Development of Novel Thin Polycaprolactone (PCL)/Clay Nanocomposite Films with Antimicrobial Activity Promoted by the Study of Mechanical, Thermal, and Surface Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Clay Nanofiller Containing Inorganic Antimicrobial Components
2.3. Intercalation of Organic Antimicrobial Component
2.4. Preparation of Thin PCL/Clay Nanocomposite Films
2.5. Sample Characterization
2.6. Antimicrobial Tests
2.6.1. Antimicrobial Tests of Clay Nanofiller Samples
2.6.2. Antimicrobial Tests of Thin PCL/Clay Nanocomposite Films
3. Results and Discussion
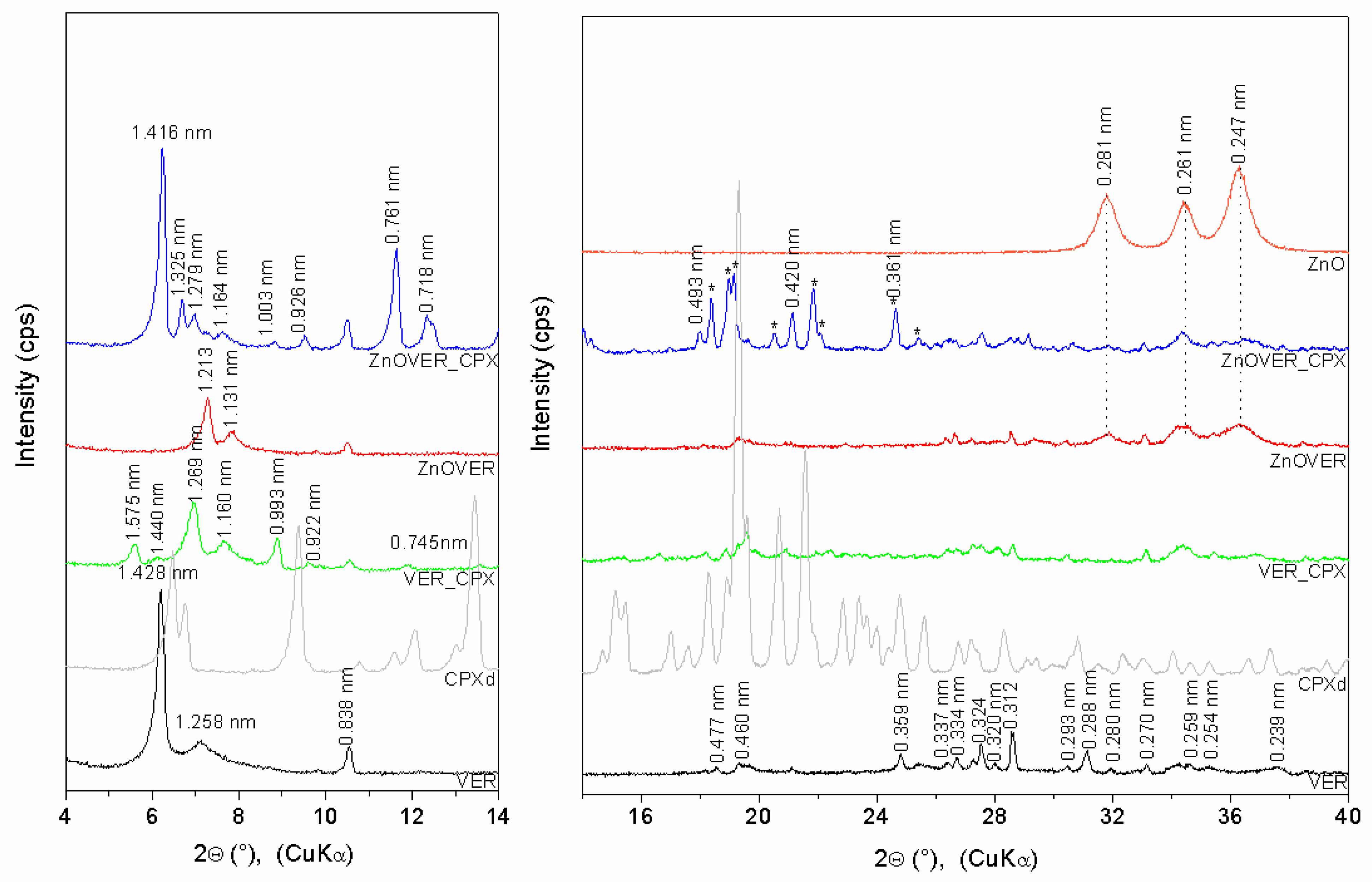
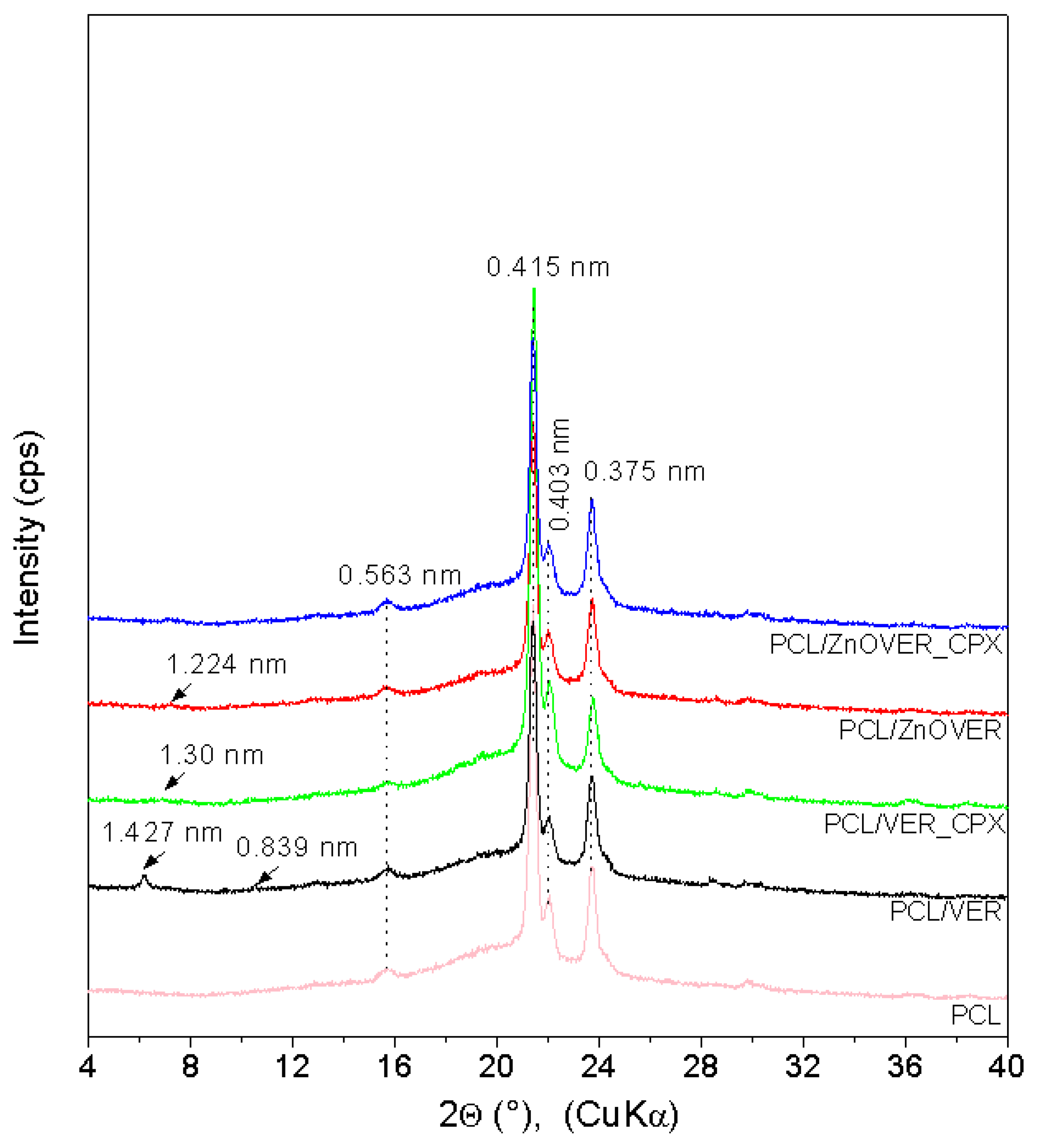
3.1. X-ray Diffraction
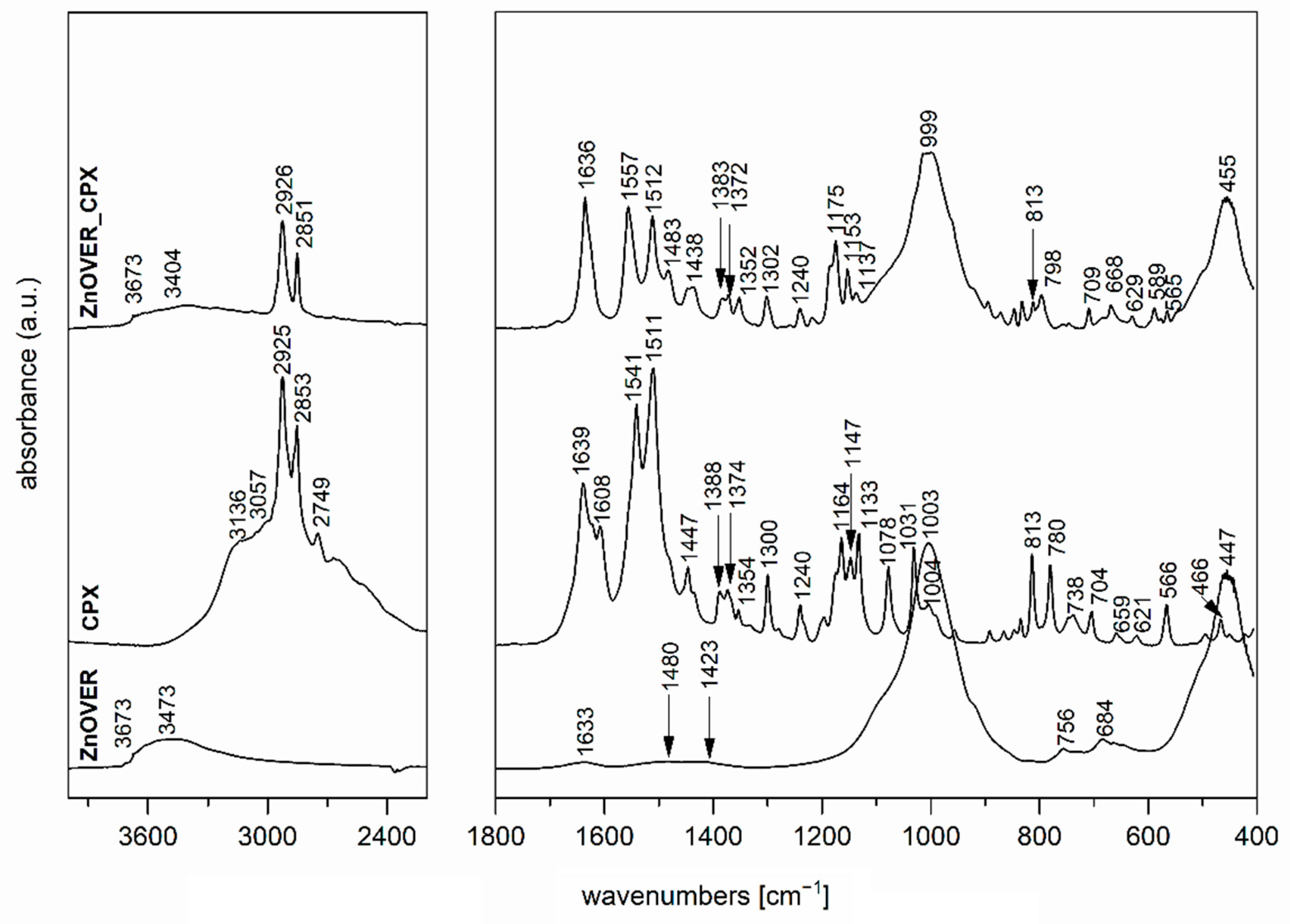
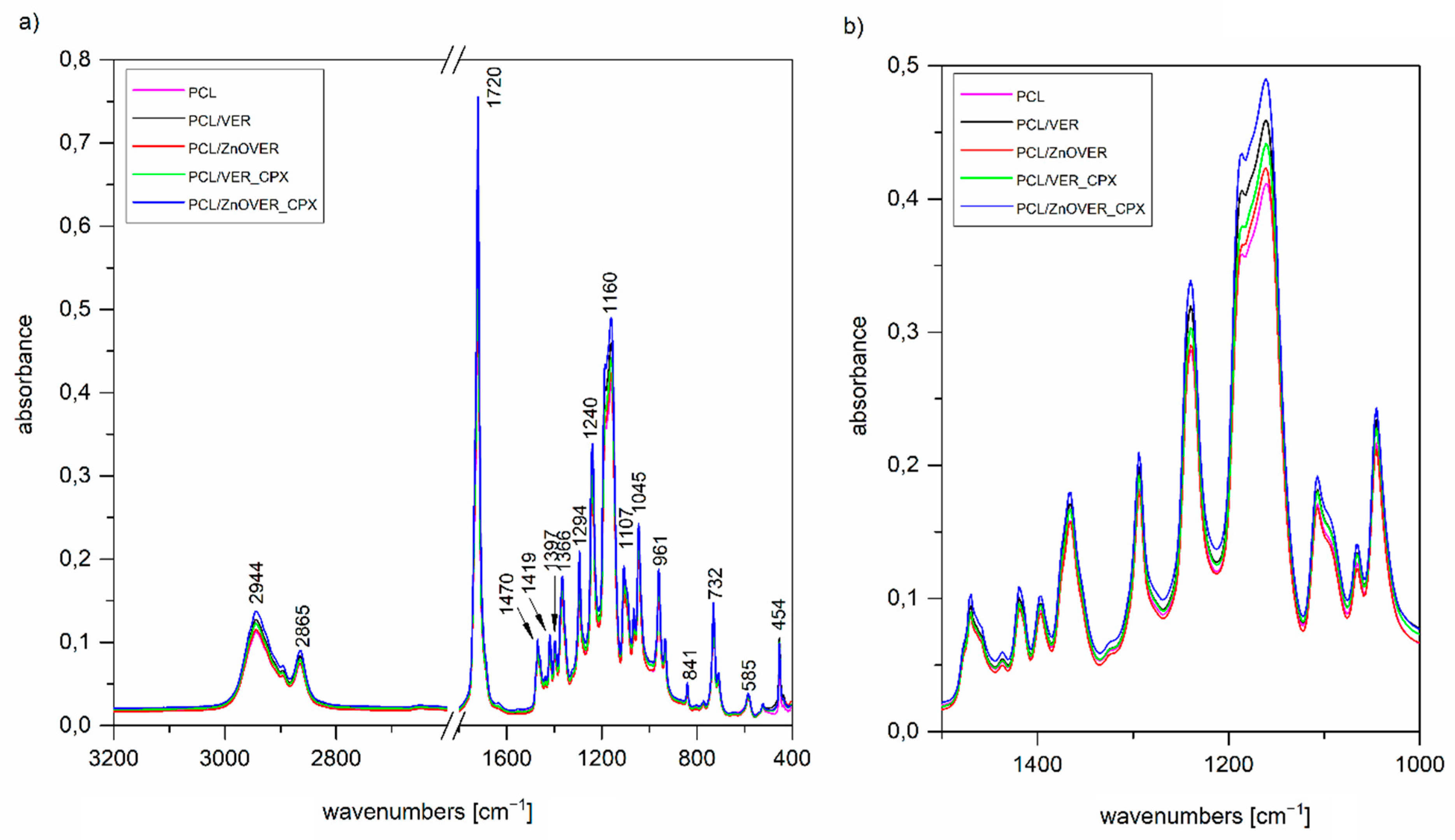
3.2. FTIR Spectroscopy
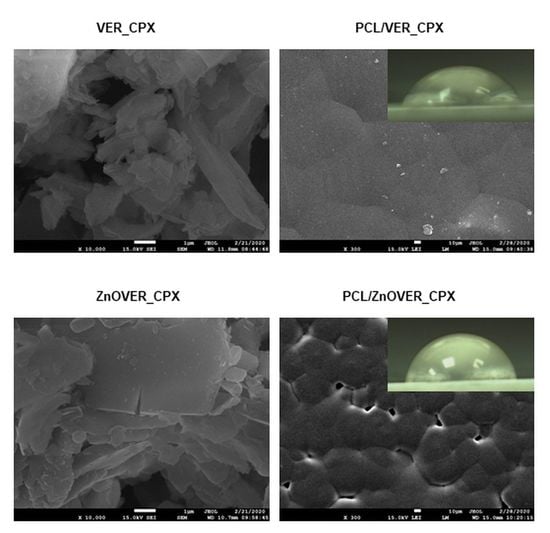
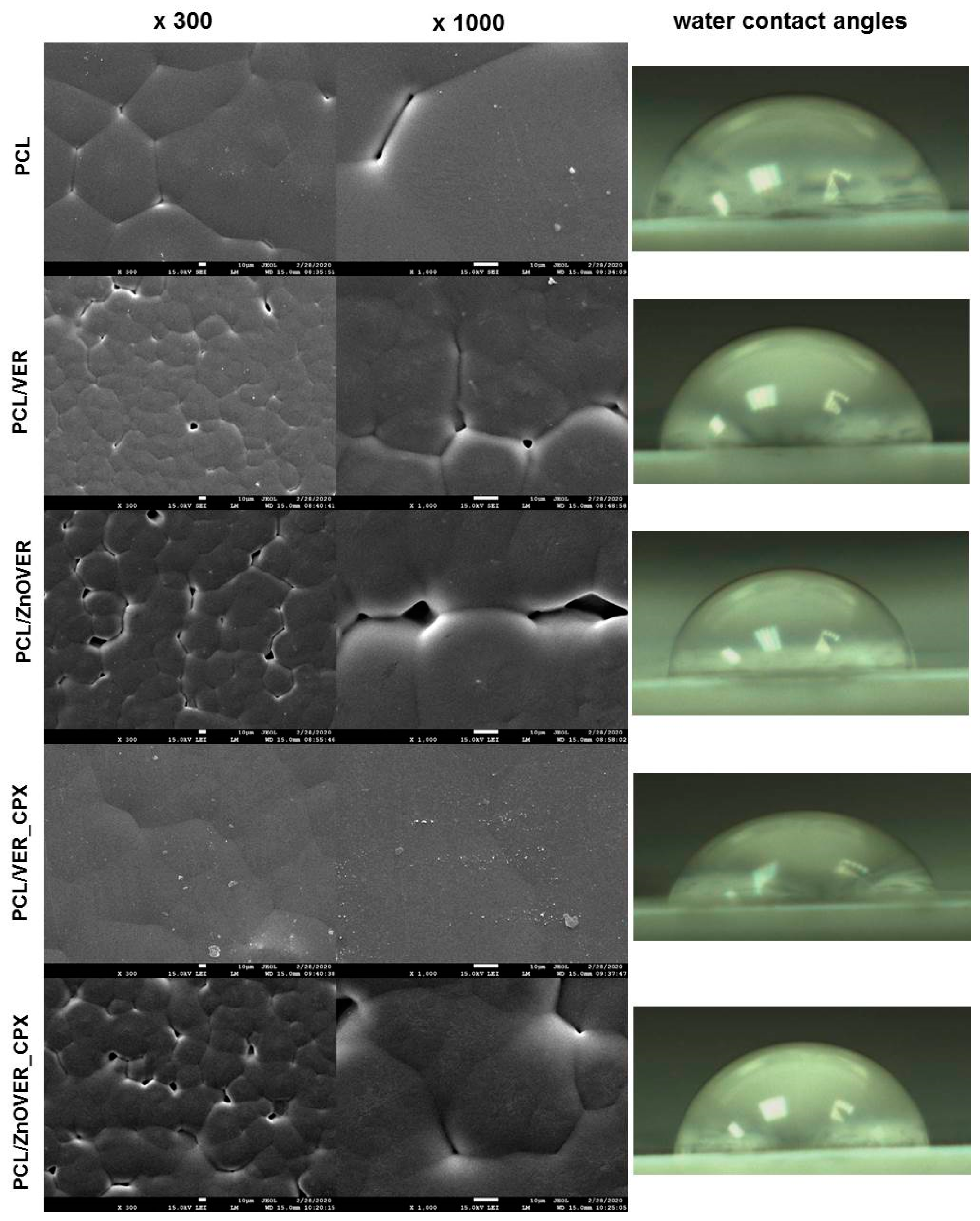
3.3. Surface Characteristics and Morphology
3.4. Thermal Analysis
3.5. Mechanical Properties of Thin PCL/Clay Nanocomposite Films
3.6. Antimicrobial Tests
3.6.1. Antimicrobial Test of Clay Nanofillers
3.6.2. Antimicrobial Test of Thin PCL/Clay Nanocomposite Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Coombes, A.G.A.; Rizzi, S.C.; Williamson, M.; Barralet, J.E.; Downes, S.; Wallace, W.A. Precipitation casting of polycaprolactone for applications in tissue engineering and drug delivery. Biomaterials 2004, 25, 315–325. [Google Scholar] [CrossRef]
- Fatih Canbolat, M.; Celebioglu, A.; Uyar, T. Drug delivery system based on cyclodextrin-naproxen inclusion complex incorporated in electrospun polycaprolactone nanofibers. Colloid. Surf. B 2014, 115, 15–21. [Google Scholar] [CrossRef]
- Schlesinger, E.; Ciaccio, N.; Desai, T.A. Polycaprolactone thin-film drug delivery systems: Empirical and predictive models for device design. Mater. Sci. Eng.: C 2015, 57, 232–239. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.H.; Lee, H.J.; Park, S.; KIM, Y.; Jeong, B. Fast Degradable Polycaprolactone for Drug Delivery. Biomacromolecules 2018, 19, 2302–2307. [Google Scholar] [CrossRef]
