Proton Exchange Membrane Fuel Cells (PEMFCs): Advances and Challenges
Abstract
:1. Introduction
2. Development of Proton Exchange Membranes (PEM)
2.1. Graphene and Carbon Nanotubes
2.2. Metal. Organic Frameworks
2.3. Ionic Liquids
2.4. Nanofibers
3. Electrocatalysts and Electrodes
3.1. Oxygen Reduction Catalysts
3.2. Hydrogen Oxidation Catalysts
4. Membrane–Electrode Assembly (MEA): Preparation and Characterization
5. Performance of Single Monocells—PEMFCs
5.1. PEMFC Performance Using Pt-Based Catalysts
5.2. PEMFC Performance Using Non-PGM Catalysts
6. PEMFC Stack Configuration and Characterization
7. PEMFC Water Management
8. Hydrophobicity of Electrodes and Gas Diffusion Layers
9. Performance Inhibition of PEMFCs by Electrodes Processes
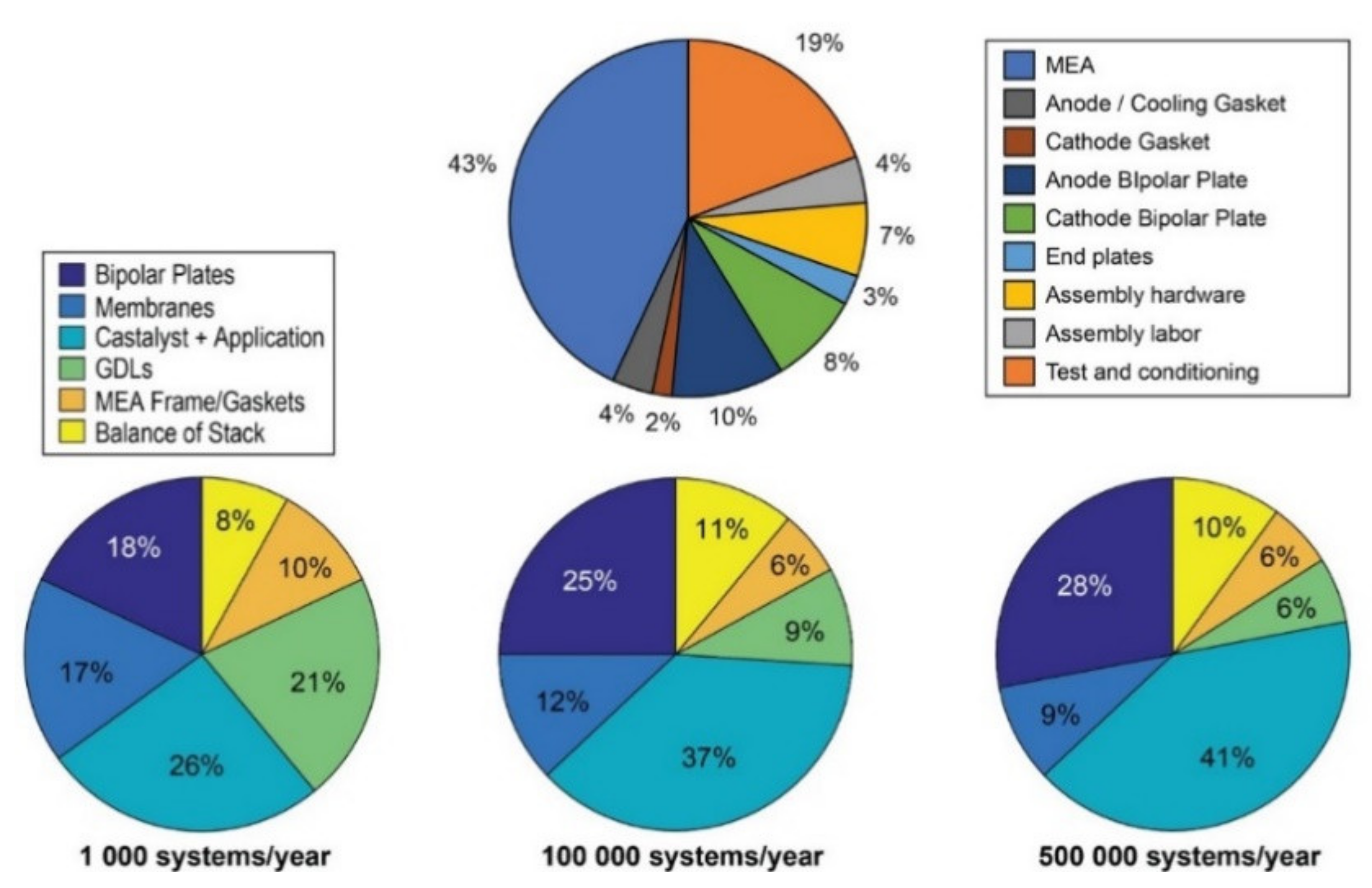
10. PEMFC Technology, Cost, Actual Applications, and Challenges
11. Conclusion and Future Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Kreuer, K.D.; Paddison, S.J.; Spohr, E.; Schuster, M. Transport in proton conductors for fuel-cell applications: Simulations, elementary reactions, and phenomenology. Chem. Rev. 2004, 104, 4637–4678. [Google Scholar] [CrossRef] [Green Version]
- Hammes-Schiffer, S.; Soudackov, A. Proton-coupled electron transfer in solution, proteins, and electrochemistry. J. Phys. Chem. B 2008, 112, 14108–14123. [Google Scholar] [CrossRef] [Green Version]
- Kraytsberg, A.; Ein-Eli, Y. Review of advanced materials for proton exchange membrane fuel cells. Energy Fuels 2014, 28, 7303–7330. [Google Scholar] [CrossRef]
- Li, Q.; Jensena, J.; Savinell, R.F.; Bjerrum, N. High temperature proton exchange membranes based on polybenzimidazoles for fuel cells. Prog. Polym. Sci. 2009, 34, 449–477. [Google Scholar] [CrossRef] [Green Version]
- Cleghorn, S.; Springer, T.; Wilson, M.; Zawodzinski, C.; Zawodzinski, T.A.; Gottesfeld, S. PEM fuel cells for transportation and stationary power generation applications. Int. J. Hydrogen Energy 1997, 22, 1137–1144. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C. Cost-effective iron-based aqueous redox flow batteries for large-scale energy storage application: A review. J. Power Sources 2021, 493, 229445. [Google Scholar] [CrossRef]
- Scott, K.; Shukla, A. Polymer electrolyte membrane fuel cells: Principles and advances. Rev. Environ. Sci. Bio/Technol. 2004, 3, 273–280. [Google Scholar] [CrossRef]
- Whittingham, M.; Savinell, R.; Zawodzinski, T. Introduction: Batteries and fuel cells. Chem. Rev. 2004, 104, 4243–4244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Shen, P.K. Recent development of polymer electrolyte membranes for fuel cells. Chem. Rev. 2012, 112, 2780–2832. [Google Scholar] [CrossRef]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Campanari, S.; Manzolini, G.; García de la Iglesia, F. Energy analysis of electric vehicles using batteries or fuel cells through well-to-wheel driving cycle simulations. J. Power Sources 2009, 186, 464–477. [Google Scholar] [CrossRef]
- Haile, S.M.; Boysen, D.; Chisholm, C.R.I.; Merle, R. Solid acids as fuel cell electrolytes. Nature 2001, 410, 910–913. [Google Scholar] [CrossRef] [Green Version]
- Pineri, M.; Eisenberg, A. Structure and Properties of Ionomers; Springer: Dordrecht, The Netherlands, 1987. [Google Scholar]
- Samms, S.R.; Wasmus, S.; Savinell, R.F. Thermal stability of nafion® in simulated fuel cell environments. J. Electrochem. Soc. 1996, 143, 1498. [Google Scholar] [CrossRef]
- Scott, K.; Xu, C.; Wu, X. Intermediate temperature proton-conducting membrane electrolytes for fuel cells. WIREs Energy Environ. 2014, 3, 24–41. [Google Scholar] [CrossRef]
- Dupis, A.C. Proton exchange membranes for fuel cells operated at medium temperatures: Materials and experimental techniques. Prog. Mater. Sci. 2011, 56, 289–327. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, C.H.; Guiver, M.D.; Lee, Y.M. Sulfonated hydrocarbon membranes for medium-temperature and low-humidity proton exchange membrane fuel cells (PEMFCs). Prog. Polym. Sci. 2011, 36, 1443–1498. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Simonsen, S.C.; Norby, T.; Chatzitakis, A. Composite membranes for high temperature PEM fuel cells and electrolysers: A critical review. Membranes 2019, 9, 83. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.S.; Maurya, S.; Kim, Y.S.; Kreller, C.R.; Wilson, M.S.; Larsen, D.; Elangovan, S.E.; Mukundan, R. Intermediate temperature fuel cells via an ion-pair coordinated polymer electrolyte. Energy Environ. Sci. 2018, 11, 979–987. [Google Scholar] [CrossRef]
- Lipman, T.; Edwards, J.; Kammen, D. Fuel cell system economics: Comparing the costs of generating power with stationary and motor vehicle PEM fuel cell systems. Energy Policy 2004, 32, 101–125. [Google Scholar] [CrossRef]
- Savinell, R.; Yeager, E.; Tryk, D.; Landau, U.; Wainright, J.; Weng, D.; Lux, K.; Litt, M.; Roger, C. A polymer electrolyte for operation at temperatures up to 200 C. J. Electrochem. Soc. 1994, 141, L46–L48. [Google Scholar] [CrossRef]
- Wainright, J.S.; Wang, J.; Weng, D.; Savinell, R.F.; Litt, M. Acid-doped polybenzimidazoles: A new polymer electrolyte. J. Electrochem. Soc. 1995, 142, L121–L123. [Google Scholar] [CrossRef]
- Li, Q.; He, R.; Jensen, J.O.; Bjerrum, N. PBI-based polymer membranes for high temperature fuel cells–preparation, characterization and fuel cell demonstration. Fuel Cells 2004, 4, 147–159. [Google Scholar] [CrossRef]
- Asensio, J.A.; Sanchez, E.; Gomez-Romero, P. Proton-conducting membranes based on benzimidazole polymers for high-temperature PEM fuel cells. A chemical quest. Chem. Soc. Rev. 2010, 39, 3210–3239. [Google Scholar] [CrossRef]
- Araya, S.S.; Zhou, F.; Liso, V.; Sahlin, S.L.; Vang, J.R.; Thomas, S.; Gao, X.; Jeppesen, C.; Kaer, S. A comprehensive review of PBI-based high temperature PEM fuel cells. Int. J. Hydrogen Energy 2016, 41, 21310–21344. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, Q.; Xia, R.; Meyer, T.J. CO2 Reduction: From homogeneous to heterogeneous electrocatalysis. Acc. Chem. Res. 2020, 53, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ren, J.; Zhang, X.; Yang, D. Elemental red phosphorus-based materials for photocatalytic water purification and hydrogen production. Nanoscale 2020, 12, 13297–13310. [Google Scholar] [CrossRef]
- Peter, S.C. Reduction of CO2 to chemicals and fuels: A solution to global warming and energy crisis. ACS Energy Lett. 2018, 3, 1557–1561. [Google Scholar] [CrossRef]
- Chen, P.; Liu, H.; Cui, W.; Lee, S.C.; Wang, L.; Dong, F. Bi-based photocatalysts for light-driven environmental and energy applications: Structural tuning, reaction mechanisms, and challenges. EcoMat 2020, 2, e12047. [Google Scholar] [CrossRef]
- Chandrashekar, S.; Nesbitt, N.T.; Smith, W.A. Electrochemical CO2 reduction over bimetallic au–sn thin films: Comparing activity and selectivity against morphological, compositional, and electronic differences. J. Phys. Chem. C 2020, 124, 14573–14580. [Google Scholar] [CrossRef]
- He, J.; Johnson, N.J.J.; Huang, A.; Berlinguette, C.P. Electrocatalytic alloys for CO2 reduction. ChemSusChem 2018, 11, 48–57. [Google Scholar] [CrossRef]
- Kim, C.; Dionigi, F.; Beermann, V.; Wang, X.; Möller, T.; Strasser, P. Alloy nanocatalysts for the electrochemical oxygen reduction (ORR) and the direct electrochemical carbon dioxide reduction reaction (CO2RR). Adv. Mater. 2018, 31, 1805617. [Google Scholar] [CrossRef]
- Anson, C.W.; Stahl, S.S. Mediated fuel cells: Soluble redox mediators and their applications to electrochemical reduction of O2 and oxidation of H2, alcohols, biomass, and complex fuels. Chem. Rev. 2020, 120, 3749–3786. [Google Scholar] [CrossRef]
- Steinfeld, A. Concentrated solar energy—The path for efficient thermal conversion to power and fuels. Sci. Bull. 2019, 64, 485–486. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Wang, W.W.; Wang, L.; Liu, D.; Zhao, F.Y. A proton exchange membrane fuel cell-compound thermoelectric system: Bidirectional modeling and energy conversion potentials. Energy Convers. Manag. 2020, 207, 112517. [Google Scholar] [CrossRef]
- He, Y.; Tan, Q.; Lu, L.; Sokolowski, J.; Wu, G. Metal-nitrogen-carbon catalysts for oxygen reduction in PEM fuel cells: Self-template synthesis approach to enhancing catalytic activity and stability. Electrochem. Energy Rev. 2019, 2, 231–251. [Google Scholar] [CrossRef]
- Pu, Z.; Zhang, G.; Hassanpour, A.; Zheng, D.; Wang, S.; Liao, S.; Chen, Z.; Sun, S. Regenerative fuel cells: Recent progress, challenges, perspectives and their applications for space energy system. Appl. Energy 2021, 283, 116376. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, W.; Zhang, X.; Tao, P.; Song, C.; Shang, W.; Deng, T.; Wu, J. Component optimization for catalyst layers in proton exchange membrane fuel cells. General Chem. 2020, 6, 200016. [Google Scholar] [CrossRef]
- Cheng, H.; Gui, R.; Liu, S.; Xie, Y.; Wu, C. Local structure engineering for active sites in fuel cell electrocatalysts. Sci. China Chem. 2020, 63, 1543–1556. [Google Scholar] [CrossRef]
- Hoang, T.T.H.; Verma, S.; Ma, S.; Fister, T.T.; Timoshenko, J.; Frenkel, A.I.; Kenis, P.J.A.; Gewirth, A.A. Nanoporous copper–silver alloys by additive-controlled electrodeposition for the selective electroreduction of CO2 to ethylene and ethanol. J. Am. Chem. Soc. 2018, 140, 5791–5797. [Google Scholar] [CrossRef]
- Morales-Guio, C.G.; Cave, E.R.; Nitopi, S.A.; Feaster, J.T.; Wang, L.; Kuhl, K.P.; Jackson, A.; Johnson, N.C.; Abram, D.N.; Hatsukade, T.; et al. Improved CO2 reduction activity towards C2+ alcohols on a tandem gold on copper electrocatalyst. Nat. Catal. 2018, 1, 764–771. [Google Scholar] [CrossRef]
- Bagotstky, V.S. Fuel Cells: Problems and Solutions, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Cruz-Martínez, H.; Tellez-Cruz, M.M.; Guerrero-Gutiérrez, O.X.; Ramírez-Herrera, C.A.; Salinas-Juárez, M.G.; Velázquez-Osorio, A.; Solorza-Feria, O. Mexican contributions for the improvement of electrocatalytic properties for the oxygen reduction reaction in PEM fuel cells. Int. J. Hydrogen Energy 2019, 44, 12477–12491. [Google Scholar] [CrossRef]
- O’Hayre, R.; Cha, S.W.; Collela, W.G.; Prinz, F.B. Fuel Cell Fundamentals; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Zhuang, W.; Li, S.; Zhang, X.; Kum, D.; Song, Z.; Yin, G.; Ju, F. A survey of powertrain configuration studies on hybrid electric vehicles. Appl. Energy. 2020, 262, 114553. [Google Scholar] [CrossRef]
- Krithika, V.; Subramani, C. A comprehensive review on choice of hybrid vehicles and power converters, control strategies for hybrid electric vehicles. Int. J. Energy Res. 2018, 42, 1789–1812. [Google Scholar] [CrossRef]
- Zhang, N.; Sutanto, D.; Muttaqi, K.M. A review of topologies of three-port DC–DC converters for the integration of renewable energy and energy storage system. Renew. Sustain. Energy Rev. 2016, 56, 388–401. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Gallard, A.; Hissel, D. A review of DC/DC converter-based electrochemical impedance spectroscopy for fuel cell electric vehicles. Renew. Energy 2019, 141, 124–138. [Google Scholar] [CrossRef]
- Wilberforce, T.; Alaswad, A.; Palumbo, A.; Dassist, M.; Olabi, A. Advances in stationary and portable fuel cell applications. Int. J. Hydrogen Energy 2016, 41, 16509–16522. [Google Scholar] [CrossRef] [Green Version]
- Revankar, S.S.; Majundar, P. Fuel Cells, Principles Design and Analysis; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Wang, Y.; Leung, D.Y.C.; Xuan, J.; Wang, H. A review on unitized regenerative fuel cell technologies, part-A: Unitized regenerative proton exchange membrane fuel cells. Renew. Sustain. Energy Rev. 2016, 65, 961–977. [Google Scholar] [CrossRef]
- Esmaeili, N.; Gray, E.M.A.; Webb, C.J. Non-fluorinated polymer composite proton exchange membranes for fuel cell applications—A review. ChemPhysChem 2019, 20, 2016–2053. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef] [Green Version]
- Mauritz, K.A.; Moore, R.B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535–4585. [Google Scholar] [CrossRef]
- Chae, K.J.; Choi, M.; Ajayi, F.F.; Park, W.; Chang, I.S.; Kim, I.S. Mass transport through a proton exchange membrane (Nafion) in microbial fuel cells. Energy Fuels 2008, 22, 169–176. [Google Scholar] [CrossRef]
- Bakonyi, P.; Koók, L.; Rózsenberszki, T.; Tóth, G.; Bélafi-Bakó, K.; Nemestóthy, N. Development and application of supported ionic liquid membranes in microbial fuel cell technology: A concise overview. Membranes 2020, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Vidakovic-Koch, T.; Gonzalez Martinez, I.; Kuwertz, R.; Kunz, U.; Turek, T.; Sundmacher, K. Electrochemical membrane reactors for sustainable chlorine recycling. Membranes 2012, 2, 510–528. [Google Scholar] [CrossRef] [Green Version]
- Ito, H.; Maeda, T.; Nakano, A.; Takenaka, H. Properties of Nafion membranes under PEM water electrolysis conditions. Int. J. Hydrogen Energy 2011, 36, 10527–10540. [Google Scholar] [CrossRef]
- Sun, C.Y.; Zhang, H. Investigation of Nafion series membranes on the performance of iron-chromium redox flow battery. Int. J. Energy Res. 2019, 43, 8739–8752. [Google Scholar] [CrossRef]
- Casciola, M.; Alberti, G.; Sganappa, M.; Narducci, R. On the decay of Nafion proton conductivity at high temperature and relative humidity. J. Power Sources 2006, 162, 141–145. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, G.; Han, M.; Li, H.; Zhang, Y.; Ni, J.; Ma, W.; Li, M.; Wang, J.; Liu, Z.; et al. Novel epoxy-based cross-linked polybenzimidazole for high temperature proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2011, 162, 8412–8421. [Google Scholar] [CrossRef]
- Carbone, A.; Pedicini, R.; Portale, G.; Longo, A.; D’Ilario, L.; Passalacqua, E. Sulphonated poly(ether ether ketone) membranes for fuel cell application: Thermal and structural characterization. J. Power Sources 2006, 163, 18–26. [Google Scholar] [CrossRef]
- Kaliaguine, S.D.; Mikhailenko, S.D.; Wang, K.P.; Xing, P.; Robertson, G.; Guiver, M. Properties of SPEEK based PEMs for fuel cell application. Catal. Today 2003, 82, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Komarov, P.V.; Veselov, I.N.; Chu, P.P.; Khalatur, P.G. Mesoscale simulation of polymer electrolyte membranes based on sulfonated poly (ether ether ketone) and Nafion. Soft Matter. 2010, 6, 3939–3956. [Google Scholar] [CrossRef]
- Krishnan, P.; Advani, S.G.; Prasad, A.K. A functional monomer to synthesize sulfonated poly(ether ether ketone) with sulfonic acid group in the pendant side chain. J. Appl. Polym. Sci. 2012, 123, 3331–3336. [Google Scholar] [CrossRef]
- Zaidi, S.M.J.; Mikhailenko, S.D.; Robertson, G.P.; Guiver, M.D.; Kaliaguine, S. Proton conducting composite membranes from polyether ether ketone and heteropolyacids for fuel cell applications. J. Membr. Sci. 2000, 173, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Shiino, K.; Otomo, T.; Yamada, T.; Arima, H.; Hiroi, K.; Takata, S.; Miyake, J.; Miyatake, K. Structural investigation of sulfonated polyphenylene ionomers for the design of better performing proton-conductive membranes. ACS Appl. Polym. Mater. 2020, 2, 5558–5565. [Google Scholar] [CrossRef]
- Sorte, E.; Paren, B.; Rodriguez, C.; Fujimoto, C.; Poirier, C.; Abbott, L.; Lynd, N.; Winey, K.; Frischknecht, A.; Alam, T. Impact of hydration and sulfonation on the morphology and ionic conductivity of sulfonated poly(phenylene) proton exchange membranes. Macromolecules 2019, 52, 857–876. [Google Scholar] [CrossRef]
- Adamski, M.; Skalski, T.J.G.; Britton, B.; Peckham, T.; Metzler, L.; Holdcroft, S. Highly stable, low gas crossover, proton-conducting phenylated polyphenylenes. Angew. Chem. Int. Ed. 2017, 129, 9186–9189. [Google Scholar] [CrossRef]
- Escorihuela, J.; Olvera-Mancilla, J.; Alexandrova, L.; del Castillo, L.; Compañ, V. Recent progress in the development of composite membranes based on polybenzimidazole for high temperature proton exchange membrane (PEM) fuel cell applications. Polymers 2020, 12, 1861. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ni, J.; Liu, D.; Gong, C.; Wang, L. Effects of branching structures on the properties of phosphoric acid-doped polybenzimidazole as a membrane material for high-temperature proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2018, 43, 16694–16703. [Google Scholar] [CrossRef]
- Liu, C.; Khan, S.; Lee, M.; Kim, K.; Akhtar, K.; Han, H. Fuel cell based on novel hyper-branched polybenzimidazole membrane. Macromol. Res. 2013, 21, 35–41. [Google Scholar] [CrossRef]
- Ni, J.; Hu, M.; Liu, D.; Xie, H.; Xiang, X.; Wang, L. Synthesis and properties of highly branched polybenzimidazoles as proton exchange membranes for high-temperature fuel cells. J. Mater. Chem. C 2016, 4, 4814–4821. [Google Scholar] [CrossRef]
- Aili, D.; Henkensmeier, D.; Martin, S.; Singh, B.; Hu, Y.; Jensen, J.O.; Cleemann, L.N.; Qingfeng, L. Polybenzimidazole-based high-temperature polymer electrolyte membrane fuel cells: New insights and recent progress. Electrochem. Energy Rev. 2020, 3, 793–845. [Google Scholar] [CrossRef]
- Escorihuela, J.; García-Bernabé, A.; Compañ, V. A deep insight into different acidic additives as doping agents for enhancing proton conductivity on polybenzimidazole membranes. Polymers 2020, 12, 1374. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Scott, K. Development of high-temperature PEMFC based on heteropolyacids and polybenzimidazole. J. Solid State Electrochem. 2010, 14, 213–219. [Google Scholar] [CrossRef]
- Pu, H.; Meyer, W.; Wegner, G. Proton transport in polybenzimidazole blended with H3PO4 or H2SO4. J. Polym. Sci. Part. B Polym. Phys. 2002, 40, 663–669. [Google Scholar] [CrossRef]
- Glipa, X.; Bonnet, B.; Mula, B.; Jones, D.; Roziere, J. Investigation of the conduction properties of phosphoric and sulfuric acid doped polybenzimidazole. J. Mater. Chem. 1999, 9, 3045–3049. [Google Scholar] [CrossRef]
- Xing, B.; Savadogo, O. The effect of acid doping on the conductivity of polybenzimidazole (PBI). J. N. Mater. Electrochem. Syst. 1999, 2, 95–101. [Google Scholar]
- Bouchet, R.; Siebert, E. Proton conduction in acid doped polybenzimidazole. Solid State Ion. 1999, 118, 287–299. [Google Scholar] [CrossRef]
- Fontanella, J.; Wintersgill, M.; Wainright, J.; Savinell, R.; Litt, M. High pressure electrical conductivity studies of acid doped polybenzimidazole. Electrochim. Acta 1998, 43, 1289–1294. [Google Scholar] [CrossRef]
- Dechnik, J.; Gascon, J.; Doonan, C.J.; Janiak, C.; Sumby, C.J. Mixed-matrix membranes. Angew. Chem. Int. Ed. 2017, 56, 9292–9310. [Google Scholar] [CrossRef] [PubMed]
- Sato, S. Graphene for nanoelectronics. Jpn. J. Appl. Phys. 2015, 54, 040102. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Tang, L.; Li, J. Graphene-based materials in electrochemistry. Chem. Soc. Rev. 2010, 39, 3157–3180. [Google Scholar] [CrossRef] [PubMed]
- Machado, B.F.; Serp, P. Graphene-based materials for catalysis. Catal. Sci. Technol. 2012, 2, 54–75. [Google Scholar] [CrossRef]
- Yam, K.M.; Guo, N.; Jiang, Z.; Li, S.; Zhang, C. Graphene-based heterogeneous catalysis: Role of graphene. Catalysts 2020, 10, 53. [Google Scholar] [CrossRef] [Green Version]
