Greener Pretreatment Approaches for the Valorisation of Natural Fibre Biomass into Bioproducts
Abstract
1. Introduction
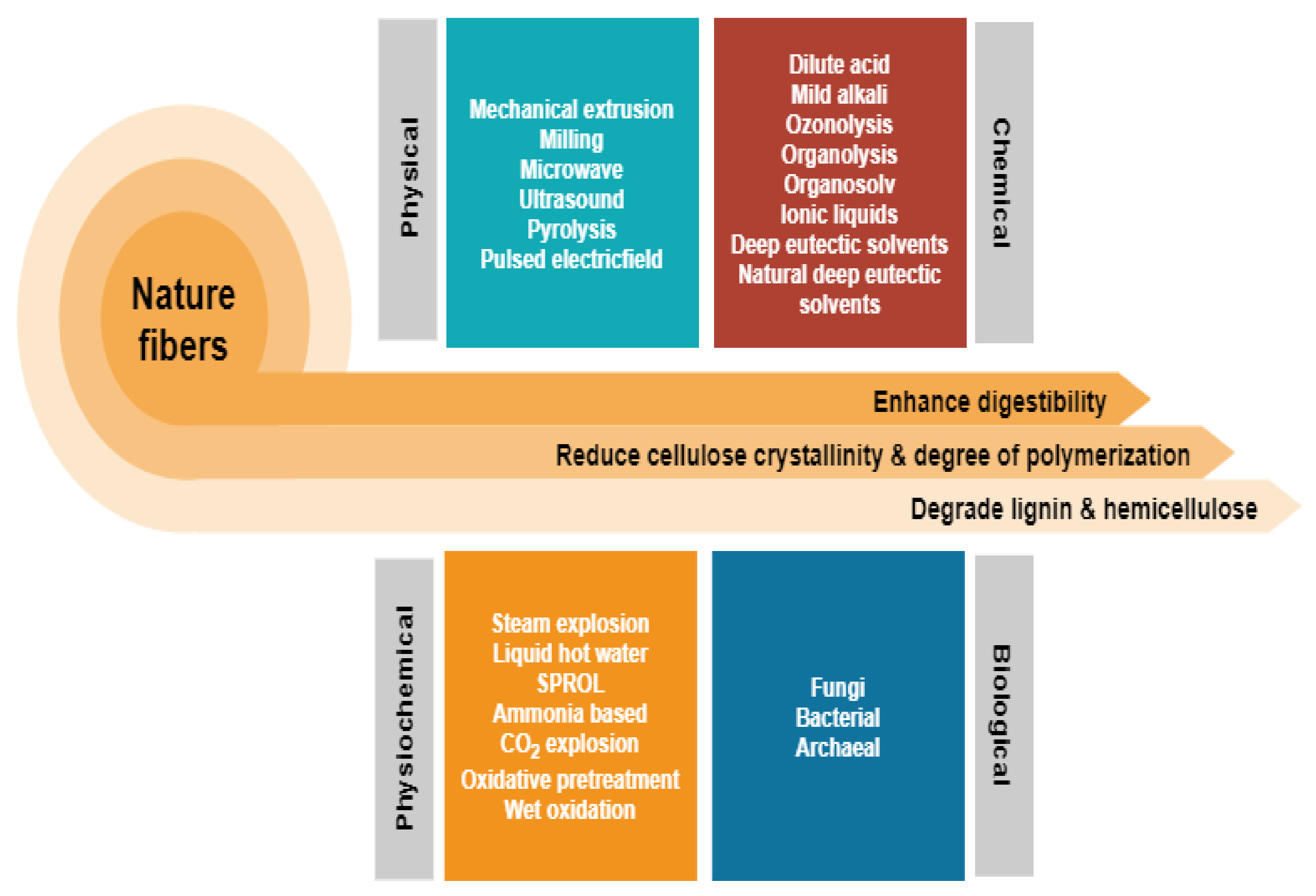
2. Physical Pretreatment
2.1. Mechanical Extrusion
2.2. Milling
2.3. Ultrasound
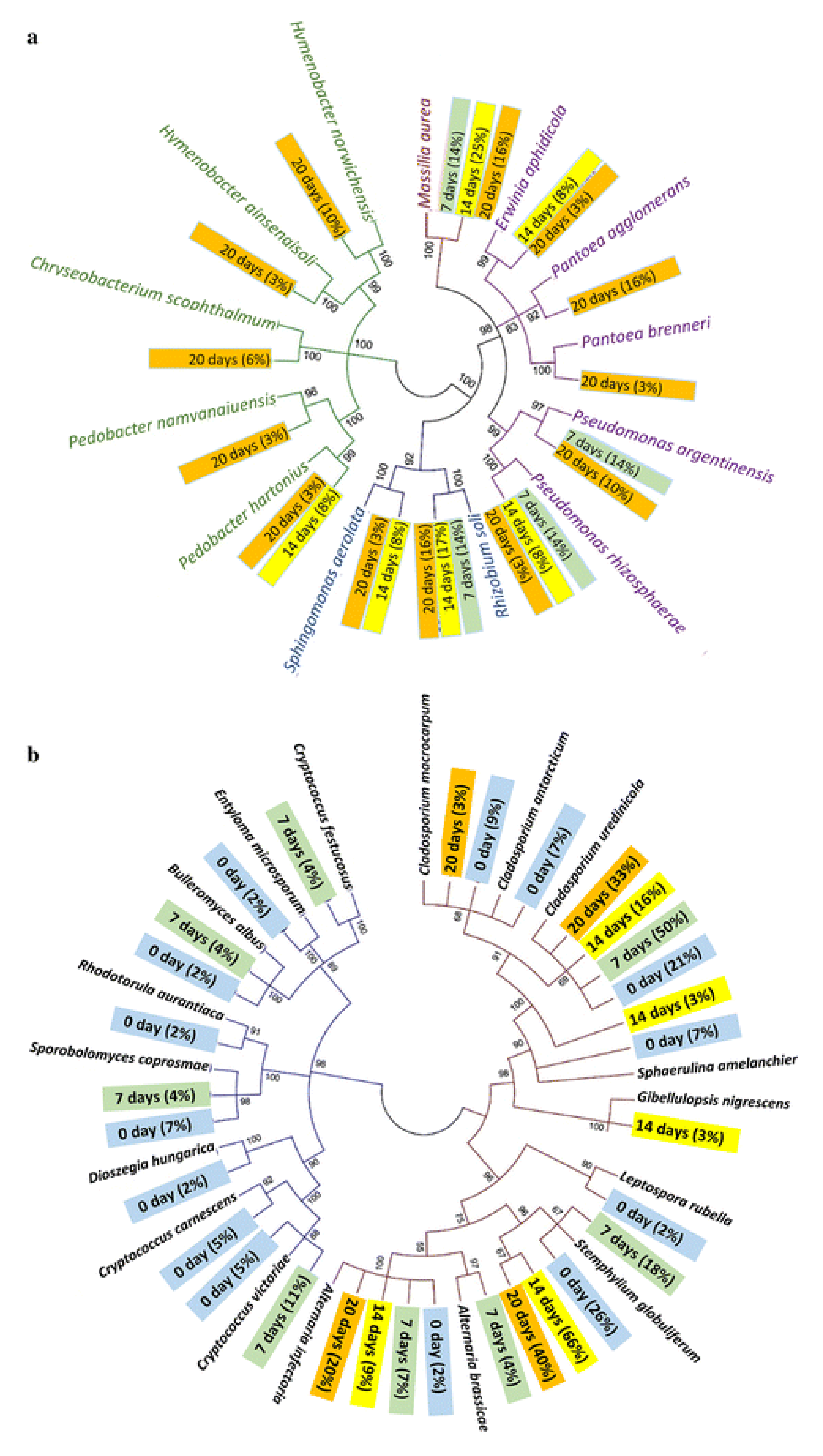
3. Biological Pretreatment
Bacterial and Fungi Interaction
4. Combination Pretreatment
4.1. Physiochemical Pretreatment
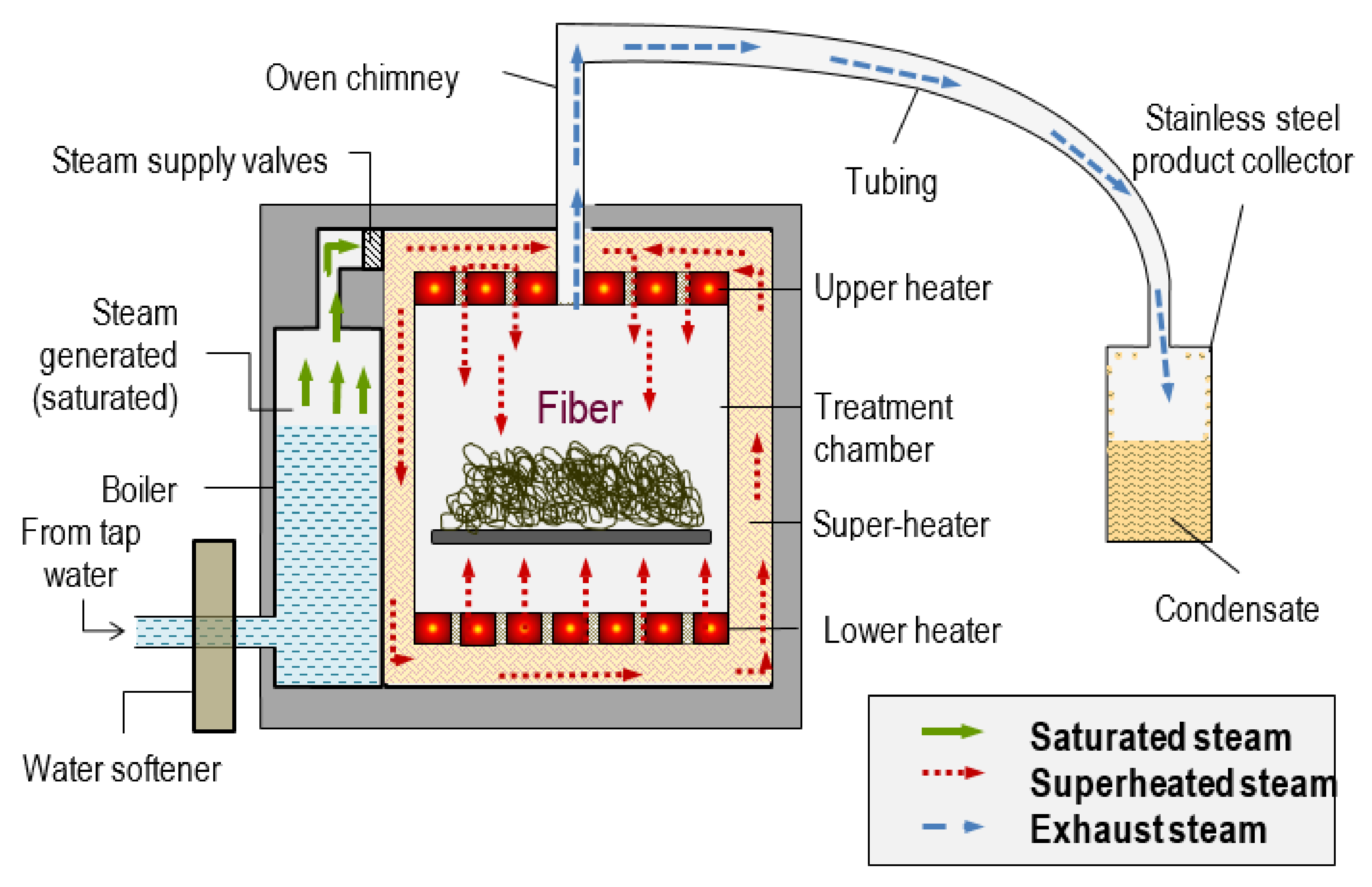
4.1.1. Superheated Steam
4.1.2. Hydrothermal
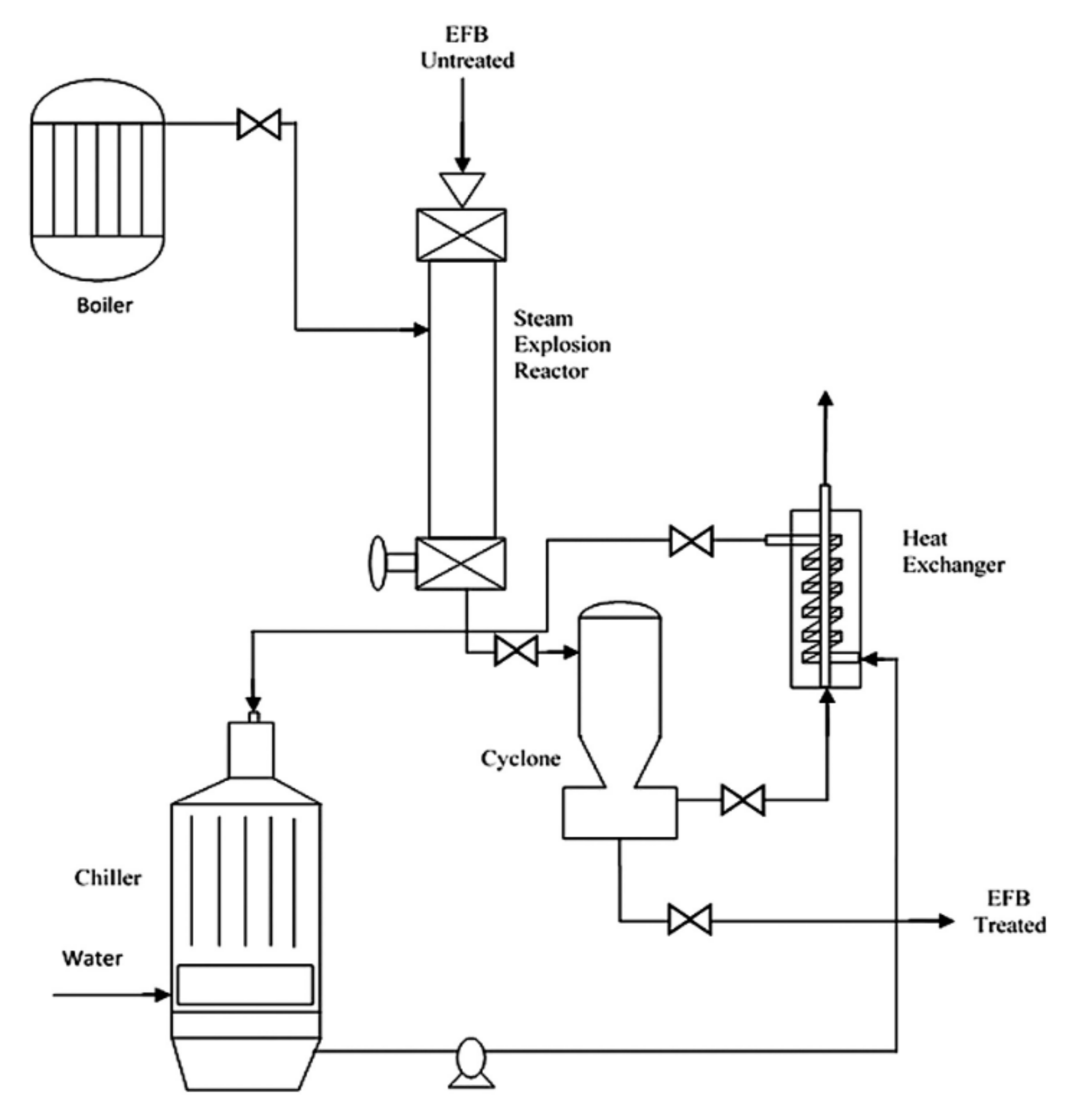
4.1.3. Steam Explosion
4.2. Biological-Chemical Pretreatment
5. The Influence of Pretreatment of Natural Fibre on Several Applications
5.1. Influence of Physical Pretreatment on Applications
5.2. Influence of Biological Pretreatment on Applications
6. Challenges and Future Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aftab, M.N.; Iqbal, I.; Riaz, F.; Karadag, A.; Tabatabaei, M. Different Pretreatment Methods of Lignocellulosic Biomass for Use in Biofuel Production. In Biomass for Bioenergy-Recent Trends and Future Challenges; IntechOpen: London, UK, 2019. [Google Scholar]
- Xiao, C.; Bolton, R.; Pan, W. Lignin from rice straw Kraft pulping: Effects on soil aggregation and chemical properties. Bioresour. Technol. 2007, 98, 1482–1488. [Google Scholar] [CrossRef]
- Himmel, M.E.; Ding, S.-Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass Recalcitrance: Engineering Plants and Enzymes for Biofuels Production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef]
- Tu, W.-C.; Hallett, J.P. Recent advances in the pretreatment of lignocellulosic biomass. Curr. Opin. Green Sustain. Chem. 2019, 20, 11–17. [Google Scholar] [CrossRef]
- Nurazzi, N.M. Treatments of natural fiber as reinforcement in polymer composites—A short review. Funct. Compos. Struct. 2021, 3, 2. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Ilyas, R.A.; Nurazzi, N.M.; Rani, M.S.A.; Atikah, M.S.N.; Shazleen, S.S. Chemical Pretreatment of Lignocellulosic Biomass for the Production of Bioproducts: An Overview. Appl. Sci. Eng. Prog. 2021. [Google Scholar] [CrossRef]
- Williams, C.L.; Emerson, R.M.; Tumuluru, J.S. Biomass Compositional Analysis for Conversion to Renewable Fuels and Chemicals. In Biomass Volume Estimation and Valorization for Energy; IntechOpen Limited: London, UK, 2017. [Google Scholar] [CrossRef]
- Mood, S.H.; Golfeshan, A.H.; Tabatabaei, M.; Jouzani, G.S.; Najafi, G.; Gholami, M.; Ardjmand, M. Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew. Sustain. Energy Rev. 2013, 27, 77–93. [Google Scholar] [CrossRef]
- Guerriero, G.; Hausman, J.F.; Strauss, J.; Ertan, H.; Siddiqui, K.S. Lignocellulosic biomass: Biosynthesis, degradation, and industrial utilization. Eng. Life Sci. 2016, 16, 1–16. [Google Scholar] [CrossRef]
- Barakat, A.; De Vries, H.; Rouau, X. Dry fractionation process as an important step in current and future lignocellulose biorefineries: A review. Bioresour. Technol. 2013, 134, 362–373. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Koupaie, E.h.; Dahadha, S.; Lakeh, A.A.B.; Azizi, A.; Elbeshbishy, E. Enzymatic pretreatment of lignocellulosic biomass for enhanced biomethane production-A review. J. Environ. Manag. 2019, 233, 774–784. [Google Scholar] [CrossRef]
- Haldar, D.; Purkait, M.K. A review on the environment-friendly emerging techniques for pretreatment of lignocellulosic biomass: Mechanistic insight and advancements. Chemosphere 2021, 264, 128523. [Google Scholar] [CrossRef]
- Zadeh, Z.E.; Abdulkhani, A.; Aboelazayem, O.; Saha, B. Recent Insights into Lignocellulosic Biomass Pyrolysis: A Critical Review on Pretreatment, Characterization, and Products Upgrading. Processes 2020, 8, 799. [Google Scholar] [CrossRef]
- Mahmood, H.; Moniruzzaman, M.; Iqbal, T.; Khan, M.J. Recent advances in the pretreatment of lignocellulosic biomass for biofuels and value-added products. Curr. Opin. Green Sustain. Chem. 2019, 20, 18–24. [Google Scholar] [CrossRef]
- Hassan, S.; Williams, G.A.; Jaiswal, A.K. Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 1–19. [Google Scholar] [CrossRef]
- Karunanithy, C.T.; Muthukumarappan, K. Influence of Extruder Temperature and Screw Speed on Pretreatment of Corn Stover while Varying Enzymes and Their Ratios. Appl. Biochem. Biotechnol. 2010, 162, 264–279. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, G.; Pan, X.; Gleisner, R. Specific surface to evaluate the efficiencies of milling and pretreatment of wood for enzymatic saccharification. Chem. Eng. Sci. 2009, 64, 474–485. [Google Scholar] [CrossRef]
- Hideno, A.; Inoue, H.; Tsukahara, K.; Fujimoto, S.; Minowa, T.; Inoue, S.; Endo, T.; Sawayama, S. Wet disk milling pretreatment without sulfuric acid for enzymatic hydrolysis of rice straw. Bioresour. Technol. 2009, 100, 2706–2711. [Google Scholar] [CrossRef]
- Bussemaker, M.J.; Zhang, D. Effect of Ultrasound on Lignocellulosic Biomass as a Pretreatment for Biorefinery and Biofuel Applications. Ind. Eng. Chem. Res. 2013, 52, 3563–3580. [Google Scholar] [CrossRef]
- Gogate, P.R.; Sutkar, V.S.; Pandit, A.B. Sonochemical reactors: Important design and scale up considerations with a special emphasis on heterogeneous systems. Chem. Eng. J. 2011, 166, 1066–1082. [Google Scholar] [CrossRef]
- Sanjay, M.R.; Siengchin, S.; Parameswaranpillai, J.; Jawaid, M.; Pruncu, C.I.; Khan, A. A comprehensive review of techniques for natural fibers as reinforcement in composites: Preparation, processing and characterization. Carbohydr. Polym. 2019, 207, 108–121. [Google Scholar] [CrossRef]
