Recent Trends and Developments in Conducting Polymer Nanocomposites for Multifunctional Applications
Abstract
1. Introduction
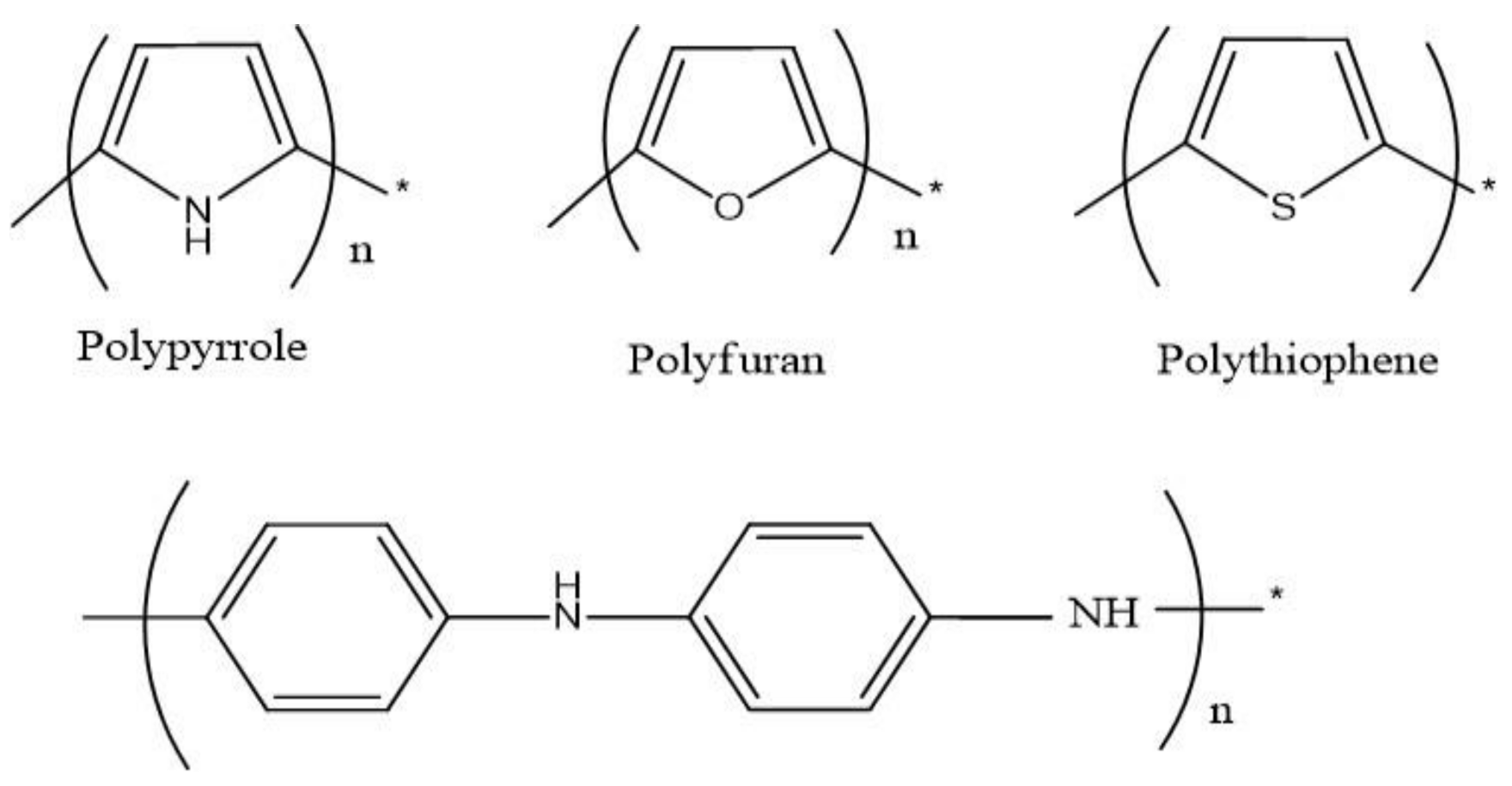
2. Synthesis of Conducting Polymers
2.1. Chemical Polymerization
2.2. Electrochemical Synthesis
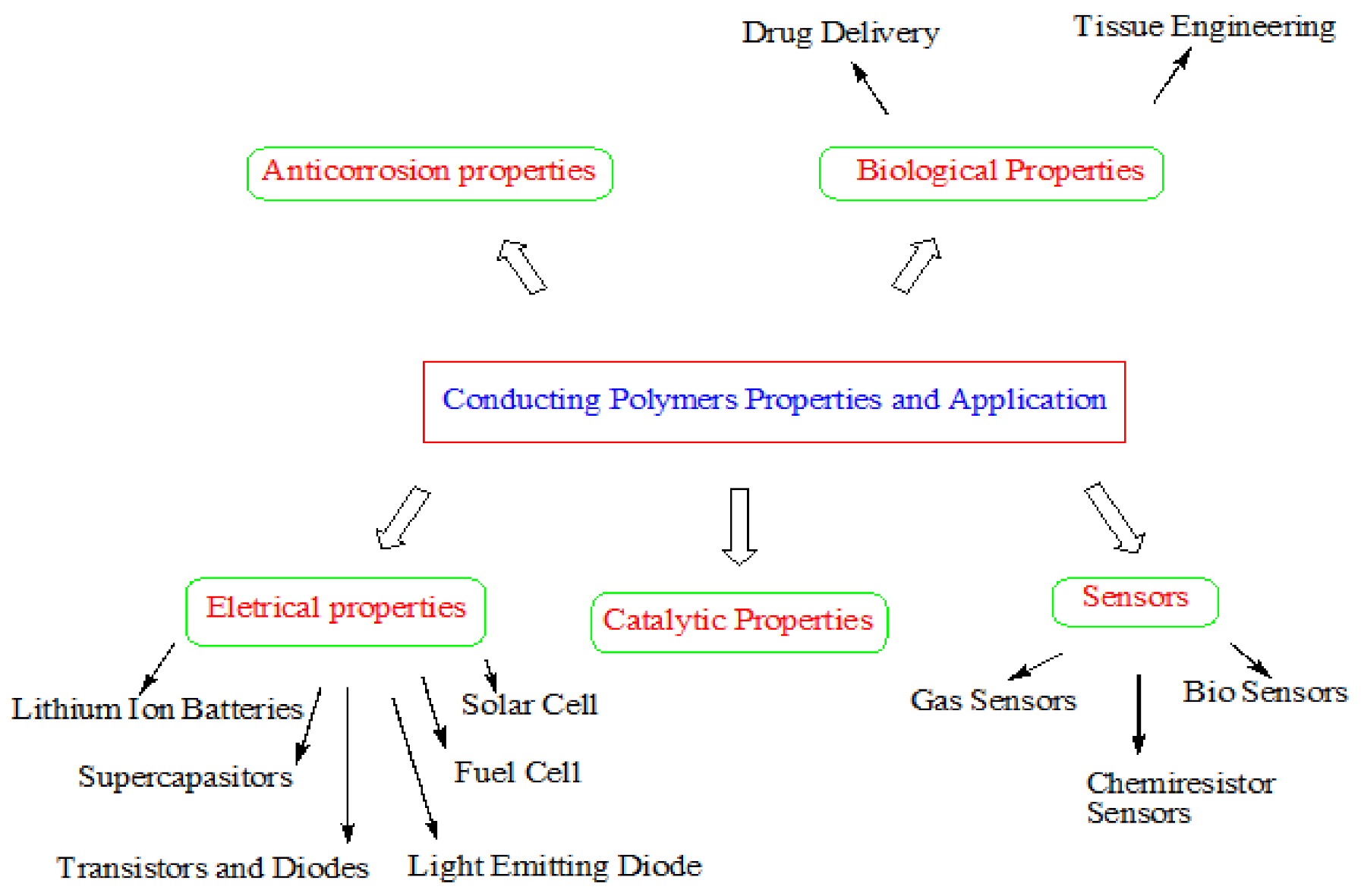
3. Properties and Multifunctional Applications of Conducting Polymers
3.1. Electrical Properties
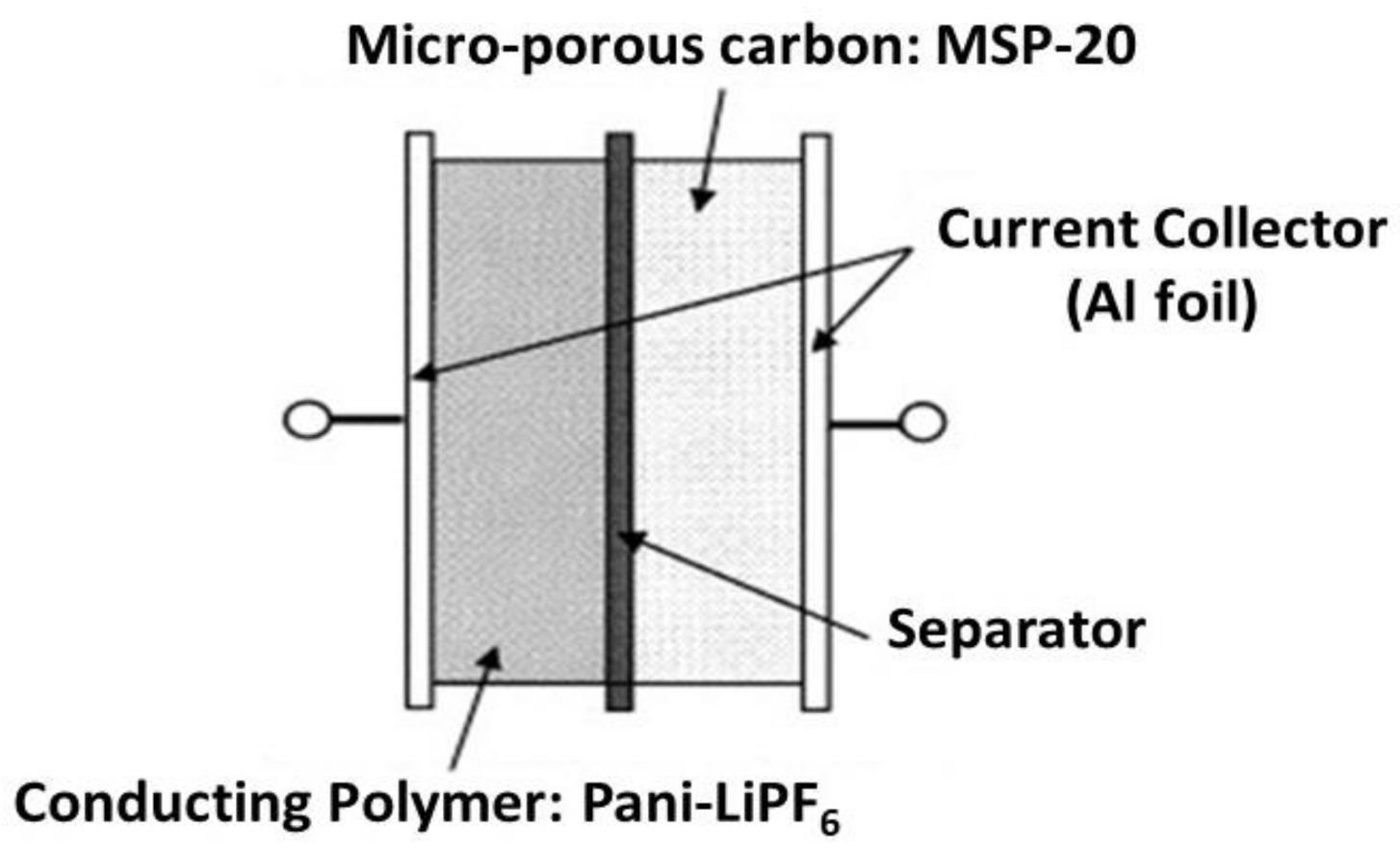
3.1.1. Lithium-Ion Batteries
3.1.2. Solar Cells
3.1.3. Fuel Cells
3.1.4. Light Emitting Diodes (LEDs)
3.1.5. Supercapacitors
3.2. Anticorrosion Properties
3.3. Catalytic Properties
3.4. Sensors
3.4.1. Gas Sensors
3.4.2. Bio Sensors
3.4.3. Chemiresistor Sensors
3.4.4. Strain Sensors
- based on structure deformation of graphene,
- based on over connected graphene sheets and finally,
- based on tunneling effect of neighboring graphene sheets.
