Highly Conductive Polyelectrolyte Membranes Poly(vinyl alcohol)/Poly(2-acrylamido-2-methyl propane sulfonic acid) (PVA/PAMPS) for Fuel Cell Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PVA/PAMPS Blend-Based Membranes
2.3. Characterization of PVA/PAMPS Blend-Based Membrane
2.3.1. FTIR
2.3.2. SEM
2.3.3. Thermogravimetric Analysis
2.3.4. Tensile Testing
2.3.5. Water Contact Angle Measurement
2.3.6. Ion Exchange Capacity
2.3.7. Methanol and Water Uptake
2.3.8. Methanol Permeability
2.3.9. Membrane Efficiency
3. Results and Discussion
3.1. ATR-FTIR Spectra
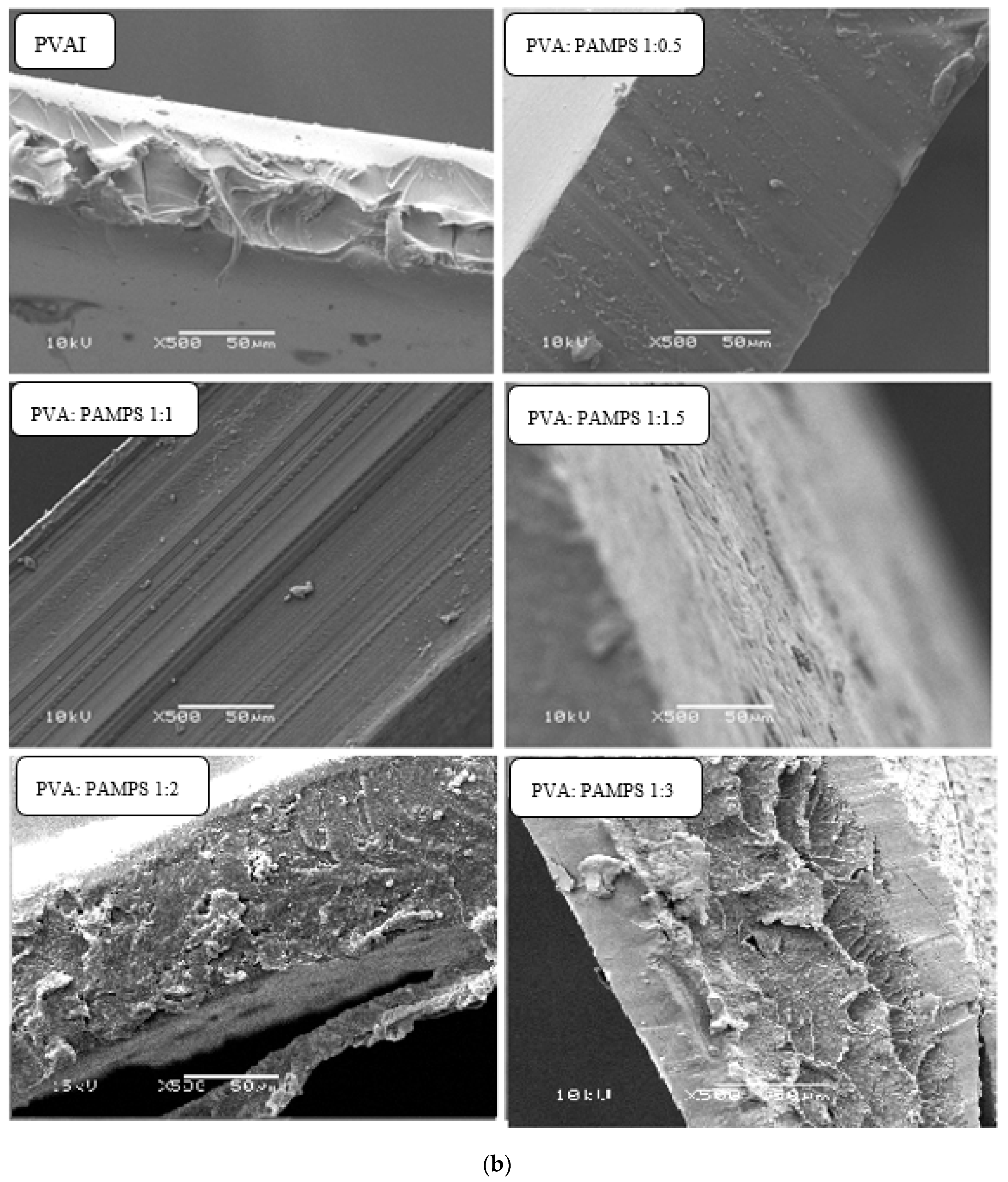
3.2. Morphological and Cross-Sectional Features
3.3. Thermal Stability
3.4. Mechanical Properties
3.5. Contact Angle
3.6. Functional Properties
3.6.1. Water and Methanol Uptake
3.6.2. Ion Exchange Capacity (IEC)
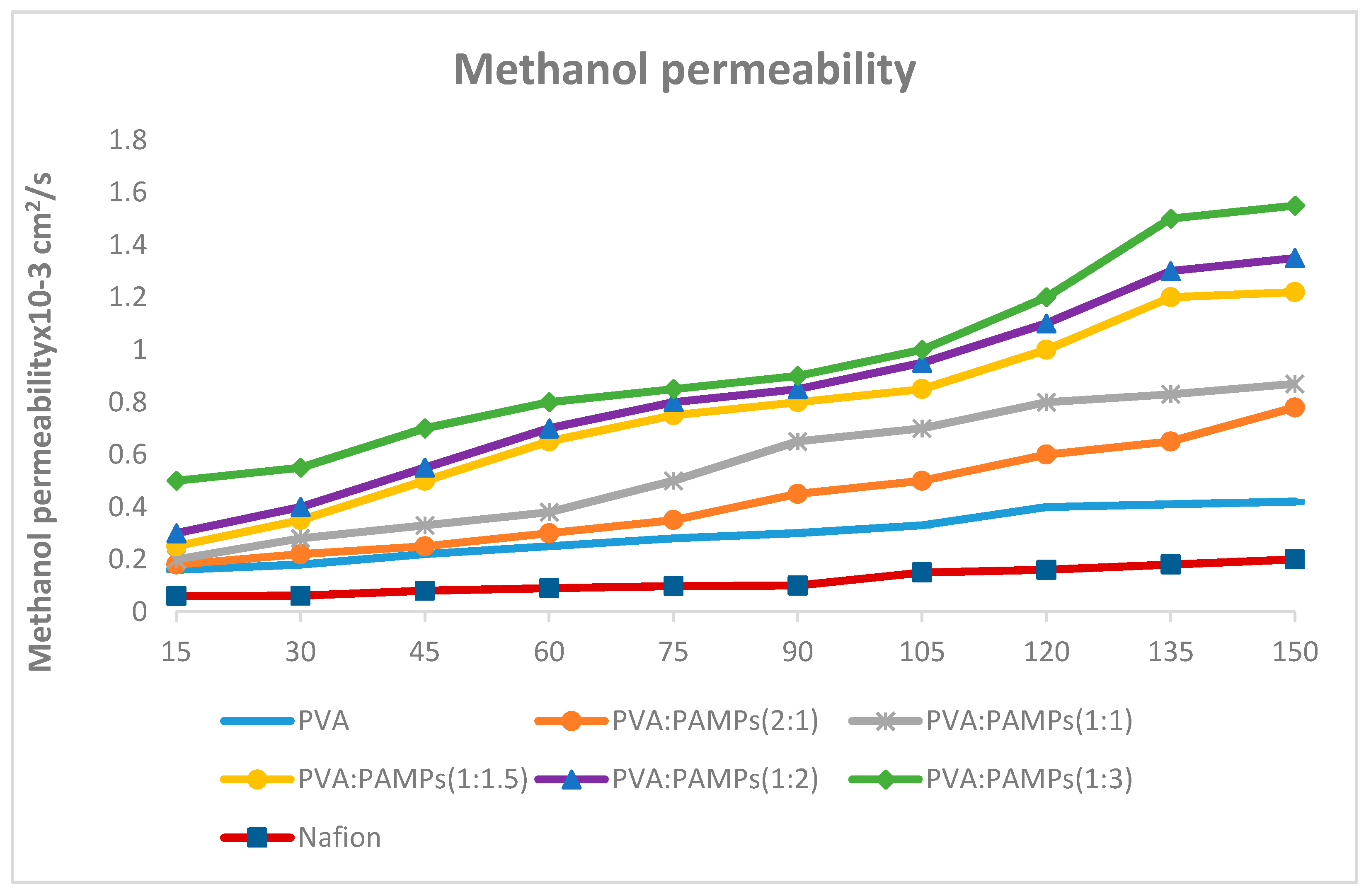
3.6.3. Methanol Permeability
3.6.4. Membrane Efficiency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sun, C.; Złotorowicz, A.; Nawn, G.; Negro, E.; Bertasi, F.; Pagot, G.; Vezzù, K.; Pace, G.; Guarnieri, M.; Di Noto, V. [Nafion/(WO3)x] hybrid membranes for vanadium redox flow batteries. Solid State Ionics 2018, 319, 110–116. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, X.; Wang, W.; Cao, D. Recent Progress in MOF-Derived, Heteroatom-Doped Porous Carbons as Highly Efficient Electrocatalysts for Oxygen Reduction Reaction in Fuel Cells. Adv. Funct. Mater. 2018, 28, 1704537. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? ACS Publications, American Chemical Society: Washington, DC, USA, 2004. [Google Scholar]
