Solubilization of Paclitaxel by Self-Assembled Amphiphilic Phospholipid-Mimetic Polymers with Varied Hydrophobicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dynamic Light Scattering Analysis
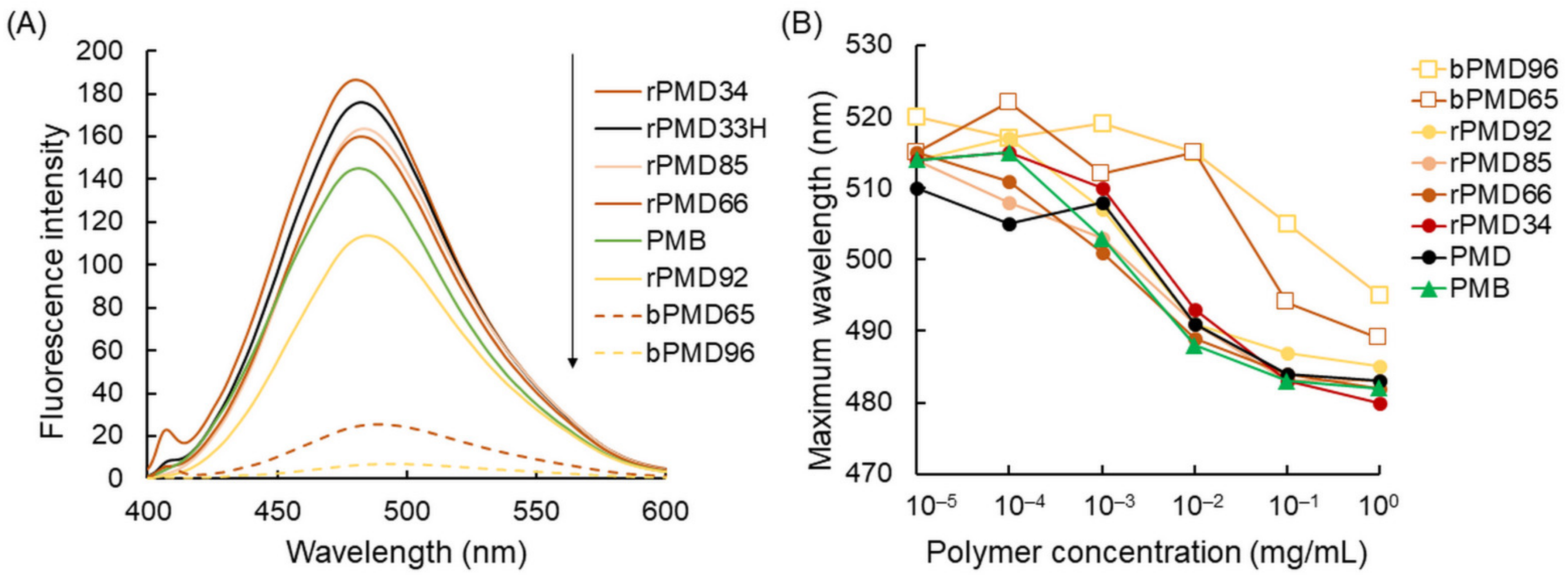
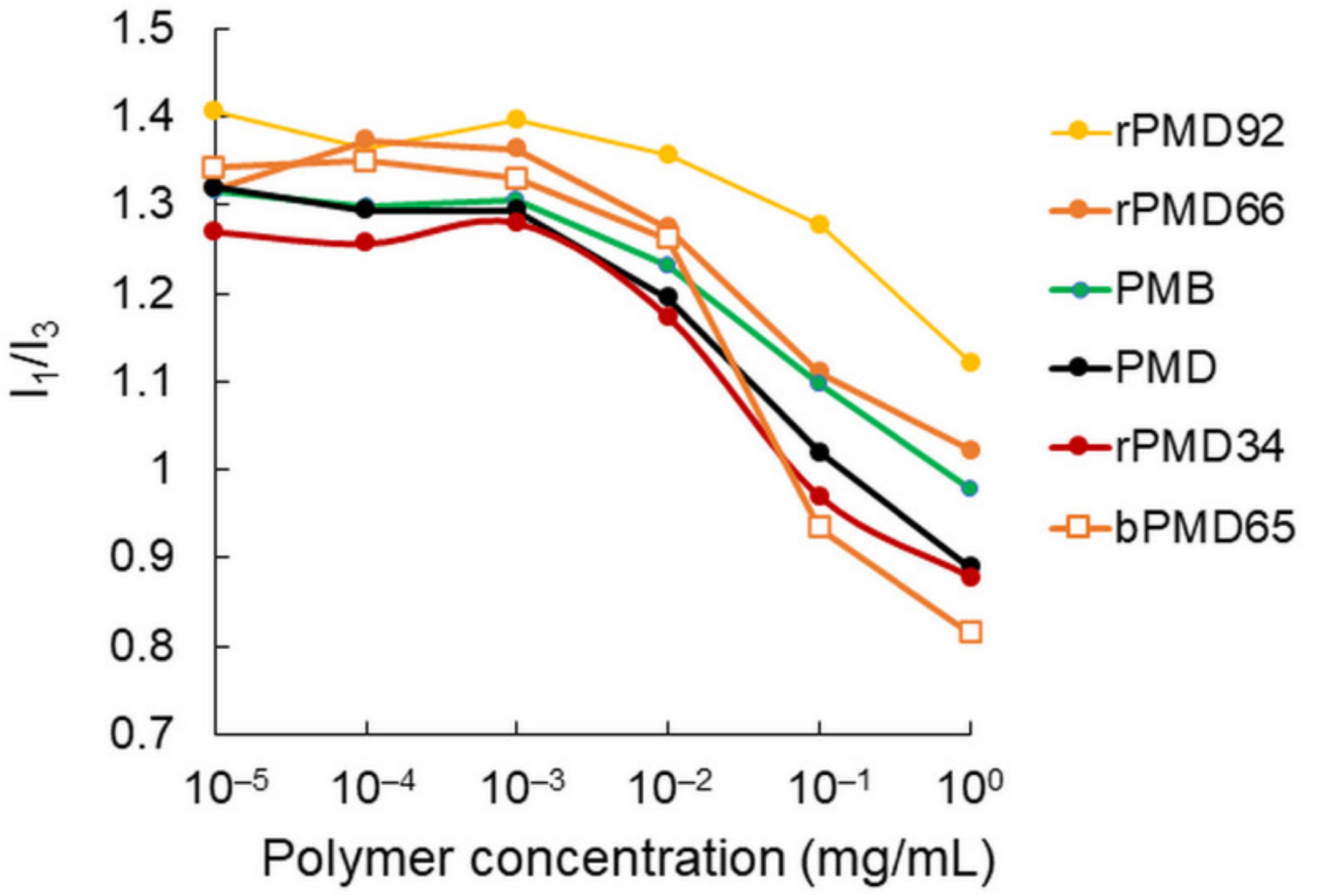
2.3. Fluorescence Spectrometry
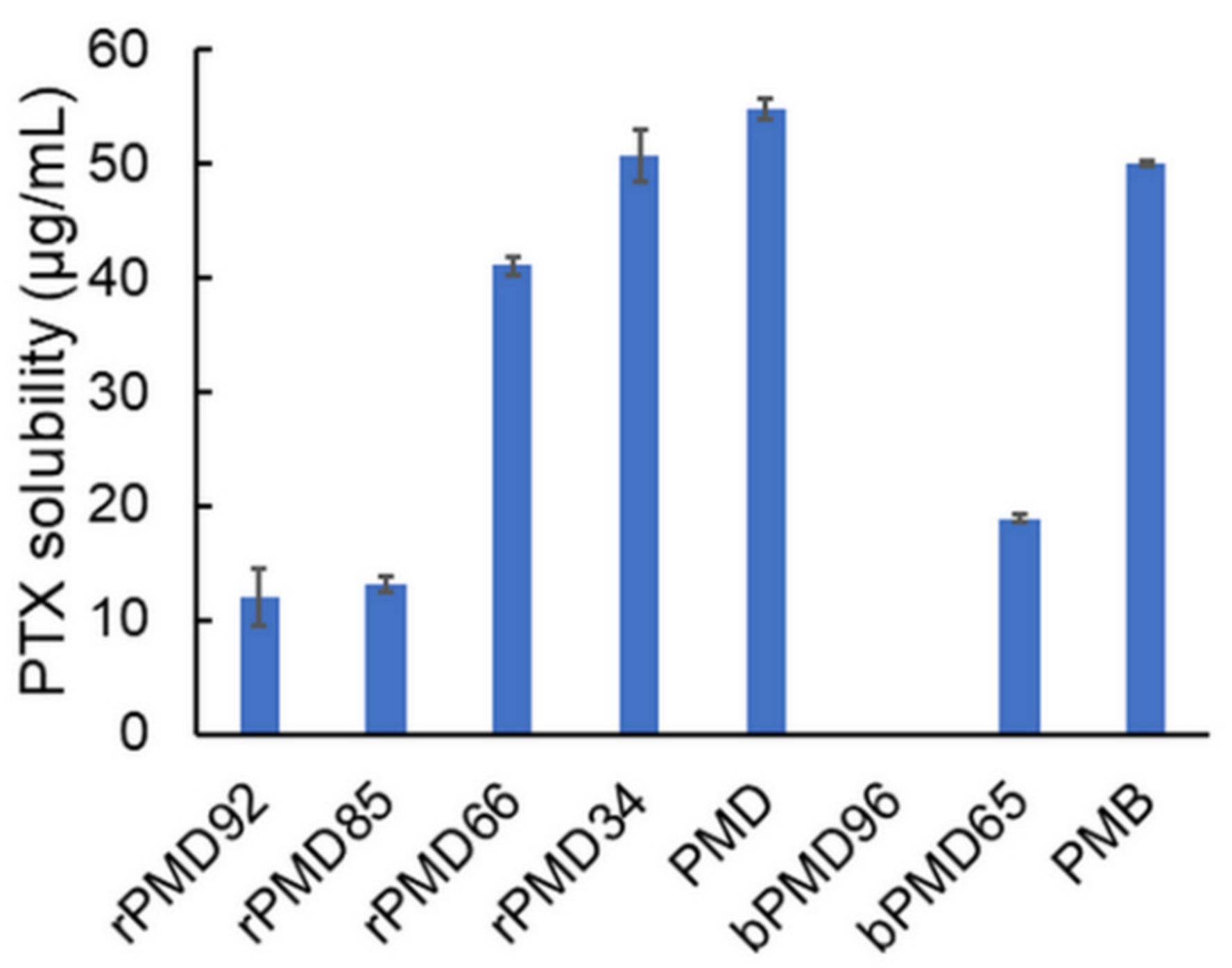
2.4. PTX Solubilization
2.5. Cytotoxicity
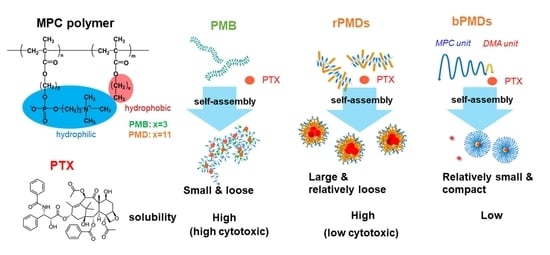
3. Results
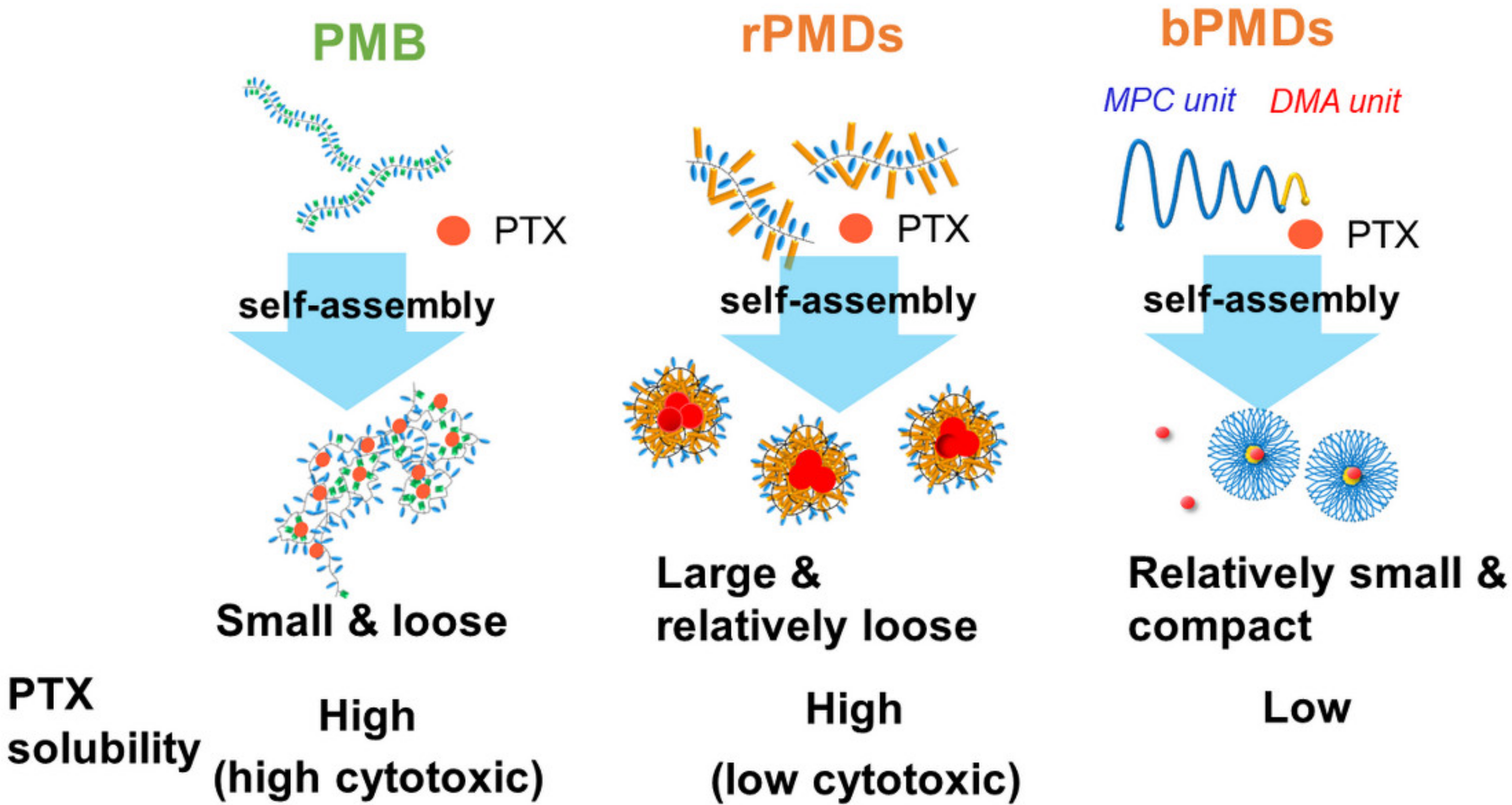
3.1. Self-Assembly of rPMDs and bPMDs
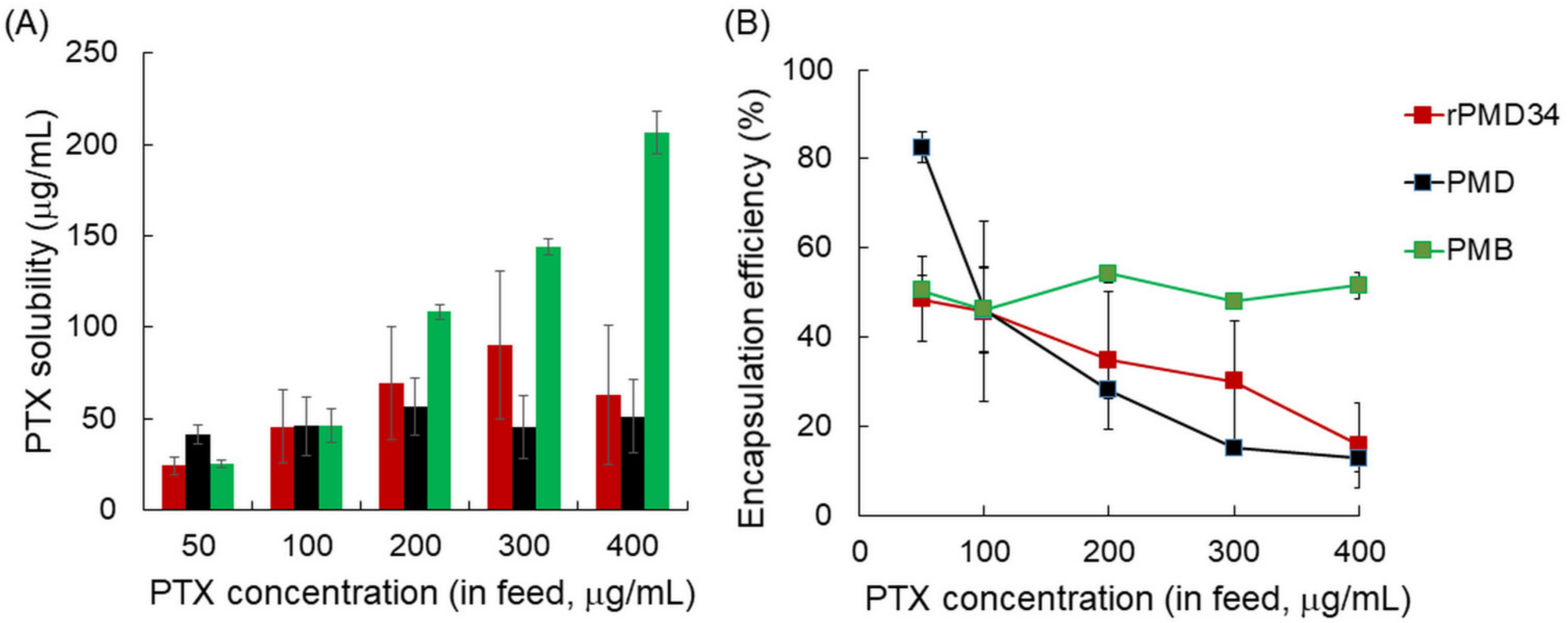
3.2. PTX Solubility of rPMDs and bPMDs
3.3. Cytotoxicity of PTX Solubilized by Different MPC Polymers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ishihara, K.; Ueda, T.; Nakabayashi, N. Preparation of phospholipid polymers and their properties as polymer hydrogel membranes. Polym. J. 1990, 22, 355–360. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Ishihara, K. Cell membrane-inspired phospholipid polymers for developing medical devices with excellent biointerfaces. Sci. Technol. Adv. Mater. 2012, 13, 064101. [Google Scholar] [CrossRef]
- Ueda, T.; Oshida, H.; Kurita, K.; Ishihara, K.; Nakabayashi, N. Preparation of 2-methacryloyloxyethyl phosphorylcholine copolymers with alkyl methacrylates and their blood compatibility. Polym. J. 1992, 24, 1259–1269. [Google Scholar] [CrossRef]