3.5. Actuators
3.6. Flexible Electronics
3.7. Shape Memory Polymers
3.8. Optical Limiting Applications
3.9. Biomedical Applications
3.9.1. Drug Delivery
3.9.2. Tissue Engineering
3.9.3. Diabetic Monitoring
4. Novel Polymer Nanocomposite Materials for Multifunctional Engineering Applications
5. Drawbacks of Conducting-Polymers
6. Concluding Remarks and Future-Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walatka, V.V.; Labes Perlstein, J.H. Polysulfur nitride—A one-dimensional chain with a metallic ground state. Phys. Rev. Lett. 1973, 31, 1139–1142. [Google Scholar] [CrossRef]
- Cheng, Q.; Pavlinek, V.; Li, C.; Lengalova, A.; He, Y.; Sáha, P. Synthesis and structural properties of polypyrrole/nano-Y2O3 conducting composite. Appl. Surf. Sci. 2006, 253, 1736–1740. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Liu, D.; Guo, J.; Liu, P. Synthesis and electrochemical performance of CeO2/PPy nanocomposites: Interfacial effect. Ind. Eng. Chem. Res. 2016, 55, 866–874. [Google Scholar] [CrossRef]
- Kumar, P.R.; Venkateswarlu, M.; Satyanarayana, N. Synthesis and ac conductivity studies of PEO+ LiClO4+ La2O3+ MoO3 nanocomposite polymer solid electrolyte. NSTI-Nanotech 2012 2011, 1, 546–549. [Google Scholar]
- Zamborini, F.P.; Alphenaar, B.W.; Kharel, P.L. Enhancing the Photovoltaic Performance of Dye-Sensitized Solar Cells with Rare-Earth Metal Oxide Nanoparticles. Meet. Abstr. 2017, 15, 879. [Google Scholar]
- Liu, P.; Wang, Y.; Wang, X.; Yang, C.; Yi, Y. Polypyrrole-coated samarium oxide nanobelts: Fabrication, characterization, and application in supercapacitors. J. Nanopart. Res. 2012, 14, 1232. [Google Scholar] [CrossRef]
- Mo, Z.; He, J.; Wang, J.; Feng, C.; Guo, R. Preparation and characterization of PPy/NanoGs/Fe3O4 conductive and magnetic nanocomposites. J. Exp. Nanosci. 2013, 8, 113–120. [Google Scholar] [CrossRef]
- Shiri, H.M.; Ehsani, A. A novel and facile route for the electrosynthesis of Ho2O3 nanoparticles and its nanocomposite with p-type conductive polymer: Characterisation and electrochemical performance. Bull. Chem. Soc. Jpn. 2016, 89, 1201–1206. [Google Scholar] [CrossRef]
- Zang, J.; Bao, S.J.; Li, C.M.; Bian, H.; Cui, X.; Bao, Q.; Sun, C.Q.; Guo, J.; Lian, K. Well-aligned cone-shaped nanostructure of polypyrrole/RuO2 and its electrochemical supercapacitor. J. Phys. Chem. C 2008, 112, 14843–14847. [Google Scholar] [CrossRef]
- Rafiqi, F.A.; Majid, K. Synthesis, characterization, luminescence properties and thermal studies of polyaniline and polythiophene composites with rare earth terbium (III) complex. Synth. Met. 2015, 202, 147–156. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Zhang, X.F.; Liu, Y.Q. Synthesis. Characterization and Properties of PANI (La-Nd Doped BaFe12O19) Composites. Key Eng. Mater. 2017, 27, 327–334. [Google Scholar] [CrossRef]
- Maji, P.; Choudhary, R.B.; Majhi, M. Effect of Y2O3 on polyindole for high frequency capacitor application. AIP Conf. Proc. 2017, 1832, 070003. [Google Scholar]
- Meng, Q.; Cai, K.; Chen, Y.; Chen, L. Research progress on conducting polymer-based supercapacitor electrode materials. Nano Energy 2017, 36, 268–285. [Google Scholar] [CrossRef]
- Le, T.H.; Kim, Y.; Yoon, H. Electrical and electrochemical properties of conducting polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Naveen, M.H.; Gurudatt, N.G.; Shim, Y.B. Applications of conducting polymer composites to electrochemical sensors: A review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- Gurunathan, K.; Murugan, A.V.; Marimuthu, R.; Mulik, U.P.; Amalnerkar, D. Pelectrochemically synthesized conducting polymeric materials for applications towards technology in electronics, optoelectronics and energy storage devices. Mater. Chem. Phys. 1999, 61, 73–191. [Google Scholar] [CrossRef]
- Shirakawa, H.; Louis, E.G.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene (CH)x. J. Chem. Soc. Chem. Commun. 1977, 16, 578–580. [Google Scholar] [CrossRef]
- Holze, R.; Inzelt, G. Conducting polymers. J. Appl. Electrochem. 2009, 39, 953–954. [Google Scholar] [CrossRef]
- Jang, J. Conducting polymer nanomaterials and their application. Emiss. Mater. Nanomater. 2006, 199, 189–260. [Google Scholar]
- Brezoi, D.V.; Ion, R.M. Phase evolution induced by polypyrrole in iron oxide polypyrrole nanocomposite. Sens. Actuators B Chem. 2005, 109, 171–175. [Google Scholar] [CrossRef]
- Babazadeh, M.; Gohari, F.R.; Olad, A. Characterization and physical properties Investigation of conducting polypyrrole/TiO2 nanocomposites prepared through one step in situ polymerization method. J. Appl. Polym. Sci. 2012, 123, 1922–1927. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Bagheri, R.; Najjar, R. Electropolymerization of polypyrrole and polypyrrole ZnO nanocomposites on mild steel and its corrosion protection performance. J. Appl. Polym. Sci. 2011, 121, 3159–3166. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Yoon, H. Recent Advances in Nanostructured Conducting polymers: From Synthesis to Practical Applications. Polymers 2016, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Meneguzzi, A.; Pham, M.C.; Lacroix, J.C.; Ferreir, C.A. Electroactive polyaromatic amine films for iron protection in sulfate medium. J. Electrochem. Soc. 2001, 148, B121. [Google Scholar] [CrossRef]
- Lenz, D.M.; Delamar, M.; Ferreira, C.A. Application of polypyrrole/TiO2 composite Films as corrosion protection of mild steel. J. Electroanal. Chem. 2003, 54, 35–44. [Google Scholar] [CrossRef]
- Lenz, D.M.; Ferreira, C.A.; Delamar, M. Distribution analysis of TiO2 and commercial zinc phosphate in polypyrrole matrix by XPS. Synth. Met. 2002, 126, 179–182. [Google Scholar] [CrossRef]