- Hickner, M.; Ghassemi, H.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Alternative Polymer Systems for Proton Exchange Membranes (PEMs). Chem. Rev. 2004, 104, 4587–4612. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, D. Conductivity and solvent uptake of proton exchange membrane based on polystyrene(ethylene–butylene)polystyrene triblock polymer. Eur. Polym. J. 2005, 41, 2644–2652. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, H. Investigation of Nafion series membranes on the performance of iron-chromium redox flow battery. Int. J. Energy Res. 2019, 43, 8739–8752. [Google Scholar] [CrossRef]
- Pivovar, B.S.; Wang, Y.; Cussler, E. Pervaporation membranes in direct methanol fuel cells. J. Membr. Sci. 1999, 154, 155–162. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Yeom, C.-K.; Kim, S.-W. Modification of poly(vinyl alcohol) membranes using sulfur-succinic acid and its application to pervaporation separation of water-alcohol mixtures. J. Appl. Polym. Sci. 1998, 68, 1717–1723. [Google Scholar] [CrossRef]
- Tsai, C.-E.; Lin, C.-W.; Hwang, B.-J. A novel crosslinking strategy for preparing poly(vinyl alcohol)-based proton-conducting membranes with high sulfonation. J. Power Sources 2010, 195, 2166–2173. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, M.; Guo, Z.; Cui, Z. Alternatively chitosan sulfate blending membrane as methanol-blocking polymer electrolyte membrane for direct methanol fuel cell. J. Membr. Sci. 2009, 337, 318–323. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Z.; Liu, C.; Xing, W.; Zhang, J. Implantation of Nafion® ionomer into polyvinyl alcohol/chitosan composites to form novel proton-conducting membranes for direct methanol fuel cells. J. Power Sources 2009, 194, 730–736. [Google Scholar] [CrossRef]
- Kudoh, Y.; Kojima, T.; Abe, M.; Oota, M.; Yamamoto, T. Proton conducting membranes consisting of poly(vinyl alcohol) and poly(styrene sulfonic acid): Crosslinking of poly(vinyl alcohol) with and without succinic acid. Solid State Ionics 2013, 253, 189–194. [Google Scholar] [CrossRef]
