Dimethylimidazolium-Functionalized Polybenzimidazole and Its Organic–Inorganic Hybrid Membranes for Anion Exchange Membrane Fuel Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Polybenzimidazole (PBI)
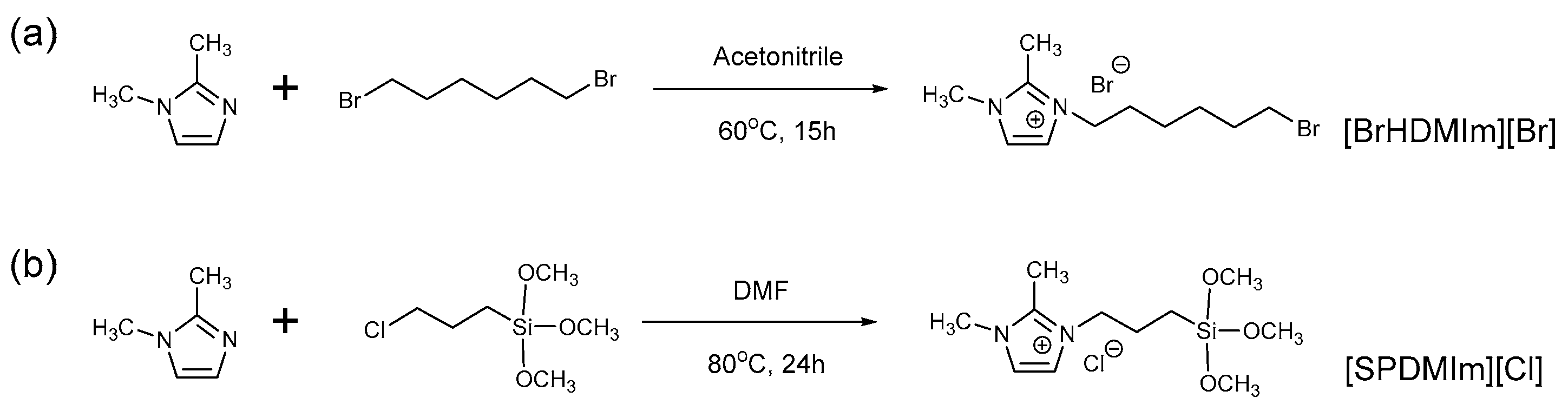
2.3. Synthesis of Bromohexyl Dimethylimidazolium Bromide ([BrHDMIm] [Br])
2.4. Synthesis of Triethoxysilylpropyl Dimethylimidazolium Chloride ([SPDMIm] [Cl])
2.5. Synthesis of Dimethylimidazolium-Functionalized Polybenzimidazole (PBI-DIm)
2.6. Fabrication of PBI-DIm Pristine Membranes and PBI-DIm-Si Hybrid Membranes
2.7. Characterization
2.8. Measurements
2.9. Hydroxide Conductivity and Alkaline Stability
2.10. Mechanical Properties and Oxidative Stability
2.11. Fuel Cell Tests
3. Results and Discussion
3.1. Synthesis and Characterization
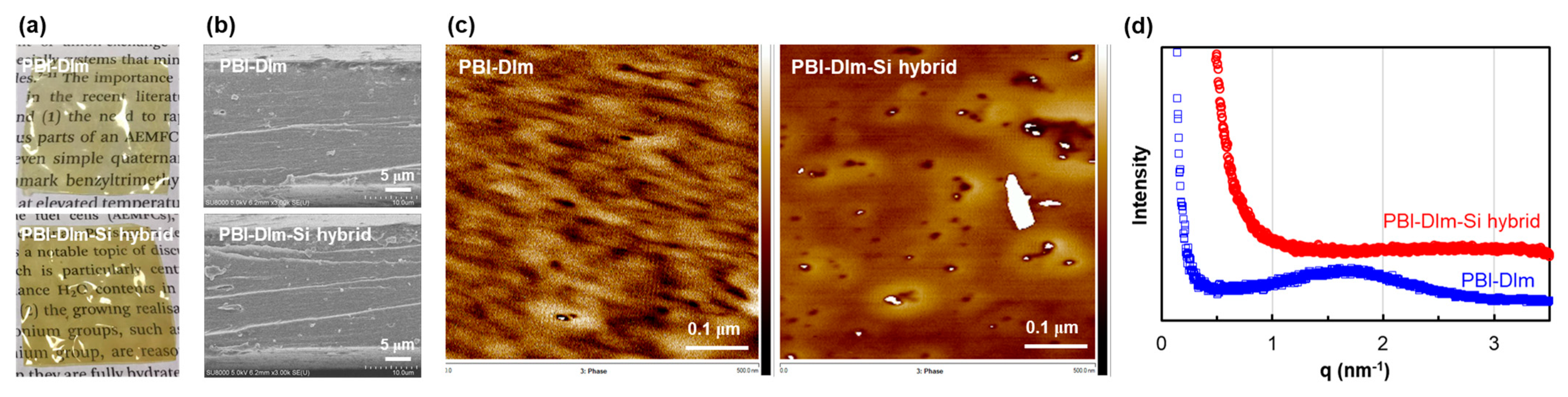
3.2. Membrane Morphology
3.3. Ion Exchange Capacity, Water Uptake and Swelling Ratio
3.4. Hydroxide Conductivity and Alkaline Stability
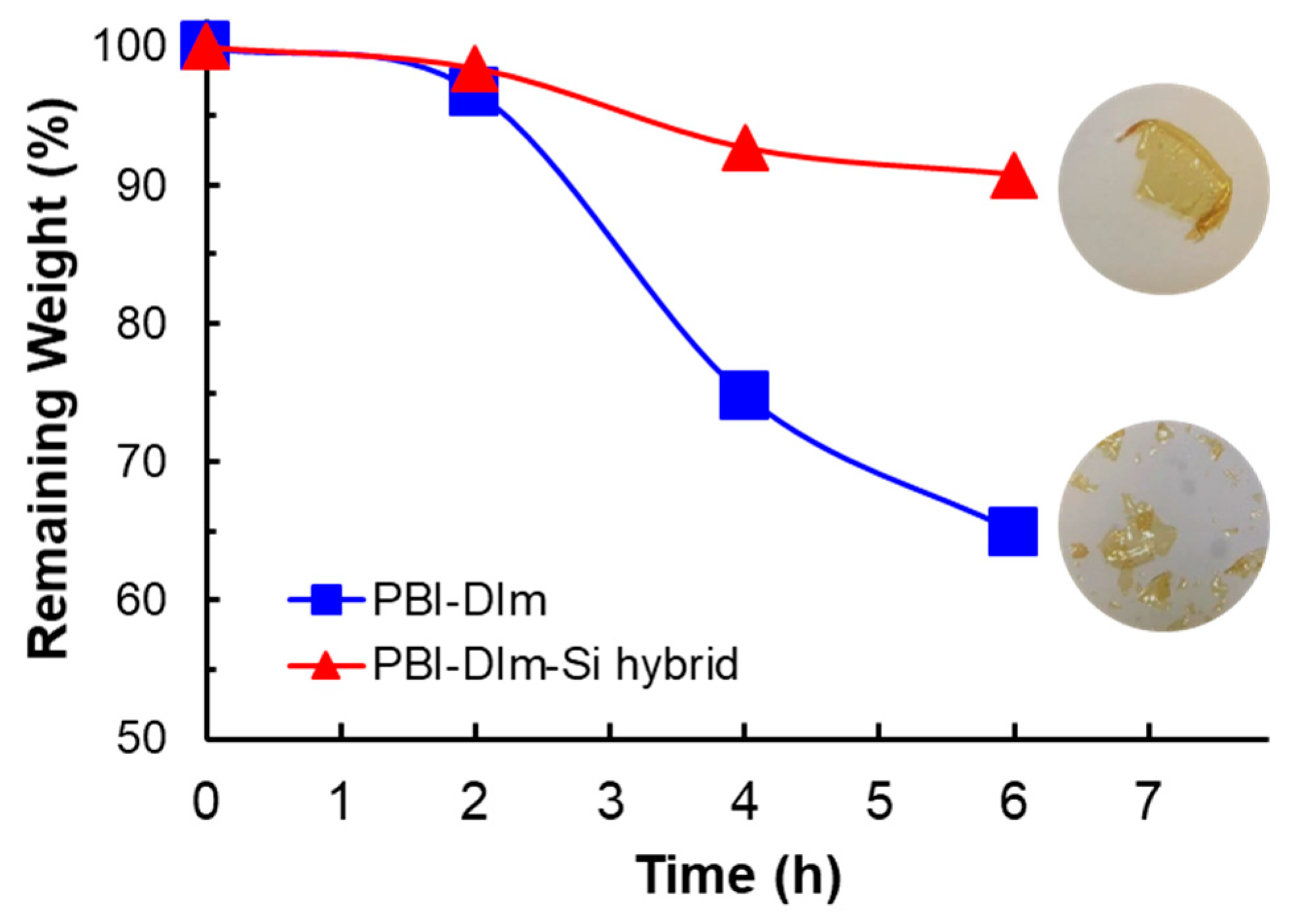
3.5. Mechanical Properties and Oxidative Stability
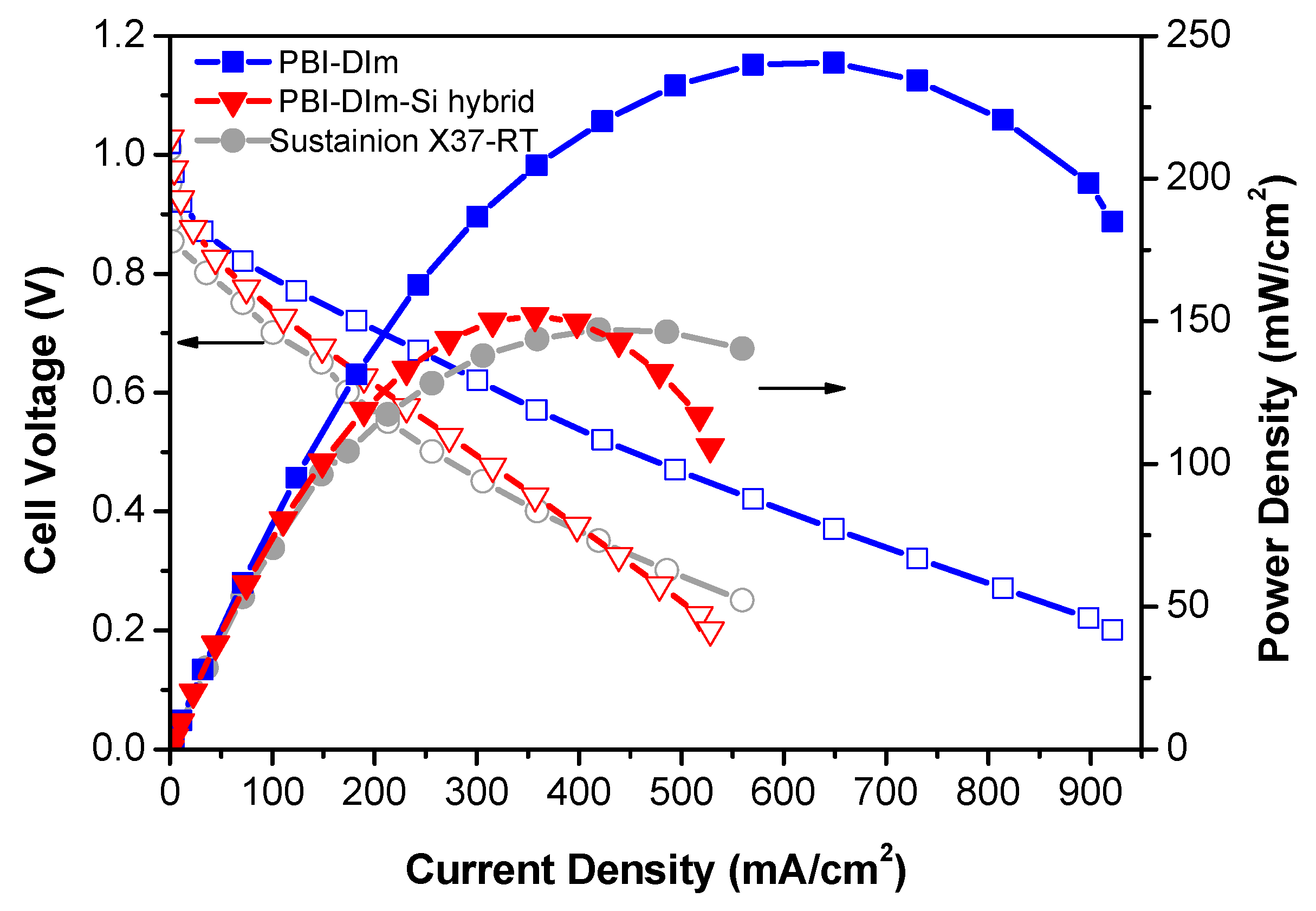
3.6. Fuel Cell Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Barbir, F.; Gómez, T. Efficiency and economics of proton exchange membrane (PEM) fuel cells. Int. J. Hydrog. Energy 1997, 22, 1027–1037. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, P.K. Recent Development of Polymer Electrolyte Membranes for Fuel Cells. Chem. Rev. 2012, 112, 2780–2832. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Liu, Y.; Xiao, B.; Yang, S.; Wang, Z.; Han, H. An analytical model for the transverse permeability of gas diffusion layer with electrical double layer effects in proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2018, 43, 17880–17888. [Google Scholar] [CrossRef]
- Liang, M.; Fu, C.; Xiao, B.; Luo, L.; Wang, Z. A fractal study for the effective electrolyte diffusion through charged porous media. Int. J. Heat Mass Transf. 2019, 137, 365–371. [Google Scholar] [CrossRef]
- Shin, D.W.; Guiver, M.D.; Lee, Y.M. Hydrocarbon-Based Polymer Electrolyte Membranes: Importance of Morphology on Ion Transport and Membrane Stability. Chem. Rev. 2017, 117, 4759–4805. [Google Scholar] [CrossRef]
- Gutru, R.; Turtayeva, Z.; Xu, F.; Maranzana, G.; Vigolo, B.; Desforges, A. A comprehensive review on water management strategies and developments in anion exchange membrane fuel cells. Int. J. Hydrog. Energy 2020, 45, 19642–19663. [Google Scholar] [CrossRef]