- Bleuze, L.; Lashermes, G.; Alavoine, G.; Recous, S.; Chabbert, B. Tracking the dynamics of hemp dew retting under controlled environmental conditions. Ind. Crop. Prod. 2018, 123, 55–63. [Google Scholar] [CrossRef]
- Fila, G.; Manici, L.M.; Caputo, F. In vitro evaluation of dew-retting of flax by fungi from southern Europe. Ann. Appl. Biol. 2001, 138, 343–351. [Google Scholar] [CrossRef]
- Repečkiene, J.; Jankauskiene, Z. Application of fungal complexes to improve flax dew-retting. Biomed. Moksl. 2009, 83, 63–71. [Google Scholar]
- Jankauskiene, Z.; Lugauskas, A.; Repeckiene, J. New Methods for the Improvement of Flax Dew Retting. J. Nat. Fibers 2007, 3, 59–68. [Google Scholar] [CrossRef]
- Liu, M.; Ale, M.T.; Kołaczkowski, B.; Fernando, D.; Daniel, G.; Meyer, A.S.; Thygesen, A. Comparison of traditional field retting and Phlebia radiata Cel 26 retting of hemp fibres for fibre-reinforced composites. AMB Express 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Fernando, D.; Thygesen, A.; Meyer, A.S.; Daniel, G. Elucidating field retting mechanisms of hemp fibres for biocomposites: Effects of microbial actions and interactions on the cellular micro-morphology and ultrastructure of hemp stems and bast fibres. BioResources 2019, 14, 4047–4084. [Google Scholar] [CrossRef]
- Farid, M.A.A.; Hassan, M.A.; Roslan, A.M.; Ariffin, H.; Norrrahim, M.N.F.; Othman, M.R.; Yoshihito, S. Improving the decolorization of glycerol by adsorption using activated carbon derived from oil palm biomass. Environ. Sci. Pollut. Res. 2021, 28, 27976–27987. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F. Superheated Steam Pretreatment of Oil Palm Biomass for Improving Nanofibrillation of Cellulose and Performance of Polypropylene/Cellulose Nanofiber Composites. Doctoral Thesis, Universiti Putra Malaysia, Selangor, Malaysia, 2018. [Google Scholar]
- Nordin, N.I.A.A.; Ariffin, H.; Andou, Y.; Hassan, M.A.; Shirai, Y.; Nishida, H.; Yunus, W.M.Z.W.; Karuppuchamy, S.; Ibrahim, N.A. Modification of Oil Palm Mesocarp Fiber Characteristics Using Superheated Steam Treatment. Molecules 2013, 18, 9132–9146. [Google Scholar] [CrossRef]
- Sharip, N.S.; Ariffin, H.; Hassan, M.A.; Nishida, H.; Shirai, Y. Characterization and application of bioactive compounds in oil palm mesocarp fiber superheated steam condensate as an antifungal agent. RSC Adv. 2016, 6, 84672–84683. [Google Scholar] [CrossRef]
- Megashah, L.N.; Ariffin, H.; Zakaria, M.R.; Hassan, M.A.; Andou, Y.; Padzil, F.N.M. Modification of cellulose degree of polymerization by superheated steam treatment for versatile properties of cellulose nanofibril film. Cellulose 2020, 27, 7417–7429. [Google Scholar] [CrossRef]
- Bahrin, E.K.; Baharuddin, A.S.; Ibrahim, M.F.; Razak, M.N.A.; Sulaiman, A.; Aziz, S.A.; Hassan, M.A.; Shirai, Y.; Nishida, H. Physicochemical property changes and enzymatic hydrolysis enhancement of oil palm empty fruit bunches treated with superheated steam. BioResources 2012, 7, 1784–1801. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Ariffin, H.; Hassan, M.A.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Nishida, H. Utilisation of superheated steam in oil palm biomass pretreatment process for reduced chemical use and enhanced cellulose nanofibre production. Int. J. Nanotechnol. 2019, 16, 668. [Google Scholar] [CrossRef]
- Norrrahim, M.; Ariffin, H.; Yasim-Anuar, T.; Hassan, M.; Ibrahim, N.; Yunus, W.; Nishida, H. Performance Evaluation of Cellulose Nanofiber with Residual Hemicellulose as a Nanofiller in Polypropylene-Based Nanocomposite. Polymers 2021, 13, 1064. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Norrrahim, M.N.F.; Hirata, S.; Hassan, M.A. Hydrothermal and wet disk milling pretreatment for high conversion of biosugars from oil palm mesocarp fiber. Bioresour. Technol. 2015, 181, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Norrrahim, M.N.F.; Ariffin, H.; Yasim-Anuar, T.A.T.; Ghaemi, F.; Hassan, M.A.; Ibrahim, N.A.; Ngee, J.L.H.; Yunus, W.M.Z.W. Superheated steam pretreatment of cellulose affects its electrospinnability for microfibrillated cellulose production. Cellulose 2018, 25, 3853–3859. [Google Scholar] [CrossRef]
- Warid, M.N.M.; Ariffin, H.; Hassan, M.A.; Shirai, Y. Optimization of Superheated Steam Treatment to Improve Surface Modification of Oil Palm Biomass Fiber. Bioresources 2016, 11, 5780–5796. [Google Scholar] [CrossRef][Green Version]
- Lei, H.; Cybulska, I.; Julson, J. Hydrothermal Pretreatment of Lignocellulosic Biomass and Kinetics. J. Sustain. Bioenergy Syst. 2013, 3, 250–259. [Google Scholar] [CrossRef][Green Version]
- Lee, J.; Park, K.Y. Impact of hydrothermal pretreatment on anaerobic digestion efficiency for lignocellulosic biomass: Influence of pretreatment temperature on the formation of biomass-degrading byproducts. Chemosphere 2020, 256, 127116. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Hirata, S.; Hassan, M.A. Hydrothermal pretreatment enhanced enzymatic hydrolysis and glucose production from oil palm biomass. Bioresour. Technol. 2015, 176, 142–148. [Google Scholar] [CrossRef]
- Rasmussen, H.; Sørensen, H.R.; Meyer, A.S. Formation of degradation compounds from lignocellulosic biomass in the biorefinery: Sugar reaction mechanisms. Carbohydr. Res. 2014, 385, 45–57. [Google Scholar] [CrossRef]
