A Multifaceted Approach for Cryogenic Waste Tire Recycling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
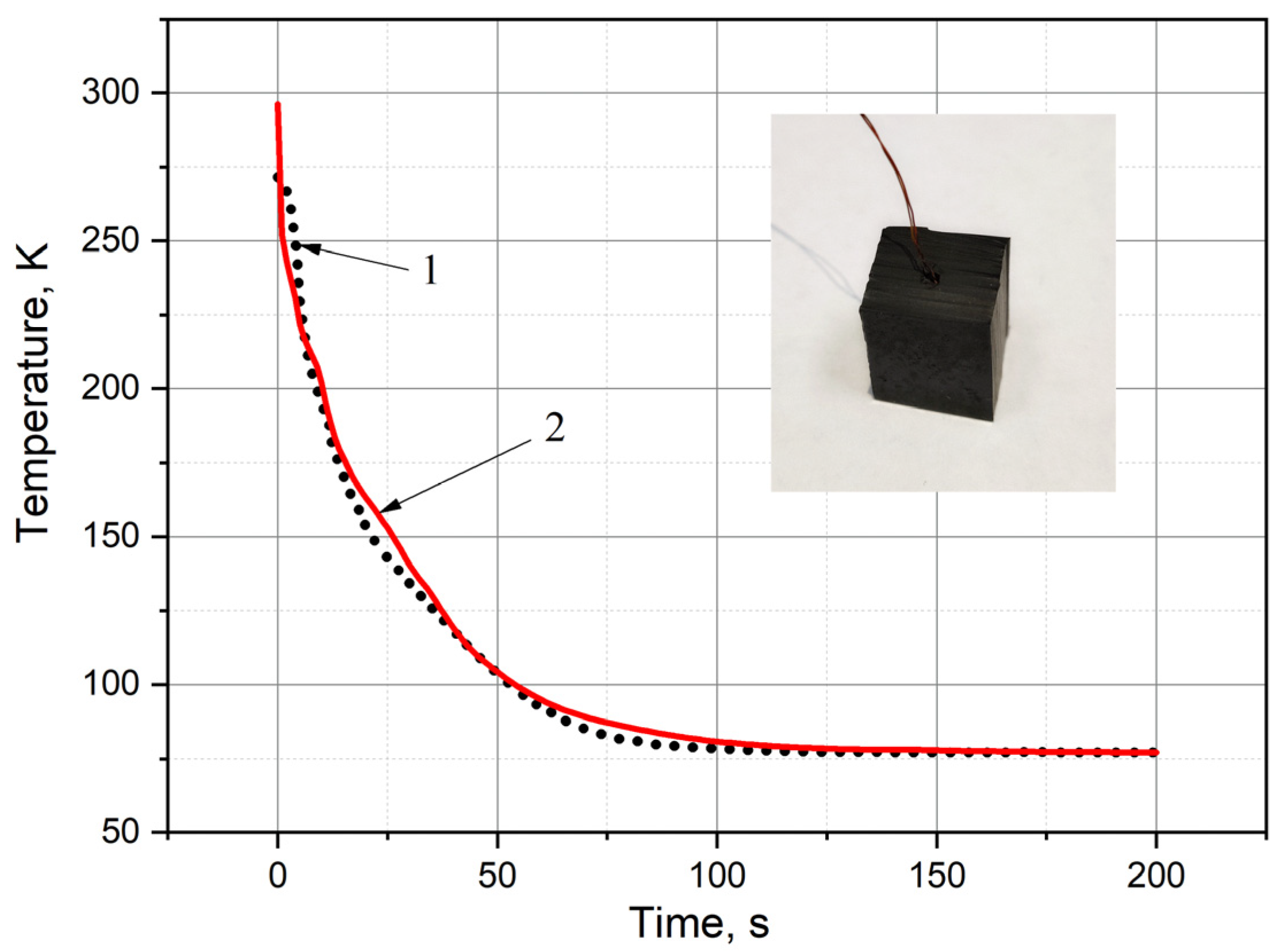
2.2. Measurement of Thermal Conductivity of Samples
2.3. Mechanical Characterization
2.4. Mathematical Model
2.5. Evaluation of Model Adequacy
2.6. Scanning Electron Microscope and Energy Dispersive X-ray Spectroscopy
3. Results and Discussion
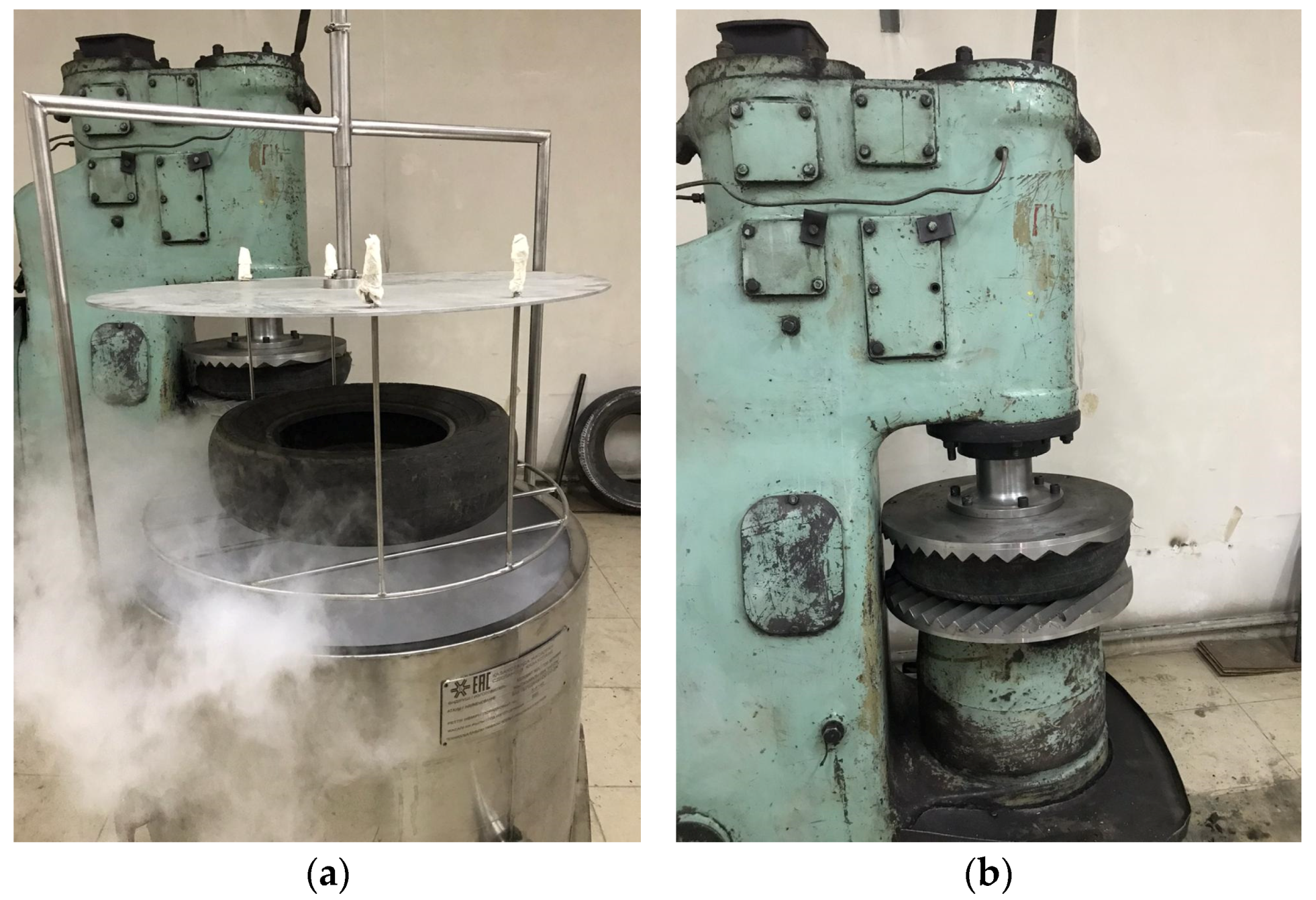
3.1. Evaluation of the Mechanical Impact Force
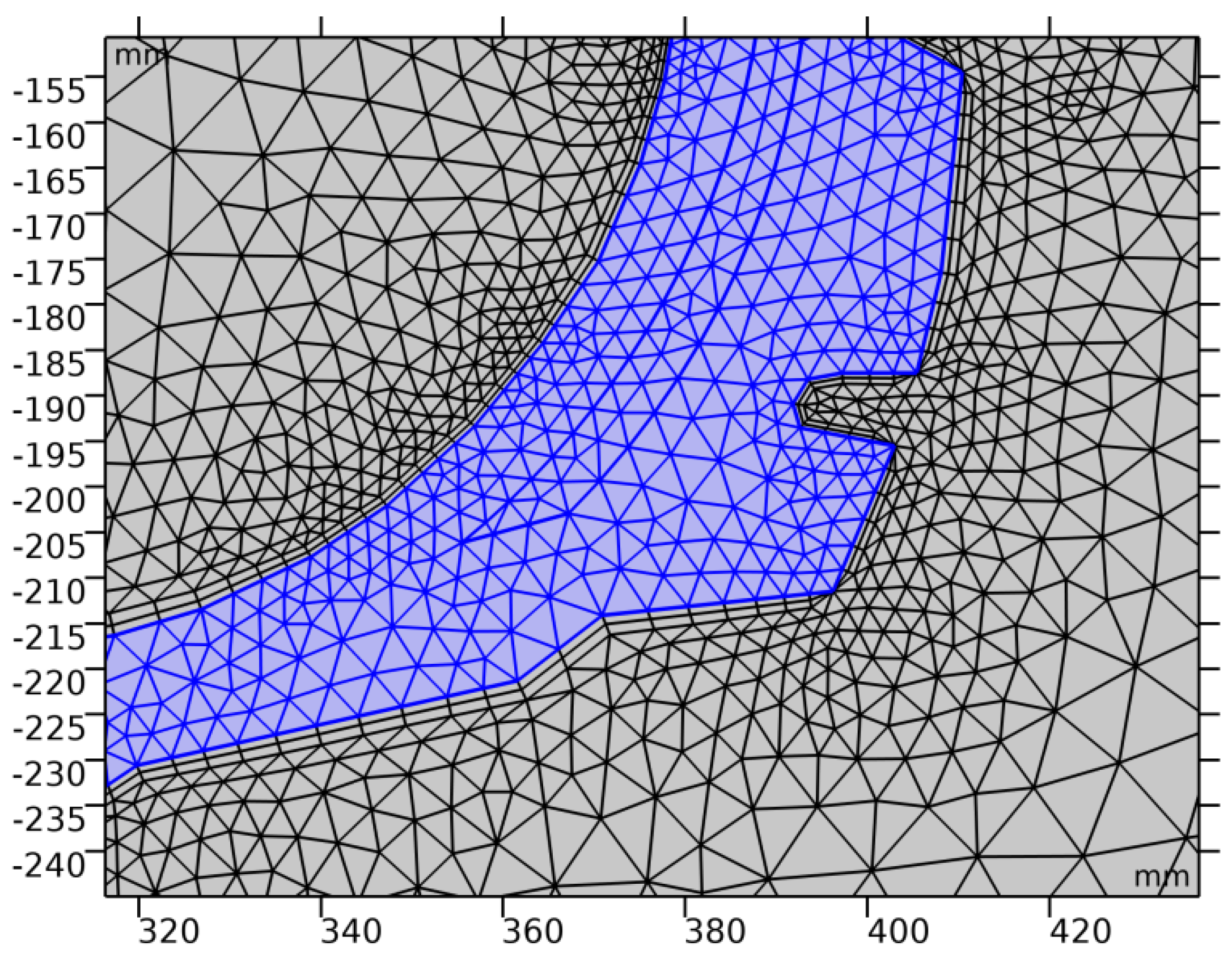
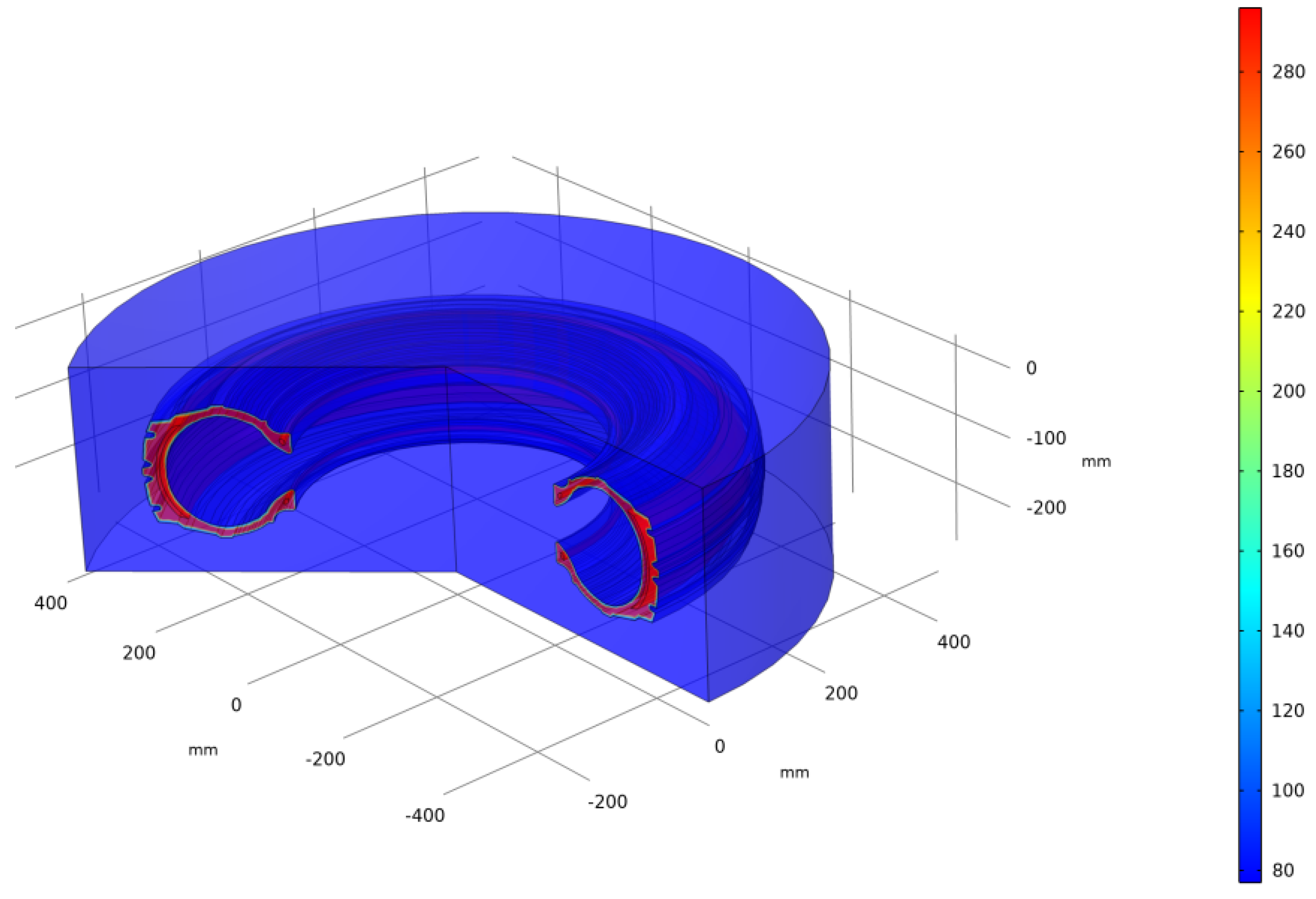
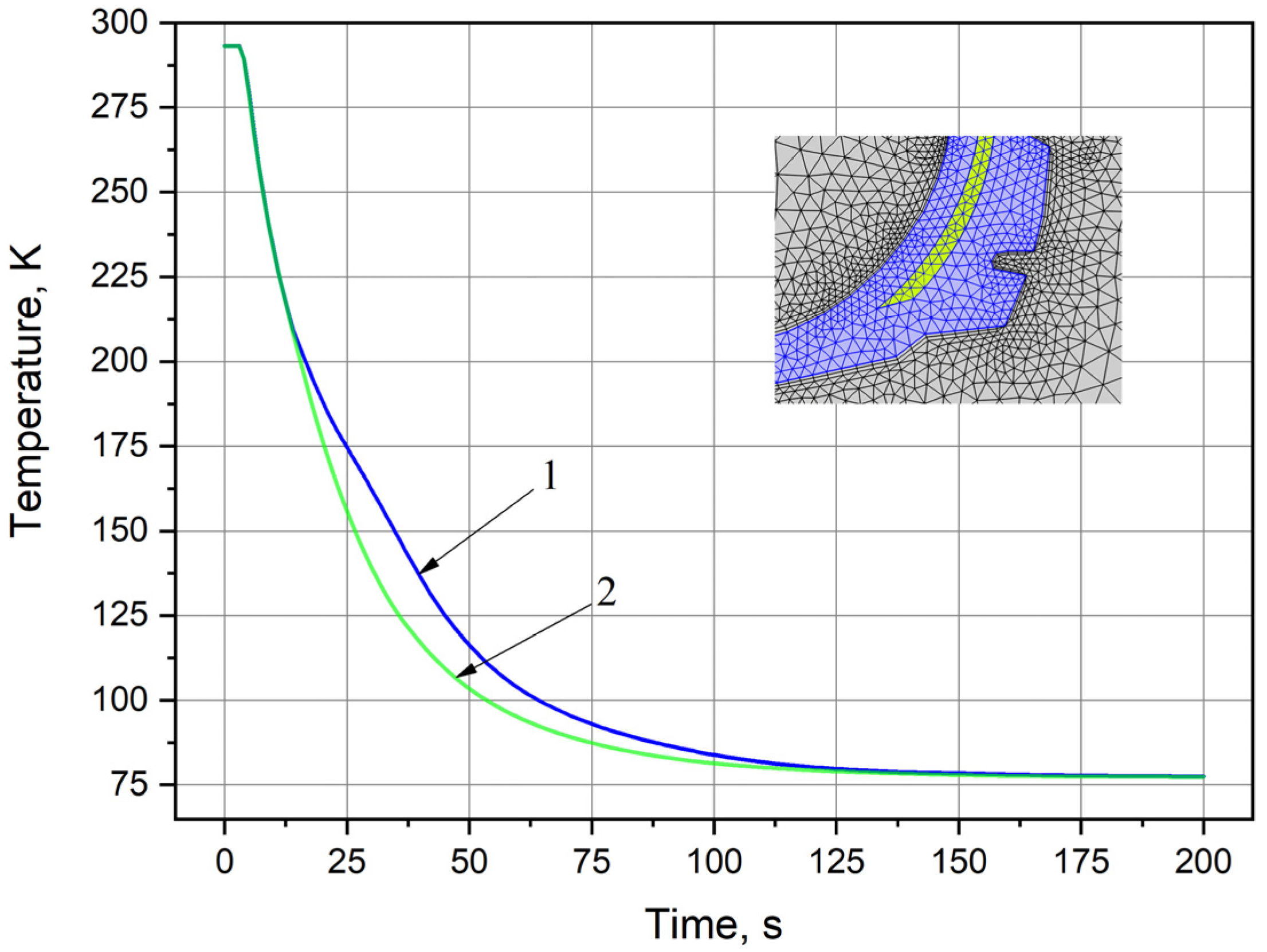
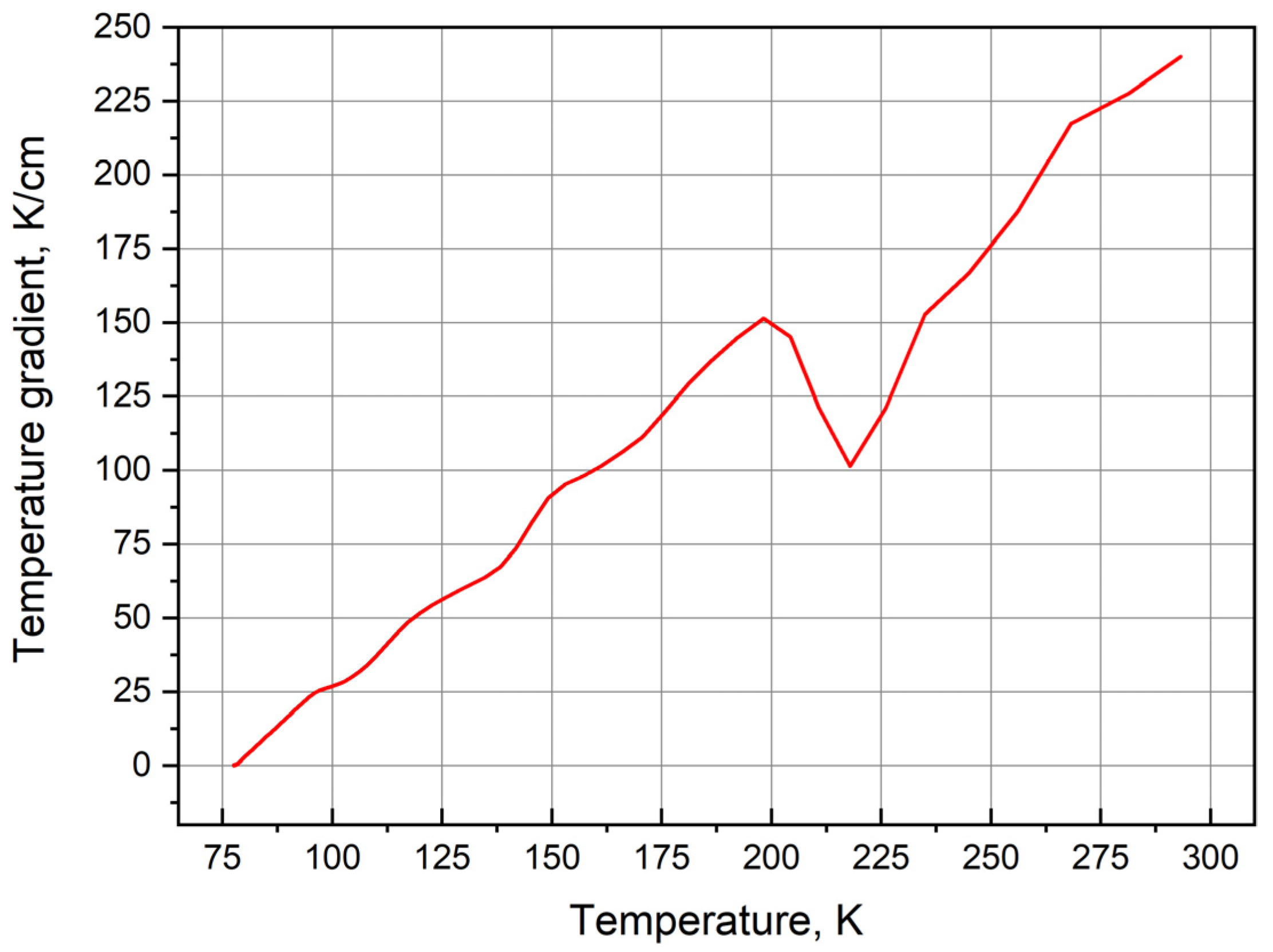
3.2. Computer Simulation
3.3. Physical and Chemical Properties
4. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Styrene Butadiene Rubber (SBR) Commodity Report; Beroe: Raleigh, NC, USA, 2020.
- Rubber Statistical Bulletin; International Rubber Study Group: Singapore, 2020.
- World Rubber; The Freedonia Group: Cleveland, OH, USA, 2016.
- Grammelis, P.; Margaritis, N.; Dallas, P.; Rakopoulos, D.; Mavrias, G. A Review on Management of End of Life Tires (ELTs) and Alternative Uses of Textile Fibers. Energies 2021, 14, 571. [Google Scholar] [CrossRef]
- Yang, X.; You, Z.; Perram, D.; Hand, D.; Ahmed, Z.; Wei, W.; Luo, S. Emission analysis of recycled tire rubber modified asphalt in hot and warm mix conditions. J. Hazard. Mater. 2019, 365, 942–951. [Google Scholar] [CrossRef]
- Marć, M.; Tsakovski, S.; Tobiszewski, M. Emissions and toxic units of solvent, monomer and additive residues released to gaseous phase from latex balloons. Environ. Res. 2021, 195, 110700. [Google Scholar] [CrossRef] [PubMed]
- Ortíz-Rodríguez, O.; Ocampo-Duque, W.; Duque-Salazar, L. Environmental Impact of End-of-Life Tires: Life Cycle Assessment Comparison of Three Scenarios from a Case Study in Valle Del Cauca, Colombia. Energies 2017, 10, 2117. [Google Scholar] [CrossRef] [Green Version]