- Cheng, J.; He, G.; Zhang, F. A mini-review on anion exchange membranes for fuel cell applications: Stability issue and addressing strategies. Int. J. Hydrog. Energy 2015, 40, 7348–7360. [Google Scholar] [CrossRef]
- An, L.; Zhao, T.S.; Shen, S.Y.; Wu, Q.X.; Chen, R. Alkaline direct oxidation fuel cell with non-platinum catalysts capable of converting glucose to electricity at high power output. J. Power Sources 2011, 196, 186–190. [Google Scholar] [CrossRef]
- Firouzjaie, H.A.; Mustain, W.E. Catalytic Advantages, Challenges, and Priorities in Alkaline Membrane Fuel Cells. ACS Catal. 2020, 10, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Maurya, S.; Shin, S.-H.; Kim, Y.; Moon, S.-H. A review on recent developments of anion exchange membranes for fuel cells and redox flow batteries. RSC Adv. 2015, 5, 37206–37230. [Google Scholar] [CrossRef]
- Danks, T.N.; Slade, R.C.T.; Varcoe, J.R. Comparison of PVDF- and FEP-based radiation-grafted alkaline anion-exchange membranes for use in low temperature portable DMFCs. J. Mater. Chem. 2002, 12, 3371–3373. [Google Scholar] [CrossRef] [Green Version]
- Pan, Z.F.; An, L.; Zhao, T.S.; Tang, Z.K. Advances and challenges in alkaline anion exchange membrane fuel cells. Prog. Energy Combust. Sci. 2018, 66, 141–175. [Google Scholar] [CrossRef]
- Xu, F.; Su, Y.; Lin, B. Progress of Alkaline Anion Exchange Membranes for Fuel Cells: The Effects of Micro-Phase Separation. Front. Mater. 2020, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.E.; Nijmeijer, K.; Scott, K.; et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Shao, R.; Chen, S.; He, X.; Qiao, J.; Zhang, J. A review of radiation-grafted polymer electrolyte membranes for alkaline polymer electrolyte membrane fuel cells. J. Power Sources 2015, 293, 946–975. [Google Scholar] [CrossRef]
- Gottesfeld, S.; Dekel, D.R.; Page, M.; Bae, C.; Yan, Y.; Zelenay, P.; Kim, Y.S. Anion exchange membrane fuel cells: Current status and remaining challenges. J. Power Sources 2018, 375, 170–184. [Google Scholar] [CrossRef]
- Hickner, M.A. Strategies for Developing New Anion Exchange Membranes and Electrode Ionomers. Electrochem. Soc. Interface 2017, 26, 69–73. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Pan, J.; Wu, L.; Zhu, Y.; Ge, X.; Ran, J.; Yang, Z.; Xu, T. A Novel Methodology to Synthesize Highly Conductive Anion Exchange Membranes. Sci. Rep. 2015, 5, 13417. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.X.; Zhuo, Y.Z.; Lai, A.N.; Zhang, Q.G.; Zhu, A.M.; Ye, M.L.; Liu, Q.L. Side-chain-type anion exchange membranes bearing pendent imidazolium-functionalized poly(phenylene oxide) for fuel cells. J. Membr. Sci. 2016, 513, 206–216. [Google Scholar] [CrossRef]