- Ng, K.W.; Achuth, H.N.; Moochhala, S.; Lim, T.C.; Hutmacher, D.W. In vivo evaluation of an ultra-thin polycaprolactone film as a wound dressing. J. Biomater. Sci.–Polym. E. 2012, 18, 925–938. [Google Scholar] [CrossRef]
- Thomas, R.; Soumya, K.R.; MATHEW, J.; Radhakrishnan, E.K. Electrospun Polycaprolactone Membrane Incorporated with Biosynthesized Silver Nanoparticles as Effective Wound Dressing Material. Appl. Biochem. Biotechnol. 2015, 176, 2213–2224. [Google Scholar] [CrossRef]
- Muwaffak, Z.; Goyanes, A.; Clark, V.; Basit, A.W.; Hilton, S.T.; Gaisford, S. Patient-specific 3D scanned and 3D printed antimicrobial polycaprolactone wound dressings. Int. J. Pharm. 2017, 527, 161–170. [Google Scholar] [CrossRef]
- Bou-Francis, A.; Piercey, M.; Al-Qatami, O.; Mazzanti, G.; Khattab, R.; Ghanem, A. Polycaprolactone blends for fracture fixation in low load-bearing applications. J. Appl. Polym. Sci. 2020, 137, 48940. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.J.; Jo, H.H.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. Fabrication and characterization of 3D-printed bone-like β-tricalcium phosphate/polycaprolactone scaffolds for dental tissue engineering. J. Ind. Eng. Chem. 2017, 46, 175–181. [Google Scholar] [CrossRef]
- Ho, C.-C.; Fang, H.-Y.; Wang, B.; Huang, T.-H.; Shie, M.-Y. The effects of Biodentine/polycaprolactone three-dimensional-scaffold with odontogenesis properties on human dental pulp cells. Int. Endod. J. 2018, 51, e291–e300. [Google Scholar] [CrossRef] [Green Version]
- Kweon, H. A novel degradable polycaprolactone networks for tissue engineering. Biomaterials 2003, 24, 801–808. [Google Scholar] [CrossRef]
- Dai, N.-T.; Williamson, M.R.; Khammo, N.; Adams, E.F.; Coombes, A.G.A. Composite cell support membranes based on collagen and polycaprolactone for tissue engineering of skin. Biomaterials 2004, 25, 4263–4271. [Google Scholar] [CrossRef]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.J.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Pei, X.; He, R.; Wan, Q.; Wang, J. Multiwall carbon nanotubes/polycaprolactone composites for bone tissue engineering application. Colloid. Surf. B. 2012, 93, 226–234. [Google Scholar] [CrossRef]