- Machida, S.; Miyata, S.; Techagumpuch, A. Chemical synthesis of high electrically conductive polypyrrole. Synth. Met. 1989, 31, 311–318. [Google Scholar] [CrossRef]
- Çolak, N.; Ozyilmaz, A. Polyaniline Coating on Iron–Synthesis and Characterization. Polym. Plast. Technol. Eng. 2005, 44, 1547–1558. [Google Scholar] [CrossRef]
- Camalet, J.L.; Lacroix, J.C.; Aeiyach, S.; Chane-Ching, K.; Lacaze, P.C. Electrode-posoition of protective polyaniline films on mild steel. J. Electroanal. Chem. 1996, 416, 179–182. [Google Scholar] [CrossRef]
- Lacroix, J.C.; Camalet, J.L.; Aeiyach, S.; Chane-Ching, K.L.; Petitjean, J.; Chauveau, E.; Lacaze, P.C. Aniline electro-polymerization on mild steel and zinc in a two step process. J. Electroanal. Chem. 2000, 481, 76–81. [Google Scholar] [CrossRef]
- Madhusudhana, G.; Santhi, R.J. Synthesis, characterization and corrosion behavior of isomers of conducting poly—Toluidine on mild steel in acid medium. Int. J. Sci. Res. 2015, 4, 1645–1650. [Google Scholar]
- Sathiyanarayanan, S.; Azim, S.S.; Venkatachari, G. Preparation of polyaniline TiO2 composite and its comparative corrosion protection performance with polyaniline. Synth. Met. 2007, 157, 205–213. [Google Scholar] [CrossRef]
- Chitte, H.K.; Bhat, N.V.; Gore, A.V.; Shind, G.N. Synthesis of polypyrrole using ammonium peroxydisulfate (APS) as oxidant together with some dopants for use in gas sensors. Mater. Sci. Appl. 2011, 2, S1491–S1498. [Google Scholar]
- Kamaraj, K.; Karpakam, V.; Sathiyanarayanan, S.; Syazim, S.; Venkatachari, G. Synthesis of tungstate doped polyaniline and its usefulness in corrosion protective coating. Electrochim. Acta 2011, 56, 9262–9268. [Google Scholar] [CrossRef]
- Taunk, M.; Kapil, A.; Chand, S. Synthesis and electrical characterization self-conducting polypyrrole-poly(vinylidene fluoride) composite films. Macromol. J. 2008, 2, 74–79. [Google Scholar] [CrossRef][Green Version]
- Ramesan, M.T. Synthesis, characterization and conductivity studies of polypyrrole copper sulfide nanocomposites. J. Appl. Polym. Sci. 2013, 128, 1540–1546. [Google Scholar] [CrossRef]
- Livingston, H.K.; Senkus, R.; Hsieh, J.T.T.; Kresta, J. The polymerization of furan on Surfaces. Macromol. Chem. Phys. 1972, 161, 101–111. [Google Scholar] [CrossRef]
- Granzow, A.; Wenedenburg, J.; Henglein, A. Die γ-radiolyse des furans and Thiophens. Z. Nat. B 1964, 19, 1015–1017. [Google Scholar] [CrossRef]
- Armour, M.; Davies, A.G.; Upadhyay, J.; Wassermann, A. Coloured electrically conducting polymers from furan, pyrrole and thiophene. J. Polym. Sci. 1967, 5, 1527–1538. [Google Scholar] [CrossRef]
- McConnell, R.; Godwin, W.R.; Baker, S.E.; Powell, K.; Baskett, A. Morara Polyfuran and copolymers a chemical synthesis. Int. J. Polym. Mater. Polym. Biomater. 2004, 53, 697–708. [Google Scholar] [CrossRef]
- Kudoh, Y. Properties of polypyrrole prepared by chemical polymerization using aqueous solution containing Fe2(SO4)3 and anionic surfactant. Synth. Met. 1996, 79, 17–22. [Google Scholar] [CrossRef]
- Jadhav, N.; Jensen, M.B.; Gelling, V. Tungstate and vanadate doped polypyrrole Aluminum flake composite coatings for the corrosion protection of aluminum 2024-T3. J. Coat. Technol. Res. 2015, 12, 259–276. [Google Scholar] [CrossRef]
- Bahrami, A.; Talib, Z.A.; Shahriari, E.; Yunus, W.M.A.; Kasim, A.; Behzad, K. Characterization of electrosynthesized conjugated polymer carbon nanotube composite optical nonlinearity and electrical property. Int. J. Mol. Sci. 2012, 13, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Hallik, A.; Alumaa, A.; Kurig, H.; Janes, A.; Lust, E.; Tamm, J. On the porosity of polypyrrole films. Synth. Met. 2007, 157, 1085–1090. [Google Scholar] [CrossRef]
- Mahmoudian, M.R.; Basirun, W.J.; Alias, Y. Synthesis and characterization of Poly (N-methypyrrole/TiO2 composites on steel. Appl. Surf. Sci. 2011, 257, 3702–3708. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Domenech, S.C.; Lacaze, P.C. Synthesis and characterization of polypyrrole/TiO2 composites on mild steel. J. Appl. Electrochem. 2001, 31, 49–56. [Google Scholar] [CrossRef]
- Subathira, A.; Meyyappan, R.M. Anticorrosion behavior of polyaniline/polypyrrole composite coating on stainless steel. Int. J. Chem. Sci. 2011, 9, 493–502. [Google Scholar]
- Akundy, G.S.; Rajagopalan, R.; Iroh, J.O. Electrochemical deposition of polyaniline polypyrrole composite coatings on aluminum. J. Appl. Polym. Sci. 2002, 83, 1970–1977. [Google Scholar] [CrossRef]
- Castagno, K.R.L.; Azambuja, D.S.; Dalmoro, V. Polypyrrole electropolymerized on aluminum alloy 1100 doped with oxalate and tungstate anions. J. Appl. Electrochem. 2009, 39, 93–100. [Google Scholar] [CrossRef]
- Sabouri, M.; Shahrabi, T.; Hosseini, M.G. Influence of tungstate ion dopants in corrosion Protection behavior of polyaniline coating on mild steel. Mater. Corros. 2008, 59, 814–818. [Google Scholar] [CrossRef]
- Karpakam, V.; Kamaraj, K.; Sathiyanarayanan, S.; Venkatachari, G.; Ramu, S. Electro synthesis of polyaniline molybdate coating on steel and its corrosion protection performance. Electrochim. Acta 2011, 56, 2165–2173. [Google Scholar] [CrossRef]
- Lenz, D.M.; Delamar, M.; Ferreira, C.A. Improvement of the anticorrosion properties of polypyrrole by zinc phosphate pigment incorporation. Prog. Org. Coat. 2007, 58, 64–69. [Google Scholar] [CrossRef]
- Aravindan, N.; Sangaranarayanan, M.V. Influence of solvent composition on the Anticorrosion performance of copper polypyrrole (Cu–PPy) coated 304 stainless steel steel. Prog. Org. Coat. 2016, 95, 38–45. [Google Scholar] [CrossRef]
- Shaktawat, V.; Jain, N.; Dixit, M.; Saxena, N.S.; Sharma, K.; Sharma, T.P. Temperature dependence of conductivity of polypyrrole doped with sulphuric acid. Indian. J. Pure Appl. Phys. 2008, 46, 427–430. [Google Scholar]