- Wei, H.; Li, Y.; Wang, S.; Tao, G.; Wang, T.; Cheng, S.; Yang, S.; Ding, Y. Side-chain-type imidazolium-functionalized anion exchange membranes: The effects of additional hydrophobic side chains and their hydrophobicity. J. Membr. Sci. 2019, 579, 219–229. [Google Scholar] [CrossRef]
- Guo, D.; Lai, A.N.; Lin, C.X.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Imidazolium-Functionalized Poly(arylene ether sulfone) Anion-Exchange Membranes Densely Grafted with Flexible Side Chains for Fuel Cells. ACS Appl. Mater. Interfaces 2016, 8, 25279–25288. [Google Scholar] [CrossRef] [Green Version]
- Swaby, S.; Ureña, N.; Pérez-Prior, M.T.; Várez, A.; Levenfeld, B. Synthesis and Characterization of Novel Anion Exchange Membranes Based on Semi-Interpenetrating Networks of Functionalized Polysulfone: Effect of Ionic Crosslinking. Polymers 2021, 13, 958. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, Z.; Tian, H.; Wang, S.; Zhang, B.; Cao, Y.; He, G.; Li, Z.; Wu, H. Preparing alkaline anion exchange membrane with enhanced hydroxide conductivity via blending imidazolium-functionalized and sulfonated poly(ether ether ketone). J. Power Sources 2015, 288, 384–392. [Google Scholar] [CrossRef]
- Lin, B.; Xu, F.; Su, Y.; Han, J.; Zhu, Z.; Chu, F.; Ren, Y.; Zhu, L.; Ding, J. Ether-Free Polybenzimidazole Bearing Pendant Imidazolium Groups for Alkaline Anion Exchange Membrane Fuel Cells Application. ACS Appl. Energy Mater. 2020, 3, 1089–1098. [Google Scholar] [CrossRef]
- Wang, X.; Chen, W.; Yan, X.; Li, T.; Wu, X.; Zhang, Y.; Zhang, F.; Pang, B.; He, G. Pre-removal of polybenzimidazole anion to improve flexibility of grafted quaternized side chains for high performance anion exchange membranes. J. Power Sources 2020, 451, 227813. [Google Scholar] [CrossRef]
- Wang, X.; Chen, W.; Li, T.; Yan, X.; Zhang, Y.; Zhang, F.; Wu, X.; Pang, B.; Li, J.; He, G. Ultra-thin quaternized polybenzimidazole anion exchange membranes with throughout OH− conducive highway networks for high-performance fuel cells. J. Mater. Chem. A 2021, 9, 7522–7530. [Google Scholar] [CrossRef]
- He, X.; Cheng, C.; Huang, S.; Zhang, F.; Duan, Y.; Zhu, C.; Guo, Y.; Wang, K.; Chen, D. Alkaline anion exchange membranes with imidazolium-terminated flexible side-chain cross-linked topological structure based on ROMP-type norbornene copolymers. Polymer 2020, 195, 122412. [Google Scholar] [CrossRef]