- Yeong, W.Y.; Sudarmadji, N.; Yu, H.Y.; Chua, C.K.; Leong, K.F.; Venkatraman, S.S.; Boey, Y.C.F.; Tan, L.P. Porous polycaprolactone scaffold for cardiac tissue engineering fabricated by selective laser sintering. Acta Biomater. 2010, 6, 2028–2034. [Google Scholar] [CrossRef]
- Patra, S.; Remy, M.; Ray, A.L.; Brouillaud, B.; Amedee, J.; Gupta, B.; Bordenave, L. A Novel Route to Polycaprolactone Scaffold for Vascular Tissue Engineering. J. Biomater. Tiss. Eng. 2013, 3, 289–299. [Google Scholar] [CrossRef]
- Choi, I.; Yoo, D.S.; Chang, Y.; Kim, S.Y.; Han, J. Polycaprolactone film functionalized with bacteriophage T4 promotes antibacterial activity of food packaging toward Escherichia coli. Food Chem. 2021, 346, 128883. [Google Scholar] [CrossRef]
- Piri, H.; Moradi, S.; Amiri, R. The fabrication of a novel film based on polycaprolactone incorporated with chitosan and rutin: Potential as an antibacterial carrier for rainbow trout packaging. Food Sci. Biotechnol. 2021, 30, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Yu, L.; Feng, M.; Meng, L.; Bai, Y.; Ali, A.; Liu, H.; Chen, L. Development and characterization of biodegradable antimicrobial packaging films based on polycaprolactone, starch and pomegranate rind hybrids. Food Packag. Shelf Life 2018, 18, 71–79. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; González-Martínez, C.; Chiralt, A. Biodegradable Antimicrobial Films for Food Packaging: Effect of Antimicrobials on Degradation. Foods 2021, 10, 1256. [Google Scholar] [CrossRef]
- Njuguna, J.; Pielichowski, K.; Desai, S. Nanofiller-reinforced polymer nanocomposites. Polym. Adv. Technol. 2008, 19, 947–959. [Google Scholar] [CrossRef] [Green Version]
- Dantas De Oliveira, A.; Augusto Gonçalves Beatrice, C. Polymer Nanocomposites with Different Types of Nanofiller. In Nanocomposites—Recent Evolutions, 1st ed.; Sivasankaran, S., Ed.; IntechOpen: London, UK, 2019; Volume 2019, pp. 1–23. [Google Scholar]