- Ishihara, K.; Iwasaki, Y. Reduced protein adsorption on novel phospholipid polymers. J. Biomater. Appl. 1998, 13, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Goda, T.; Ishihara, K.; Miyahara, Y. Critical update on 2-methacryloyloxyethyl phosphorylcholine (MPC) polymer science. J. Appl. Polym. Sci. 2015, 132, 41766. [Google Scholar] [CrossRef]
- Moro, T.; Takatori, Y.; Ishihara, K.; Konno, T.; Takigawa, Y.; Matsushita, T.; Chung, U.I.L.; Nakamura, K.; Kawaguchi, H. Surface grafting of artificial joints with a biocompatible polymer for preventing periprosthetic osteolysis. Nat. Mater. 2004, 3, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.P.; Nakabayashi, N.; Iwasaki, Y.; Boland, T.; LeBerge, M. Frictional properties of poly(MPC-co-BMA) phospholipid polymer for catheter applications. Biomaterials 2003, 24, 5121–5129. [Google Scholar] [CrossRef]
- Lewis, A.L.; Hughes, P.D.; Kirkwood, L.C.; Leppard, S.W.; Redman, R.P.; Tolhurst, L.A.; Stratford, P.W. Synthesis and characterization of phosphorylcholine-based polymers useful for coating blood filtration devices. Biomaterials 2000, 21, 1847–1859. [Google Scholar] [CrossRef]