- Wang, C.; Mo, B.; He, Z.; Shao, Q.; Pan, D.; Wujick, E.; Guo, J.; Xie, X.; Xie, X.; Guo, Z. Crosslinked norbornene copolymer anion exchange membrane for fuel cells. J. Membr. Sci. 2018, 556, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, A.D.; Ryu, C.Y.; Kim, Y.S.; Bae, C. Stable Elastomeric Anion Exchange Membranes Based on Quaternary Ammonium-Tethered Polystyrene-b-poly(ethylene-co-butylene)-b-polystyrene Triblock Copolymers. Macromolecules 2015, 48, 7085–7095. [Google Scholar] [CrossRef]
- Park, H.-S.; Hong, C.-K. Anion Exchange Membrane Based on Sulfonated Poly (Styrene-Ethylene-Butylene-Styrene) Copolymers. Polymers 2021, 13, 1669. [Google Scholar] [CrossRef]
- Mohanty, A.D.; Tignor, S.E.; Krause, J.A.; Choe, Y.-K.; Bae, C. Systematic Alkaline Stability Study of Polymer Backbones for Anion Exchange Membrane Applications. Macromolecules 2016, 49, 3361–3372. [Google Scholar] [CrossRef]
- Hou, H.; Wang, S.; Jiang, Q.; Jin, W.; Jiang, L.; Sun, G. Durability study of KOH doped polybenzimidazole membrane for air-breathing alkaline direct ethanol fuel cell. J. Power Sources 2011, 196, 3244–3248. [Google Scholar] [CrossRef]
- Aili, D.; Hansen, M.K.; Renzaho, R.F.; Li, Q.; Christensen, E.; Jensen, J.O.; Bjerrum, N.J. Heterogeneous anion conducting membranes based on linear and crosslinked KOH doped polybenzimidazole for alkaline water electrolysis. J. Membr. Sci. 2013, 447, 424–432. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.X.; Pan, Z.F.; An, L. Recent advances in alkali-doped polybenzimidazole membranes for fuel cell applications. Renew. Sustain. Energy Rev. 2018, 89, 168–183. [Google Scholar] [CrossRef]
- Qu, C.; Zhang, H.; Zhang, F.; Liu, B. A high-performance anion exchange membrane based on bi-guanidinium bridged polysilsesquioxane for alkaline fuel cell application. J. Mater. Chem. 2012, 22, 8203–8207. [Google Scholar] [CrossRef]
- He, X.; Han, Z.; Yang, Y.; Wang, S.; Tu, G.; Huang, S.; Zhang, F.; Chen, D. The preparation and application of a ROMP-type epoxy-functionalized norbornene copolymer and its hybrid alkaline anion exchange membranes. RSC Adv. 2017, 7, 55977–55985. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Cheng, J.; Liu, Y.; Gong, S.; He, G.; Li, L.; Li, S.; Zhang, F. Improved conductivity and stability of anion exchange membrane modified with bi-phenylguanidinium bridged silsesquioxane. Int. J. Hydrog. Energy 2017, 42, 21016–21026. [Google Scholar] [CrossRef]