- Shankar, K.; Mor, G.K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Effect of device geometry on the performance of TiO2 nanotube array-organic semiconductor double heterojunction solar cells. J. Non-Cryst. Solids 2008, 354, 2767–2771. [Google Scholar] [CrossRef]
- Abidian, M.R.; Martin, D.C. Experimental and theoretical characterization of Implantable neural microelectrodes modified with conducting polymers nanotubes. Biomaterials 2008, 28, 1273–1283. [Google Scholar] [CrossRef]
- Zeng, T.W.; Lo, H.H.; Lin, Y.Y.; Chen, C.W.; Su, W.F. Hybrid poly(3-hexylthio Phene)/titanium dioxide nanorods material for solar cell applications. Sol. Energy Mater. Sol. Cell 2009, 93, 952–957. [Google Scholar] [CrossRef]
- Rupali, G.; Amitabha, D. Conducting polymer nanocomposites: A Brief Overview. Chem. Mater. 2000, 12, 608–622. [Google Scholar]
- Cao, Y.; Qiu, J.; Smith, P. Effect of solvent and co-solvents on the processability of polyaniline-spectroscopic and diffraction studies. Synth. Met. 1995, 69, 187–190. [Google Scholar] [CrossRef]
- Reghu, M.; Yoon, C.O.; Yang, C.Y.; Moses, D.; Heger, A.J. Superlocalization of the electronic wave functions in conductive polymer blends at concentrations near the percolation threshold. Macromolecules 1993, 26, 7245–7249. [Google Scholar] [CrossRef]
- Olinga, T.E.; Fraysse, J.; Travers, J.P.; Dufresne, A.; Pron, A. Highly conducting and solution-processable polyaniline obtained via protonation with a new sulfonic acid containing plasticizing functional groups. Macromolecules 2000, 33, 2107–2113. [Google Scholar] [CrossRef]
- Zhan, L.; Chen, H.; Fang, J.; Wang, S.; Ding, L.X.; Li, Z.; Ashman, P.J.; Wang, H. Coaxial Co3O4 polypyrrole core-shell nanowire arrays for high performance lithium ion. Electrochim. Acta 2016, 209, 192–200. [Google Scholar] [CrossRef]
- McGehee, D.G.; Topinka, M.A. Conducting Polymers Applications for Electronic Devices and Sensors. Nat. Mater. 2006, 5, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, P.; Srinivasan, S. Quantum jumps in the PEMFC science and techno-logy from the 1960s to the year 2000 Part II. Engineering, technology development and application aspects. J. Power Sources 2001, 102, 253–269. [Google Scholar] [CrossRef]
- Quartarone, E.; Angioni, S.; Mustarelli, P. Polymer and Composite Membranes for Proton-Conducting High-Temperature Fuel Cells. Materials 2017, 10, 687. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, B.; Thampi, K.R.; Bonard, J.M.; Mathieu, J.H.; Xanthopoulos, S.N.; Viswanathan, S. Conducting polymeric nanotubules as high-performance methanol oxidation catalyst catalyst Support. Chem. Commun. 2003, 16, 2022–2023. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.; Spreitzer, H.; Kreuder, W.; Kluge, E.; Schenk, H.; Parker, I.; Cao, Y. Soluble PPVs with enhanced performance A mechanistic approach. Adv. Mater. 2000, 12, 42–48. [Google Scholar] [CrossRef]
- Lee, S.H.; Jang, B.B.; Tsutsui, T. Sterically hindered fluorenyl substituted poly (p-phenylenevinylenes) for light-emitting diodes. Macromolecules 2002, 35, 1356–1364. [Google Scholar] [CrossRef]
- Jin, Y.; Kang, J.H.; Song, S.; Park, S.H.; Moon, J.; Woo, H.Y.; Lee, K.H.; Suh, H.S. Poly-(phenylenevinylene) derivatives containing a new electron-withdrawing phenyl group for LEDs. Bull. Korean Chem. Soc. 2008, 29, 139–147. [Google Scholar]
- Sokolik, I.; Yang, Z.; Karasz, F.E.; Morton, D.C. Blue-light electroluminescence from phenylene vinylene-based copolymers. J. Appl. Phys. 1993, 74, 3584–3586. [Google Scholar] [CrossRef]
- Pasquier, A.D.; Laforgue, A.; Simon, P.; Amatucci, G.G.; Fauvarque, J.F. A non-aqueous Asymmetric Hybrid Li4Ti5O12/Poly (fluorophenylthiophene) Energy Storage Device. J. Electrochem. Soc. 2002, 149, A302–A306. [Google Scholar] [CrossRef]
- Sarangapani, S.; Tilak, B.V.; Chen, C.P. Materials for electrochemical capacitors. J. Electrochem. Soc. 1996, 143, 3791–3799. [Google Scholar] [CrossRef]
- Faggioli, E.; Rena, P.; Danel, V.; Andrieu, X.; Mallant, R.; Kahlen, H. Supercapacitors for the energy management of electric vehicles. J. Power Sources 1999, 84, 261–269. [Google Scholar] [CrossRef]
- Ates, M.; Sarac, A.S. Electrochemical impedance spectroscopic study of poly-thiophene on carbon materials. Polym. Plast. Technol. Eng. 2011, 50, 1130–1148. [Google Scholar] [CrossRef]
- Ryu, K.S.; Lee, Y.; Has, K.S.; Park, Y.J.; Kang, M.G.; Park, N.G.; Chang, S.H. Electrochemial supercapacitor based on polyaniline doped with lithium salt and active carbon electrodes. Solid State Ion. 2004, 175, 765–769. [Google Scholar] [CrossRef]
- Garcia, M.L.A.; Smit, M.A. Study of electrodeposited polypyrrole coatings for the corrosion protection of stainless-steel bipolar plates for the PEM fuel cell. J. Power Sources 2006, 158, 397–402. [Google Scholar] [CrossRef]
- Ren, Y.J.; Chen, J.; Zeng, C.L. Corrosion protection of type 304 stainless steel bipolar plates of proton-exchange membrane fuel cells by doped polyaniline coating. J. Power Sources 2010, 195, 1914–1919. [Google Scholar] [CrossRef]
- Kilmartin, P.A.; Trier, L.; Wright, G.A. Corrosion inhibition of polyaniline and poly (o-methoxyaniline) on stainless steels. Synth. Met. 2002, 131, 99–109. [Google Scholar] [CrossRef]
- Ocon, P.; Ibanez, A.; Fatas, E. Electrochemical and mechanical properties of poly-pyrrole coatings on steel. Electrochim. Acta 2004, 49, 3693–3699. [Google Scholar]
- Tuken, T.; Ozyilmaz, A.T.; Yazic, B.; Erbil, M. Electrochemical synthesis of polyaniline on mild steel in acetonitrile-LiClO4 and corrosion performance. Appl. Surf. Sci. 2004, 236, 292–305. [Google Scholar] [CrossRef]
- Santros, J.R.; Mattoso, L.H.C.; Mothed, A.J. Investigation of corrosion protection of steel by polyaniline films. Electrochim. Acta 1998, 43, 309–313. [Google Scholar] [CrossRef]
