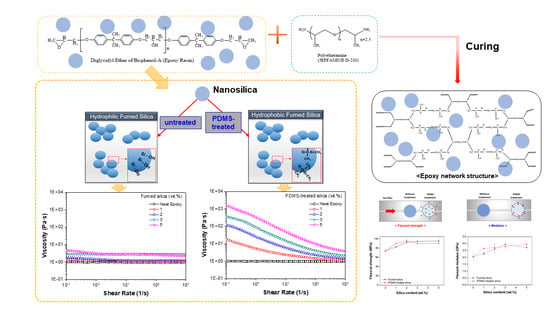
Enhancing Thermo-Mechanical Properties of Epoxy Composites Using Fumed Silica with Different Surface Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Epoxy Fumed Silica Composites
2.3. Characterization and Measurements
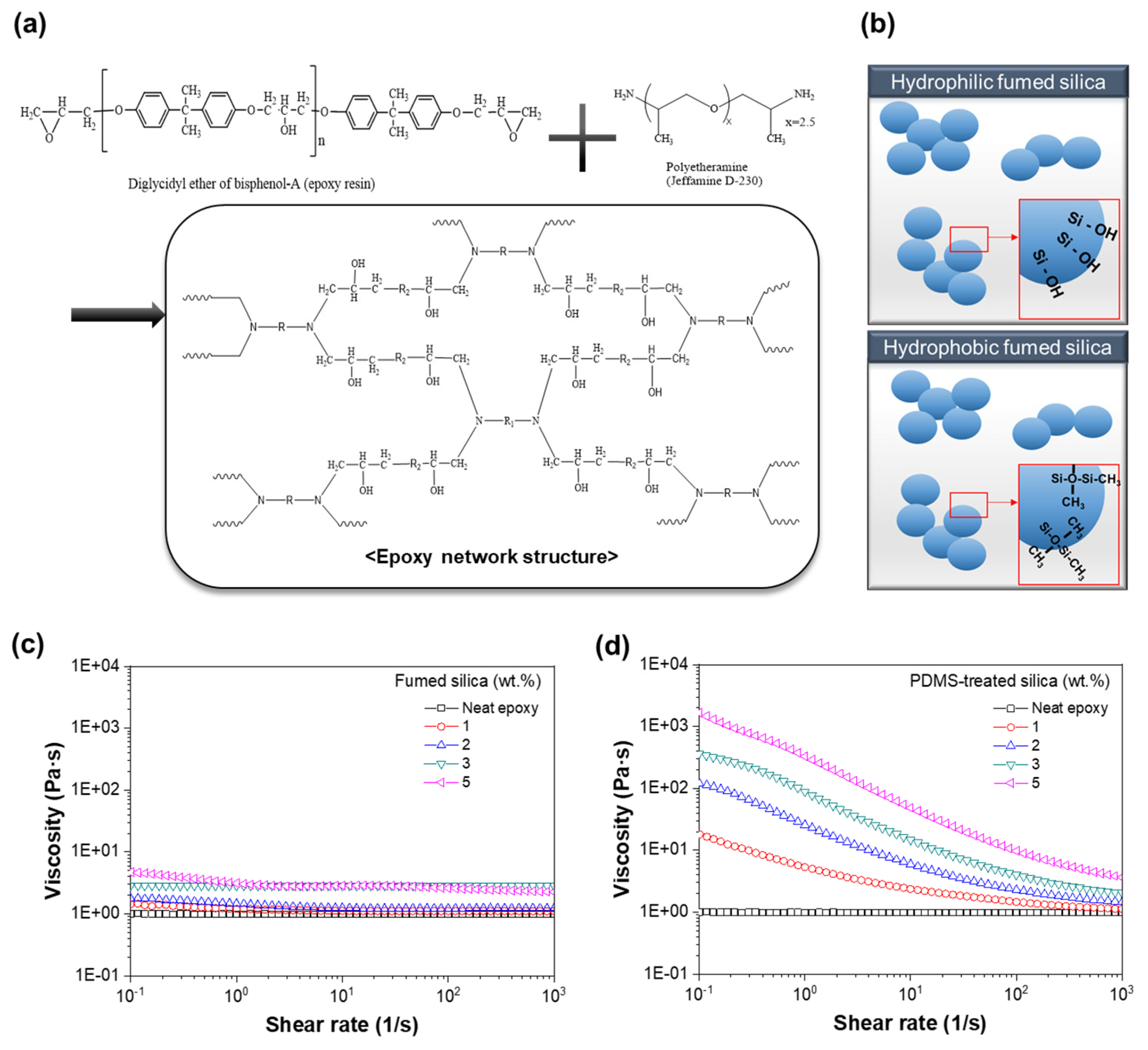
3. Results and Discussion
3.1. Characterization
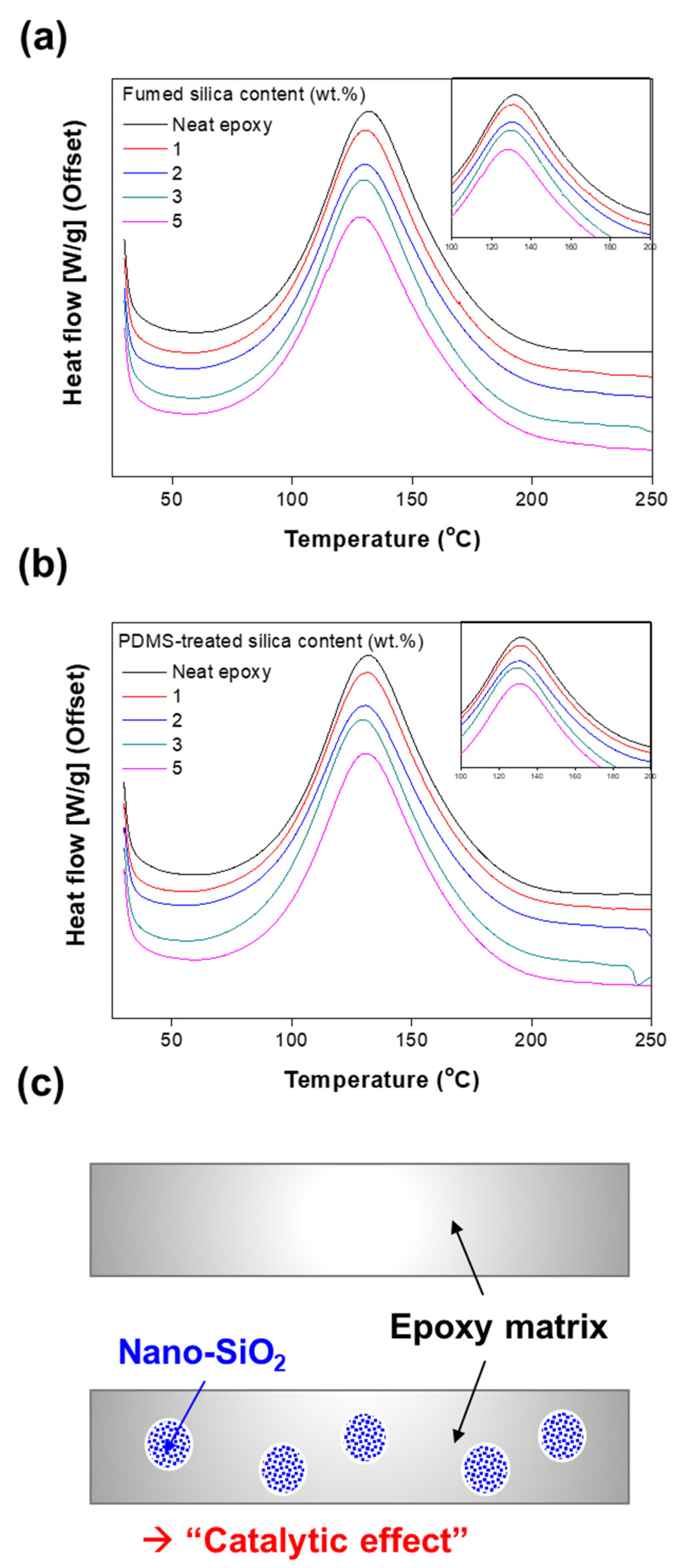
3.2. Curing Behavior
3.3. Morphology
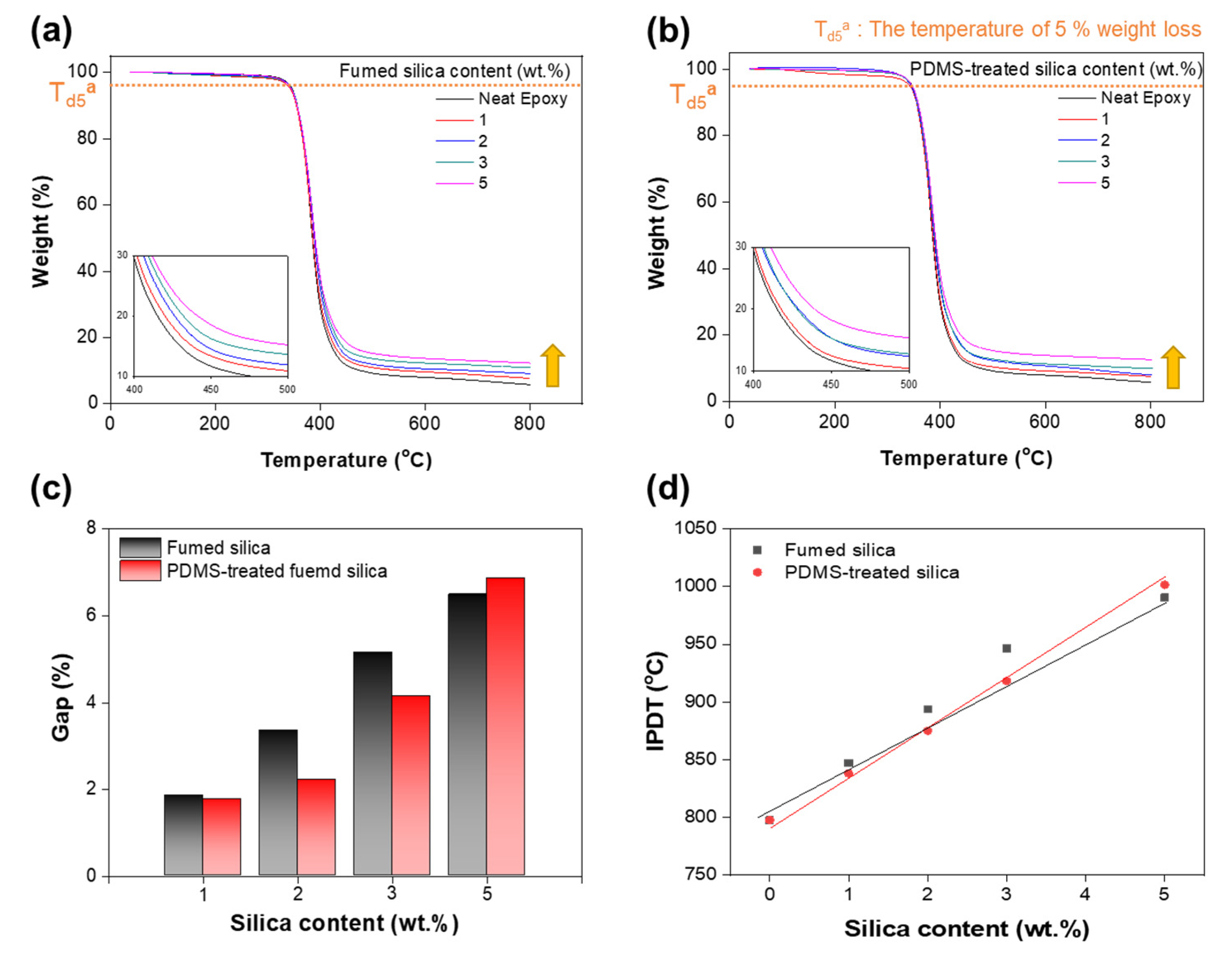
3.4. Thermal Stability
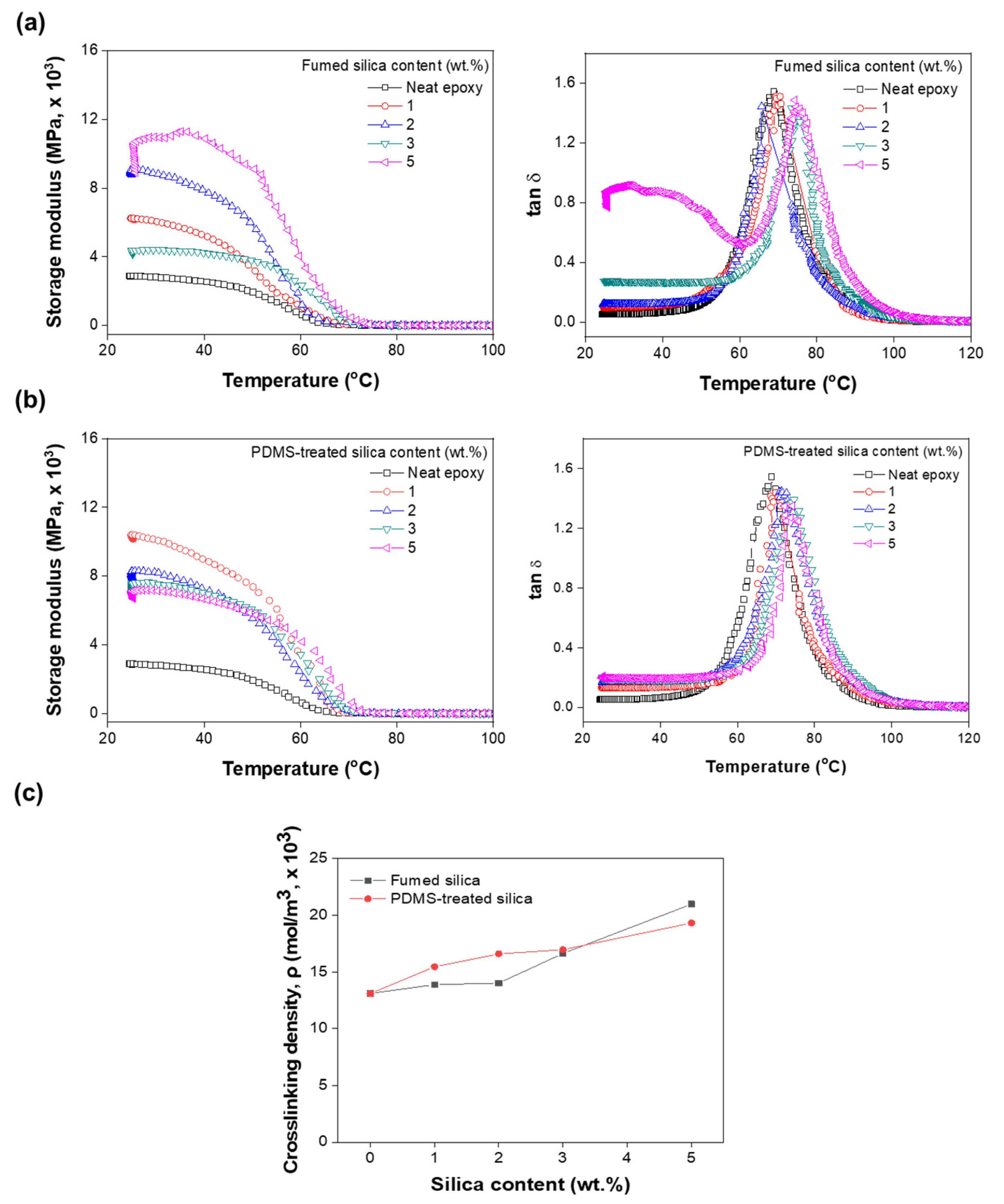
3.5. Thermo-Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balguri, P.K.; Samuel, D.G.H.; Thumu, U. A review on mechanical properties of epoxy nanocomposites. Mater. Today Proc. 2021, 44, 346–355. [Google Scholar] [CrossRef]
- Jancar, J.; Douglas, J.F.; Starr, F.W.; Kumar, S.K.; Cassagnau, P.; Lesser, A.J.; Sternstein, S.S.; Buehler, M.J. Current issues in research on structure-property relationships in polymer nanocomposites. Polymer 2010, 51, 3321–3343. [Google Scholar] [CrossRef]
- Gomez-Romero, P. Hybrid organic-inorganic materials—In search of synergic activity. Adv. Mater. 2001, 13, 163–174. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic-inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Mammeri, F.; Le Bourhis, E.; Rozes, L.; Sanchez, C. Mechanical properties of hybrid organic-inorganic materials. J. Mater. Chem. 2005, 15, 3787–3811. [Google Scholar] [CrossRef]
- Woldemariam, M.H.; Belingardi, G.; Koricho, E.G.; Reda, D.T. Effects of nanomaterials and particles on mechanical properties and fracture toughness of composite materials: A short review. AIMS Mater. Sci. 2019, 6, 1191–1212. [Google Scholar] [CrossRef]
