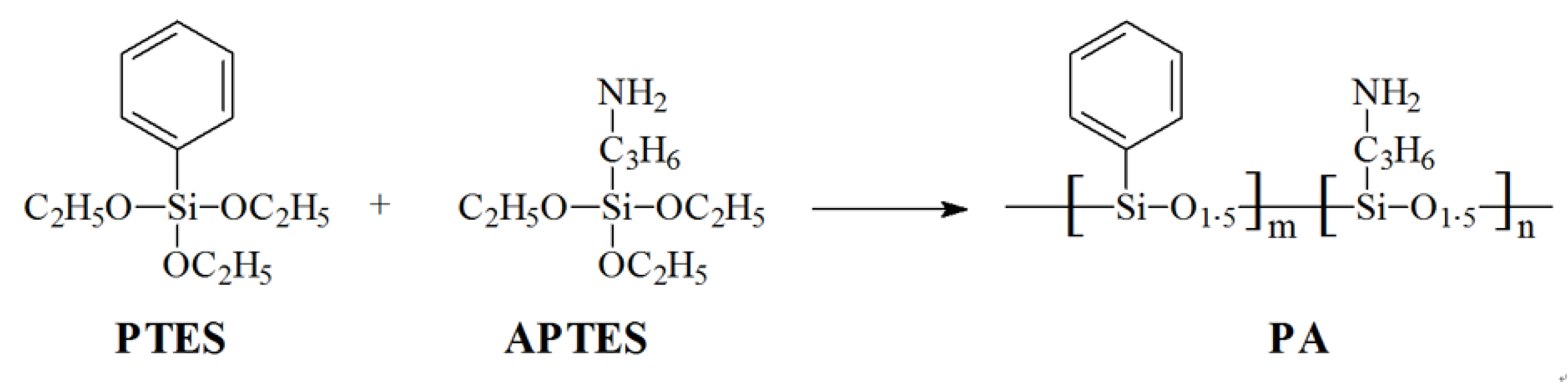
2.2. Synthesis of Polysilicone (PA)
2.3. Functionalization of CNTs
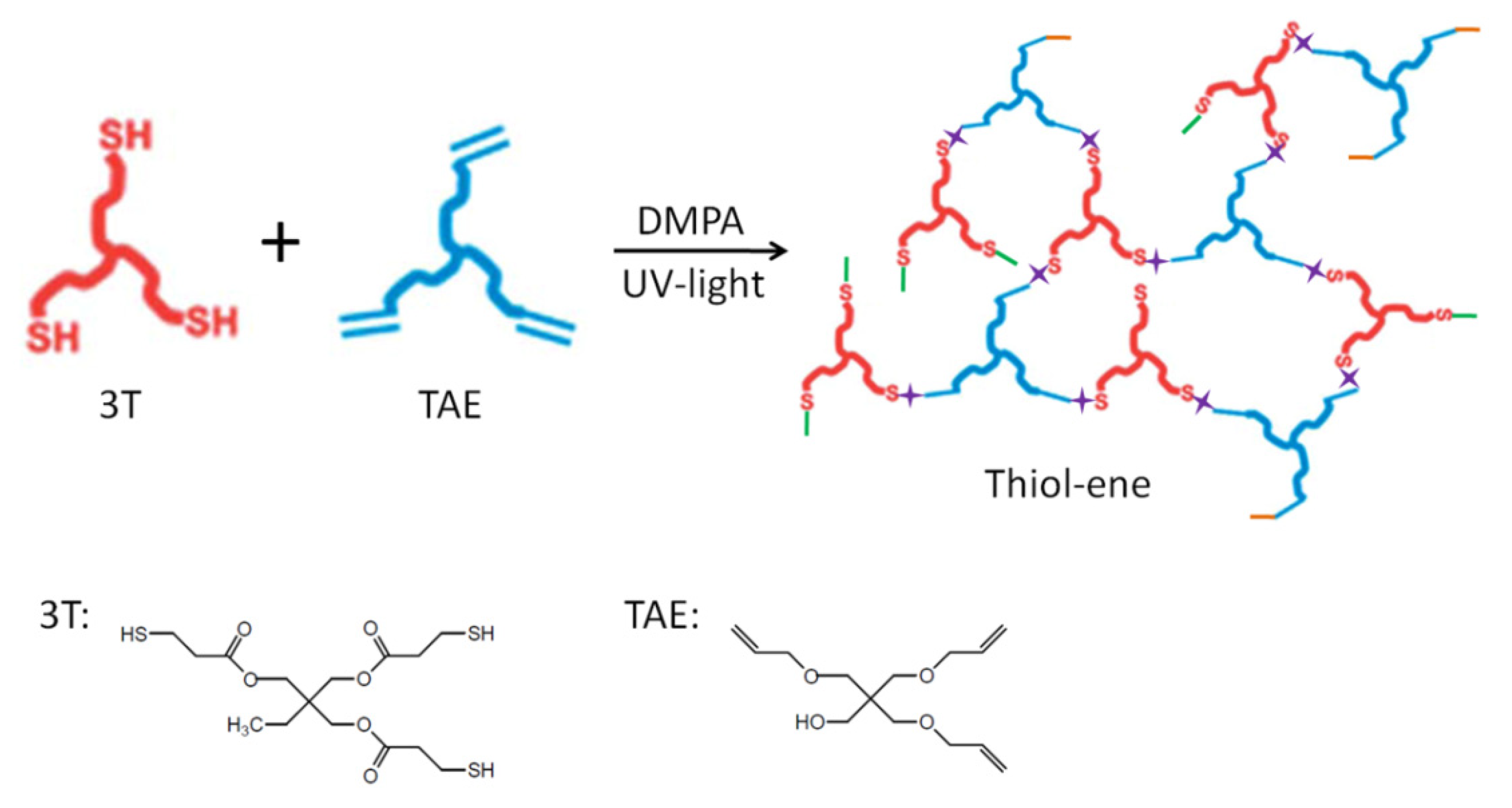
2.4. Preparation of Thiol-Ene Nanocomposites
2.5. Characterization and Measurement
3. Resultsand Discussion
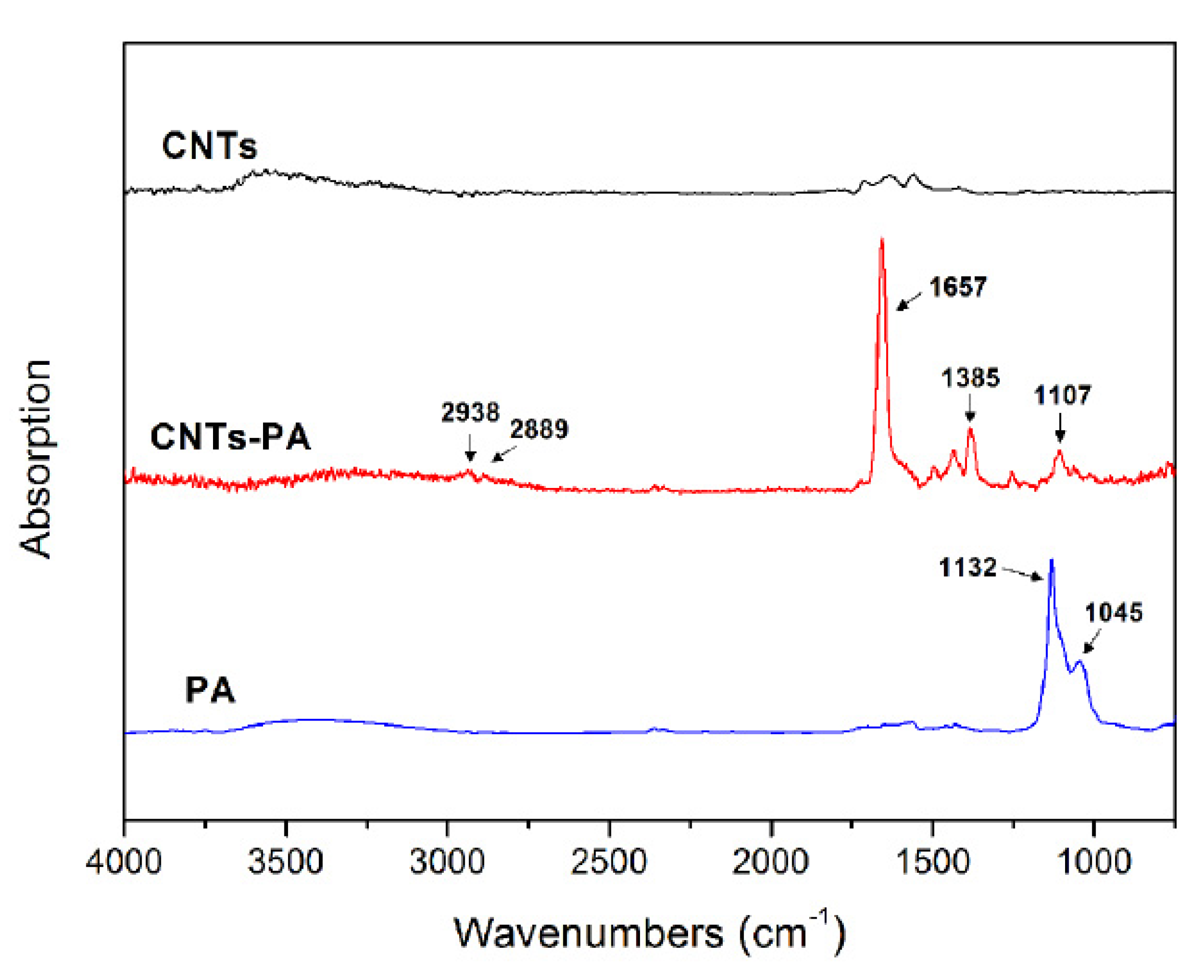
3.1. Structural Characterization
3.2. Flame Retardancy
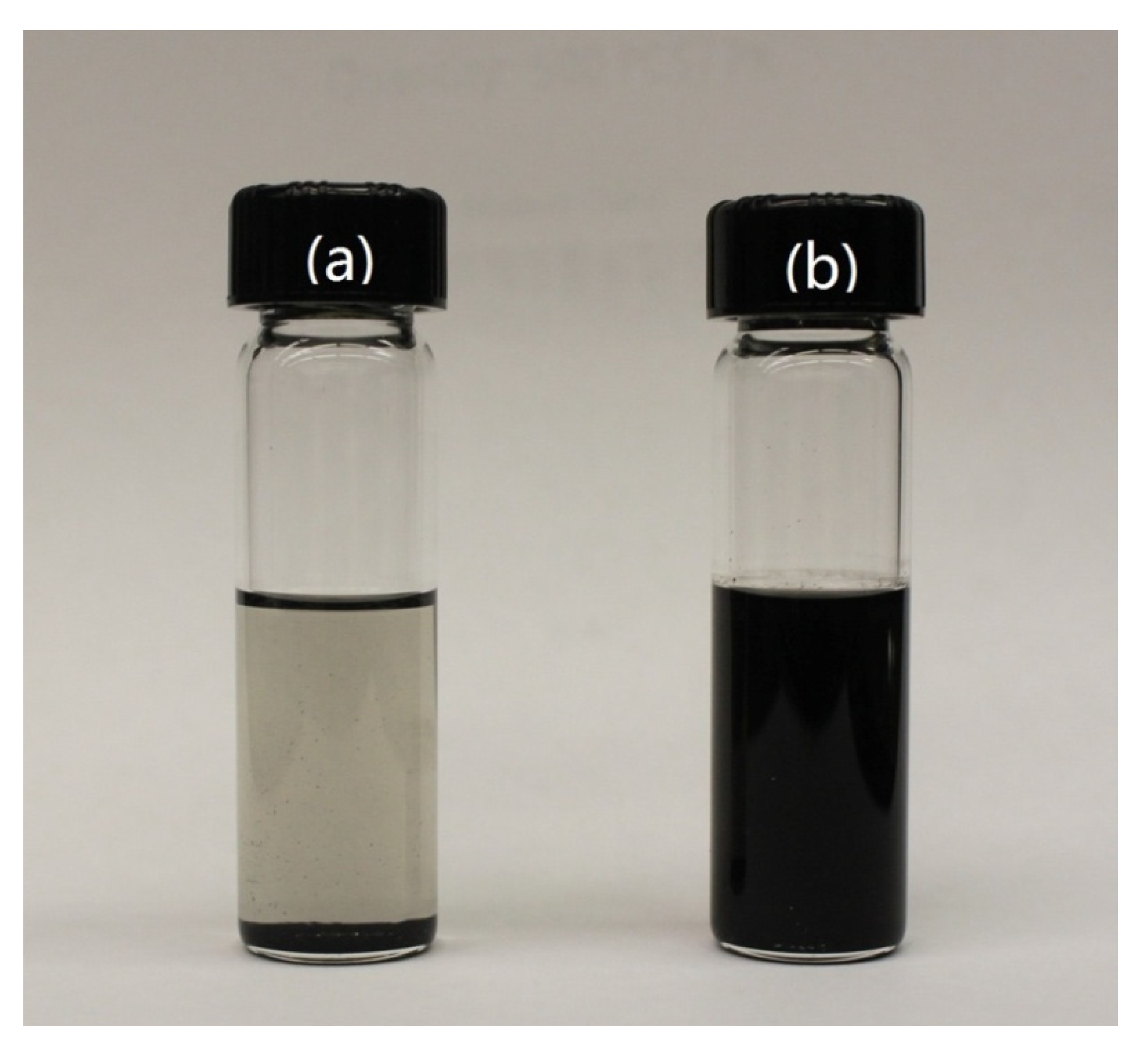
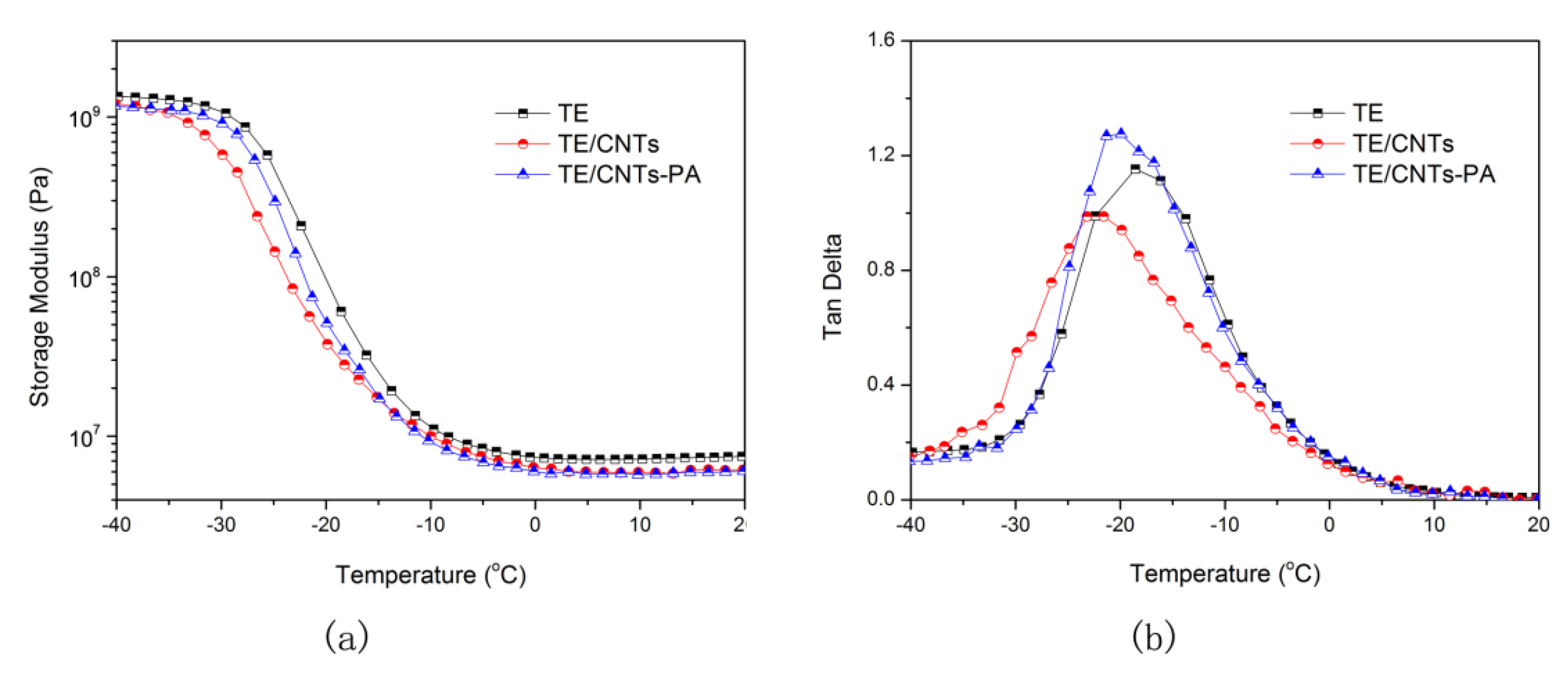
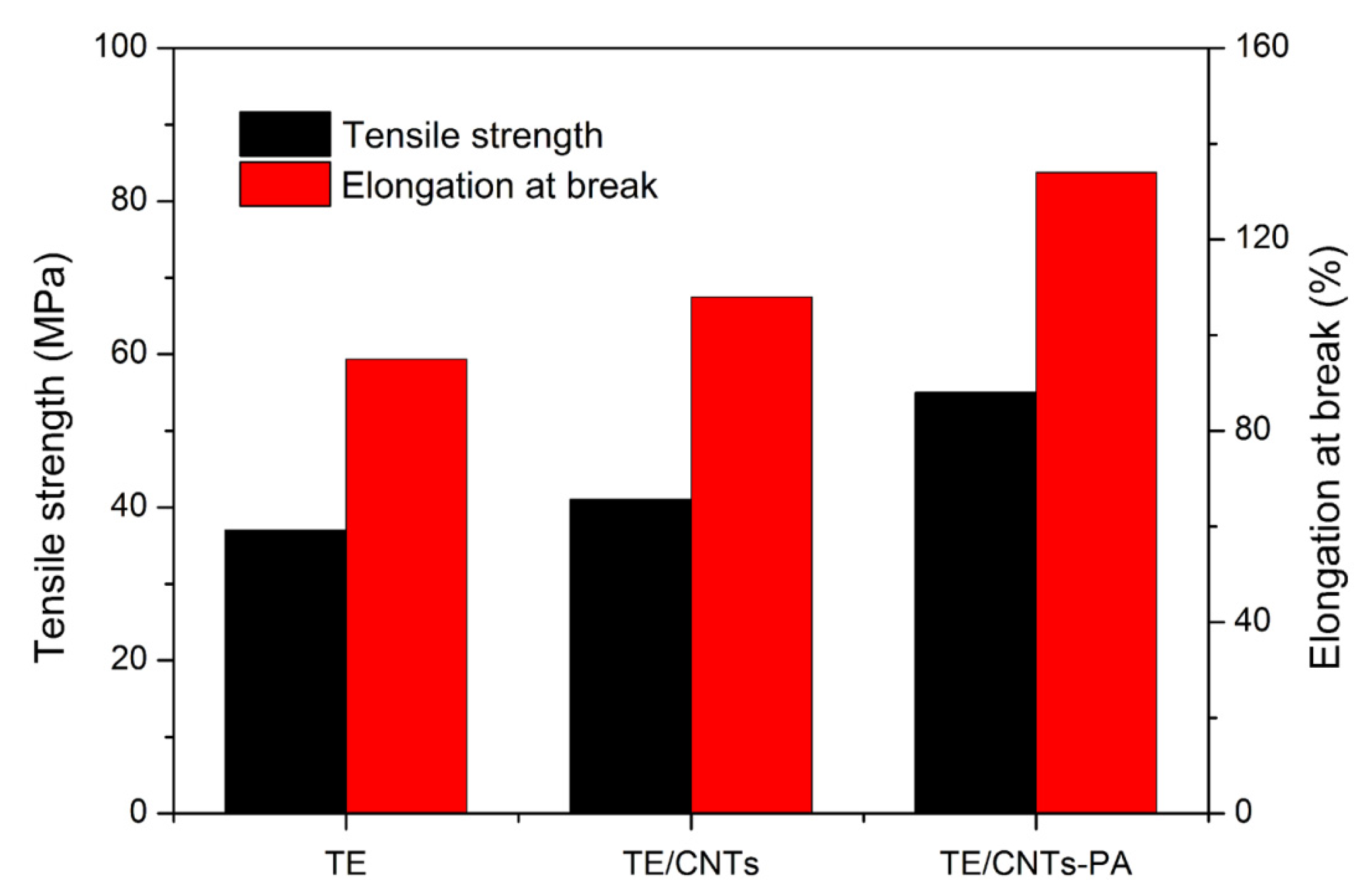
3.3. Dispersionand Mechanical Properties
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goetz, J.; Kwisnek, L.; Nazarenko, S. From gas barriers to high gas flux membranes: UV-cured thiol-ene networks for transport applications. Radtech. Rep. 2014, 4, 27–32. [Google Scholar]
