Abstract
Bone tissue engineering commonly encompasses the use of three-dimensional (3D) scaffolds to provide a suitable microenvironment for the propagation of cells to regenerate damaged tissues or organs. 3D printing technology has been extensively applied to allow direct 3D scaffolds manufacturing. Polycaprolactone (PCL) has been widely used in the fabrication of 3D scaffolds in the field of bone tissue engineering due to its advantages such as good biocompatibility, slow degradation rate, the less acidic breakdown products in comparison to other polyesters, and the potential for loadbearing applications. PCL can be blended with a variety of polymers and hydrogels to improve its properties or to introduce new PCL-based composites. This paper describes the PCL used in developing state of the art of scaffolds for bone tissue engineering. In this review, we provide an overview of the 3D printing techniques for the fabrication of PCL-based composite scaffolds and recent studies on applications in different clinical situations. For instance, PCL-based composite scaffolds were used as an implant surgical guide in dental treatment. Furthermore, future trend and potential clinical translations will be discussed.
1. Introduction
Bone tissue, as one of the most important organs, plays multiple roles in daily life [1]. Lots of patients are suffering from bone disease resulting from tumor resections, trauma, infections, cysts, and injuries caused by accidents. It has been reported that over four million operations using bone grafts are performed each year to treat bone defects [2]. Autogenous bone transplantation and replacement are the main traditional options for patients with bone defects [3,4]. However, the potential risks of tissue grafts including complications and secondary injuries remain a major clinical challenge [5,6].
To overcome this shortage, bone tissue engineering is one of the most proposing alternative methods. Bone tissue engineering focuses on the main processes including cell growth and the customized construction of human bone tissue [7,8,9]. Further, 3D printing has multiple advantages, including precise deposition, cost-effectiveness, simplicity, and cell distribution controllability [10]. The developments and applications of 3D printing have been increasing constantly over the past few years.
In the field of bone tissue regeneration, polycaprolactone (PCL) is one of the most common materials in fabricating scaffolds. PCL is a Food and Drug Administration (FDA) approved linear polyester with good biocompatibility, slow degradation rate, less acidic breakdown products in comparison to other polyesters, and has the potential for loadbearing applications [11,12]. The slow degradation of PCL allows time for bone remodeling and can also be manipulated to adjust the polymer’s biodegradation rates [13,14]. Additionally, PCL is one of the most preferred polymers for extrusion-based 3D printing due to its melting temperature of 55–60 °C [13]. It exhibits good mechanical properties with high flexibility and great elongation, conducive to the preparation of scaffolds for craniofacial bone repair [9]. However, pure PCL has no osteogenic potential to induce bone regeneration [15]. Thus, researchers combine PCL with various polyesters, inorganic substances, metal elements, or collagen to improve the properties of the scaffolds. This review discusses and summarizes recent advancements in PCL-based composite scaffolds, focusing on the fabrication and functionalization methods and their application to promote bone growth in vitro and in vivo. Further, the future trends and potential clinical translations will be discussed.
2. Fabrication Techniques of Three-Dimensional Printing for Bone Scaffolds
The availability of desired properties for 3D printed scaffolds relies on the printing technology that is used. Generally, 3D printing is a process of layer-by-layer fabrication using powder, liquid, or solid material substrates. Starting from the bottom and building up, each newly formed layer is triggered to adhere to the previous layer, gradually increasing the size of the construct [16]. The techniques using in 3D printing include stereolithography (SLA), selective laser sintering (SLS), and fused deposition modelling (FDM). Because of the themoplasticity of PCL, the most common technique used for 3D printing is FDM [4,16,17,18,19]. FDM uses a temperature controlled printhead to deposit thermoplastic material onto a platform in a layer-by-layer manner to build up a 3D construct. PCL begins to melt by being driven into a heated printhead, allowing thin layers to be deposited precisely and sequentially. The molten PCL cools in the air of the printing environment, allowing it to rapidly fuse together to create a scaffold [16]. However, the elevated temperatures limit the inclusion of biomolecules and hydrogels.
Multiple studies have focused on cell behaviors on PCL scaffolds the scales of which usually range from hundreds of microns to millimeters fabricated by FDM [20]. However, there is a limitation of the techniques that can be used for the fabrication of fibrous micro-environments to study cell behaviors [21]. Electrohydrodynamic (EHD) printing, also named Melt Electrospinning Writing (MEW), is a recently developed technology to overcome the above limitations [22,23]. As shown in Figure 1, the design of MEW devices combines the advantages of conventional electrospinning and 3D printing [21]. This technology allows micron to sub-micron fiber fabrication [24]. Kim [25] fabricated fibrous scaffolds with the EHD technique and demonstrated significantly high metabolic activity and mineralization of the cells cultured on the micro-fiber PCL scaffolds. The comparison of PCL scaffolds fabricated by FDM and by MEW is shown in Figure 2. The micro-fiber PCL scaffolds printed by MEW directly affected the cell adhesion morphology. SLS is another technique for PCL-based scaffold fabrication. The basic design of a SLS printer is a housing that has a powder bed, a laser, a piston to move down in the vertical direction, and a roller to spread a new layer of powder. The computer-controlled laser beam sinters the powder, and the remaining powder works as a structural support for the scaffold being constructed [26]. With laser assistance, SLS is more accurate but more expensive than FDM.
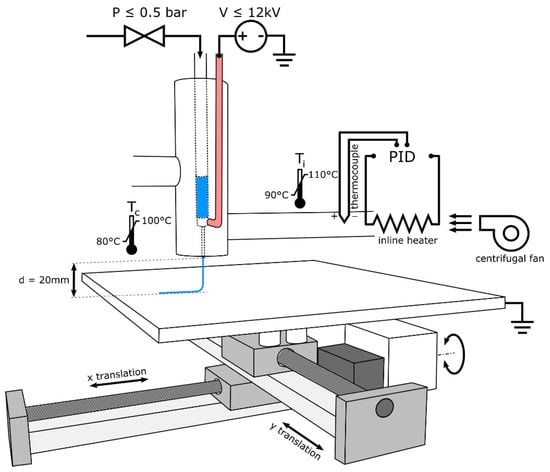
Figure 1.
Schematic of MEW device design illustrating the use of heated air to control syringe and needle temperature while using air pressure and high voltage to draw PCL and to produce electrospun fibers [21]. Copyright 2018 Elsevier.
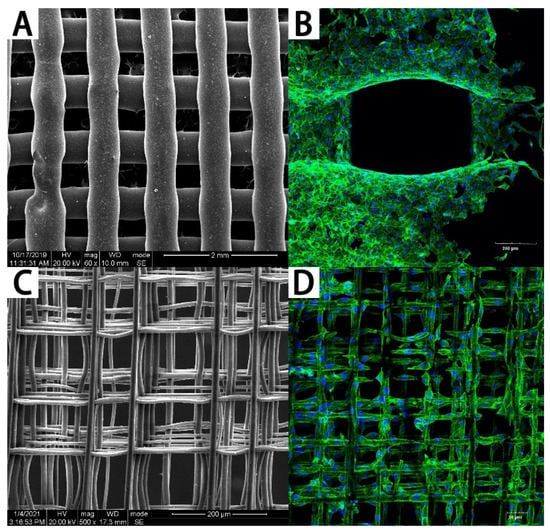
Figure 2.
The PCL scaffolds fabricated by FDM and MEW. (A) The PCL scaffold fabricated by FDM and (B) cells cultured on it. (C) The PCL scaffold fabricated by MEW and (D) cells cultured on it.
Bioprinting is another advanced technology which has aroused wide interest in recent years. Bioprinting can be used to deposit living cells and other biomaterials to build complex tissue constructs [16,27]. For the bioprinting of PCL-based composite scaffolds, researchers usually combine PCL with hydrogels that load living cells. Bioprinters have multiple print nozzles, one for PCL scaffolds printing, and the others for cell-loaded biomaterial printing simultaneously or separately. With this technology, mesenchymal stem cells (MSCs) or human umbilical vein endothelial cells (HUVECs) were usually loaded on the scaffolds to improve the vascularization of the printed structure [28,29,30,31]. The comparison of the 3D printing techniques for PCL-based scaffold fabrication is shown in Table 1.

Table 1.
Comparison of 3D printing technique for PCL-based scaffold fabrication.
3. PCL-Based Composite 3D Scaffolds
3.1. The Advanced Properties of PCL-Based Composite 3D Scaffolds
Numerous scaffolds produced from a variety of biomaterials have been used in the field in attempts to regenerate different tissues and organs [16]. Generally, 3D scaffolds are designed to imitate the extracellular matrix (ECM). These scaffolds are required to possess bioactive characteristics as follows and as reported [33,34]: a porous structure for the transport of nutrients, waste products, and for the communication with other cells; good biocompatibility with the controlled degradation and absorption rate of cell/tissue growth in vitro and/or in vivo; suitable surface chemistry stimulating cell ingrowth, cell attachment, and cell differentiation; and properties matching the individual clinical environments of bone defects.