- Justino, C.I.; Gomes, A.R.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A. Graphene based sensors and biosensors. TrAC Trends Anal. Chem. 2017, 91, 53–66. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Choma, J.; Jaroniec, M. Gas adsorption properties of graphene-based materials. Adv. Colloid Interface Sci. 2017, 243, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhengjie, L.; Yaqian, D.; Quan-Hong, Y.; Feiyu, K. Graphene-based materials for electrochemical energy storage devices: Opportunities and challenges. Energy Storage Mater. 2016, 2, 107–138. [Google Scholar]
- Tapas, K.; Saswata, B.; Ananta, K.M.; Partha, K.; Nam, H.K.; Joong, H.L. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar]
- Yan, X.H.; Ruizhe, W.; Xu, J.B.; Zhengtang, L.; Zhao, T.S. A monolayer graphene—Nafion sandwich membrane for direct methanol fuel cells. J. Power Sources 2016, 311, 188–194. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhass, K.M.; Zimmey, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.B.T.; Ruoff, S.R. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.C.; Yang, H.N.; Park, S.H.; Kim, W.J. Nafion/graphene oxide composite membranes for low humidifying polymer electrolyte membrane fuel cell. Membr. Sci. 2014, 452, 20–28. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Kim, A.R.; Yoo, D.J. Sulfonated graphene oxide/Nafion composite membranes for high temperature and low humidity proton exchange membrane fuel cells. RSC Adv. 2018, 8, 7494–7508. [Google Scholar] [CrossRef] [Green Version]
- Sahu, A.K.; Ketpang, K.; Shanmugam, S.; Kwon, O.; Lee, S.; Kim, H. Sulfonated graphene–nafion composite membranes for polymer electrolyte fuel cells operating under reduced relative humidity. J. Phys. Chem. C 2016, 120, 15855–15866. [Google Scholar] [CrossRef]
- Cao, N.; Zhou, C.; Wang, Y.; Ju, H.; Tan, D.; Li, J. Synthesis and characterization of sulfonated graphene oxide reinforced sulfonated poly (ether ether ketone) (SPEEK) composites for proton exchange membrane materials. Materials 2018, 11, 516. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Rodriguez, J.L.; Escorihuela, J.; Garcia-Bernabé, A.; Giménez, E.; Solorza-Feria, O.; Compañ, V. Proton conducting electrospun sulfonated polyether ether ketone graphene oxide composite membranes. RSC Adv. 2017, 7, 53481–53491. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Xiao, L.; Jiang, Z.; Tian, X.; Luo, L.; Liu, W.; Xu, Z.; Yang, H.; Jiang, Z. Sulfonic acid functionalized graphene oxide paper sandwiched in sulfonated poly(ether ether ketone): A proton exchange membrane with high performance for semi-passive direct methanol fuel cells. Int. J. Hydrogen Energy 2017, 42, 16731–16740. [Google Scholar] [CrossRef]
- Kumar, R.; Mamlouka, M.; Scott, K. Sulfonated polyether ether ketone—Sulfonated graphene oxide composite membranes for polymer electrolyte fuel cells. RSC Adv. 2014, 4, 617–623. [Google Scholar] [CrossRef]
- Üregen, N.; Pehlivanoğlu, K.; Özdemir, Y.; Devrim, Y. Development of polybenzimidazole/graphene oxide composite membranes for high temperature PEM fuel cells. Int. J. Hydrogen Energy 2017, 42, 2636–2647. [Google Scholar] [CrossRef]
- Yusoff, Y.N.; Loh, K.S.; Wong, W.Y.; Daud, W.; Lee, T.K. Sulfonated graphene oxide as an inorganic filler in promoting the properties of a polybenzimidazole membrane as a high temperature proton exchange membrane. Int. J. Hydrogen Energy 2020, 45, 27510–27526. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.; Ko, T.; Han, J.; Lee, J.C. Polybenzimidazole composite membranes containing imidazole functionalized graphene oxide showing high proton conductivity and improved physicochemical properties. Int. J. Hydrogen Energy 2021, 46, 12254–12262. [Google Scholar] [CrossRef]
- Zadehnazari, A.; Takassi, M.A. Synthesis of modified multi-walled carbon nanotube poly(benzimidazole-imide) composites: Assessment of morphological and thermo-mechanical properties, Compos. Interfaces 2016, 23, 909–924. [Google Scholar] [CrossRef]
- Park, M.J.; Lee, J.K.; Lee, B.S.; Lee, Y.W.; Choi, I.S.; Lee, S. Covalent modification of multiwalled carbon nanotubes with imidazolium-based ionic liquids: Effect of anions on solubility. Chem. Mater. 2006, 18, 1546–1551. [Google Scholar] [CrossRef]
- Guerrero Moreno, N.; Gervasio, D.; Godínez García, A.; Pérez Robles, J.F. Polybenzimidazole-multiwall carbon nanotubes composite membranes for polymer electrolyte membrane fuel cells. J. Power Sources 2015, 300, 229–237. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Baumann, A.E.; Burns, D.A.; Liu, B.; Thoi, V.S. Metal-organic framework functionalization and design strategies for advanced electrochemical energy storage devices. Commun. Chem. 2019, 2, 86. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Li, Z.; Wang, D.; Li, Z.; Peng, Z.X.; Liu, X.C.; Zheng, P. Metal organic frameworks modified proton exchange membranes for fuel cells. Front. Chem. 2020, 8, 694. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Nie, P.; Dou, H.; Ding, B.; Li, L.; Zhang, L.X. Exploring metal organic frameworks for energy storage in batteries and supercapacitors. Mater. Today 2017, 20, 191–209. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, F.; Feng, W.; Zou, X.; Zhao, C.; Na, H.; Liu, C.; Sun, F.; Zhu, G. From metal–organic framework (MOF) to MOF–polymer composite membrane: Enhancement of low-humidity proton conductivity. Chem. Sci. 2013, 4, 983–992. [Google Scholar] [CrossRef]
- Cai, K.; Sun, F.; Liang, X.; Liu, C.; Zhao, N.; Zou, X.; Zhu, G. An acid-stable hexaphosphate ester based metal–organic framework and its polymer composite as proton exchange membrane. J. Mater. Chem. A 2017, 5, 12943–12950. [Google Scholar] [CrossRef]
- Sen, U.; Erkartal, M.; Kung, C.; Ramani, V.; Hupp, J.T.; Farha, O.K. Proton conducting self-assembled metal–organic framework/polyelectrolyte hollow hybrid nanostructures. ACS Appl. Mater. Interfaces 2016, 8, 23015–23021. [Google Scholar] [CrossRef]
- Furukawa, H.; Gándara, F.; Zhang, Y.; Jiang, J.; Queen, W.L.; Hudson, M.R.; Yaghi, O.M. Water adsorption in porous metal–organic frameworks and related materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. [Google Scholar] [CrossRef]
- Luo, H.-B.; Wang, M.; Liu, S.-X.; Xue, C.; Tian, Z.-F.; Zou, Y.; Ren, X.-M. Proton conductance of a superior water-stable metal–organic framework and its composite membrane with poly (vinylidene fluoride). Inorg. Chem. 2017, 56, 4169–4175. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Y.; Li, J.J.; Han, Z.; Duan, P.G.; Li, L.K.; Zang, S.Q. Tuning the functional substituent group and guest of metal–organic frameworks in hybrid membranes for improved interface compatibility and proton conduction. J. Mater. Chem. A 2017, 5, 3464–3474. [Google Scholar] [CrossRef]
- Vega, J.; Andrio, A.; Lemus, A.A.; del Castillo, L.F.; Compañ, V. Conductivity study of Zeolitic Imidazolate Frameworks, Tetrabutylammonium hydroxide doped with Zeolitic Imidazolate Frameworks, and mixed matrix membranes of Polyetherimide/Tetrabutylammonium hydroxide doped with Zeolitic Imidazolate Frameworks for proton. Electrochim. Acta 2017, 258, 153–166. [Google Scholar] [CrossRef]
- Xin, C.; Gang, L. Proton conductive Zr-based MOFs. Inorg. Chem. Front. 2020, 7, 3765–3784. [Google Scholar]
- Ferey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surble, S.; Margiolaki, I. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Chongshan, Y.; Chunqing, H.; Qicheng, L.; Bangyun, X.; Xiaowei, Z.; Libing, Q.; Jingjing, L.; Yawei, Z. Free volume, gas permeation, and proton conductivity in MIL-101-SO 3 H/Nafion composite membranes. Phys. Chem. Chem. Phys. 2019, 21, 25982–25992. [Google Scholar]
- Rao, Z.; Tang, B.B.; Wu, P.Y. Proton conductivity of proton exchange membrane synergistically promoted by different functionalized metal–organic frameworks. ACS Appl. Mater. Interfaces 2017, 9, 22597–22603. [Google Scholar] [CrossRef]
- Donnadio, A.; Narducci, R.; Casciola, M.; Marmottini, F.; D’Amato, R.; Jazestani, M.; Chiniforoshan, H.; Costantino, F. Mixed Membrane matrices based on nafion/UiO-66/SO3H-UiO-66 Nano-MOFs: Revealing the effect of crystal size, sulfonation, and filler loading on the mechanical and conductivity properties. ACS Appl. Mater. Interfaces 2017, 9, 42239–42246. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, G.W.; Zhao, Y.N.; Cao, Y.; Wu, H.; Li, Y.; Jiang, Z. Enhanced proton conductivity of proton exchange membranes by incorporating sulfonated metal-organic frameworks. J. Power Sources 2014, 262, 372–379. [Google Scholar] [CrossRef]
- Zhang, B.; Cao, Y.; Li, Z.; Wu, H.; Yin, Y.H.; Cao, L.; He, X.Y.; Jiang, Z.Y. Proton exchange nanohybrid membranes with high phosphotungstic acid loading within metal-organic frameworks for PEMFC applications. Electrochim. Acta 2017, 240, 186–194. [Google Scholar] [CrossRef]
- Sun, H.; Tang, B.; Wu, P. Development of hybrid ultrafiltration membranes with improved water separation properties using modified superhydrophilic metal-organic framework nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 21473–21484. [Google Scholar] [CrossRef]
- Da Trindade, L.G.; Borba, K.M.; Zanchet, L.; Lima, D.W.; Trench, A.B.; Rey, F.; Martini, E.M. SPEEK-based proton exchange membranes modified with MOF-encapsulated ionic liquid. Mater. Chem. Phys. 2019, 236, 121792. [Google Scholar] [CrossRef]
- Ahmadian-Alam, L.; Mahdavi, H. A novel polysulfone-based ternary nanocomposite membrane consisting of metal-organic framework and silica nanoparticles: As proton exchange membrane for polymer electrolyte fuel cells. Renew. Energy 2018, 126, 630–639. [Google Scholar] [CrossRef] [Green Version]
- Ru, C.; Li, Z.; Zhao, C.; Duan, Y.; Zhuang, Z.; Bu, F.; Na, H. Enhanced proton conductivity of sulfonated hybrid poly(arylene ether ketone) membranes by incorporating an amino–sulfo bifunctionalized metal–organic framework for direct methanol fuel cells. ACS Appl. Mater. Interfaces 2018, 10, 7963–7973. [Google Scholar] [CrossRef] [PubMed]
- Escorihuela, J.; Sahuquillo, Ó.; García-Bernabé, A.; Giménez, E.; Compañ, V. Phosphoric acid doped polybenzimidazole (pbi)/zeolitic imidazolate framework composite membranes with significantly enhanced proton conductivity under low humidity conditions. Nanomaterials 2018, 8, 775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subhabrata, M.; Anupam, D.; Tushar, J.; Samar, K. Fabricating a MOF Material with Polybenzimidazole into an efficient proton exchange membrane. ACS Appl. Energy Mater. 2020, 3, 7964–7977. [Google Scholar]
- Sun, H.Z.; Tang, B.B.; Wu, P.Y. Two-dimensional zeolitic imidazolate framework/carbon nanotube hybrid networks modified proton exchange membranes for improving transport properties. ACS Appl. Mater. Interfaces 2017, 9, 35075–35085. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Yang, F.; Zhou, L.L.; Yin, B.; Wang, P.; Wang, L. Achieving high power density and excellent durability for high temperature proton exchange membrane fuel cells based on crosslinked branched polybenzimidazole and metal-organic frameworks. J. Membr. Sci. 2021, 630, 119288. [Google Scholar] [CrossRef]
- Sandip, K.S.; Anthony, W.S. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar]
- Earle, M.J.; Seddon, K.R. Ionic liquids: Green solvents for the future. In Clean Solvents Alternative Media for Chemical Reactions and Processing; Abraham, M.A., Moens, L., Eds.; Clean Solvents; American Chemical Society: Washington, DC, USA, 2002. [Google Scholar] [CrossRef] [Green Version]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Zhang, J.; Huang, C.; Lei, Z. Ionic liquids in selective oxidation: Catalysts and solvents. Chem. Rev. 2017, 117, 6929–6983. [Google Scholar] [CrossRef] [PubMed]
- González, L.; Escorihuela, J.; Altava, B.; Burguete, M.I.; Luis, S. Chiral room temperature ionic liquids as enantioselective promoters for the asymmetric aldol reaction. Eur. Org. Chem. 2014, 24, 5356–5363. [Google Scholar] [CrossRef]
- González-Mendoza, L.; Escorihuela, J.; Altava, B.; Burguete, M.I.; Luis, S.V. Application of optically active chiral bis-(imidazolium) salts as potential receptors of chiral dicarboxylate salts of biological relevance. Org. Biomol. Chem. 2015, 13, 5450–5459. [Google Scholar] [CrossRef] [PubMed]
- Valls, A.; Altava, B.; Burguete, M.I.; Escorihuela, J.; Martí-Centelles, V.; Luis, S.V. Supramolecularly assisted synthesis of chiral tripodal imidazolium compounds. Org. Chem. Front. 2019, 6, 1214–1225. [Google Scholar] [CrossRef]
- Marrucho, I.M.; Branco, L.; Rebelo, L. Ionic liquids in pharmaceutical applications. Chem. Biomol. Eng. 2014, 5, 527–546. [Google Scholar] [CrossRef]
- González-Mendoza, L.; Altava, B.; Burguete, M.I.; Escorihuela, J.; Hernando, E.; Luis, S.; Vicent, C.; Quesada, R. Bis(imidazolium) salts derived from amino acids as receptors and transport agents for chloride anions. RSC Adv. 2015, 5, 34415–34423. [Google Scholar] [CrossRef] [Green Version]
- Noshadi, I.; Walker, B.; Portillo-Lara, R.; Shirzaei Sani, E.; Gomes, N.; Aziziyan, M.R.; Annabi, N. Engineering biodegradable and biocompatible bio-ionic liquid conjugated hydrogels with tunable conductivity and mechanical properties. Sci. Rep. 2017, 7, 4345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, S.; Haumann, M.; Wasserscheid, P. Ionic liquids in chemical engineering. Ann. Rev. Chem. Biomol. Eng. 2010, 1, 203–230. [Google Scholar] [CrossRef]
- Opallo, M.; Lesniewski, A. A review on electrodes modified with ionic liquids. J. Electroanal. Chem. 2011, 656, 2–16. [Google Scholar] [CrossRef]
- Shiddiky, M.J.; Torriero, A.A. Application of ionic liquids in electrochemical sensing systems. Biosens. Bioelectron. 2011, 26, 1775–1787. [Google Scholar] [CrossRef]
- Chen, D.; Ying, W.; Guo, Y.; Ying, Y.; Peng, X. Enhanced gas separation through nanoconfined ionic liquid in laminated MoS2 membrane. ACS Appl. Mater. Interfaces 2017, 9, 44251–44257. [Google Scholar] [CrossRef] [PubMed]
- Pedersen-Bjergaard, S.; Rasmussen, K. Liquid-liquid-liquid microextraction for sample preparation of biological fluids prior to capillary electrophoresis. Anal. Chem. 1999, 71, 2650–2656. [Google Scholar] [CrossRef]
- Liu, J.F.; Jiang, G.B.; Chi, Y.G.; Cai, Y.Q.; Zhou, Q.X.; Hu, J.T. Use of ionic liquids for liquid-phase microextraction of polycyclic aromatic hydrocarbons. Anal. Chem. 2003, 75, 5870–5876. [Google Scholar] [CrossRef] [PubMed]
- Farnoush, F.; Atefeh, S. Ionic liquids based polymeric membrane drug sensors. Curr. Anal. Chem. 2017, 13, 52–61. [Google Scholar]
- Liu, H.; Liu, Y.; Li, J. Ionic liquids in surface electrochemistry. Phys. Chem. Chem. Phys. 2010, 12, 1685–1697. [Google Scholar] [CrossRef] [PubMed]
- Masayoshi, W.; Morgan, L.T.; Shiguo, Z.; Kazuhide, U.; Tomohiro, Y.; Kaoru, D. Application of ionic liquids to energy storage and conversion materials and devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar]
- Doyle, M.; Choi, S.; Proulx, G. High-temperature proton conducting membranes based on perfluorinated ionomer membrane-ionic liquid composites. J. Electrochem. Soc. 2000, 147, 34–37. [Google Scholar] [CrossRef]
- Schäfer, T.; Di Paolo, R.E.; Franco, R.; Cresp, J.G. Elucidating interactions of ionic liquids with polymer films using confocal Raman spectroscopy. Chem. Commun. 2005, 20, 2594–2596. [Google Scholar]
- Yang, J.; Che, Q.; Zhou, L.; He, R.; Savinell, R.F. Studies of a high temperature proton exchange membrane based on incorporating an ionic liquid cation 1-butyl-3-methylimidazolium into a Nafion matrix. Electrochim. Acta 2011, 56, 5940–5946. [Google Scholar] [CrossRef]
- Tigelaar, D.M.; Waldecker, J.R.; Peplowski, K.M. Study of the incorporation of protic ionic liquids into hydrophilic and hydrophobic rigid-rod elastomeric polymers. Polymer 2006, 47, 4269–4275. [Google Scholar] [CrossRef]
- Schmidt, C.; Glück, T.; Schmidt-Naake, G. Modification of Nafion Membranes by Impregnation with Ionic Liquids. Chem. Eng. Technol. 2008, 31, 13–22. [Google Scholar] [CrossRef]
- Mondal, A.N.; Tripathi, B.P.; Shahi, V.K. Highly stable aprotic ionic-liquid doped anhydrous proton-conducting polymer electrolyte membrane for high-temperature applications. J. Mater. Chem. 2011, 21, 4117–4124. [Google Scholar] [CrossRef]
- Che, Q.; Yue, J. Polymerized imidazolium ionic liquids crosslinking sulfonated poly (ether ether ketone) (SPEEK) for high-temperature proton exchange membrane. RSC Adv. 2016, 6, 111729–111738. [Google Scholar] [CrossRef]
- Stumphauser, T.; Kasza, G.; Domjá, A.; Wacha, A.; Varga, Z.; Thomann, Y.; Thomann, R.; Pásztói, B.; Trötschler, T.M.; Kerscher, B.; et al. Nanoconfined crosslinked poly(ionic liquid)s with unprecedented selective swell. Polymers 2020, 12, 2292. [Google Scholar] [CrossRef] [PubMed]
- Mališ, J.; Mazúr, P.; Schauer, J.; Paidar, M.; Bouzek, K. Polymer-supported 1-butyl-3-methylimidazolium trifluoromethanesulfonate and 1-ethylimidazolium trifluoromethanesulfonate as electrolytes for the high temperature PEM-type fuel cell. Int. J. Hydrogen Energy 2013, 38, 4697–4704. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, W.; Wang, J.; Zhang, T.; Shi, B.; Liu, J.; Cao, S. Enhanced anhydrous proton conductivity of polymer electrolyte membrane enabled by facile ionic liquid-based hoping pathways. J. Membr. Sci. 2015, 476, 136–147. [Google Scholar] [CrossRef]
- Wang, X.Y.; Jin, M.S.; Li, X.; Zhao, L.H. The influence of various ionic liquids on the properties of SPEEK membrane doped with mesoporous silica. Electrochim. Acta. 2017, 257, 290–300. [Google Scholar] [CrossRef]
- Elwan, H.A.; Mamlouk, M.; Scott, K. A review of proton exchange membranes based on protic ionic liquid/polymer blends for polymer electrolyte membrane fuel cells. J. Power Sources 2020, 484, 229197. [Google Scholar] [CrossRef]
- Wang, J.T.W.; Hsu, S.L.C. Enhanced high-temperature polymer electrolyte membrane for fuel cells based on polybenzimidazole and ionic liquids. Electrochim. Acta. 2011, 56, 2842–2846. [Google Scholar] [CrossRef]
- Van de Ven, E.; Chairuna, A.; Merle, G.; Pacheco Benito, S.; Borneman, S.; Nijmeijer, K. Ionic liquid doped polybenzimidazole membranes for high temperature proton exchange membrane fuel cell applications. J. Power Sources 2013, 222, 202–209. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, L.; Wang, P.; Zhang, F.; Yu, S.; Shao, S.Z.; Yi, B. Ionic-liquid-based proton conducting membranes for anhydrous H2/Cl2 fuel-cell applications. ACS Appl. Mater. Interfaces 2014, 6, 3195–3200. [Google Scholar] [CrossRef] [PubMed]
- Escorihuela, J.; García-Bernabé, A.; Montero, A.; Sahuquillo, O.; Giménez, E.; Compañ, V. Ionic liquid composite polybenzimidazol membranes for high temperature PEMFC applications. Polymers 2019, 11, 732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compañ, V.; Escorihuela, J.; Olvera, J.; García-Bernabé, A.; Andrio, A. Influence of the anion on diffusivity and mobility of ionic liquids composite polybenzimidazol membranes. Electrochim. Acta 2020, 354, 136666. [Google Scholar] [CrossRef]
- Song, X.; Ding, L.; Wang, L.; He, M.; Han, X. Polybenzimidazole membranes embedded with ionic liquids for use in high proton selectivity vanadium redox flow batteries. Electrochim Acta 2019, 295, 1034–1043. [Google Scholar] [CrossRef]
- Lin, J.; Korte, C. PBI-type polymers and acidic proton conducting ionic liquids–conductivity and molecular interactions. Fuel Cells 2020, 20, 461–468. [Google Scholar] [CrossRef]