- Clarke, S.; Davies, M.C.; Roberts, C.J.; Tendler, S.J.B.; Williams, P.M.; O’Byrne, V.; Lewis, A.L.; Russell, J. Surface mobility of 2-methacryloyloxyethyl phosphorylcholine-co-lauryl methacrylate polymers. Langmuir 2000, 16, 5116–5122. [Google Scholar] [CrossRef]
- Lewis, A.L.; Tolhurst, L.A.; Stratford, P.W. Analysis of a phosphorylcholine-based polymer coating on a coronary stent pre- and post-implantation. Biomaterials 2002, 23, 1697–1706. [Google Scholar] [CrossRef]
- Katayama, R.; Ikeda, M.; Shiraishi, K.; Matsumoto, A.; Kojima, C. Formation of hydrophobic domains on the poly(MPC-co-dodecyl methacrylate)-coated surface recognized by macrophage-like cells. Langmuir 2019, 35, 12229–12235. [Google Scholar] [CrossRef]
- Katayama, R.; Tanaka, N.; Takagi, Y.; Shiraishi, K.; Tanaka, Y.; Matsumoto, A.; Kojima, C. Characterization of hydration process of phospholipid-mimetic polymers using the air injection-meditated liquid exclusion methods. Langmuir 2020, 36, 5626–5632. [Google Scholar] [CrossRef]
- Kojima, C.; Katayama, R.; Nguyen, T.L.; Oki, Y.; Tsujimoto, A.; Yusa, S.; Shiraishi, K.; Matsumoto, A. Different antifouling effects of random and block copolymers comprising 2-methacryloyloxyethyl phosphorylcholine and dodecyl methacrylate. Eur. Polym. J. 2020, 136, 109932. [Google Scholar] [CrossRef]