Owing to its brilliant biocompatibility and easy processability, PCL has been extensively used in scaffold fabrication. However, the poor hydrophilia and low bioactivity of pure PCL systems limit their applications in the biomedical field [34]. Combining the PCL matrix with bioactive inorganic particles as fillers provides a promising way to overcome these shortcomings [34,35]. Metals, oxides, polymers, and carbon-based materials have all been applied to PCL scaffolds for property improvement [16]. A summary of recent researches and the advances in PCL-based composite scaffolds and property improvements is shown in Table 2.

Table 2.
A summary of recent research and advances in PCL-based composite scaffolds and property improvement.
The effects of different materials on the performance of composite scaffolds were also compared. Ethan [65] focused on the comparison of PCL-based scaffolds combined with tricalcium phosphate (TCP), hydroxyapatite (HA), Bio-Oss (BO), or decellularized bone matrix (DCB). They concluded that PCL-BO and PCL-DCB hybrids were superior to PCL-HA or PCL-TCP blends for bone healing applications. Marco [66] found that different diameters of hydroxyapatite blended in the printed scaffolds had distinct performance. PCL-nano-HA scaffolds showed higher levels of alkaline phosphatase activity compared to PCL-micro-HA structures. Differing from physical mixing process, Chen [67] synthesized poly(l-lactide-co-caprolactone-co-acryloyl carbonate)(poly(LLA-CL-AC)) by ring-opening polymerization in the presence of Sn(Oct)2 as a catalyst and octanol as an initiator for the first time. They found that the stiffness of the scaffolds increased after UV irradiation cross-linking.
3.2. The Architecture Structure of PCL-Based Composite Scaffolds
The standard approach in bone tissue engineering is to seed and grow cells on scaffolds in vitro. Typical scaffolds are 3D porous structures temporarily mimicking the natural extracellular matrix of bone [68]. Ideally, 3D scaffolds should be highly porous, have well-interconnected pore networks, and have consistent and adequate pore size for cell migration and infiltration [69]. Scaffold architecture design can significantly influence both mechanical properties and cell behaviors. The common structures designed for printed scaffolds are shown in Table 3.

Table 3.
The structure design of printed scaffolds.
A lot of researchers have focused on the outer morphology of printed scaffolds. Cylindrical and cube-shaped structures are common 3D printing shapes in preliminary studies. Similarly, circular, sinusoidal, and conventional orthogonal models were also fabricated and compared in previous studies, as shown in Figure 3 [70]. The results demonstrated that less orthogonal elements enhanced osteogenic performance. Further, the scaffold shapes are usually designed to match the shape of bone defect area for clinical application.
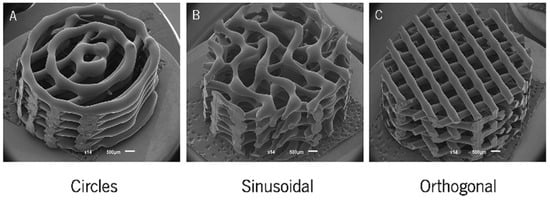
Figure 3.
SEM micrographs of the (A) circle, (B) sinusoidal, and (C) orthogonal scaffolds produced [70]. Copyright 2018 MDPI.
Numbers of studies have examined the inner structure inducement on cells behavior. First, the different deposit angle, which usually has an effect on mechanical property and porosity of scaffolds, has been studied [16]. The scaffold porosity is an important factor affecting the performance of scaffolds. Shim [71] found that 3D printed PCL GBR membranes with 30% porosity (130 μm pore size) were excellent for calvarial regeneration. Second, the pore structure has also been studied. Yang [72] developed compatible scaffolds which included macropores, medium-sized pores, and small pores, and these scaffolds are tailored to be similar to that of natural extracellular matrix (ECM). Adeola [73] focused on the effects of pore geometry on modulating mechanical behavior of PCL scaffolds. Lee [74] found that the kagome structure obviously improved the mechanical properties of PCL scaffolds compared to the grid structure. Abigail [75] fabricated artificial models that mimic the microstructure of bone, improving the accuracy of bone grafts.
In the field of tissue engineering, personalized medicine highlights the use of specifically designed scaffolds. The customized scaffolds can optimize the repairing process in cases of irregular-shaped wounds and tissue defects, especially for orthopedic, oral, and maxillofacial surgery [37]. With the aid of computed tomography (CT) or magnetic resonance imaging (MRI) data, the fabrication of patient-customized scaffolds using 3D printing technology is realizable. In this way, the mechanical properties, pore size, and porosity of the scaffold can be controlled [88]. Bae [89] successfully conducted the implant process using PCL-based scaffolds in the beagle model (Figure 4). These scaffolds restored the original volume and shape of the alveolar ridge in the defect site and performed well as a surgical guide to place the implant at the proper location and depth.
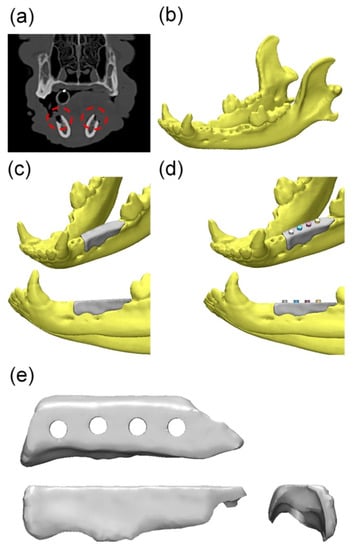
Figure 4.
Overall modeling process of the implant guide scaffold: (a) Red dashed line: alveolar bone defect of mandible; (b) 3D modeling process of CT image; (c) 3D scaffold cover of the defect area; (d) 4 thru holes for inserting implant fixture; (e) Final model of implant-guided scaffold [89]. Copyright 2017 MDPI.
3.3. Cell-Laden PCL-Based Composite Scaffolds
Naturally based hydrogels can offer excellent cellular interaction and biocompatibility, but they suffer from poor mechanical properties. On the other hand, PCL printed scaffolds possess compressive strengths within the range of cortical bone [90,91]. This has aroused great interest in the 3D bioprinting of cell laden hydrogel bioinks reinforced with stiffer PCL fibers [92,93,94]. Combining these two materials, cells can be diffused on the scaffolds accurately with the bioprinting process. Researchers demonstrated that the cells which were injected into the pores of scaffolds before clinical implantation, indicating promising osteogenesis enhancement in vivo [88].
There are a number of hydrogels that can be used for cell loading medium, such as matrigel [95], alginate [30,96], agarose [92], hyaluronic acid [78,92], and GelMA [31,92]. In the study of Caroline Murphy [95], they presented a scaffold with a mixture extrudable paste of PCL and borate glass. Human adipose stem cells suspended in matrigel were then ejected inside of the scaffold as droplets. They found a controlled release of the bioactive glass for up to 14 days with degradation of the scaffolds. The results showed a high level of angiogenesis in the interior the scaffold. Stichler [78] prepared hyaluronic acid/poly(glycolic acid) mixed hydrogels using UV light cross-linking. After loading human and equine mesenchymal stem cells, the PCL-based composite scaffolds were prepared using double-nozzle 3D printing technology. The cells showed a good chondrogenic differentiation prospect after 21 days. The combination of PCL and hydrogel improves the mechanical properties. At the same time, the existence of hydrogel is more suitable for the growth and reproduction of cells than pure PCL. Due to the high plasticity of PCL and the advantages of 3D printing technology, construction of personalized biomimetic tissue has become practical.
It has been well-established that lack of vascularization within the engineered bone grafts is a major barrier to bone healing [97,98]. Aiming to overcome this problem, Mitchell [28] presented a hydrogel-based prevascularization strategy to generate prevascularized bone scaffolds. They coated co-culture PCL/HA scaffolds with hydrogels, which encapsulated ADMSC and HUVEC. This co-culture system promoted vascularization in vitro and in vivo. Similarly, Wen [29] fabricated a PCL/polydopamine-modified calcium silicate scaffold loading with ADMSC and HUVEC. Xie [30] incorporated mesenchymal stem cell-derived microvesicles into alginate/ PCL constructs for angiogenesis promotion.
3.4. Carrier Function of PCL-Based Composite Scaffolds
Scaffold bioactivity can be increased by adding components that are able to interact with or bind to living tissues [99]. For a better clinical effect, PCL-based scaffolds with therapeutic agents added during the 3D process can be used as a carrier to load drugs and other bioactive substances to realize a long-term release.