- Ye, H.; Huang, J.; Xu, J.; Kodiweera, N.; Jayakody, J.R.P.; Greenbaum, S.G. New membranes based on ionic liquids for PEM fuel cells at elevated temperatures. J. Power Sources 2008, 178, 651–660. [Google Scholar] [CrossRef]
- Hyun, J.K.; Hyun, J.K.; Yong, G.S.; Hak, S.H. Nafion–Nafion/polyvinylidene fluoride–Nafion laminated polymer membrane for direct methanol fuel cells. J. Power Sources 2004, 135, 66–71. [Google Scholar]
- Ki-Yun, C.; Ho-Young, J.; Kyung, A.S.; Wan-Keun, K.; Shi-Joon, S.; Jung-Ki, P. Preparation and charateristics of Nafion membrane coated with a PVdF copolymer/recast Nafion blend for direct methanol fuel cell. J. Power Sources 2006, 159, 524–528. [Google Scholar]
- Min-Kyu, S.; Young-Taek, K.; James, M.; Russell, K.; HeeWoo, R. Chemically-modified Nafionl/poly (vinylidene fluoride) blend ionomers for proton exchange membrane fuel cells. J. Power Sources 2003, 117, 14–21. [Google Scholar]
- Sood, R.; Cavaliere, S.; Jones, D.J.; Rozière, J. Electrospun nanofibre composite polymer electrolyte fuel cell and electrolysis membranes. Nano Energy 2016, 26, 729–745. [Google Scholar] [CrossRef] [Green Version]
- Parashuram, K.; Numan, Y.; Heechul, C. Nanofiber-based proton exchange membranes: Development of aligned electrospun nanofibers for polymer electrolyte fuel cell applications. ACS Sustain. Chem. Eng. 2019, 7, 1808–1825. [Google Scholar]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Takemori, R.; Ito, G.; Tanaka, M.; Kawakami, H. Ultra-high proton conduction in electrospun sulfonated polyimide nanofibers. RSC Adv. 2014, 4, 20005–20009. [Google Scholar] [CrossRef]
- Ballengee, J.B.; Pintauro, P.N. Composite fuel cell membranes from dual-nanofiber electrospun mats. Macromolecules 2011, 44, 7307–7314. [Google Scholar] [CrossRef]
- Dong, B.; Gwee, L.; Salas-de la Cruz, D.; Winey, K.I.; Yossef, A.; Elabd, Y.A. Super proton conductive high-purity Nafion nanofibers. Nano Lett. 2010, 10, 3785–3790. [Google Scholar] [CrossRef]
- Rozière, J.; Jones, D. Non-fluorinated polymer materials for proton exchange membrane fuel cells. Annu. Rev. Mater. Res. 2003, 33, 503–555. [Google Scholar] [CrossRef]
- Jiang, R.; Kunz, H.; Fenton, J. Investigation of membrane property and fuel cell behavior with sulfonated poly(ether ether ketone) electrolyte: Temperature and relative humidity effects. J. Power Sources 2005, 150, 120–128. [Google Scholar] [CrossRef]
- Do, K.N.T.; Kim, D. Comparison of homogeneously and heterogeneously sulfonated polyetheretherketone membranes in preparation, properties and cell performance. J. Power Sources 2008, 185, 63–69. [Google Scholar] [CrossRef]
- Wu, H.-L.; Ma, C.-C.M.; Liu, F.-Y.; Chen, C.-Y.; Lee, S.-J.; Chiang, C.-L. Preparation and characterization of poly(ether sulfone)/sulfonated poly(ether ether ketone) blend membranes. Eur. Polym. J. 2006, 42, 1688–1695. [Google Scholar] [CrossRef]
- Manea, C.; Mulder, M. Characterization of polymer blends of polyethersulfone/sulfonated polysulfone and polyethersulfone/sulfonated polyetheretherketone for direct methanol fuel cell applications. J. Membr. Sci. 2002, 206, 443–453. [Google Scholar] [CrossRef]
- Wilhelm, F.G.; Pünt, I.G.M.; van der Vegt, N.; Strathmann, H.; Wessling, M. Cation permeable membranes from blends of sulfonated poly (ether ether ketone) and poly (ether sulfone). J. Membrane Sci. 2002, 199, 167–176. [Google Scholar] [CrossRef]
- Tsai, J.-C.; Cheng, H.-P.; Kuo, J.F.; Huang, Y.H.; Chen, C.Y. Blended Nafion®/SPEEK direct methanol fuel cell membranes for reduced methanol permeability. J. Power Sources 2009, 189, 958–965. [Google Scholar] [CrossRef]
- Wei, G.; Xua, L.; Huang, C.; Wang, Y. SPE water electrolysis with SPEEK/PES blend membrane. Int. J. Hydrogen Energy 2010, 35, 7778–7783. [Google Scholar] [CrossRef]
- Mikhailenko, S.D.; Wang, K.; Kaliaguine, S.; Xing, P.; Robertson, G.P.; Guiver, M.D. Proton conducting membranes based on cross-linked sulfonated poly (ether ether ketone)(SPEEK). J. Membrane Sci. 2004, 233, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Zhong, S.; Fu, T.; Dou, Z.; Zhao, C.; Na, H. Preparation and evaluation of a proton exchange membrane based on crosslinkable sulfonated poly (ether ether ketone) s. J. Power Sources 2006, 162, 51–57. [Google Scholar] [CrossRef]
- Mikhailenko, S.D.; Robertson, G.P.; Guiver, M.D.; Kaliaguine, S. Properties of PEMs based on cross-linked sulfonated poly (ether ether ketone). J. Membr. Sci. 2006, 285, 306–316. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Gogel, V.; Friedrich, K.; Kerres, J. Novel covalently cross-linked poly (etheretherketone) ionomer membranes. J. Power Sources 2006, 155, 3–12. [Google Scholar] [CrossRef]
- Yang, T. Preliminary study of SPEEK/PVA blend membranes for DMFC applications. Int. J. Hydrogen Energy 2008, 33, 6772–6779. [Google Scholar] [CrossRef]
- Li, H.; Zhang, G.; Wu, J.; Zhao, C.; Zhang, Y.; Shao, K.; Han, M.; Lin, H.; Zhu, J.; Na, H. A novel sulfonated poly (ether ether ketone) and cross-linked membranes for fuel cells. J. Power Sources. 2010, 195, 6443–6449. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, G.; Xu, D.; Zhao, C.; Ma, W.; Li, H.; Zhang, Y.; Xu, S.; Jiang, H.; Sun, H.; et al. Cross-linked membranes based on sulfonated poly (ether ether ketone)(SPEEK)/Nafion for direct methanol fuel cells (DMFCs). Int. J. Hydrogen Energy 2011, 36, 11025–11033. [Google Scholar] [CrossRef]
- Zhu, Y.; Zieren, S.; Manthiram, A. Novel crosslinked membranes based on sulfonated poly (ether ether ketone) for direct methanol fuel cells. Chem. Commun. 2011, 47, 7410–7412. [Google Scholar] [CrossRef] [PubMed]
- Kerres, J.A. Development of ionomer membranes for fuel cells. J. Membr. Sci. 2001, 185, 3–27. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Z.; Bi, D.; Lin, H.; Shao, K.; Fu, T.; Zhong, S.; Na, H. Blend membranes based on disulfonated poly (aryl ether ether ketone) s (SPEEK) and poly (amide imide)(PAI) for direct methanol fuel cell usages. Polymer 2007, 48, 3090–3097. [Google Scholar] [CrossRef]
- Chen, J.; Maekawa, Y.; Asano, M.; Yoshida, M. Double crosslinked polyetheretherketone-based polymer electrolyte membranes prepared by radiation and thermal crosslinking techniques. Polymer 2007, 48, 6002–6009. [Google Scholar] [CrossRef]
- Silva, V.; Mendes, A.; Madeira, L.; Nunes, S. Pre-treatment effect on the transport properties of sulfonated poly (ether ether ketone) membranes for DMFC applications. Desalination 2006, 200, 645–647. [Google Scholar] [CrossRef]
- Robertson, G.P.; Mikhailenko, S.D.; Wang, K.; Xing, P.; Guiver, M.D.; Kaliaguine, S. Casting solvent interactions with sulfonated poly (ether ether ketone) during proton exchange membrane fabrication. J. Membr. Sci. 2003, 219, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Guan, R.; Dai, H.; Li, C.; Liu, J.; Xu, J. Effect of casting solvent on the morphology and performance of sulfonated polyethersulfone membranes. Membr. Sci. 2006, 277, 148–156. [Google Scholar] [CrossRef]
- Yu, D.M.; Yoon, S.; Kim, T.H.; Lee, J.Y.; Lee, J.; Hong, Y.T. Properties of sulfonated poly (arylene ether sulfone)/electrospun nonwoven polyacrylonitrile composite membrane for proton exchange membrane fuel cells. J. Membr. Sci. 2013, 446, 212–219. [Google Scholar] [CrossRef]
- Lee, C.; Jo, S.M.; Choi, J.; Baek, K.-Y.; Truong, Y.B.; Kyratzis, I.L.; Shul, Y.-G. SiO 2/sulfonated poly ether ether ketone (SPEEK) composite nanofiber mat supported proton exchange membranes for fuel cells. J. Mater. Sci. 2013, 48, 3665–3671. [Google Scholar] [CrossRef]
- Li, H.Y.; Liu, Y.L. Polyelectrolyte composite membranes of polybenzimidazole and crosslinked polybenzimidazole-polybenzoxazine electrospun nanofibers for proton exchange membrane fuel cells. J. Mater. Chem. A 2013, 1, 1171–1178. [Google Scholar] [CrossRef]
- Muthuraja, P.; Prakash, S.; Manisankar, P. Stable nanofibrous poly (aryl sulfone ether benzimidazole) membrane with high conductivity for high temperature PEM fuel cells. Solid State Ion. 2018, 317, 201–209. [Google Scholar] [CrossRef]
- Escorihuela, J.; García-Bernabé, A.; Montero, A.; Andrio, A.; Sahuquillo, O.; Giménez, E.; Compañ, V. Proton conductivity through polybenzimidazole composite membranes containing silica nanofiber mats. Polymers 2019, 11, 1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escorihuela, J.; Bañuls, M.J.; García-Castelló, J.; Toccafondo, V.; García-Rupérez, J.; Puchades, R.; Maquieira, A. Chemical silicon surface modification and bioreceptor attachment to develop competitive integrated photonic biosensors. Anal. Bioanal. Chem. 2012, 404, 2831–2840. [Google Scholar] [CrossRef]
- Bañuls, M.J.; Jiménez-Meneses, P.; Meyer, A.; Vasseur, J.; Morvan, F.; Escorihuela, J.; Puchades, R.; Maquieira, Á. Improved performance of DNA microarray multiplex hybridization using probes anchored at several points by Thiol-Ene or Thiol-Yne click chemistry. Bioconjugate Chem. 2017, 28, 496–506. [Google Scholar] [CrossRef]
- Escorihuela, J.; Bañus, M.; Puchades, R.; Maquieira, A. Site-specific immobilization of DNA on silicon surfaces by using the thiol–yne reaction. J. Mater. Chem. B. 2014, 2, 8510–8517. [Google Scholar] [CrossRef] [PubMed]
- Escorihuela, J.; Bañus, M.; Grijalvo, S.; Eritja, R.; Puchades, R.; Maquieira, A. Direct covalent attachment of DNA microarrays by rapid thiol−ene “Click” chemistry. Bioconjugate Chem. 2014, 25, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Escorihuela, J.; Pujari, S.P.; Zuilhof, H. Organic monolayers by B(C6F5)3-catalyzed siloxanation of oxidized silicon surfaces. Langmuir 2017, 33, 2185–2193. [Google Scholar] [CrossRef] [Green Version]
- Escorihuela, J.; Zuilhof, H. Rapid surface functionalization of hydrogen-terminated silicon by alkyl silanols. J. Am. Chem. Soc. 2017, 139, 5870–5876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.G.; Kang, Y.; Huang, J. Fluidized electrocatalysis. CCS Chem. 2020, 2, 31–41. [Google Scholar] [CrossRef]
- Perez, A.E.; Ribadeneira, R. Modeling with DFT and chemical descriptors approach for the development of catalytic alloys for PEMFCsi. In Density Functional Theory; Glossman-Mitnik, D., Ed.; Intechopen Limited: London, UK, 2019; pp. 33–54. [Google Scholar] [CrossRef] [Green Version]
- Prithi, J.A.; Shanmugam, R.; Rao, R.G.; Rajalakshmi, N. Experimental and theoretical study on SO2 tolerance of Pt electrocatalysts: Role of carbon support. Electroanalysis 2020, 32, 2555–2563. [Google Scholar] [CrossRef]
- Lee, J.M.; Han, H.; Jin, S.; Choi, S.M.; Kim, H.J.; Seo, M.H.; Kim, W.B. A review on recent progress in the aspect of stability of oxygen reduction electrocatalysts for proton-exchange membrane fuel cell: Quantum mechanics. Energy Technol. 2019, 7, 1900312. [Google Scholar] [CrossRef]
- Zhang, L.L.; Li, L.; Chen, H.; Wei, Z. Recent progress in precious metal-free carbon-based materials towards the oxygen reduction reaction: Activity, stability, and anti-poisoning. Chem. Eur. J. 2020, 26, 3973–3990. [Google Scholar] [CrossRef]
- Liu, W.; Li, M.; Jiang, G.; Li, G.; Zhu, J.; Xiao, M.; Zhu, Y.; Gao, R.; Yu, A.; Feng, M.; et al. Graphene quantum dots-based advanced electrode materials: Design, synthesis and their applications in electrochemical energy storage and electrocatalysis. Chen. Adv. Energy Mater. 2020, 10, 2001275. [Google Scholar] [CrossRef]
- Wang, J.; Xu, H.; Huo, Y.; Wang, Y.; Dong, M. Progress of electrospray and electrospinning in energy applications. Nanotechnology 2020, 31, 132001. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yin, Y.; Yang, D.; Zhang, C.; Ming, P.; Li, B. Efficient synthesis of Pt–Co nanowires as cathode catalysts for proton exchange membrane fuel cells. RSC Adv. 2020, 10, 6287–6296. [Google Scholar] [CrossRef] [Green Version]
- Shao, M.; Chang, Q.; Dodelet, J.P.; Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, X.; Lu, X.F.; Xia, B.Y.; Lou, X.W.D. Advanced electrocatalysts for the oxygen reduction reaction in energy conversion technologies. Joule 2020, 4, 45–68. [Google Scholar] [CrossRef]
- Wang, X.X.; Swihart, M.T.; Wu, G. Non-aqueous solution synthesis of Pt-based nanostructures for fuel cell catalysts. Nat. Catal. 2019, 2, 578–589. [Google Scholar] [CrossRef]
- Lang, P.; Yuan, N.; Jiang, Q.; Zhang, Y.; Tang, J. Recent advances and prospects of metal-based catalysts for oxygen reduction reaction. Energy Technol. 2020, 8, 1900984. [Google Scholar] [CrossRef]
- Gilroy, K.D.; Ruditskiy, A.; Peng, H.C.; Qin, D.; Xia, Y. Bimetallic nanocrystals: Syntheses, properties, and applications. Chem. Rev. 2016, 116, 10414–10472. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Jiang, H.; Bao, X.; Zou, S.; Liao, J.; Zhang, Z. Recent progress of Pt-based catalysts for oxygen reduction reaction in preparation strategies and catalytic mechanism. J. Electroanal. Chem. 2019, 848, 113279. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, C.; Liu, Z.; Huang, J.; Wu, M.; Liu, H.; Huang, Y. Pt-based nanocrystal for electrocatalytic oxygen reduction. Adv. Mater. 2019, 31, 1808115. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Cano, Z.P.; Yu, A.; Chen, Z.; Jiang, G.; Fu, X.; Lu, J. Enhancing oxygen reduction activity of pt-based electrocatalysts: From theoretical mechanisms to practical methods. Angew. Chem. Int. Ed. 2020, 59, 18334–18348. [Google Scholar] [CrossRef] [PubMed]
- Kitchin, J.R.; Nørskov, J.K.; Barteau, M.A.; Chen, J.G. Role of strain and ligand effects in the modification of the electronic and chemical properties of bimetallic surfaces. Phys. Rev. Lett. 2004, 93, 156801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Shao, Q.; Huang, X. Amorphous oxide nanostructures for advanced electrocatalysis. Chem. Eur. J. 2020, 26, 3943–3960. [Google Scholar] [CrossRef]
- Esfahani, R.A.M.; Vankova, S.K.; Easton, E.B.; Ebralidze, I.I.; Specchia, S. A hybrid Pt/NbO/CNTs catalyst with high activity and durability for oxygen reduction reaction in PEMFC. Renew. Energy 2020, 154, 913–924. [Google Scholar]
- Nechiyil, D.; Garapati, M.S.; Shende, R.C.; Joulié, S.; Neumeyer, D.; Bacsa, R.; Puecha, P.; Ramaprabhub, S.; Bacsa, W. Optimizing metal-support interphase for efficient fuel cell oxygen reduction reaction catalyst. J. Colloid Interface Sci. 2020, 561, 439–448. [Google Scholar] [CrossRef]
- Park, C.; Lee, E.; Lee, G.; Tak, Y. Superior durability and stability of Pt electrocatalyst on N-doped graphene-TiO2 hybrid material for oxygen reduction reaction and polymer electrolyte membrane fuel cells. Appl. Catal. B Environ. 2020, 268, 118414. [Google Scholar] [CrossRef]
- Ham, K.; Chung, S.; Lee, J. Narrow size distribution of Pt nanoparticles covered by an S-doped carbon layer for an improved oxygen reduction reaction in fuel cells. J. Power Sources 2020, 450, 227650. [Google Scholar] [CrossRef]
- Xu, C.; Yang, J.; Liu, E.; Jia, Q.; Veith, G.M.; Nair, G.; DiPrieto, S.; Sun, K.; Chen, J.; Pietrasz, P.; et al. Physical vapor deposition process for engineering Pt based oxy. J. Power Sources 2020, 451, 227709. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, X.; Yu, Y.; Chen, L.; Cheng, T.; Huang, J.; Wang, Y.; Liu, Y.; Harada, M.; Ishihara, A.; et al. Indium oxide supported Pt–In alloy nanocluster catalysts with enhanced catalytic performance toward oxygen reduction. J. Power Sources 2020, 446, 227332. [Google Scholar] [CrossRef]
- Fortunato, G.V.; Cardoso, E.S.; Martini, B.K.; Maia, G. Ti/Pt−Pd-based nanocomposite: Effects of metal oxides on the oxygen reduction reaction. ChemElectroChem 2020, 7, 1610–1618. [Google Scholar] [CrossRef]
- Wei, L.; Shi, J.; Cheng, G.; Lu, L.; Xu, H.; Li, Y. Pt/TiN–TiO2 catalyst preparation and its performance in oxygen reduction reaction. J. Power Sources 2020, 454, 227934. [Google Scholar] [CrossRef]
- Yin, S.; Xie, Z.; Deng, X.; Xuan, W.; Duan, Y.; Zhang, S.; Liang, Y. Simple synthesis of ordered platinum-gold nanoparticles with the enhanced catalytic activity for oxygen reduction reaction. J. Electroanal. Chem. 2020, 856, 113707. [Google Scholar] [CrossRef]
- Wang, Q.; Tao, H.; Li, Z.; Wang, G. Effect of iron precursor on the activity and stability of PtFe/C catalyst for oxygen reduction reaction. J. Alloys Compd. 2020, 814, 152212. [Google Scholar] [CrossRef]
- Reyes-Rodríguez, J.L.; Velázquez-Osorio, A.; Bahena-Uribe, D.; Soto-Guzmán, A.B.; Leyva, M.A.; Rodríguez-Castellanos, A.; Citalán-Cigarroa, S.; Solorza-Feria, O. Tailoring the morphology of Ni–Pt nanocatalysts through the variation of oleylamine and oleic acid: A study on oxygen reduction from synthesis to fuel cell application. Catal. Sci. Technol. 2019, 9, 2630–2650. [Google Scholar] [CrossRef]
- Wu, D.; Shen, X.; Zhou, L.Q.; Nagai, T.; Pan, Y.; Yao, L.; Zulevi, B.; Lubers, A.; Jia, H.; Peng, Z. A vacuum impregnation method for synthesizing octahedral Pt2CuNi nanoparticles on mesoporous carbon support and the oxygen reduction reaction electrocatalytic properties. J. Colloid Interface Sci. 2020, 564, 245–253. [Google Scholar] [CrossRef]
- Dionigi, F.; Weber, C.C.; Primbs, M.; Gocyla, M.; Bonastre, A.; Spöri, C.; Schmies, H.; Hornberger, E.; Drnec, S.K.J.; Heggen, M.; et al. Controlling near-surface Ni composition in octahedral PtNi (Mo) nanoparticles by Mo doping for a highly active oxygen reduction reaction catalyst. Nano Lett. 2019, 19, 6876–6885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nan, H.; Su, Y.Q.; Tang, C.; Cao, R.; Li, D.; Yu, J.; Liu, Q.; Deng, Y.; Tian, X. Engineering the electronic and strained interface for high activity of PdMcore@ Ptmonolayer electrocatalysts for oxygen reduction reaction. Sci. Bull. 2020, 65, 1396–1404. [Google Scholar] [CrossRef]
- Liu, S.; Li, Z.; Wang, C.; Tao, W.; Huang, M.; Zuo, M.; Tang, Y.; Yang, K.; Zhang, L.; Chen, S.; et al. Turning main-group element magnesium into a highly active electrocatalyst for oxygen reduction reaction. Nat. Commun. 2020, 11, 938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, C.; Duan, X.; Huang, Y. Molecular design of single-atom catalysts for oxygen reduction reac molecular design of single-atom catalysts for oxygen reduction reactiontion. Adv. Energy Mater. 2020, 10, 1903815. [Google Scholar] [CrossRef]
- Huang, X.; Shen, T.; Zhang, T.; Qiu, H.; Gu, X.; Ali, Z.; Hou, Y. Efficient oxygen reduction catalysts of porous carbon nanostructures decorated with transition metal species. Adv. Energy Mater. 2020, 10, 1900375. [Google Scholar] [CrossRef]
- Xiao, M.; Zhu, J.; Li, G.; Li, N.; Li, S.; Cano, Z.P.; Ma, L.; Cui, P.; Xu, P.; Jiang, G.; et al. A single-atom iridium heterogeneous catalyst in oxygen reduction reaction. Angew. Chem. Int. Ed. 2019, 58, 9640–9645. [Google Scholar] [CrossRef]
- Qiao, M.; Wang, Y.; Wang, Q.; Hu, G.; Mamat, X.; Zhang, S.; Wang, S. Hierarchically ordered porous carbon with atomically dispersed FeN4 for ultraefficient oxygen reduction reaction in proton-exchange membrane fuel cells. Angew. Chem. Int. Ed. 2020, 59, 2688–2694. [Google Scholar] [CrossRef]
- Venegas, R.; Muñoz-Becerra, K.; Candia-Onfray, C.; Marco, J.F.; Zagal, J.H.; Recio, F. Experimental reactivity descriptors of MNC catalysts for the oxygen reduction reaction. Electrochim. Acta. 2020, 332, 13534. [Google Scholar] [CrossRef]
- García, Á.; Retuerto, M.; Dominguez, C.; Pascual, L.; Ferrer, P.; Gianolio, D.; Serrano, A.; Aßmann, P.; Sanchez, D.; Peña, M.; et al. Fe doped porous triazine as efficient electrocatalysts for the oxygen reduction reaction in acid electrolyte. Appl. Catal. B Environ. 2020, 264, 118507. [Google Scholar] [CrossRef]