- Bianco, F.; Şenol, H.; Papirio, S. Enhanced lignocellulosic component removal and biomethane potential from chestnut shell by a combined hydrothermal–alkaline pretreatment. Sci. Total. Environ. 2021, 762, 144178. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Huang, G.; Yang, Z.; Han, L. Understanding the synergistic effect and the main factors influencing the enzymatic hydrolyzability of corn stover at low enzyme loading by hydrothermal and/or ultrafine grinding pretreatment. Bioresour. Technol. 2018, 264, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Phuttaro, C.; Sawatdeenarunat, C.; Surendra, K.; Boonsawang, P.; Chaiprapat, S.; Khanal, S.K. Anaerobic digestion of hydrothermally-pretreated lignocellulosic biomass: Influence of pretreatment temperatures, inhibitors and soluble organics on methane yield. Bioresour. Technol. 2019, 284, 128–138. [Google Scholar] [CrossRef]
- Megashah, L.N. Development of Efficient Processing Method for the Production of Cellulose Nanofibrils from Oil Palm Biomass. Doctoral Thesis, Universiti Putra Malaysia, Selangor, Malaysia, 2020. [Google Scholar]
- Sarker, T.R.; Pattnaik, F.; Nanda, S.; Dalai, A.K.; Meda, V.; Naik, S. Hydrothermal pretreatment technologies for lignocellulosic biomass: A review of steam explosion and subcritical water hydrolysis. Chemosphere 2021, 284, 131372. [Google Scholar] [CrossRef]
- Marques, F.P.; Soares, A.K.L.; Lomonaco, D.; e Silva, L.M.A.; Santaella, S.T.; Rosa, M.D.F.; Leitão, R.C. Steam explosion pretreatment improves acetic acid organosolv delignification of oil palm mesocarp fibers and sugarcane bagasse. Int. J. Biol. Macromol. 2021, 175, 304–312. [Google Scholar] [CrossRef]
- Medina, J.D.C.; Woiciechowski, A.; Filho, A.Z.; Nigam, P.S.; Ramos, L.P.; Soccol, C.R. Steam explosion pretreatment of oil palm empty fruit bunches (EFB) using autocatalytic hydrolysis: A biorefinery approach. Bioresour. Technol. 2016, 199, 173–180. [Google Scholar] [CrossRef]
- Abraham, E.; Deepa, B.; Pothan, L.A.; Jacob, M.; Thomas, S.; Cvelbar, U.; Anandjiwala, R. Extraction of nanocellulose fibrils from lignocellulosic fibres: A novel approach. Carbohydr. Polym. 2011, 86, 1468–1475. [Google Scholar] [CrossRef]
- Marques, F.P.; Silva, L.M.A.; Lomonaco, D.; Rosa, M.D.F.; Leitão, R.C. Steam explosion pretreatment to obtain eco-friendly building blocks from oil palm mesocarp fiber. Ind. Crop. Prod. 2020, 143, 111907. [Google Scholar] [CrossRef]
- Meenakshisundaram, S.; Fayeulle, A.; Leonard, E.; Ceballos, C.; Pauss, A. Fiber degradation and carbohydrate production by combined biological and chemical/physicochemical pretreatment methods of lignocellulosic biomass—A review. Bioresour. Technol. 2021, 331, 125053. [Google Scholar] [CrossRef]
- Ariffin, H.; Norrrahim, M.N.F.; Yasim-Anuar, T.A.T.; Nishida, H.; Hassan, M.A.; Ibrahim, N.A.; Yunus, W.M.Z.W. Oil Palm Biomass Cellulose-Fabricated Polylactic Acid Composites for Packaging Applications. In Bionanocomposites for Packaging Applications; Springer Science and Business Media LLC.: Berlin, Germany, 2017; pp. 95–105. [Google Scholar]
- Ilyas, R.; Sapuan, S.; Nurazzi, N.M.; Norrrahim, M.N.F.; Ibrahim, R.; Atikah, M.; Huzaifah, M.; Radzi, A.; Izwan, S.; Azammi, A.N.; et al. Macro to nanoscale natural fiber composites for automotive components: Research, development, and application. In Biocomposite and Synthetic Composites for Automotive Applications; Elsevier BV: Amsterdam, The Netherlands, 2021; pp. 51–105. [Google Scholar]
- Ilyas, R.; Sapuan, S.; Harussani, M.; Hakimi, M.; Haziq, M.; Atikah, M.; Asyraf, M.; Ishak, M.; Razman, M.; Nurazzi, N.; et al. Polylactic Acid (PLA) Biocomposite: Processing, Additive Manufacturing and Advanced Applications. Polymers 2021, 13, 1326. [Google Scholar] [CrossRef]
- Sharip, N.S.; Yasim-Anuar, T.A.T.; Norrrahim, M.N.F.; Shazleen, S.S.; Nurazzi, N.M.; Sapuan, S.M.; Ilyas, R.A. A Review on Nanocellulose Composites in Biomedical Application. In Composites in Biomedical Applications; CRC Press: Boca Raton, FL, USA, 2020; pp. 161–190. [Google Scholar]
- Norrrahim, M.N.F.; Kasim, N.A.M.; Knight, V.F.; Ujang, F.A.; Janudin, N.; Razak, M.A.I.A.; Shah, N.A.A.; Noor, S.A.M.; Jamal, S.H.; Ong, K.K.; et al. Nanocellulose: The next super versatile material for the military. Mater. Adv. 2021, 2, 1485–1506. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Kasim, N.A.M.; Knight, V.F.; Misenan, M.S.M.; Janudin, N.; Shah, N.A.A.; Kasim, N.; Yusoff, W.Y.W.; Noor, S.A.M.; Jamal, S.H.; et al. Nanocellulose: A bioadsorbent for chemical contaminant remediation. RSC Adv. 2021, 11, 7347–7368. [Google Scholar] [CrossRef]
- Yasim-Anuar, T.A.T. Well-Dispersed Cellulose Nanofiber in Low Density Polyethylene Nanocomposite by Liquid-Assisted Extrusion. Polymers 2020, 12, 927. [Google Scholar] [CrossRef] [PubMed]
- Norrrahim, M.N.F.; Nurazzi, N.M.; Jenol, M.A.; Farid, M.A.A.; Janudin, N.; Ujang, F.A.; Yasim-Anuar, T.A.T.; Najmuddin, S.U.F.S.; Ilyas, R.A. Emerging development of nanocellulose as an antimicrobial material: An overview. Mater. Adv. 2021, 2, 3538–3551. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Asyraf, M.R.M.; Rayung, M.; Norrrahim, M.N.F.; Shazleen, S.S.; Rani, M.S.A.; Shafi, A.R.; Aisyah, H.A.; Radzi, M.H.M.; Sabaruddin, F.A.; et al. Thermogravimetric Analysis Properties of Cellulosic Natural Fiber Polymer Composites: A Review on Influence of Chemical Treatments. Polymers 2021, 13, 2710. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F. Cationic Nanocellulose as Promising Candidate for Filtration Material of COVID-19: A Perspective. Appl. Sci. Eng. Prog. 2021. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Yasim-Anuar, T.A.T.; Sapuan, S.; Ilyas, R.; Hakimi, M.I.; Najmuddin, S.U.F.S.; Jenol, M.A. Nanocellulose Reinforced Polypropylene and Polyethylene Composite for Packaging Application. In Bio-based Packaging; Wiley: Hoboken, NJ, USA, 2021; pp. 133–150. [Google Scholar]
- Lee, C.H.; Lee, S.H.; Padzil, F.N.M.; Ainun, Z.M.A.; Norrrahim, M.N.F.; Chin, K.L. Biocomposites and Nanocomposites. In Composite Materials; Informa UK Limited: London, UK, 2021; pp. 29–60. [Google Scholar]
