LDPE/Bismuth Oxide Nanocomposite: Preparation, Characterization and Application in X-ray Shielding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
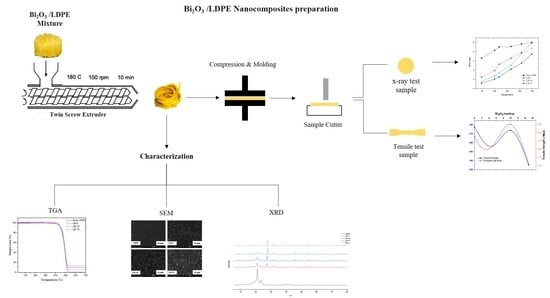
2.2. Nanocomposite Preparation
3. Characterization
3.1. X-ray Differentiation
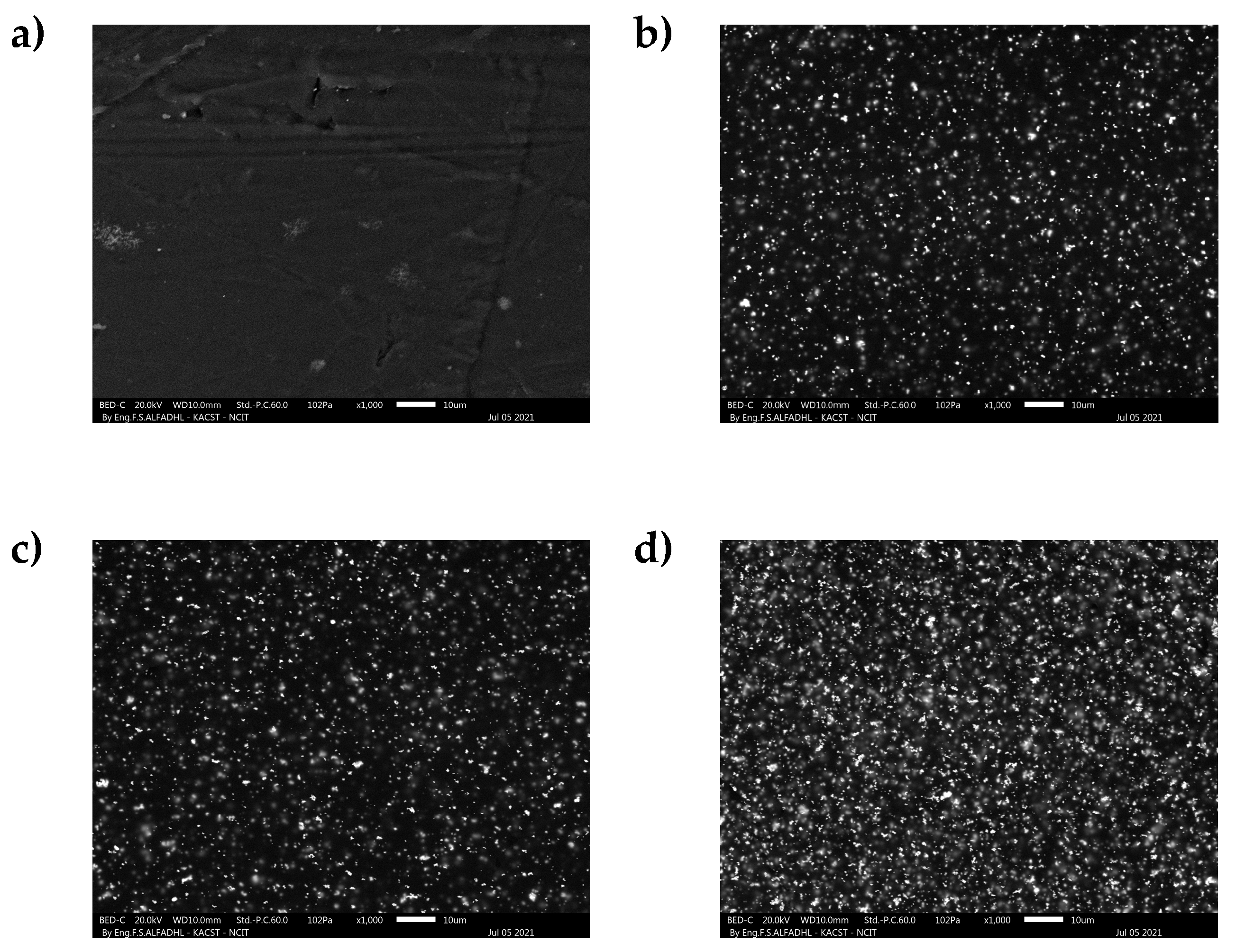
3.2. Scanning Electron Microscope (SEM)
3.3. Density Measurements
3.4. Tensile Testing
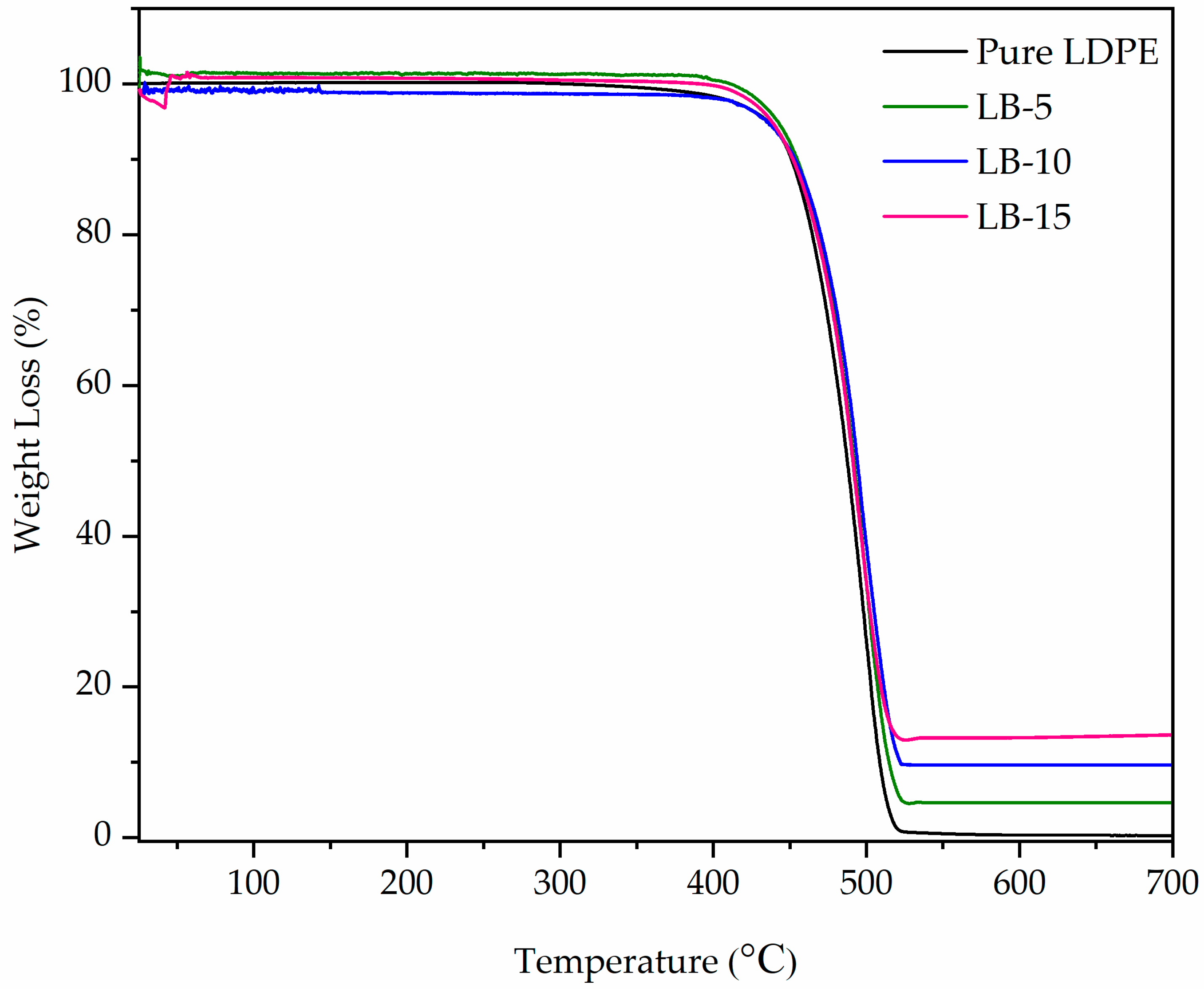
3.5. Thermal Analysis by TGA
4. Results and Discussion
4.1. Density Measurements
4.2. Thermal Stability
4.3. Mechanical Properties
4.4. X-ray Diffraction
4.5. Morphology
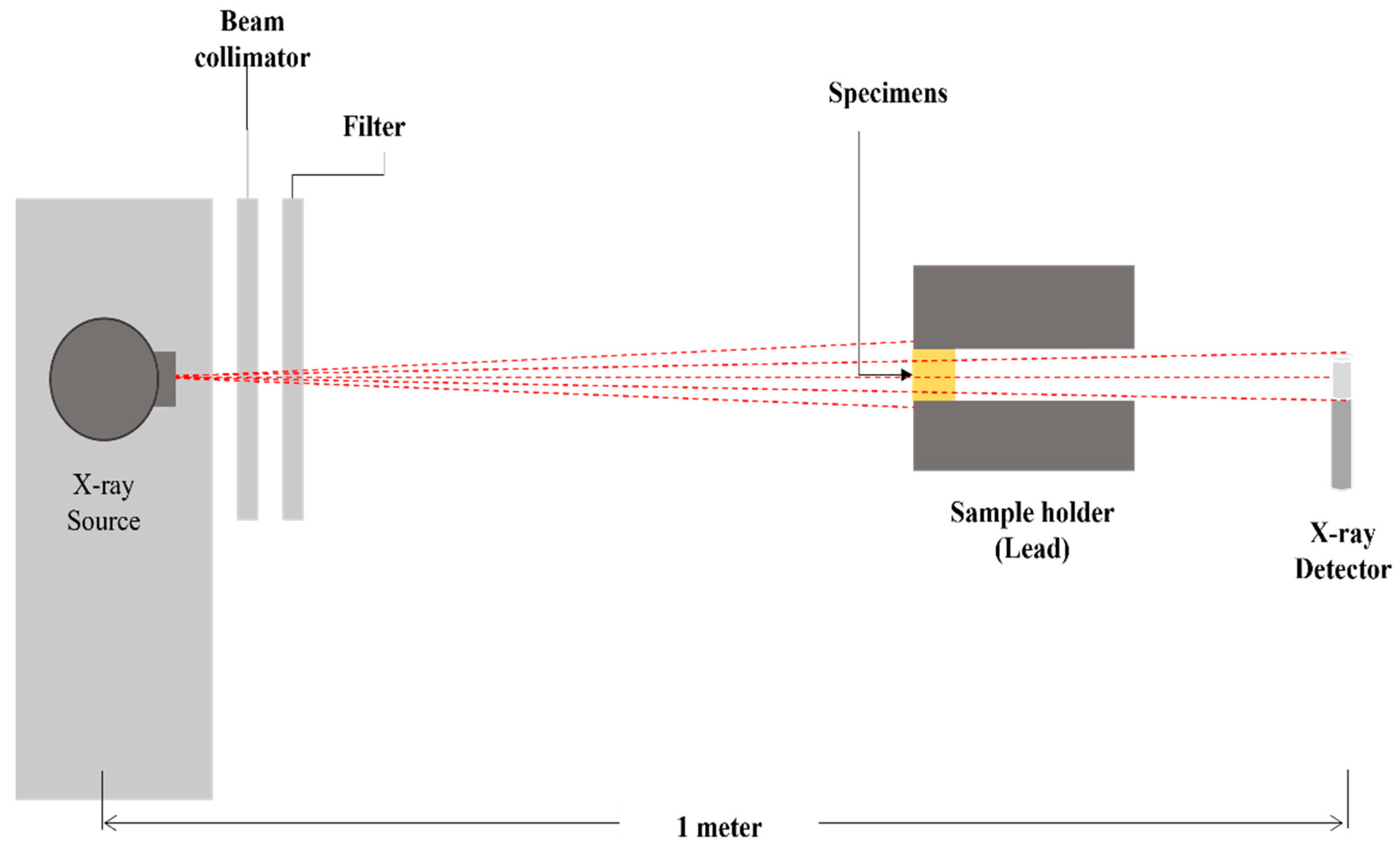
5. Shielding Properties and Application
5.1. Shielding Efficiency of the Polymer Composites in Low Energies Applications
5.2. Shielding Ability Investigation of the Polymer Composites
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alavian, H.; Tavakoli-Anbaran, H. Comparative study of mass attenuation coefficients for LDPE/metal oxide composites by Monte Carlo simulations. Eur. Phys. J. Plus 2020, 135, 82. [Google Scholar] [CrossRef]
- Harish, V.; Nagaiah, N.; Kumar, H. Lead oxides filled isophthalic resin polymer composites for gamma radiation shielding applications. Indian J. Pure Appl. Phys. 2012, 50, 847–850. [Google Scholar]
- Martin, J.E. Physics for radiation protection: A handbook. John Wiley & Sons, Inc.: Ann Arbor, MI, USA, 2006; pp. 367–421. [Google Scholar]
- Ambika, M.; Nagaiah, N.; Harish, V.; Lokanath, N.; Sridhar, M.; Renukappa, N.; Suman, S. Preparation and characterisation of Isophthalic-Bi2O3 polymer composite gamma radiation shields. Radiat. Phys. Chem. 2017, 130, 351–358. [Google Scholar] [CrossRef]
- Atashi, P.; Rahmani, S.; Ahadi, B.; Rahmati, A. Efficient, flexible and lead-free composite based on room temperature vulcanizing silicone rubber/W/Bi2O3 for gamma ray shielding application. J. Mater. Sci.: Mater. Electron. 2018, 29, 12306–12322. [Google Scholar] [CrossRef]