Multiple studies have investigated the pharmacokinetic of 3D printed scaffolds. Hoang [100] used porogen leaching and 3D printing techniques and created microporous PCL scaffolds with micropors for drug loading and releasing control. They found that microscale porosity avoided the burst release of drugs and maintained relatively long-lasting drug concentrations. A summary of drug loading in PCL-based composite scaffolds is presented in Table 4.

Table 4.
A summary of drug loading in PCL-based composite scaffolds.
Biomaterials are designed to release bioactive substances at the injury site to stimulate bone repair [1,105]. The modification of bioactive components on 3D scaffolds, which enable bone cells to function in a sustainable manner, has aroused great interest worldwide. To mimic the physiological bone hierarchy, the bioactive molecules were added to PCL-based scaffolds for osseointegration (Table 5).

Table 5.
Bioactive molecule inclusion in PCL-based scaffolds for osseointegration.
Bone morphogenetic proteins (BMPs) are considered to be the most eminent in advancing bone growth by inducing osteogenic differentiation [1,6,15]. In Jang’s study [106], they made alginate/BMP-2/umbilical cord serum (UCS) coated on 3D-printed PCL scaffolds and demonstrated that the simultaneous use of low-dose BMP-2 and UCS significantly increased osteogenesis based in vitro and in vivo. To promote successful bone regeneration, efficient vascularization is a pre-requisite. Therefore, the angiogenic growth factor VEGF and its controlled delivery play a vital part in bone regeneration [107]. Additionally, Eric [108] constructed PCL scaffolds seeded with microspheres containing VEGF and VEGF with either BMP-2 or FGF-2 and observed significantly higher vascular ingrowth and vessel penetration than the controls. Collagen type I (COLI) can also be coated to the 3D printed structure, promoting the proliferation of chondrocytes [109]. Moreover, Won [110] compared the ability of promoting cell activity and mineralization between rhBM-2 and platelet-rich plasma (PRP) in scaffolds and concluded that rhBMP-2 was more efficient. As for mental molecule modification, gold nanoparticles (GNPs) grown on the polydopamine (PDA) coating of scaffolds have been demonstrated to be effective for bone regeneration [111]. Further, Michal [112] confirmed that osteoclast activity was greatly suppressed by the lithium release of printed PCL scaffolds.
4. PCL-Based Composite Scaffolds Utilized in Different Situations
For clinical application, three types of clinical applications for 3D-printed technologies are defined in previous studies [125]. The first is for prosthetic rehabilitation (improve patient aesthetic), the second is for reconstruction (tissue grafting), and last is for tissue regeneration (recapitulate native tissue structure and function). PCL-based scaffolds can be used in many kinds of tissue engineering, such as skin regeneration [126], skeletal muscle tissue regeneration [127], and tendon regeneration [73]. In the field of bone defect therapy, there are reconstruction and regeneration processes for cartilage tissue and bone tissue.
4.1. Reconstruction and Regeneration of Cartilage Tissue Using PCL-Based Composite Scaffolds
PCL is reported as a popular material in cartilage regeneration. Combining PCL with agarose and GelMA or MECM, engineered meniscus were constructed, which had the potential for use as a substitute for total meniscus replacement [10,128]. Zhang [129] and his colleagues constructed scaffolds for total meniscal substitution in a rabbit model. With the aid of multidetector CT and computer design, 3D scaffolds for use in total ear reconstruction were successfully fabricated [130]. Additionally, 3D printing techniques were also used for trachea engineering. For instance, Parket [131], Shan [86], and Gao [132] have successfully fabricated trachea scaffolds. Interestingly, Parket implanted the tracheal scaffold into the omentum before tracheal scaffold implantation in rabbits and concluded that the omentum-culture of the tracheal scaffold was beneficial for rapid the re-epithelialization and revascularization of the scaffold. Further, it also prevented postoperative luminal stenosis.
4.2. Reconstruction and Regeneration of Bone Tissue Using PCL-Based Composite Scaffolds
For animal surgery, Carla [133] conducted a surgical therapy of a chronic oronasal fistula in a cat using autologous platelet-rich fibrin and bone marrow loaded printed PCL scaffold. A CT scan revealed complete healing after a six-month follow-up. In Lee’s research [87], a customized scaffold matched with an 8-shaped bone defect on the rabbit calvarium model was designed according to 3D computed tomography. they then implanted the scaffold in the defect area, which showed excellent mechanical robustness and enhanced osteoconductivity. Rebecca Chung [134] proposed a patient-specific 3D printed bioresorbable graft substitute for segmental bone replacement.
In the dental application field, it is difficult to fabricate a scaffold matching the complex shape and functions of the nature craniomaxillofacial (CMF) bones. With the CT multidetector data, we can construct patient-customized structure 3D scaffolds for bone tissue regeneration using 3D printing [16,18]. Joshua printed an anatomically shaped scaffold that closely resembled the 3D models [135]. A custom scaffold can be used simultaneously as an implant surgical guide and as a bone graft in a large bone defect site. Upon dental implant surgery, successful implant placement is reliant on adequate alveolar bone volume at the implant site, which can provide mechanical stability for dental implants. It is important to augment the alveolar ridge for enabling the placement of dental implants and thus to restore both functionality and esthetic appearance [90]. Rider [90] and Vaquette [136] proposed that printed scaffolds showed potential in transferring to alveolar vertical bone augmentation (Figure 5). Due to the high compressive strength of the printed structure, these scaffolds may be applicable for procedures involving simultaneous implant placement and ridge augmentation.
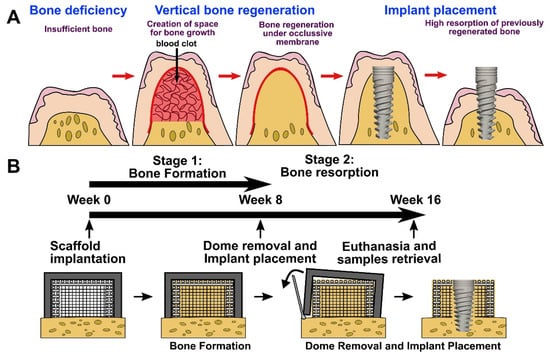
Figure 5.
Vertical bone augmentation with a 3D printing approach. (A) Description of the clinical problem following surgical re-entry in previously elevated bone, resulting in significant bone resorption. (B) Timeline of the experimental approach involving a two-staged strategy; bone formation following surgical re-entry and implant placement [136]. Copyright 2021 Elsevier.
5. Conclusions
PCL is a common polymer with unique biomedical and mechanical properties that make it favorable for a wide range of bone tissue engineering applications. Its low degeneration allows the imperative periods needed for new bone regeneration. With the development of three-dimensional printing techniques, various 3D structures can be fabricated successfully. Numbers of materials were utilized in the studies on PCL-based composite scaffolds, and composite scaffolds demonstrated superior performance to pure PCL scaffolds in recent studies. Cells can be also printed into the scaffolds by blending with hydrogel, which provides a compatible medium for cell proliferation. Further, in vivo studies of PCL scaffolds used in bone or cartilage tissue engineering applications have proven their osteogenic potential. Nevertheless, these studies were mostly conducted in small animals (usually rats and rabbits), which may not sufficiently predict clinical application in humans. Almost none of the researchers have proceeded to the phase of human trials yet. Thus, the advantages of PCL-based tissue engineering remain distant for patients in hospital. However, PCL is still a promising biomaterial. Future work should focus on PCL-based scaffolds in large animal models as well as in human clinical trials. We expect to develop custom-made 3D composite scaffolds that can be grafted directly with stem cells in clinical practice.