- He, X.; Jiang, X.; Wang, Z.; Deng, Y.; Han, Z.; Yang, Y.; Chen, D. Crosslinked hydroxyl-conductive copolymer/silica composite membranes based on addition-type polynorbornene for alkaline anion exchange membrane fuel cell applications. Polym. Eng. Sci. 2018, 58, 13–21. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Wang, J.; Wang, L. Preparation and characterization of a sol-gel derived silica/PVA-Py hybrid anion exchange membranes for alkaline fuel cell application. J. Electroanal. Chem. 2020, 873, 114342. [Google Scholar] [CrossRef]
- Jheng, L.C.; Hsu, S.L.C.; Lin, B.Y.; Hsu, Y.L. Quaternized polybenzimidazoles with imidazolium cation moieties for anion exchange membrane fuel cells. J. Membr. Sci. 2014, 460, 160–170. [Google Scholar] [CrossRef]
- Lin, X.; Varcoe, J.R.; Poynton, S.D.; Liang, X.; Ong, A.L.; Ran, J.; Li, Y.; Xu, T. Alkaline polymer electrolytes containing pendant dimethylimidazolium groups for alkaline membrane fuel cells. J. Mater. Chem. A 2013, 1, 7262–7269. [Google Scholar] [CrossRef]
- Sun, Z.; Pan, J.; Guo, J.; Yan, F. The Alkaline Stability of Anion Exchange Membrane for Fuel Cell Applications: The Effects of Alkaline Media. Adv. Sci. 2018, 5, 1800065. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.-W.; Hsu, S.L.-C. Synthesis and properties of a new fluorine-containing polybenzimidazole for high-temperature fuel-cell applications. J. Polym. Sci. Part. A Polym. Chem. 2006, 44, 4508–4513. [Google Scholar] [CrossRef]
- Jheng, L.-C.; Rosidah, A.A.; Hsu, S.L.-C.; Ho, K.-S.; Pan, C.-J.; Cheng, C.-W. Nanocomposite membranes of polybenzimidazole and amine-functionalized carbon nanofibers for high temperature proton exchange membrane fuel cells. RSC Adv. 2021, 11, 9964–9976. [Google Scholar] [CrossRef]
- Dai, J.; He, G.; Ruan, X.; Zheng, W.; Pan, Y.; Yan, X. Constructing a rigid crosslinked structure for enhanced conductivity of imidazolium functionalized polysulfone hydroxide exchange membrane. Int. J. Hydrog. Energy 2016, 41, 10923–10934. [Google Scholar] [CrossRef]
- Chen, J.; Shen, C.; Gao, S.; Yuan, Y.; Ren, X. Novel imidazole-grafted hybrid anion exchange membranes based on poly (2,6-dimethyl-1,4-phenylene oxide) for fuel cell applications. J. Appl. Polym. Sci. 2018, 135, 46034. [Google Scholar] [CrossRef]
- Jheng, L.-C.; Tai, C.-K.; Hsu, S.L.-C.; Lin, B.-Y.; Chen, L.; Wang, B.-C.; Chiang, L.-K.; Ko, W.-C. Study on the alkaline stability of imidazolium and benzimidazolium based polyelectrolytes for anion exchange membrane fuel cells. Int. J. Hydrog. Energy 2017, 42, 5315–5326. [Google Scholar] [CrossRef]
- Ngo, H.L.; Lecompte, K.; Hargens, L.; McEwen, A.B. Thermal properties of imidazolium ionic liquids. Thermochim. Acta 2000, 357, 97–102. [Google Scholar] [CrossRef]