- Nambiar, S.; Yeow, J.T. Polymer-composite materials for radiation protection. ACS Appl. Mater. Interfaces 2012, 4, 5717–5726. [Google Scholar] [CrossRef] [PubMed]
- Shik, N.A.; Gholamzadeh, L. X-ray shielding performance of the EPVC composites with micro-or nanoparticles of WO3, PbO or Bi2O3. Appl. Radiat. Isot. 2018, 139, 61–65. [Google Scholar] [CrossRef]
- Fontainha, C.; Baptista-Neto, A.; Faria, L. Polymer-based Nanocomposites of P(VDF-TrFE)/Bi2O3 Applied to X-ray Shielding. J. Mater. Sci. 2016, 4, 16–24. [Google Scholar]
- Jamil, M.; Hazlan, M.H.; Ramli, R.M.; Azman, N.Z.N. Study of electrospun PVA-based concentrations nanofibre filled with Bi2O3 or WO3as potential x-ray shielding material. Radiat. Phys. Chem. 2019, 156, 272–282. [Google Scholar] [CrossRef]
- Khan, W.S.; Hamadneh, N.N.; Khan, W.A. Polymer nanocomposites–synthesis techniques, classification and properties. In Science and Applications of Tailored Nanostructures; One Central Press (OCP): Cheshire, UK, 2016; pp. 50–67. [Google Scholar]
- Higgins, M.C.M.; Radcliffe, N.A.; Toro-González, M.; Rojas, J.V. Gamma ray attenuation of hafnium dioxide-and tungsten trioxide-epoxy resin composites. J. Radioanal. Nucl. Chem. 2019, 322, 707–716. [Google Scholar] [CrossRef]
- Plionis, A.; Garcia, S.; Gonzales, E.; Porterfield, D.; Peterson, D. Replacement of lead bricks with non-hazardous polymer-bismuth for low-energy gamma shielding. J. Radioanal. Nucl. Chem. 2009, 282, 239–242. [Google Scholar] [CrossRef]
- El-Fiki, S.; El Kameesy, S.; Nashar, D.; Abou-Leila, M.; El-Mansy, M.; Ahmed, M. Influence of bismuth contents on mechanical and gamma ray attenuation properties of silicone rubber composite. Int. J. Adv. Res. 2015, 3, 1035–1041. [Google Scholar]
- Aghaz, A.; Faghihi, R.; Mortazavi, S.; Haghparast, A.; Mehdizadeh, S.; Sina, S. Radiation attenuation properties of shields containing micro and Nano WO3 in diagnostic X-ray energy range. Int. J. Radiat. Res. 2016, 14, 127–131. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; El-Khatib, A.M.; Badawi, M.S.; Rashad, A.R.; El-Sharkawy, R.M.; Thabet, A.A. Fabrication, characterization and gamma rays shielding properties of nano and micro lead oxide-dispersed-high density polyethylene composites. Radiat. Phys. Chem. 2018, 145, 160–173. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; El-Khatib, A.M.; Badawi, M.S.; Rashad, A.R.; El-Sharkawy, R.M.; Thabet, A.A. Recycled high-density polyethylene plastics added with lead oxide nanoparticles as sustainable radiation shielding materials. J. Clean. Prod. 2018, 176, 276–287. [Google Scholar] [CrossRef]
- Afshar, M.; Morshedian, J.; Ahmadi, S. Radiation attenuation capability and flow characteristics of HDPE composite loaded with W, MoS2, and B4C. Polym. Compos. 2019, 40, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Lsayed, Z.; Badawi, M.S.; Awad, R.; Thabet, A.A.; El-Khatib, A.M. Study of some γ-ray attenuation parameters for new shielding materials composed of nano ZnO blended with high density polyethylene. Nucl. Technol. Radiat. Prot. 2019, 34, 342–352. [Google Scholar] [CrossRef] [Green Version]
- El-Khatib, A.M.; Abbas, M.I.; Abd Elzaher, M.; Badawi, M.S.; Alabsy, M.T.; Alharshan, G.A.; Aloraini, D.A. Gamma attenuation coefficients of nano cadmium oxide/high density polyethylene composites. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, D.; Yang, G.; Bourham, M.; Moneghan, D. Gamma radiation shielding properties of poly (methyl methacrylate)/Bi2O3 composites. Nucl. Eng. Technol. 2020, 52, 2613–2619. [Google Scholar] [CrossRef]
- Kaçal, M.; Polat, H.; Oltulu, M.; Akman, F.; Agar, O.; Tekin, H. Gamma shielding and compressive strength analyses of polyester composites reinforced with zinc: An experiment, theoretical, and simulation based study. Appl.Phys. A. 2020, 126, 1–15. [Google Scholar] [CrossRef]
- Saboori, A.; Dadkhah, M.; Fino, P.; Pavese, M. An overview of metal matrix nanocomposites reinforced with graphene nanoplatelets; mechanical, electrical and thermophysical properties. Metals 2018, 8, 423. [Google Scholar] [CrossRef] [Green Version]
