Emerging Developments Regarding Nanocellulose-Based Membrane Filtration Material against Microbes
Abstract
:1. Introduction
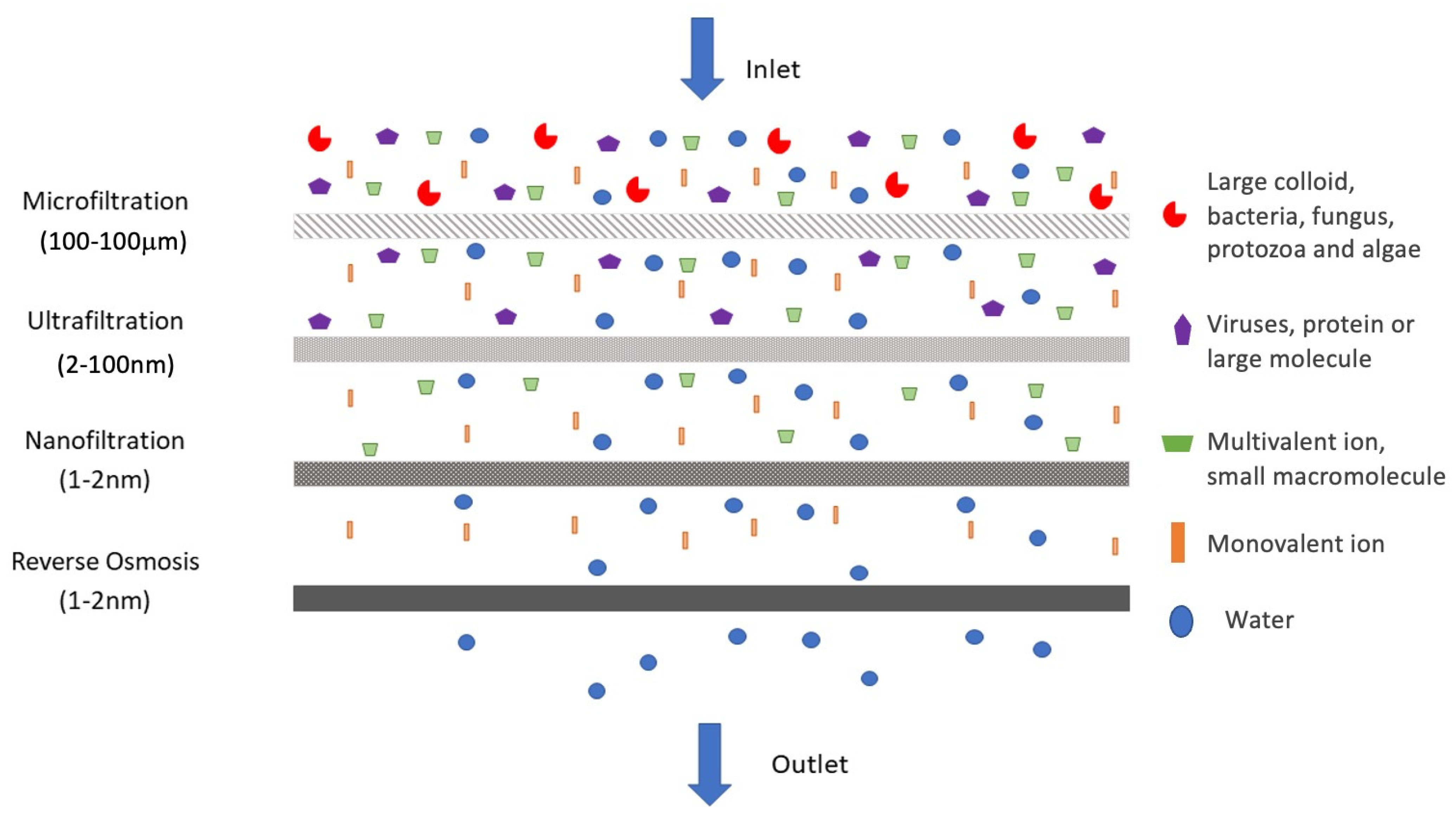
2. Types and Rejection Mechanisms of Membrane Filters
| Source of CNF | Modulus (GPa) | Tensile Strength (MPa) | Strain to Failure (%) | Reference |
|---|---|---|---|---|
| Pulp | 10.4–13.7 | 129–214 | 3.3–10.1 | [45] |
| Kraft pulp | 17 | 250 | 2–6 | [46] |
| Wood | 6.2–6.9 | 222–233 | 7.0–7.6 | [47] |
| Wood | 13 | 223 | - | [48] |
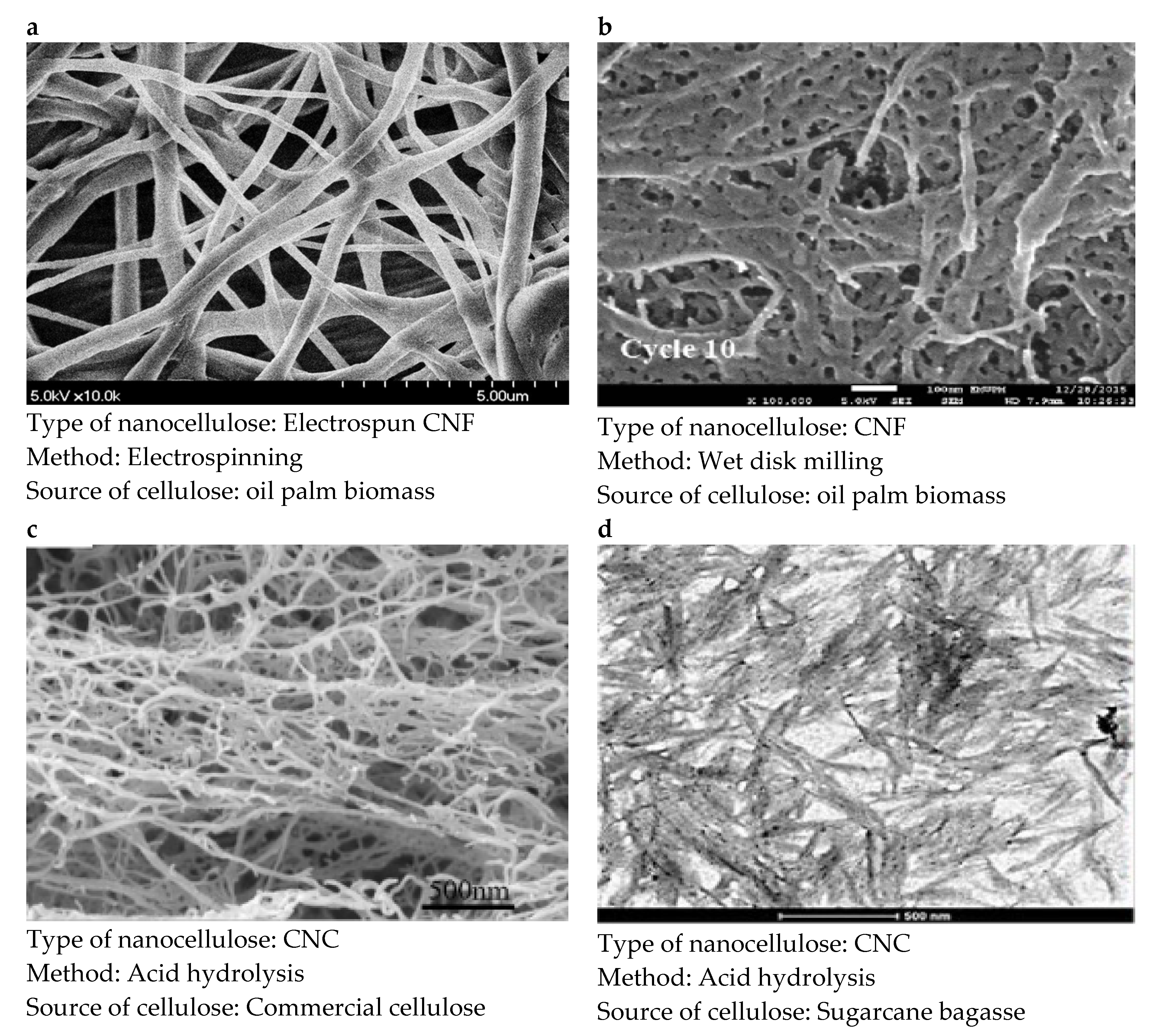
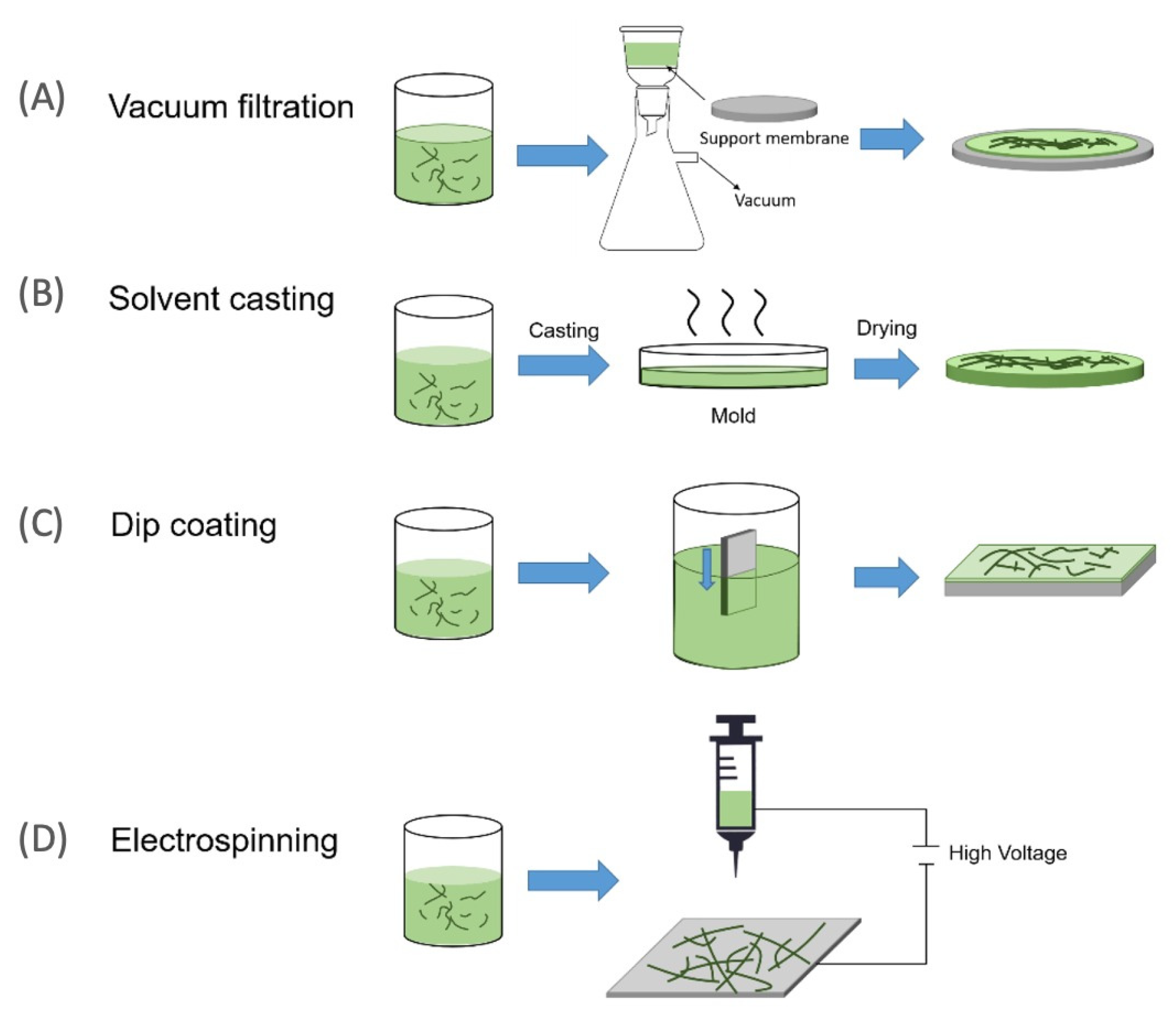
2.1. Fabrication of Nanocellulose Membrane
2.1.1. Vacuum Filtration
2.1.2. Casting Evaporation and Coating Self-Standing
2.1.3. Electrospinning
3. Attributes of Nanocellulose Membrane Filtration of Microbes
| Characteristic | PP | Nanocellulose |
|---|---|---|
| Fibre length (nm) | - | 400 |
| Diameter (nm) | 25,000 | 1–100 |
| Efficiency against pathogens | ~100% | 99.9980–99.9995% |
| Tensile modulus (GPa) | 1.5 | 145 |
| Tensile strength (GPa) | 0.02 | 7.5 |
| Poison’s ratio | 0.4 | 0.3 |
| Property | Advantages | Reference |
|---|---|---|
| Nanoporosity | Good virus filtration using size-exclusion method. Typically, the pore size of nanocellulose is below 100 nm. | [15] |
| Surface functionalization | Functionalization nanocellulose with several compounds to make it cationic charged causes an increase in its binding affinity towards viruses. | [73] |
| High specific surface area | Provides a large surface area for functionalization. Thereby increasing interaction efficiency. | [74] |
| Renewable | Nanocellulose can be easily sourced from plant bio-waste. Its use can eliminate the use of other non-renewable polymers as mentioned in the Introduction section. | [49,66] |
| Biodegradability | An important aspect to save the environment. It is biodegradable in landfills. Hence, current environment issues from used and discarded surgical masks can be reduced or even eliminated. | [75] |
| High mechanical strength | High strength membrane filters can be fabricated using it. | [76] |
| Stability in water | It can reduce biofouling of membrane filters. This is important for application in membrane filters for wastewater. | [77] |
| Nanocellulose | Abbreviation | Sources | Main Treatments | Dimensions |
|---|---|---|---|---|
Cellulose nanofiber | CNF | Plants | Mechanical fibrillation | Diameter: 5–50 nm Length: Several µm |
Cellulose nanocrystals nanowhiskers/nanorods | CNC | Plants | Acid hydrolysis | Diameter: 2–20 nm Length: 100 nm to several µm |
Bacterial nanocellulose/biocellulose | BNC | Microorganisms | Polymerization and crystallization | Diameter: 2–4 nm Length: 100µm |
4. Modifications on Nanocellulose to Improve Filter Efficiency