- Khan, S.; Lorenzelli, L.; Dahiya, R.S. Technologies for Printing Sensors and Electronics Over Large Flexible Substrates: A Review. IEEE Sens. J. 2015, 15, 3164–3185. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Song, M.H.; Lee, J.-Y.; Lee, H.-K.; Yoo, D.J. Amine functionalized carbon nanotube (ACNT) filled in sulfonated poly(ether ether ketone) membrane: Effects of ACNT in improving polymer electrolyte fuel cell performance under reduced relative humidity. Compos. Part. B Eng. 2020, 188, 107890. [Google Scholar] [CrossRef]
- Dietrich, P.M.; Streeck, C.; Glamsch, S.; Ehlert, C.; Lippitz, A.; Nutsch, A.; Kulak, N.; Beckhoff, B.; Unger, W.E.S. Quantification of Silane Molecules on Oxidized Silicon: Are there Options for a Traceable and Absolute Determination? Anal. Chem. 2015, 87, 10117–10124. [Google Scholar] [CrossRef]
- Hossain, M.A.; Jang, H.; Lee, S.; Hong, T.; Jin, L.; Tan, F.; Kim, D.; Kim, W. Comparison of properties of anion conductive Parmax membranes containing imidazolium cation and quaternary ammonium. Int. J. Hydrog. Energy 2015, 40, 1324–1332. [Google Scholar] [CrossRef]
- Feng, T.; Lin, B.; Zhang, S.; Yuan, N.; Chu, F.; Hickner, M.A.; Wang, C.; Zhu, L.; Ding, J. Imidazolium-based organic–inorganic hybrid anion exchange membranes for fuel cell applications. J. Membr. Sci. 2016, 508, 7–14. [Google Scholar] [CrossRef]
- Feng, C.; He, P.F. Moisture and thermal expansion properties and mechanism of interaction between ions of a Nafion-based membrane electrode assembly. RSC Adv. 2017, 7, 34556–34566. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Qiao, X.; Liu, M.; Liu, L.; Li, N. The effect of –NH− on quaternized polybenzimidazole anion exchange membranes for alkaline fuel cells. J. Membr. Sci. 2021, 626, 119178. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Q.; Wang, C.; Lee, Y.M.; Guiver, M.D. Phenyltrimethylammonium Functionalized Polysulfone Anion Exchange Membranes. Macromolecules 2012, 45, 2411–2419. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Tse, Y.-L.S.; Lindberg, G.E.; Knight, C.; Voth, G.A. Hydroxide Solvation and Transport in Anion Exchange Membranes. J. Am. Chem. Soc. 2016, 138, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, K.H.; Chu, J.Y.; Kim, A.R.; Yoo, D.J. Enhanced Hydroxide Conductivity and Dimensional Stability with Blended Membranes Containing Hyperbranched PAES/Linear PPO as Anion Exchange Membranes. Polymers 2020, 12, 3011. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Chu, J.Y.; Kim, A.R.; Yoo, D.J. Fabrication of High-Alkaline Stable Quaternized Poly(arylene ether ketone)/Graphene Oxide Derivative Including Zwitterion for Alkaline Fuel Cells. ACS Sustain. Chem. Eng. 2021, 9, 8824–8834. [Google Scholar] [CrossRef]