- Eid, G.A.; Kany, A.; El-Toony, M.; Bashter, I.; Gaber, F. Application of epoxy/Pb3O4 composite for gamma ray shielding. Arab. J. Nucl. Sci. Appl. 2013, 46, 226–233. [Google Scholar]
- Kim, J.; Seo, D.; Lee, B.C.; Seo, Y.S.; Miller, W.H. Nano-W Dispersed Gamma Radiation Shielding Materials. Adv. Eng. Mater. 2014, 16, 1083–1089. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazemi, F.; Malekie, S. A Monte Carlo study on the shielding properties of a novel polyvinyl alcohol (PVA)/WO3 composite, against gamma rays, using the MCNPX code. J. Biomed. Phys. Eng. 2019, 9, 465. [Google Scholar] [CrossRef] [PubMed]
- Gavrish, V.; Baranov, G.; Chayka, T.; Derbasova, N.; Lvov, A.; Matsuk, Y. Tungsten nanoparticles influence on radiation protection properties of polymers. In IOP Conference Series: Materials Science and Engineering; IOP publishing: Tomsk, Russia, 2015; Volume 110, pp. 1–6. [Google Scholar]
- Liao, Y.-C.; Xu, D.-G.; Zhang, P.-C. Preparation and characterization of Bi2O3/XNBR flexible films for attenuating gamma rays. Nucl. Sci. Tech. 2018, 29, 1–12. [Google Scholar] [CrossRef]
- Azman, N.N.; Siddiqui, S.; Hart, R.; Low, I.-M. Effect of particle size, filler loadings and x-ray tube voltage on the transmitted x-ray transmission in tungsten oxide—epoxy composites. Appl. Radiat. Isot. 2013, 71, 62–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belgin, E.E.; Aycik, G. Preparation and radiation attenuation performances of metal oxide filled polyethylene based composites for ionizing electromagnetic radiation shielding applications. J. Radiational Nucl. Chem. 2015, 306, 107–117. [Google Scholar] [CrossRef]
- Ambika, M.; Nagaiah, N.; Suman, S. Role of bismuth oxide as a reinforcer on gamma shielding ability of unsaturated polyester based polymer composites. J. Appl. Polym. Sci. 2017, 134, 1–7. [Google Scholar] [CrossRef]
- Pavlenko, V.; Cherkashina, N.; Yastrebinsky, R. Synthesis and radiation shielding properties of polyimide/Bi2O3 composites. Heliyon 2019, 5, e01703. [Google Scholar] [CrossRef] [Green Version]