- Nurazzi, N.; Asyraf, M.; Athiyah, S.F.; Shazleen, S.; Rafiqah, S.; Harussani, M.; Kamarudin, S.; Razman, M.; Rahmah, M.; Zainudin, E.; et al. A Review on Mechanical Performance of Hybrid Natural Fiber Polymer Composites for Structural Applications. Polymers 2021, 13, 2170. [Google Scholar] [CrossRef]
- Nurazzi, N.; Asyraf, M.; Khalina, A.; Abdullah, N.; Aisyah, H.; Rafiqah, S.; Sabaruddin, F.; Kamarudin, S.; Norrrahim, M.; Ilyas, R.; et al. A Review on Natural Fiber Reinforced Polymer Composite for Bullet Proof and Ballistic Applications. Polymers 2021, 13, 646. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Moset, V.; Xavier, C.D.A.N.; Feng, L.; Wahid, R.; Møller, H. Combined low thermal alkali addition and mechanical pre-treatment to improve biogas yield from wheat straw. J. Clean. Prod. 2018, 172, 1391–1398. [Google Scholar] [CrossRef]
- Khan, A.S.; Man, Z.; Bustam, M.A.; Kait, C.F.; Khan, M.I.; Muhammad, N.; Nasrullah, A.; Ullah, Z.; Ahmad, P. Impact of Ball-Milling Pretreatment on Pyrolysis Behavior and Kinetics of Crystalline Cellulose. Waste Biomass-Valorization 2016, 7, 571–581. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Pan, X.; Zalesny, R.S. Pretreatment of woody biomass for biofuel production: Energy efficiency, technologies, and recalcitrance. Appl. Microbiol. Biotechnol. 2010, 87, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Jȩdrzejczyk, M.; Soszka, E.; Czapnik, M.; Ruppert, A.M.; Grams, J. Physical and Chemical Pretreatment of Lignocellulosic Biomass. In Second and Third Generation of Feedstocks: The Evolution of Biofuels; Elsevier: Amsterdam, The Netherlands, 2019; pp. 143–196. [Google Scholar] [CrossRef]
- De la Rubia, M.A.; Fernández-Cegrí, V.; Raposo, F.; Borja, R. Influence of particle size and chemical composition on the performance and kinetics of anaerobic digestion process of sunflower oil cake in batch mode. Biochem. Eng. J. 2011, 58–59, 162–167. [Google Scholar] [CrossRef]
- Ramos, L.P. The chemistry involved in the steam treatment of lignocellulosic materials. Química Nova 2003, 26, 863–871. [Google Scholar] [CrossRef]
- Takács, E.; Wojnárovits, L.; Földváry, C.; Hargittai, P.; Borsa, J.; Sajó, I. Effect of combined gamma-irradiation and alkali treatment on cotton-cellulose. Radiat. Phys. Chem. 2000, 57, 399–403. [Google Scholar] [CrossRef]
- Naimi, L.J.; Sokhansanj, S. Data-based equation to predict power and energy input for grinding wheat straw, corn stover, switchgrass, miscanthus, and canola straw. Fuel Process. Technol. 2018, 173, 81–88. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, N.; García-Bernet, D.; Domínguez, J.M. Faster methane production after sequential extrusion and enzymatic hydrolysis of vine trimming shoots. Environ. Chem. Lett. 2018, 16, 295–299. [Google Scholar] [CrossRef]
- Zhang, S.-C.; Lai, Q.-H.; Lu, Y.; Liu, Z.-D.; Wang, T.; Zhang, C.; Xing, X.-H. Enhanced biohydrogen production from corn stover by the combination of Clostridium cellulolyticum and hydrogen fermentation bacteria. J. Biosci. Bioeng. 2016, 122, 482–487. [Google Scholar] [CrossRef]
- He, L.; Huang, H.; Lei, Z.; Liu, C.; Zhang, Z. Enhanced hydrogen production from anaerobic fermentation of rice straw pretreated by hydrothermal technology. Bioresour. Technol. 2014, 171, 145–151. [Google Scholar] [CrossRef]
- Baeta, B.; Lima, D.R.S.; Adarme, O.F.H.; Gurgel, L.; de Aquino, S.F. Optimization of sugarcane bagasse autohydrolysis for methane production from hemicellulose hydrolyzates in a biorefinery concept. Bioresour. Technol. 2016, 200, 137–146. [Google Scholar] [CrossRef]
- Jackowiak, D.; Bassard, D.; Pauss, A.; Ribeiro, T. Optimisation of a microwave pretreatment of wheat straw for methane production. Bioresour. Technol. 2011, 102, 6750–6756. [Google Scholar] [CrossRef]
- Li, L.; Kong, X.; Yang, F.; Li, D.; Yuan, Z.; Sun, Y. Biogas Production Potential and Kinetics of Microwave and Conventional Thermal Pretreatment of Grass. Appl. Biochem. Biotechnol. 2012, 166, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Simona, M.; Gianfranco, A.; Jody, G.; Paolo, B. Energetic assessment of extrusion as pre-treatment to improve the anaerobic digestion of agricultural ligno-cellulosic biomasses. In Proceedings of the 15th International Conference Ramiran 2013, Versailles, France, 3–5 June 2013; Volume 1. [Google Scholar]
- Bauer, A.; Lizasoain, J.; Theuretzbacher, F.; Agger, J.W.; Rincón, M.; Menardo, S.; Saylor, M.K.; Enguídanos, R.; Nielsen, P.J.; Potthast, A.; et al. Steam explosion pretreatment for enhancing biogas production of late harvested hay. Bioresour. Technol. 2014, 166, 403–410. [Google Scholar] [CrossRef]
- Alinia, R.; Zabihi, S.; Esmaeilzadeh, F.; Kalajahi, J.F. Pretreatment of wheat straw by supercritical CO2 and its enzymatic hydrolysis for sugar production. Biosyst. Eng. 2010, 107, 61–66. [Google Scholar] [CrossRef]
- Chandra, R.P.; Chu, Q.; Hu, J.; Zhong, N.; Lin, M.; Lee, J.-S.; Saddler, J. The influence of lignin on steam pretreatment and mechanical pulping of poplar to achieve high sugar recovery and ease of enzymatic hydrolysis. Bioresour. Technol. 2016, 199, 135–141. [Google Scholar] [CrossRef]
- DeMartini, J.D.; Foston, M.; Meng, X.; Jung, S.; Kumar, R.; Ragauskas, A.J.; Wyman, C.E. How chip size impacts steam pretreatment effectiveness for biological conversion of poplar wood into fermentable sugars. Biotechnol. Biofuels 2015, 8, 1–16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walch, E.; Zemann, A.; Schinner, F.; Bonn, G.; Bobleter, O. Enzymatic saccharification of hemicellulose obtained from hydrothermally pretreated sugar cane bagasse and beech bark. Bioresour. Technol. 1992, 39, 173–177. [Google Scholar] [CrossRef]