- Crosby, A.J.; Lee, J.-Y. Polymer Nanocomposites: The “Nano” Effect on Mechanical Properties. Polym. Rev. 2007, 47, 217–229. [Google Scholar] [CrossRef]
- Šupová, M.; Simha Martynková, G.; Barabaszová, K. Effect of Nanofillers Dispersion in Polymer Matrices: A Review. Sci. Adv. Mater. 2011, 3, 1–25. [Google Scholar] [CrossRef]
- Okada, A.; Usuki, A. Twenty Years of Polymer-Clay Nanocomposites. Macromol. Mater. Eng. 2006, 291, 1449–1476. [Google Scholar] [CrossRef]
- Salam, H.; Dong, Y.; Davies, I. Development of biobased polymer/clay nanocomposites. In Fillers and Reinforcements for Advanced Nanocomposites, 1st ed.; Dong, Y., Umer, R., Kin-Tak Lau, A., Eds.; Woodhead Publishing, Elsevier: Cambridge, UK, 2015; Volume 2015, pp. 101–132. [Google Scholar]
- Mallakpour, S.; Rashidimoghadam, S. Recent developments in the synthesis of hybrid polymer/clay nanocomposites. In Hybrid Polymer Composite Materials, 1st ed.; Thakur, K.V., Thakur, M.K., Pappu, A., Eds.; Woodhead Publishing, Elsevier: Cambridge, UK, 2017; Volume 2017, pp. 227–265. [Google Scholar]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Babu, S.S.; Mathew, S.; Kalarikkal, N.; Thomas, S.; Radhakrishnan, E.K. Antimicrobial, antibiofilm, and microbial barrier properties of poly (ε-caprolactone)/cloisite 30B thin films. 3 Biotech 2016, 6, 249. [Google Scholar] [CrossRef] [Green Version]
- Babu, S.S.; Kalarikkal, N.; Thomas, S.; Radhakrishnan, E.K. Enhanced antimicrobial performance of cloisite 30B/poly (ε-caprolactone) over cloisite 30B/poly (L-lactic acid) as evidenced by structural features. Appl. Clay Sci. 2018, 153, 198–204. [Google Scholar] [CrossRef]
- Cesur, S.; Köroğlu, C.; Yalçin, H.T. Antimicrobial and biodegradable food packaging applications of polycaprolactone/organo nanoclay/chitosan polymeric composite films. J. Vinyl Addit. Technol. 2018, 24, 376–387. [Google Scholar] [CrossRef]
- Yahiaoui, F.; Benhacine, F.; Ferfera-Harrar, H.; Habi, A.; Hadj-Hamou, A.S.; Grohens, Y. Development of antimicrobial PCL/nanoclay nanocomposite films with enhanced mechanical and water vapor barrier properties for packaging applications. Polym. Bull. 2015, 72, 235–254. [Google Scholar] [CrossRef]
