Chemical Oxidative Polymerization of Methylene Blue: Reaction Mechanism and Aspects of Chain Structure
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
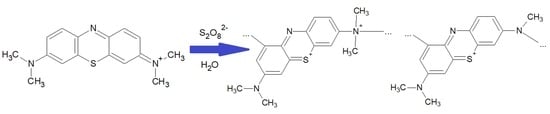
2.2. Synthesis of Polymethylene Blue
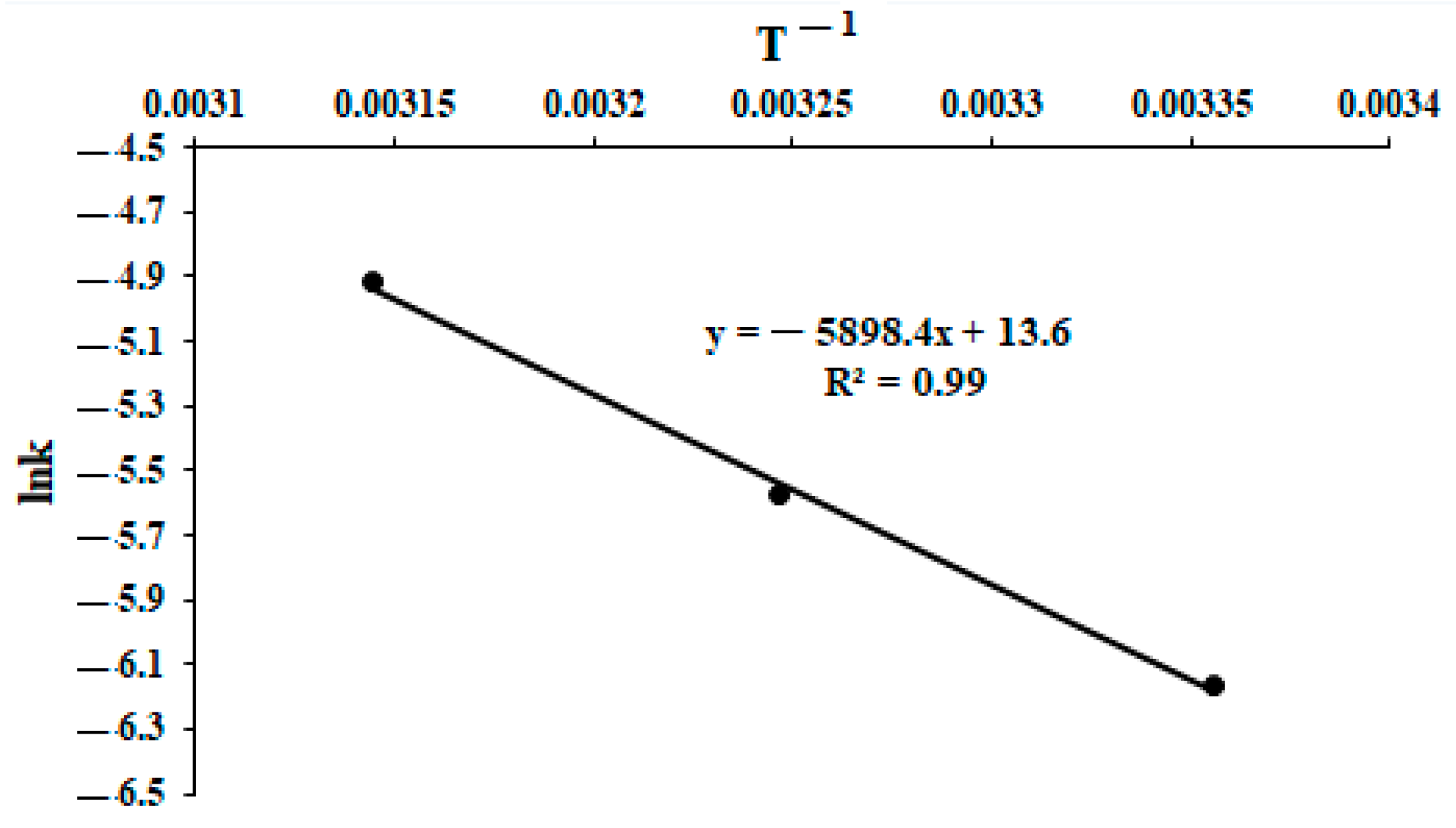
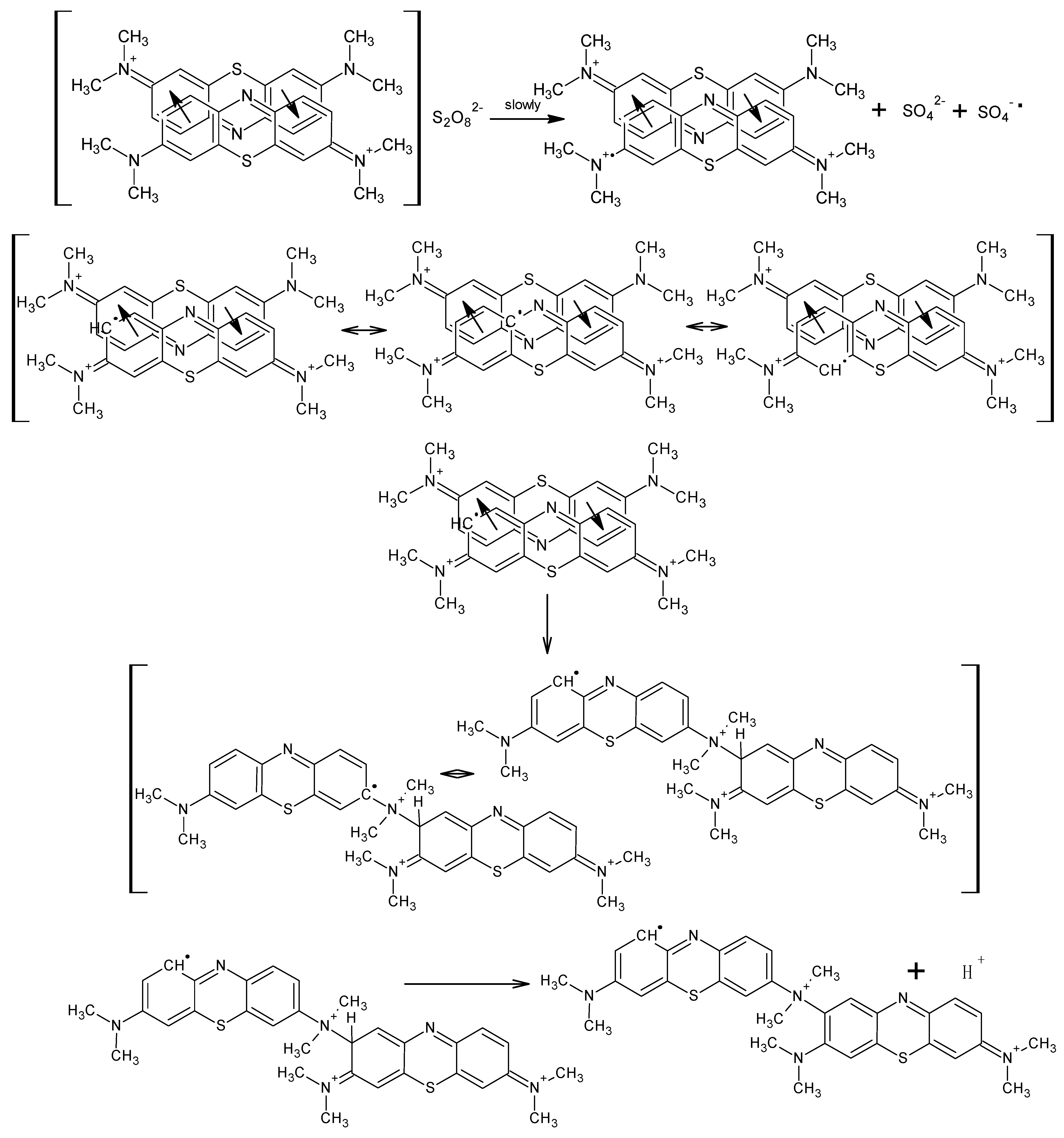
2.3. Kinetics of Polymerization of Methylene Blue
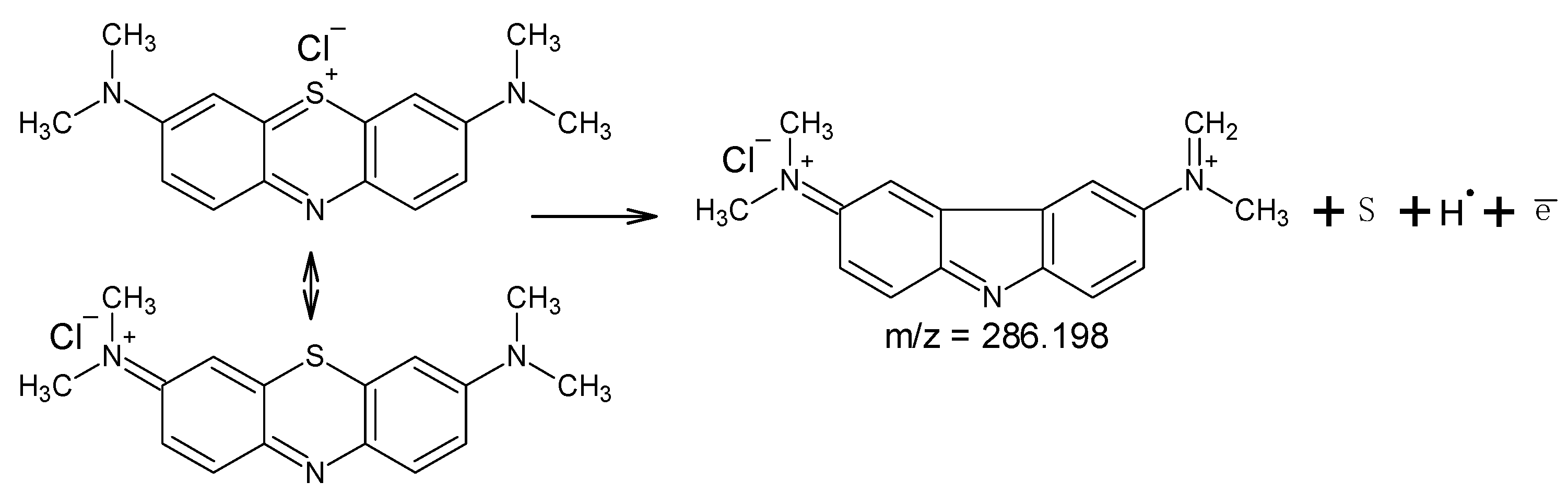