- Qiao, J.; Hamaya, T.; Okada, T. Chemically Modified Poly(vinyl alcohol)−Poly(2-acrylamido-2-methyl-1-propanesulfonic acid) as a Novel Proton-Conducting Fuel Cell Membrane. Chem. Mater. 2005, 17, 2413–2421. [Google Scholar] [CrossRef]
- Hamaya, T.; Inoue, S.; Qiao, J.; Okada, T. Novel proton-conducting polymer electrolyte membranes based on PVA/PAMPS/PEG400 blend. J. Power Sources 2006, 156, 311–314. [Google Scholar] [CrossRef]
- Zhu, Y.; Xia, S.; Liu, G.; Jin, W. Preparation of ceramic-supported poly(vinyl alcohol)–chitosan composite membranes and their applications in pervaporation dehydration of organic/water mixtures. J. Membr. Sci. 2010, 349, 341–348. [Google Scholar] [CrossRef]
- Li, H.Q.; Liu, X.J.; Wang, H.; Yang, H.; Wang, Z.; He, J. Proton exchange membranes with cross-linked interpenetrating network of sulfonated polyvinyl alcohol and poly(2-acrylamido-2-methyl-1-propanesulfonic acid): Excellent relative selectivity. J. Membr. Sci. 2020, 595, 117511. [Google Scholar] [CrossRef]
- Yang, J.M.; Chiu, H.C. Preparation and characterization of polyvinyl alcohol/chitosan blended membrane for alkaline direct methanol fuel cells. J. Membr. Sci. 2012, 419–420, 65–71. [Google Scholar] [CrossRef]
- Abu-Saied, M.A.; Fontananova, E.; Drioli, E.; Eldin, M.S.M. Sulphonated poly (glycidyl methacrylate) grafted cellophane membranes: Novel application in polyelectrolyte membrane fuel cell (PEMFC). J. Polym. Res. 2013, 20, 1–13. [Google Scholar] [CrossRef]
- Abu-Saied, M.; Fahmy, A.; Morgan, N.; Qutop, W.; Abdelbary, H.; Friedrich, J.F. Enhancement of Poly(vinyl chloride) Electrolyte Membrane by Its Exposure to an Atmospheric Dielectric Barrier Discharge Followed by Grafting with Polyacrylic Acid. Plasma Chem. Plasma Process. 2019, 39, 1499–1517. [Google Scholar] [CrossRef]