- Holešová, S.; Čech Barabaszová, K.; Hundáková, M.; Plevová, E.; Kalendová, A. Novel LDPE /vermiculite/ciclopiroxolamine hybrid nanocomposites: Structure, surface properties, and antifungal activity. J. Appl. Polym. Sci. 2021, 138, 50232. [Google Scholar] [CrossRef]
- Čech Barabaszová, K.; Holešová, S.; Hundáková, M.; Kalendová, A. Tribo-Mechanical Properties of the Antimicrobial Low-Density Polyethylene (LDPE) Nanocomposite with Hybrid ZnO–Vermiculite–Chlorhexidine Nanofillers. Polymers 2020, 12, 2811. [Google Scholar] [CrossRef]
- Čech Barabaszová, K.; Holešová, S.; Hundáková, M.; Mohyla, V. Vermiculite in polycaprolactone films prepared with the used of ultrasound. Mater. Today-Proc. 2021, 37, 13–20. [Google Scholar] [CrossRef]
- Holešová, S.; Reli, M.; Hundáková, M.; Čech Barabaszová, K.; Ritz, M.; Plevová, E.; Pazdziora, E. Synthesis and Antimicrobial Activity of Polyethylene/Chlorhexidine/Vermiculite Nanocomposites. J. Nanosci. Nanotechnol. 2019, 19, 2925–2933. [Google Scholar] [CrossRef] [PubMed]
- Yam, W.Y.; Ismail, J.; Kammer, H.W.; SCHMIDT, H.; Kummerlöwe, C. Polymer blends of poly(ϵ-caprolactone) and poly(vinyl methyl ether) – thermal properties and morphology. Polymer 1999, 40, 5545–5552. [Google Scholar] [CrossRef]
- Kneiflová, J. Hodnocení baktericidní účinnosti dezinfekčních prostředků suspenzní mikrometodou. Čs. Epidemiol. Mikrobiol. Imunol. 1988, 37, 97–104. [Google Scholar]
- Marcos, C.; Arango, Y.C.; Rodriguez, I. X-ray diffraction studies of the thermal behaviour of commercial vermiculites. Appl. Clay Sci. 2009, 42, 368–378. [Google Scholar] [CrossRef]
- Sani, H.A.; Ahmad, M.B.; Hussein, M.Z.; Ibrahim, N.A.; Musa, A.; Saleh, T.A. Nanocomposite of ZnO with montmorillonite for removal of lead and copper ions from aqueous solutions. Process Saf. Environ. Protect. 2007, 109, 97–105. [Google Scholar] [CrossRef]
- Tarawneh, R.T.; Hamdan, I.I.; Bani-Jaber, A.; Darwish, R.M. Physicochemical studies on ciclopirox olamine complexes with divalent metal ions. Int. J. Pharm. 2005, 289, 179–187. [Google Scholar] [CrossRef]