- Talo, A.; Forsen, O.; Yeassani, S. Corrosion protective polyaniline epoxy blend coating on mild steel. Synth. Met. 1999, 102, 1394–1396. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Zhang, D.; Tang, Y.; Cang, H. Electrosynthesis of PANI/PPy coating doped by phosphotungstate on mild steel and their corrosion resistances. Prog. Org. Coat. 2015, 88, 84–91. [Google Scholar] [CrossRef]
- Sazou, D.; Georgolios, C. Formation of conducting polyaniline coatings on iron surfaces by electropolymerization of aniline in aqueous solutions. J. Electroanal. Chem. 1997, 429, 81–93. [Google Scholar] [CrossRef]
- Sazou, D. Electrodeposition of ring-substituted polyanilines on Fe surfaces from aqueous oxalic acid solutions and corrosion protection of Fe. Synth. Met. 2001, 118, 133–147. [Google Scholar] [CrossRef]
- Keles, H.; Solmaz, R.; Ozcan, M.; Kardas, G.; Dehri, I. Copper modified poly-6-amino m-cresol (poly-AmC/Cu) coating for mild steel protection. Surf. Coat. Technol. 2009, 203, 1469–1473. [Google Scholar] [CrossRef]
- Zhou, Q.; Shi, G. Conducting Polymer-Based Catalysts. J. Am. Chem. Soc. 2016, 138, 2868–2876. [Google Scholar] [CrossRef]
- Gao, F.; Hou, X.; Wang, A.; Chu, G.; Wu, W.; Chen, J.; Zou, H. Preparation of Polyyrrole/TiO2 nanocomposites with enhanced photocatalytic performance. Particuology 2016, 26, 73–78. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Wu, J.; Xin, B. One step preparation of Fe3O4/Pd@ polypyrrole composites with enhanced catalytic activity and stability. J. Colloid Interface Sci. 2016, 476, 214–221. [Google Scholar] [CrossRef]
- Hopkins, A.R.; Lewis, N.S. Detection and classification characteristics of arrays of carbon black/organic polymer composite chemiresistive vapor detectors for the nerve agent stimulants dimethyl methyl phosphonate and di isopropyl methyl phosponate. Anal. Chem. 2001, 73, 884–892. [Google Scholar] [CrossRef][Green Version]
- Doleman, B.J.; Lewis, N.S. Comparison of odor detection thresholds and odor discriminablities of a conducting polymer composite electronic nose versus mammalian olfaction. Sens. Actuators B 2001, 72, 41–50. [Google Scholar] [CrossRef]
- Jin, G.; Norrish, J.; Too, C.; Wallace, G. Polypyrrole filament sensors for gases and vapors. Curr. Appl. Phys. 2004, 4, 366–369. [Google Scholar] [CrossRef]
- Fang, Q.; Chetwynd, D.G.; Gardner, J.W. Conducting polymer films by UV photo Processing. Sens. Actuators A Phys. 2002, 99, 74–77. [Google Scholar] [CrossRef]
- Mashat, L.A.; Tran, H.D.; Wlodarski, W.; Kaner, R.B.; Zadeh, K.K. Conductometric hydrogen gas sensors based on polypyrrole nanofibers. Sens. J. 2008, 4, 365–370. [Google Scholar] [CrossRef]
- Nambiar, S.; Yeow, J. Conductive polymer-based sensors for biomedical applications. Biosens. Bioelectron. 2011, 26, 1825–1832. [Google Scholar] [CrossRef]
- Umana, M.; Waller, J. Protein modified electrodes: The glucose oxidase/polypyrrole system. Anal. Chem. 1986, 58, 2979–2983. [Google Scholar] [CrossRef]
- Osaka, T.; Komaba, S.; Fujino, Y.; Matsuda, T.; Satoh, I. High sensitivity flow injection analysis of urea using composite electropolymerized polypyrrole-polyion complex film. J. Electrochem. Soc. 1999, 146, 615–619. [Google Scholar] [CrossRef]
- Marco, M.P.; Barcelo, D. Environment applications of analytical biosensors. Meas. Sci. Technol. 1996, 7, 1547–1572. [Google Scholar] [CrossRef]
- Zheng, W. Effect of organic vapors on the molecular conformation of non-doped polyaniline. Synth. Met. 1997, 84, 63–64. [Google Scholar] [CrossRef]
- Selvakumar, S.; Somanathan, N.; Reddy, K.A. Chemi resistor sensors based on conducting polymers for hypergolic propellants and acidic vapors of rocket exhaust plumes—A review. Propellants Explos. Pyrotech. 2013, 38, 176–189. [Google Scholar] [CrossRef]
- Fu, X.W.; Liao, Z.M.; Zhou, J.X.; Zhou, Y.B.; Wu, H.C.; Zhang, R.; Yu, D. Strain dependent resistance in chemical vapor deposition grown graphene. Appl. Phys. Lett. 2011, 99, 213107. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.; Shi, Z.; Zhang, L.; Shi, D.; Wang, E.; Zhang, G. Super-elastic graphene ripples for flexible strain sensors. ACS Nano 2011, 5, 3645–3650. [Google Scholar] [CrossRef] [PubMed]
- Diaconu, I.; Dorohoi, D.O.; Topoliceanu, F. Electrostriction of a polyurethane elastomer-based polyester. IEEE Sens. J. 2006, 6, 876–880. [Google Scholar] [CrossRef]
- Shahinpoor, M. Ionic polymer–conductor composites as biomimetic sensors, robotic actuators and artificial muscles—A review. Electrochim. Acta 2003, 48, 2343–2353. [Google Scholar] [CrossRef]
- Chen, T.; Qiu, J.; Zhu, K.; He, X.; Kang, X.; Dong, E.L. Poly (methyl methacrylate)-functionalized graphene/polyurethane dielectric elastomer composites with superior electric field induced strain. Mater. Lett. 2014, 128, 19–22. [Google Scholar] [CrossRef]
- Zhu, S.E.; Shabani, R.; Rho, J.; Kim, Y.; Hong, B.H.; Ahn, J.H.; Cho, H.J. Graphene-based bimorph microactuators. Nano Lett. 2011, 11, 977–981. [Google Scholar] [CrossRef]
- Liang, J.; Huang, L.; Li, N.; Huang, Y.; Wu, Y.; Fang, S.; Baughman, R. Electromechanical actuator with controllable motion, fast response rate, and high-frequency resonance based on graphene and polydiacetylene. ACS Nano 2012, 6, 4508–4519. [Google Scholar] [CrossRef] [PubMed]
- Sen, I.; Seki, Y.; Sarikanat, M.; Cetin, L.; Gurses, B.O.; Ozdemir, O.; Yilmaz, O.C.; Sever, K.; Akar, E.; Mermer, O. Electroactive behavior of graphene nanoplatelets loaded cellulose composite actuators. Compos. Part B Eng. 2015, 69, 369–377. [Google Scholar] [CrossRef]
- Jung, J.H.; Jeon, J.H.; Sridhar, V.; Oh, I.K. Electro-active graphene–Nafion actuators. Carbon 2011, 49, 1279–1289. [Google Scholar] [CrossRef]