- Lee, J.; Lee, Y.; Park, S.; Ha, K. Preparation and properties of thiol-ene UV cured nanocomposites with methacrylate-grafted cellulose nanocrystals as fillers. Polym. Korea 2019, 43, 612–620. [Google Scholar] [CrossRef]
- Wu, F.; Bao, X.; Xu, H.; Kong, D.; Wang, J. Functionalization of graphene oxide with polysilicone: Synthesis, characterization and fire retardancy in thiol-ene systems. J. Macromolec. Sci. B 2021, 60, 339–349. [Google Scholar] [CrossRef]
- Clark, T.; Kwisnek, L.; Hoyle, C.; Nazarenko, S. Photopolymerization of thiol-ene systems based on oligomeric thiols. J. Polym. Sci. Polym. Chem. 2009, 47, 14–24. [Google Scholar] [CrossRef]
- Bastürk, E.; Oktay, B.; Kahraman, M.; Apohan, N. UV cured thiol-ene flame retardant hybrid coatings. Prog. Org. Coat. 2013, 76, 936–943. [Google Scholar] [CrossRef]
- Akmak, E.; Mülazim, Y.; Kahraman, M.; Apohan, N. Preparation and characterization of boron containing thiol-ene photocured hybrid coatings. Prog. Org. Coat. 2012, 75, 28–32. [Google Scholar]
- De Volder, M.; Tawfick, S.; Baughman, R. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, S.; Lu, W.; Wu, R.; Fan, W.; Guo, C.; Chen, G. Poly(3,4-ethylenedioxythiophene)/Te/single-walled carbon nanotube composites with high thermoelectric performance promoted by electropolymerization. ACS Appl. Mater. Interfaces 2020, 12, 3547–3553. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, L.; Shao, Q.; Huang, F.; Huang, Y. Mn3O4 nanocrystals anchored on multi-walled carbon nanotubes as high-performance anode materials for lithium-ion batteries. Mater. Lett. 2012, 80, 110–113. [Google Scholar] [CrossRef]