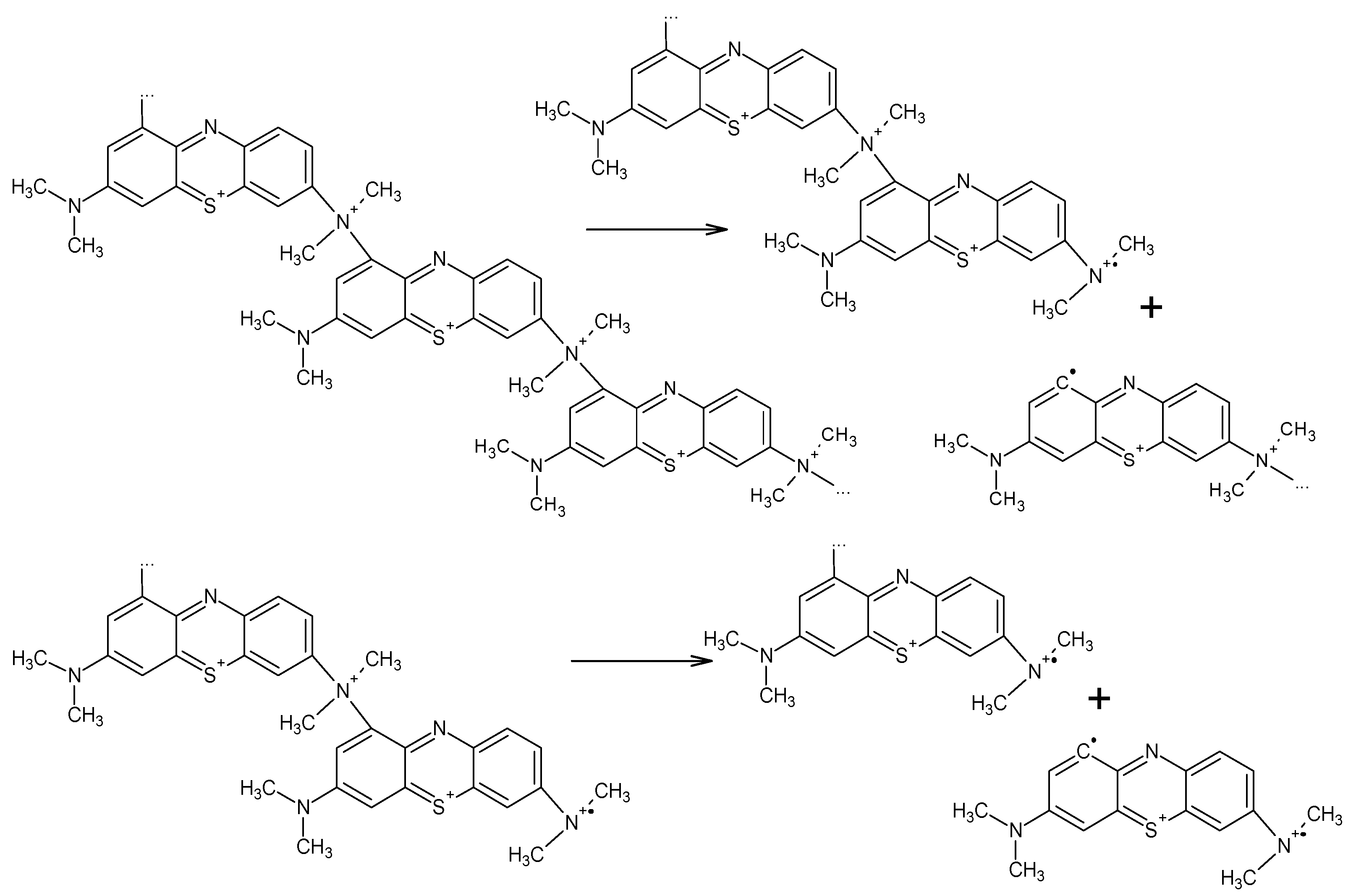
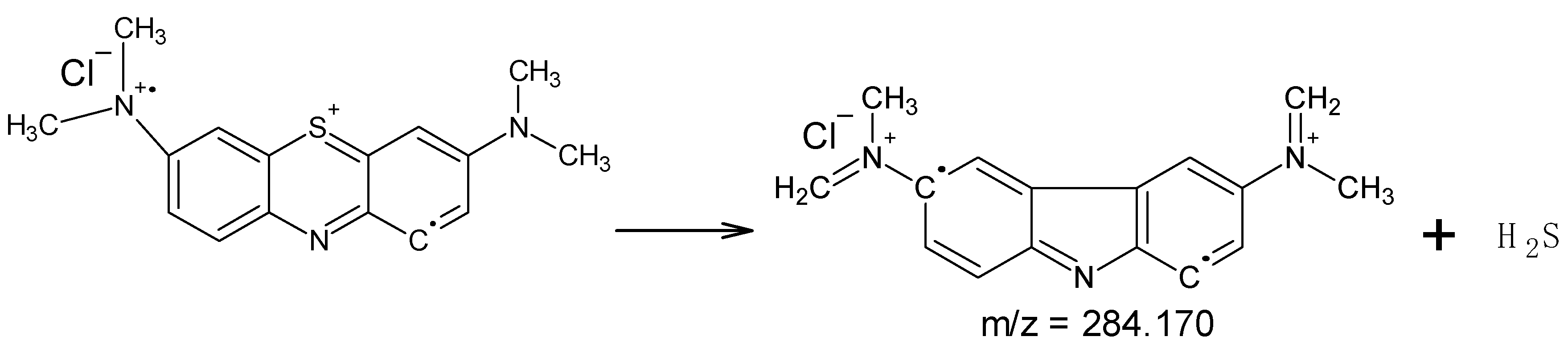
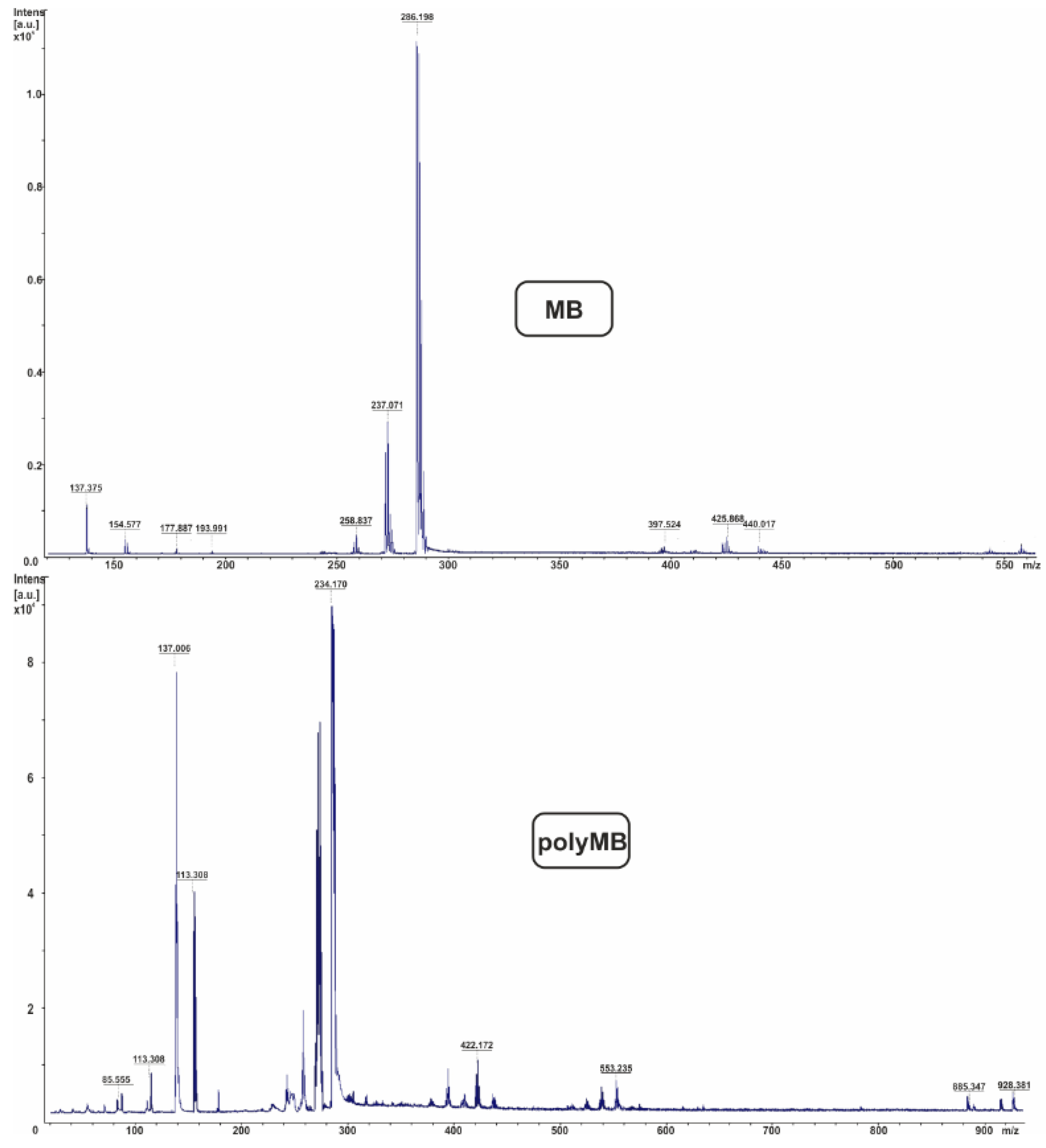
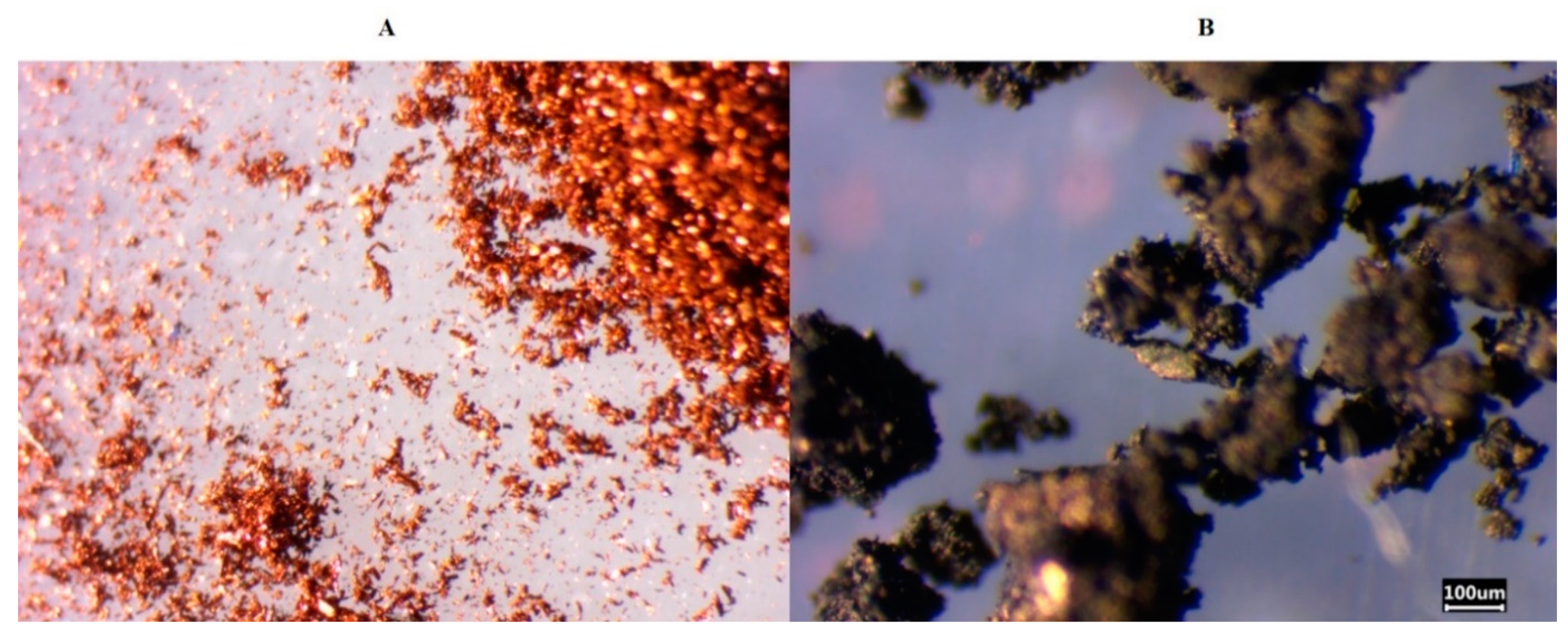
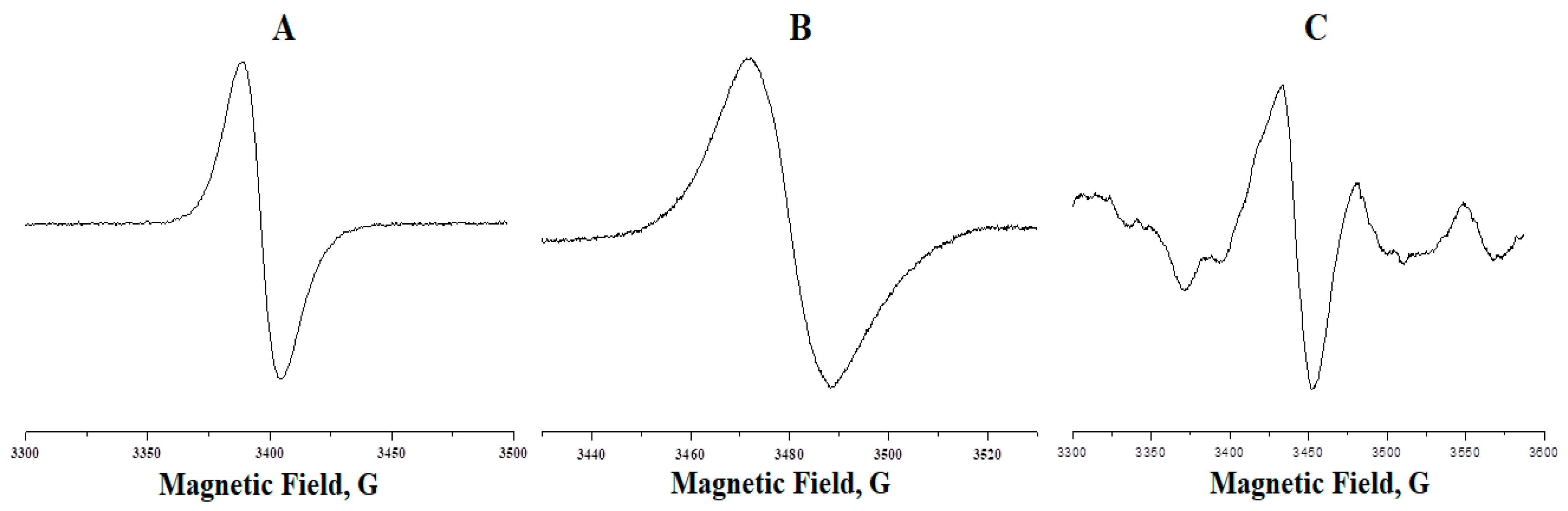