- Ståhl, M.; Nieminen, K.; Sixta, H. Hydrothermolysis of pine wood. Biomass-Bioenergy 2018, 109, 100–113. [Google Scholar] [CrossRef]
- Pińkowska, H.; Krzywonos, M.; Wolak, P. Valorization of Rapeseed Meal by Hydrothermal Treatment—Effect of Reaction Parameters on Low Molecular Products Distribution. Cellul. Chem. Technol. 2019, 53, 755–765. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Zhang, L.; Zhan, P.; Liu, N.; Wu, Z. Preparation of nanocellulose from steam exploded poplar wood by enzymolysis assisted sonication Preparation of nanocellulose from steam exploded poplar wood by enzymolysis assisted sonication. Mater. Res. Express 2020, 7, 035010. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, X.; Li, J.; Wang, Q.; Wang, F.; Kong, L. Homogeneous Isolation of Nanocellulose from Cotton Cellulose by High Pressure Homogenization. J. Mater. Sci. Chem. Eng. 2013, 1, 49–52. [Google Scholar] [CrossRef]
- Li, J.; Wei, X.; Wang, Q.; Chen, J.; Chang, G.; Kong, L.; Su, J.; Liu, Y. Homogeneous isolation of nanocellulose from sugarcane bagasse by high pressure homogenization. Carbohydr. Polym. 2012, 90, 1609–1613. [Google Scholar] [CrossRef]
- Hatakka, A. Pretreatment of wheat straw by white-rot fungi for enzymic saccharification of cellulose. Appl. Microbiol. Biotechnol. 1983, 18, 350–357. [Google Scholar] [CrossRef]
- Çağrı, A.; Ince, O.; Bozan, M.; Ozbayram, G.; Ince, B. Biological pretreatment with Trametes versicolor to enhance methane production from lignocellulosic biomass: A metagenomic approach. Ind. Crop. Prod. 2019, 140. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, X.; Singh, D.; Yu, H.; Yang, X. Biological pretreatment of lignocellulosics: Potential, progress and challenges. Biofuels 2010, 1, 177–199. [Google Scholar] [CrossRef]
- Tanahashi, M. Characterization and degradation mechanisms of wood components by steam explosion and utilization of exploded wood. In Bulletin of the Wood Research Institute Kyoto University; Kyoto University Research Information Repository: Kyoto, Japan, 1990; Volume 77, pp. 49–117. [Google Scholar]
- Gregg, D.; Saddler, J.N. A Techno-Economic Assessment of the Pretreatment and Fractionation Steps of a Biomass-to-Ethanol Process. In Seventeenth Symposium on Biotechnology for Fuels and Chemicals; Humana Press: Totowa, NJ, USA, 1996; Volume 57, pp. 711–727. [Google Scholar] [CrossRef]
- De Souza, C.G.M.; Tychanowicz, G.K.; De Souza, D.F.; Peralta, R.M. Production of laccase isoforms byPleurotus pulmonarius in response to presence of phenolic and aromatic compounds. J. Basic Microbiol. 2004, 44, 129–136. [Google Scholar] [CrossRef]
- Galbe, M.; Zacchi, G. A review of the production of ethanol from softwood. Appl. Microbiol. Biotechnol. 2002, 59, 618–628. [Google Scholar] [CrossRef]
- Mtui, G.Y.S. Trends in industrial and environmental biotechnology research in Tanzania. Afr. J. Biotechnol. 2007, 6, 2860–2867. [Google Scholar] [CrossRef][Green Version]
- Ubalua, A.O. Cassava wastes: Treatment options and value addition alternatives. Afr. J. Biotechnol. 2007, 6, 2065–2073. [Google Scholar] [CrossRef]
- Demirbaş, A. Utilization of Urban and Pulping Wastes to Produce Synthetic Fuel via Pyrolysis. Energy Sources 2002, 24, 205–213. [Google Scholar] [CrossRef]
- Guerra, A.; Mendonça, R.; Ferraz, A. Molecular weight distribution of wood components extracted from Pinus taeda biotreated by Ceriporiopsis subvermispora. Enzym. Microb. Technol. 2003, 33, 12–18. [Google Scholar] [CrossRef]
- Maijala, P.; Kleen, M.; Westin, C.; Poppius-Levlin, K.; Herranen, K.; Lehto, J.; Reponen, P.; Mäentausta, O.; Mettälä, A.; Hatakka, A. Biomechanical pulping of softwood with enzymes and white-rot fungus Physisporinus rivulosus. Enzym. Microb. Technol. 2008, 43, 169–177. [Google Scholar] [CrossRef]
- Wan, C.; Li, Y. Microbial pretreatment of corn stover with Ceriporiopsis subvermispora for enzymatic hydrolysis and ethanol production. Bioresour. Technol. 2010, 101, 6398–6403. [Google Scholar] [CrossRef]
- Oyeleke, S.B.; Dauda, B.E.N.; Oyewole, O.A.; Okoliegbe, I.N.; Ojebode, T. Production of bioethanol from cassava and sweet potato peels. Ad. Environ. Biol 2011, 5, 3729–3733. [Google Scholar]
- Taha, M.; Shahsavari, E.; Al-Hothaly, K.; Mouradov, A.; Smith, A.; Ball, A.; Adetutu, E.M. Enhanced Biological Straw Saccharification Through Coculturing of Lignocellulose-Degrading Microorganisms. Appl. Biochem. Biotechnol. 2015, 175, 3709–3728. [Google Scholar] [CrossRef]
- Song, L.; Yu, H.; Ma, F.; Zhang, X. Biological Pretreatment under Non-sterile Conditions for Enzymatic Hydrolysis of Corn Stover. Bioresources 2013, 8, 3802–3816. [Google Scholar] [CrossRef]
- Du, W.; Yu, H.; Song, L.; Zhang, J.; Weng, C.; Ma, F.; Zhang, X. The promoting effect of byproducts from Irpex lacteus on subsequent enzymatic hydrolysis of bio-pretreated cornstalks. Biotechnol. Biofuels 2011, 4, 37. [Google Scholar] [CrossRef]
- Zhang, Q.; He, J.; Tian, M.; Mao, Z.; Tang, L.; Zhang, J.; Zhang, H. Enhancement of methane production from cassava residues by biological pretreatment using a constructed microbial consortium. Bioresour. Technol. 2011, 102, 8899–8906. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.; Hom-Diaz, A.; Blanquez, P.; Vicent, T.; Ferrer, I. Improving biogas production from microalgae by enzymatic pretreatment. Bioresour. Technol. 2016, 199, 347–351. [Google Scholar] [CrossRef]