- Wang, S.; Xin, F.; Chen, Y.; Qian, L.; Chen, Y. Phosphorus-nitrogen containing polymer wrapped carbon nanotubes and their flame-retardant effect on epoxy resin. Polym. Degrad. Stab. 2016, 129, 133–141. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Gruke, E.; Hilding, J.; Groth, K.; Harris, R.; Awad, W.; Douglas, J. Thermal degradation and flammability properties of poly(propylene)/carbon nanotube composites. Macromol. Rapid. Commun. 2002, 23, 761–765. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Du, F.; Douglas, J.; Winey, K.; Harris, R.; Shields, J. Nanoparticle networks reduce the flammability of polymer nanocomposites. Nat. Mater. 2005, 4, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Siddiqui, N.; Marom, G.; Kim, J. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Wang, G.; Qu, Z.; Liu, L.; Shi, Q.; Guo, H. Study of SMA graft modified MWNT/PVC composite materials. Mater. Sci. Eng. A-Struct. 2008, 472, 136–139. [Google Scholar] [CrossRef]
- Qu, Z.; Wang, G. Effective chemical oxidation on the structure of multiwalled carbon nanotubes. J. Nanosci. Nanotechnol. 2012, 12, 105–111. [Google Scholar] [PubMed]
- Shen, Z.; Bateman, S.; Wu, D.; McMahon, P.; Dell’Olio, M.; Gotama, J. The effects of carbon nanotubes on mechanical and thermal properties of woven glass fibre reinforced polyamide-6 nanocomposites. Compos. Sci. Technol. 2009, 69, 239–244. [Google Scholar] [CrossRef]
- Ma, H.; Tong, L.; Xu, Z.; Fang, Z. Functionalizing carbon nanotubes by grafting on intumescent flame retardant: Nanocomposite synthesis, morphology, rheology, and flammability. Adv. Funct. Mater. 2008, 18, 414–421. [Google Scholar] [CrossRef]
- Song, P.; Shen, Y.; Du, B.; Guo, Z.; Fang, Z. Fabrication of fullerene-decorated carbon nanotubes and their application in flame-retarding polypropylene. Nanoscale 2009, 1, 118–121. [Google Scholar] [CrossRef]