- Wei, X.; Luo, X.; Wang, H.; Gu, W.; Cai, W.; Lin, Y.; Zhu, C. Highly-defective Fe-NC catalysts towards pH-universal oxygen reduction reaction. Appl. Catal. B Environ. 2020, 263, 118347. [Google Scholar] [CrossRef]
- Wang, X.; Fang, J.; Liu, X.; Zhang, X.; Lv, Q.; Xu, Z.; Zhang, X.; Zhu, W.; Zhuang, Z. Converting biomass into efficient oxygen reduction reaction catalysts for proton exchange membrane fuel cells. Sci. China Mater. 2020, 63, 524–532. [Google Scholar] [CrossRef] [Green Version]
- Chong, L.; Wen, J.; Kubal, J.; Sen, F.G.; Zou, J.; Greeley, J.; Chan, M.; Barkholtz, H.; Ding, W.; Liu, D.J. Ultralow-loading platinum-cobalt fuel cell catalysts derived from imidazolate frameworks. Science 2018, 362, 1276–1281. [Google Scholar] [CrossRef]
- Lu, F.; Xia, B.Y.; Zang, S.Q.; Lou, X.W. Metal–organic frameworks based electrocatalysts for the oxygen reduction reaction. Angew. Chem. Int. 2020, 59, 4634–4650. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Nayak, A.K.; Choi, H.; Ali, G.; Kwon, J.; Choi, S.; Paik, U.; Song, T. Partial dehydration in hydrated tungsten oxide nanoplates leads to excellent and robust bifunctional oxygen reduction and hydrogen evolution reactions in acidic media. ACS Sustain. Chem. Eng. 2020, 8, 9507–9518. [Google Scholar] [CrossRef]
- Chisaka, M. Creation of oxygen reduction reaction active sites on titanium oxynitride without increasing the nitrogen doping level. Phys. Chem. Chem. Phys. 2018, 20, 15613–15617. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Luo, J.; Nan, H.; Fu, Z.; Zeng, J.; Liao, S.J. Binary transition metal nitrides with enhanced activity and durability for the oxygen reduction reaction. Mater. Chem. A 2015, 3, 16801–16809. [Google Scholar] [CrossRef]
- Sun, T.; Wu, Q.; Che, R.; Bu, Y.; Jiang, Y.; Li, Y.; Wang, X.; Hu, Z. Alloyed Co–Mo nitride as high-performance electrocatalyst for oxygen reduction in acidic medium. ACS Catal. 2015, 5, 1857–1862. [Google Scholar] [CrossRef]
- Cao, B.; Neuefeind, J.; Adzic, R.; Khalifah, P. Molybdenum nitrides as oxygen reduction reaction catalysts: Structural and electrochemical studies. Inorg. Chem. 2015, 54, 2128–2136. [Google Scholar] [CrossRef]
- Tian, X.L.; Wang, L.; Chi, B.; Xu, Y.; Zaman, S.; Qi, K.; Liu, H.; Liao, S.; Xia, B.Y. Formation of a tubular assembly by ultrathin Ti0.8Co0.2N nanosheets as efficient oxygen reduction electrocatalysts for hydrogen–/Metal–Air F. ACS Catal. 2018, 8, 8970–8975. [Google Scholar] [CrossRef]
- Durst, J.; Simon, C.; Hasché, F.; Gasteiger, H.A. Hydrogen oxidation and evolution reaction kinetics on carbon supported Pt, Ir, Rh, and Pd electrocatalysts in acidic media. J. Electrochem. Soc. 2014, 162, F190. [Google Scholar] [CrossRef]
- Rebollar, L.; Intikhab, S.; Snyder, J.D.; Tang, M.H. Kinetic isotope effects quantify pH-sensitive water dynamics at the Pt electrode interface. J. Phys. Chem. Lett. 2020, 11, 2308–2313. [Google Scholar] [CrossRef] [PubMed]
- Elbert, K.; Hu, J.; Ma, Z.; Zhang, Y.; Chen, G.; An, W.; Liu, P.; Isaacs, H.; Wang, J.X. Elucidating hydrogen oxidation/evolution kinetics in base and acid by enhanced activities at the optimized Pt shell thickness on the Ru core. ACS Catal. 2015, 5, 6764–6772. [Google Scholar] [CrossRef]
- Sheng, W.; Zhuang, Z.; Gao, M.; Zheng, J.; Chen, J.G.; Yan, Y. Correlating hydrogen oxidation and evolution activity on platinum at different pH with measured hydrogen binding energy. Nat. Commun. 2015, 6, 5848. [Google Scholar] [CrossRef]
- Marković, N.M.; Grgur, B.N.; Ross, P.N. Temperature-dependent hydrogen electrochemistry on platinum low-index single-crystal surfaces in acid solutions. J. Phys. Chem. B 1997, 101, 5405–5413. [Google Scholar] [CrossRef]
- Zalitis, C.M.; Kucernak, A.R.; Sharman, J.; Wright, E. Design principles for platinum nanoparticles catalysing electrochemical hydrogen evolution and oxidation reactions: Edges are much more active than facets. J. Mater. Chem. A 2017, 5, 23328–23338. [Google Scholar] [CrossRef] [Green Version]
- Liao, W.; Yau, S. Au (111)-supported Pt monolayer as the most active electrocatalyst toward hydrogen oxidation and evolution reactions in sulfuric acid. J. Phys. Chem. C 2017, 121, 19218–19225. [Google Scholar] [CrossRef]
- Xie, T.W.H.; Chen, M.; D’Aloia, A.; Cho, J.; Wu, G.; Li, Q. Precious metal-free approach to hydrogen electrocatalysis for energy conversion: From mechanism understanding to catalyst design. Nano Energy 2017, 42, 69–89. [Google Scholar]
- Yang, Y.; Luo, M.; Zhang, W.; Sun, Y.; Chen, X.; Guo, S. Metal surface and interface energy electrocatalysis: Fundamentals, performance engineering, and opportunities. Chem 2018, 4, 2054–2083. [Google Scholar] [CrossRef] [Green Version]
- Gasteiger, H.A.; Markovic, N.M.; Ross, P.N., Jr. Electrooxidation of CO and H2/CO mixtures on a well-characterized Pt3Sn electrode surface. J. Phys. Chem. 1995, 99, 8945–8949. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Markovic, N.M.; Ross, P.N., Jr. H2 and CO electrooxidation on well-characterized Pt, Ru, and Pt-Ru. 1. Rotating disk electrode studies of the pure gases including temperature effects. J. Phys. Chem. 1995, 99, 8290–8301. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Markovic, N.M.; Ross, P.N., Jr. H2 and CO electrooxidation on well-characterized Pt, Ru, and Pt-Ru. 2. rotating disk electrode studies of CO/H2 mixtures at 62. degree. C. J. Phys. Chem. 1995, 99, 16757–16767. [Google Scholar] [CrossRef]
- Lee, S.J.; Mukerjee, S.; Ticianelli, E.A.; McBreen, J. Electrocatalysis of CO tolerance in hydrogen oxidation reaction in PEM fuel cells. Electrochim. Acta. 1995, 44, 3283–3293. [Google Scholar] [CrossRef]
- Innocente, A.F.; Angelo, A.C.D. Electrocatalysis of oxidation of hydrogen on platinum ordered intermetallic phases: Kinetic and mechanistic studies. J. Power Sources 2006, 162, 151–159. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, O.H.; Park, I.S.; Cho, Y.H.; Sung, Y.E. Excellent performances of modified ruos bimetallic materials as anode catalysts for polymer electrolyte membrane fuel cells. Electrocatalysis 2018, 9, 352–358. [Google Scholar] [CrossRef]
- Hasannaeimi, V.; Mukherjee, S. Noble-metal based metallic glasses as highly catalytic materials for hydrogen oxidation reaction in fuel cells. Sci. Rep. 2019, 9, 12136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stühmeier, B.M.; Selve, S.; Patel, M.U.; Geppert, T.N.; Gasteiger, H.A.; El-Sayed, H.A. Highly Selective Pt/TiOx catalysts for the hydrogen oxidation reaction. ACS Appl. Energy Mater. 2019, 2, 5534–5539. [Google Scholar] [CrossRef]
- Hassan, A.; Paganin, V.A.; Ticianelli, E.A. Pt modified tungsten carbide as anode electrocatalyst for hydrogen oxidation in proton exchange membrane fuel cell: CO tolerance and stability. Appl. Catal. B Environ. 2015, 165, 611–619. [Google Scholar] [CrossRef]
- Tzorbatzoglou, F.; Brouzgou, A.; Jing, S.; Wang, Y.; Song, S.; Tsiakaras, P. Oxygen reduction and hydrogen oxidation reaction on novel carbon supported PdxIry electrocatalysts. Int. J. Hydrogen Energy 2018, 43, 11766–11777. [Google Scholar] [CrossRef]
- Kucernak, A.R.; Fahy, K.F.; Sundaram, V.N. Facile synthesis of palladium phosphide electrocatalysts and their activity for the hydrogen oxidation, hydrogen evolutions, oxygen reduction and formic acid oxidation reactions. Catal. Today. 2016, 262, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Liao, J.; Ding, W.; Tao, S.; Nie, Y.; Li, W.; Wu, G.; Chen, S.; Li, L.; Wei, Z. Carbon supported IrM (M= Fe, Ni, Co) alloy nanoparticles for the catalysis of hydrogen oxidation in acidic and alkaline medium. Chinese J. Catal. 2016, 37, 1142–1148. [Google Scholar] [CrossRef]
- Ghosh, A.; Chandran, P.; Ramaprabhu, S. Palladium-nitrogen coordinated cobalt alloy towards hydrogen oxidation and oxygen reduction reactions with high catalytic activity in renewable energy generations of proton exchange membrane fuel cell. Appl. Energy. 2017, 208, 37–48. [Google Scholar] [CrossRef]
- Chandran, P.; Ramaprabhu, S. Catalytic performance of non-platinum-based hybrid carbon hetero-structure for oxygen reduction and hydrogen oxidation reactions in proton exchange membrane fuel cell. Int. J. Hydrog. Energy. 2018, 43, 18477–18487. [Google Scholar] [CrossRef]
- Pu, Z.; Liu, T.; Zhao, W.; Shi, X.; Liu, Y.; Zhang, G.; Hu, W.; Sun, S.; Liao, S. Versatile Route To Fabricate Precious-Metal Phosphide Electrocatalyst for Acid-Stable Hydrogen Oxidation and Evolution Reactions. ACS Appl. Mater. Interfaces. 2020, 12, 11737–11744. [Google Scholar] [CrossRef]
- Kundu, M.K.; Mishra, R.; Bhowmik, T.; Barman, S. Rhodium metal–rhodium oxide (Rh–Rh2O3) nanostructures with Pt-like or better activity towards hydrogen evolution and oxidation reactions (HER, HOR) in acid and base: Correlating its HOR/HER activity with hydrogen binding energy and oxophilicity of the catalyst. J. Mater. Chem. A. 2018, 6, 23531–23541. [Google Scholar]
- Mittermeier, T.; Madkikar, P.; Wang, X.; Gasteiger, H.A.; Piana, M. Probing transition-metal silicides as PGM-free catalysts for hydrogen oxidation and evolution in acidic medium. Materials 2017, 10, 661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.; Kwon, Y.; Kwon, S.; Seo, J.G. Substrate effect of platinum-decorated carbon on enhanced hydrogen oxidation in PEMFC. ACS Omega 2020, 5, 26902–26907. [Google Scholar] [CrossRef]
- Xiong, B.; Zhao, W.; Chen, L.; Shi, J. One-step synthesis of W2C@N, P-C nanocatalysts for efficient hydrogen electrooxidation across the whole pH range. Adv. Funct. Mater. 2019, 29, 1902505. [Google Scholar] [CrossRef]
- Zeng, H.; Chen, S.; Jin, Y.Q.; Li, J.; Song, J.; Le, Z.; Liang, G.; Zhang, H.; Xie, F.; Chen, J.; et al. Electron density modulation of metallic MoO2 by Ni doping to produce excellent hydrogen evolution and oxidation activities in acid. ACS Energy Lett. 2020, 5, 1908–1915. [Google Scholar] [CrossRef]
- Liu, E.; Jiao, L.; Li, J.; Stracensky, T.; Sun, Q.; Mukerjee, S.; Jia, Q. Interfacial water shuffling the intermediates of hydrogen oxidation and evolution reactions in aqueous media. Energy Environ. Sci. 2020, 13, 3064–3074. [Google Scholar] [CrossRef]
- Gentil, S.; Lalaoui, N.; Dutta, A.; Nedellec, Y.; Cosnier, S.; Shaw, W.J.; Artero, V.; Le Goff, A. Carbon-nanotube-supported bio-inspired nickel catalyst and its integration in hybrid hydrogen/air fuel cells. Angew. Chem. 2017, 129, 1871–1875. [Google Scholar] [CrossRef]
- Reuillard, B.; Blanco, M.; Calvillo, L.; Coutard, N.; Ghedjatti, A.; Chenevier, P.; Agnoli, S.; Otyepka, M.; Granozzi, G.; Artero, V. Noncovalent integration of a bioinspired Ni catalyst to graphene acid for reversible electrocatalytic hydrogen oxidation. ACS Appl. Mater. Interfaces 2020, 12, 5805–5811. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Wang, D.; Zhang, Y.; Tao, L.; Wang, T.; Zou, Y.; Wang, Y.; Chen, R.; Wang, S. Defect engineering for fuel-cell electrocatalysts. Adv. Mater. 2020, 43, 1907879. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Rui, L.; Dandan, S.; Yu, Z. Gram-scale synthesis of well-dispersed shape-controlled Pt− Ni/C as high-performance catalysts for the oxygen reduction reaction. ACS Appl. Mater. Interfaces 2019, 11, 29689–29697. [Google Scholar]
- Talukdar, K.; Gazdzicki, P.; Friedrich, K. Comparative investigation into the performance and durability of long and short side chain ionomers in polymer electrolyte membrane fuel cells. J. Power Sources 2019, 439, 227078. [Google Scholar] [CrossRef]
- Colin, B. Manufacturing of Low Cost, Durable Membrane Electrode Assemblies Engineered for Rapid Conditioning; WL Gore & Associates Inc.: Newark, DE, USA, 2017. [Google Scholar]
- Staehler, M.; Staehler, A.; Scheepers, F.; Carmo, M.; Stolten, D. A completely slot die coated membrane electrode assembly. Int. J. Hydrogen Energy 2019, 44, 7053–7058. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, S.; Kim, O.-H.; Ahn, C.-Y.; Kim, M.-J.; Kang, S.Y.; Jeon, T.I.; Shim, J.-G.; Lee, D.; Lee, J.H.; et al. Ultra-low loading of IrO2 with an inverse-opal structure in a polymer-exchange membrane water electrolysis. Nano Energy 2019, 58, 158–166. [Google Scholar] [CrossRef]
- Marinoiu, A.; Andrulevicius, M.; Tamuleviciene, A.; Tamulevicius, T.; Carcadea, E.; Raceanu, M.; Varla, M. High performance catalytic system with enhanced durability in PEM fuel cell. Int. J. Hydrogen Energy 2020, 45, 10409–10422. [Google Scholar] [CrossRef]
- Wang, G.; Zou, L.; Huang, Q.; Zou, Z.; Yang, H. Multidimensional nanostructured membrane electrode assemblies for proton exchange membrane fuel cell applications. Mater. Chem. A Mater. Energy Sustain. 2019, 7, 9447–9477. [Google Scholar] [CrossRef]
- Wang, M.; Park, J.H.; Kabir, S.; Neyerlin, K.C.; Kariuki, N.N.; Lv, H.; Stamenkovic, V.R.; Myers, D.; Ulsh, M.; Mauger, S.A. Impact of catalyst ink dispersing methodology on fuel cell performance using in-situ x-ray scattering. ACS Appl. Energy Mater. 2019, 2, 6417–6427. [Google Scholar] [CrossRef]
- Liu, M.; Wang, L.; Zhao, K.; Shi, S.; Shao, Q.; Zhang, L.; Sun, X.; Zhao, Y.; Zhang, J. Atomically dispersed metal catalysts for the oxygen reduction reaction: Synthesis, characterization, reaction mechanisms. Energy Environ. Sci. 2019, 12, 2890–2923. [Google Scholar] [CrossRef]
- Mauger, S.; Neyerlin, K.; Alia, S.; Ngo, C.; Babu, S.; Hurst, S.K.E.; Pylypenko, S.; Litster, S.; Pivovar, B. Fuel cell performance implications of membrane electrode assembly fabrication with platinum-nickel nanowire catalysts. J. Electrochem. Soc. 2018, 165, F238–F245. [Google Scholar] [CrossRef]
- Millington, B.; Whipple, V.; Pollet, B. A novel method for preparing proton exchange membrane fuel cell electrodes by the ultrasonic-spray technique. J. Power Sources 2011, 196, 8500–8508. [Google Scholar] [CrossRef]
- Ban, H.J.; Kim, M.; Kim, D.; Lim, J.; Kim, T.; Jeong, C.; Kim, Y.-A.; Kim, H.-S. Electrochemical characteristics of solid polymer electrode fabricated with low IrO2 loading for water electrolysis. J. Electrochem. Sci. Technol. 2019, 10, 22–28. [Google Scholar]
- Orfanidi, A.; Rheinlaender, P.; Schulte, N.; Gasteiger, H. Ink solvent dependence of the ionomer distribution in the catalyst layer of a PEMFC. J. Electrochem. Soc. 2018, 165, F1254. [Google Scholar] [CrossRef]
- Banham, D.; Ye, S. Current status and future development of catalyst materials and catalyst layers for proton exchange membrane fuel cells: An industrial perspective. ACS Energy Lett. 2017, 2, 629–638. [Google Scholar] [CrossRef]
- Moghadari, M.R.; Shojaeefard, M.H.; Molaeimanesh, G.R. Impact of carbon paper anisotropy on water droplet movement through the electrodes of proton-exchange membrane fuel cells. Energy Fuels 2020, 34, 10039–10049. [Google Scholar] [CrossRef]
- Talukdar, K.; Helmly, S.; Schulze, M.; Sanchez, D.G.; Handl, M.; Hiesgen, R.; Kraut, J.; Friedrich, K.A. Enveloping of catalyst powder by ionomer for dry spray coating in polymer electrolyte membrane fuel cells. J. Power Sources 2019, 424, 82–90. [Google Scholar] [CrossRef]
- Jabbari, Z.; Nassernejad, B.; Fallah, N.; Javanbakht, M.; Afsham, N. Fabrication of novel binderless anode via electrophoretic deposition for HT-PEMFC. Surf. Eng. 2019, 35, 1013–1020. [Google Scholar] [CrossRef]
- Hwang, C.K.; Kim, J.; Hwang, S.; Kim, J.-H.; Sung, C.-H.; Moon, B.-M.; Chae, K.; Singh, J.; Jitend, J.P.; Kim, S.-H.; et al. Porous strained Pt nanostructured thin-film electrocatalysts via dealloying for PEM fuel cells. Adv. Mater. Interfaces 2020, 7, 1901326. [Google Scholar] [CrossRef]
- Sui, P.-C.; Zhu, X.; Djilali, N. Modeling of PEM fuel cell catalyst layers: Status and outlook. Electrochem. Energy Rev. 2019, 2, 428–466. [Google Scholar] [CrossRef]
- Hbilate, Z.; Naimi, Y.; Takky, D. Modelling operation of proton exchange membrane fuel cells—A brief review of current status. Mater. Today: Proc. 2019, 13, 13889–13898. [Google Scholar] [CrossRef]
- Yi, P.; Zhang, D.; Qiu, D.; Peng, L.; Lai, X. Carbon-based coatings for metallic bipolar plates used in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2019, 44, 6813–6843. [Google Scholar] [CrossRef]
- Stein, T.; Ein-Eli, Y. Challenges and perspectives of metal-based proton exchange membrane’s bipolar plates: Exploring durability and longevity. Energy Technol. 2020, 8, 2000007. [Google Scholar] [CrossRef]
- Bott-Neto, J.; Asset, T.; Maillard, F.; Dubau, L.; Ahmad, Y.; Guerin, K.; Berthon-Fabry, S.; Mosdale, A.; Mosdale, R.; Ticianelli, E.A.; et al. Utilization of graphitized and fluorinated carbon as platinum nanoparticles supports for application in proton exchange membrane fuel cell cathodes. J. Power Sources 2018, 404, 28–38. [Google Scholar] [CrossRef]
- Koray Celik, M.; Oeztuerk, A.; Selim Coegenli, M.; Yurtcan, A.B. Evaluation of low and high surface area TiO₂ and Al₂O₃ metal oxides-carbon hybrids in terms of polymer electrolyte membrane fuel cell catalyst support. J. Nanosci. Nanotechnol. 2020, 20, 1189–1208. [Google Scholar] [CrossRef]
- Liu, S.; Li, S.; Wang, R.; Rao, Y.; Zhong, Q.; Hong, K.; Pan, M. Preparation of high performance and ultra-low platinum loading membrane electrode assembly for PEMFC commercial application. J. Electrochem. Soc. 2019, 166, F1308–F1313. [Google Scholar] [CrossRef]
- Godinez-Salomon, F.; Rhodes, C.P.; Suarez Alcantara, K.; Zhu, Q.; Canton, S.E.; Calderon, H.A.; Reyes-Rodríguez, J.L.; Leyva, M.A.; Solorza-Feria, O. Tuning the oxygen reduction activity and stability of Ni (OH) 2@ Pt/C catalysts through controlling Pt surface composition, strain, and electronic structure. Electrochim. Acta. 2017, 247, 958–969. [Google Scholar] [CrossRef]
- Sapkota, P.; Boyer, C.; Dutta, R.; Cazorla, C.; Aguey-Zinsou, K.F. Planar polymer electrolyte membrane fuel cells: Powering portable devices from hydrogen. Sustain. Energy Fuels 2020, 4, 439–468. [Google Scholar] [CrossRef]
- Chen, J.; Yan, X.; Fu, C.; Feng, Y.; Lin, C.; Li, X.; Shen, S.; Ke, C.; Zhang, J. Insight into the rapid degradation behavior of nonprecious metal fe–n–c electrocatalyst-based proton exchange membrane fuel cells. ACS Appl. Mater. Interfaces 2019, 11, 37779–37786. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Yang, Y. Graphitic carbon nitride for electrochemical energy conversion and storage. ACS Energy Lett. 2018, 3, 2796–2815. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, G.-L.; Sun, C.-J.; Hwang, I.; Xu, M.; Wu, H.-W.; Wei, Z.; Pan, X.; Amine, K.; Shao, M. Durable hybrid electrocatalysts for proton exchange membrane fuel cells. Nano Energy 2020, 77, 105192. [Google Scholar] [CrossRef]
- Song, Z.; Norouzi Banis, M.; Liu, H.; Zhang, L.; Zhao, Y.; Li, J.; Doyle-Davis, K.; Li, R.; Knights, S.; Ye, S.; et al. Ultralow loading and high-performing pt catalyst for a polymer electrolyte membrane fuel cell anode achieved by atomic layer deposition. ACS Catal. 2019, 9, 5365–5374. [Google Scholar] [CrossRef]
- Zheng, H.B.; An, L.; Zheng, Y.; Qu, C.; Fang, Y.; Liu, Q.; Dang, D. Tuning the catalytic activity of Ir@ Pt nanoparticles through controlling Ir core size on cathode performance for PEM fuel cell application. Front. Chem. 2018, 6, 299. [Google Scholar] [CrossRef] [PubMed]
- Devrim, Y.; Arica, E. Multi-walled carbon nanotubes decorated by platinum catalyst for high temperature PEM fuel cell. Int. J. Hydrogen Energy 2019, 44, 18951–18966. [Google Scholar] [CrossRef]