- Spange, S. Silica surface modification by cationic polymerization and carbenium intermediates. Prog. Polym. Sci. 2000, 25, 781–849. [Google Scholar] [CrossRef]
- Sindorf, D.W.; Maciel, G.E. Solid-State NMR Studies of the Reactions of Silica Surfaces with Polyfunctional Chloromethylsilanes and Ethoxymethylsilanes. J. Am. Chem. Soc. 1983, 105, 3767–3776. [Google Scholar] [CrossRef]
- Guan, M.; Hao, L.; Chen, L.; Gao, F.; Qiu, S.; Zhou, H.; Chen, H.; Zhou, X. Facile Mechanical-Induced Functionalization of Hexagonal Boron Nitride and Its Application as Vehicles for Antibacterial Essential Oil. ACS Sustain. Chem. Eng. 2020, 8, 15120–15133. [Google Scholar] [CrossRef]
- Tarrío-Saavedra, J.; López-Beceiro, J.; Naya, S.; Artiaga, R. Effect of silica content on thermal stability of fumed silica/epoxy composites. Polym. Degrad. Stab. 2008, 93, 2133–2137. [Google Scholar] [CrossRef]
- Guo, Q.; Zhu, P.; Li, G.; Huang, L.; Zhang, Y.; Lu, D.D.; Sun, R.; Wong, C. One-pot synthesis of bimodal silica nanospheres and their effects on the rheological and thermal-mechanical properties of silica-epoxy composites. RSC Adv. 2015, 5, 50073–50081. [Google Scholar] [CrossRef]
- Ochi, M.; Matsumura, T. Thermomechanical properties and phase structure of epoxy/silica nano-hybrid materials constructed from a linear silicone oligomer. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 1631–1639. [Google Scholar] [CrossRef]