Author Contributions
Conceptualization, X.Y.; writing review and editing, X.Y. and Y.W.; revising and editing, X.Y., J.C. and Y.Z.; supervision, J.C. and Q.W. Moreover, J.C. and Q.W. contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (81970948, 81901060).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bhattacharjee, P.; Kundu, B.; Naskar, D.; Kim, H.-W.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Silk scaffolds in bone tissue engineering: An overview. Acta Biomater. 2017, 63, 1–17. [Google Scholar] [CrossRef]
- Samorezov, J.E.; Alsberg, E. Spatial regulation of controlled bioactive factor delivery for bone tissue engineering. Adv. Drug Deliv. Rev. 2014, 84, 45–67. [Google Scholar] [CrossRef] [Green Version]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.-C.; Reid, R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Huang, K.-H.; Wang, C.-Y.; Chen, C.-Y.; Hsu, T.-T.; Lin, C.-P. Incorporation of Calcium Sulfate Dihydrate into a Mesoporous Calcium Silicate/Poly-ε-Caprolactone Scaffold to Regulate the Release of Bone Morphogenetic Protein-2 and Accelerate Bone Regeneration. Biomedicines 2021, 9, 128. [Google Scholar] [CrossRef]
- Cao, Y.; Cheng, P.; Sang, S.; Xiang, C.; An, Y.; Wei, X.; Shen, Z.; Zhang, Y.; Li, P. Mesenchymal stem cells loaded on 3D-printed gradient poly(ε-caprolactone)/methacrylated alginate composite scaffolds for cartilage tissue engineering. Regen. Biomater. 2021, 8, rbab019. [Google Scholar] [CrossRef]
- Dias, D.; Vale, A.C.; Cunha, E.P.F.; Paiva, M.C.; Reis, R.L.; Vaquette, C.; Alves, N.M. 3D -printed cryomilled poly(ε-caprolactone)/graphene composite scaffolds for bone tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 961–972. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Nagarajan, S.; Belaid, H.; Farha, C.; Iatsunskyi, I.; Coy, E.; Soussan, L.; Huon, V.; Bares, J.; Belkacemi, K.; et al. Fabrication of 3D printed antimicrobial polycaprolactone scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2021, 118, 111525. [Google Scholar] [CrossRef]
- Guo, W.; Chen, M.; Wang, Z.; Tian, Y.; Zheng, J.; Gao, S.; Li, Y.; Zheng, Y.; Li, X.; Huang, J.; et al. 3D-printed cell-free PCL–MECM scaffold with biomimetic micro-structure and micro-environment to enhance in situ meniscus regeneration. Bioact. Mater. 2021, 6, 3620–3633. [Google Scholar] [CrossRef]
- Lee, H.; Yeo, M.; Ahn, S.; Kang, D.-O.; Jang, C.H.; Lee, H.; Park, G.-M.; Kim, G.H. Designed hybrid scaffolds consisting of polycaprolactone microstrands and electrospun collagen-nanofibers for bone tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 97, 263–270. [Google Scholar] [CrossRef]
- Zimmerling, A.; Yazdanpanah, Z.; Cooper, D.M.L.; Johnston, J.D.; Chen, X. 3D printing PCL/nHA bone scaffolds: Exploring the influence of material synthesis techniques. Biomater Res. 2021, 25, 3. [Google Scholar] [CrossRef]
- Seyedsalehi, A.; Daneshmandi, L.; Barajaa, M.; Riordan, J.; Laurencin, C.T. Fabrication and characterization of mechanically competent 3D printed polycaprolactone-reduced graphene oxide scaffolds. Sci. Rep. 2020, 10, 22210. [Google Scholar] [CrossRef]
- Cakmak, A.M.; Unal, S.; Sahin, A.; Oktar, F.N.; Sengor, M.; Ekren, N.; Gunduz, O.; Kalaskar, D.M. 3D Printed Polycaprolactone/Gelatin/BacterialCellulose/Hydroxyapatite Composite Scaffold for Bone Tissue Engineering. Polymers 2020, 12, 1962. [Google Scholar] [CrossRef]
- Park, S.A.; Lee, H.J.; Kim, S.Y.; Kim, K.S.; Jo, D.W.; Park, S.Y. Three-dimensionally printed polycaprolactone/beta-tricalcium phosphate scaffold was more effective as an rhBMP-2 carrier for new bone formation than polycaprolactone alone. J. Biomed. Mater. Res. Part A 2021, 109, 840–848. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno Madrid, A.P.; Mariel Vrech, S.; Alejandra Sanchez, M.; Paola Rodriguez, A. Advances in additive manufacturing for bone tissue engineering scaffolds. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 100, 631–644. [Google Scholar] [CrossRef]
- Arealis, G.; Nikolaou, V.S. Bone printing: New frontiers in the treatment of bone defects. Injury 2015, 46, S20–S22. [Google Scholar] [CrossRef]
- Wu, G.H.; Hsu, S.H. Review: Polymeric-Based 3D Printing for Tissue Engineering. J. Med. Biol. Eng. 2015, 35, 285–292. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Gao, Q.; Wang, P.; Shao, L.; Yuan, H.; Fu, J.; Chen, W.; He, Y. Structure-induced cell growth by 3D printing of heterogeneous scaffolds with ultrafine fibers. Mater. Des. 2019, 181, 108092. [Google Scholar] [CrossRef]
- Eichholz, K.F.; Hoey, D.A. Mediating human stem cell behaviour via defined fibrous architectures by melt electrospinning writing. Acta Biomater. 2018, 75, 140–151. [Google Scholar] [CrossRef]
- He, J.; Xu, F.; Cao, Y.; Liu, Y.; Li, D. Towards microscale electrohydrodynamic three-dimensional printing. J. Phys. D Appl. Phys. 2016, 49, 055504. [Google Scholar] [CrossRef]
- Kade, J.C.; Dalton, P.D. Polymers for Melt Electrowriting. Adv. Healthc. Mater. 2021, 10, e2001232. [Google Scholar] [CrossRef] [PubMed]
- Hochleitner, G.; Jungst, T.; Brown, T.D.; Hahn, K.; Moseke, C.; Jakob, F.; Dalton, P.D.; Groll, J. Additive manufacturing of scaffolds with sub-micron filaments via melt electrospinning writing. Biofabrication 2015, 7, 035002. [Google Scholar] [CrossRef]
- Kim, M.; Yun H-s Kim, G.H. Electric-field assisted 3D-fibrous bioceramic-based scaffolds for bone tissue regeneration: Fabrication, characterization, and in vitro cellular activities. Sci. Rep. 2017, 7, 3166. [Google Scholar] [CrossRef]
- Youssef, A.; Hollister, S.J.; Dalton, P.D. Additive manufacturing of polymer melts for implantable medical devices and scaffolds. Biofabrication 2017, 9, 1. [Google Scholar] [CrossRef]
- Swetha, S.; Lavanya, K.; Sruthi, R.; Selvamurugan, N. An insight into cell-laden 3D-printed constructs for bone tissue engineering. J. Mater Chem. B 2020, 8, 9836–9862. [Google Scholar] [CrossRef]
- Kuss, M.A.; Wu, S.; Wang, Y.; Untrauer, J.B.; Li, W.; Lim, J.Y.; Duan, B. Prevascularization of 3D printed bone scaffolds by bioactive hydrogels and cell co-culture. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1788–1798. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Shen, Y.-F.; Ho, C.-C.; Yu, J.; Wu, Y.-H.A.; Wang, K.; Shih, C.-T.; Shie, M.-Y. Osteogenic and angiogenic potentials of the cell-laden hydrogel/mussel-inspired calcium silicate complex hierarchical porous scaffold fabricated by 3D bioprinting. Mater. Sci. Eng. C 2018, 91, 679–687. [Google Scholar] [CrossRef]
- Xie, H.; Wang, Z.; Zhang, L.; Lei, Q.; Zhao, A.; Wang, H.; Li, Q.; Chen, Z.; Zhang, W. Development of an angiogenesis-promoting microvesicle-alginate-polycaprolactone composite graft for bone tissue engineering applications. PeerJ 2016, 4, e2040. [Google Scholar] [CrossRef]
- Jian, Z.; Zhuang, T.; Qinyu, T.; Liqing, P.; Kun, L.; Xujiang, L.; Diaodiao, W.; Zhen, Y.; Shuangpeng, J.; Xiang, S.; et al. 3D bioprinting of a biomimetic meniscal scaffold for application in tissue engineering. Bioact. Mater. 2021, 6, 1711–1726. [Google Scholar] [CrossRef]
- Do, A.V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef] [Green Version]