- Eldin, M.S.M.; Elzatahry, A.A.; El-Khatib, K.M.; Hassan, E.A.; El-Sabbah, M.M.; Abu-Saied, M.A. Novel grafted nafion membranes for proton-exchange membrane fuel cell applications. J. Appl. Polym. Sci. 2010, 119, 120–133. [Google Scholar] [CrossRef]
- Abu-Saied, M.A.; Taha, T.H.; Elnaggar, E.M.; Amer, R.A.; Mansy, A.E.; Elkady, G.M. Green production of bio-ethanol from cellulosic fiber waste and its separation using polyacrylonitrile-co-poly methyl acrylate membrane. Cellulose 2018, 25, 6621–6644. [Google Scholar] [CrossRef]
- Taha, T.H.; Abu-Saied, M.A.; Elnaggar, E.; Amer, R.A.; Mansy, A.E.; Elkady, G.M. Bio-Ethanol Production from Environmental Waste followed by Polymeric Separation of Formed Ethanol/Water Mixture. Al-Azhar Bull. Sci. 2018, 29, 71–80. [Google Scholar]
- Eldin, M.S.M.; Tamer, T.M.; Abu Saied, M.A.; Soliman, E.A.; Madi, N.K.; Ragab, I.M.; Fadel, I. Click Grafting of Chitosan onto PVC Surfaces for Biomedical Applications. Adv. Polym. Technol. 2015, 37, 38–49. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Abu-Saied, M.; Doma, A.; Menzel, H.; Chen, X. Influence of degree of substitution and folic acid coinitiator on pullulan-HEMA hydrogel properties crosslinked under visible-light initiating system. Int. J. Biol. Macromol. 2018, 116, 1175–1185. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Bang, Y.H.; Di Noto, V. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries. Electrochimica Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Abu-Saied, M.; Taha, T.H.; El-Deeb, N.M.; Hafez, E. Polyvinyl alcohol/Sodium alginate integrated silver nanoparticles as probable solution for decontamination of microbes contaminated water. Int. J. Biol. Macromol. 2018, 107, 1773–1781. [Google Scholar] [CrossRef]