- Battistella, M.; Cascione, M.; Fiedler, B.; Wichmann, M.H.G.; Quaresimin, M.; Schulte, K. Fracture behaviour of fumed silica/epoxy nanocomposites. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1851–1858. [Google Scholar] [CrossRef]
- Shirono, H.; Amano, Y.; Kawaguchi, M.; Kato, T. Characteristics of alkyltrimethoxysilane-treated fumed silicas and rheological behavior of fumed silica suspensions in an epoxy resin. J. Colloid Interface Sci. 2001, 239, 555–562. [Google Scholar] [CrossRef]
- El-Fattah, M.A.; El Saeed, A.M.; El-Ghazawy, R.A. Chemical interaction of different sized fumed silica with epoxy via ultrasonication for improved coating. Prog. Org. Coat. 2019, 129, 1–9. [Google Scholar] [CrossRef]
- Ghosh, S.; Goswami, S.K.; Mathias, L.J. Surface modification of nano-silica with amides and imides for use in polyester nanocomposites. J. Mater. Chem. A 2013, 1, 6073–6080. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C.; Zhang, Q.; Huang, Z. Synthesis of Fumed Silica Treated with Organosilane and Its Effect on Epoxy Resin. Polym. Plast. Technol. Eng. 2013, 52, 145–148. [Google Scholar] [CrossRef]
- Choi, J.R.; Park, S.J. A study on thermal conductivity and fracture toughness of alumina nanofibers and powders-filled epoxy matrix composites. Polym. Korea 2013, 37, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.K.; Yang, G.; Yang, J.P.; Fu, S.Y.; Ye, L.; Huang, Y.G. Simultaneously increasing cryogenic strength, ductility and impact resistance of epoxy resins modified by n-butyl glycidyl ether. Polymer 2009, 50, 1316–1323. [Google Scholar] [CrossRef]