5. Recent Developments on Nanocellulose as a Filtration Material against Microbes
5.1. Viruses
5.2. Bacteria
5.3. Other Types of Microbes
6. Challenges and Future Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Norrrahim, M.N.F.; Norizan, M.N.; Jenol, M.A.; Farid, M.A.A.; Janudin, N.; Ujang, F.A.; Yasim-Anuar, T.A.T.; Najmuddin, S.U.F.S.; Ilyas, R.A. Emerging Development on Nanocellulose as Antimicrobial Material: An Overview. Mater. Adv. 2021, 2, 3538–3551. [Google Scholar] [CrossRef]
- Atkinson, S.K.; Sadofsky, L.R.; Morice, A.H. How does rhinovirus cause the common cold cough? BMJ Open Respir. Res. 2016, 3, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamont, R.F.; Sobel, J.D.; Carrington, D.; Mazaki-Tovi, S.; Kusanovic, J.P.; Vaisbuch, E.; Romero, R. Varicella-zoster virus (chickenpox) infection in pregnancy. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 1155–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, A.K.; Hon, K.L.; Leong, K.F. Rubella (German measles) revisited. Hong Kong Med. J. 2019, 25, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Hegerle, N.; Guiso, N. Epidemiology of whooping cough & typing of Bordetella pertussis. Future Microbiol. 2013, 8, 1391–1403. [Google Scholar] [PubMed]
- Spyrou, M.A.; Tukhbatova, R.I.; Wang, C.C.; Valtueña, A.A.; Lankapalli, A.K.; Kondrashin, V.V.; Tsybin, V.A.; Khokhlov, A.; Kühnert, D.; Herbig, A.; et al. Analysis of 3800-year-old Yersinia pestis genomes suggests Bronze Age origin for bubonic plague. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gagneux, S. Ecology and evolution of Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2018, 16, 202–213. [Google Scholar] [CrossRef]
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.E.; Moyes, C.L.; Henry, A.; Eckhoff, P.A.; et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015, 526, 207–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furlan, K.C.; Kakizaki, P.; Chartuni, J.C.N.; Valente, N.Y.S. Sycosiform tinea barbae caused by trichophyton rubrum and its association with autoinoculation. Bras. Dermatol. 2017, 92, 160–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makola, N.F.; Magongwa, N.M.; Matsaung, B.; Schellack, G.; Schellack, N. Managing athlete’s foot. S. Afr. Fam. Pract. 2018, 60, 37–41. [Google Scholar] [CrossRef]
- Fahimirad, S.; Fahimirad, Z.; Sillanpää, M. Efficient removal of water bacteria and viruses using electrospun nanofibers. Sci. Total Environ. 2020, 751, 141673. [Google Scholar] [CrossRef]
- Sussman, E.M. Escherichia coli: Mechanisms of Virulence. J. R. Soc. Med. 1997, 90, 466. [Google Scholar]
- World Health Organization Drinking-Water. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 20 September 2021).