- Zedler, Ł.; Kowalkowska-Zedler, D.; Colom, X.; Cañavate, J.; Saeb, M.R.; Formela, K. Reactive Sintering of Ground Tire Rubber (GTR) Modified by a Trans-Polyoctenamer Rubber and Curing Additives. Polymers 2020, 12, 3018. [Google Scholar] [CrossRef]
- Karaağaç, B.; Turan, H.O.; Oral, D.D. Use of ground EPDM wastes in EPDM-based rubber compounds. J. Elastomers Plast. 2015, 47, 117–135. [Google Scholar] [CrossRef]
- Araujo-Morera, J.; Verdejo, R.; López-Manchado, M.A.; Hernández Santana, M. Sustainable mobility: The route of tires through the circular economy model. Waste Manag. 2021, 126, 309–322. [Google Scholar] [CrossRef]
- Shulman, V.L. Tire Recycling. In Waste; Academic Press: London, UK, 2019; pp. 489–515. [Google Scholar]
- Fang, Y.; Zhan, M.; Wang, Y. The status of recycling of waste rubber. Mater. Des. 2001, 22, 123–128. [Google Scholar] [CrossRef]
- Adhikari, B. Reclamation and recycling of waste rubber. Prog. Polym. Sci. 2000, 25, 909–948. [Google Scholar] [CrossRef]
- Fazli, A.; Rodrigue, D. Waste Rubber Recycling: A Review on the Evolution and Properties of Thermoplastic Elastomers. Materials 2020, 13, 782. [Google Scholar] [CrossRef] [Green Version]
- Sathiskumar, C.; Karthikeyan, S. Recycling of waste tires and its energy storage application of by-products—A review. Sustain. Mater. Technol. 2019, 22, e00125. [Google Scholar] [CrossRef]
- Simon, D.Á.; Pirityi, D.; Tamás-Bényei, P.; Bárány, T. Microwave devulcanization of ground tire rubber and applicability in SBR compounds. J. Appl. Polym. Sci. 2020, 137, 48351. [Google Scholar] [CrossRef] [Green Version]