- Fedorovich, N.E.; Alblas, J.; Hennink, W.E.; Oner, F.C.; Dhert, W.J. Organ printing: The future of bone regeneration? Trends Biotechnol. 2011, 29, 601–606. [Google Scholar] [CrossRef]
- Liao, G.; Jiang, S.; Xu, X.; Ke, Y. Electrospun aligned PLLA/PCL/HA composite fibrous membranes and their in vitro degradation behaviors. Mater. Lett. 2012, 82, 159–162. [Google Scholar] [CrossRef]
- Guarino, V.; Causa, F.; Taddei, P.; Di Foggia, M.; Ciapetti, G.; Martini, D.; Fagnano, C.; Baldini, N.; Ambrosio, L. Polylactic acid fibre-reinforced polycaprolactone scaffolds for bone tissue engineering. Biomaterials 2008, 29, 3662–3670. [Google Scholar] [CrossRef]
- Doyle, S.; Henry, L.; McGennisken, E.; Onofrillo, C.; Bella, C.; Duchi, S.; O’Connell, C.; Pirogova, E. Characterization of Polycaprolactone Nanohydroxyapatite Composites with Tunable Degradability Suitable for Indirect Printing. Polymers 2021, 13, 295. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Yang, G.; Hu, X.; Wang, L.; Liu, B.; Wang, J.; Zhang, S. Delivering Proangiogenic Factors from 3D-Printed Polycaprolactone Scaffolds for Vascularized Bone Regeneration. Adv. Health Mater. 2020, 9, e2000727. [Google Scholar] [CrossRef]
- Wu, Y.-H.A.; Chiu, Y.-C.; Lin, Y.-H.; Ho, C.-C.; Shie, M.-Y.; Chen, Y.-W. 3D-Printed Bioactive Calcium Silicate/Poly-epsilon-Caprolactone Bioscaffolds Modified with Biomimetic Extracellular Matrices for Bone Regeneration. Int. J. Mol. Sci. 2019, 20, 942. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Junior, J.R.P.; Nalesso, P.R.; Musson, D.; Cornish, J.; Mendonça, F.; Caetano, G.F.; Bartolo, P. Engineered 3D printed poly(ε-caprolactone)/graphene scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 759–770. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Enhanced cell functions on graphene oxide incorporated 3D printed polycaprolactone scaffolds. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 102, 1–11. [Google Scholar] [CrossRef]
- Park, J.; Park, S.; Kim, J.E.; Jang, K.J.; Seonwoo, H.; Chung, J.H. Enhanced Osteogenic Differentiation of Periodontal Ligament Stem Cells Using a Graphene Oxide-Coated Poly(epsilon-caprolactone) Scaffold. Polymers 2021, 13, 797. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Vyas, C.; Roberts, I.; Poutrel, Q.-A.; Chiang, W.-H.; Blaker, J.J.; Huang, Z.; Bártolo, P. Fabrication and characterisation of 3D printed MWCNT composite porous scaffolds for bone regeneration. Mater. Sci. Eng. C 2019, 98, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Rogowska-Tylman, J.; Locs, J.; Salma, I.; Woźniak, B.; Pilmane, M.; Zalite, V.; Wojnarowicz, J.; Kędzierska-Sar, A.; Chudoba, T.; Szlazak, K.; et al. In vivo and in vitro study of a novel nanohydroxyapatite sonocoated scaffolds for enhanced bone regeneration. Mater. Sci. Eng. C 2019, 99, 669–684. [Google Scholar] [CrossRef]
- Bas, O.; Hanßke, F.; Lim, J.; Ravichandran, A.; Kemnitz, E.; Teoh, S.-H.; Hutmacher, D.W.; Börner, H.G. Tuning mechanical reinforcement and bioactivity of 3D printed ternary nanocomposites by interfacial peptide-polymer conjugates. Biofabrication 2019, 11, 035028. [Google Scholar] [CrossRef]
- Petretta, M.; Gambardella, A.; Boi, M.; Berni, M.; Cavallo, C.; Marchiori, G.; Maltarello, M.; Bellucci, D.; Fini, M.; Baldini, N.; et al. Composite Scaffolds for Bone Tissue Regeneration Based on PCL and Mg-Containing Bioactive Glasses. Biology 2021, 10, 398. [Google Scholar] [CrossRef]
- Rumiński, S.; Ostrowska, B.; Jaroszewicz, J.; Skirecki, T.; Włodarski, K.; Swieszkowski, W.; Lewandowska-Szumieł, M. Three-dimensional printed polycaprolactone-based scaffolds provide an advantageous environment for osteogenic differentiation of human adipose-derived stem cells. J. Tissue Eng. Regen. Med. 2018, 12, e473–e485. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhang, S.; Lan, Y.; Huang, C.; Wang, C.; Lai, X.; Chen, H.; Ao, N. 3D printed porous polycaprolactone/oyster shell powder (PCL/OSP) scaffolds for bone tissue engineering. Mater. Res. Express 2018, 5, 045403. [Google Scholar] [CrossRef]
- Moncal, K.K.; Heo, D.N.; Godzik, K.P.; Sosnoski, D.M.; Mrowczynski, O.D.; Rizk, E.; Ozbolat, V.; Tucker, S.M.; Gerhard, E.M.; Dey, M.; et al. 3D printing of poly(ε-caprolactone)/poly(d,l-lactide-co-glycolide)/hydroxyapatite composite constructs for bone tissue engineering. J. Mater. Res. 2018, 33, 1972–1986. [Google Scholar] [CrossRef]
- Rindone, A.N.; Nyberg, E.; Grayson, W.L. 3D-Printing Composite Polycaprolactone-Decellularized Bone Matrix Scaffolds for Bone Tissue Engineering Applications. Methods Mol. Biol. 2018, 1577, 209–226. [Google Scholar]
- Hung, B.P.; Naved, B.A.; Nyberg, E.L.; Dias, M.; Holmes, C.; Elisseeff, J.H.; Dorafshar, A.; Grayson, W.L. Three-Dimensional Printing of Bone Extracellular Matrix for Craniofacial Regeneration. ACS Biomater. Sci. Eng. 2016, 2, 1806–1816. [Google Scholar] [CrossRef]
- Park, S.A.; Lee, S.J.; Seok, J.M.; Lee, J.H.; Kim, W.D.; Kwon, I.K. Fabrication of 3D Printed PCL/PEG Polyblend Scaffold Using Rapid Prototyping System for Bone Tissue Engineering Application. J. Bionic. Eng. 2018, 15, 435–442. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Ahn, G.; Kim, C.; Lee, J.-S.; Lee, I.-G.; An, S.-H.; Yun, W.-S.; Kim, S.-Y.; Shim, J.-H. Synergistic Effects of Beta Tri-Calcium Phosphate and Porcine-Derived Decellularized Bone Extracellular Matrix in 3D-Printed Polycaprolactone Scaffold on Bone Regeneration. Macromol. Biosci. 2018, 18, e1800025. [Google Scholar] [CrossRef]
- Vella, J.B.; Trombetta, R.P.; Hoffman, M.D.; Inzana, J.; Awad, H.; Benoit, D.S.W. Three-dimensional printed calcium phosphate and poly(caprolactone) composites with improved mechanical properties and preserved microstructure. J. Biomed. Mater. Res. Part A 2018, 106, 663–672. [Google Scholar] [CrossRef]
- Tamjid, E. Three-dimensional polycaprolactone-bioactive glass composite scaffolds: Effect of particle size and volume fraction on mechanical properties and in vitro cellular behavior. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 1005–1015. [Google Scholar] [CrossRef]
- Neufurth, M.; Wang, X.; Wang, S.; Steffen, R.; Ackermann, M.; Haep, N.D.; Schröder, H.C.; Müller, W.E. 3D printing of hybrid biomaterials for bone tissue engineering: Calcium-polyphosphate microparticles encapsulated by polycaprolactone. Acta Biomater. 2017, 64, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.-S.; Choi, J.-W.; Kim, J.-H.; Chung, H.Y.; Jin, S.; Shim, J.-H.; Yun, W.-S.; Jeong, C.-M.; Huh, J.-B. Comparative Efficacies of Collagen-Based 3D Printed PCL/PLGA/β-TCP Composite Block Bone Grafts and Biphasic Calcium Phosphate Bone Substitute for Bone Regeneration. Materials 2017, 10, 421. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Lee, S.J.; Jo, H.H.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. Fabrication and characterization of 3D-printed bone-like β-tricalcium phosphate/polycaprolactone scaffolds for dental tissue engineering. J. Ind. Eng. Chem. 2017, 46, 175–181. [Google Scholar] [CrossRef]
- Shim, K.-S.; Kim, S.E.; Yun, Y.-P.; Jeon, D.I.; Kim, H.J.; Park, K.; Song, H.-R. Surface immobilization of biphasic calcium phosphate nanoparticles on 3D printed poly(caprolactone) scaffolds enhances osteogenesis and bone tissue regeneration. J. Ind. Eng. Chem. 2017, 55, 101–109. [Google Scholar] [CrossRef]