- Abu-Saied, M.; Wycisk, R.; Abbassy, M.M.; El-Naim, G.A.; El-Demerdash, F.; Youssef, M.; Bassuony, H.; Pintauro, P.N. Sulfated chitosan/PVA absorbent membrane for removal of copper and nickel ions from aqueous solutions—Fabrication and sorption studies. Carbohydr. Polym. 2017, 165, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.; Im, H.; Kim, J. The effect of sulfonated graphene oxide on Sulfonated Poly (Ether Ether Ketone) membrane for direct methanol fuel cells. J. Membr. Sci. 2013, 425–426, 11–22. [Google Scholar] [CrossRef]
- Abu-Saied, M.; El-Desouky, E.; Soliman, E.; El-Naim, G.A. Novel sulphonated poly (vinyl chloride)/poly (2-acrylamido-2-methylpropane sulphonic acid) blends-based polyelectrolyte membranes for direct methanol fuel cells. Polym. Test. 2020, 89, 106604. [Google Scholar] [CrossRef]
- Higa, M.; Mehdizadeh, S.; Feng, S.; Endo, N.; Kakihana, Y. Cell performance of direct methanol alkaline fuel cell (DMAFC) using anion exchange membranes prepared from PVA-Based block copolymer. J. Membr. Sci. 2020, 597, 117618. [Google Scholar] [CrossRef]
- Pagidi, A.; Arthanareeswaran, G.; Seepana, M.M. Synthesis of highly stable PTFE-ZrP-PVA composite membrane for high-temperature direct methanol fuel cell. Int. J. Hydrogen Energy 2019, 45, 7829–7837. [Google Scholar] [CrossRef]
- Nagar, H.; Sahu, N.; Rao, V.B.; Sridhar, S. Surface modification of sulfonated polyethersulfone membrane with polyaniline nanoparticles for application in direct methanol fuel cell. Renew. Energy 2020, 146, 1262–1277. [Google Scholar] [CrossRef]
- Ghosh, M.K.; Uddin, N.; Choi, C.H. Hydrophobic and Hydrophilic Associations of a Methanol Pair in Aqueous Solution. J. Phys. Chem. B 2012, 116, 14254–14260. [Google Scholar] [CrossRef] [PubMed]