- Preghenella, M.; Pegoretti, A.; Migliaresi, C. Thermo-mechanical characterization of fumed silica-epoxy nanocomposites. Polymer 2005, 46, 12065–12072. [Google Scholar] [CrossRef]
- Sasidharan, S.; Anand, A. Epoxy-Based Hybrid Structural Composites with Nanofillers: A Review. Ind. Eng. Chem. Res. 2020, 59, 12617–12631. [Google Scholar] [CrossRef]
- Li, Z.; Okamoto, K.; Ohki, Y.; Tanaka, T. Effects of nano-filler addition on partial discharge resistance and dielectric breakdown strength of Micro-Al2O3/epoxy composite. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 653–661. [Google Scholar] [CrossRef]
- Patil, P.N.; Roilo, D.; Brusa, R.S.; Miotello, A.; Checchetto, R. Influence of nano-level molecular packing on the gas transport properties in amine-modified epoxy resins. Polymer 2015, 58, 130–138. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; Wang, Q.; Hahn, H.T.; Lee, S.G.; Lee, K.H.; Guo, Z. Epoxy resin nanocomposites reinforced with ionized liquid stabilized carbon nanotubes. Int. J. Smart Nano Mater. 2011, 2, 176–193. [Google Scholar] [CrossRef] [Green Version]
- Mai, V.D.; Lee, D.-I.; Park, J.H.; Lee, D.S. Rheological properties and thermal conductivity of epoxy resins filled with a mixture of alumina and boron nitride. Polymers 2019, 11, 597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.; Jin, F.L.; Park, S.J. Thermo-mechanical behaviors of epoxy resins reinforced with nano-Al 2O 3 particles. J. Ind. Eng. Chem. 2012, 18, 594–596. [Google Scholar] [CrossRef]
- Jin, F.L.; Park, S.J. Thermal properties of epoxy resin/filler hybrid composites. Polym. Degrad. Stab. 2012, 97, 2148–2153. [Google Scholar] [CrossRef]