- Chiu, Y.-C.; Fang, H.-Y.; Hsu, T.-T.; Lin, C.-Y.; Shie, M.-Y. The Characteristics of Mineral Trioxide Aggregate/Polycaprolactone 3-dimensional Scaffold with Osteogenesis Properties for Tissue Regeneration. J. Endod. 2017, 43, 923–929. [Google Scholar] [CrossRef]
- Daly, A.C.; Cunniffe, G.M.; Sathy, B.N.; Jeon, O.; Alsberg, E.; Kelly, D.J. 3D Bioprinting of Developmentally Inspired Templates for Whole Bone Organ Engineering. Adv. Healthc. Mater. 2016, 5, 2353–2362. [Google Scholar] [CrossRef]
- Miao, S.; Zhu, W.; Castro, N.J.; Leng, J.; Zhang, L.G. Four-Dimensional Printing Hierarchy Scaffolds with Highly Biocompatible Smart Polymers for Tissue Engineering Applications. Tissue Eng. Part C-Methods 2016, 22, 952–963. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, E.M.; Oliveira, F.; Silva, R.; Neto, M.A.; Fernandes, M.H.; Amaral, M.; Vallet-Regí, M.; Vila, M. Three-dimensional printed PCL-hydroxyapatite scaffolds filled with CNTs for bone cell growth stimulation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1210–1219. [Google Scholar] [CrossRef]
- Karimipour-Fard, P.; Jeffrey, M.P.; JonesTaggart, H.; Pop-Iliev, R.; Rizvi, G. Development, processing and characterization of Polycaprolactone/Nano-Hydroxyapatite/Chitin-Nano-Whisker nanocomposite filaments for additive manufacturing of bone tissue scaffolds. J. Mech. Behav. Biomed. Mater 2021, 120, 104583. [Google Scholar] [CrossRef]
- Wang, C.; Xu, H.; Liu, C.; Peng, Z.; Min, R.; Zhang, Z.; Li, J.; Jin, Y.; Wang, Y.; Li, Z.; et al. CaO2/gelatin oxygen slow-releasing microspheres facilitate tissue engineering efficiency for the osteonecrosis of femoral head by enhancing the angiogenesis and survival of grafted bone marrow mesenchymal stem cells. Biomater. Sci. 2021, 9, 3005–3018. [Google Scholar] [CrossRef]
- Nyberg, E.; Rindone, A.; Dorafshar, A.; Grayson, W.L. Comparison of 3D-Printed Poly-epsilon-Caprolactone Scaffolds Functionalized with Tricalcium Phosphate, Hydroxyapatite, Bio-Oss, or Decellularized Bone Matrix. Tissue Eng. Part A 2017, 23, 503. [Google Scholar] [CrossRef]
- Domingos, M.; Gloria, A.; Coelho, J.; Bartolo, P.; Ciurana, J. Three-dimensional printed bone scaffolds: The role of nano/micro-hydroxyapatite particles on the adhesion and differentiation of human mesenchymal stem cells. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2017, 231, 555–564. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Hochleitner, G.; Woodfield, T.; Groll, J.; Dalton, P.D.; Amsden, B.G. Additive Manufacturing of a Photo-Cross-Linkable Polymer via Direct Melt Electrospinning Writing for Producing High Strength Structures. Biomacromolecules 2016, 17, 208–214. [Google Scholar] [CrossRef]
- Butscher, A.; Bohner, M.; Hofmann, S.; Gauckler, L.; Muller, R. Structural and material approaches to bone tissue engineering in powder-based three-dimensional printing. Acta Biomater 2011, 7, 907–920. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, D.R.; Sobreiro-Almeida, R.; Sol, P.C.; Neves, N.M. Development of non-orthogonal 3D-printed scaffolds to enhance their osteogenic performance. Biomater. Sci. 2018, 6, 1569–1579. [Google Scholar] [CrossRef]
- Shim, J.-H.; Jeong, J.-H.; Won, J.-Y.; Bae, J.-H.; Ahn, G.; Jeon, H.; Yun, W.-S.; Bae, E.-B.; Choi, J.-W.; Lee, S.-H.; et al. Porosity effect of 3D-printed polycaprolactone membranes on calvarial defect model for guided bone regeneration. Biomed. Mater. 2017, 13, 015014. [Google Scholar] [CrossRef]
- Yang, T.; Hu, Y.; Wang, C.; Binks, B.P. Fabrication of Hierarchical Macroporous Biocompatible Scaffolds by Combining Pickering High Internal Phase Emulsion Templates with Three-Dimensional Printing. ACS Appl. Mater. Interfaces 2017, 9, 22950–22958. [Google Scholar] [CrossRef] [PubMed]
- Olubamiji, A.D.; Izadifar, Z.; Si, J.L.; Cooper, D.M.L.; Eames, B.F.; Chen, D.X.B. Modulating mechanical behaviour of 3D-printed cartilage-mimetic PCL scaffolds: Influence of molecular weight and pore geometry. Biofabrication 2016, 8, 025020. [Google Scholar] [CrossRef]
- Lee, S.-H.; Cho, Y.S.; Hong, M.W.; Lee, B.-K.; Park, Y.; Park, S.-H.; Kim, Y.Y.; Cho, Y.-S. Mechanical properties and cell-culture characteristics of a polycaprolactone kagome-structure scaffold fabricated by a precision extruding deposition system. Biomed. Mater. 2017, 12, 055003. [Google Scholar] [CrossRef]
- Feasibility of Fabricating Personalized 3D-Printed Bone Grafts Guided by High-Resolution Imaging. Available online: https://www.spiedigitallibrary.org/conference-proceedings-of-spie/10138/101380O/Feasibility-of-fabricating-personalized-3D-printed-bone-grafts-guided-by/10.1117/12.2254475.short?SSO=1 (accessed on 27 June 2021).
- Barbara, O.; Andrea, D.L.; Lorenzo, M.; Wojciech, S. Influence of internal pore architecture on biological and mechanical properties of three-dimensional fiber deposited scaffolds for bone regeneration. Biomed. Mater Res. A. 2016, 104, 991–1001. [Google Scholar]
- Roh, H.-S.; Lee, C.-M.; Hwang, Y.-H.; Kook, M.-S.; Yang, S.-W.; Lee, D.; Kim, B.-H. Addition of MgO nanoparticles and plasma surface treatment of three-dimensional printed polycaprolactone/hydroxyapatite scaffolds for improving bone regeneration. Mater. Sci. Eng. C 2017, 74, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Stichler, S.; Böck, T.; Paxton, N.; Bertlein, S.; Levato, R.; Schill, V.; Smolan, W.; Malda, J.; Tessmar, J.; Blunk, T.; et al. Double printing of hyaluronic acid/poly(glycidol) hybrid hydrogels with poly(ε-caprolactone) for MSC chondrogenesis. Biofabrication 2017, 9, 044108. [Google Scholar] [CrossRef]
- Zheng, P.; Yao, Q.; Mao, F.; Liu, N.; Xu, Y.; Wei, B.; Wang, L. Adhesion, proliferation and osteogenic differentiation of mesenchymal stem cells in 3D printed poly-ε-caprolactone/hydroxyapatite scaffolds combined with bone marrow clots. Mol. Med. Rep. 2017, 16, 5078–5084. [Google Scholar] [CrossRef] [Green Version]
- Szlazak, K.; Jaroszewicz, J.; Ostrowska, B.; Nabiałek, M.; Szota, M.; Swieszkowski, W. Characterization of Three-Dimensional Printed Composite Scaffolds Prepared with Different Fabrication Methods. Arch. Met. Mater. 2016, 61, 645–650. [Google Scholar] [CrossRef]
- Theodoridis, K.; Aggelidou, E.; Vavilis, T.; Manthou, M.E.; Tsimponis, A.; Demiri, E.C.; Boukla, A.; Salpistis, C.; Bakopoulou, A.; Mihailidis, A.; et al. Hyaline cartilage next generation implants from adipose-tissue-derived mesenchymal stem cells: Comparative study on 3D-printed polycaprolactone scaffold patterns. J. Tissue Eng. Regen. Med. 2019, 13, 342–355. [Google Scholar] [CrossRef]
- Yang, G.H.; Kim, M.; Kim, G. Additive-manufactured polycaprolactone scaffold consisting of innovatively designed microsized spiral struts for hard tissue regeneration. Biofabrication 2016, 9, 15005. [Google Scholar] [CrossRef]
- Yang, G.-H.; Lee, H.; Kim, G. Preparation and characterization of spiral-like micro-struts with nano-roughened surface for enhancing the proliferation and differentiation of preosteoblasts. J. Ind. Eng. Chem. 2018, 61, 244–254. [Google Scholar] [CrossRef]