- Elkady, M.; Abu-Saied, M.; Rahman, A.A.; Soliman, E.; Elzatahry, A.; Yossef, M.E.; Eldin, M.M. Nano-sulphonated poly (glycidyl methacrylate) cations exchanger for cadmium ions removal: Effects of operating parameters. Desalination 2011, 279, 152–162. [Google Scholar] [CrossRef]


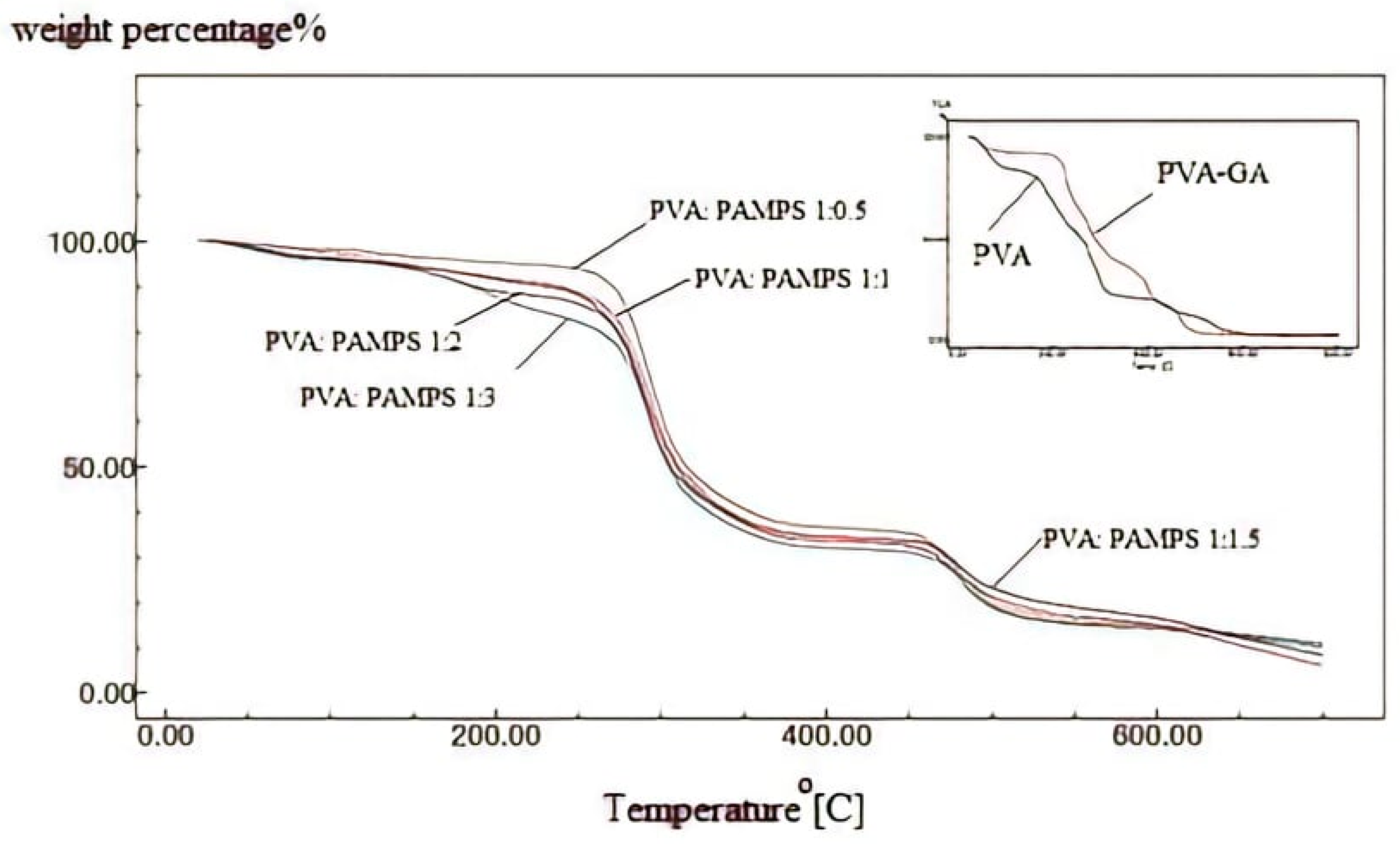
| Temp. (°C) | Residual Weight Percentage | ||||||
|---|---|---|---|---|---|---|---|
| Non-Cross-Linked PVA | GA-Cross-Linked PVA | GA-Cross-Linked Polymer Blends at Different PVA/PAMPS Ratio | |||||
| 1:0.5 | 1:1 | 1:1.5 | 1:2 | 1:3 | |||
| 100 | 90.15 | 92.51 | 85.72 | 83.33 | 77.24 | 74.36 | 70.02 |
| 200 | 85.17 | 90.91 | 81.86 | 80.33 | 75.54 | 70.66 | 68.70 |
| 300 | 55.90 | 60.12 | 57.24 | 50.16 | 46.61 | 44.02 | 40.73 |
| 400 | 38.14 | 34.54 | 30.86 | 29.37 | 30.15 | 22.42 | 21.95 |
| 500 | 25.80 | 20.11 | 25.5 | 24.51 | 22.08 | 24.46 | 23.91 |
| 600 | 10.51 | 12.96 | 10.34 | 8.20 | 7.99 | 7.76 | 5.15 |
| Membrane | Thickness (μm) | TS (MPa) | Elongation at Break (%) | Contact Angle | WU (%) | MU (%) | IEC (meq/gm) | Methanol Permeability Coefficient (cm2 s−1) | Efficiency Factor |
|---|---|---|---|---|---|---|---|---|---|
| GA-cross-linked PVA | 181 ± 0.8 | 20.00 ± 1.6 | 53.97 ± 1.6 | 34.05 ± 1.6 | 60 ± 4 | 17.54 ± 1.6 | 0.077 ± 0.01 | 4.8 × 10−7 | 1.10 × 105 |
| PVA:PAMPS 1:0.5 | 184 ± 1.6 | 10.52 ± 0.81 | 109.08 ± 4 | 31.52 ± 2.4 | 65.26 ± 3.2 | 15.14 ± 2.4 | 1.23 ± 0.08 | 2 × 10−8 | 1.78 × 105 |
| PVA:PAMPS 1:1 | 187 ± 1.6 | 9.18 ± 0.81 | 126.34 ± 3.3 | 25.42 ± 1.6 | 70.51 ± 4 | 13.04 ± 1.6 | 2.64 ± 0.16 | 2.5 × 10−8 | 4.00 × 105 |
| PVA:PAMPS 1:1.5 | 188 ± 2.4 | 8.37 ± 0.41 | 162.18 ± 4 | 23.15 ± 0.8 | 73.36 ± 2.4 | 11.88 ± 2.4 | 3.587 ± 0.16 | 2.8 × 10−8 | 4.10 × 105 |