- Chu, J.Y.; Lee, K.H.; Kim, A.R.; Yoo, D.J. Graphene-mediated organic-inorganic composites with improved hydroxide conductivity and outstanding alkaline stability for anion exchange membranes. Compos. Part. B Eng. 2019, 164, 324–332. [Google Scholar] [CrossRef]
- Yoshimura, K.; Hiroki, A.; Yu, H.-C.; Zhao, Y.; Shishitani, H.; Yamaguchi, S.; Tanaka, H.; Maekawa, Y. Alkaline durable 2-methylimidazolium containing anion-conducting electrolyte membranes synthesized by radiation-induced grafting for direct hydrazine hydrate fuel cells. J. Membr. Sci. 2019, 573, 403–410. [Google Scholar] [CrossRef]
- Dekel, D.R.; Amar, M.; Willdorf, S.; Kosa, M.; Dhara, S.; Diesendruck, C.E. Effect of Water on the Stability of Quaternary Ammonium Groups for Anion Exchange Membrane Fuel Cell Applications. Chem. Mater. 2017, 29, 4425–4431. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, S.; Pagels, M.K.; Jeon, J.Y.; Bae, C. Ionomers for electrochemical energy conversion & storage technologies. Polymer 2020, 211, 123080. [Google Scholar]
- Hübner, G.; Roduner, E. EPR investigation of HO/ radical initiated degradation reactions of sulfonated aromatics as model compounds for fuel cell proton conducting membranes. J. Mater. Chem. 1999, 9, 409–418. [Google Scholar] [CrossRef]
- Zhu, L.; Zimudzi, T.J.; Wang, Y.; Yu, X.; Pan, J.; Han, J.; Kushner, D.I.; Zhuang, L.; Hickner, M.A. Mechanically Robust Anion Exchange Membranes via Long Hydrophilic Cross-Linkers. Macromolecules 2017, 50, 2329–2337. [Google Scholar] [CrossRef]
- Wang, X.; Wang, P.; Sun, Y.; Wang, J.; Fang, H.; Yang, S.; Wei, H.; Ding, Y. A mechanically strong and tough anion exchange membrane engineered with non-covalent modalities. Chem. Commun. 2017, 53, 12369–12372. [Google Scholar] [CrossRef]
- Liu, F.; Yi, B.; Xing, D.; Yu, J.; Zhang, H. Nafion/PTFE composite membranes for fuel cell applications. J. Membr. Sci. 2003, 212, 213–223. [Google Scholar] [CrossRef]
- Changkhamchom, S.; Kunanupatham, P.; Phasuksom, K.; Sirivat, A. Anion exchange membranes composed of quaternized polybenzimidazole and quaternized graphene oxide for glucose fuel cell. Int. J. Hydrog. Energy 2021, 46, 5642–5652. [Google Scholar] [CrossRef]
- Abdi, Z.G.; Chiu, T.-H.; Pan, Y.-Z.; Chen, J.-C. Anion exchange membranes based on ionic polybenzimidazoles crosslinked by thiol-ene reaction. React. Funct. Polym. 2020, 156, 104719. [Google Scholar] [CrossRef]