- Novikova, K.; Kuriganova, A.; Leontyev, I.; Gerasimova, E.; Maslova, O.; Rakhmatullin, A.; Smirnova, N.; Dobrovolsky, Y. Influence of carbon support on catalytic layer performance of proton exchange membrane fuel cells. Electrocatalysis 2018, 9, 22–30. [Google Scholar] [CrossRef]
- Yamamoto, O.; Takeda, Y.; Kanno, R.; Noda, M. Perovskite-type oxides as oxygen electrodes for high temperature oxide fuel cells. Solid State Ion. 1987, 22, 241–246. [Google Scholar] [CrossRef]
- Mohamedazeem, M.; Liu, Y.; Ramakrishna, S. Recent progress of carbon dots and carbon nanotubes applied in oxygen reduction reaction of fuel cell for transportation. Appl. Energy 2020, 257, 114027. [Google Scholar]
- Holst-Olesen, K.; Reda, M.; Hansen, H.A.; Vegge, T.; Arenz, M. Enhanced oxygen reduction activity by selective anion adsorption on non-precious-metal catalysts. ACS Catal. 2018, 8, 7104–7112. [Google Scholar] [CrossRef]
- Chen, Q.; Ding, Y.; Chen, M. Nanoporous metal by dealloying for electrochemical energy conversion and storage. MRS Bull. 2018, 43, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Dai, X.; Zhang, T.; Tan, Q.; Chen, Y.; Asefa, T. Ordered nanoporous nitrogen-and oxygen-codoped carbon nanospheres as electrocatalysts for oxygen-reduction reaction in direct methanol fuel cells. ACS Appl. Nano Mater. 2020, 3, 5139–5148. [Google Scholar] [CrossRef]
- Yang, C.; Tao, S.; Huang, N.; Duan, J.; Makiura, R. Heteroatom-doped carbon electrocatalysts derived from nanoporous two-dimensional covalent organic frameworks for oxygen reduction and hydrogen evolution. ACS Appl. Nano Mater. 2020, 3, 5481–5488. [Google Scholar] [CrossRef]
- Zhang, K.; Kirlikovali, K.O.; Varma, R.; Jin, Z.; Jang, H.; Farha, O.K.; Shokouhimehr, M. Covalent organic frameworks: Emerging organic solid materials for energy and electrochemical applications. ACS Appl. Mater. Interfaces 2020, 12, 27821–27852. [Google Scholar] [CrossRef]
- Yousaf, A.B.; Monnier, J.R.; Weidner, J.; Hassan, M.K.; Zaidi, S.J.; Kasak, P. A precious-metal-free Fe-intercalated carbon nitride porous-network with enhanced activity for the oxygen reduction reaction and methanol-tolerant oxygen reduction reaction. Sustain. Energy Fuels 2020, 4, 5050–5060. [Google Scholar] [CrossRef]
- Diaz-Duran, A.K.; Viva, F.; Roncaroli, F. High durability fuel cell cathodes obtained from cobalt metal organic frameworks. Electrochim. Acta. 2019, 320, 134623. [Google Scholar] [CrossRef]
- Madkikar, P.; Menga, D.; Harzer, G.S.; Mittermeier, T.; Siebel, A.; Wagner, F.E.; Merz, M.; Schuppler, S.; Nagel, P.; Munoz-Garcia, A.; et al. Nanometric Fe-substituted ZrO2 on carbon black as PGM-free ORR catalyst for PEMFCs. J. Electrochem. Soc. 2019, 166, F3032–F3043. [Google Scholar] [CrossRef]
- Song, M.; Song, Y.; Yanhui, S.; Sha, W.; Xu, B.; Guo, J.; Wu, Y. Recent advances in non-precious transition metal/nitrogen-doped carbon for oxygen reduction electrocatalysts in PEMFCs. Catalysts 2020, 10, 141. [Google Scholar] [CrossRef] [Green Version]
- Zhou, N.; Wang, N.; Wu, Z.; Li, L. Probing active sites on metal-free, nitrogen-doped carbons for oxygen electroreduction: A review. Catalysts 2018, 8, 509. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.-X.; Zhu, M.; Zuo, M.; Chu, S.-Q.; Zhang, J.; Wu, Y.; Liang, H.-W.; Feng, X. Identification of catalytic sites for oxygen reduction in metal/nitrogen-doped carbons with encapsulated metal nanoparticles. Angew. Chem. Int. Ed. 2020, 59, 1627–1633. [Google Scholar] [CrossRef]
- Mun, Y.; Lee, S.; Kim, K.; Kim, S.; Lee, S.; Han, J.; Lee, J. Versatile strategy for tuning ORR activity of a single Fe-N4 site by controlling electron-withdrawing/donating properties of a carbon plane. J. Am. Chem. Soc. 2019, 141, 6254–6262. [Google Scholar] [CrossRef]
- Zang, J.; Wang, F.; Cheng, Q.; Wang, G.; Ma, L.; Chen, C.; Yang, L.; Zou, Z.; Xie, D.; Yang, H. Cobalt/zinc dual-sites coordinated with nitrogen in nanofibers enabling efficient and durable oxygen reduction reaction in acidic fuel cells. J. Mater. Chem. A 2020, 8, 3686–3691. [Google Scholar] [CrossRef]
- Zhan, Y.; Zeng, H.; Xie, F.; Zhang, H.; Zhang, W.; Jin, Y.; Zhang, Y.; Chen, J.; Meng, H. Templated growth of Fe/N/C catalyst on hierarchically porous carbon for oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 2019, 431, 31–39. [Google Scholar] [CrossRef]
- Sazali, N.; Norharyati, W.; Salleh, W.; Jamaludin, A.; Razali, M. New perspectives on fuel cell technology: A brief review. Membranes 2020, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Haile, A.S.; Yohannes, W.; Mekonnen, Y. Pyridinic-Type N-doped graphene on cobalt substrate as efficient electrocatalyst for oxygen reduction reaction in acidic solution in fuel cell. RSC Adv. 2020, 10, 27346–27356. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Cheng, C.; Yang, Z.; Hermansson, K. Tuning the ORR activity of Pt-based Ti 2 CO 2 MXenes by varying the atomic cluster size and doping with metals. Nanoscale 2020, 12, 12497–12507. [Google Scholar] [CrossRef]
- Dhali, S.; Karakoti, M.; Pandey, S.; SanthiBhushan, B.; Verma, R.; Ravindra, K.; Srivastava, A.; Verma, R.; Ravindra, K.; Srivastava, A.; et al. Graphene oxide supported Pd-Fe nanohybrid as an efficient electrocatalyst for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2020, 45, 18704–18715. [Google Scholar] [CrossRef]
- Hsieh, S.; Yang, Y.; Feng, S. Characterization of the operational parameters of a H2/air micro PEMFC with different flow fields by impedance spectroscopy. J. Power Sources 2006, 162, 262–270. [Google Scholar] [CrossRef]
- Petrone, R.; Vitagliano, C.; Pera, M.-C.; Chamagne, D.; Sorrentino, M. Characterization of an H2/O2 PEMFC short-stack performance aimed to health-state monitoring and diagnosis. Fuel Cells 2018, 18, 3279–3286. [Google Scholar] [CrossRef]
- Lin, C.; Yan, X.; Wei, G.; Ke, C.; Shen, S.; Zhang, J. Optimization of configurations and cathode operating parameters on liquid-cooled proton exchange membrane fuel cell stacks by orthogonal method. Appl. Energy 2019, 253, 113496. [Google Scholar] [CrossRef]
- Kim, M.; Lee, D. Development of the anode bipolar plate/membrane assembly unit for air breathing PEMFC stack using silicone adhesive bonding. J. Power Sources 2016, 315, 86–95. [Google Scholar] [CrossRef]
- Yan, W.; Zeng, M.; Yang, T.; Chen, C.Y.; Amani, M.; Amani, P. Performance improvement of air-breathing proton exchange membrane fuel cell stacks by thermal management. Int. J. Hydrogen Energy 2020, 45, 22324–22339. [Google Scholar] [CrossRef]
- Abdul Rasheed, R.K.; Chan, S.H.; Hwa, S. Analysis of steady state heating configuration for high-temperature proton exchange membrane fuel cell based on multi-physical numerical modelling. Electrochim. Acta. 2016, 222, 280–292. [Google Scholar] [CrossRef]
- Liu, W.; Peng, Z.; Kim, Z.B.; Gao, B.; Pei, Y. Development of a PEMFC dynamic model and the application to the analysis of fuel cell vehicle performance. Mater. Sci. Eng. 2019, 628, 12006. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Xu, L.; Chang, H.; Tu, Z.; Chan, S. Partial flooding and its effect on the performance of a proton exchange membrane fuel cell. Energy Convers. Manag. 2020, 207, 112537. [Google Scholar] [CrossRef]
- Li, T.; Wang, K.; Wang, J.; Liu, Y.; Han, Y.; Song, J.; Hu, H.; Lin, G.; Liu, Y. Preparation of hierarchical-pore gas diffusion layer for fuel cell. J. Mater. Sci. 2020, 55, 4558–4569. [Google Scholar] [CrossRef]
- Shojaeefard, M.; Molaeimanesh, G.; Moqaddari, M.R. Effects of compression on the removal of water droplet from GDLs of PEM fuel cells. Fuel Cells 2019, 19, 675–684. [Google Scholar] [CrossRef]
- Omrani, R.; Seif, M.S.; Mafinejad, Y.; Paul, B.; Islam, R.; Shabani, B. PEMFC purging at low operating temperatures: An experimental approach. Int. J. Energy Res. 2019, 43, 7496–7507. [Google Scholar] [CrossRef]
- Tardy, E.; Courtois, F.; Chandesris, M.; Poirot-Crouvezier, J.-P.; Morin, A.; Bultel, Y. Investigation of liquid water heterogeneities in large area PEM fuel cells using a pseudo-3D multiphysics model. Int. J. Heat Mass Transf. 2019, 145, 118720. [Google Scholar] [CrossRef]
- Ijaodola, O.; El-Hassan, Z.; Ogungbemi, E.; Khatib, F.; Wilberforce, T.; Thompson, J.; Olabi, A. Energy efficiency improvements by investigating the water flooding management on proton exchange membrane fuel cell (PEMFC). Energy 2019, 179, 246–267. [Google Scholar] [CrossRef]
- Chen, Z.; Shuang, X.; Wei, L.; Haijiang, W. Comprehensive anode parameter study for an open-cathode PEMFC. Energy Fuels 2020, 34, 7582–7590. [Google Scholar]
- Jiang, M.; Zhou, B. Droplet behaviors on inclined surfaces with dynamic contact angle. Int. J. Hydrogen Energy 2019, 45, 29848–29860. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, B.; Jiang, M. Dynamic contact angle effects on gas-liquid behaviors in the cathode of proton exchange membrane fuel cell with stirred tank reactor design. Int. J. Green Energy 2019, 16, 386–400. [Google Scholar] [CrossRef]
- He, P.; Chen, L.; Mu, Y.-T.; Tao, W.-Q. Modeling of the effect of ionomer volume fraction on water management for proton exchange membrane fuel cell. Energy Procedia 2019, 158, 2139–2144. [Google Scholar] [CrossRef]
- Xu, L.; Fang, C.; Cheng, J.H.S.; Li, J.; Ouyang, M.; Lehnert, W. Self-humidification of a polymer electrolyte membrane fuel cell system with cathodic exhaust gas recirculation. J. Electrochem. Energy Convers. Storage 2018, 15, 2021003. [Google Scholar] [CrossRef]
- Jiang, M.; Zhou, B. Numerical study of flow regimes in microchannel with dynamic contact angle. Int. J. Hydrogen Energy 2020, 45, 29782–29790. [Google Scholar] [CrossRef]
- Nanadegani, F.; Lay, E.; Sunden, B. Effects of an MPL on water and thermal management in a PEMFC. Int. J. Energy Res. 2019, 43, 274–296. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Shangguan, X.; Ma, X.; Zhang, J.; Qin, Y. Influence of corner structure of fuel cell serpentine channel on water removal. Int. J. Hydrogen Energy 2020, 45, 29812–29823. [Google Scholar] [CrossRef]
- Zhou, S.; Dhupia, J. Online adaptive water management fault diagnosis of PEMFC based on orthogonal linear discriminant analysis and relevance vector machine. Int. J. Hydrogen Energy 2020, 45, 7005–7014. [Google Scholar] [CrossRef]
- Qin, Y.; Yin, Y.; Jiao, K.; Du, Q. Effects of needle orientation and gas velocity on water transport and removal in a modified PEMFC gas flow channel having a hydrophilic needle. Int. J. Energy Res. 2019, 43, 72538–72549. [Google Scholar] [CrossRef]
- Wang, S.; Qin, Y.; Wu, S.; Shangguan, X.; Zhang, J.; Yin, Y. Numerical and experimental investigation of baffle plate arrangement on proton exchange membrane fuel cell performance. J. Power Sources 2020, 457, 228034. [Google Scholar] [CrossRef]
- Hou, J.; Yang, M.; Zhang, J. Active and passive fuel recirculation for solid oxide and proton exchange membrane fuel cells. Renew. Energy 2020, 155, 1355–1371. [Google Scholar] [CrossRef]
- Mocoteguy, P.; Ludwig, B.; Beretta, D.; Pedersen, T. Study of the impact of water management on the performance of PEMFC commercial stacks by impedance spectroscopy. Int. J. Hydrogen Energy 2020, 45, 16724–16737. [Google Scholar] [CrossRef]
- Mohseninia, A.; Kartouzian, D.; Schlumberger, R.; Markoetter, H.; Wilhelm, F.; Scholta, J.; Manke, I. Enhanced water management in PEMFCs: Perforated catalyst layer and microporous layers. ChemSusChem 2020, 13, 2931–2934. [Google Scholar] [CrossRef]
- Liu, H.C.; Yang, W.M.; Tan, J.; Cheng, L.S. A fin-shaped flow channel enhances water removal performance in a proton exchange membrane fuel cell. Fuel Cells 2019, 19, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.; Park, H.-Y.; Jung, J.; Lee, J.; Park, H.-Y.; Jang, J.H.; Kim, S.M.; Yoo, S.J. Enhanced water management of three-dimensional graphene-Ni foam with patterned wettability in a polymer electrolyte membrane fuel cell. ACS Sustain. Chem. Eng. 2019, 7, 15487–15494. [Google Scholar] [CrossRef]
- Atyabi, S.A.; Afshari, E. Three-dimensional multiphase model of proton exchange membrane fuel cell with honeycomb flow field at the cathode side. J. Clean. Prod. 2019, 214, 738–748. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Karthikeyan, P.; Muthukumar, M.; Magesh Kannan, V.; Thanarajan, T.; Maiyalagan, T.; Hong, C.-W.; Jothi, V.; Yi, S.-C. Adoption of novel porous inserts in the flow channel of pem fuel cell for the mitigation of cathodic flooding. Int. J. Hydrogen Energy 2020, 54, 7863–7872. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.; Shui, J. Effect of catalyst layer hydrophobicity on Fe−N−C proton exchange membrane fuel cells. ChemElectroChem 2020, 7, 1775–1780. [Google Scholar] [CrossRef]
- Ungan, H.; Yurtcan, A.B. PEMFC catalyst layer modification with the addition of different amounts of PDMS polymer in order to improve water management. Int. J. Energy Res. 2019, 43, 5946–5958. [Google Scholar] [CrossRef]
- Ungan, H.; Yurtcan, A.B. Water management improvement in PEM fuel cells via addition of PDMS or APTES polymers to the catalyst layer. J. Chem. 2020, 44, 1227–1243. [Google Scholar] [CrossRef]
- Ozturk, A.; Yurtcan, A.B. Investigation of synergetic effect of PDMS polymer hydrophobicity and polystyrene-silica particles roughness in the content of microporous layer on water management in PEM fuel cell. Appl. Surf. Sci. 2020, 511, 145415. [Google Scholar] [CrossRef]
- Weerathunga, D.; Jayawickrama, S.; Phua, Y.; Nobori, K.; Fujigaya, T. Effect of polytetrafluoroethylene particles in cathode catalyst layer based on carbon nanotube for polymer electrolyte membrane fuel cells. Bull. Chem. Soc. Jpn. 2019, 92, 2038–2042. [Google Scholar] [CrossRef]
- Lu, C.; Shi, F.; Jin, J.; Peng, X. Study on the properties of vertical carbon nanotube films grown on stainless steel bipolar plates. Materials 2019, 12, 899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Han, Y.; Shi, J.; Li, T.; Wang, H.; Wang, X.; Sun, J. ZrC coating modified Ti bipolar plate for proton exchange membrane fuel cell. Fuel Cells 2020, 20, 540–546. [Google Scholar] [CrossRef]
- Simon, C.; Endres, J.; Nefzger-Loders, B.; Wilhelm, F.; Gasteiger, H. Interaction of pore size and hydrophobicity/hydrophilicity for improved oxygen and water transport through microporous layers. J. Electrochem. Soc. 2019, 166, F1022–F1035. [Google Scholar] [CrossRef]
- Mariani, M.; Latorrata, S.; Stampino, S.G.; Dotelli, G. Evaluation of graphene nanoplatelets as a microporous layer material for PEMFC: Performance and durability analysis. Fuel Cells 2019, 19, 685–694. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Li, X.; Jiang, L. Review on design and evaluation of environmental photocatalysts. Prog. Nat. Sci. Mater. Int. 2020, 30, 846–854. [Google Scholar] [CrossRef]
- Chen, L.; Lin, R.; Chen, X.; Hao, Z.; Diao, X.; Froning, D.; Tang, S. Microporous layers with different decorative patterns for polymer electrolyte membrane fuel cells. ACS Appl. Mater. Int. 2020, 12, 24048–24058. [Google Scholar] [CrossRef]
- Malhotra, S.; Ghosh, S. Effect of gas diffusion layer surface wettability gradient on water behavior in a serpentine gas flow channel of proton exchange membrane fuel cell. J. Fluids Eng. 2018, 140, 81302. [Google Scholar] [CrossRef]
- Malhotra, S.; Ghosh, S. Numerical investigation of drop dynamics in presence of wettability gradient inside a serpentine channel of proton exchange membrane fuel cell. Int. J. Energy Res. 2020, 44, 6964–6980. [Google Scholar] [CrossRef]
- Arif, M.; Cheung, S.C.P.; Andrews, J. Influence of hydrophobicity and porosity of the gas diffusion layer on mass transport losses in PEM fuel cells: A simulation study supported by experiments. Energy Fuels 2020, 34, 13010–13022. [Google Scholar] [CrossRef]
- Yong, K.; Ganesan, P.; Kazi, S.; Ramesh, S.; Sandaran, S. Computational modelling of droplet dynamics behaviour in polymer electrolyte membrane fuel cells: A review. J. Electrochem. Sci. Tech. 2019, 10, 345–360. [Google Scholar] [CrossRef]
- Zhao, J.; Shahgaldi, S.; Ozden, A.; Alaefour, I.E.; Li, X.; Hamdullahpur, F. Effect of catalyst deposition on electrode structure, mass transport and performance of polymer electrolyte membrane fuel cells. Appl. Energy 2019, 255, 113802. [Google Scholar] [CrossRef]
- Gómez–Marín, A.M.; Ticianelli, E.A. A reviewed vision of the oxygen reduction reaction mechanism on Pt-based catalysts. Curr. Opin. Electrochem. 2018, 9, 129–136. [Google Scholar] [CrossRef]
- Gómez-Marín, A.M.; Feliu, J.M. Oxygen reduction at platinum electrodes: The interplay between surface and surroundings properties. Curr. Opin. Electrochem. 2018, 9, 166–172. [Google Scholar] [CrossRef]
- Holton, O.; Stevenson, J. The role of platinum in proton exchange membrane fuel cells. Platin. Met. Rev. 2013, 57, 259–271. [Google Scholar] [CrossRef]
- Sandbeck, D.; Inaba, M.; Quinson, J.; Bucher, J.; Zana, A.; Arenz, M.; Cherevko, S. Particle size effect on platinum dissolution: Practical considerations for fuel cells. ACS Appl. Mater. Interfaces 2020, 12, 25718–25727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bai, H.; Yan, W.; Zhang, J.; Wang, H.; Xiang, Y.; Lu, S. Enhancing cell performance and durability of high temperature polymer electrolyte membrane fuel cells by inhibiting the formation of cracks in catalyst layers. J. Electrochem. Soc. 2020, 167, 114501. [Google Scholar] [CrossRef]
- Mongird, K.; Viswanathan, V.; Balducci, P.; Alam, J.; Fotedar, V.; Koritarov, V. An evaluation of energy storage cost and performance characteristics. Energies 2020, 13, 3307. [Google Scholar] [CrossRef]
- Wilson, A.; Kleen, G.; Papageorgopoulos, D.; Ahluwalia, R.; James, B.; Houchins, C.; Huya-Kouad, J. Hydrogen and Fuel Cells Program Record Title: Fuel Cell System Cost-2017. DOE Hydrogen and Fuel Cells Program Record. 2017. Available online: https://www.hydrogen.energy.gov/pdfs/17007_fuel_cell_system_cost_2017.pdf (accessed on 10 January 2021).
- U.S. Department of Energy. Manufacturing Cost Analysis of PEM Fuel Cell Systems for 5- and 10-KW Backup Power Applications. Available online: www.energy.gov/sites/prod/files/2016/12/f34/fcto_cost_analysis_pem_fc_5-10kw_backup_power_0.pdf (accessed on 10 January 2021).
- Qing, L.; Baoguo, W.; Peican, W.; Shuai, L. Hydrogen generation with acid/alkaline amphoteric water electrolysis. Energy Chem. 2019, 38, 162–169. [Google Scholar] [CrossRef]
- Whiston, M.M.; Azevedo, I.L.; Litster, S.; Whitefoot, K.S.; Samaras, C.; Whitacre, J.F. Expert assessments of the cost and expected future performance of proton exchange membrane fuel cells for vehicles. Proc. Natl. Acad. Sci. USA 2019, 116, 4899–4904. [Google Scholar] [CrossRef] [Green Version]
- Miller, E.L.; Thompson, S.; Randolph, K.; Hulvey, Z.; Rustagi, N.; Satyapal, S. US Department of Energy hydrogen and fuel cell technologies perspectives. MRS Bull. 2020, 45, 57–64. [Google Scholar] [CrossRef]
- Argonne National Laboratory. Light Duty Electric Drive Vehicles Monthly Update. Available online: www.anl.gov/es/light-duty-electric-drive-vehicles-monthly-salesupdates (accessed on 10 January 2020).
- Office of Energy Efficiency & Renewable Energy. Fuel Cell Technical Team Roadmap. Available online: www.vehicles.energy.gov/about/partnerships/usdrive.html (accessed on 10 November 2020).