- Ali, S.S.; Al-Tohamy, R.; Manni, A.; Luz, F.C.; Elsamahy, T.; Sun, J. Enhanced digestion of bio-pretreated sawdust using a novel bacterial consortium: Microbial community structure and methane-producing pathways. Fuel 2019, 254, 115604. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, Y.; Chen, X.; Shao, Q.; Qin, W. Pretreatment of Miscanthus with biomass-degrading bacteria for increasing delignification and enzymatic hydrolysability. Microb. Biotechnol. 2019, 12, 787–798. [Google Scholar] [CrossRef]
- Machado, A.D.S.; Ferraz, A. Biological pretreatment of sugarcane bagasse with basidiomycetes producing varied patterns of biodegradation. Bioresour. Technol. 2017, 225, 17–22. [Google Scholar] [CrossRef]
- Castoldi, R.; Bracht, A.; de Morais, G.R.; Baesso, M.L.; Corrêa, R.C.G.; Peralta, R.; Moreira, R.; Polizeli, M.D.L.; de Souza, C.G.M.; Peralta, R.M. Biological pretreatment of Eucalyptus grandis sawdust with white-rot fungi: Study of degradation patterns and saccharification kinetics. Chem. Eng. J. 2014, 258, 240–246. [Google Scholar] [CrossRef]
- Kumar, M.N.; Ravikumar, R.; Sankar, M.K.; Thenmozhi, S. New insight into the effect of fungal mycelia present in the bio-pretreated paddy straw on their enzymatic saccharification and optimization of process parameters. Bioresour. Technol. 2018, 267, 291–302. [Google Scholar] [CrossRef]
- Jagtap, S.; Dhiman, S.; Kim, T.-S.; Li, J.; Kang, Y.C.; Lee, J.-K. Characterization of a β-1,4-glucosidase from a newly isolated strain of Pholiota adiposa and its application to the hydrolysis of biomass. Biomass-Bioenergy 2013, 54, 181–190. [Google Scholar] [CrossRef]
- Dhiman, S.S.; Haw, J.-R.; Kalyani, D.; Kalia, V.C.; Kang, Y.C.; Lee, J.-K. Simultaneous pretreatment and saccharification: Green technology for enhanced sugar yields from biomass using a fungal consortium. Bioresour. Technol. 2015, 179, 50–57. [Google Scholar] [CrossRef]
- Jagtap, S.; Dhiman, S.; Kim, T.-S.; Li, J.; Lee, J.-K.; Kang, Y.C. Enzymatic hydrolysis of aspen biomass into fermentable sugars by using lignocellulases from Armillaria gemina. Bioresour. Technol. 2013, 133, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Y.; Sabo, R.; Luo, X. Integrated production of nano-fibrillated cellulose and cellulosic biofuel (ethanol) by enzymatic fractionation of wood fibers. Green Chem. 2011, 13, 1339–1344. [Google Scholar] [CrossRef]
- Henriksson, M.; Berglund, L.; Lindström, T. An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur. Polym. J. 2007, 43, 3434–3441. [Google Scholar] [CrossRef]
- Tsukamoto, J.; Durán, N.; Tasic, L. Nanocellulose and Bioethanol Production from Orange Waste using Isolated Microorganisms. J. Braz. Chem. Soc. 2013, 24, 1537–1543. [Google Scholar] [CrossRef]
- de Camargo, L.A.; Pereira, S.C.; Correa, A.C.; Farinas, C.S.; Marconcini, J.M.; Mattoso, L.H.C. Feasibility of Manufacturing Cellulose Nanocrystals from the Solid Residues of Second-Generation Ethanol Production from Sugarcane Bagasse. BioEnergy Res. 2016, 9, 894–906. [Google Scholar] [CrossRef]
- Martelli-Tosi, M.; Torricillas, M.D.S.; Martins, M.A.; De Assis, O.B.G.; Tapia-Blácido, D.R. Using Commercial Enzymes to Produce Cellulose Nanofibers from Soybean Straw. J. Nanomater. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Beltramino, F.; Roncero, M.B.; Vidal, T.; Torres, A.L.; Valls, C. Increasing yield of nanocrystalline cellulose preparation process by a cellulase pretreatment. Bioresour. Technol. 2015, 192, 574–581. [Google Scholar] [CrossRef] [PubMed]

| No. | Title | Highlights of Review | Ref. |
|---|---|---|---|
| 1. | Enzymatic pretreatment of lignocellulosic biomass for enhanced biomethane production-A review |
| [12] |
| 2. | A review on the environment-friendly emerging techniques for pretreatment of lignocellulosic biomass: Mechanistic insight and advancement |
| [13] |
| 3. | Recent Insights into Lignocellulosic Biomass Pyrolysis: A Critical Review on Pretreatment, Characterization, and Products Upgrading |
| [14] |
| 4. | Recent advances in the pretreatment of lignocellulosic biomass for biofuels and value-added products |
| [15] |
| 5. | Emerging technologies for the pretreatment of lignocellulosic biomass |
| [16] |
| Retting Period | 0 Days | 7 Days | 14–20 Days | After 50 Days |
|---|---|---|---|---|
| Changes in the hemp stem’s and fibre’s ultrastructure | (i) Stem with a well-preserved layered structure (ii) Un-collapsed, unbroken cells with their original cell geometry (iii) Living cells with cytoplasm (iv) Cuticle and trichomes are unharmed on the clear surface. (v) Chloroplasts in abundance in the upper epidermis | (i) The structure as a whole is in good condition. (ii) Fungal growth on the outside of the stems and inside the stems (iii) With damaged epidermis and parenchyma, cellular architecture is less stable. | (i) Cuticle has seriously deteriorated. (ii) Changes in cellular anatomy, as well as significant loss of live cells (iii) Fibre bundles were isolated from each other and the epidermis. (iv) Thick-walled cells populate seldom; parenchyma degrades completely, although chlorenchyma suffers less harm. (v) Bast fibres with sporadic moderate attacks (vi) Fungi colonisation and decay morphology were both affected by fibre morphology. | (i) The structure of hemp was severely harmed and dissolved. (ii) The epidermis and cambium were heavily invaded by dominating bacteria. (iii) In the bast regions, the parenchyma cells have been destroyed, and the structural integrity has been lost. (iv) All cell types, including fibre cells, have hyphae inside their lumina. (v) BFIs are more intense inside the stem. (vi) Anatomy and ultrastructure have been severely harmed. (vii) Bast fibres with a thick wall and degradation properties (viii) Effects on the ultrastructure of the fibre wall.