- World Health Organization. Modes of Transmission of Virus Causing COVID-19: Implications for IPC Precaution Recommendations. Available online: https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations (accessed on 20 September 2021).
- Metreveli, G.; Wågberg, L.; Emmoth, E.; Belák, S.; Strømme, M.; Mihranyan, A. A Size-Exclusion Nanocellulose Filter Paper for Virus Removal. Adv. Healthc. Mater. 2014, 3, 1546–1550. [Google Scholar] [CrossRef]
- Hai, F.I.; Riley, T.; Shawkat, S.; Magram, S.F.; Yamamoto, K. Removal of pathogens by membrane bioreactors: A review of the mechanisms, influencing factors and reduction in chemical disinfectant dosing. Water 2014, 6, 3603–3630. [Google Scholar] [CrossRef]
- Das, O.; Neisiany, R.E.; Capezza, A.J.; Hedenqvist, M.S.; Försth, M.; Xu, Q.; Jiang, L.; Ji, D.; Ramakrishna, S. The need for fully bio-based facemasks to counter coronavirus outbreaks: A perspective. Sci. Total Environ. 2020, 736, 1–7. [Google Scholar] [CrossRef]
- Ilyas, R.; Sapuan, S.; Norrrahim, M.N.F.; Yasim-Anuar, T.A.T.; Kadier, A.; Kalil, M.S.; Atikah, M.; Ibrahim, R.; Asrofi, M.; Abral, H.; et al. Nanocellulose/starch biopolymer nanocomposites: Processing, manufacturing, and applications. In Advanced Processing, Properties, and Applications of Starch and Other Bio-Based Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 65–88. [Google Scholar]
- Norrrahim, M.N.F.; Huzaifah, M.R.M.; Farid, M.A.A.; Shazleen, S.S.; Misenan, M.S.M.; Yasim-Anuar, T.A.T.; Naveen, J.; Nurazzi, N.M.; Rani, M.S.A.; Hakimi, M.I.; et al. Greener Pretreatment Approaches for the Valorisation of Natural Fibre Biomass into Bioproducts. Polymers 2021, 13, 2971. [Google Scholar] [CrossRef] [PubMed]
- Norrrahim, M.N.F.; Ilyas, R.A.; Nurazzi, N.M.; Rani, M.S.A.; Atikah, M.S.N.; Shazleen, S.S. Chemical Pretreatment of Lignocellulosic Biomass for the Production of Bioproducts: An Overview. Appl. Sci. Eng. Prog. 2021, 13, 2971. [Google Scholar]
- Lee, C.H.; Lee, S.H.; Padzil, F.N.M.; Ainun, Z.M.A.; Norrrahim, M.N.F.; Chin, K.L. Biocomposites and Nanocomposites. In Composite Materials; CRC Press: Boca Raton, FL, USA, 2021; pp. 29–60. [Google Scholar]
- Yasim-Anuar, T.A.T.; Ariffin, H.; Norrrahim, M.N.F.; Hassan, M.A.; Andou, Y.; Tsukegi, T.; Nishida, H. Well-Dispersed Cellulose Nanofiber in Low Density Polyethylene Nanocomposite by Liquid-Assisted Extrusion. Polymers 2020, 12, 927. [Google Scholar] [CrossRef] [Green Version]
- Norrrahim, M.N.F.; Ariffin, H.; Yasim-Anuar, T.A.T.; Hassan, M.A.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Nishida, H. Performance Evaluation of Cellulose Nanofiber with Residual Hemicellulose as a Nanofiller in Polypropylene-Based Nanocomposite. Polymers 2021, 13, 1064. [Google Scholar] [CrossRef] [PubMed]
- Misenan, M.S.M.; Ali, E.S.; Khiar, A.S.A. Conductivity, dielectric and modulus study of chitosan-methyl cellulose-BMIMTFSI polymer electrolyte doped with cellulose nano crystal. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018; Volume 1972. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Yasim-Anuar, T.A.T.; Jenol, M.A.; Mohd Nurazzi, N.; Ilyas, R.A.; Sapuan, S. Performance Evaluation of Cellulose Nanofiber Reinforced Polypropylene Biocomposites for Automotive Applications. In Biocomposite and Synthetic Composites for Automotive Applications; Woodhead Publishing Series: Amsterdam, The Netherlands, 2020; pp. 119–215. [Google Scholar]
- Sharip, N.S.; Yasim-Anuar, T.A.T.; Norrrahim, M.N.F.; Shazleen, S.S.; Nurazzi, N.M.; Sapuan, S.M.; Ilyas, R.A. A review on nanocellulose composites in biomedical application. In Composites in Biomedical Applications; CRC Press: Boca Raton, FL, USA, 2020; pp. 161–190. [Google Scholar]
- Ilyas, R.A.; Sapuan, S.M.; Harussani, M.M.; Hakimi, M.Y.A.Y.; Haziq, M.Z.M.; Atikah, M.S.N.; Asyraf, M.R.M.; Ishak, M.R.; Razman, M.R.; Nurazzi, N.M.; et al. Polylactic Acid (PLA) Biocomposite: Processing, Additive Manufacturing and Advanced Applications. Polymers 2021, 13, 1326. [Google Scholar] [CrossRef]
- Ilyas, R.; Sapuan, S.; Atikah, M.; Asyraf, M.; Rafiqah, S.A.; Aisyah, H.; Nurazzi, N.M.; Norrrahim, M. Effect of hydrolysis time on the morphological, physical, chemical, and thermal behavior of sugar palm nanocrystalline cellulose (Arenga pinnata (Wurmb.) Merr). Text. Res. J. 2021, 91, 152–167. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Mohd Nurazzi, N.; Norrrahim, M.N.F.; Ibrahim, R.; Atikah, M.S.N.; Huzaifah, M.R.M.; Radzi, A.M.; Izwan, S.; Noor Azammi, A.M.; et al. Macro to Nanocscale Natural Fibre Composites for Automotive Components: Research, Development, and Application. In Biocomposite and Synthetic Composites for Automotive Applications; Woodhead Publishing Series: Amsterdam, The Netherlands, 2020; pp. 51–105. [Google Scholar]