- Ohshio, M.; Ishihara, K.; Yusa, S.-I. Self-association behavior of cell membrane-inspired amphiphilic random copolymers in water. Polymers 2019, 11, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, M.; Konno, T.; Inoue, Y.; Ishihara, K. Solubilization of poorly water-soluble compounds using amphiphilic phospholipid polymers with different molecular architectures. Colloids. Surf. B. 2017, 158, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Yusa, S.-I.; Fukuda, K.; Yamamoto, T.; Ishihara, K.; Morishima, Y. Synthesis of well-defined amphiphilic block copolymers having phospholipid polymer sequences as a novel biocompatible polymer micelle reagent. Biomacromolecules 2005, 6, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Chen, Y.; Wang, Y.; Ji, J. Zwitterionic drug nanocarriers: A biomimetic strategy for drug delivery. Colloids Surf. B 2014, 124, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Raval, N.; Maheshwari, R.; Shukla, H.; Kalia, K.; Torchilin, V.P.; Tekade, R.K. Multifunctional polymeric micellar nanomedicine in the diagnosis and treatment of cancer. Mater. Sci. Eng. C 2021, 126, 112186. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. J Control. Release 2021, 332, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; He, Y.; Zhang, J.; Wang, L.; Wang, Z.; Ji, F.; Chen, S. Highly hemocompatible zwitterionic micelles stabilized by reversible cross-linkage for anti-cancer drug delivery. Colloids Surf. B 2014, 115, 384–390. [Google Scholar] [CrossRef]
- Cao, J.; Xie, X.; Lu, A.; He, B.; Chen, Y.; Gu, Z.; Luo, X. Cellular internalization of doxorubicin loaded star-shaped micelles with hydrophilic zwitterionic sulfobetaine segments. Biomaterials 2014, 35, 4517–4524. [Google Scholar] [CrossRef]
- Sill, K.N.; Sullivan, B.; Carie, A.; Semple, J.E. Synthesis and characterization of micelle-forming PEG-poly(amino acid) copolymers with iron-hydroxamate cross-linkable blocks for encapsulation and release of hydrophobic drugs. Biomacromolecules 2017, 18, 1874–1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crown, J.; O’Leary, M. The taxanes: An update. Lancet 2000, 355, 1176–1178. [Google Scholar] [CrossRef]
- Weiss, R.B.; Donehower, R.C.; Wiernik, P.H.; Ohnuma, T.; Gralla, R.J.; Trump, D.L.; Baker, J.R., Jr.; Van Echo, D.A.; Von Hoff, D.D.; Leyland-Jones, B. Hypersensitivity reactions from taxol. J. Clin. Oncol. 1990, 8, 1263–1268. [Google Scholar] [CrossRef]