| PVA:PAMPS 1:2 | 190 ± 1.6 | 7.73 ± 0.41 | 32.27 ± 1.6 | 20.73 ± 0.8 | 75.86 ± 4 | 10.54 ± 3.3 | 3.77 ± 0.32 | 3.0 × 10−8 | 4.57 × 105 |
| PVA:PAMPS 1:3 | 192 ± 1.6 | 3.01 ± 0.33 | 14.56 ± 0.8 | 18.13 ± 1.60 | 90.16 ± 3.27 | 9.51 ± 4 | 3.93 ± 0.36 | 3.2 × 10−8 | 4.70 × 105 |
| Nafion-117 | 185 | 18.20 | 12.2 | 110 | 18.94 | 22 | 0.91 | 3.39 × 10−6 | 2.6 × 105 |
| Sample (Sigma-Aldrich) | Nafion 117 | PVA | PAMPS | PVA–PAMPS 1:1 (10 × 10 cm) |
|---|---|---|---|---|
| Cost | (10 × 10 cm)/$33.00 | (25 G)/$47.07 | (100 G)/$136.36 | $3.2464 |
| (1 G)/$1.882 | (1 G)/$1.3636 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Saied, M.A.; Soliman, E.A.; Abualnaj, K.M.; El Desouky, E. Highly Conductive Polyelectrolyte Membranes Poly(vinyl alcohol)/Poly(2-acrylamido-2-methyl propane sulfonic acid) (PVA/PAMPS) for Fuel Cell Application. Polymers 2021, 13, 2638. https://doi.org/10.3390/polym13162638
Abu-Saied MA, Soliman EA, Abualnaj KM, El Desouky E. Highly Conductive Polyelectrolyte Membranes Poly(vinyl alcohol)/Poly(2-acrylamido-2-methyl propane sulfonic acid) (PVA/PAMPS) for Fuel Cell Application. Polymers. 2021; 13(16):2638. https://doi.org/10.3390/polym13162638
Chicago/Turabian StyleAbu-Saied, M. A., Emad Ali Soliman, Khamael M. Abualnaj, and Eman El Desouky. 2021. "Highly Conductive Polyelectrolyte Membranes Poly(vinyl alcohol)/Poly(2-acrylamido-2-methyl propane sulfonic acid) (PVA/PAMPS) for Fuel Cell Application" Polymers 13, no. 16: 2638. https://doi.org/10.3390/polym13162638
APA StyleAbu-Saied, M. A., Soliman, E. A., Abualnaj, K. M., & El Desouky, E. (2021). Highly Conductive Polyelectrolyte Membranes Poly(vinyl alcohol)/Poly(2-acrylamido-2-methyl propane sulfonic acid) (PVA/PAMPS) for Fuel Cell Application. Polymers, 13(16), 2638. https://doi.org/10.3390/polym13162638