- Omrani, A.; Simon, L.C.; Rostami, A.A. The effects of alumina nanoparticle on the properties of an epoxy resin system. Mater. Chem. Phys. 2009, 114, 145–150. [Google Scholar] [CrossRef]
- Huang, G.C.; Lee, J.K. Isothermal cure characterization of fumed silica/epoxy nanocomposites: The glass transition temperature and conversion. Compos. Part A Appl. Sci. Manuf. 2010, 41, 473–479. [Google Scholar] [CrossRef]
- Chae, G.S.; Park, H.W.; Kwon, K.; Shin, S. Comparative study of the impact wedge-peel performance of epoxy structural adhesives modified with functionalized silica nanoparticles. Polymers 2021, 13, 469. [Google Scholar] [CrossRef]
- Dittanet, P.; Pearson, R.A. Effect of silica nanoparticle size on toughening mechanisms of filled epoxy. Polymer 2012, 53, 1890–1905. [Google Scholar] [CrossRef]
- Chan, C.M.; Wu, J.; Li, J.X.; Cheung, Y.K. Polypropylene/calcium carbonate nanocomposites. Polymer 2002, 43, 2981–2992. [Google Scholar] [CrossRef]
- Verma, V.; Sayyed, A.H.M.; Sharma, C.; Shukla, D.K. Tensile and fracture properties of epoxy alumina composite: Role of particle size and morphology. J. Polym. Res. 2020, 27, 388. [Google Scholar] [CrossRef]
- Kumar, R.; Mohanty, S.; Nayak, S.K. Study on epoxy resin-based thermal adhesive filled with hybrid expanded graphite and graphene nanoplatelet. SN Appl. Sci. 2019, 1, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Mariappan, T.; Wilkie, C.A. Flame retardant epoxy resin for electrical and electronic applications. Fire Mater. 2014, 38, 588–598. [Google Scholar] [CrossRef]