- Cherkashina, N.; Pavlenko, A. Synthesis of polymer composite based on polyimide and Bi12SiO20 sillenite. Polym. Plast. Technol. Eng. 2018, 57, 1923–1931. [Google Scholar] [CrossRef]
- Roy, P.; Surekha, P.; Raman, R.; Rajagopal, C. Investigating the role of metal oxidation state on the degradation behaviour of LDPE. Polym. Degrad. Stab. 2009, 94, 1033–1039. [Google Scholar] [CrossRef]
- Gupta, R.; Badel, B.; Gupta, P.; Bucknall, D.G.; Flynn, D.; Pancholi, K. Flexible Low-Density Polyethylene–BaTiO3 Nanoparticle Composites for Monitoring Leakage Current in High-Tension Equipment. ACS Appl. Nano Mater. 2021, 4, 2413–2422. [Google Scholar] [CrossRef]
- Klinkova, L.; Nikolaichik, V.; Barkovskii, N.; Fedotov, V. Thermal stability of Bi2O3. Russ. J. Inorg. Chem. 2007, 52, 1822–1829. [Google Scholar] [CrossRef]
- Sheela, M.; Kamat, V.A.; Kiran, K.; Eshwarappa, K. Preparation and characterization of bismuth-filled high-density polyethylene composites for gamma-ray shielding. Radiat. Prot. Environ. 2019, 42, 180–186. [Google Scholar]
- Chee, C.Y.; Song, N.; Abdullah, L.C.; Choong, T.S.; Ibrahim, A.; Chantara, T. Characterization of mechanical properties: Low-density polyethylene nanocomposite using nanoalumina particle as filler. J. Nanomater. 2012, 2012, 215978. [Google Scholar] [CrossRef]
- Inci, B.; Wagener, K.B. Decreasing the alkyl branch frequency in precision polyethylene: Pushing the limits toward longer run lengths. J. Am. Chem. Soc. 2011, 133, 11872–11875. [Google Scholar] [CrossRef]
- Trujillo, M.; Arnal, M.; Müller, A.; Laredo, E.; Bredeau, S.; Bonduel, D.; Dubois, P. Thermal and morphological characterization of nanocomposites prepared by in-situ polymerization of high-density polyethylene on carbon nanotubes. Macromolecules 2007, 40, 6268–6276. [Google Scholar] [CrossRef]
- Sabri, J.H.; Mahdi, K.H. A Comparative Study for Micro and Nano shield of (PbO) composite for gamma Radiation. Energy Procedia 2019, 157, 802–814. [Google Scholar] [CrossRef]