- Renou, L.; Coste, S.; Cartigny, Y.; Petit, M.N.; Vincent, C.; Schneider, J.M.; Coquerel, G. Mechanism of hydration and dehydration of ciclopirox ethanolamine (1:1). Cryst. Growth Des. 2009, 9, 3918–3927. [Google Scholar] [CrossRef]
- Fadaie, M.; Mirzaei, E. Nanofibrillated chitosan/polycaprolactone bionanocomposite scaffold with improved tensile strength and cellular behaviour. Nanomed. J. 2018, 5, 77–89. [Google Scholar]
- Ciardelli, G.; Chiono, V.; Vozzi, G.; Pracella, M.; Ahluwalia, A.; Barbani, N.; Cristallini, C.; Giusti, P. Blends of poly-(epsilon-caprolactone) and polysaccharides in tissue engineering applications. Biomacromolecules 2005, 6, 1961–1976. [Google Scholar] [CrossRef] [PubMed]
- Farmer, V.C. The Layer Silicates. In The Infrared Spectra of Minerals, 1st ed.; Farmer, V.C., Ed.; The Mineralogical Society of Great Britain and Ireland: London, UK, 1974; Volume 4, pp. 331–364. [Google Scholar]
- Silverstein, R.M.; Basser, G.C.; Morrill, T. Infrared Spectrometry. In Spectrometric identification of organic compounds, 2nd ed.; Silverstein, R.M., Basser, G.C., Morrill, T., Eds.; John Wiley & Sons Inc.: New York, NY, USA, 1991; pp. 72–126. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, Tables and Charts, 3rd ed.; John Wiley & Sons Inc.: Chichester, UK, 2001; p. 364. [Google Scholar]
- Elzein, T.; Nasser-Eddine, M.; Delaite, C.; Bistac, S.; Dumas, P. FTIR study of polycaprolactone chain organization at interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Čech Barabaszová, K.; Valášková, M. Characterization of vermiculite particles after different milling techniques. Powder Technol. 2013, 239, 277–283. [Google Scholar] [CrossRef]
- Crist, B.; Schultz, J.M. Polymer spherulites: A critical review. Prog. Polym. Sci. 2016, 56, 1–63. [Google Scholar] [CrossRef]
- Ludueña, L.N.; Vazquez, A.; Alvarez, V.A. Crystallization of polycaprolactone–clay nanocomposites. J. Appl. Polym. Sci. 2008, 109, 3148–3156. [Google Scholar] [CrossRef]