- Norrrahim, M.N.F.; Yasim-Anuar, T.A.T.; Sapuan, S.M.; Ilyas, R.A.; Hakimi, M.I.; Najmuddin, S.U.F.S.; Jenol, M.A. Nanocellulose Reinforced Polypropylene and Polyethylene Composite for Packaging Application. In Bio-Based Packaging: Material Environmental and Economic Aspects; Sapuan, S.M., Ilyas, R.A., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; pp. 133–150. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Kasim, N.A.M.; Knight, V.F.; Misenan, M.S.M.; Janudin, N.; Shah, N.A.A.; Kasim, N.; Yusoff, W.Y.W.; Noor, S.A.M.; Jamal, S.H.; et al. Nanocellulose: A bioadsorbent for chemical contaminant remediation. RSC Adv. 2021, 11, 7347–7368. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Kasim, N.A.M.; Knight, V.F.; Ujang, F.A.; Janudin, N.; Razak, M.A.I.A.; Shah, N.A.A.; Noor, S.A.M.; Jamal, S.H.; Ong, K.K.; et al. Nanocellulose: The Next Super Versatile Material for the Military. Mater. Adv. 2021, 2, 1485–1506. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Kasim, N.A.M.; Knight, V.F.; Halim, N.A.; Shah, N.A.A.; Noor, S.A.M.; Jamal, S.H.; Ong, K.K.; Yunus, W.M.Z.W.; Farid, M.A.A.; et al. Performance Evaluation of Cellulose Nanofiber Reinforced Polymer Composites. Funct. Compos. Struct. 2021, 3. [Google Scholar] [CrossRef]
- Misenan, M.S.M.; Janudin, N.; Idayu, M.A.; Norrrahim, M.N.F.; Jamal, S.H.; Wan Yusoff, W.Y.; Kasim, N.; Yunus, W.M.D.Z.W.; Ernest, V.F.K.V.; Kasim, N.A.M. Cellulose Nanofiber as Potential Absorbent Material for Chloride Ion. Solid State Phenom. 2021, 317, 263–269. [Google Scholar] [CrossRef]
- Ariffin, H.; Tengku Yasim-Anuar, T.A.; Norrrahim, M.N.F.; Hassan, M.A. Synthesis of Cellulose Nanofiber from Oil Palm Biomass by High Pressure Homogenization and Wet Disk Milling. In Nanocellulose: Synthesis, Structure, Properties and Applications; World Scientific: Singapore, 2021; pp. 51–64. [Google Scholar]
- Jasmania, L. Preparation of nanocellulose and its potential application. Int. J. Nanomater. Nanotechnol. Nanomed. 2018, 4, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.L.; Fadel, S.M.; Abouzeid, R.E.; Elseoud, W.S.A.; Hassan, E.A.; Berglund, L.; Oksman, K. Water purification ultrafiltration membranes using nanofibers from unbleached and bleached rice straw. Sci. Rep. 2020, 10, 11278. [Google Scholar] [CrossRef]
- Ma, H.; Burger, C.; Hsiao, B.S.; Chu, B. Ultra-fine cellulose nanofibers: New nano-scale materials for water purification. J. Mater. Chem. 2011, 21, 7507–7510. [Google Scholar] [CrossRef]
- Thakur, V.K.; Voicu, S.I. Recent Advances in Cellulose and Chitosan Based Membranes for Water Purification: A Concise Review. Carbohydr. Polym. 2016, 146, 148–165. [Google Scholar] [CrossRef]
- Suman; Kardam, A.; Gera, M.; Jain, V.K. A novel reusable nanocomposite for complete removal of dyes, heavy metals and microbial load from water based on nanocellulose and silver nano-embedded pebbles. Environ. Technol. 2014, 36, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Abou-Zeid, R.; Hassan, E.; Berglund, L.; Aitomäki, Y.; Oksman, K. Membranes Based on Cellulose Nanofibers and Activated Carbon for Removal of Escherichia coli Bacteria from Water. Polymers 2017, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Norrrahim, M.N.F.; Kasim, N.A.M.; Knight, V.F.; Ong, K.K.; Noor, S.A.M.; Jamal, S.H.; Shah, N.A.A.; Halim, N.A.; Ilyas, R.A.; Yunus, W.M.Z.W. Cationic Nanocellulose as Promising Candidate for Filtration Material of COVID-19: A Perspective. Appl. Sci. Eng. Prog. 2021. [Google Scholar] [CrossRef]
- Du, N.; Park, H.B.; Dal-Cin, M.M.; Guiver, M.D. Advances in high permeability polymeric membrane materials for CO2 separations. Energy Environ. Sci. 2012, 5, 7306–7322. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.Y.; Haagenson, D.; Wiesenborn, D.P. Cellulose nanocrystals vs. Cellulose nanofibrils: A comparative study on their microstructures and effects as polymer reinforcing agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef]
- Henriksson, M.; Berglund, L.A.; Isaksson, P.; Lindström, T.; Nishino, T. Cellulose nanopaper structures of high toughness. Biomacromolecules 2008, 9, 1579–1585. [Google Scholar] [CrossRef]
- Syverud, K.; Stenius, P. Strength and barrier properties of MFC films. Cellulose 2009, 16, 75–85. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem.-Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Nogi, M.; Iwamoto, S.; Nakagaito, A.N.; Yano, H. Optically Transparent Nanofiber Paper. Adv. Mater. 2009, 21, 1595–1598. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Ariffin, H.; Yasim-Anuar, T.A.T.; Ghaemi, F.; Hassan, M.A.; Ibrahim, N.A.; Ngee, J.L.H.; Yunus, W.M.Z.W. Superheated steam pretreatment of cellulose affects its electrospinnability for microfibrillated cellulose production. Cellulose 2018, 25, 3853–3859. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F. Superheated Steam Pretreatment of Oil Palm Biomass for Improving Nanofibrillation of Cellulose and Performance of Polypropylene/Cellulose Nanofiber Composites. Ph.D. Thesis, Universiti Putra Malaysia, Selangor, Malaysia, 2018. [Google Scholar]