- ISO 4037-1:2019. Radiological Protection—X and Gamma Reference Radiation for Calibrating Dosemeters and Doserate Meters and for Determining Their Response as a Function of Photon Energy—Part 1: Radiation Characteristics and Production Methods; ISO: Geneva, Switzerland, 2019; ISC, 17, 17.240. [Google Scholar]
- Berger, M.J.; Hubbell, J.H.; Seltzer, S.M.; Chang, J.; Coursey, J.S.; Sukumar, R.D.; Zucker, S.; Olsen, K. XCOM: Photon Cross Sections on a Personal Computer; NIST, PML, Radiation Physics Division: Washington, DC, USA, 1987; pp. 87–3597. [Google Scholar] [CrossRef]
- El-Sayed, A.; Ali, M.A.M.; Ismail, M.R. Natural fibre high-density polyethylene and lead oxide composites for radiation shielding. Radiat. Phys. Chem. 2003, 66, 185–195. [Google Scholar] [CrossRef]
- Almurayshid, M.; Alsagabi, S.; Alssalim, Y.; Alotaibi, Z.; Almsalam, R. Feasibility of polymer-based composite materials as radiation shield. Radiat. Phys. Chem. 2021, 183, 109425. [Google Scholar] [CrossRef]
- Alavian, H.; Tavakoli-Anbaran, H. Study on gamma shielding polymer composites reinforced with different sizes and proportions of tungsten particles using MCNP code. Prog. Nucl. Energy 2019, 115, 91–98. [Google Scholar] [CrossRef]
- Gurler, O.; Akar-Tarim, U. Determination of radiation shielding properties of some polymer and plastic materials against gamma-rays. Acta Phys. Pol. A 2016, 130, 236–238. [Google Scholar] [CrossRef]



| Sample Code | Composition (wt%) | Density (g cm−3) (Experimental) | Density (g cm−3) (Calculated) | Error (%) |
|---|---|---|---|---|
| Pure LDPE | Pure LDPE | 0.926 | 0.930 | 0.430 |
| LB-5 | LDPE (95%) + Bi2O3 (5%) | 0.961 | 0.973 | 1.293 |
| LB-10 | LDPE (90%) + Bi2O3 (10%) | 1.010 | 1.021 | 3.270 |
| LB-15 | LDPE (85%) + Bi2O3 (15%) | 1.060 | 1.074 | 8.762 |
| Sample | Bi2O3 Loading (wt%) | Onset Temp (°C) | High Peak Temp (°C) | Weight Loss at 600 °C (%) |
|---|---|---|---|---|
| Pure LDPE | 0 | 459 | 500 | 0.3 |
| LB-5 | 5 | 459 | 500.2 | 4.6 |
| LB-10 | 10 | 461 | 501.2 | 9.6 |
| LB-15 | 15 | 454 | 500.6 | 13.2 |
| Sample | Bi2O3 Loading (wt%) | Error (%) | |