- Surana, K.; Singh, P.K.; Bhattacharya, B.; Verma, C.S.; Mehra, R.M. Synthesis of graphene oxide coated Nafion membrane for actuator application. Ceram. Int. 2015, 41, 5093–5099. [Google Scholar] [CrossRef]
- Loomis, J.; King, B.; Burkhead, T.; Xu, P.; Bessler, N.; Terentjev, E.; Panchapakesan, B. Graphene-nanoplatelet-based photomechanical actuators. Nanotechnology 2012, 23, 045501. [Google Scholar] [CrossRef]
- Loomis, J.; King, B.; Panchapakesan, B. Layer dependent mechanical responses of graphene composites to near-infrared light. Appl. Phys. Lett. 2012, 100, 073108. [Google Scholar] [CrossRef]
- Ansari, S.; Rahima, C.; Muralidharan, M.N. Photomechanical Characteristics of Thermally Reduced Graphene Oxide–Polydimethylsiloxane Nanocomposites. Polym. Plast. Technol. Eng. 2013, 52, 1604–1610. [Google Scholar] [CrossRef]
- Ansari, S.; Muralidharan, M.N.; Ushus, D. Graphene/poly (styrene--b--isoprene--b--styrene) nanocomposite optical actuators. J. Appl. Polym. Sci. 2013, 130, 3902–3908. [Google Scholar] [CrossRef]
- Deng, T.; Yoon, C.; Jin, Q.; Li, M.; Liu, Z.; Gracias, D.H. Selffolding graphene-polymer bilayers. Appl. Phys. Lett. 2015, 106, 203108. [Google Scholar] [CrossRef]
- Song, B.; Wu, Z.; Zhu, Y.; Moon, K.S.; Wong, C.P. Three-dimensional graphene-based composite for flexible electronic applications. In Proceedings of the 2015 IEEE 65th Electronic Components and Technology Conference (ECTC), San Diego, CA, USA, 26–29 May 2015; pp. 1803–1807. [Google Scholar]
- Kim, Y.J.; Park, H.C.; Kim, B.K. Triple shape-memory effect bysilanized polyurethane/silane-functionalized graphene oxide nanocomposites bilayer. High Perform. Polym. 2015, 27, 886–897. [Google Scholar] [CrossRef]
- Tan, L.; Gan, L.; Hu, J.; Zhu, Y.; Han, J. Functional shape memory composite nanofibers with graphene oxide filler. Compos. Part A Appl. Sci. Manuf. 2015, 76, 115–123. [Google Scholar] [CrossRef]
- Ponnamma, D.; Sadasivuni, K.K.; Strankowski, M.; Moldenaers, P.; Thomas, S.; Grohens, Y. Interrelated shape memory and Payne effect in polyurethane/graphene oxide nanocomposites. RSC Adv. 2013, 3, 16068–16079. [Google Scholar] [CrossRef]
- Tao, L.; Zhou, B.; Bai, G.; Wang, Y.; Yu, S.F.; Lau, S.P.; Xu, D. Fabrication of covalently functionalized graphene oxide incorporated solid-state hybrid silica gel glasses and their improved nonlinear optical response. J. Phys. Chem. C 2013, 117, 23108–23116. [Google Scholar] [CrossRef]
- Du, Y.; Dong, N.; Zhang, M.; Zhu, K.; Na, R.; Zhang, S.; Wang, J. Covalent functionalization of graphene oxide with porphyrin and porphyrin incorporated polymers for optical limiting. Phys. Chem. Chem. Phys. 2017, 19, 2252–2260. [Google Scholar] [CrossRef]
- Gan, Y.; Feng, M.; Zhan, H. Enhanced optical limiting effects of graphene materials in polyimide. Appl. Phys. Lett. 2014, 104, 171105. [Google Scholar] [CrossRef]
- Husaini, S.; Slagle, J.E.; Murray, J.M.; Guha, S.; Gonzalez, L.P.; Bedford, R.G. Broadband saturable absorption and optical limiting in graphene-polymer composites. Appl. Phys. Lett. 2013, 102, 191112. [Google Scholar] [CrossRef]
- Elie, A.G. Electroconductive hydrogels: Synthesis, characterization and biomedical applications. Biomaterials 2010, 31, 2701–2716. [Google Scholar] [CrossRef]
- Owens, D.R.; Zinman, B.; Bolli, G. Alternative routes of insulin delivery. Diabet. Med. 2003, 20, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.C.; Moulton, S.E.; Ding, J.; Richardson, R.; Cameron, A.; Leary, S.O.; Wallace, G.G.; Clark, G.M. Optimizing the incorporation and release of a neurotrophic factor using conducting polypyrrole. J. Control Release 2006, 116, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Matranga, C.; Tan, S.; Alba, N.; Cui, X.T. Carbon nanotube nano-reservoir for controlled release of anti-inflammatory dexamethasone. Biomaterials 2011, 32, 6316–6323. [Google Scholar] [CrossRef]
- Herrasti, P.; Kulak, A.N.; Bavykin, D.V.; Ponce, C.; Walsh, F.C. Electrodeposition of polypyrrole titanate nanotube composites coatings and their corrosion resistance. Electrochim. Acta 2011, 56, 1323–1328. [Google Scholar] [CrossRef]
- Massoumi, B.; Entezami, A.A. Electrochemically stimulated 2-ethyl hexyl phosphate (EHP) release through redox switching of conducting polypyrrole film and polypyrrole/poly (N-methylpyrrole) self-doped polyaniline bilayers. Polym. Int. J. 2002, 51, 555–560. [Google Scholar] [CrossRef]
- Miller, L.L.; Zhou, X.U. Poly (N-methyl pyrrolylium) poly (styrene sulfonate) a conductive electrically switchable cation exchanger that cathodically binds and anodically releases dopamine. Macromolecules 1987, 20, 1594–1597. [Google Scholar] [CrossRef]
- Bendrea, A.D.; Cianga, L.; Cianga, I. Progress in the field of conducting polymers for tissue engineering applications. J. Biomater. Appl. 2011, 26, 3–84. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, L.; Li, L.; Cheng, W.; Xu, L.; Ping, X.; Pan, L.; Shi, Y. Conducting Polymers and Their Applications in Diabetes Management. Sensors 2016, 16, 1787. [Google Scholar] [CrossRef]
- Gupta, K.; Jana, P.; Meikap, A.; Nath, T. Synthesis of La0.67Sr0.33MnO3 and polyaniline nanocomposite with its electrical and magneto-transport properties. J. Appl. Phys. 2010, 107, 073704. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, S.; Li, H.; Zhang, S.; Tan, Z.-C. Thermal stability of several polyaniline/rare earth oxide composites: Part IV. Polyaniline/La2O3 and polyaniline/Sm2O3 composites. J. Therm. Anal. Calorim. 2014, 115, 259–266. [Google Scholar] [CrossRef]
- Wang, S.; Huang, Z.; Wang, J.; Li, Y.; Tan, Z. Thermal stability of several polyaniline/rare earth oxide composites (I): Polyaniline/CeO2 composites. J. Therm. Anal. Calorim. 2012, 107, 1199–1203. [Google Scholar] [CrossRef]