- Zhang, T.; Zhang, Y.; Jiang, H.; Wang, X. Adjusting the pore size of nano-cellulose aerogel by heat treatment in the gel stage. Mater. Res. Express 2019, 6, 075027. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Characterization of cellulose nanocrystals produced by acid-hydrolysis from sugarcane bagasse as agro-waste. J. Mater. Phys. Chem. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Wu, L.; Manukyan, L.; Mantas, A.; Mihranyan, A. Nanocellulose-Based Nanoporous Filter Paper for Virus Removal Filtration of Human Intravenous Immunoglobulin. ACS Appl. Nano Mater. 2019, 2, 6352–6359. [Google Scholar] [CrossRef]
- Ottoson, J.; Hansen, A.; Westrell, T.; Johansen, K.; Norder, H.; Stenström, T.A. Removal of noro-and enteroviruses, giardia cysts, cryptosporidium oocysts, and fecal indicators at four secondary wastewater treatment plants in Sweden. Water Environ. Res. 2006, 78, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Francy, D.S.; Stelzer, E.A.; Bushon, R.N.; Brady, A.M.; Williston, A.G.; Riddell, K.R.; Borchardt, M.A.; Spencer, S.K.; Gellner, T.M. Comparative effectiveness of membrane bioreactors, conventional secondary treatment, and chlorine and UV disinfection to remove microorganisms from municipal wastewaters. Water Res. 2012, 46, 4164–4178. [Google Scholar] [CrossRef]
- Held, D.; Kilz, P. Size-exclusion chromatography as a useful tool for the assessment of polymer quality and determination of macromolecular properties. Chem. Teach. Int. 2021, 3, 77–103. [Google Scholar] [CrossRef]
- Fornasiero, F.; Park, H.G.; Holt, J.K.; Stadermann, M.; Grigoropoulos, C.P.; Noy, A.; Bakajin, O. Mechanism of ion exclusion by sub-2nm carbon nanotube membranes. Mater. Res. Soc. Symp. Proc. 2008, 1106, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Harinarayan, C.J.; Mueller, A.; Ljunglöf, R.; Fahrner, J.; Van Alstine, R. An Exclusion Mechanism in Ion Exchange Chromatography. Biotechnol. Bioeng. 2006, 95, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Thielemans, W. Thermodynamics of Adsorption on Nanocellulose Surfaces; Springer: Dordrecht, The Netherlands, 2019; Volume 26. [Google Scholar]
- Dai, Z.; Ottesen, V.; Deng, J.; Helberg, R.M.L.; Deng, L. A brief review of nanocellulose based hybrid membranes for CO2 separation. Fibers 2019, 7, 40. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Q.; Ye, D.; Chang, C.; Zhang, L. Facile fabrication of superhydrophilic membranes consisted of fibrous tunicate cellulose nanocrystals for highly efficient oil/water separation. J. Membr. Sci. 2017, 525, 1–8. [Google Scholar] [CrossRef]
- Ansaloni, L.; Salas-Gay, J.; Ligi, S.; Baschetti, M.G. Nanocellulose-based membranes for CO2 capture. J. Membr. Sci. 2017, 522, 216–225. [Google Scholar] [CrossRef]
- Dai, Z.; Deng, J.; Yu, Q.; Helberg, R.M.L.; Janakiram, S.; Ansaloni, L.; Deng, L. Fabrication and Evaluation of Bio-Based Nanocomposite TFC Hollow Fiber Membranes for Enhanced CO2 Capture. ACS Appl. Mater. Interfaces 2019, 11, 10874–10882. [Google Scholar] [CrossRef] [PubMed]
- Hutten, I.M. CHAPTER 1-Introduction to Nonwoven Filter Media. In Handbook of Nonwoven Filter Media; Hutten, I.M.B.T.-H., Ed.; Butterworth-Heinemann: Oxford, UK, 2007; pp. 1–28. ISBN 978-1-85617-441-1. [Google Scholar]
- Good, T.; Hospital, S. Evaluation of the Efficiency of Medical Masks and the Creation of New Medical Masks. J. Int. Med. Res. 2007, 35, 213–223. [Google Scholar]
- Voisin, H.; Bergström, L.; Liu, P.; Mathew, A. Nanocellulose-Based Materials for Water Purification. Nanomaterials 2017, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Ariffin, H.; Norrrahim, M.N.F.; Yasim-Anuar, T.A.T.; Nishida, H.; Hassan, M.A.; Ibrahim, N.A.; Yunus, W.M.Z.W. Oil Palm Biomass Cellulose-Fabricated Polylactic Acid Composites for Packaging Applications. In Bionanocomposites for Packaging Applications; Springer: Berlin/Heidelberg, Germany, 2018; pp. 95–105. ISBN 978-331-967-3-196. [Google Scholar]
- Norrrahim, M.N.F.; Ariffin, H.; Yasim-Anuar, T.A.T.; Hassan, M.A.; Nishida, H.; Tsukegi, T. One-pot nanofibrillation of cellulose and nanocomposite production in a twin-screw extruder. IOP Conf. Ser. Mater. Sci. Eng. 2018, 368, 1–9. [Google Scholar] [CrossRef]
- Halib, N.; Perrone, F.; Cemazar, M.; Dapas, B.; Farra, R.; Abrami, M.; Chiarappa, G.; Forte, G.; Zanconati, F.; Pozzato, G.; et al. Potential applications of nanocellulose-containing materials in the biomedical field. Materials 2017, 10, 977. [Google Scholar] [CrossRef] [Green Version]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile application of nanocellulose: From industry to skin tissue engineering and wound healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef] [Green Version]
- Fareez, I.M.; Jasni, A.H.; Norrrahim, M.N.F. Nanofibrillated Cellulose Based Bio-phenolic Composites. In Phenolic Polymers Based Composite Materials; Springer: Singapore, 2020; pp. 139–151. [Google Scholar]
- Norrrahim, M.N.F.; Ariffin, H.; Hassan, M.A.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Nishida, H. Utilisation of superheated steam in oil palm biomass pretreatment process for reduced chemical use and enhanced cellulose nanofibre production. Int. J. Nanotechnol. 2019, 16, 668–679. [Google Scholar] [CrossRef]