- Carmo, M.; Doubek, G.; Sekol, R.C.; Linardi, M.; Taylor, A.D. Development and electrochemical studies of membrane electrode assemblies for polymer electrolyte alkaline fuel cells using FAA membrane and ionomer. J. Power Sources 2013, 230, 169–175. [Google Scholar] [CrossRef]
- Lin, C.; Wang, J.; Shen, G.; Duan, J.; Xie, D.; Cheng, F.; Zhang, Y.; Zhang, S. Construction of crosslinked polybenz imidazole-based anion exchange membranes with ether-bond-free backbone. J. Membr. Sci. 2019, 590, 117303. [Google Scholar] [CrossRef]







| AEM | Theoretical IEC 1 (mmol/g) | Experimental IEC 4 (mmol/g) | WU 5 (%) | SR 5 (%) | λ | Insoluble Solid Content (wt%) |
|---|---|---|---|---|---|---|
| PBI-DIm | 2.02 2 | 1.85 | 62.7 | 26.7 | 18.8 | 62.4 |
| PBI-DIm-Si hybrid | 2.05 3 | 1.87 | 30.9 | 10.0 | 9.2 | 78.8 |
| State | AEM | Elastic Modulus (GPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|
| Dry | PBI-DIm | 0.63 ± 0.14 | 26.0 ± 3.5 | 15.2 ± 3.6 |
| PBI-Dim-Si hybrid | 0.64 ± 0.11 | 32.9 ± 3.7 | 16.9 ± 4.2 | |
| Fully hydrated | PBI-DIm | 0.18 ± 0.04 | 17.0 ± 0.7 | 31.9 ± 4.0 |
| PBI-Dim-Si hybrid | 0.51 ± 0.04 | 28.2 ± 2.5 | 27.2 ± 4.83 | |
| Dry, alkaline degraded 1 | PBI-DIm | 0.64 ± 0.12 | 25.8 ± 3.8 | 15.9 ± 3.5 |
| PBI-Dim-Si hybrid | 0.66 ± 0.09 | 33.1 ± 4.1 | 18.3 ± 4.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jheng, L.-C.; Cheng, C.-W.; Ho, K.-S.; Hsu, S.L.-C.; Hsu, C.-Y.; Lin, B.-Y.; Ho, T.-H. Dimethylimidazolium-Functionalized Polybenzimidazole and Its Organic–Inorganic Hybrid Membranes for Anion Exchange Membrane Fuel Cells. Polymers 2021, 13, 2864. https://doi.org/10.3390/polym13172864
Jheng L-C, Cheng C-W, Ho K-S, Hsu SL-C, Hsu C-Y, Lin B-Y, Ho T-H. Dimethylimidazolium-Functionalized Polybenzimidazole and Its Organic–Inorganic Hybrid Membranes for Anion Exchange Membrane Fuel Cells. Polymers. 2021; 13(17):2864. https://doi.org/10.3390/polym13172864
Chicago/Turabian StyleJheng, Li-Cheng, Cheng-Wei Cheng, Ko-Shan Ho, Steve Lien-Chung Hsu, Chung-Yen Hsu, Bi-Yun Lin, and Tsung-Han Ho. 2021. "Dimethylimidazolium-Functionalized Polybenzimidazole and Its Organic–Inorganic Hybrid Membranes for Anion Exchange Membrane Fuel Cells" Polymers 13, no. 17: 2864. https://doi.org/10.3390/polym13172864
APA StyleJheng, L.-C., Cheng, C.-W., Ho, K.-S., Hsu, S. L.-C., Hsu, C.-Y., Lin, B.-Y., & Ho, T.-H. (2021). Dimethylimidazolium-Functionalized Polybenzimidazole and Its Organic–Inorganic Hybrid Membranes for Anion Exchange Membrane Fuel Cells. Polymers, 13(17), 2864. https://doi.org/10.3390/polym13172864