- Chiang, C.L.; Hsu, S.W. Synthesis, characterization and thermal properties of novel epoxy/expandable graphite composites. Polym. Int. 2010, 59, 119–126. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Wang, Z.; Sun, G.; Hu, P. Thermal stability of flexible polyurethane foams containing modified layered double hydroxides and zinc borate. Int. J. Polym. Anal. Charact. 2020, 25, 499–516. [Google Scholar] [CrossRef]
- Liu, S.H.; Shen, M.Y.; Kuan, C.F.; Kuan, H.C.; Ke, C.Y.; Chiang, C.L. Improving thermal stability of polyurethane through the addition of hyperbranched polysiloxane. Polymers 2019, 11, 697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.L.; Wei, W.L.; Hsu, K.Y.; Ho, W.H. Thermal stability of epoxy-silica hybrid materials by thermogravimetric analysis. Thermochim. Acta 2004, 412, 139–147. [Google Scholar] [CrossRef]
- Marunaka, R.; Kawaguchi, M. Rheological behavior of hydrophobic fumed silica suspensions in aromatic dispersion media. J. Dispers. Sci. Technol. 2017, 38, 223–228. [Google Scholar] [CrossRef]
- Kang, S.; Hong, S.I.; Choe, C.R.; Park, M.; Rim, S.; Kim, J. Preparation and characterization of epoxy composites filled with functionalized nanosilica particles obtained via sol-gel process. Polymer 2001, 42, 879–887. [Google Scholar] [CrossRef]
- Keller, A.; Chong, H.M.; Taylor, A.C.; Dransfeld, C.; Masania, K. Core-shell rubber nanoparticle reinforcement and processing of high toughness fast-curing epoxy composites. Compos. Sci. Technol. 2017, 147, 78–88. [Google Scholar] [CrossRef]