- Du, B.; Fang, Z. The preparation of layered double hydroxide wrapped carbon nanotubes and their application as a flame retardant for polypropylene. Nanotechnology 2010, 21, 315603–315608. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Fang, P. Functionalization of multi-walled carbon nanotubes grafted with self-generated functional groups and their polyamide 6 composites. Carbon 2010, 48, 721–729. [Google Scholar] [CrossRef]
- Yu, H.; Liu, J.; Wen, X.; Jiang, Z.; Wang, Y.; Wang, L.; Zheng, J.; Fu, S.; Tang, T. Charing polymer wrapped carbon nanotubes for simultaneously improving the flame retardancy and mechanical properties of epoxy resin. Polymer 2011, 52, 4891–4898. [Google Scholar] [CrossRef]
- Muleja, A.; Mbianda, X.; Krause, R. Synthesis, characterization and thermal decomposition behaviour of triphenylphosphine-linked multiwalled carbon nanotubes. Carbon 2012, 50, 2741–2751. [Google Scholar] [CrossRef]
- Liu, S.; Lang, X.; Ye, H.; Zhang, S.; Zhao, J. Preparation and characterization of copolymerized aminopropyl/phenylsilsesquioxane microparticles. Eur. Polym. J. 2005, 41, 996–1001. [Google Scholar] [CrossRef]
- Meng, H.; Sui, G.; Fang, P.; Yang, R. Effects of acid- and diamine modified MWNTs on the mechanical properties and crystallization behavior of polyamide 6. Polymer 2008, 49, 610–620. [Google Scholar] [CrossRef]

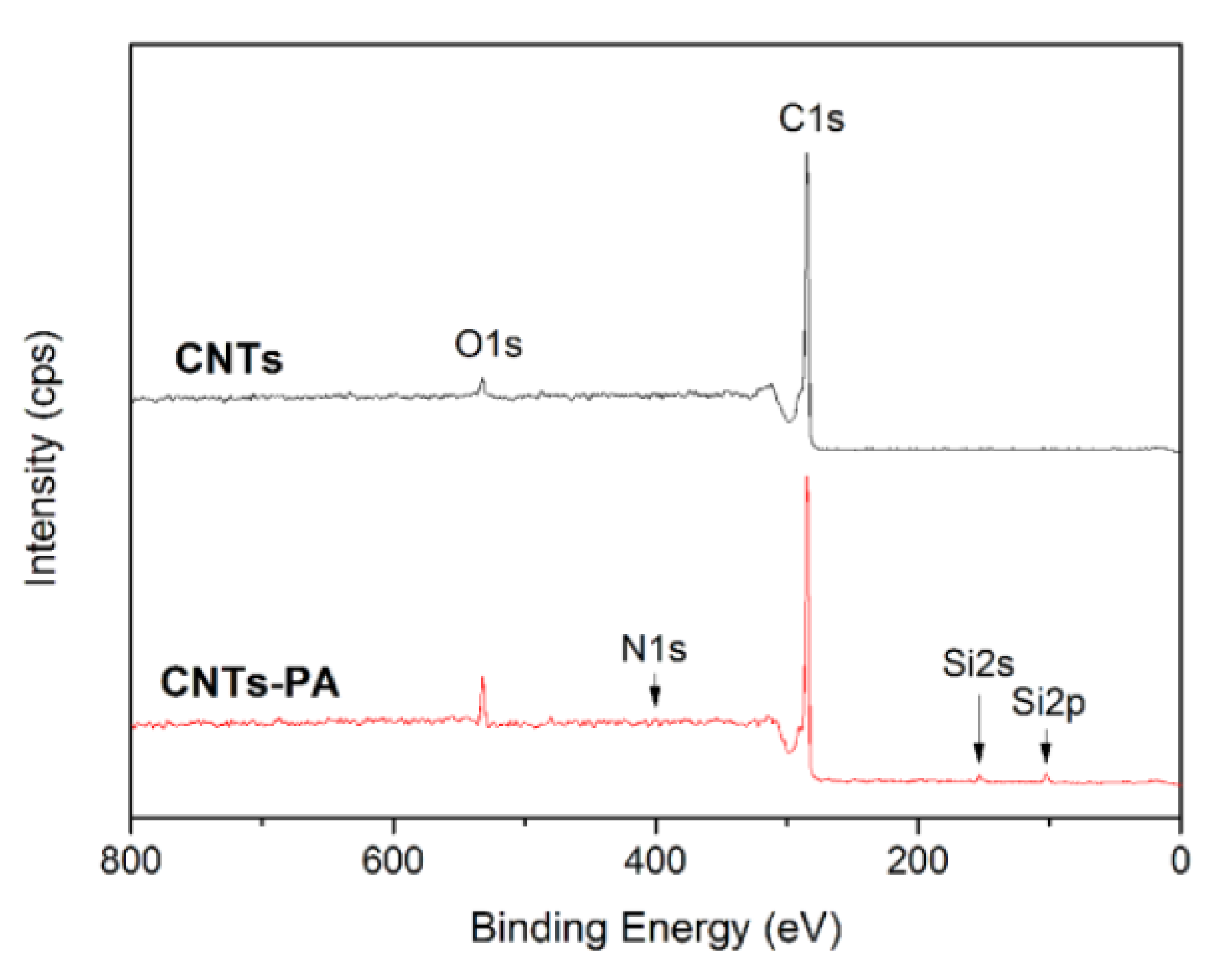
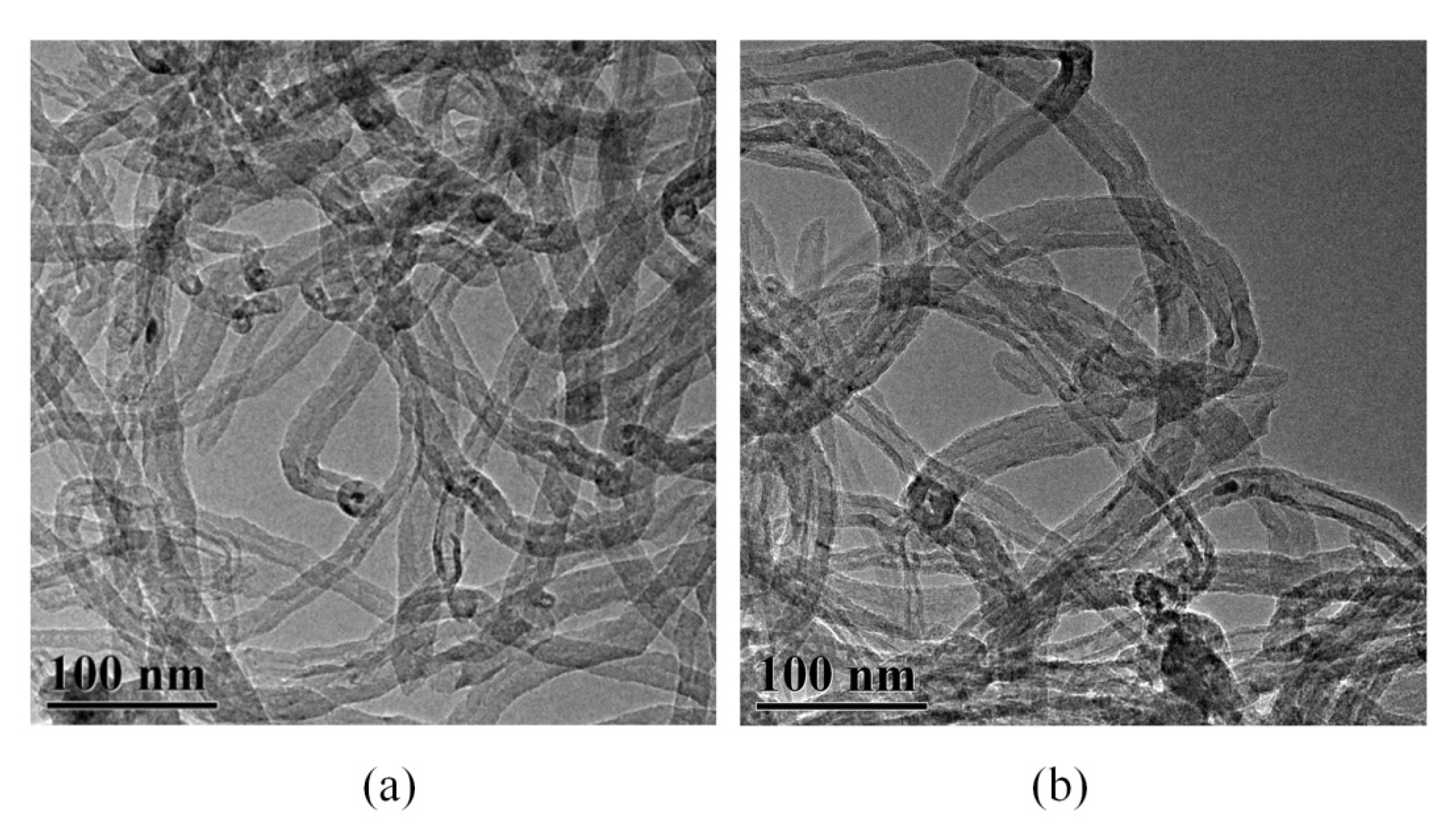
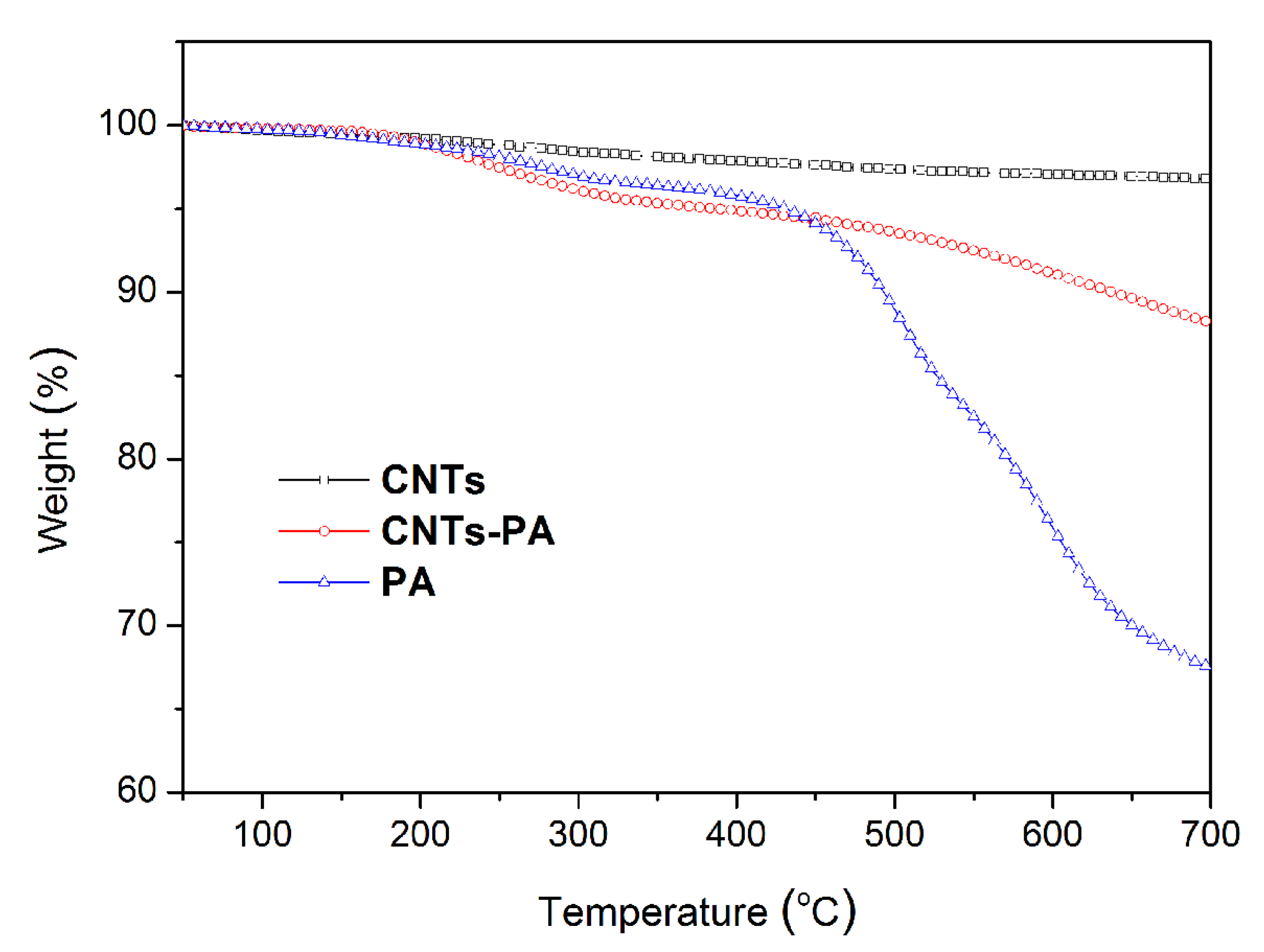
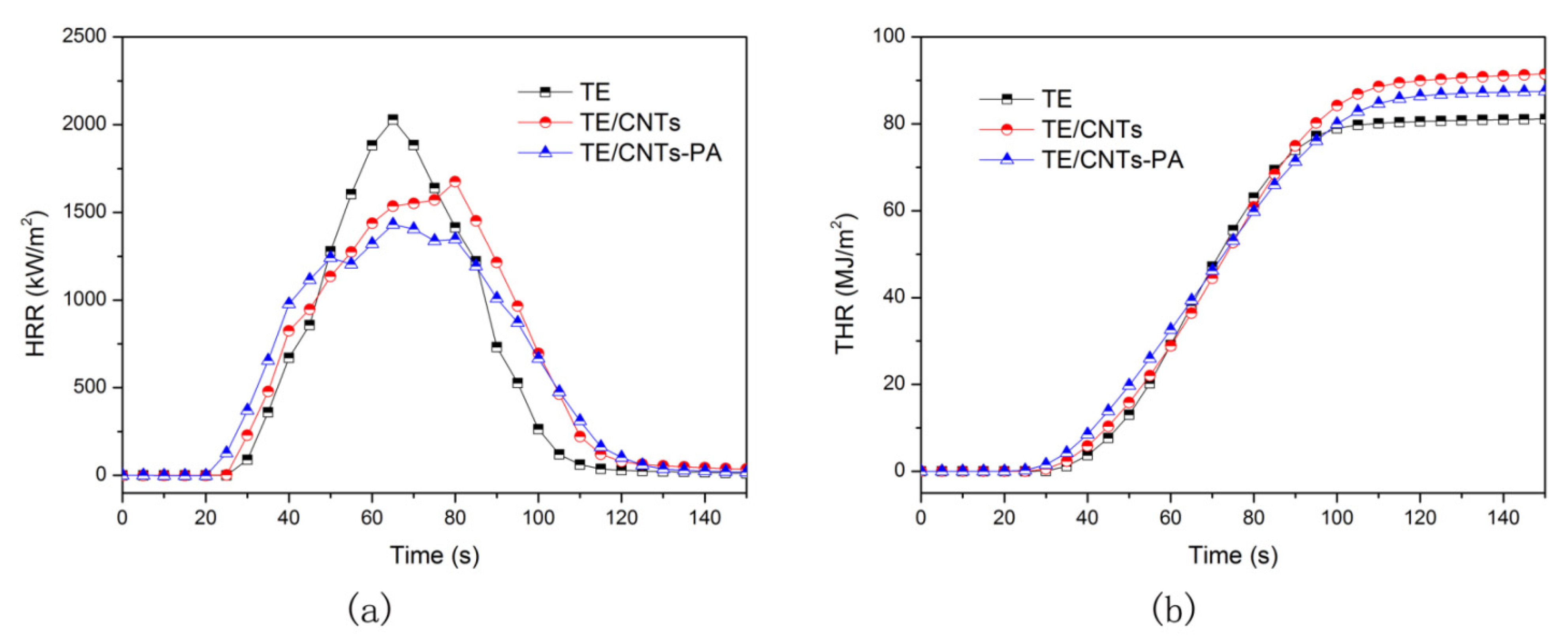

| Element (At.%) | CNTs | CNTs-PA |
|---|---|---|
| C | 96.25 | 93.65 |
| O | 3.75 | 4.68 |
| Si | - | 1.08 |
| N | - | 0.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J. Flame Retardancy and Dispersion of Functionalized Carbon Nanotubes in Thiol-Ene Nanocomposites. Polymers 2021, 13, 3308. https://doi.org/10.3390/polym13193308
Wang J. Flame Retardancy and Dispersion of Functionalized Carbon Nanotubes in Thiol-Ene Nanocomposites. Polymers. 2021; 13(19):3308. https://doi.org/10.3390/polym13193308
Chicago/Turabian StyleWang, Jiangbo. 2021. "Flame Retardancy and Dispersion of Functionalized Carbon Nanotubes in Thiol-Ene Nanocomposites" Polymers 13, no. 19: 3308. https://doi.org/10.3390/polym13193308
APA StyleWang, J. (2021). Flame Retardancy and Dispersion of Functionalized Carbon Nanotubes in Thiol-Ene Nanocomposites. Polymers, 13(19), 3308. https://doi.org/10.3390/polym13193308