- Markl, E.; Lackner, M. Devulcanization Technologies for Recycling of Tire-Derived Rubber: A Review. Materials 2020, 13, 1246. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, C.; Manjare, S.; Rajan, S.K. Recycling of waste tire by pyrolysis to recover carbon black: Alternative & environment-friendly reinforcing filler for natural rubber compounds. Compos. Part B Eng. 2020, 200, 108346. [Google Scholar] [CrossRef]
- Bing, W.; Hongbin, Z.; Zeng, D.; Yuefeng, F.; Yu, Q.; Rui, X. Microwave-assisted fast pyrolysis of waste tires: Effect of microwave power on products composition and quality. J. Anal. Appl. Pyrolysis 2021, 155, 104979. [Google Scholar] [CrossRef]
- Xu, J.; Yu, J.; Xu, J.; Sun, C.; He, W.; Huang, J.; Li, G. High-value utilization of waste tires: A review with focus on modified carbon black from pyrolysis. Sci. Total Environ. 2020, 742, 140235. [Google Scholar] [CrossRef]
- Yang, B.; Chen, M. Influence of Interactions among Polymeric Components of Automobile Shredder Residue on the Pyrolysis Temperature and Characterization of Pyrolytic Products. Polymers 2020, 12, 1682. [Google Scholar] [CrossRef]
- Van Hoek, H.; Noordermeer, J.; Heideman, G.; Blume, A.; Dierkes, W. Best Practice for De-Vulcanization of Waste Passenger Car Tire Rubber Granulate Using 2-2′-dibenzamidodiphenyldisulfide as De-Vulcanization Agent in a Twin-Screw Extruder. Polymers 2021, 13, 1139. [Google Scholar] [CrossRef]
- Bockstal, L.; Berchem, T.; Schmetz, Q.; Richel, A. Devulcanisation and reclaiming of tires and rubber by physical and chemical processes: A review. J. Clean. Prod. 2019, 236, 117574. [Google Scholar] [CrossRef]
- Bowles, A.J.; Fowler, G.D.; O’Sullivan, C.; Parker, K. Sustainable rubber recycling from waste tyres by waterjet: A novel mechanistic and practical analysis. Sustain. Mater. Technol. 2020, 25, e00173. [Google Scholar] [CrossRef]
- Saxena, N.S.; Pradeep, P.; Mathew, G.; Thomas, S.; Gustafsson, M.; Gustafsson, S.E. Thermal conductivity of styrene butadiene rubber compounds with natural rubber prophylactics waste as filler. Eur. Polym. J. 1999, 35, 1687–1693. [Google Scholar] [CrossRef]