- Wang, F.; Porter, M.; Konstantopoulos, A.; Zhang, P.; Cui, H. Preclinical development of drug delivery systems for paclitaxel-based cancer chemotherapy. J Control. Release 2017, 267, 100–118. [Google Scholar] [CrossRef]
- Konno, T.; Watanabe, J.; Ishihara, K. Enhanced solubility of paclitaxel using water-soluble and biocompatible 2-methacryloyloxyethyl phosphorylcholine polymers. J. Biomed. Mater. Res. A 2003, 65A, 209–214. [Google Scholar] [CrossRef]
- Wada, M.; Jinno, H.; Ueda, M.; Ikeda, T.; Kitajima, M.; Konno, T.; Watanabe, J.; Ishihara, K. Efficacy of an MPC-BMA co-polymer as a nanotransporter for paclitaxel. Anticancer Res. 2007, 27, 1431–1435. [Google Scholar] [PubMed]
- Kojima, C.; Suehiro, T.; Watanabe, K.; Ogawa, M.; Fukuhara, A.; Nishisaka, E.; Harada, A.; Kono, K.; Inui, T.; Magata, Y. Doxorubicin-conjugated dendrimer/collagen hybrid gels for metastasis-associated drug delivery systems. Acta Biomater. 2013, 9, 5673–5680. [Google Scholar] [CrossRef]


| Polymer | Sequence | Composition (mol%) | Mn ×104 (Da) | Mw ×104 (Da) | Mw/Mn | Diameter e (nm) | |
|---|---|---|---|---|---|---|---|
| MPC | BMA/DMA | ||||||
| PMB | random | 30 | 70 | NA | 46 b | NA | ND |
| PMD | random | 33 | 66 | NA | 42 b | NA | 740 ± 170 |
| rPMD34 | random | 34 | 64 | - a | - a | NA | 700 ± 80 |
| rPMD66 | random | 66 | 34 | 4.8 c | 8.1 | 1.7 | 240 ± 20 |