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z.; et al. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef]
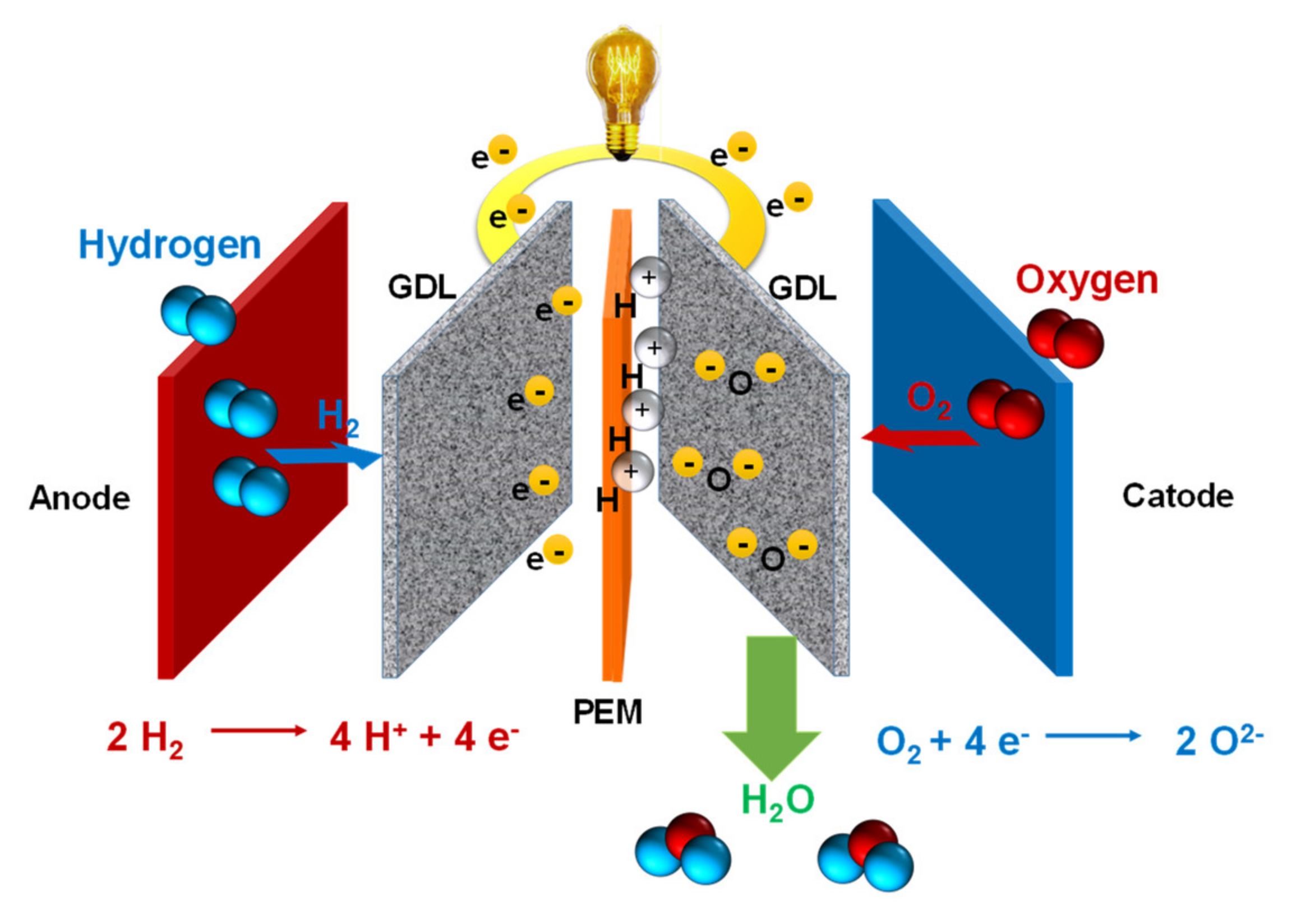
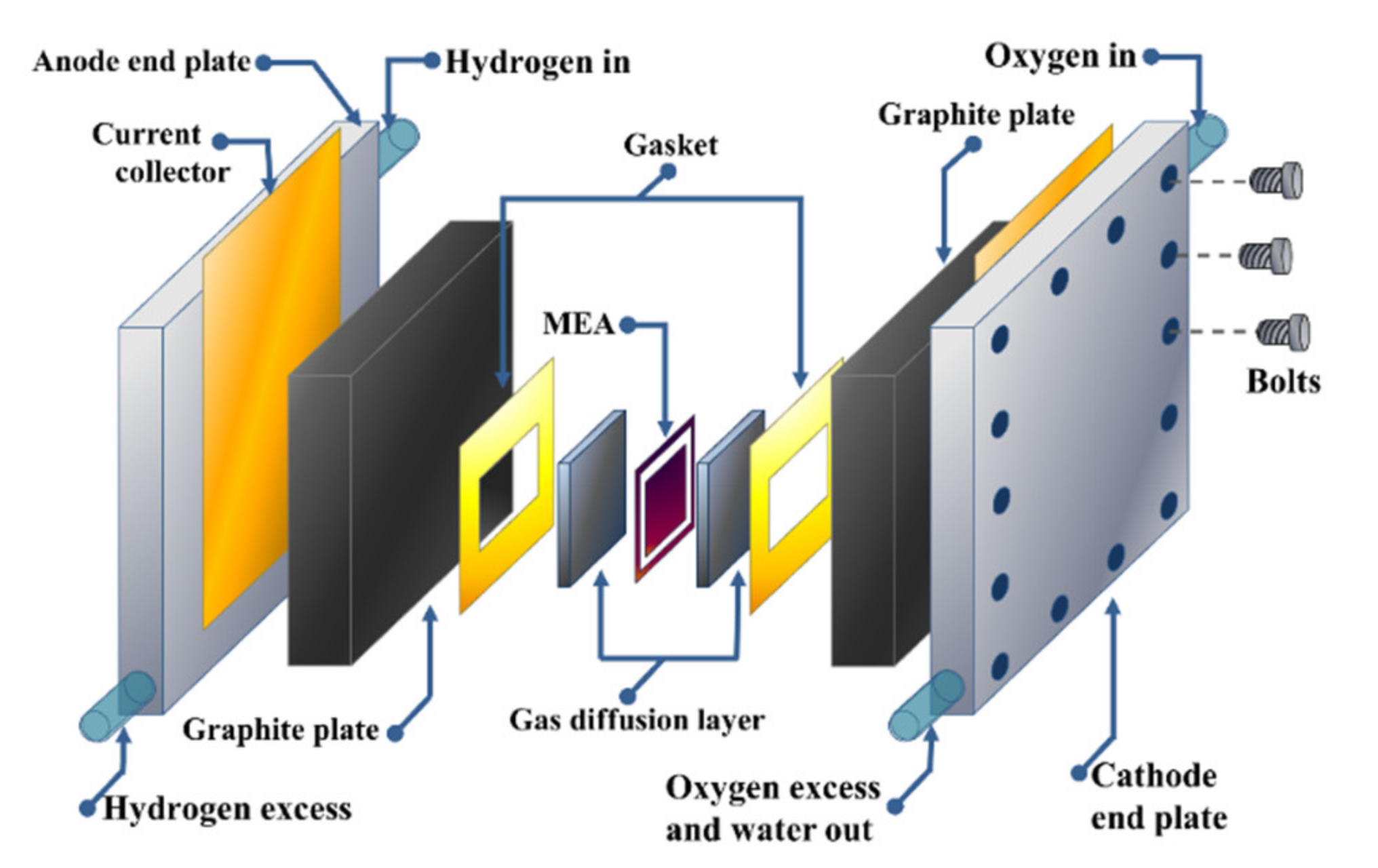
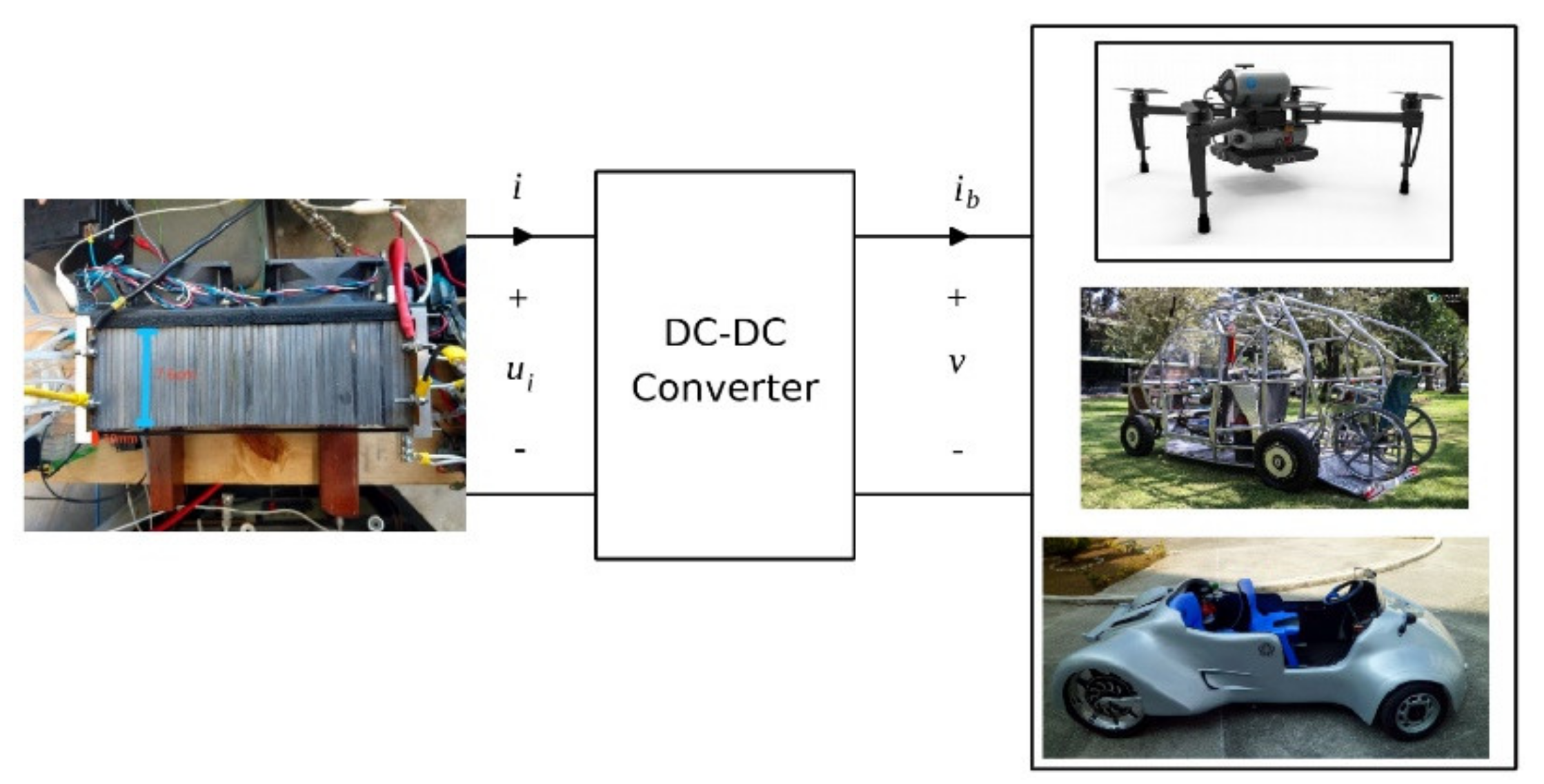
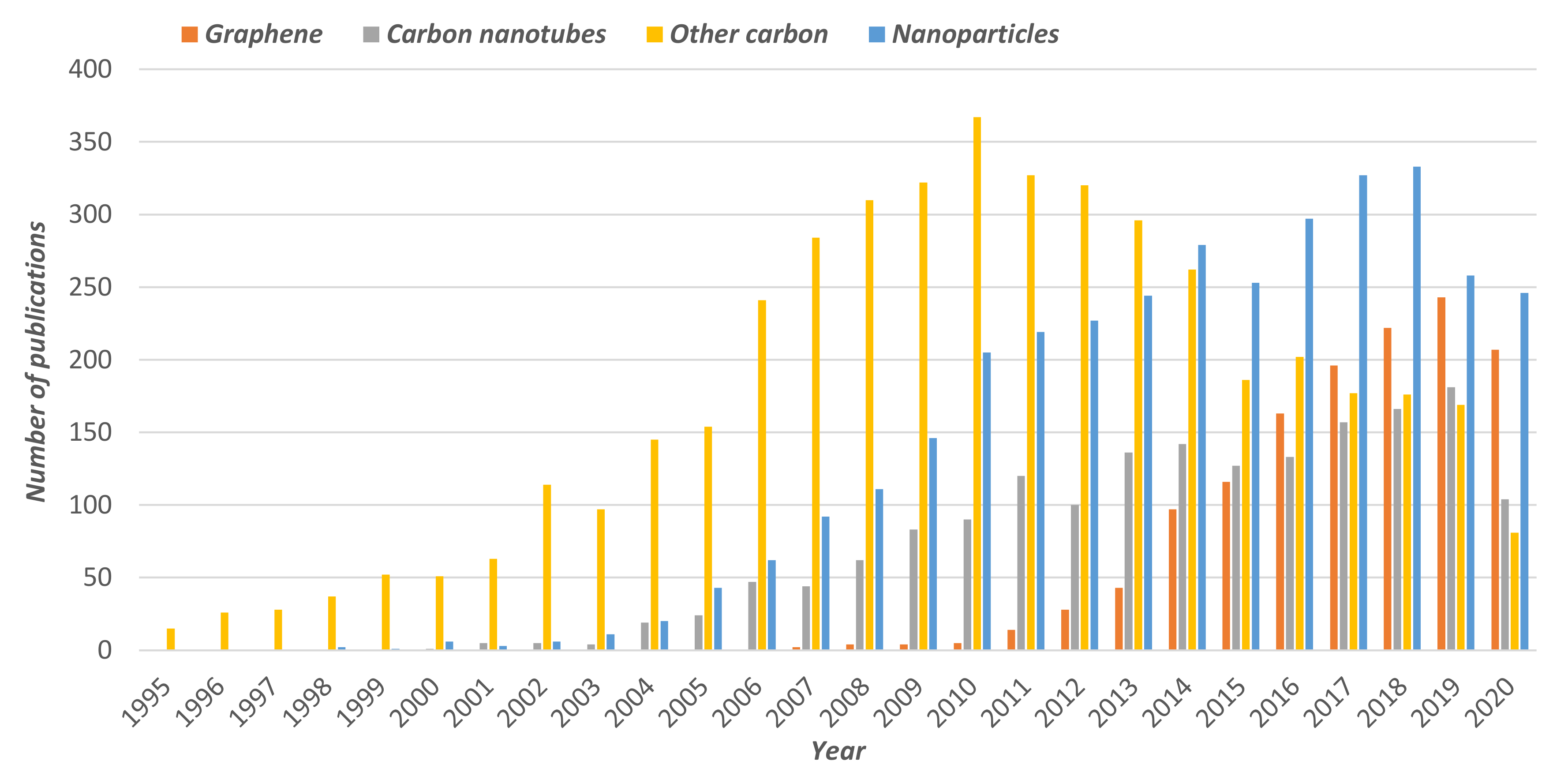
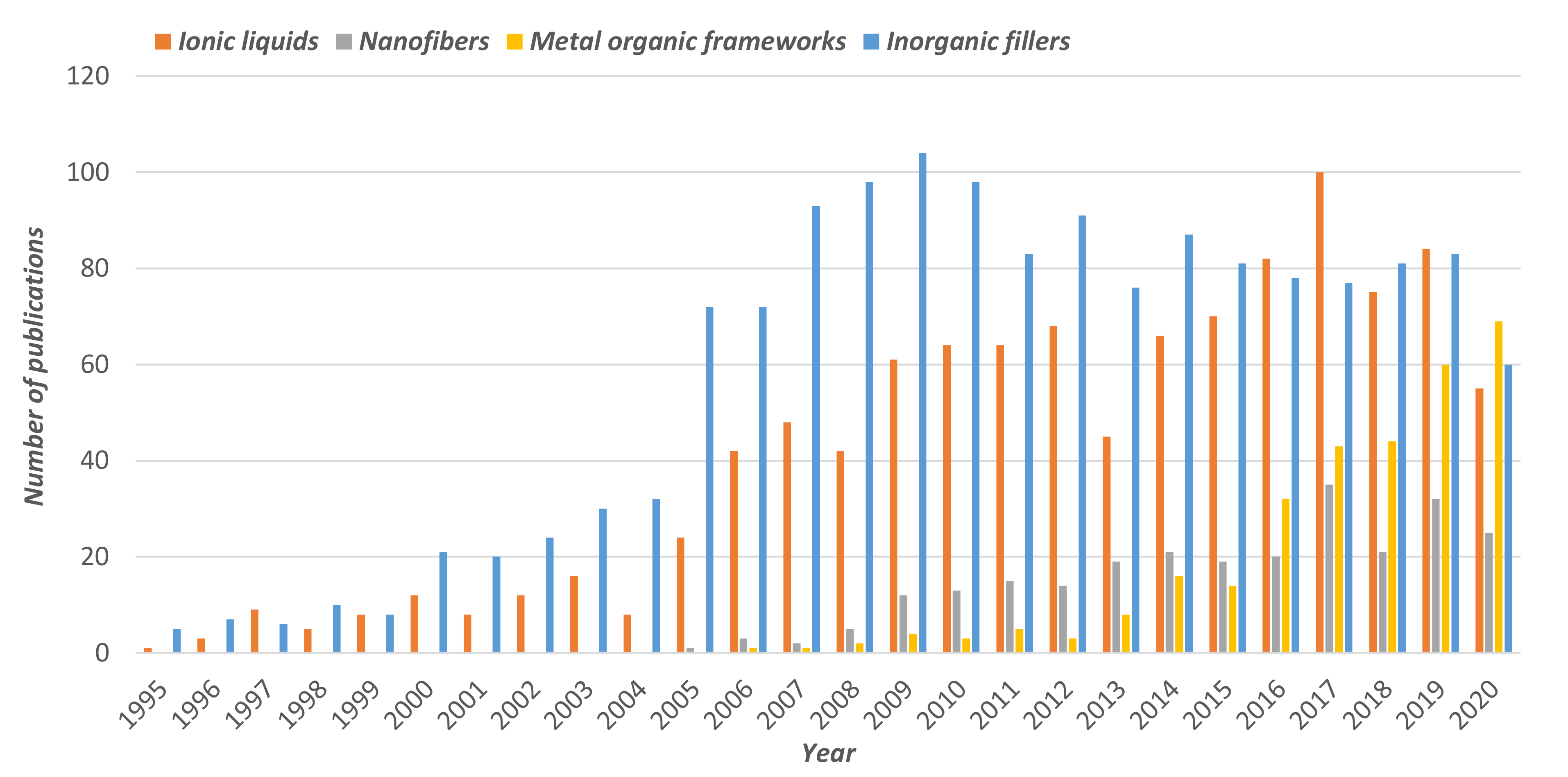


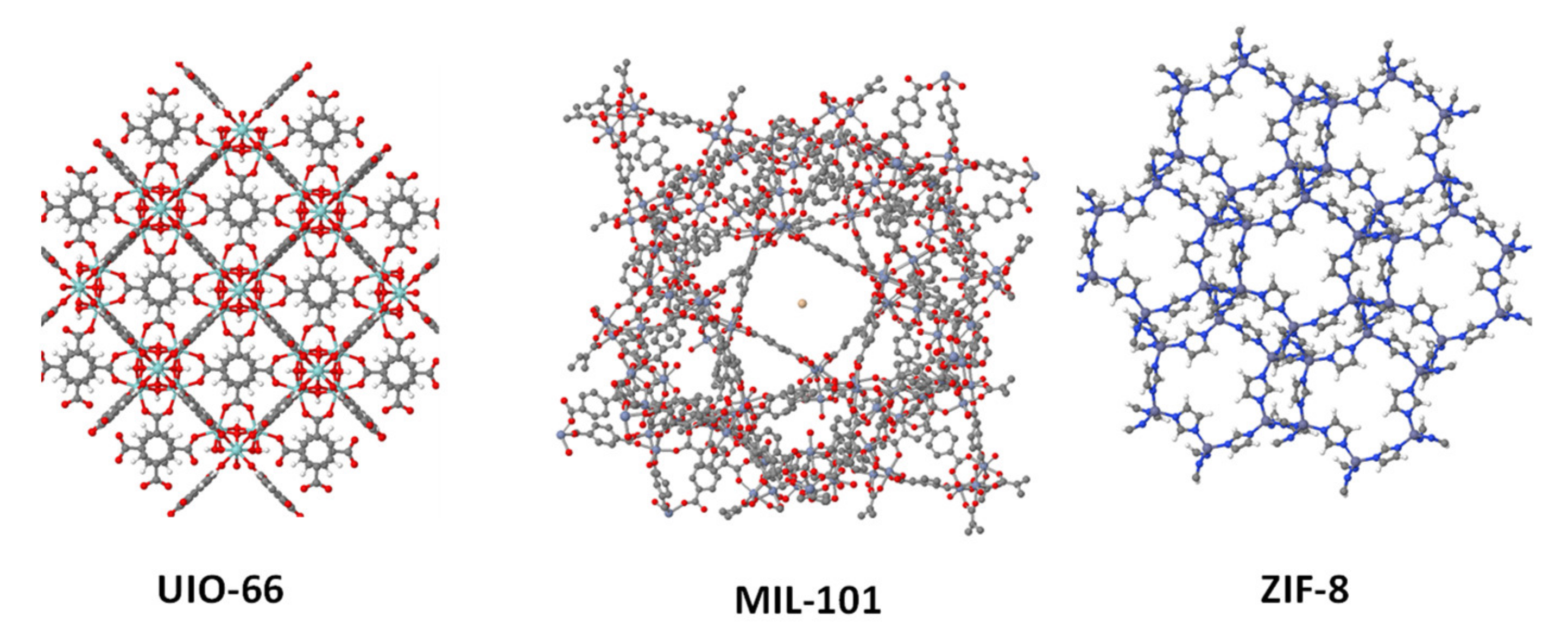
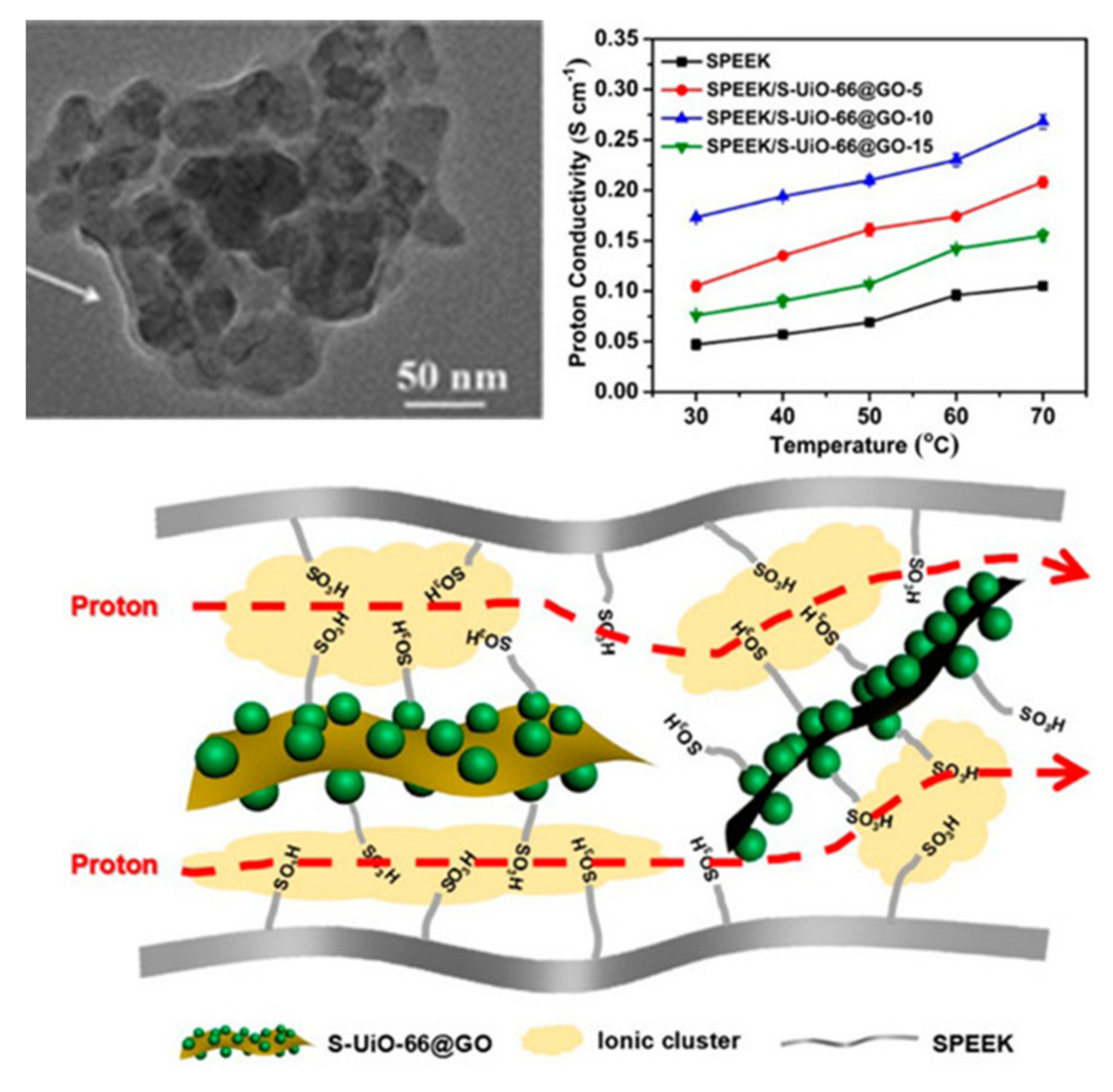
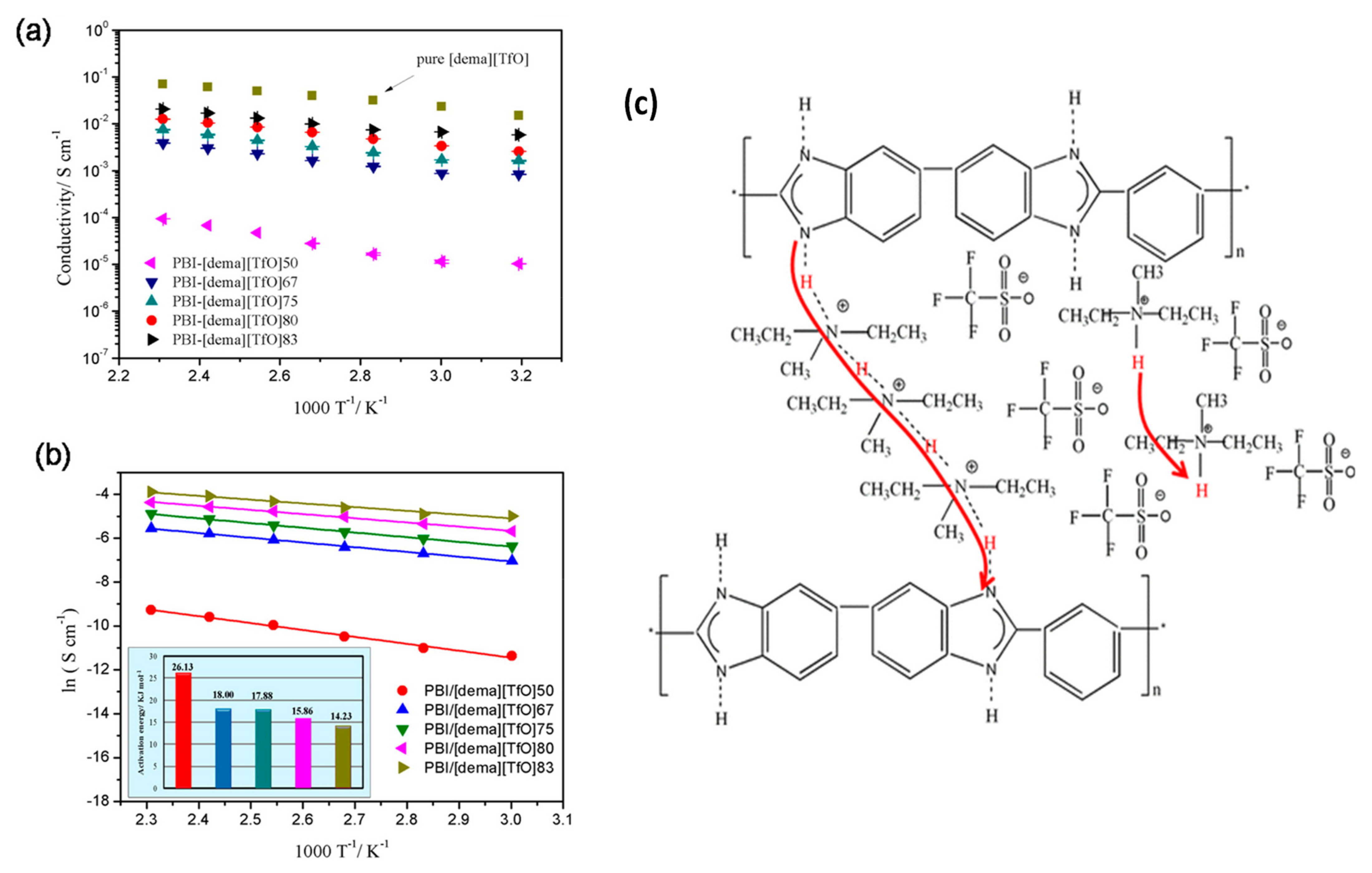
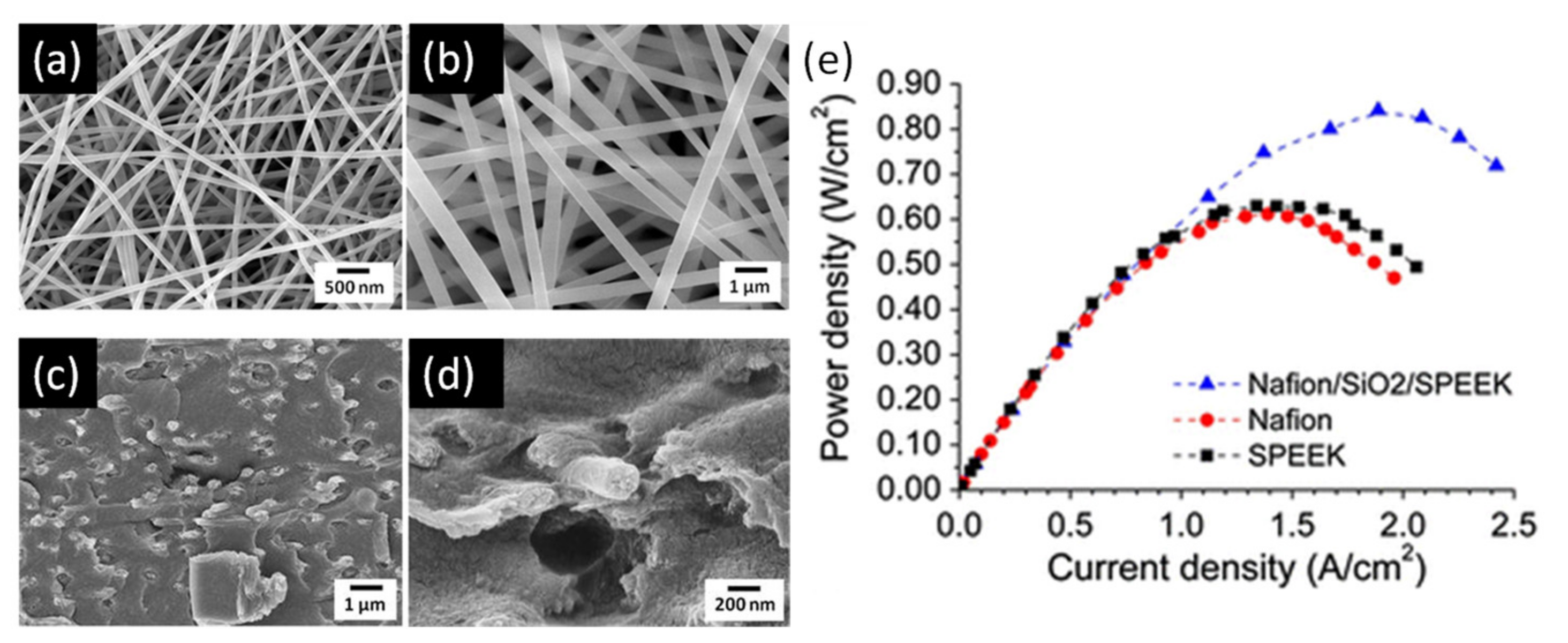
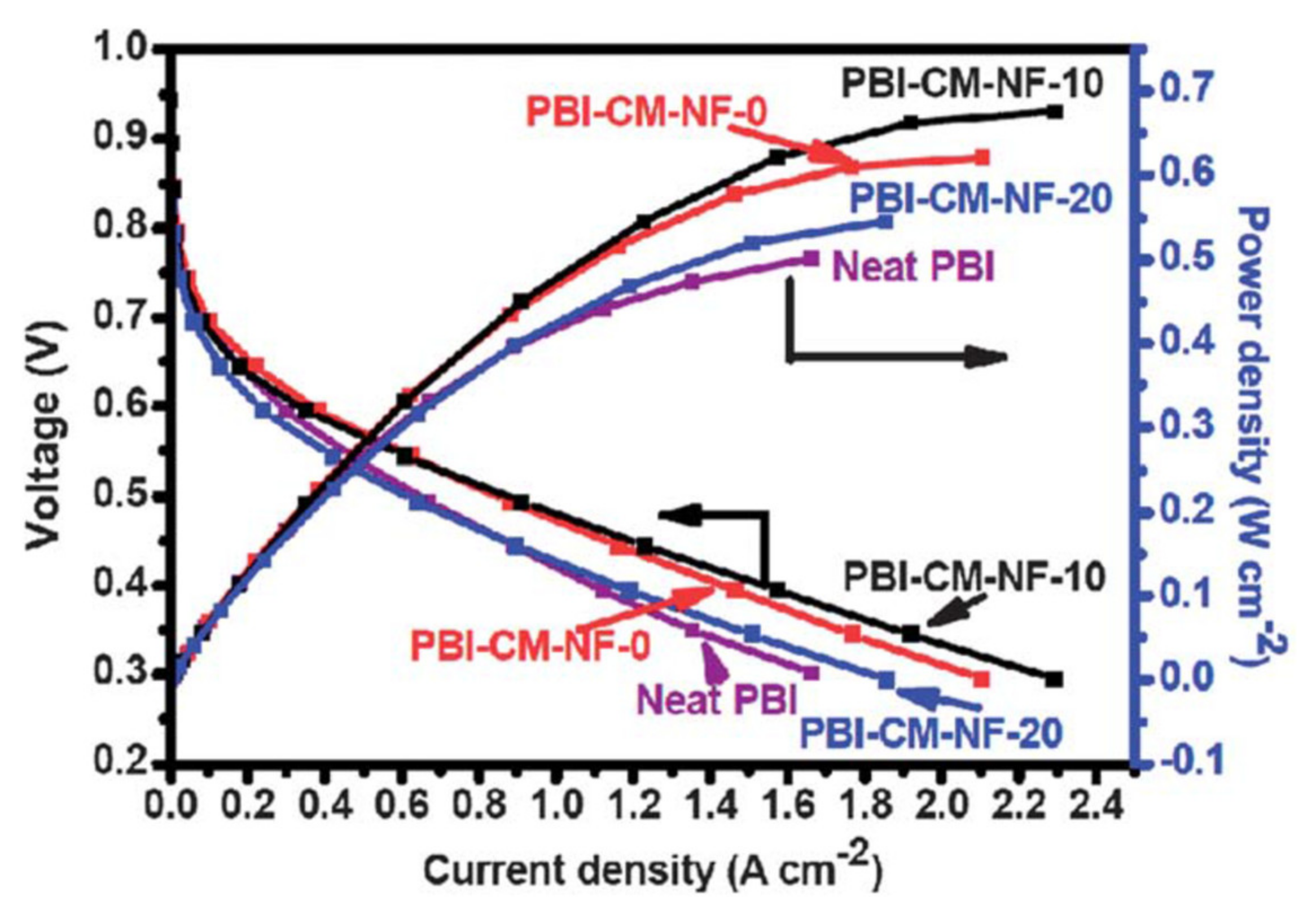
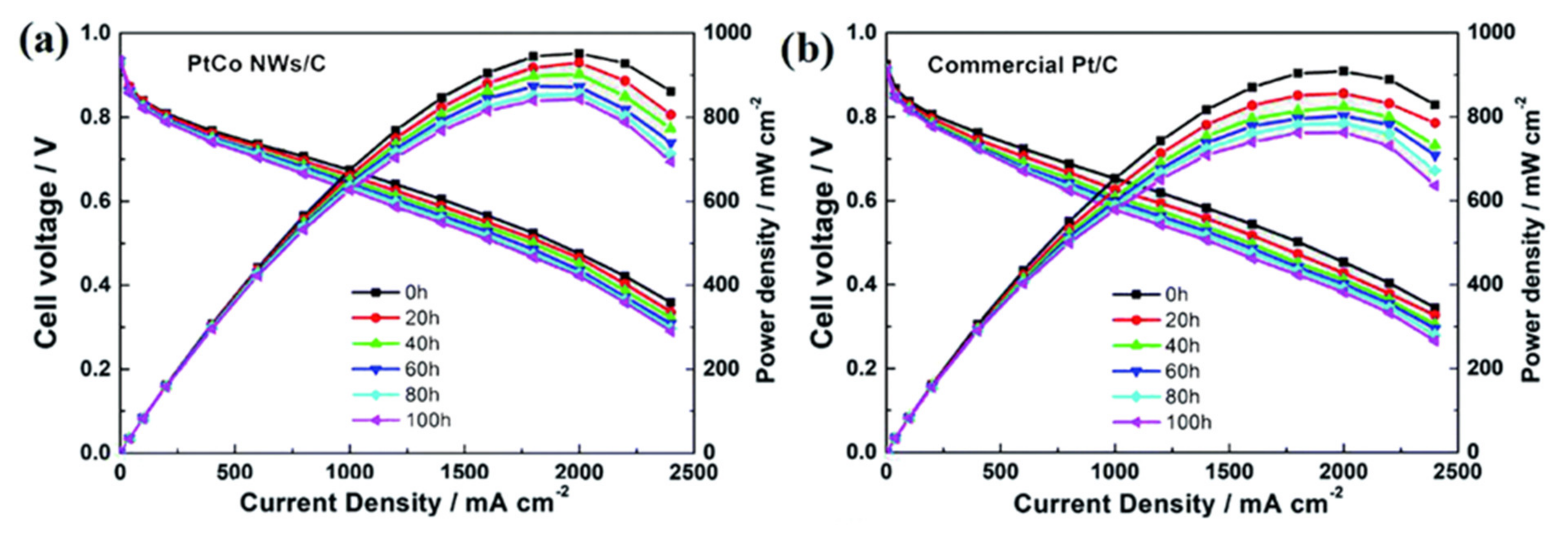
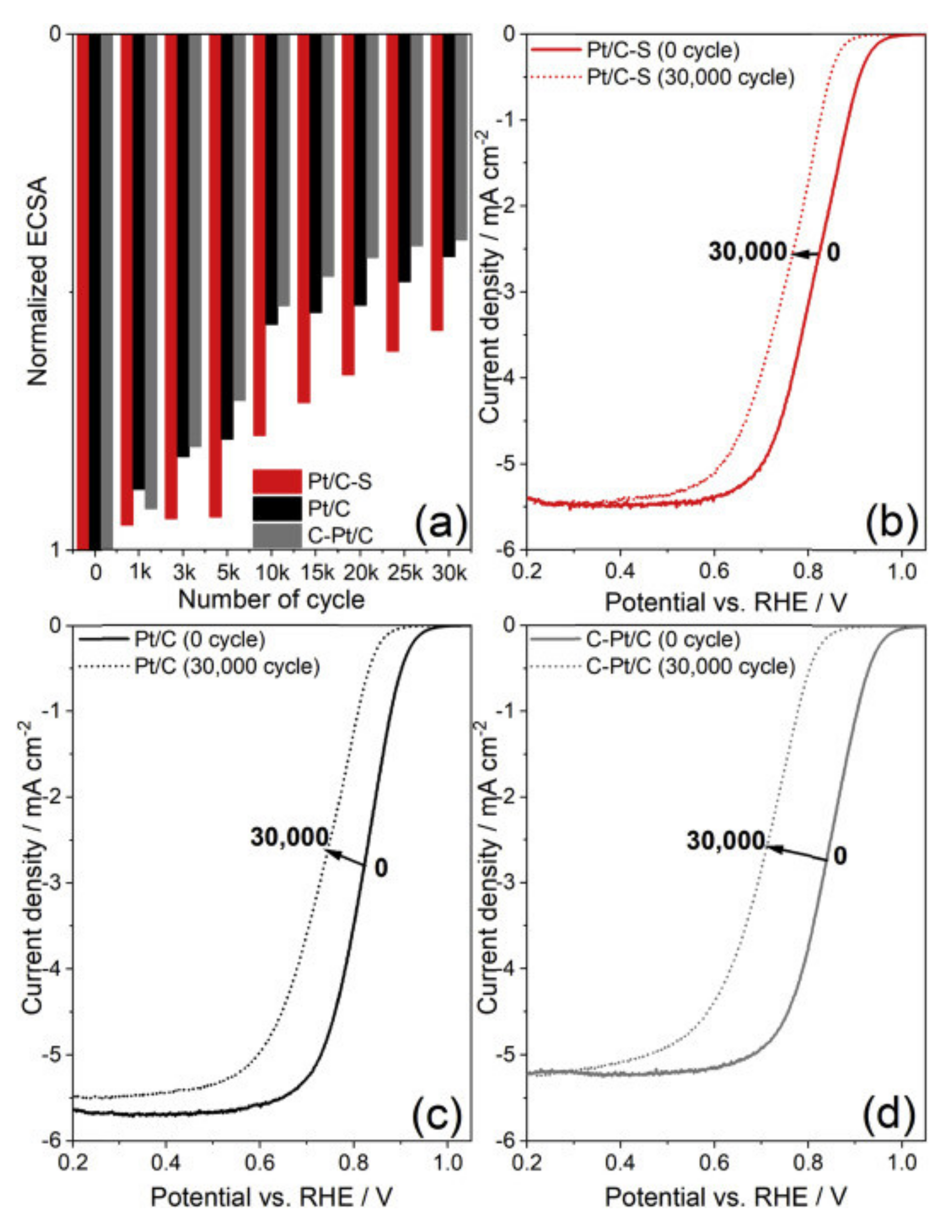
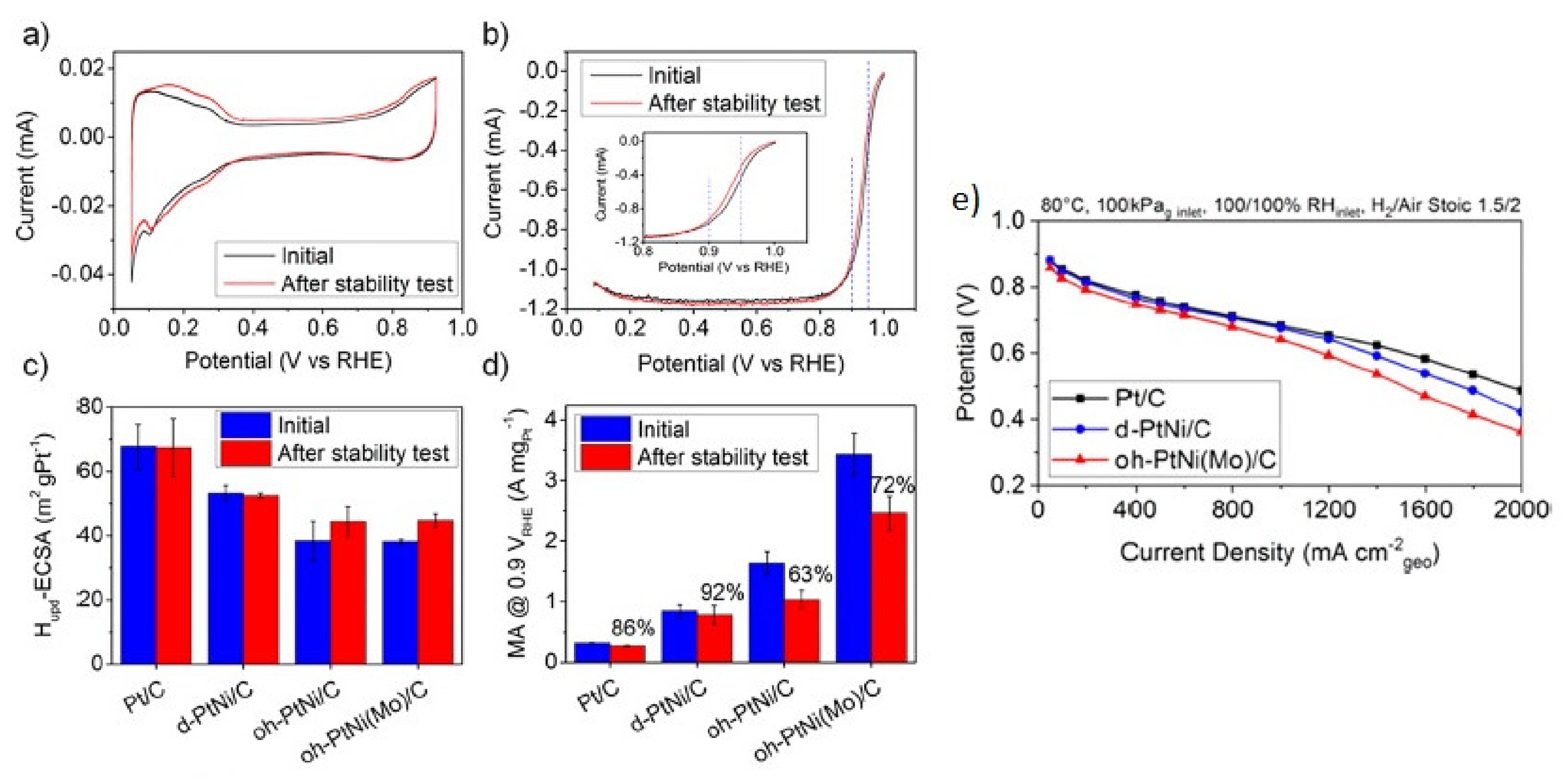
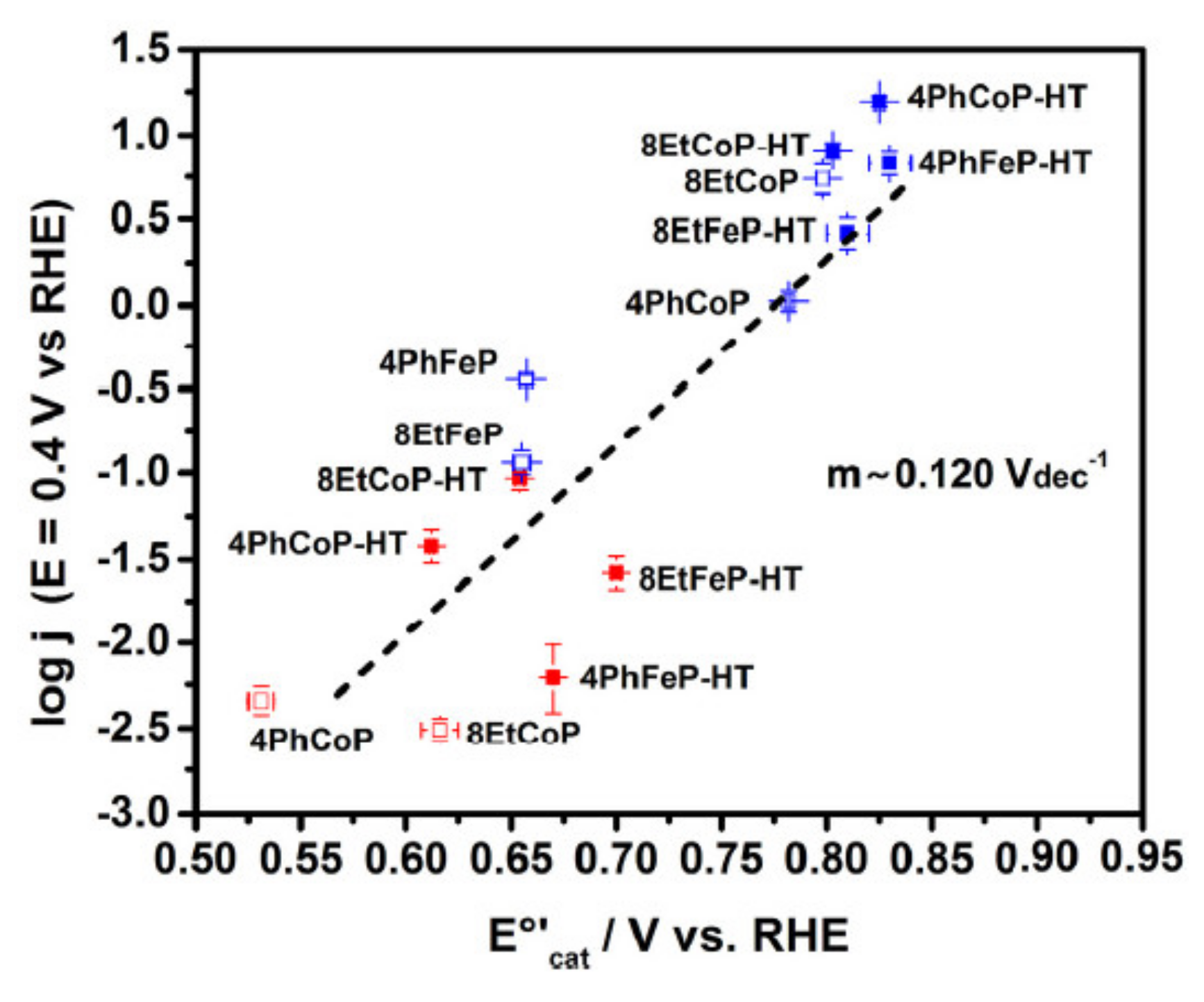
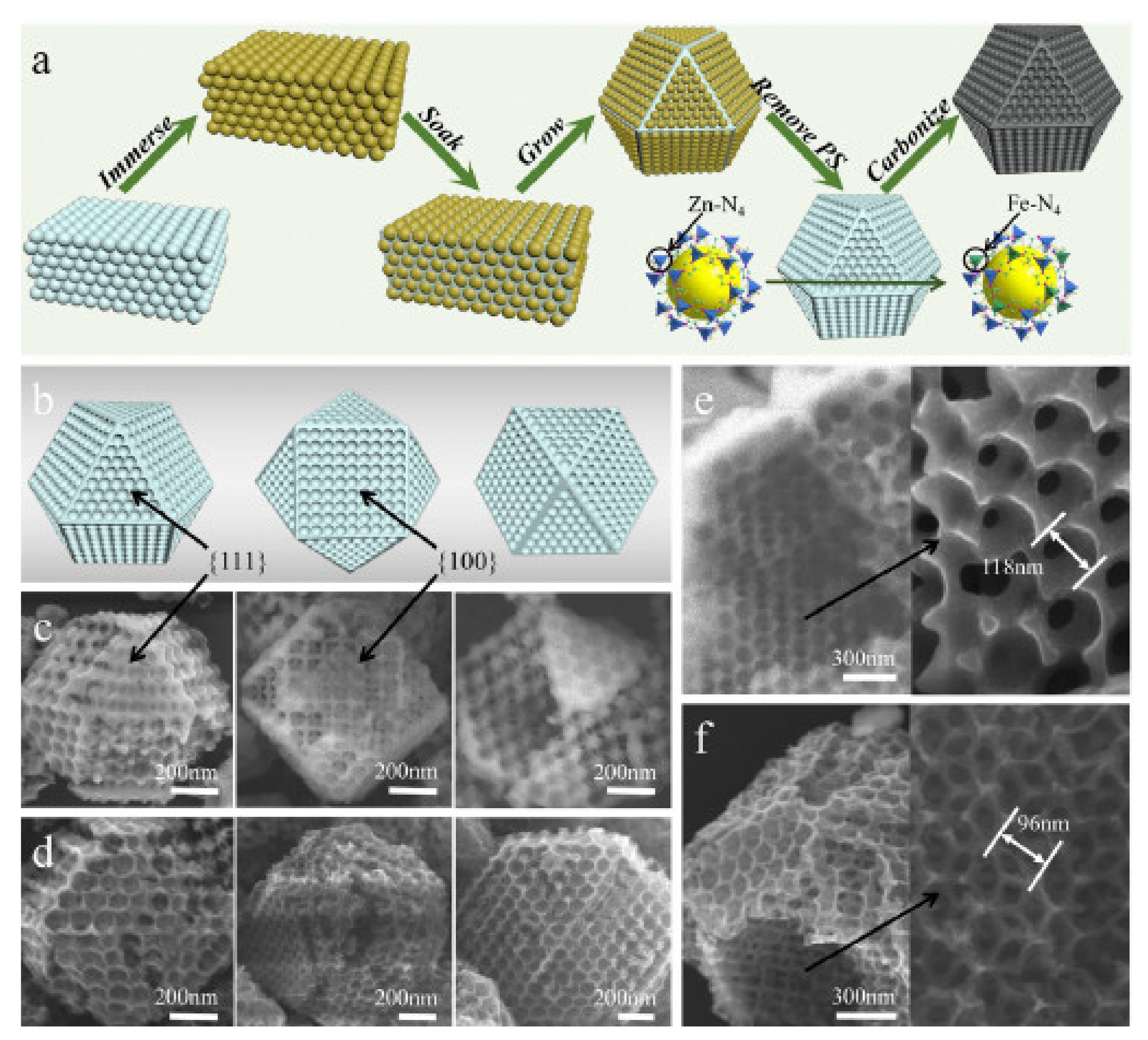

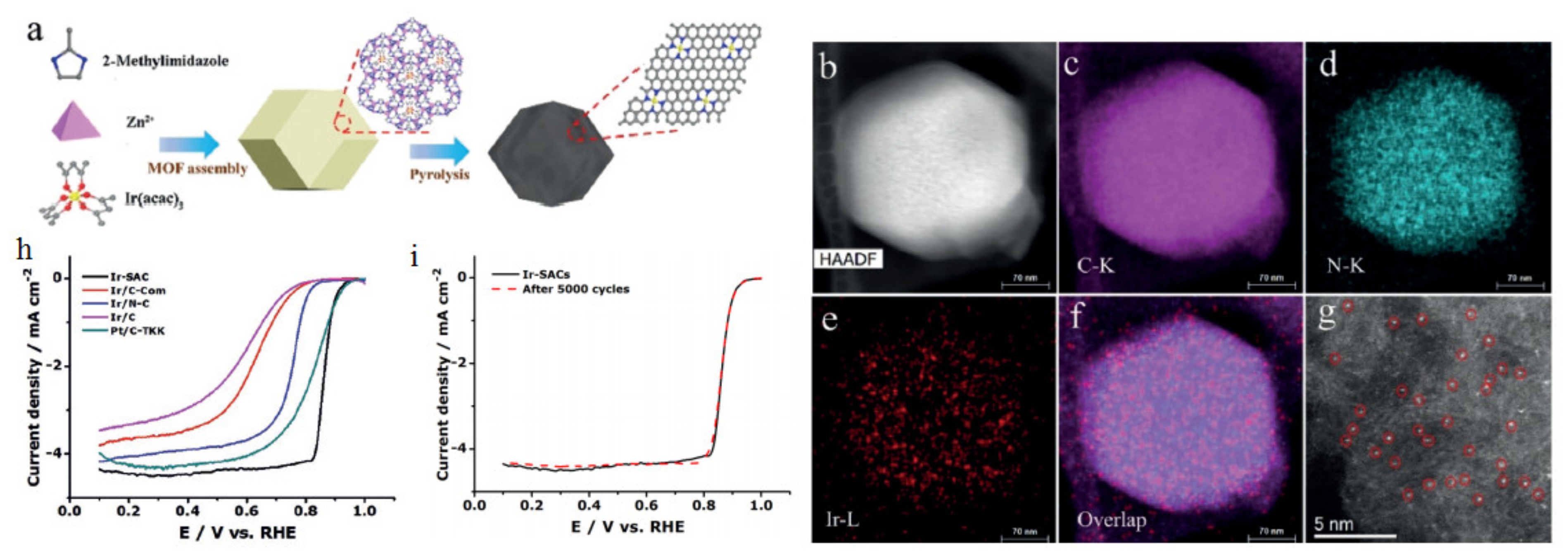
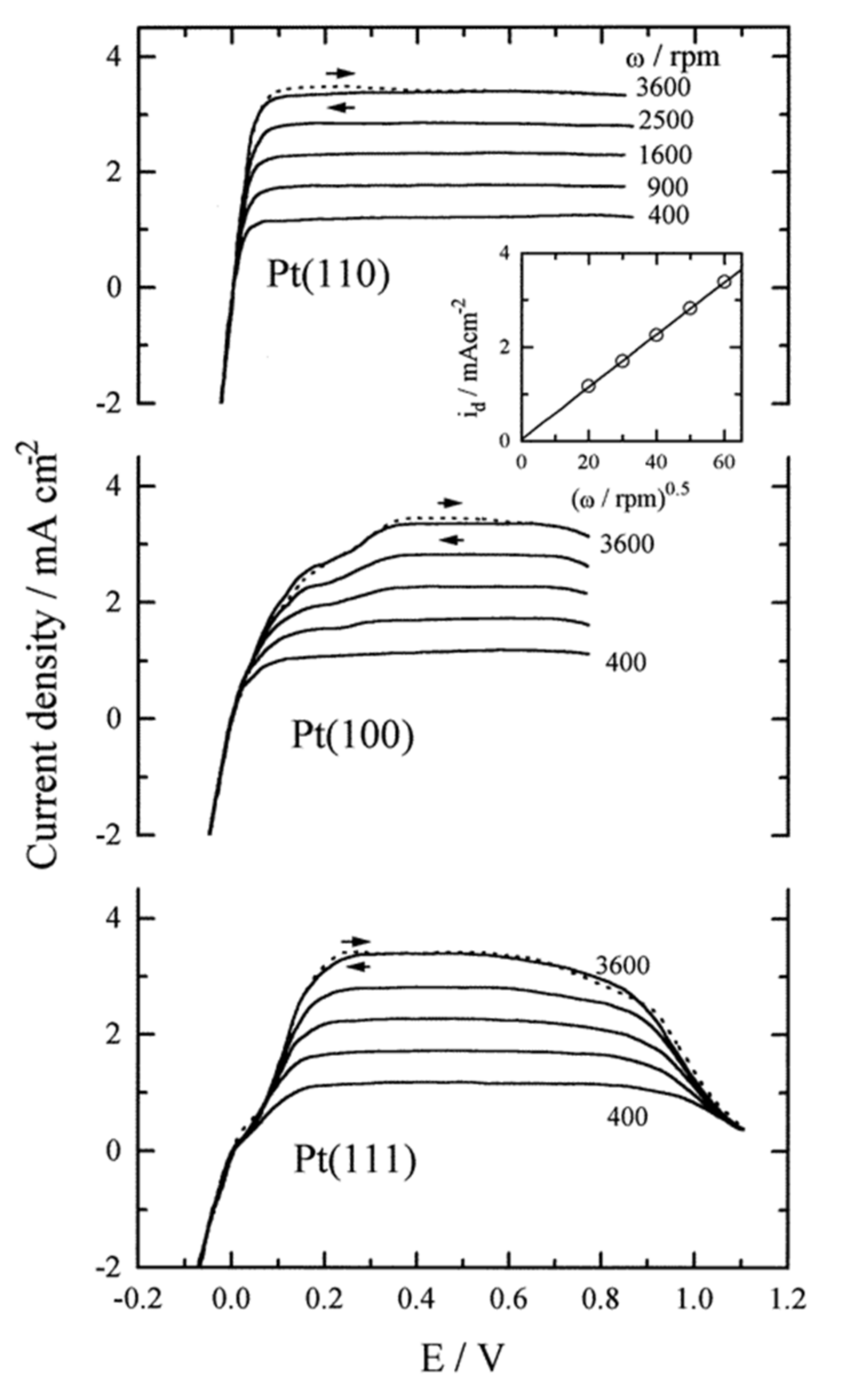
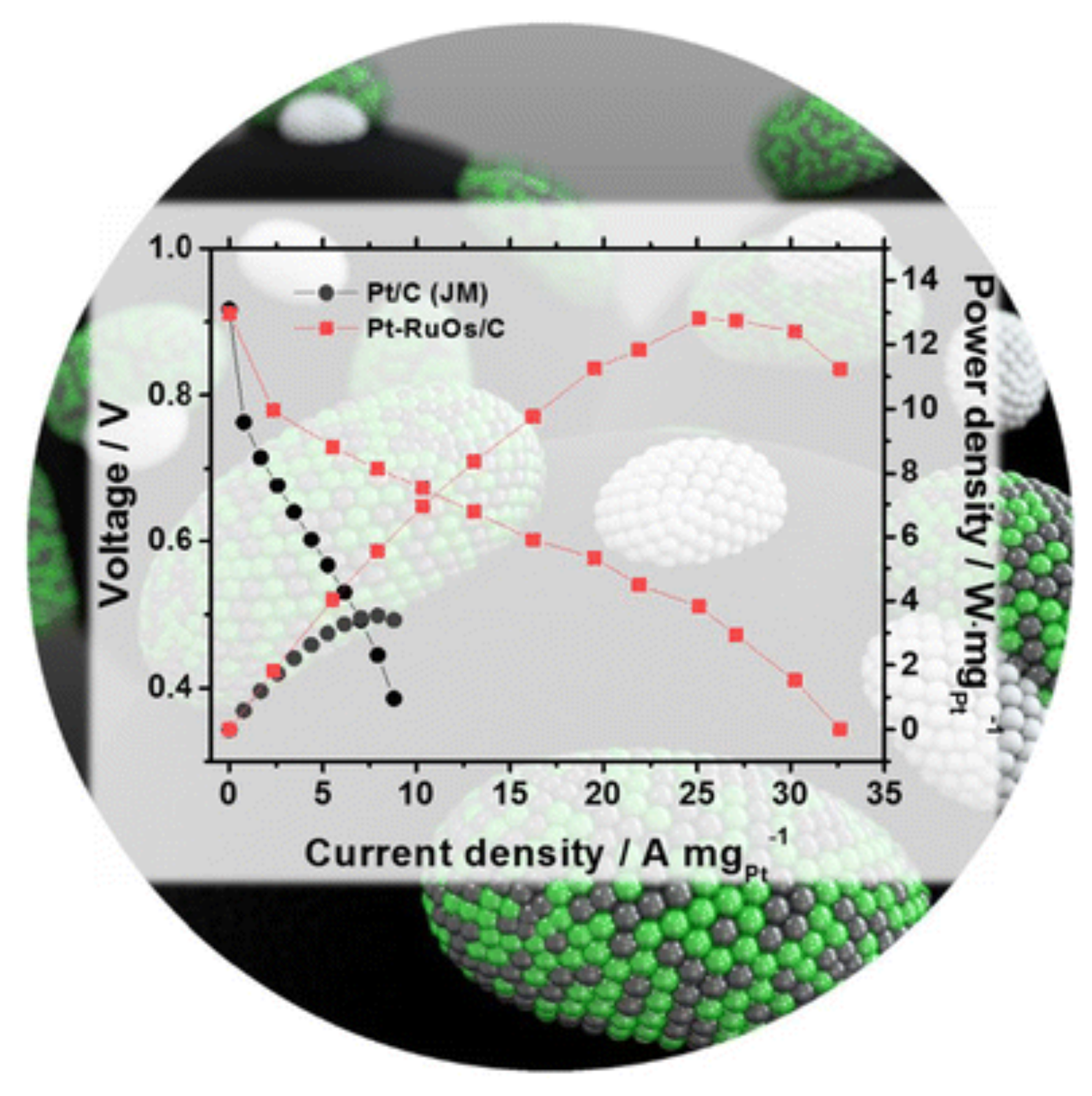
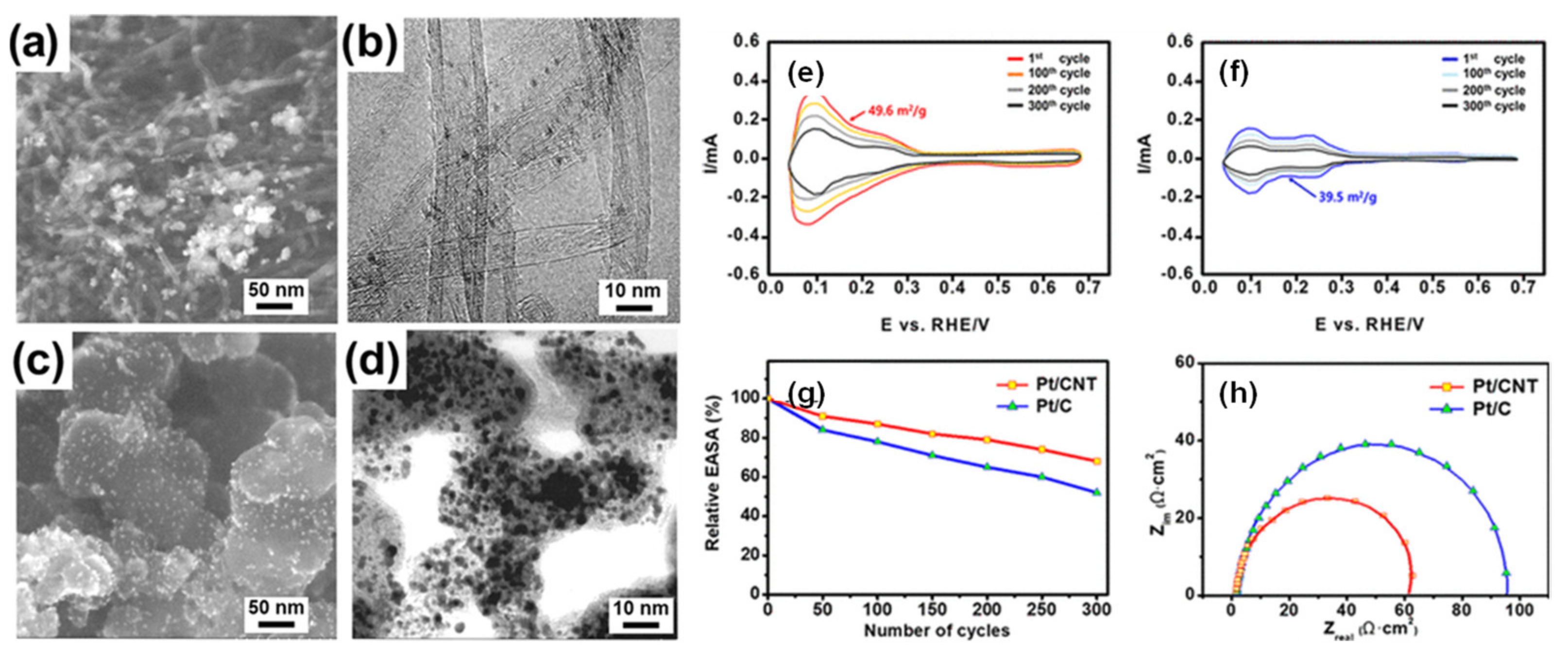
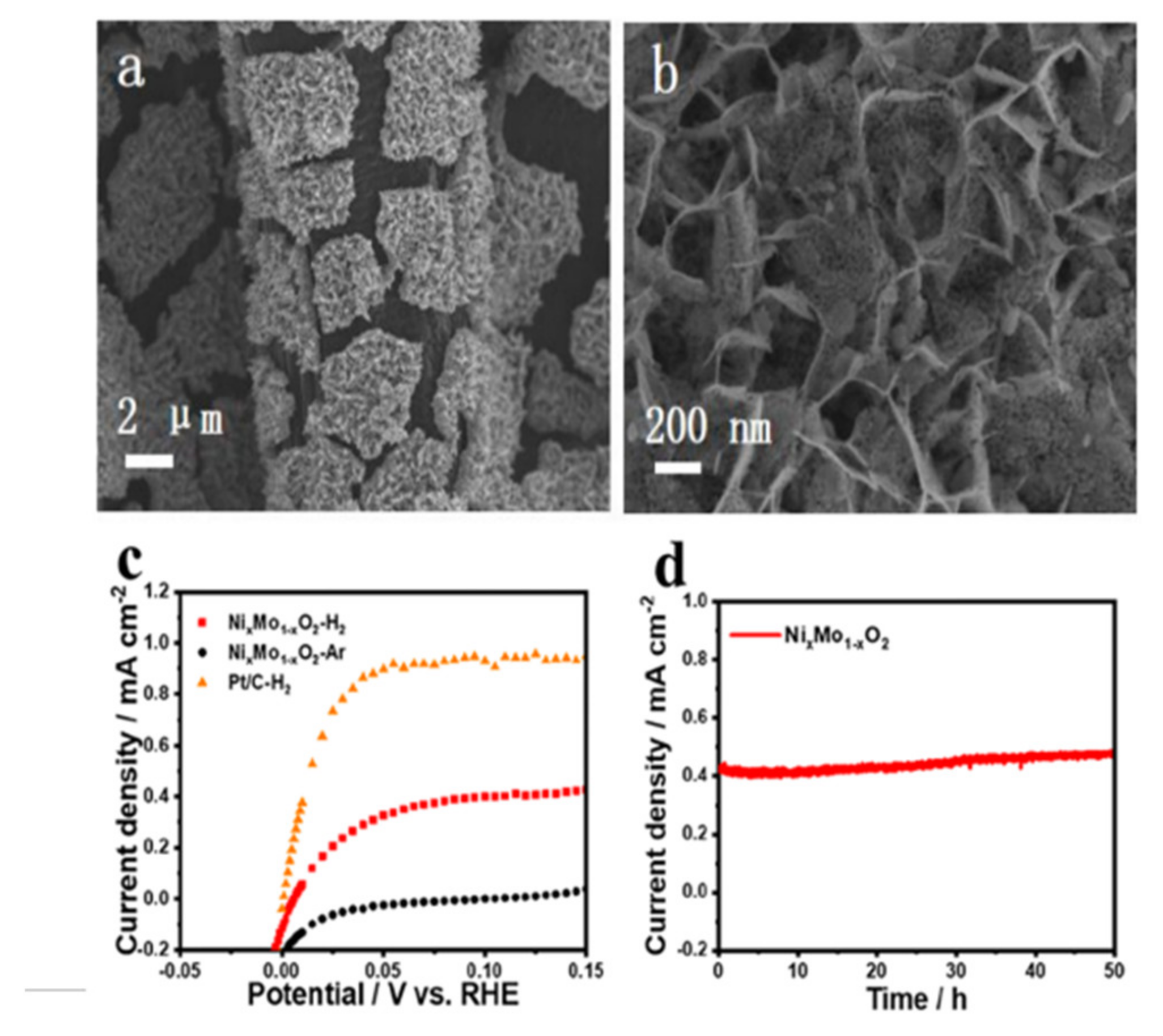
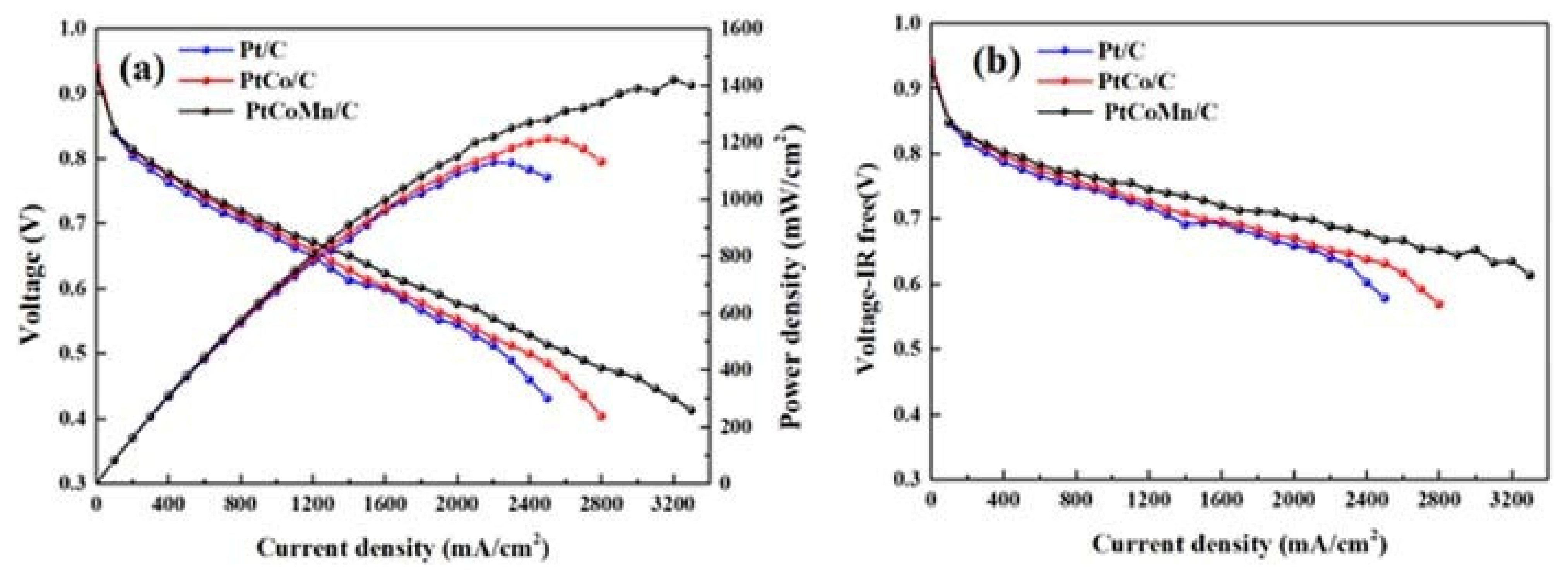
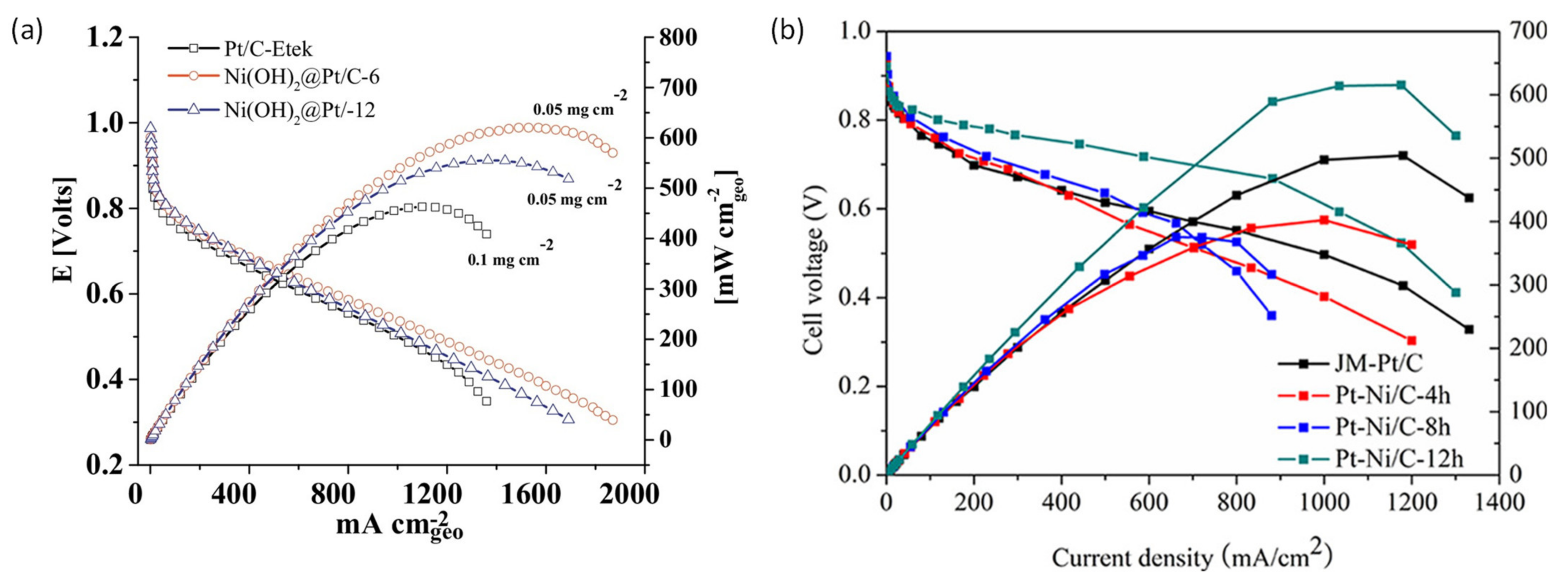

| Material | Ik (mAcm−2) |
|---|---|