- Cho, Y.S.; Hong, M.W.; Quan, M.; Kim, S.-Y.; Lee, S.-H.; Lee, S.-J.; Kim, Y.Y.; Cho, Y.-S. Assessments for bone regeneration using the polycaprolactone SLUP (salt-leaching using powder) scaffold. J. Biomed. Mater. Res. Part A 2017, 105, 3432–3444. [Google Scholar] [CrossRef]
- Gupta, D.; Singh, A.K.; Kar, N.; Dravid, A.; Bellare, J. Modelling and optimization of NaOH-etched 3-D printed PCL for enhanced cellular attachment and growth with minimal loss of mechanical strength. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 98, 602–611. [Google Scholar] [CrossRef]
- Shan, Y.; Wang, Y.; Fan, Y.; Yang, J.; Ren, W.; Yu, X.; Li, J.; Shi, H. Biomechanical properties and cellular biocompatibility of 3D printed tracheal graft. Bioprocess Biosyst. Eng. 2017, 40, 1813–1823. [Google Scholar] [CrossRef]
- Lee, S.-H.; Lee, K.-G.; Hwang, J.-H.; Cho, Y.S.; Jeong, H.-J.; Park, S.-H.; Park, Y.; Cho, Y.-S.; Lee, B.-K. Evaluation of mechanical strength and bone regeneration ability of 3D printed kagome-structure scaffold using rabbit calvarial defect model. Mater. Sci. Eng. C 2019, 98, 949–959. [Google Scholar] [CrossRef]
- Lee, J.W.; Chu, S.G.; Kim, H.T.; Choi, K.Y.; Oh, E.J.; Shim, J.-H.; Yun, W.-S.; Huh, J.B.; Moon, S.H.; Kang, S.S.; et al. Osteogenesis of Adipose-Derived and Bone Marrow Stem Cells with Polycaprolactone/Tricalcium Phosphate and Three-Dimensional Printing Technology in a Dog Model of Maxillary Bone Defects. Polymers 2017, 9, 450. [Google Scholar] [CrossRef] [Green Version]
- Bae, J.C.; Lee, J.-J.; Shim, J.-H.; Park, K.-H.; Lee, J.-S.; Bae, E.-B.; Choi, J.-W.; Huh, J.-B. Development and Assessment of a 3D-Printed Scaffold with rhBMP-2 for an Implant Surgical Guide Stent and Bone Graft Material: A Pilot Animal Study. Materials 2017, 10, 1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rider, P.; Kacarevic, Z.P.; Alkildani, S.; Retnasingh, S.; Schnettler, R.; Barbeck, M. Additive Manufacturing for Guided Bone Regeneration: A Perspective for Alveolar Ridge Augmentation. Int. J. Mol. Sci. 2018, 19, 3308. [Google Scholar] [CrossRef] [Green Version]
- Bartolo, P.; Domingos, M.; Gloria, A.; Ciurana, J. BioCell Printing: Integrated automated assembly system for tissue engineering constructs. CIRP Ann. 2011, 60, 271–274. [Google Scholar] [CrossRef]
- Daly, A.C.; Critchley, S.E.; Rencsok, E.M.; Kelly, D.J. A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage. Biofabrication 2016, 8, 045002. [Google Scholar] [CrossRef]
- Critchley, S.; Sheehy, E.; Cunniffe, G.; Diaz-Payno, P.; Carroll, S.F.; Jeon, O.; Alsberg, E.; Brama, P.A.; Kelly, D.J. 3D printing of fibre-reinforced cartilaginous templates for the regeneration of osteochondral defects. Acta Biomater. 2020, 113, 130–143. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Q.; Zhang, Y.; Dai, K.; Wei, Y. 3D-bioprinted gradient-structured scaffold generates anisotropic cartilage with vascularization by pore-size-dependent activation of HIF1α/FAK signaling axis. Nanomedicine 2021, 102426. [Google Scholar] [CrossRef]
- Murphy, C.; Kolan, K.; Li, W.; Semon, J.; Day, D.; Leu, M. 3D bioprinting of stem cells and polymer/bioactive glass composite scaffolds for bone tissue engineering. Int. J. Bioprinting 2017, 3, 54–64. [Google Scholar] [CrossRef]
- Kim, Y.B.; Lee, H.; Yang, G.-H.; Choi, C.H.; Lee, D.; Hwang, H.; Jung, W.-K.; Yoon, H.; Kim, G.H. Mechanically reinforced cell-laden scaffolds formed using alginate-based bioink printed onto the surface of a PCL/alginate mesh structure for regeneration of hard tissue. J. Colloid Interface Sci. 2016, 461, 359–368. [Google Scholar] [CrossRef]
- Garcia, J.R.; Garcia, A.J. Biomaterial-mediated strategies targeting vascularization for bone repair. Drug Deliv. Trans. L Res. 2016, 6, 77–95. [Google Scholar] [CrossRef] [Green Version]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Differential analysis of peripheral blood- and bone marrow-derived endothelial progenitor cells for enhanced vascularization in bone tissue engineering. J. Orthop. Res. 2012, 30, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Nettleship, I.; Schmelzer, E.; Gerlach, J.C.; Zhang, M.X.; Wang, J.; Liu, C. Tissue Engineering and Regenerative Medicine Therapies for Cell Senescence in Bone and Cartilage. Tissue Eng. Part B Rev. 2020, 26, 64–78. [Google Scholar] [CrossRef] [Green Version]
- Dang, H.P.; Shabab, T.; Shafiee, A.; Peiffer, Q.C.; Fox, K.; Tran, N.; Dargaville, T.; Hutmacher, D.W.; A Tran, P. 3D printed dual macro-, microscale porous network as a tissue engineering scaffold with drug delivering function. Biofabrication 2019, 11, 035014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govender, M.; Indermun, S.; Kumar, P.; Choonara, Y.E.; Pillay, V. 3D Printed, PVA–PAA Hydrogel Loaded-Polycaprolactone Scaffold for the Delivery of Hydrophilic In-Situ Formed Sodium Indomethacin. Materials 2018, 11, 1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, J.; Ma, J.; Lin, L.; Wang, B.; Jansen, J.A.; Walboomers, X.F.; Zuo, Y.; Yang, F. Three-Dimensional Printing of Drug-Loaded Scaffolds for Antibacterial and Analgesic Applications. Tissue Eng. Part C Methods 2019, 25, 222–231. [Google Scholar] [CrossRef]
- Kim, S.E.; Yun, Y.-P.; Shim, K.-S.; Kim, H.J.; Park, K.; Song, H.-R. 3D printed alendronate-releasing poly(caprolactone) porous scaffolds enhance osteogenic differentiation and bone formation in rat tibial defects. Biomed. Mater. 2016, 11, 055005. [Google Scholar] [CrossRef]
- Puppi, D.; Piras, A.M.; Pirosa, A.; Sandreschi, S.; Chiellini, F. Levofloxacin-loaded star poly(ε-caprolactone) scaffolds by additive manufacturing. J. Mater. Sci. Mater. Med. 2016, 27, 44. [Google Scholar] [CrossRef] [PubMed]
- Bessa, P.C.; Balmayor, E.R.; Azevedo, H.; Nürnberger, S.; Casal, M.; van Griensven, M.; Reis, R.L.; Redl, H. Silk fibroin microparticles as carriers for delivery of human recombinant BMPs. Physical characterization and drug release. J. Tissue Eng. Regen. Med. 2010, 4, 349–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, C.H.; Lee, J.; Kim, G. Synergistic effect of alginate/BMP-2/Umbilical cord serum-coated on 3D-printed PCL biocomposite for mastoid obliteration model. J. Ind. Eng. Chem. 2019, 72, 432–441. [Google Scholar] [CrossRef]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Wagner, E.; Parry, J.; Dadsetan, M.; Bravo, D.; Riester, S.M.; Van Wijnen, A.J.; Yaszemski, M.J.; Kakar, S. VEGF-mediated angiogenesis and vascularization of a fumarate-crosslinked polycaprolactone (PCLF) scaffold. Connect. Tissue Res. 2018, 59, 542–549. [Google Scholar] [CrossRef]
- He, Y.; Liu, W.; Guan, L.; Chen, J.; Duan, L.; Jia, Z.; Huang, Z.; Li, W.; Liu, J.; Xiong, Z.; et al. A 3D-Printed PLCL Scaffold Coated with Collagen Type I and Its Biocompatibility. Biomed Res. Int. 2018, 2018, 5147156. [Google Scholar] [CrossRef]
- Kim, W.; Jang, C.H.; Kim, G. Optimally designed collagen/polycaprolactone biocomposites supplemented with controlled. release of HA/TCP/rhBMP-2 and HA/TCP/PRP for hard tissue regeneration. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 78, 763–772. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, H.-J.; Kim, S.-Y.; Seok, J.M.; Lee, J.H.; Kim, W.D.; Kwon, I.K.; Park, S.-Y.; A Park, S. In situgold nanoparticle growth on polydopamine-coated 3D-printed scaffolds improves osteogenic differentiation for bone tissue engineering applications:in vitroandin vivostudies. Nanoscale 2018, 10, 15447–15453. [Google Scholar] [CrossRef] [Green Version]