- Reese, W. Thermal Properties of Polymers at Low Temperatures. J. Macromol. Sci. Part A Chem. 1969, 3, 1257–1295. [Google Scholar] [CrossRef]
- Adhikari, J.; Das, A.; Sinha, T.; Saha, P.; Kim, J.K. Grinding of Waste Rubber. In Rubber Recycling Challenges and Developments; Kim, J.K., Saha, P., Thomas, S., Haponiuk, J.T., Aswathi, M.K., Eds.; The Royal Society of Chemistry: London, UK, 2019; p. 8. [Google Scholar]
- Dierkes, W. Untreated and treated rubber powders. In Rubber Recycling; De, S.K., Khait, K., Isayev, A.I., Eds.; CRC Press: Boca Raton, FL, USA, 2005; p. 3. [Google Scholar]
- Sienkiewicz, M.; Kucinska-Lipka, J.; Janik, H.; Balas, A. Progress in used tyres management in the European Union: A review. Waste Manag. 2012, 32, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- Fukumori, K. Recycling technology of tire rubber. JSAE Rev. 2002, 23, 259–264. [Google Scholar] [CrossRef]
- Harrison, K.; Tong, S.; Hilyard, N. An economic evaluation of cryogenic-grinding of scrap automotive tyres. Conserv. Recycl. 1986, 9, 1–14. [Google Scholar] [CrossRef]
- Daborn, G.R.; Derry, R. Cryogenic communition in scrap recycling. Resour. Conserv. Recycl. 1988, 1, 49–63. [Google Scholar] [CrossRef]
- Allen, D.H.; Biddulph, M.W. The economic evaluation of cryopulverising. Conserv. Recycl. 1978, 2, 255–261. [Google Scholar] [CrossRef]
- Burford, R.P. Cryogenic regrinding of rubber. Conserv. Recycl. 1981, 4, 219–233. [Google Scholar] [CrossRef]
- Piotrowska, A.; Aszklar, K.; Dzidek, A.; Ptaszek, B.; Czerwińska-Ledwig, O.; Pilch, W. The impact of a single whole body cryostimulation treatment on selected skin properties of healthy young subjects. Cryobiology 2021, 100, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Ioan Faur, C.; Abu-Awwad, A.; Pop, D.L.; Zamfir, C.L.; Gurgus, D.; Hoinoiu, T.; Motoc, A.; Haivas, C.; Grigoraș, M.L.; Folescu, R. Liquid Nitrogen Efficiency in Treatment of Giant Cell Tumor of Bone and Prevention of Recurrence. Appl. Sci. 2020, 10, 6310. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, G.; Zhu, G.; Jia, B.; Zhang, P. Applicability of liquid nitrogen fire extinguishing in urban underground utility tunnel. Case Stud. Therm. Eng. 2020, 21, 100657. [Google Scholar] [CrossRef]
- Gao, F.; Cai, C.; Yang, Y. Experimental research on rock fracture failure characteristics under liquid nitrogen cooling conditions. Results Phys. 2018, 9, 252–262. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, S.; Yang, R.; Wu, X.; Li, R.; Zhang, H.; Hung, P. A review of liquid nitrogen fracturing technology. Fuel 2020, 266, 117040. [Google Scholar] [CrossRef]
- Saxena, S.N.; Barnwal, P.; Balasubramanian, S.; Yadav, D.N.; Lal, G.; Singh, K.K. Cryogenic grinding for better aroma retention and improved quality of Indian spices and herbs: A review. J. Food Process. Eng. 2018, 41, e12826. [Google Scholar] [CrossRef]
- Khadatkar, R.M.; Kumar, S.; Pattanayak, S.C. Cryofreezing and cryofreezer. Cryogenics 2004, 44, 661–678. [Google Scholar] [CrossRef]
- Burfoot, D.; Hall, J.; Nicholson, K.; Holmes, K.; Hanson, C.; Handley, S.; Mulvey, E. Effect of rapid surface cooling on Campylobacter numbers on poultry carcasses. Food Control 2016, 70, 293–301. [Google Scholar] [CrossRef]
- Fenton, D.L.; Callahan, C.W.; Elansari, A.M. Refrigeration. In Postharvest Technology of Perishable Horticultural Commodities; Elhadi, Y., Ed.; Woodhead Publishing: London, UK, 2019; pp. 209–270. [Google Scholar]
- Huang, C.; Qian, X.; Yang, R. Thermal conductivity of polymers and polymer nanocomposites. Mater. Sci. Eng. R Rep. 2018, 132, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Cai, G.; Liu, X. Investigation of thermal conductivity and prediction model of recycled tire rubber-sand mixtures as lightweight backfill. Constr. Build. Mater. 2020, 248, 118657. [Google Scholar] [CrossRef]