- Sunasee, R.; Hemraz, U.D. Synthetic strategies for the fabrication of cationic surface-modified cellulose nanocrystals. Fibers 2018, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Lin, N.; Dufresne, A. Nanoscale Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: A review. Nanoscale 2012, 4, 3274–3294. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Boufi, S. Nanocellulose as a novel nanostructured adsorbent for environmental remediation: A review. Cellulose 2017, 24, 1171–1197. [Google Scholar] [CrossRef]
- Rusli, R.; Eichhorn, S.J. Determination of the stiffness of cellulose nanowhiskers and the fiber- matrix interface in a nanocomposite using Raman spectroscopy Determination of the stiffness of cellulose nanowhiskers and the fiber-matrix interface in a nanocomposite using Raman spe. Appl. Phys. Lett. 2008, 93, 1–3. [Google Scholar] [CrossRef]
- Nguyen, T.; Roddick, F.A.; Fan, L. Biofouling of Water Treatment Membranes: A Review of the Underlying Causes, Monitoring Techniques and Control Measures. Membranes 2012, 2, 804–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, H.P.S.A.; Davoudpour, Y.; Islam, N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef]
- Kongruang, S. Bacterial Cellulose Production by Acetobacter xylinum Strains from Agricultural Waste Products. Appl. Biochem. Biotechnol. 2008, 148, 245–256. [Google Scholar] [CrossRef]
- Ahmad, I.; Thomas, S.; Dufresne, A.; Huang, J.; Lin, N. Advances in cellulose nanomaterials. Cellulose 2018, 25, 2151–2189. [Google Scholar] [CrossRef]
- Islam, N.; Rahman, F. Production and modification of nanofibrillated cellulose composites and potential applications. In Green Composites for Automotive Applications; Elsevier Ltd: Amsterdam, The Netherlands, 2019; pp. 115–141. ISBN 978-008-102-1-774. [Google Scholar]
- Abol-fotouh, D.; Hassan, M.A.; Shokry, H.; Roig, A.; Azab, M.S.; Kashyout, A.E.B. Bacterial nanocellulose from agro-industrial wastes: Low-cost and enhanced production by Komagataeibacter saccharivorans. Sci. Rep. 2020, 10, 3491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.T.; Alam, M.M.; Zoccola, M. Review on modification of nanocellulose for application in composites. Int. J. Innov. Res. Sci. 2013, 2, 5444–5451. [Google Scholar]
- Quellmalz, A.; Mihranyan, A. Citric Acid Cross-Linked Nanocellulose-Based Paper for Size-Exclusion Nanofiltration. ACS Biomater. Sci. Eng. 2015, 1, 271–276. [Google Scholar] [CrossRef]
- Wei, L.; Agarwal, U.P.; Hirth, K.C.; Matuana, L.M.; Sabo, R.C.; Stark, N.M. Chemical modification of nanocellulose with canola oil fatty acid methyl ester. Carbohydr. Polym. 2017, 169, 108–116. [Google Scholar] [CrossRef] [Green Version]
- Radhakrishnan, A.; Nahi, J.; Beena, B. Synthesis and characterization of multi-carboxyl functionalized nanocellulose/graphene oxide-zinc oxide composite for the adsorption of uranium (VI) from aqueous solutions: Kinetic and equilibrium profiles. Mater. Today Proc. 2020, 41, 557–563. [Google Scholar] [CrossRef]
- Li, L.; Yu, C.; Yu, C.; Chen, Q.; Yu, S. Nanocellulose as template to prepare rough-hydroxy rich hollow silicon mesoporous nanospheres (R-nCHMSNs) for drug delivery. Int. J. Biol. Macromol. 2021, 180, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhou, Z.; Yang, J.; Zhang, M.; Cai, F.; Lu, P. ZnO nanoparticles stabilized oregano essential oil Pickering emulsion for functional cellulose nanofibrils packaging films with antimicrobial and antioxidant activity. Int. J. Biol. Macromol. 2021, 190, 433–440. [Google Scholar] [CrossRef]
- Fan, W.; Wang, J.; Zhang, Z.; Li, Z. Synergistic enhancement of UV-resistance and electrical conductivity of waterborne polyurethane composite with combination of functionalized 2D graphene oxide and 1D nanocellulose. Prog. Org. Coat. 2021, 151, 106017. [Google Scholar] [CrossRef]
- Henschen, J. Bio-Based Preparation of Nanocellulose and Functionalization Using Polyelectrolytes. Ph.D. Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2019. [Google Scholar]
- Lebrun, G.J.L. Cellulose-based virus-retentive filters: A review. Rev. Environ. Sci. Biotechnol. 2017, 16, 455–489. [Google Scholar] [CrossRef]
- Burnouf, T.; Radosevich, M. Nanofiltration of plasma-derived biopharmaceutical products. Haemophilia 2003, 19, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Kalbfuss, B.; Wolff, M.; Morenweiser, R.; Reichl, U. Purification of cell culture-derived human influenza A virus by size-exclusion and anion-exchange chromatography. Biotechnol. Bioeng. 2007, 96, 932–944. [Google Scholar] [CrossRef]
- Gustafsson, O.; Manukyan, L.; Mihranyan, A. High-Performance Virus Removal Filter Paper for Drinking Water Purification. Glob. Chall. 2018, 2, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manukyan, L.; Li, P.; Gustafsson, S.; Mihranyan, A. Growth media filtration using nanocellulose-based virus removal filter for upstream biopharmaceutical processing. J. Membr. Sci. 2019, 572, 464–474. [Google Scholar] [CrossRef]