- Bartnikowski, M.; Moon, H.-J.; Ivanovski, S. Release of lithium from 3D printed polycaprolactone scaffolds regulates macrophage and osteoclast response. Biomed. Mater. 2018, 13, 065003. [Google Scholar] [CrossRef] [Green Version]
- Hamlet, S.M.; Vaquette, C.; Shah, A.; Hutmacher, D.W.; Ivanovski, S. 3-Dimensional functionalized polycaprolactone-hyaluronic acid hydrogel constructs for bone tissue engineering. J. Clin. Periodontol. 2017, 44, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, D.; Yoon, T.R.; Kim, H.K.; Jo, H.H.; Park, J.S.; Lee, J.H.; Kim, W.D.; Kwon, I.K.; A Park, S. Surface modification of 3D-printed porous scaffolds via mussel-inspired polydopamine and effective immobilization of rhBMP-2 to promote osteogenic differentiation for bone tissue engineering. Acta Biomater. 2016, 40, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Scocozza, F.; Perteghella, S.; Sorlini, M.; Auricchio, F.; Torre, M.; Conti, M. 3D Bioprinted Scaffolds Containing Mesenchymal Stem/Stromal Lyosecretome: Next Generation Controlled Release Device for Bone Regenerative Medicine. Pharmacrutics 2021, 13, 515. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Li, J.; Zhang, J.; Pan, Z.; Liu, Y.; Zhou, F.; Hong, Y.; Hu, Y.; Gu, Y.; Ouyang, H.; et al. An interleukin-4-loaded bi-layer 3D printed scaffold promotes osteochondral regeneration. Acta Biomater. 2020, 117, 246–260. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Wei, X.; Hao, Y.; Wang, J. Evaluation of 3D-Printed Polycaprolactone Scaffolds Coated with Freeze-Dried Platelet-Rich Plasma for Bone Regeneration. Materials 2017, 10, 831. [Google Scholar] [CrossRef] [Green Version]
- Cunniffe, G.M.; Gonzalez-Fernandez, T.; Daly, A.; Sathy, B.N.; Jeon, O.; Alsberg, E.; Kelly, D. Three-Dimensional Bioprinting of Polycaprolactone Reinforced Gene Activated Bioinks for Bone Tissue Engineering. Tissue Eng. Part A 2017, 23, 891–900. [Google Scholar] [CrossRef]
- Wei, P.; Xu, Y.; Gu, Y.; Yao, Q.; Li, J.; Wang, L. IGF-1-releasing PLGA nanoparticles modified 3D printed PCL scaffolds for cartilage tissue engineering. Drug Deliv. 2020, 27, 1106–1114. [Google Scholar] [CrossRef]
- Mostafavi, A.; Abudula, T.; Russell, C.S.; Mostafavi, E.; Williams, T.J.; Salah, N.; Alshahrie, A.; Harris, S.; Basri, S.M.M.; Mishra, Y.K.; et al. In situ printing of scaffolds for reconstruction of bone defects. Acta Biomater. 2021, 127, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Xie, K.; Guo, Y.; Tan, J.; Wu, J.; Yang, Y.; Fu, P.; Wang, L.; Jiang, W.; Hao, Y. Fabrication and Biological Activity of 3D-Printed Polycaprolactone/Magnesium Porous Scaffolds for Critical Size Bone Defect Repair. ACS Biomater. Sci. Eng. 2020, 6, 5120–5131. [Google Scholar] [CrossRef]
- Hansske, F.; Bas, O.; Vaquette, C.; Hochleitner, G.; Groll, J.; Kemnitz, E.; Hutmacher, D.W.; Börner, H.G. Via precise interface engineering towards bioinspired composites with improved 3D printing processability and mechanical properties. J. Mater. Chem. B 2017, 5, 5037–5047. [Google Scholar] [CrossRef]
- Yang, L.; Ullah, I.; Yu, K.; Zhang, W.; Zhou, J.; Sun, T.; Shi, L.; Yao, S.; Chen, K.; Zhang, X.; et al. Bioactive Sr(2+)/Fe(3+)co-substituted hydroxyapatite in cryogenically 3D printed porous scaffolds for bone tissue engineering. Biofabrication 2021, 13, 035007. [Google Scholar] [CrossRef] [PubMed]
- Poh, P.S.P.; Hutmacher, D.W.; Holzapfel, B.M.; Solanki, A.K.; Stevens, M.M.; Woodruff, M.A. In vitro and in vivo bone formation potential of surface calcium phosphate-coated polycaprolactone and polycaprolactone/bioactive glass composite scaffolds. Acta Biomater. 2016, 30, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, E.L.; Farris, A.L.; Hung, B.P.; Dias, M.; Garcia, J.R.; Dorafshar, A.; Grayson, W.L. 3D-Printing Technologies for Craniofacial Rehabilitation, Reconstruction, and Regeneration. Ann. Biomed. Eng. 2017, 45, 45–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VijayaVenkataRaman, S.; Lu, W.F.; Fuh, J.Y.H. 3D bioprinting of skin: A state-of-the-art review on modelling, materials, and processes. Biofabrication 2016, 8, 032001. [Google Scholar] [CrossRef] [PubMed]
- Yeo, M.; Lee, H.; Kim, G.H. Combining a micro/nano-hierarchical scaffold with cell-printing of myoblasts induces cell alignment and differentiation favorable to skeletal muscle tissue regeneration. Biofabrication 2016, 8, 035021. [Google Scholar] [CrossRef] [PubMed]
- Bahcecioglu, G.; Hasirci, N.; Bilgen, B.; Hasirci, V. A 3D printed PCL/hydrogel construct with zone-specific biochemical composition mimicking that of the meniscus. Biofabrication 2018, 11, 025002. [Google Scholar] [CrossRef]
- Zhang, Z.-Z.; Wang, S.-J.; Zhang, J.-Y.; Jiang, W.-B.; Huang, A.-B.; Qi, Y.-S.; Ding, J.-X.; Chen, X.-S.; Jiang, D.; Yu, J.-K. 3D-Printed Poly(ε-caprolactone) Scaffold Augmented With Mesenchymal Stem Cells for Total Meniscal Substitution: A 12- and 24-Week Animal Study in a Rabbit Model. Am. J. Sports Med. 2017, 45, 1497–1511. [Google Scholar] [CrossRef]
- Jung, B.K.; Kim, J.Y.; Kim, Y.S.; Roh, T.S.; Seo, A.; Park, K.-H.; Shim, J.-H.; Yun, I.S. Ideal scaffold design for total ear reconstruction using a three-dimensional printing technique. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1295–1303. [Google Scholar] [CrossRef]
- Park, H.S.; Lee, J.S.; Jung, H.; Kim, D.Y.; Kim, S.W.; Sultan, T.; Park, C.H. An omentum-cultured 3D-printed artificial trachea: In Vivo bioreactor. Artif. Cells Nanomed. Biotechnol. 2018, 46, S1131–S1140. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, H.; Dong, W.; Bai, J.; Gao, B.; Xia, D.; Feng, B.; Chen, M.; He, X.; Yin, M.; et al. Tissue-engineered trachea from a 3D-printed scaffold enhances whole-segment tracheal repair. Sci. Rep. 2017, 7, 5246. [Google Scholar] [CrossRef] [Green Version]
- Soares, C.S.; Barros, L.C.; Saraiva, V.; Gomez-Florit, M.; Babo, P.S.; Dias, I.; Reis, R.L.; Carvalho, P.P.; E Gomes, M. Bioengineered surgical repair of a chronic oronasal fistula in a cat using autologous platelet-rich fibrin and bone marrow with a tailored 3D printed implant. J. Feline Med. Surg. 2018, 20, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.; Kalyon, D.M.; Yu, X.; Valdevit, A. Segmental bone replacement via patient-specific, three-dimensional printed bioresorbable graft substitutes and their use as templates for the culture of mesenchymal stem cells under mechanical stimulation at various frequencies. Biotechnol. Bioeng. 2018, 115, 2365–2376. [Google Scholar] [CrossRef] [PubMed]
- Temple, J.P.; Hutton, D.L.; Hung, B.P.; Huri, P.Y.; Cook, C.A.; Kondragunta, R.; Jia, X.; Grayson, W.L. Engineering anatomically shaped vascularized bone grafts with hASCs and 3D-printed PCL scaffolds. J. Biomed. Mater. Res. Part A 2014, 102, 4317–4325. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Mitchell, J.; Fernandez-Medina, T.; Kumar, S.; Ivanovski, S. Resorbable additively manufactured scaffold imparts dimensional stability to extraskeletally regenerated bone. Biomaterials 2021, 269, 120671. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).