|---|---|---|---|
| The Theoretical Values | The Experimental Values | ||
| Pure LDPE | 0 | 0 | 0 |
| LB-5 | 5.00 | 4.49 | 10.2 |
| LB-10 | 10.0 | 9.16 | 8.37 |
| LB-15 | 15.0 | 14.5 | 3.39 |
| Sample | Bi2O3 Loading (wt%) | Tensile Strength (MPa) | Elongation @ Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|---|
| Pure LDPE | 0 | 14.77 ± 0.60 | 378 ± 19 | 355 ± 36 |
| LB-5 | 5 | 14.19 ± 1.29 | 356 ± 18 | 355 ± 13 |
| LB-10 | 10 | 15.51 ± 0.43 | 374 ± 22 | 378 ± 16 |
| LB-15 | 15 | 13.09 ± 0.98 | 335 ± 48 | 350 ± 14 |
| Sample | 2θ | Scherrer Crystallite Size (nm) | FWHM |
|---|---|---|---|
| Pure LDPE | 21 | 9.52 | 0.8400 |
| Pure Bi2O3 | 27.8 | 18.41 | 0.4400 |
| LB-5 | 27.4 | 18.39 | 0.4400 |
| LB-10 | 27.4 | 18.39 | 0.4400 |
| LB-15 | 27.8 | 18.41 | 0.4400 |
| Shortened Name | Tube Potential (kV) | Effective Energy (keV) | Additional Filtration Thickness | |||
|---|---|---|---|---|---|---|
| mm Pb | mm Sn | mm Cu | mm Al | |||
| N-60 | 60 | 47.9 | - | - | 0.631 | 3.912 |
| N-100 | 100 | 83.3 | - | - | 5.027 | 3.920 |
| N-120 | 120 | 100 | - | 1.013 | 5.027 | 3.950 |
| N-150 | 150 | 118 | - | 2.605 | - | 3.903 |
| N-200 | 200 | 165 | 1.028 | 3.004 | 2.032 | 3.901 |
| N-250 | 250 | 207 | 3.099 | 2.062 | - | 3.925 |
| N-300 | 300 | 248 | 5.152 | 3.016 | - | 3.929 |
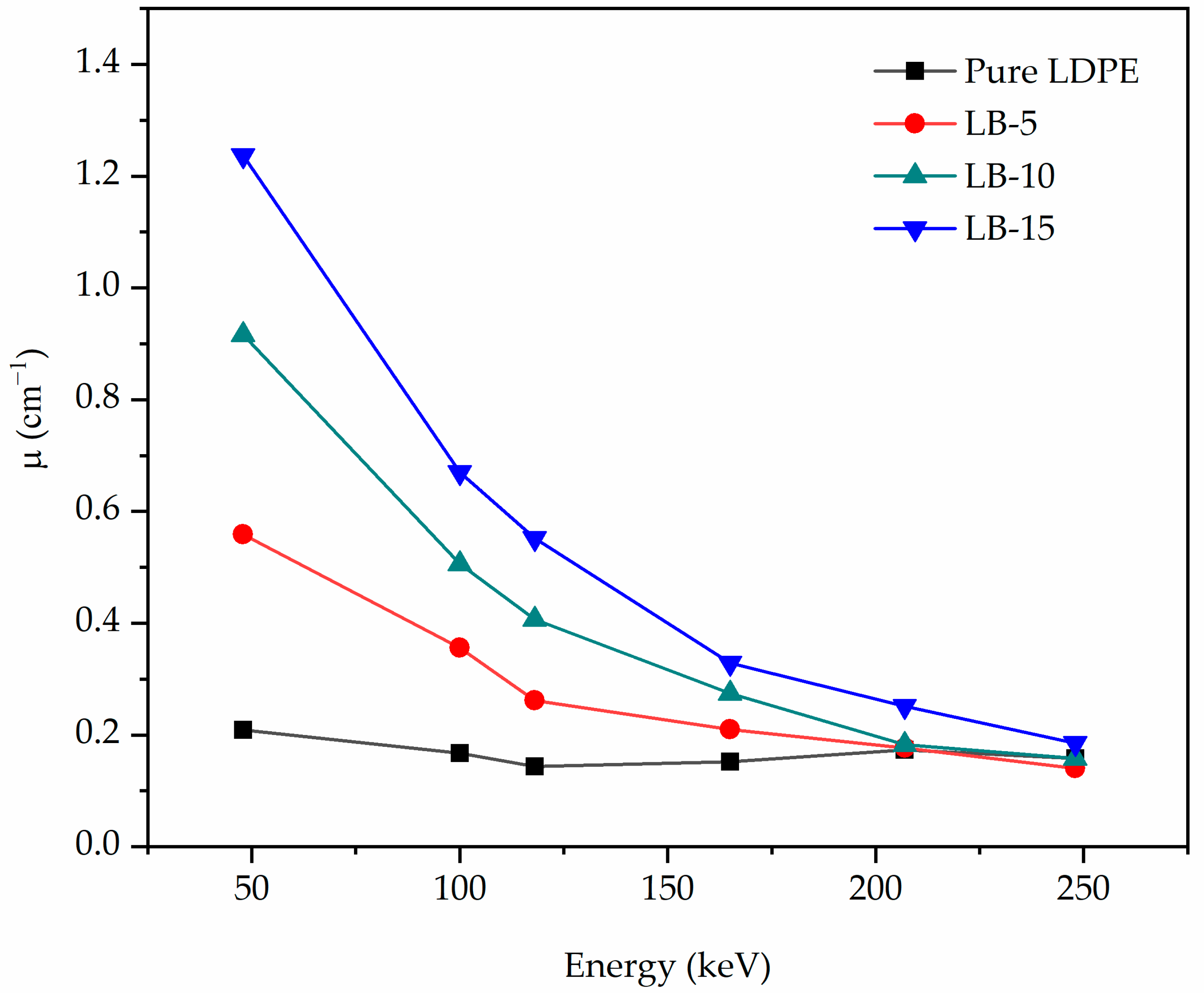
| Energy (keV) | Pure LDPE | LB-5 | LB-10 | LB-15 | ||||
|---|---|---|---|---|---|---|---|---|
| Exp. | Cal. | Exp. | Cal. | Exp. | Cal. | Exp. | Cal. | |
| 47.90 | 0.2094 ± 0.0035 | 0.1987 | 0.5589 ± 0.0041 | 0.5775 | 0.9164 ± 0.0062 | 0.9562 | 1.2369 ± 0.0077 | 1.3350 |
| 100.00 | 0.1671 ± 0.0024 | 0.1687 | 0.3560 ± 0.0030 | 0.4086 | 0.5061 ± 0.0030 | 0.6485 | 0.6696 ± 0.0032 | 0.8883 |
| 118.00 | 0.1535 ± 0.0022 | 0.1620 | 0.2619 ± 0.0027 | 0.3171 | 0.4067 ± 0.0028 | 0.4721 | 0.5520 ± 0.0029 | 0.6271 |
| 165.00 | 0.1520 ± 0.0017 | 0.1478 | 0.2010 ± 0.0019 | 0.2109 | 0.2745 ± 0.0025 | 0.2739 | 0.3287 ± 0.0024 | 0.3369 |
| 207.00 | 0.1432 ± 0.0011 | 0.1378 | 0.1768 ± 0.0017 | 0.1716 | 0.1824 ± 0.0025 | 0.2053 | 0.2515 ± 0.0023 | 0.2391 |