- Thayyil, S.; Al-Maghrabi, M.; Bahuleyan, B.; Ramesan, M.T. Synthesis, characterization, thermal properties, conductivity and sensor application study of polyaniline/cerium-doped titanium dioxide nanocomposites. J. Mater. Sci. 2018, 53, 591–603. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, S.; Huang, Z.; Li, Y.; Tan, Z. A kinetic analysis of thermal decomposition of polyaniline and its composites with rare earth oxides. J. Therm. Anal. Calorim. 2014, 119, 1853–1860. [Google Scholar] [CrossRef]
- Parvatikar, N.; Jain, S.; Khasim, S.; Revansiddappa, M.; Bhoraskar, S.; Prasad, M. Electrical and humidity sensing properties of polyaniline/WO3 composites. Sens. Actuators B Chem. 2006, 114, 599–603. [Google Scholar] [CrossRef]
- Khasim, S.; Ansari, J.; Parveen, A.; Al-Hartomy, O.; Khattar, Z.; Badi, N.; Roy, A. Synthesis, characterization, dielectric and rectification properties of PANI/Nd2O3:Al2O3 nanocomposites. Polym. Adv. Technol. 2016, 27, 1064–1071. [Google Scholar] [CrossRef]
- Hoshino, K.; Yazawa, N.; Tanaka, Y.; Chiba, T.; Izumizawa, T.; Kubo, M. Polycarbazole nanocomposites with conducting metal oxides for transparent electrode applications. ACS Appl. Mater. Interfaces 2010, 22, 413–424. [Google Scholar] [CrossRef]
- Sever, E.; Unal, H. Colloidal properties of surface functionalized nanocube-TiO2/poly(3-octylthiophene) core/shell conducting nanocomposite. Appl. Surf. Sci. 2015, 355, 1028–1036. [Google Scholar] [CrossRef]
- Erdönmez, S.; Karabul, Y.; Kilic, M.; Özdemir, Z.; Esmer, K. Structural characterization and dielectric parameters of Polyindole/WO3 nanocomposites. Polym. Compos. 2021, 42, 1347–1355. [Google Scholar] [CrossRef]
- Galembeck, A.; Alves, O. Chemical polymerization of pyrrole on CeO2 films. Synth. Met. 1997, 84, 151–152. [Google Scholar] [CrossRef]
- Karimi, A.; Husain, S.W.; Hosseini, M.; Azar, P.A.; Ganjali, M.R. Rapid and sensitive detection of hydrogen peroxide in milk by Enzyme-free electrochemiluminescence sensor based on a polypyrrole-cerium oxide nanocomposite. Sens. Actuators B Chem. 2018, 271, 90–96. [Google Scholar] [CrossRef]
- Seemaa, S.; Ambika Prasad, M.V.N. Synthesis, Characterisation and Conductivity Studies of Polypyrrole-Nb2O5 Composites. Int. J. Ethics Eng. Manag. 2014, 1, 270–272. [Google Scholar]
- Vishnuvardhan, T.K.; Kulkarni, V.R.; Basavaraja, C.; Raghavendra, S.C. Synthesis, characterization and A.C. conductivity of polypyrrole/Y2O3 composites. Bull. Mater. Sci. 2006, 29, 77–83. [Google Scholar] [CrossRef]
- Sun, W.; Mo, Z. Polypyrrole coated graphene nanoplatelets and the effect of rare earth ions with nanocomposites. J. Polym. Res. 2014, 21, 516. [Google Scholar] [CrossRef]
- Majumder, M.; Choudhary, R.; Thakur, A.; Karbhal, I. Impact of rare-earth metal oxide (Eu2O3) on the electrochemical properties of a polypyrrole/CuO polymeric composite for supercapacitor applications. RSC Adv. 2017, 7, 20037–20048. [Google Scholar] [CrossRef]
- Reddy, M.O.; Chandra Babu, B. Structural, Optical, Electrical, and Magnetic Properties of PVA: Gd3+ and PVA: Ho3+ Polymer Films. Indian J. Mater. Sci. 2015, 2015, 927364. [Google Scholar] [CrossRef][Green Version]
- Mohanapriya, M.K.; Deshmukh, K.; Ahamed, M.B.; Chidambaram, K.; Khadheer Pasha, S.K. Influence of cerium oxide (CeO2) nanoparticles on the structural, Morphological, mechanical and dielectric properties of PVA/PPy blend Nanocomposites. Mater. Today Proc. 2016, 3, 1864–1873. [Google Scholar] [CrossRef]
- Song, J.; Lu, C.; Xu, D.; Ni, Y.; Liu, Y.; Xu, Z.; Liu, J. The effect of lanthanum oxide (La2O3) on the structure and crystallization of poly(vinylidene fluoride). Polym. Int. 2010, 59, 954–960. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Asyraf, M.R.M.; Dayana, D.A.Z.N.; Amelia, J.J.N.; Rani, M.S.A.; Norrrahim, M.N.F.; Nurazzi, N.M.; Aisyah, H.A.; Sharma, S.; et al. Polymer Composites Filled with Metal Derivatives: A Review of Flame Retardants. Polymers 2021, 13, 1701. [Google Scholar] [CrossRef] [PubMed]
- Chohan, J.S.; Mittal, N.; Kumar, R.; Singh, S.; Sharma, S.; Dwivedi, S.P.; Saxena, A.; Chattopadhyaya, S.; Ilyas, R.A.; Le, C.H.; et al. Optimization of FFF Process Parameters by Naked Mole-Rat Algorithms with Enhanced Exploration and Exploitation Capabilities. Polymers 2021, 13, 1702. [Google Scholar] [CrossRef]
- Chohan, J.S.; Mittal, N.; Kumar, R.; Singh, S.; Sharma, S.; Singh, J.; Rao, K.V.; Mia, M.; Pimenov, D.Y.; Dwivedi, S.P. Mechanical Strength Enhancement of 3D Printed Acrylonitrile Butadiene Styrene Polymer Components Using Neural Network Optimization Algorithm. Polymers 2020, 12, 2250. [Google Scholar] [CrossRef]
- Singh, Y.; Singh, J.; Sharma, S. Multi-objective Optimization of Kerf-taper and Surface-roughness Quality Characteristics for Cutting-operation On Coir and Carbon Fibre Reinforced Epoxy Hybrid Polymeric Composites During CO2-Pulsed Laser-cutting Using RSM. Lasers Manuf. Mater. Process. 2021, 8, 157–182. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, J.; Kumar, H.; Sharma, A.; Aggarwal, V.; Gill, A.; Jayarambabu, N.; Kailasa, S.; Rao, K.V. Utilization of rapid prototyping technology for the fabrication of an orthopedic shoe inserts for foot pain reprieve using thermo-softening viscoelastic polymers: A novel experimental approach. Meas. Control 2020, 53, 519–530. [Google Scholar] [CrossRef]
- Singh, Y.; Singh, J.; Sharma, S.; Sharma, A.; Chohan, J. Process Parameter Optimization in Laser Cutting of Coir Fiber Reinforced Epoxy Composite—A Review. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Chohan, J.S.; Kumar, R.; Singh, T.B.; Singh, S.; Sharma, S.; Singh, J.; Mia, M.; Pimenov, D.Y.; Chattopadhyaya, S.; Dwivedi, S.P.; et al. Taguchi S/N and TOPSIS Based Optimization of Fused Deposition Modelling and Vapor Finishing Process for Manufacturing of ABS Plastic Parts. Materials 2020, 13, 5176. [Google Scholar] [CrossRef] [PubMed]
- Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Rahimian Koloor, S.S.; Petrů, M. Micro- and Nanocellulose in Polymer Composite Materials: A Review. Polymers 2021, 13, 231. [Google Scholar] [CrossRef]
- Prabhakaran, S.; Vijayan, K.; Sharma, S.; Mouleeswaran, S.K.; Ramasamy, J.K.; Redoune, Z. Experimental study on thermal and morphological analysis of Green composite sandwich made of Flax and agglomerated cork. J. Therm. Anal. Calorim. 2020, 139, 3003–3012. [Google Scholar] [CrossRef]
- Singh, Y.; Singh, J.; Sharma, S.; Lam, T.D.; Nguyen, D.N. Fabrication and characterization of coir/carbon-fiber reinforced epoxy-based hybrid composite for helmet shells and sports-good applications: Influence of fiber surface modifications on the mechanical, thermal and morphological properties. J. Mater. Res. Technol. 2020, 9, 15593–15603. [Google Scholar] [CrossRef]
- Li, H.-Y.; Huang, D.-N.; Ji, J. Inorganic-polymer composite coatings for biomedical devices. Smart Mater. Med. 2021, 2, 1–14. [Google Scholar] [CrossRef]
- Rikhari, B.; Mani, S.P.; Rajendran, N. Polypyrrole/graphene oxide composite coating on Ti implants: A promising material for biomedical applications. J. Mater. Sci. 2020, 55, 5211–5229. [Google Scholar] [CrossRef]
- Nautiyal, A.; Qiao, M.; Cook, J.; Zhang, X.; Huang, T. High Performance Polypyrrole Coating for Corrosion Protection and Biocidal Applications. Appl. Surf. Sci. 2018, 427, 922–930. [Google Scholar] [CrossRef]
- Yadav, R.; Tirumali, M.; Wang, X.; Naebe, M.; Kandasubramanian, B. Polymer composite for antistatic application in aerospace. Def. Technol. 2020, 16, 107–118. [Google Scholar] [CrossRef]
- Zamiri, G.; Haseeb, A.S.M.A. Recent Trends and Developments in Graphene/Conducting Polymer Nanocomposites Chemiresistive Sensors. Materials 2020, 13, 3311. [Google Scholar] [CrossRef]
- Ghosh, S.; Das, S.; Mosquera, M.E.G. Conducting Polymer-Based Nanohybrids for Fuel Cell Application. Polymers 2020, 12, 2993. [Google Scholar] [CrossRef]
- Sharma, S.; Sudhakara, P.; Singh, J.; Ilyas, R.A.; Asyraf, M.R.M.; Razman, M.R. Critical Review of Biodegradable and Bioactive Polymer Composites for Bone Tissue Engineering and Drug Delivery Applications. Polymers 2021, 13, 2623. [Google Scholar] [CrossRef]


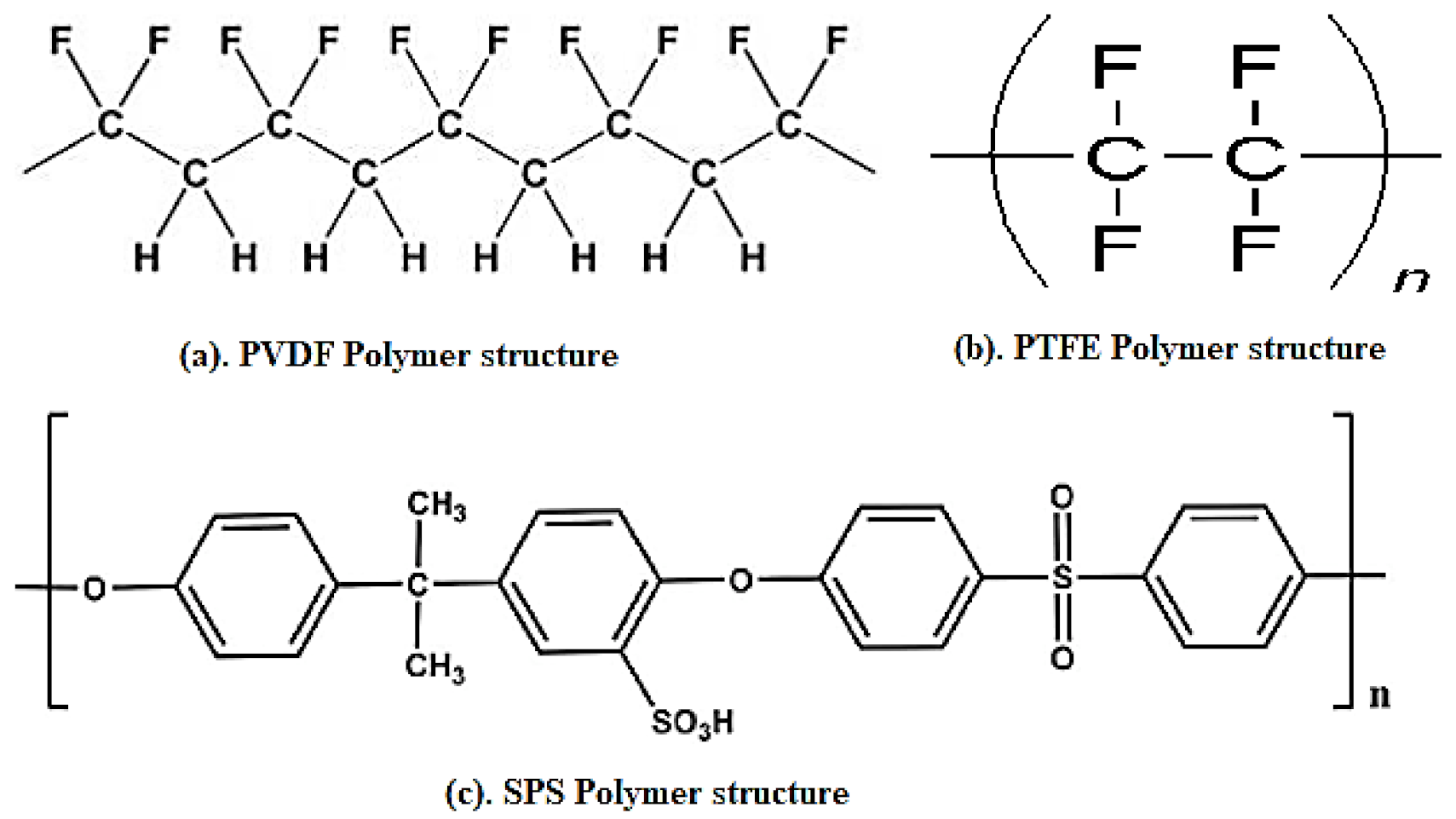

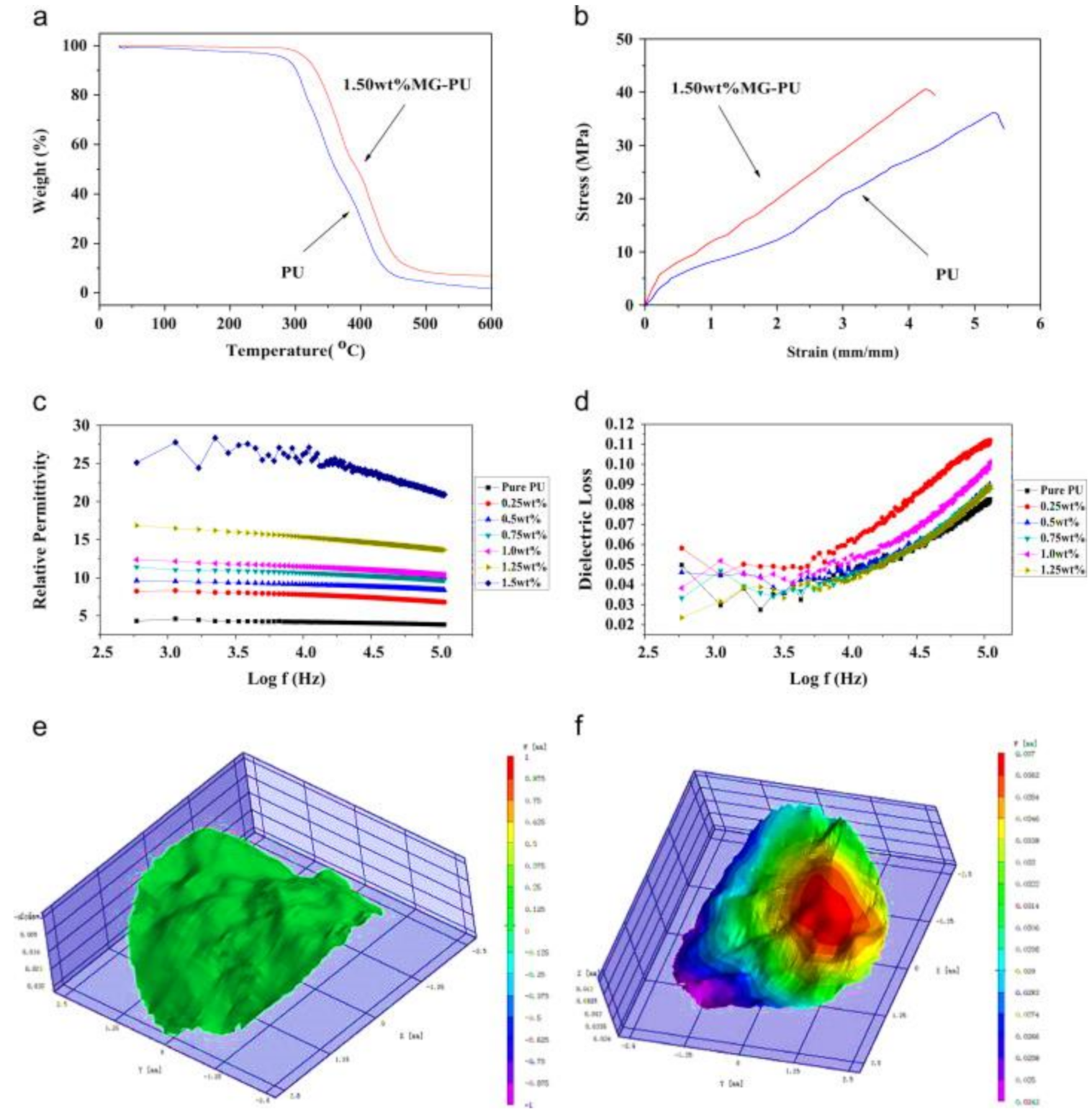

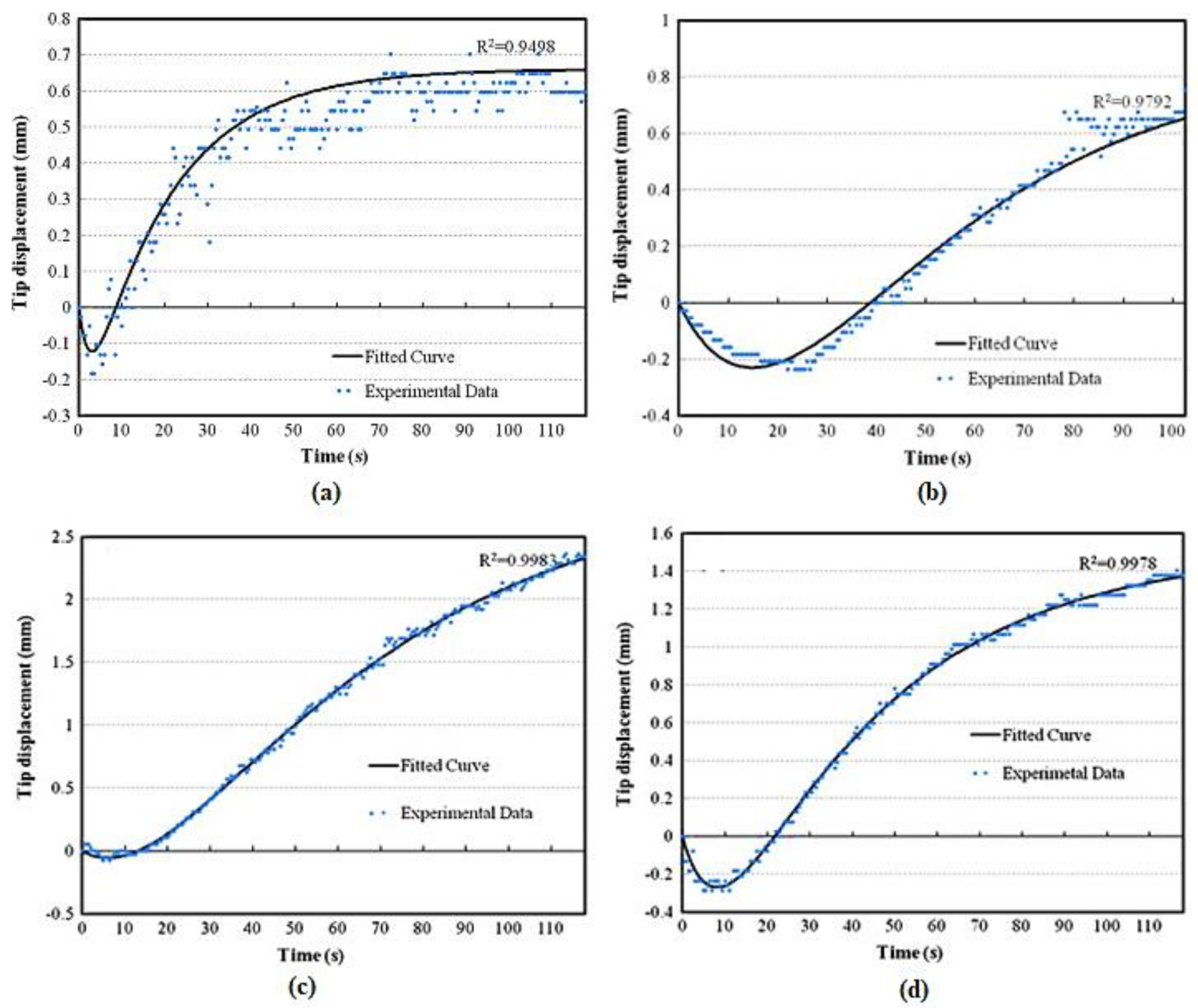
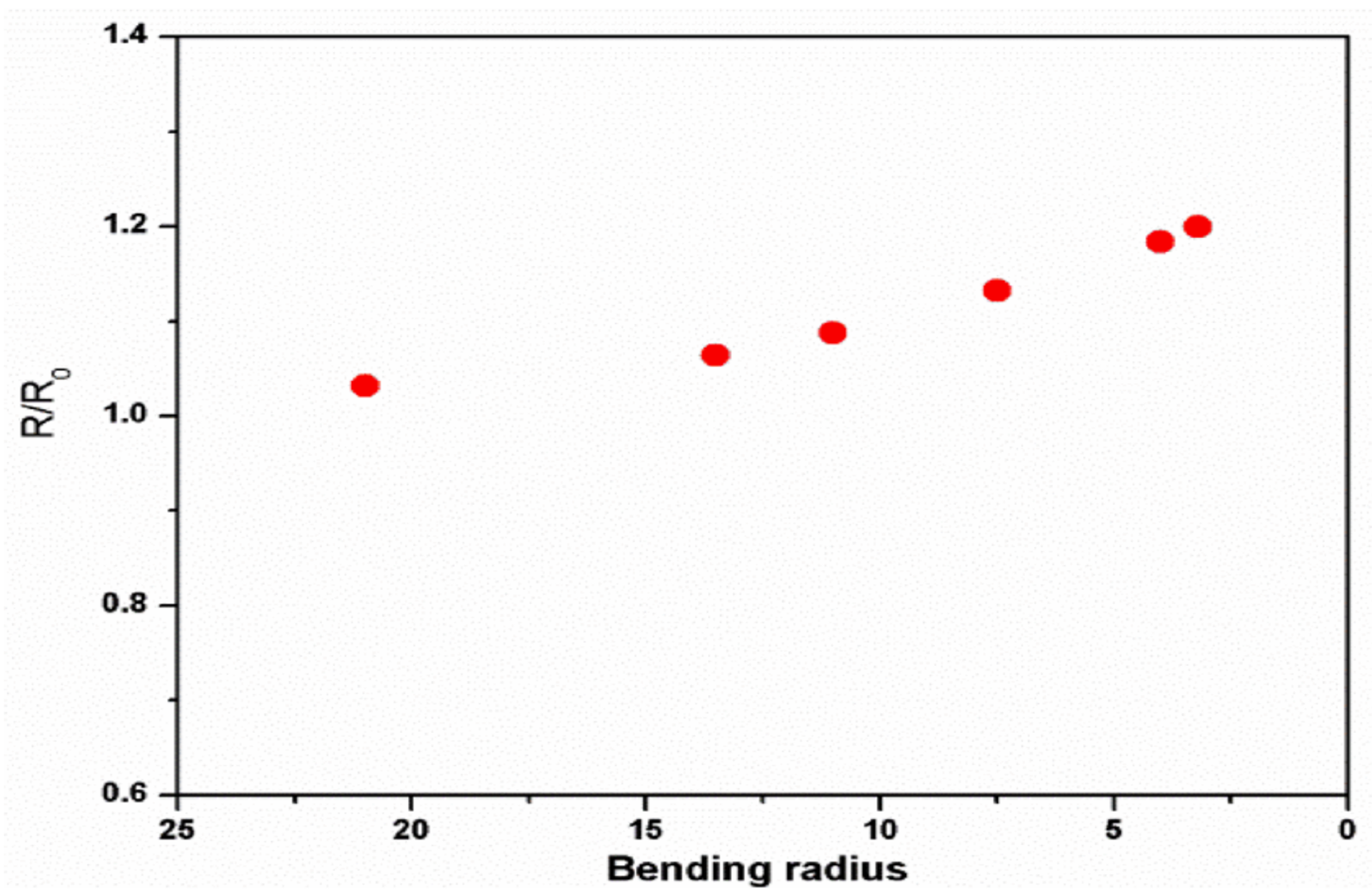
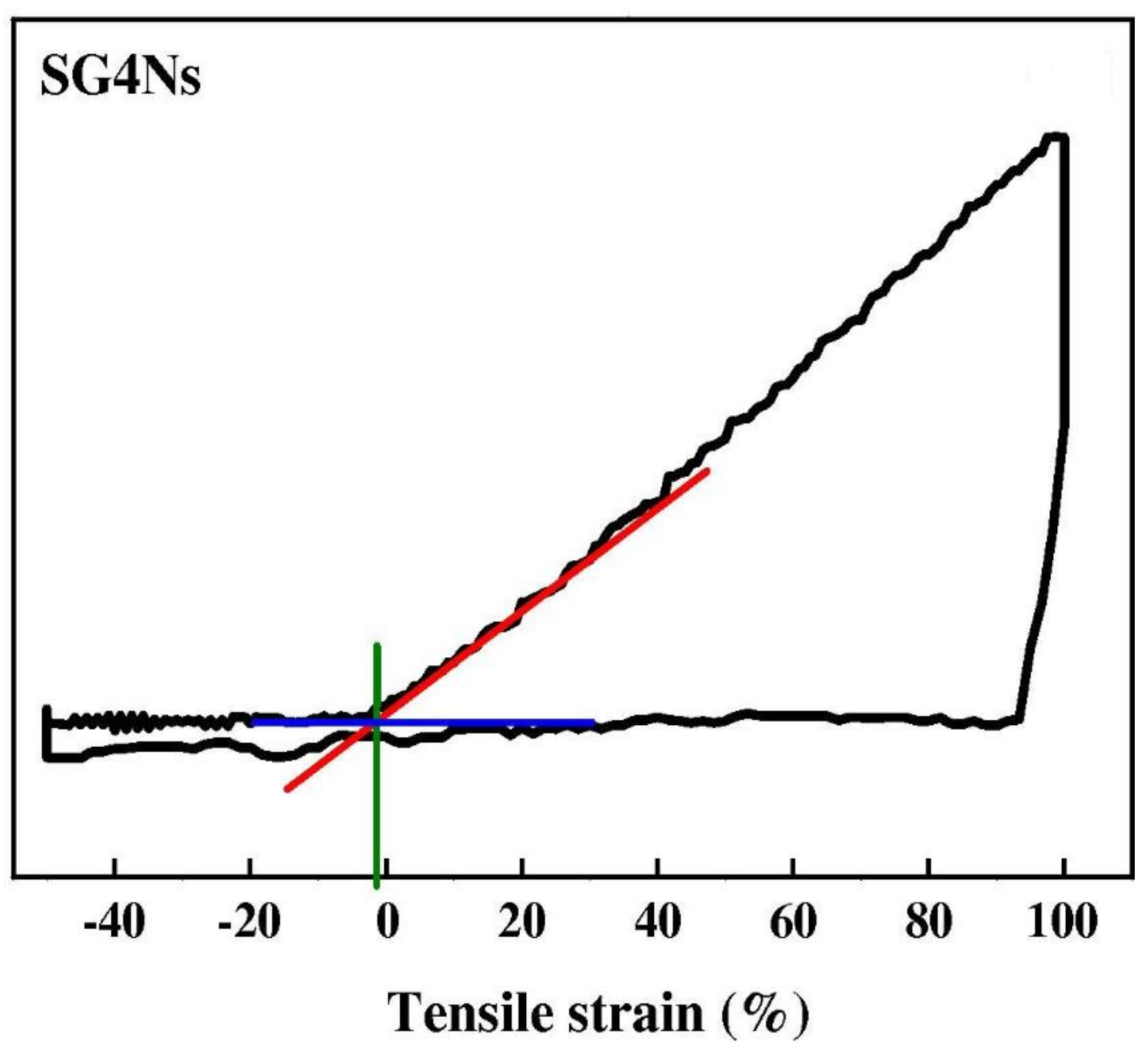
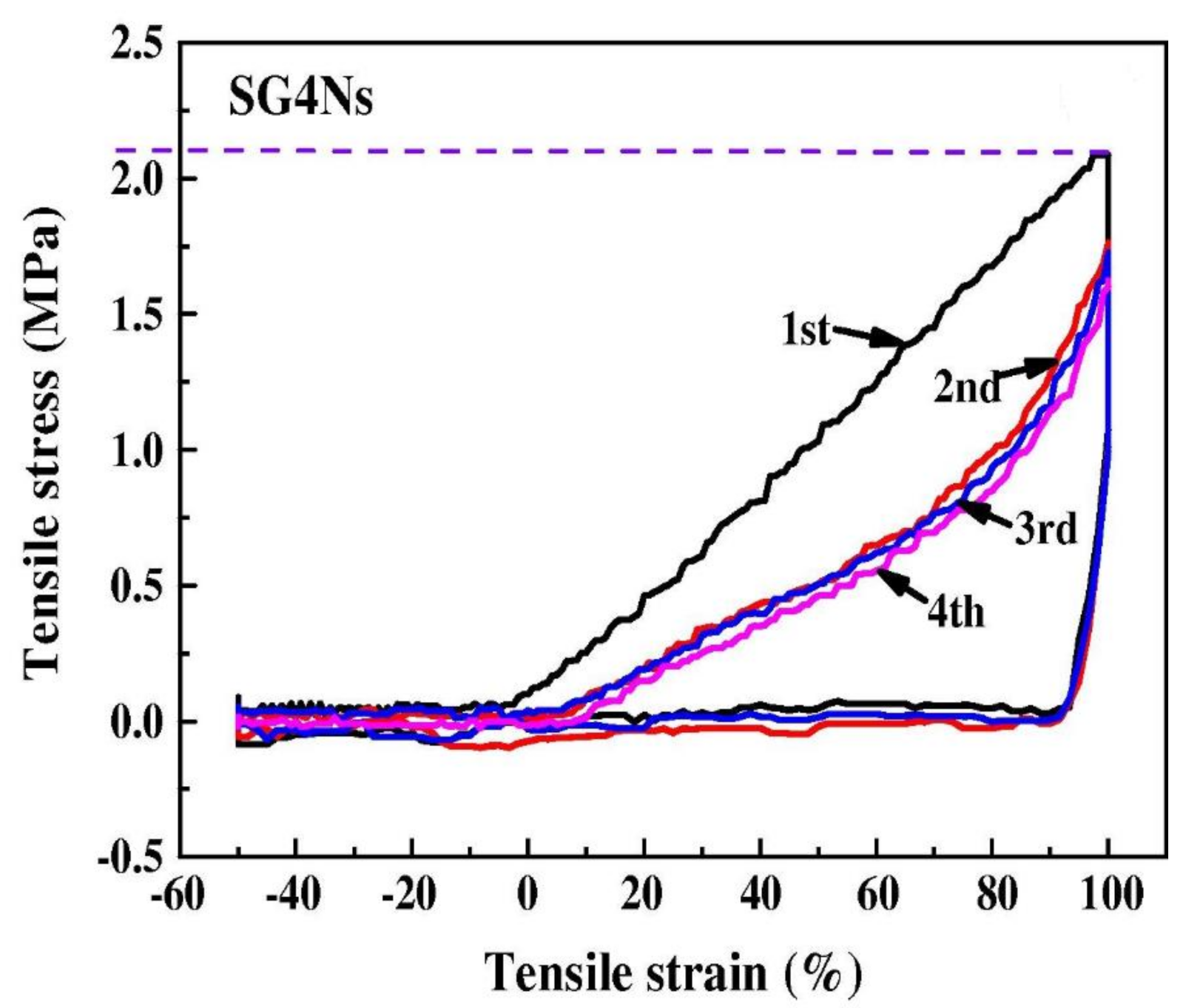

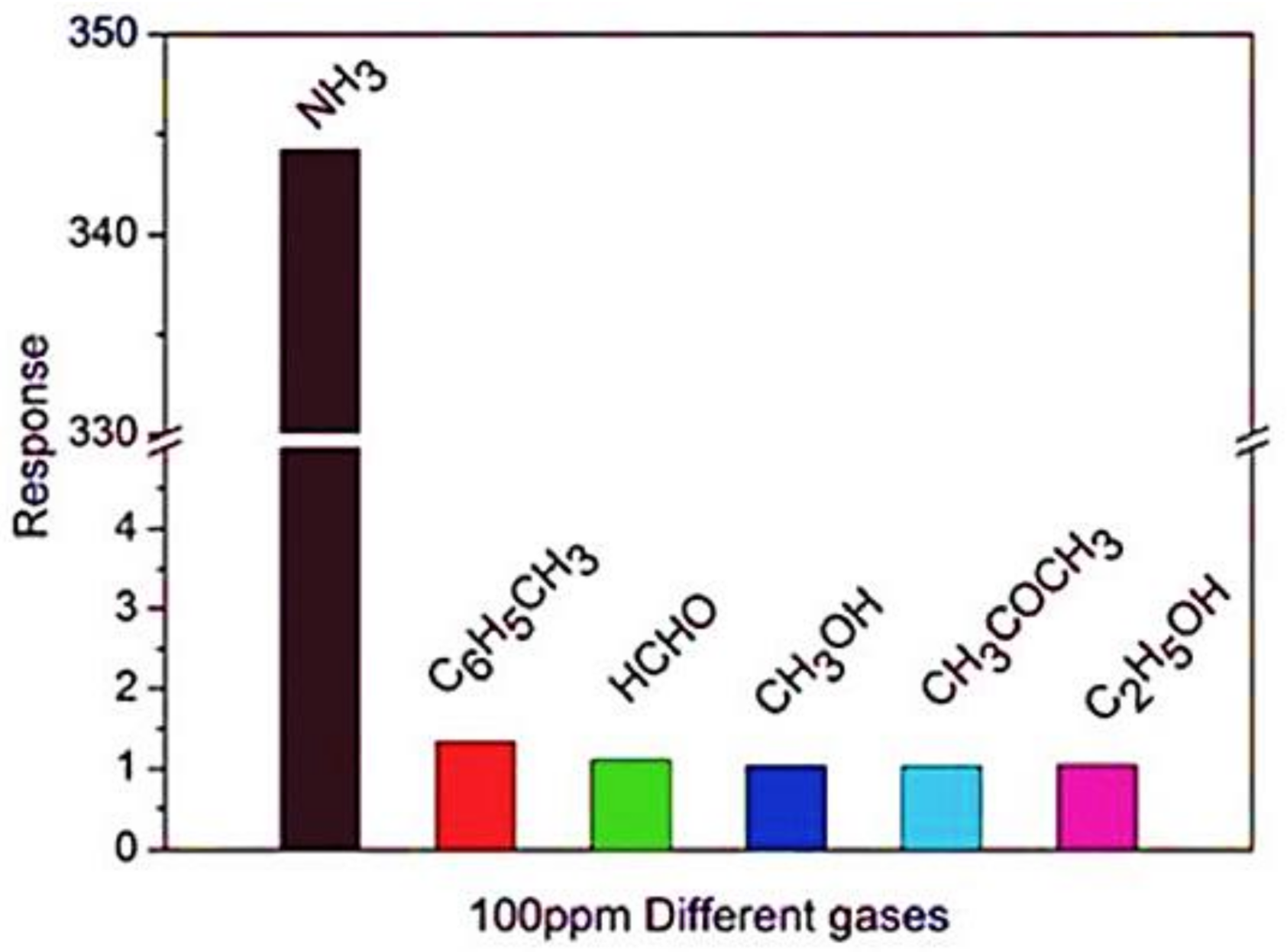
| Polymer | REO | Applications | References |
|---|---|---|---|
| Poly(ethylene oxide) (PEO) | La2O3 | Semiconductor and Solid Polymer Electrolyte (SPE) | [4] |
| Polyaniline (PANI) | La0.67Sr0.33MnO3 | Sensor | [110] |
| PANI | Sm2O3, La2O3 | Thermally stable material | [111] |
| PANI | CeO2 | Thermally stable material | [112] |
| PANI | CeO2 | Semiconductor and supercapacitor | [112] |
| PANI | La-Nd | Electromagnetic Interference | [11] |
| PANI | Ce-TiO2 | Sensor | [113] |
| PANI | CeO2, Dy2O3 | Thermally stable material | [114] |
| PANI | Terbium(iii) | Light Emitting Diode | [10] |
| PANI | WO3 | Sensing | [115] |
| PANI | Nd2O3:Al2O3 | Dielectric constant | [116] |
| Polycarbazole | - | Semiconductor | [117] |
| Polyindole (PIN) | TiO2 | Semiconductor | [118] |
| PIN | Y2O3 | Dielectric constant | [119] |
| Polypyrrole (PPY) | CeO2 | Semiconductor | [120] |
| PPY | CeO2 | Sensor | [121] |
| PPY | Nb2O5 | Semiconductor | [122] |
| PPY | Y2O3 | Semiconductor | [2] |
| PPY | Sm2O3 | Supercapacitor | [6] |
| PPY | Y2O3 | Batteries, sensors and actuators | [123] |
| PPY | La3+, Sm3+, Tb3+, Eu3+ | Supercapacitor | [124] |
| PPY | RuO2 | Supercapacitor | [9] |
| PPY | Eu2O3 | Supercapacitor | [125] |
| PPY | Y2O3 | Dielectric constant | [123] |
| Polyvinyl Alcohol (PVA) | Ho3+, Gd3+ | Optical display | [126] |
| PVA/PPY | - | Dielectric | [127] |
| Polyvinylidene fluoride (PVDF) | La2O3 | Thermally stable material | [128] |
| Chemical Polymerization | Electrochemical Polymerization |
|---|---|
| Yield of the product is large in amount | Yield is less, and synthesis of the thin film is possible |
| Synthesis is difficult | Synthesis is quite easy |
| They do not offer control of polymerization and doping level | In this method, polymerization and doping levels can be controlled |
| Doping and polymerization do not occur simultaneously | Doping and polymerization occur simultaneously |
| Polymer is easily collected and packed | Difficult to remove the film from the electrode surface |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Sudhakara, P.; Omran, A.A.B.; Singh, J.; Ilyas, R.A. Recent Trends and Developments in Conducting Polymer Nanocomposites for Multifunctional Applications. Polymers 2021, 13, 2898. https://doi.org/10.3390/polym13172898
Sharma S, Sudhakara P, Omran AAB, Singh J, Ilyas RA. Recent Trends and Developments in Conducting Polymer Nanocomposites for Multifunctional Applications. Polymers. 2021; 13(17):2898. https://doi.org/10.3390/polym13172898
Chicago/Turabian StyleSharma, Shubham, P. Sudhakara, Abdoulhdi A. Borhana Omran, Jujhar Singh, and R. A. Ilyas. 2021. "Recent Trends and Developments in Conducting Polymer Nanocomposites for Multifunctional Applications" Polymers 13, no. 17: 2898. https://doi.org/10.3390/polym13172898
APA StyleSharma, S., Sudhakara, P., Omran, A. A. B., Singh, J., & Ilyas, R. A. (2021). Recent Trends and Developments in Conducting Polymer Nanocomposites for Multifunctional Applications. Polymers, 13(17), 2898. https://doi.org/10.3390/polym13172898