| Pt | 55 |
| Pt-Mn | 15 |
| Pt-Pb | 0.93 |
| Pt-Sb | 115 |
| Pt-Sn | 69 |
| Catalyst | Synthesis Method | Size Particle (nm) | io (mAcm−2) | Specific Activity@ 0.1 V (A/gIr) | Ref. |
|---|---|---|---|---|---|
| Pd/C | Pulse microwave assisted polyol | 4.3 ± 0.3 | 0.35 | N.R. | [281] |
| Pd3Ir/C | 4.5 ± 0.3 | 0.7 | N.R. | ||
| PdIr/C | 4.4 ± 0.3 | 1.6 | N.R. | ||
| PdIr3/C | 5.3 ± 0.3 | 1 | N.R. | ||
| Ir/C | 5.7 ± 0.3 | 0.2 | N.R. | ||
| Pd/C | Thermal synthesis (300°C, Ar, 1h) | 4.5 | N.R. | N.R. | [284] |
| PdP2 | 5 | N.R. | N.R. | ||
| Pd5P2 | 5.5 | N.R. | N.R. | ||
| IrFe/C | Solvent vaporization + hydrogen reduction method | 3.8 ± 0.2 | N.R. | 146.9 | [285] |
| IrCo/C | 2.6 ± 0.2 | N.R. | 133 | ||
| IrNi/C | 3.4 ± 0.2 | N.R. | 152 | ||
| Pt | N.R. | N.R. | 16 |
| Catalyst | Synthesis Method | Size Particle (nm) | Max. Power Density (mWcm−2) | Ref. |
|---|---|---|---|---|
| Pd-Co/gCN | Thermal condensation+ polyol reduction | 10 | 290 | [282] |
| Pd3Co/PCNT | CCVD+ modified Hummers method+ polyol reduction | N.R. | 327 | [283] |
| Pd3Co/CNT | N.R. | |||