|
| The dynamics and activity of microbes | Fungi (i) Rarely seen Bacteria (ii) Not observed Fungi | Fungi (i) Mycelia with sparse growth (ii) Less variety (iii) Outside of the cortical layers, colonisation occurs largely in live cells. (iv) Trichomes near to the surface trichomes have dense colonisation. (v) Dependence on readily available food (vi) Damage to cell walls is reduced. Bacteria (i) Less abundant | Fungi (i) Extensive and plentiful (ii) Mycelia densely covering the cuticle (iii) diverse population (iv) a large number of spores (v) Interactions and activities that are intense Bacteria (i) Abundant (ii) Diverse population iii) Over the cuticle, colonies (iv) Associated with hyphae and fungal spores (v) After 20 days, there are more noticeable activity (vi) Cuticle has severely deteriorated | Fungi (i) Less abundant on the outside of the stem (ii) Mycelia on the surface is dead, but there are active hyphae inside the stem (iii) Mycelia, an invading bacteria’s sole source of nourishment, showed bacterial mycophagy (i.e., extracellular and endocellular biotrophic and extracellular necrotrophic activities). Bacteria (i) Highly abundant inside and outside the stems (ii) Highly dominant and diverse role. (iii) Visible as dense overlay representing (a) Biofilms (b) Morphologically different colonies (c) Randomly scattered cells (iv) Showed strong BFIs (v) Using fungal highways, bacterial movement occurs over and inside the hemp stem. (vi) Cutinolytic and cellulolytic activities were improved. |
| Substrate | Conditions | Component’s Degradation (%) | |||
|---|---|---|---|---|---|
| 1st Step | 2nd Step | Lignin | Hemicellulose | Cellulose | |
| Biological—alkaline pretreatment | |||||
| Corn stalks | Irpex lacteus (28 °C, 15 d) | 0.25 M NaOH solution (75 °C, 2 h) | 80 | 51.37 | 6.62 |
| Populus tomentosa | Trametes velutina D10149 (28 °C, 28 d) | 70% (v/v) ethanol aqueous solution containing 1%(w/v) NaOH (75 °C, 3 h) | 23.08 | 22.22 | 18.91 |
| Willow sawdust | Leiotrametes menziesii (27 °C, 30 d) | 1% (w/v) NaOH (80 °C, 24 h) | 59.8 | 68.1 | 51.2 |
| Abortiporus biennis (27 °C, 30 d) | 54.2 | 51.8 | 29.1 | ||
| Biological—acid pretreatment | |||||
| Populus tomentosa | Trametes velutina D1014 (28 °C, 56 d) | 1% sulphuric acid (140 °C, 1 h) | 23.82 | 75.96 | (+) 18.74 |
| Oil palm empty fruit bunches | Pleurotus floridanus LIPIMC996 (31 °C, 28 d) | Ball milled at 29.6/s for 4 min. Phosphoric acid treatment (50 °C, 5 h) | (+) 8.29 | 60.63 | (+) 37.52 |
| Olive tree biomass | Irpex lacteus (Fr.238 617/93) (30 °C, 28 d) | 2% w/v H2SO4 (130 °C, 1.5 h) | (+) 105.82 | 75.29 | (+) 62.95 |
| Biological—oxidative pretreatment | |||||
| Corn Straw | Echinodontium taxodii (25 °C, 15 d) | 0.0016% NaOH and 3% H2O2 (25 °C, 16 h) | 52.00 | 23.64 | (+) 45.45 |
| Hemp chips | Pleurotus eryngii (28 °C, 21 d) | 3% NaOH and 3% (v/v) H2O2 (40 °C, 24 h) | 55.7 | 23.2 | 25.1 |
| Biological—organosolv pretreatment | |||||
| Sugarcane straw | Ceriporiopsis subvermispora (27 °C, 15 d) | Acetosolv pulping (Acetic acid with 0.3% w/w HCl) (120 °C, 5 h | 86.8 | 93.8 | 32.1 |
| Pinus radiata | Gloeophyllum trabeum (27 °C, 28 d) | 60% ethanol in water solvent (200 °C, 1 h) | 74.26 | 80.74 | - |
| Biological—liquid hot water (LHW) pretreatment | |||||
| Soybean | Liquid Hot water (170 °C, 3 min, 400 rpm, 110 psi, solid to liquid ratio of 1:10) | Ceriporiopsis subvermispora (28 °C, 18 d) | 36.69 | 41.34 | 0.84 |
| Corn stover | 41.99 | 42.91 | 7.09 | ||
| Wheat straw | Hot water extraction (HWE) (85 °C, 10 min, solid to liquid ratio of 1:20) | Ceriporiopsis subvermispora (28 °C, 18 d) | 24.87 | 13.19 | 1.86 |
| Corn stover | 30.09 | 28.14 | 4.96 | ||
| Soybean | 0.09 | 0.09 | 0.09 | ||
| Biological—steam explosion pretreatment | |||||
| Beech woodmeal | Phanerochaete chrysosporium (37 °C, 28 d) | Steam explosion (215 °C, 6.5 min) | 42.00 | - | - |
| Sawtooth oak, corn and bran | Lentinula edodes (120 d) | Steam explosion (214 °C, 5 min, 20 atm) | 17.1 | 80.43 | (+) 5.19 |
| Pretreatments | Preferred Natural Fibres | Purposes | Advantages | Disadvantages |
|---|---|---|---|---|
| Physical | Hardwoods and agricultural residues | Enhance the digestibility of lignocellulosic biomass by increase the available specific surface area, and reduce both the degree of polymerisation and cellulose crystallinity | (1) No recycling cost (2) No chemical usage (3) Increase biogas, bioethanol and biohydrogen yields | (1) Excessive size reduction decreases biofuel production (2) Formation of fermentation inhibitors at high temperature (3) Incomplete digestion of lignin-carbohydrate matrix (4) The need to wash the hydrolysate decreases sugar yield (5) High energy requirement |
| Biological | Hardwoods, softwoods, and agricultural residues | Leverage the action of fungi capable of producing enzymes that can degrade lignin, hemicellulose, and polyphenols | (1) The depolymerisation is very selective and efficient (2) Low-capital cost (3) Low energy requirement (4) No chemicals requirement (5) Mild process conditions | (1) The rate of biological pretreatment is too slow for industrial purposes (10–14 days) (2) Require careful growth conditions and a large amount of space (3) A fraction of carbohydrate is consumed by the microbes, thus reduces the sugar yield |
| Bioproducts | Natural Fibres | Pretreatments | Conditions | Yield Improvement/Product Properties | References |
|---|---|---|---|---|---|
| Biohydrogen | Corn stover | Steam explosion | 1.5 Mpa and 198 °C for 1.5 min | 51.9 L H2 kg−1 TS * | [79] |
| Rice straw | Hydrothermal | pH 7.0, 210 °C, 15.4 °C min−1, and 20% TS | 28.0 mL H2 g−1 VS * | [80] | |
| Biomethane | Sugarcane bagasse | Hydrolysis | 178.6 °C, 43.4 min, and solid to liquid ratio of 0.24 | 1.56 Nm3 CH4 kg−1 TOC * | [81] |
| Wheat straw | Microwave irradiation | 260 °C, 33 bars, 3 min | 28% | [82] | |
| Pennisetum hybrid | 12% | [83] | |||