- Aldiyarov, A.; Sokolov, D.; Nurmukan, A.; Korshikov, E. The study of thermophysical properties of rubber and plastic household waste to determine the temperature conditions of cryoprocessing. Appl. Surf. Sci. 2020, 511, 145487. [Google Scholar] [CrossRef]
- Eiermann, K. Thermal conductivity of high polymers. J. Polym. Sci. Part C Polym. Symp. 2007, 6, 157–165. [Google Scholar] [CrossRef]
- Goyanes, S.; Lopez, C.C.; Rubiolo, G.H.; Quasso, F.; Marzocca, A.J. Thermal properties in cured natural rubber/styrene butadiene rubber blends. Eur. Polym. J. 2008, 44, 1525–1534. [Google Scholar] [CrossRef]
- Hughes, T.J.R.; Franca, L.P.; Mallet, M. A new finite element formulation for computational fluid dynamics: I. Symmetric forms of the compressible Euler and Navier-Stokes equations and the second law of thermodynamics. Comput. Methods Appl. Mech. Eng. 1986, 54, 223–234. [Google Scholar] [CrossRef]
- Couchman, P.R. Compositional Variation of Glass-Transition Temperatures. 2. Application of the Thermodynamic Theory to Compatible Polymer Blends. Macromolecules 1978, 11, 1156–1161. [Google Scholar] [CrossRef]
- Zeggai, N.; Bouberka, Z.; Dubois, F.; Bouchaour, T.; Dali Youcef, B.; Delarace, L.; Potier, J.; Supiot, P.; Maschke, U. Effect of structure on the glass transition temperatures of linear and crosslinked poly (isobornylacrylate-co-isobutylacrylate). J. Appl. Polym. Sci. 2021, 138, 50449. [Google Scholar] [CrossRef]
- Mark, J.; Ngai, K.; Graessley, W.; Mandelkern, L.; Samulski, E.; Koenig, J.; Wignall, G. The rubber elastic state. In Physical Properties of Polymers; Cambridge University Press: Cambridge, UK, 2004; pp. 3–71. [Google Scholar]