- Mallakpour, S.; Nouruzi, N. Effects of citric acid-functionalized ZnO nanoparticles on the structural, mechanical, thermal and optical properties of polycaprolactone nanocomposite films. Mater. Chem. Phys. 2017, 197, 129–137. [Google Scholar] [CrossRef]



| Sample | SSA [m2·g−1] | dm [µm] | d43 [µm] | ζ-Potential [mV] |
|---|---|---|---|---|
| VER | 32.03 | 4.75 | 4.64 | −58.7 |
| ZnOVER | 29.11 | 0.34; 6.72 | 5.87 | −34.8 |
| VER_CPX | 9.97 | 7.18 | 12.80 | −44.1 |
| ZnOVER_CPX | 9.16 | 0.34; 11.52 | 13.25 | −36.5 |
| Sample | WCA [°] |
|---|---|
| PCL | 70.75 |
| PCL/VER | 80.78 |
| PCL/ZnOVER | 77.17 |
| PCL/VER_CPX | 76.97 |
| PCL/ZnOVER_CPX | 75.65 |
| Sample | ∆ m (%) | Td (°C) | Tmax (°C) | ΔHm [J/g] | Χc [%] |
|---|---|---|---|---|---|
| PCL | 99.9 | 380.0 | 413.6 | 85.8 | 63.0 |
| PCL/VER | 97.6 | 379.8 | 412.4 | 83.4 | 61.9 |
| PCL/ZnOVER | 97.3 | 371.8 | 404.0 | 79.9 | 59.3 |
| PCL/VER_CPX | 99.6 | 283.0, 376.0 | 327.5, 412.0 | 71.4 | 53.0 |
| PCL/ZnOVER_CPX | 100.0 | 286.5, 378.7 | 330.9, 407.3 | 90.3 | 67.0 |
| Sample | Young’s Modulus E (MPa) | Tensile Strength Rm (MPa) | Maximum Force Fmax (N) | Maximum Strain Smax (mm/mm) |
|---|---|---|---|---|
| PCL | 129 ± 11 | 23.9 ± 5.0 | 41.0 ± 8.5 | 8.0 ± 0.7 |
| PCL/VER | 107 ± 8 | 14.8 ± 1.1 | 25.5 ± 2.1 | 5.3 ± 0.9 |
| PCL/ZnOVER | 144 ± 5 | 12.4 ± 2.2 | 21.5 ± 3.5 | 1.2 ± 0.6 |
| PCL/VER_CPX | 128 ± 5 | 13.8 ± 2.3 | 23.5 ± 3.5 | 3.6 ± 2.7 |
| PCL/ZnOVER_CPX | 96 ± 6 | 10.7 ± 1.3 | 18.5 ± 2.1 | 1.2 ± 0.3 |
| Strain | Sample | MIC [% w/v] | |||||
|---|---|---|---|---|---|---|---|
| 30 min | 120 min | 300 min | 1 day | 3 days | 5 days | ||
| S. aureus | VER | - | - | - | - | - | - |
| ZnOVER | - | - | - | - | 1.11 | 1.11 | |
| VER_CPX | 10 | 10 | 10 | 1.11 | 0.014 | 0.014 | |
| ZnOVER_CPX | - | - | - | 0.37 | 0.37 | 0.37 | |
| E. coli | VER | - | - | - | - | - | - |
| ZnOVER | - | - | - | 1.11 | 1.11 | 1.11 | |
| VER_CPX | 3.33 | 3.33 | 3.33 | 3.33 | 0.014 | 0.014 | |
| ZnOVER_CPX | - | - | - | 3.33 | 1.11 | 1.11 | |
| Candida a. | VER | - | - | - | - | - | - |
| ZnOVER | - | - | - | - | 10 | 1.11 | |
| VER_CPX | 3.33 | 1.11 | 0.37 | 0.014 | 0.014 | 0.014 | |
| ZnOVER_CPX | - | - | - | 10 | 0.37 | 0.37 | |