- Asper, M.; Hanrieder, T.; Quellmalz, A.; Mihranyan, A. Removal of xenotropic murine leukemia virus by nanocellulose based filter paper. Biologicals 2015, 43, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Mautner, A.; Bismarck, A. Bacterial nanocellulose papers with high porosity for optimized permeance and rejection of nm-sized pollutants. Carbohydr. Polym. 2021, 251, 117130. [Google Scholar] [CrossRef]
- Leung, W.W.F.; Sun, Q. Electrostatic charged nanofiber filter for filtering airborne novel coronavirus (COVID-19) and nano-aerosols. Sep. Purif. Technol. 2020, 250, 116886. [Google Scholar] [CrossRef]
- Artika, I.M.; Dewantari, A.K.; Wiyatno, A. Molecular biology of coronaviruses: Current knowledge. Heliyon 2020, 6, e04743. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Albukhari, S.M.; Heldt, C.L.; Heiden, P.A. Virus and chlorine adsorption onto guanidine modified cellulose nanofibers using covalent and hydrogen bonding. Carbohydr. Res. 2020, 498, 108153. [Google Scholar] [CrossRef] [PubMed]
- Meingast, C.; Heldt, C.L. Arginine-enveloped virus inactivation and potential mechanisms. Biotechnol. Prog. 2020, 30, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Rosilo, H.; McKee, J.R.; Kontturi, E.; Koho, T.; Hytönen, V.P.; Ikkala, O.; Kostiainen, M.A. Cationic polymer brush-modified cellulose nanocrystals for high-affinity virus binding. Nanoscale 2014, 6, 11871–11881. [Google Scholar] [CrossRef]
- Rivière, G.N.; Korpi, A.; Sipponen, M.H.; Zou, T.; Kostiainen, M.A.; Österberg, M. Agglomeration of Viruses by Cationic Lignin Particles for Facilitated Water Purification. ACS Sustain. Chem. Eng. 2020, 8, 4167–4177. [Google Scholar] [CrossRef]
- Sun, M.; Wang, H.; Li, X. Modification of cellulose microfibers by polyglutamic acid and mesoporous silica nanoparticles for Enterovirus 71 adsorption. Mater. Lett. 2020, 277, 128320. [Google Scholar] [CrossRef]
- Gustafsson, S.; Lordat, P.; Hanrieder, T.; Asper, M.; Schaefer, O.; Mihranyan, A. Mille-feuille paper: A novel type of filter architecture for advanced virus separation applications. Mater. Horiz. 2016, 3, 320–327. [Google Scholar] [CrossRef]
- Wang, R.; Guan, S.; Sato, A.; Wang, X.; Wang, Z.; Yang, R.; Hsiao, B.S.; Chu, B. Nanofibrous microfiltration membranes capable of removing bacteria, viruses and heavy metal ions. J. Membr. Sci. 2013, 446, 376–382. [Google Scholar] [CrossRef]
- Otoni, C.G.; Figueiredo, J.S.L.; Capeletti, L.B.; Cardoso, M.B.; Bernardes, J.S.; Loh, W. Tailoring the Antimicrobial Response of Cationic Nanocellulose-Based Foams through Cryo-Templating. ACS Appl. Bio Mater. 2019, 2, 1975–1986. [Google Scholar] [CrossRef]
- Gouda, M.; Hebeish, A.A.; Al-Omair, M.A. Development of silver-containing nanocellulosics for effective water disinfection. Cellulose 2014, 21, 1965–1974. [Google Scholar] [CrossRef]
- Ottenhall, A.; Henschen, J.; Illergård, J.; Ek, M. Cellulose-based water purification using paper filters modified with polyelectrolyte multilayers to remove bacteria from water through electrostatic interactions. Environ. Sci. Water Res. Technol. 2018, 4, 2070–2079. [Google Scholar] [CrossRef] [Green Version]
- Nath, B.K.; Chaliha, C.; Kalita, E.; Kalita, M.C. Synthesis and characterization of ZnO:CeO2:nanocellulose:PANI bionanocomposite. A bimodal agent for arsenic adsorption and antibacterial action. Carbohydr. Polym. 2016, 148, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, O.; Gustafsson, S.; Manukyan, L.; Mihranyan, A. Significance of Brownian motion for nanoparticle and virus capture in nanocellulose-based filter paper. Membranes 2018, 8, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelal, I.; Ali, U.; Nadir, D. Filtration and Antibacterial Properties of Bacterial Cellulose Membranes for Textile Wastewater Treatment. AVvicenna J. Environ. Health Eng. 2018, 5, 106–114. [Google Scholar]
- Barud, H.S.; Regiani, T.; Marques, R.F.C.; Lustri, W.R.; Messaddeq, Y.; Ribeiro, S.J.L. Antimicrobial Bacterial Cellulose-Silver Nanoparticles Composite Membranes. J. Nanomater. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Fang, Y.; Chen, W. Preparation of Silver/Bacterial Cellulose Composite Membrane and Study on Its Antimicrobial Activity. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2013, 43, 907–913. [Google Scholar] [CrossRef]
- Aguilar-Sanchez, A.; Jalvo, B.; Mautner, A.; Nameer, S.; Pöhler, T.; Tammelin, T.; Mathew, A.P. Waterborne nanocellulose coatings for improving the antifouling and antibacterial properties of polyethersulfone membranes. J. Membr. Sci. 2020, 620, 118842. [Google Scholar] [CrossRef]
- Sen, B.; Tahir, M.; Sonmez, F.; Turan Kocer, M.A.; Canpolat, O. Relationship of Algae to Water Pollution and Waste Water Treatment. In Water Treatment; IntechOpen: London, UK, 2013; pp. 335–354. [Google Scholar]
- Gopakumar, D.A.; Arumughan, V.; Pasquini, D.; Leu, S.Y.; Abdul Khalil, H.P.S.; Thomas, S. Nanocellulose-Based Membranes for Water Purification; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128139271. [Google Scholar]