- Mark, J.; Ngai, K.; Graessley, W.; Mandelkern, L.; Samulski, E.; Koenig, J.; Wignall, G. The glass transition and the glassy state. In Physical Properties of Polymers; Cambridge University Press: Cambridge, UK, 2004; pp. 72–152. [Google Scholar]
- Zuoguang, Z.; Chenghong, H.; Yubin, L.; Zhijie, S. Dynamic Viscoelasticity of Carbon Fibre Reinforced Polymers under High Load: Effects of Static and Dynamic Loads. Polym. Polym. Compos. 2007, 15, 297–305. [Google Scholar] [CrossRef]
- Kim, J.K.; Saha, P.; Thomas, S.; Haponiuk, J.T.; Aswathi, M.K.; Thomas, S. (Eds.) Rubber Recycling; Green Chemistry Series; Royal Society of Chemistry: Cambridge, UK, 2018; ISBN 978-1-78801-084-9. [Google Scholar]
- Wen, J. Heat Capacities of Polymers. In Physical Properties of Polymers Handbook; Springer: New York, NY, USA, 2007; pp. 145–154. [Google Scholar]
- Orwoll, R.A. Densities, Coefficients of Thermal Expansion, and Compressibilities of Amorphous Polymers. In Physical Properties of Polymers Handbook; Springer: New York, NY, USA, 2007; pp. 93–101. [Google Scholar]
- Nitrogen. In Handbook of Compressed Gases / Compressed Gasses Association; Springer: Boston, MA, USA, 1999; pp. 528–534.
- Properties of Some Metals and Alloys; The International Nickel Company Inc.: Toronto, ON, Canada, 1982.
- Newton, I. Scala graduum caloris. Philos. Trans. R. Soc. (Lond.) 1701, 22, 824–829. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, S.; Moriya, S. Newton’s Law of Cooling: Follow up and exploration. Int. J. Heat Mass Transf. 2021, 164, 120544. [Google Scholar] [CrossRef]
- Yang, Y. Thermal Conductivity. In Physical Properties of Polymers Handbook; Springer: New York, NY, USA, 2007; pp. 155–163. [Google Scholar]
- Uher, C. Thermal Conductivity of Metals. In Thermal Conductivity; Springer: Boston, MA, USA; pp. 21–91.