| 248.00 | 0.1382 ± 0.0020 | 0.1298 | 0.1403 ± 0.0014 | 0.1501 | 0.1579 ± 0.0022 | 0.1704 | 0.1849 ± 0.0020 | 0.1908 |
| Energy (keV) | Pure LDPE | LB-5 | LB-10 | LB-15 |
|---|---|---|---|---|
| 47.90 | 17.11 | 44.17 | 76.52 | 79.16 |
| 100.00 | 14.02 | 23.17 | 46.95 | 40.24 |
| 118.00 | 13.90 | 31.02 | 55.08 | 57.22 |
| 165.00 | 12.06 | 23.90 | 47.43 | 50.34 |
| 207.00 | 12.73 | 19.66 | 35.21 | 34.08 |
| 248.00 | 14.37 | 16.84 | 25.05 | 27.31 |
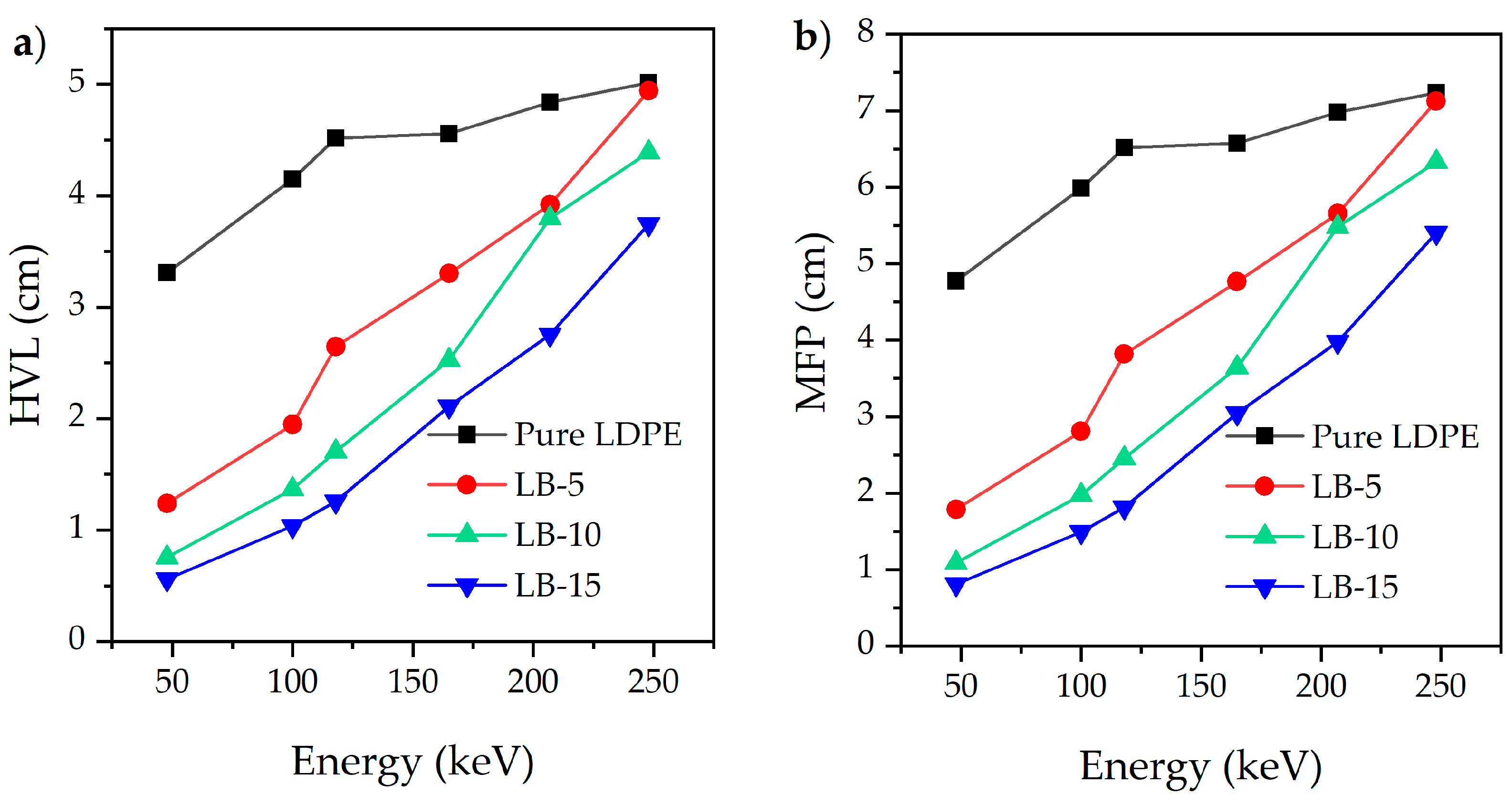
| Study | Composites | HVL (cm) | MFP (cm) |
|---|---|---|---|
| This study | LDPE 85% + Bi2O3 15% | 1.03 | 1.50 |
| Almurayshid, et al. 2021 [45] | HDPE 85% + W 15% | 1.18 | 1.63 |
| Almurayshid, et al. 2021 [45] | HDPE 85% + Mo 15% | 2.29 | 3.30 |
| (Alavian and Tavakoli-Anbaran, 2019) [46] | LDPE 99% + W 1% | - | 4.27 |
| (Gurler and Akar-Tarim, 2016) [47] | Concrete (NBS) | 1.81 | 2.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshahri, S.; Alsuhybani, M.; Alosime, E.; Almurayshid, M.; Alrwais, A.; Alotaibi, S. LDPE/Bismuth Oxide Nanocomposite: Preparation, Characterization and Application in X-ray Shielding. Polymers 2021, 13, 3081. https://doi.org/10.3390/polym13183081
Alshahri S, Alsuhybani M, Alosime E, Almurayshid M, Alrwais A, Alotaibi S. LDPE/Bismuth Oxide Nanocomposite: Preparation, Characterization and Application in X-ray Shielding. Polymers. 2021; 13(18):3081. https://doi.org/10.3390/polym13183081
Chicago/Turabian StyleAlshahri, Saad, Mohammed Alsuhybani, Eid Alosime, Mansour Almurayshid, Alhanouf Alrwais, and Salha Alotaibi. 2021. "LDPE/Bismuth Oxide Nanocomposite: Preparation, Characterization and Application in X-ray Shielding" Polymers 13, no. 18: 3081. https://doi.org/10.3390/polym13183081
APA StyleAlshahri, S., Alsuhybani, M., Alosime, E., Almurayshid, M., Alrwais, A., & Alotaibi, S. (2021). LDPE/Bismuth Oxide Nanocomposite: Preparation, Characterization and Application in X-ray Shielding. Polymers, 13(18), 3081. https://doi.org/10.3390/polym13183081