| rPMD85 | random | 85 | 15 | 4.8 c | 13.3 | 2.8 | ND |
| rPMD92 | random | 92 | 8 | 4.9 c | 11.8 | 2.4 | ND |
| bPMD65 | block | 65 | 35 | 5.1 d | NA | NA | 130 ± 20 |
| bPMD96 | block | 96 | 4 | 3.1 d | NA | NA | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kojima, C.; Hirose, T.; Katayama, R.; Matsumoto, A. Solubilization of Paclitaxel by Self-Assembled Amphiphilic Phospholipid-Mimetic Polymers with Varied Hydrophobicity. Polymers 2021, 13, 2805. https://doi.org/10.3390/polym13162805
Kojima C, Hirose T, Katayama R, Matsumoto A. Solubilization of Paclitaxel by Self-Assembled Amphiphilic Phospholipid-Mimetic Polymers with Varied Hydrophobicity. Polymers. 2021; 13(16):2805. https://doi.org/10.3390/polym13162805
Chicago/Turabian StyleKojima, Chie, Tomoka Hirose, Risa Katayama, and Akikazu Matsumoto. 2021. "Solubilization of Paclitaxel by Self-Assembled Amphiphilic Phospholipid-Mimetic Polymers with Varied Hydrophobicity" Polymers 13, no. 16: 2805. https://doi.org/10.3390/polym13162805
APA StyleKojima, C., Hirose, T., Katayama, R., & Matsumoto, A. (2021). Solubilization of Paclitaxel by Self-Assembled Amphiphilic Phospholipid-Mimetic Polymers with Varied Hydrophobicity. Polymers, 13(16), 2805. https://doi.org/10.3390/polym13162805