- Camaño, E.; Martire, N.; Goyanes, S.N.; Marzocca, A.J.; Rubiolo, G.H. Evaluation of the thermal diffusivity of rubber compounds through the glass transition range. J. Appl. Polym. Sci. 1997, 63, 157–162. [Google Scholar] [CrossRef]
- Vergnaud, J.-M.; Rosca, I.-D. Rubber Curing and Properties; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781420085235. [Google Scholar]
- Bieliński, D.M.; Klajn, K.; Gozdek, T.; Kruszyński, R.; Świątkowski, M. Influence of n-ZnO Morphology on Sulfur Crosslinking and Properties of Styrene-Butadiene Rubber Vulcanizates. Polymers 2021, 13, 1040. [Google Scholar] [CrossRef]




| T, K | λ, W/m·K |
|---|---|
| 95 | 0.142 ± 0.003 |
| 105 | 0.187 ± 0.017 |
| 115 | 0.166 ± 0.005 |
| 125 | 0.177 ± 0.005 |
| 135 | 0.185 ± 0.009 |
| 145 | 0.142 ± 0.006 |
| 155 | 0.164 ± 0.005 |
| 165 | 0.174 ± 0.011 |
| 175 | 0.155 ± 0.011 |
| 185 | 0.157 ± 0.006 |
| 195 | 0.154 ± 0.004 |
| 205 | 0.236 ± 0.005 |
| 215 | 0.331 ± 0.012 |
| 225 | 0.184 ± 0.006 |
| 235 | 0.209 ± 0.011 |
| 245 | 0.218 ± 0.003 |
| 255 | 0.173 ± 0.001 |
| 265 | 0.148 ± 0.001 |
| 275 | 0.124 ± 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yerezhep, D.; Tychengulova, A.; Sokolov, D.; Aldiyarov, A. A Multifaceted Approach for Cryogenic Waste Tire Recycling. Polymers 2021, 13, 2494. https://doi.org/10.3390/polym13152494
Yerezhep D, Tychengulova A, Sokolov D, Aldiyarov A. A Multifaceted Approach for Cryogenic Waste Tire Recycling. Polymers. 2021; 13(15):2494. https://doi.org/10.3390/polym13152494
Chicago/Turabian StyleYerezhep, Darkhan, Aliya Tychengulova, Dmitriy Sokolov, and Abdurakhman Aldiyarov. 2021. "A Multifaceted Approach for Cryogenic Waste Tire Recycling" Polymers 13, no. 15: 2494. https://doi.org/10.3390/polym13152494
APA StyleYerezhep, D., Tychengulova, A., Sokolov, D., & Aldiyarov, A. (2021). A Multifaceted Approach for Cryogenic Waste Tire Recycling. Polymers, 13(15), 2494. https://doi.org/10.3390/polym13152494