| Strain | Sample | Number of CFU | ||
|---|---|---|---|---|
| Time of Contact [h] | ||||
| 24 | 72 | 96 | ||
| S. aureus | PCL/VER | >330 | >330 | >330 |
| PCL/ZnOVER | 197 | >330 | >330 | |
| PCL/VER_CPX | 165 | >330 | >330 | |
| PCL/ZnOVER_CPX | 26 | >330 | 199 | |
| E. coli | PCL/VER | >330 | >330 | 50 |
| PCL/ZnOVER | >330 | >330 | 58 | |
| PCL/VER_CPX | >330 | >330 | 298 | |
| PCL/ZnOVER_CPX | 0 | 0 | 0 | |
| Candida a. | PCL/VER | 71 | >330 | >330 |
| PCL/ZnOVER | 93 | >330 | >330 | |
| PCL/VER_CPX | 135 | 74 | 0 | |
| PCL/ZnOVER_CPX | 22 | 0 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holešová, S.; Čech Barabaszová, K.; Hundáková, M.; Ščuková, M.; Hrabovská, K.; Joszko, K.; Antonowicz, M.; Gzik-Zroska, B. Development of Novel Thin Polycaprolactone (PCL)/Clay Nanocomposite Films with Antimicrobial Activity Promoted by the Study of Mechanical, Thermal, and Surface Properties. Polymers 2021, 13, 3193. https://doi.org/10.3390/polym13183193
Holešová S, Čech Barabaszová K, Hundáková M, Ščuková M, Hrabovská K, Joszko K, Antonowicz M, Gzik-Zroska B. Development of Novel Thin Polycaprolactone (PCL)/Clay Nanocomposite Films with Antimicrobial Activity Promoted by the Study of Mechanical, Thermal, and Surface Properties. Polymers. 2021; 13(18):3193. https://doi.org/10.3390/polym13183193
Chicago/Turabian StyleHolešová, Sylva, Karla Čech Barabaszová, Marianna Hundáková, Michaela Ščuková, Kamila Hrabovská, Kamil Joszko, Magdalena Antonowicz, and Bożena Gzik-Zroska. 2021. "Development of Novel Thin Polycaprolactone (PCL)/Clay Nanocomposite Films with Antimicrobial Activity Promoted by the Study of Mechanical, Thermal, and Surface Properties" Polymers 13, no. 18: 3193. https://doi.org/10.3390/polym13183193
APA StyleHolešová, S., Čech Barabaszová, K., Hundáková, M., Ščuková, M., Hrabovská, K., Joszko, K., Antonowicz, M., & Gzik-Zroska, B. (2021). Development of Novel Thin Polycaprolactone (PCL)/Clay Nanocomposite Films with Antimicrobial Activity Promoted by the Study of Mechanical, Thermal, and Surface Properties. Polymers, 13(18), 3193. https://doi.org/10.3390/polym13183193