- Kim, T.-I.; Park, H.; Han, M. Development of algae removal method based on positively charged bubbles. KSCE J. Civ. Eng. 2017, 21, 2567–2572. [Google Scholar] [CrossRef]
- Ge, S.; Champagne, P.; Wang, H.; Jessop, P.G.; Cunningham, M.F. Microalgae Recovery from Water for Biofuel Production Using CO2-Switchable Crystalline Nanocellulose. Environ. Sci. Technol. 2016, 50, 7896–7903. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Wang, L.; Champagne, P.; Cao, G.; Chen, Z.; Wang, S.; Ge, S. Effects of crystalline nanocellulose on wastewater-cultivated microalgal separation and biomass composition. Appl. Energy 2019, 239, 207–217. [Google Scholar] [CrossRef]
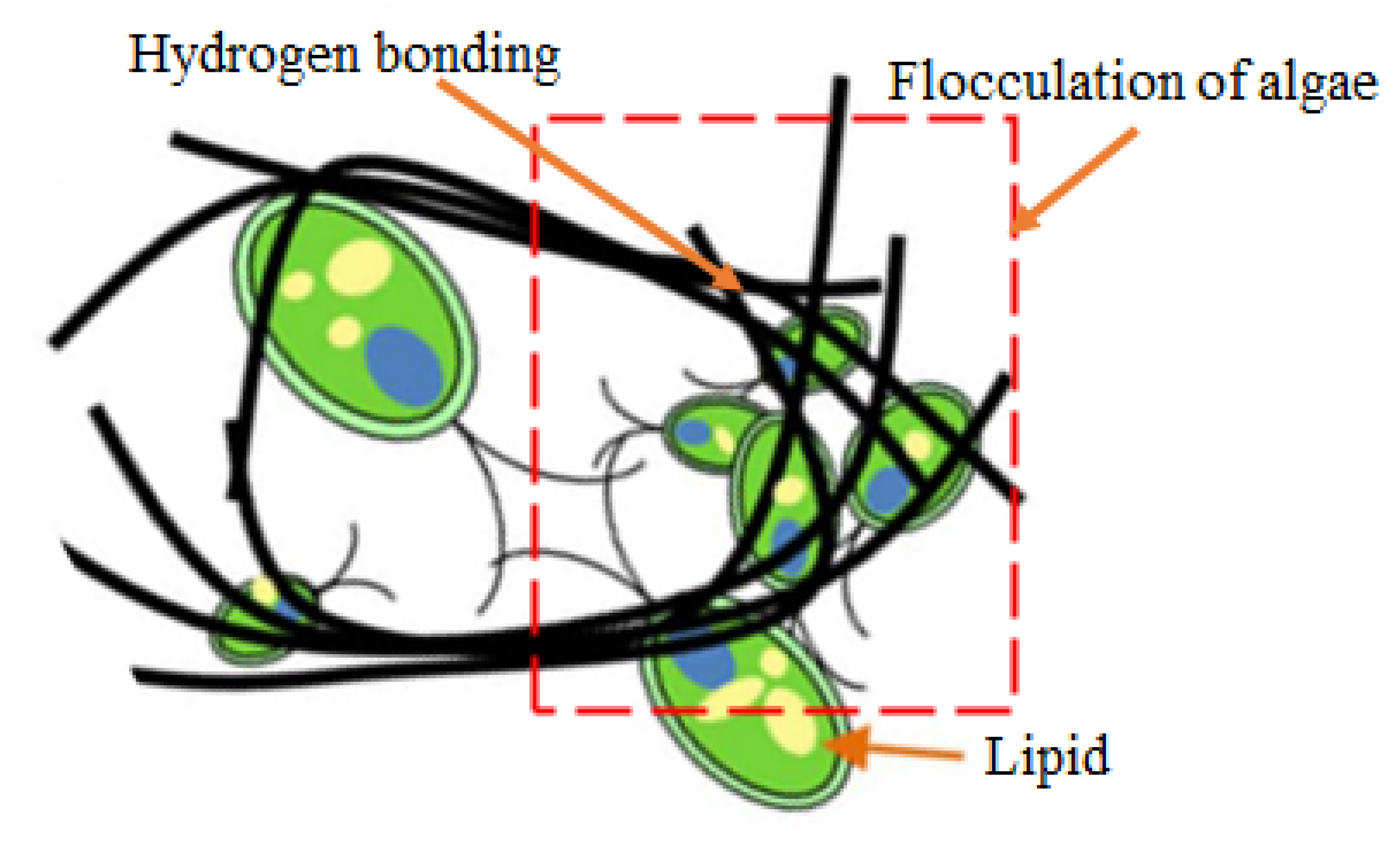
- Yu, S., II; Min, S.K.; Shin, H.S. Nanocellulose size regulates microalgal flocculation and lipid metabolism. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Patankar, S.C.; Renneckar, S. Greener synthesis of nanofibrillated cellulose using magnetically separable TEMPO nanocatalyst. Green Chem. 2017, 19, 4792–4797. [Google Scholar] [CrossRef] [Green Version]


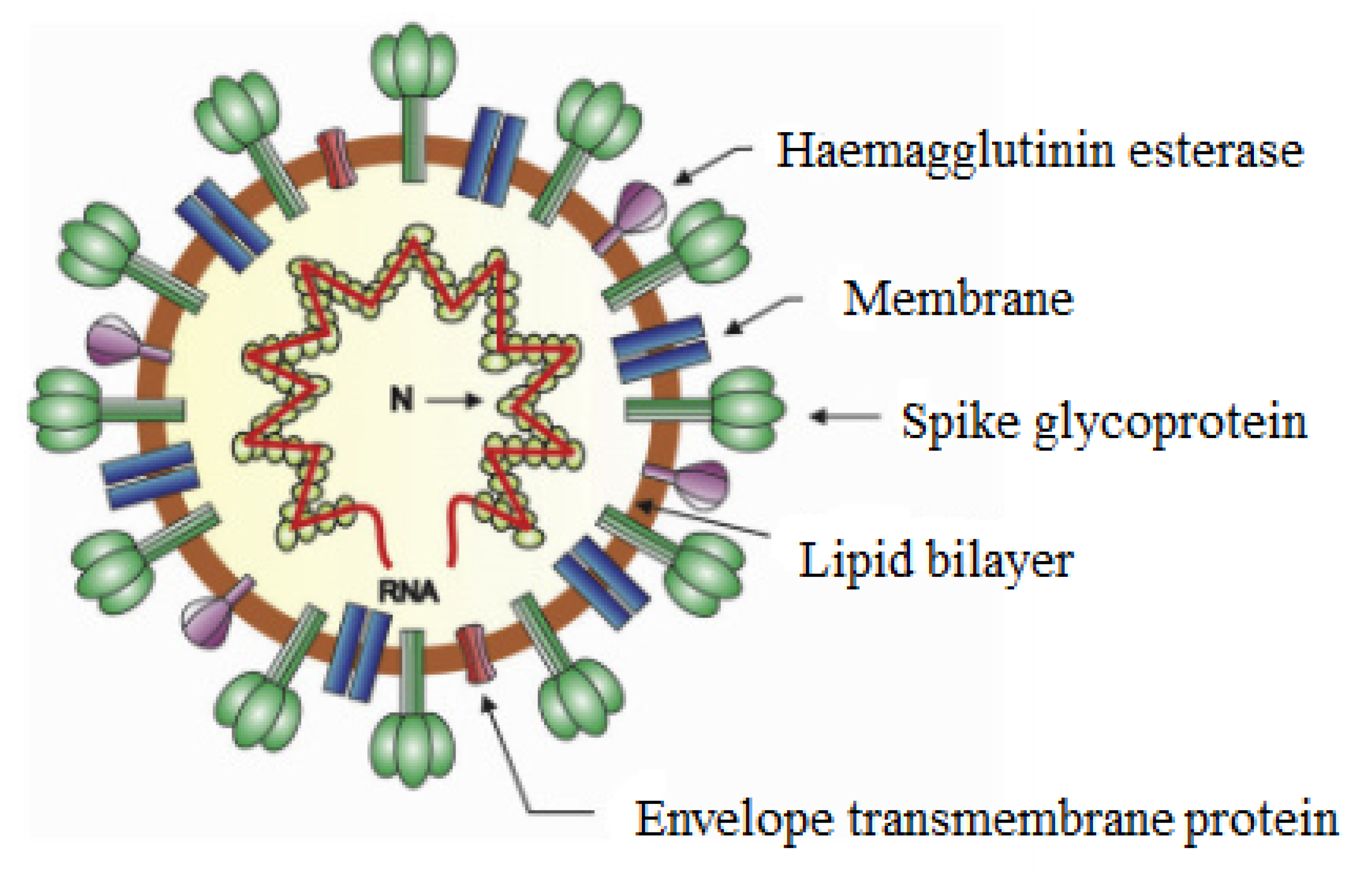

| Infectious Disease | Microbe That Causes the Disease | Type of Microbe | Reference |
|---|---|---|---|
| Coronavirus (COVID-19) | Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) | Virus | [1] |
| Cold | Rhinovirus | Virus | [2] |
| Chickenpox | Varicella zoster | Virus | [3] |
| German measles | Rubella | Virus | [4] |
| Whooping cough | Bordatella pertussis | Bacteria | [5] |
| Bubonic plague | Yersinia pestis | Bacteria | [6] |
| TB (Tuberculosis) | Mycobacterium tuberculosis | Bacteria | [7] |
| Malaria | Plasmodium falciparum | Protozoa | [8] |
| Tinea barbae (dermatophyte infection) | Trichophyton rubrum | Fungus | [9] |
| Athletes’ foot | Trichophyton mentagrophytes | Fungus | [10] |
| Functional Group | Chemical Structure |
|---|---|
| a. Functional group: aminoethyl methacrylate or poly(N-(2-aminoethylmethacrylamide) | 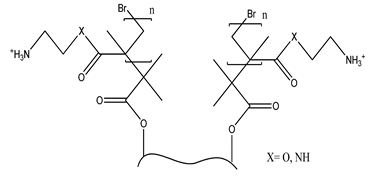 |
| b. Functional group: 2,3-epoxypropyl trimethylammonium chloride | 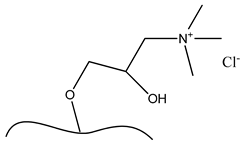 |
| c. Imidazolium |  |
| d. Pyridinium | 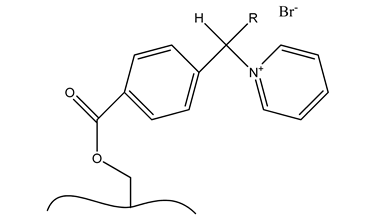 |
| e. e-vinylpyidine | 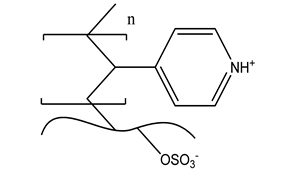 |
 |
| Microbes | Type of Nanocellulose | Functionalization | Findings | Reference |
|---|---|---|---|---|
| A/swine/Sweden/9706/2010 (H1N2)—Swine influenza | BNC | Not applicable |
| [15] |
| Xenotropic murine | BNC | Not applicable |
| [97] |
| MS2 viruses | BNC | Not applicable |
| [53] |
| ColiphagesΦX174 | BNC | Not applicable |
| [53] |
| Parvoviruses | BNC | Not applicable |
| [106] |
| EV71 | CNF | Polyglutamic acid and mesoporous silica nanoparticles |
| [105] |
| Sindbis virus | CNC | Guanidine |
| [101] |
| Porcine parvo virus | CNC | Guanidine |
| [101] |
| Microbes | Type of Nanocellulose | Functionalization | Findings | Reference |
|---|---|---|---|---|
| Escherichia coli | CNC | Silver nanoparticles |
| [40] |
| Bacillus subtilis and Escherichia coli | CNF | ZnO and CeO2 |
| [111] |
| Escherichia coli | BNC | Not applicable |
| [112] |
| Escherichia coli, Staphylococcus aureus | CNF | Activated carbon |
| [41] |
| Escherichia coli | BNC | Silver nanoparticle |
| [113] |
| Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa | BNC | Silver nanoparticle |
| [114] |
| Escherichia coli, Staphylococcus aureus | BNC | Silver nanoparticle |
| [115] |
| Escherichia coli | CNF | Polyethersulfone (PES) membranes |
| [116] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norrrahim, M.N.F.; Mohd Kasim, N.A.; Knight, V.F.; Ong, K.K.; Mohd Noor, S.A.; Abdul Halim, N.; Ahmad Shah, N.A.; Jamal, S.H.; Janudin, N.; Misenan, M.S.M.; et al. Emerging Developments Regarding Nanocellulose-Based Membrane Filtration Material against Microbes. Polymers 2021, 13, 3249. https://doi.org/10.3390/polym13193249
Norrrahim MNF, Mohd Kasim NA, Knight VF, Ong KK, Mohd Noor SA, Abdul Halim N, Ahmad Shah NA, Jamal SH, Janudin N, Misenan MSM, et al. Emerging Developments Regarding Nanocellulose-Based Membrane Filtration Material against Microbes. Polymers. 2021; 13(19):3249. https://doi.org/10.3390/polym13193249
Chicago/Turabian StyleNorrrahim, Mohd Nor Faiz, Noor Azilah Mohd Kasim, Victor Feizal Knight, Keat Khim Ong, Siti Aminah Mohd Noor, Norhana Abdul Halim, Noor Aisyah Ahmad Shah, Siti Hasnawati Jamal, Nurjahirah Janudin, Muhammad Syukri Mohamad Misenan, and et al. 2021. "Emerging Developments Regarding Nanocellulose-Based Membrane Filtration Material against Microbes" Polymers 13, no. 19: 3249. https://doi.org/10.3390/polym13193249
APA StyleNorrrahim, M. N. F., Mohd Kasim, N. A., Knight, V. F., Ong, K. K., Mohd Noor, S. A., Abdul Halim, N., Ahmad Shah, N. A., Jamal, S. H., Janudin, N., Misenan, M. S. M., Ahmad, M. Z., Yaacob, M. H., & Wan Yunus, W. M. Z. (2021). Emerging Developments Regarding Nanocellulose-Based Membrane Filtration Material against Microbes. Polymers, 13(19), 3249. https://doi.org/10.3390/polym13193249