- Gu, H.; Ma, C.; Gu, J.; Guo, J.; Yan, X.; Huang, J.; Zhang, Q.; Guo, Z. An overview of multifunctional epoxy nanocomposites. J. Mater. Chem. C 2016, 4, 5890–5906. [Google Scholar] [CrossRef]
- Yu, J.; Huang, X.; Wu, C.; Wu, X.; Wang, G.; Jiang, P. Interfacial modification of boron nitride nanoplatelets for epoxy composites with improved thermal properties. Polymer 2012, 53, 471–480. [Google Scholar] [CrossRef]


| Sample | Epoxy Resin (g) | Curing Agent (g) | Filler | |
|---|---|---|---|---|
| (g) | (wt.%) | |||
| Pure epoxy | 10 | 0 | 0 | |
| Ep/F1 | 0.14 | 1 | ||
| Ep/F2 | 4 | 0.29 | 2 | |
| Ep/F3 | 0.43 | 3 | ||
| Ep/F5 | 0.74 | 5 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-M.; Kim, H.; Kim, H.-J. Enhancing Thermo-Mechanical Properties of Epoxy Composites Using Fumed Silica with Different Surface Treatment. Polymers 2021, 13, 2691. https://doi.org/10.3390/polym13162691
Kim K-M, Kim H, Kim H-J. Enhancing Thermo-Mechanical Properties of Epoxy Composites Using Fumed Silica with Different Surface Treatment. Polymers. 2021; 13(16):2691. https://doi.org/10.3390/polym13162691
Chicago/Turabian StyleKim, Kyung-Min, Hoon Kim, and Hyun-Joong Kim. 2021. "Enhancing Thermo-Mechanical Properties of Epoxy Composites Using Fumed Silica with Different Surface Treatment" Polymers 13, no. 16: 2691. https://doi.org/10.3390/polym13162691
APA StyleKim, K.-M., Kim, H., & Kim, H.-J. (2021). Enhancing Thermo-Mechanical Properties of Epoxy Composites Using Fumed Silica with Different Surface Treatment. Polymers, 13(16), 2691. https://doi.org/10.3390/polym13162691