| Blend of maize, ryegrass, and rice straw | Extrusion | Exit slit opened at 60% | 11.5–13.4% | [84] | |
| Hay | Steam explosion | 220 °C for 15 min | 16% | [85] | |
| Vine trimming shoot | Extrusion | 200 g h−1 feed rate | 51–58% hemicellulose reduction, 15.7–21.4% CH4 increased | [78] | |
| Biosugar | Wheat straw | Supercritical CO2 & steam explosion | A steam explosion at 200 °C for 15 min and supercritical CO2 of 12 MPa at 190 °C for 60 min | 36.5% | [86] |
| Poplar wood chips | Mechanical pulping & steam | Disc clearance set 0.5–0.1 mm for mechanical pulping and steam pretreatment at 210 °C for 5 min | 76% | [87] | |
| Poplar wood | Steam explosion | 180 °C and 18 min | 94% | [88] | |
| Cane bagasse | Hydrothermal | 200 °C | 4 mg xylose ml−1 * | [89] | |
| Pinewood | 240 °C and 10 min | 32% ** | [90] | ||
| Rapeseed meal | 260 °C and 10 min | 51 g glucose kg−1 * | [91] | ||
| Nanocellulose | Poplar wood | Steam explosion | 2 MPa for 180 s | 13.2% | [92] |
| Cotton | High-pressure homogenization | 80 MPa for 30 HPH | 10–20 nm in diameter, reduced thermal stability, and crystallinity | [93] | |
| Sugarcane bagasse | 10–20 nm in diameter, reduced thermal stability, and crystallinity | [94] | |||
| Oil palm biomass | Superheated steam | 260 °C for 30 min | <100 nm diameter, 27% crystallinity reduced | [35] |
| Bioproducts | Natural Fibres | Type of Microbes/Enzymes | Hydrolysis Conditions | Yield Improvement/Product Properties | References |
|---|---|---|---|---|---|
| Biohydrogen | Corn stover | Clostridium cellulolyticum and hydrogen fermentation bacteria | 20 mL of medium, 5% (v/v) inoculum, 10 g L−1 carbon source, at 37 °C for 96 hrs | 40.3 L H2 kg−1 TS * | [79] |
| Bioethanol | Corn stover | Ceriporiopsis subvermispora | 28 °C for 18 days | 57.8% yield increased | [107] |
| Corn stover | Ceriporiopsis subvermispora | 28 °C for 35 days | 66.6% yield increased | [107] | |
| Potato and cassava peel | Gloeophyllum sepiarium and Pleurotus ostreatus | 28 °C for 7 days | 26% yield increased | [108] | |
| Straw | Neosartorya fischeri–Myceliophthora thermophila and Aeromonas hydrophila–Pseudomonas poae | 30–55 °C for 6 days | 7-fold yield increased | [109] | |
| Corn stover | Irpex lacteus | 28 °C for 42 days | 66.9% yield increased | [110] | |
| Corn stalks | 28 °C for 28 days | 82% yield increased | [111] | ||
| Biomethane | Wheat straw | Trametes versicolor | Laccase at 500 U/L, 25 °C for 6 days | 10–18% yield increase | [96] |
| Cassava | Yeast and cellulolytic bacteria | 100 mL of PCS medium, at 55 °C for 12 h | 96.6% yield increased | [112] | |
| Microalgae | Enzyme mix (cellulase, glucohydrolase and xylanase) | 1% enzyme mix, 37 °C for 24 hrs | 15% yield increased | [113] | |
| Sawdust | Methanobrevibacter thaueri MB-1, Methanosarcina acetivorans MB-2, and Methanococcus voltae MB 3. | 60 °C for 6 days | 92.2% yield increased | [114] | |
| Biosugar | Corn stover | Ceriporiopsis subvermispora | 28 °C for 5–7 days | 57–67% yield increase | [107] |
| Silver grass | Bacillus, Pseudomonas, Exiguobacterium, and Aeromonas | 37 °C for 7 days | 2.2-fold yield increased | [115] | |
| Sugarcane bagasse | Ceriporiopsis submervispora | 27 °C for 60 days | 47% yield increased | [116] | |
| Sawdust | Pleurotus pulmonarius | 28 °C for 30 days | 94.8% yield increased | [117] | |
| Paddy straw | Pleurotus florida | 25–29 °C for 28 days | 75.3% yield increased | [118] | |
| Rice straw | Pholiota adiposa | 25 °C for 120 h | 716 mg g−1 * | [119] | |
| Pholiota adipose and Armillaria gemina | 27 °C for 45 days | 74.2% yield increased | [120] | ||
| Populus tomentiglandulosa | Armillaria gemina SKU2114 | 30 °C for 48 h | 62% yield increased | [121] | |
| Nanocellulose | Eucalyptus | Endoglucanase and cellobiohydrolase | 7 pH, 50 °C for 48 h | 20 nm diameter, >500 nm length | [122] |
| Wood fibre | Endoglucanase | 4.8 pH, 50 °C for 2 h | 5–30 nm diameter | [123] | |
| Orange residues | ß-glucosidase | 4 pH, 50 °C for 48 h | 180 nm diameter, 1.3 mm length | [124] | |
| Sugarcane bagasse | ß-glucosidase and endoglucanase | 5 pH, 50 °C for 24 h | 14–18 nm diameter, 195–250 nm length | [125] | |
| Maple pulp | Cellic CTec 2 and Cellic HTec 2 (commercial enzymes) | 4.8 pH, 50 °C for 72 h | 5–10 nm diameter, 1 μm length | [126] | |
| Cotton linters | Cellulase | 5 pH, 55 °C for 24 h | 35 nm diameter, 0.3 mm length | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norrrahim, M.N.F.; Huzaifah, M.R.M.; Farid, M.A.A.; Shazleen, S.S.; Misenan, M.S.M.; Yasim-Anuar, T.A.T.; Naveen, J.; Nurazzi, N.M.; Rani, M.S.A.; Hakimi, M.I.; et al. Greener Pretreatment Approaches for the Valorisation of Natural Fibre Biomass into Bioproducts. Polymers 2021, 13, 2971. https://doi.org/10.3390/polym13172971
Norrrahim MNF, Huzaifah MRM, Farid MAA, Shazleen SS, Misenan MSM, Yasim-Anuar TAT, Naveen J, Nurazzi NM, Rani MSA, Hakimi MI, et al. Greener Pretreatment Approaches for the Valorisation of Natural Fibre Biomass into Bioproducts. Polymers. 2021; 13(17):2971. https://doi.org/10.3390/polym13172971
Chicago/Turabian StyleNorrrahim, Mohd Nor Faiz, Muhammad Roslim Muhammad Huzaifah, Mohammed Abdillah Ahmad Farid, Siti Shazra Shazleen, Muhammad Syukri Mohamad Misenan, Tengku Arisyah Tengku Yasim-Anuar, Jesuarockiam Naveen, Norizan Mohd Nurazzi, Mohd Saiful Asmal Rani, Mohd Idham Hakimi, and et al. 2021. "Greener Pretreatment Approaches for the Valorisation of Natural Fibre Biomass into Bioproducts" Polymers 13, no. 17: 2971. https://doi.org/10.3390/polym13172971
APA StyleNorrrahim, M. N. F., Huzaifah, M. R. M., Farid, M. A. A., Shazleen, S. S., Misenan, M. S. M., Yasim-Anuar, T. A. T., Naveen, J., Nurazzi, N. M., Rani, M. S. A., Hakimi, M. I., Ilyas, R. A., & Jenol, M. A. (2021). Greener Pretreatment Approaches for the Valorisation of Natural Fibre Biomass into Bioproducts. Polymers, 13(17), 2971. https://doi.org/10.3390/polym13172971