| IrP2/rGO | Modified Hummers method+ solvothermal method | 10 | N.R. | [286] |
| Rh-Rh2O3/C | Polyol method+ thermal treatment | 10-15 | N.R. | [287] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tellez-Cruz, M.M.; Escorihuela, J.; Solorza-Feria, O.; Compañ, V. Proton Exchange Membrane Fuel Cells (PEMFCs): Advances and Challenges. Polymers 2021, 13, 3064. https://doi.org/10.3390/polym13183064
Tellez-Cruz MM, Escorihuela J, Solorza-Feria O, Compañ V. Proton Exchange Membrane Fuel Cells (PEMFCs): Advances and Challenges. Polymers. 2021; 13(18):3064. https://doi.org/10.3390/polym13183064
Chicago/Turabian StyleTellez-Cruz, Miriam M., Jorge Escorihuela, Omar Solorza-Feria, and Vicente Compañ. 2021. "Proton Exchange Membrane Fuel Cells (PEMFCs): Advances and Challenges" Polymers 13, no. 18: 3064. https://doi.org/10.3390/polym13183064
APA StyleTellez-Cruz, M. M., Escorihuela, J., Solorza-Feria, O., & Compañ, V. (2021). Proton Exchange Membrane Fuel Cells (PEMFCs): Advances and Challenges. Polymers, 13(18), 3064. https://doi.org/10.3390/polym13183064