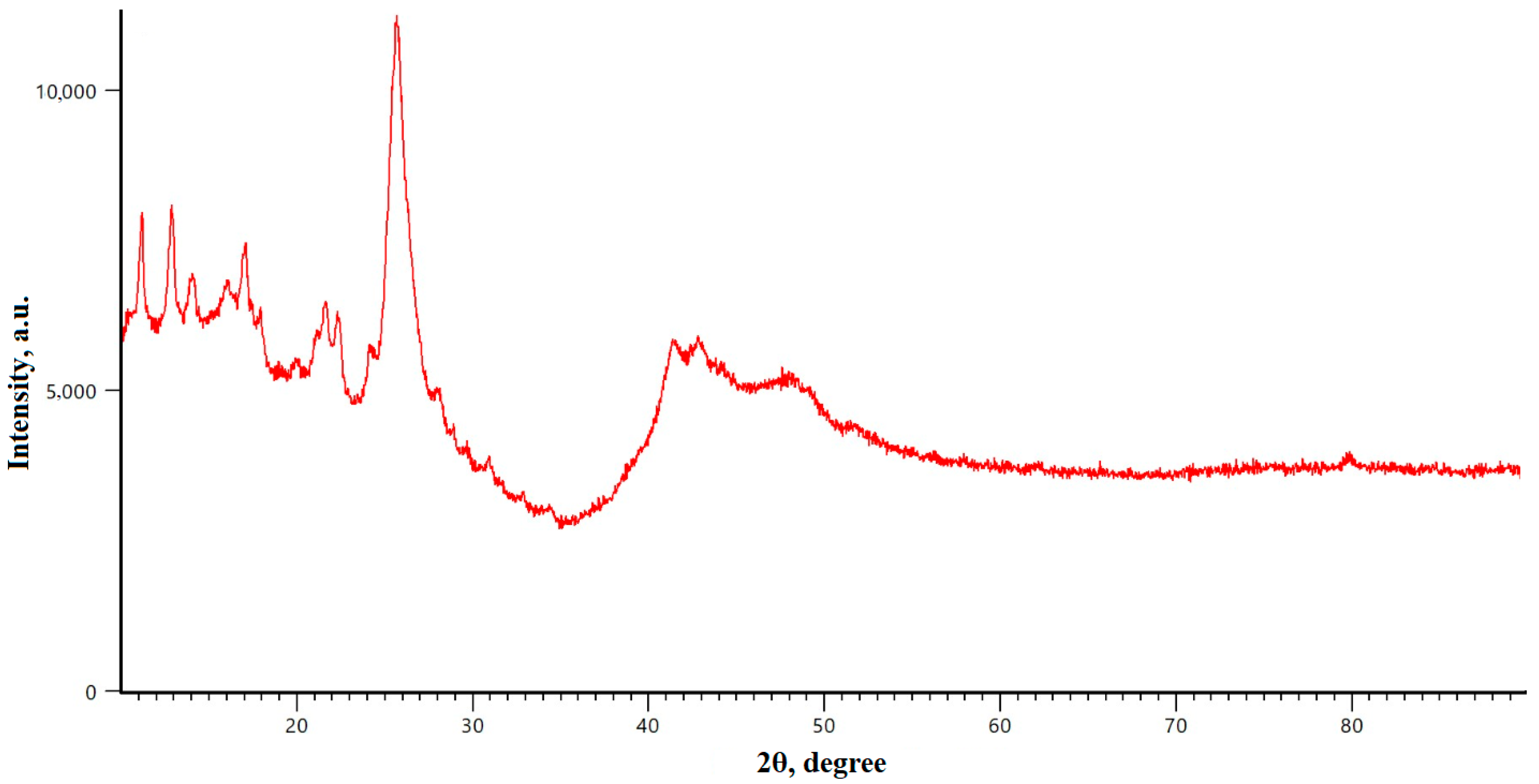
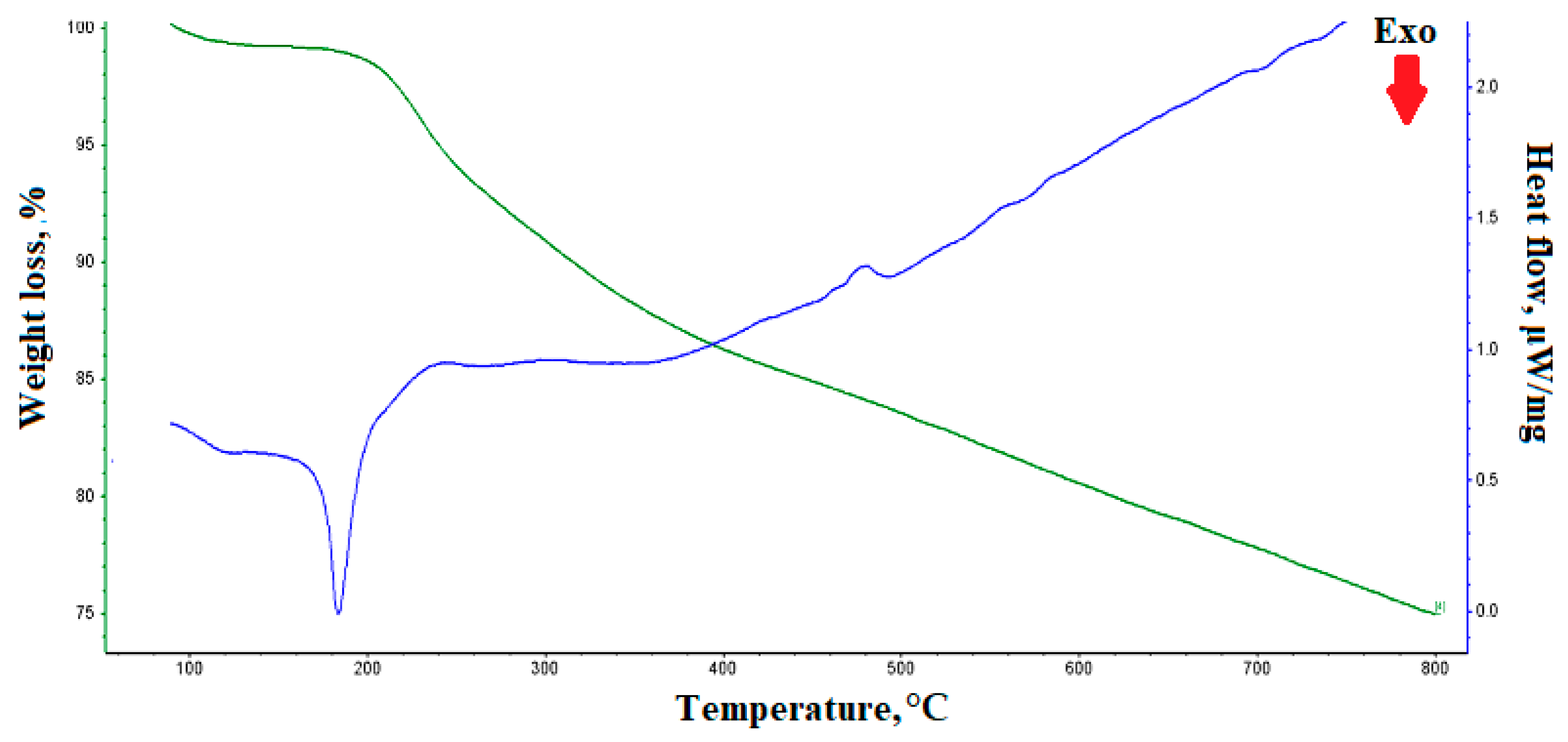
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaplan, İ.H.; Dağcı, K.; Alanyalıoğlu, M. Nucleation and Growth Mechanism of Electropolymerization of Methylene Blue: The Effect of Preparation Potential on Poly(methylene blue) Structure. Electroanalysis 2010, 22, 2694–2701. [Google Scholar] [CrossRef]
- Liu, B.; Cang, H.; Cui, L.; Zhang, H. Electrochemical Polymerization of Methylene Blue on Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2017, 12, 9907–9913. [Google Scholar] [CrossRef]
- Choi, H.N.; Lee, W.Y. Amperometric Determination of Nitrite at Poly(Methylene Blue)-Modified Glassy Carbon Electrode. Bull. Korean Chem. Soc. 2012, 33, 415–419. [Google Scholar]
- Giribabu, K.; Suresh, R.; Manigandan, R.; Munusamy, S.; Kumar, S.P.; Muthamizh, S.; Narayanan, V. Nanomolar determination of 4-nitrophenol based on a poly(methylene blue)-modified glassy carbon electrode. Analyst 2013, 138, 5811–5818. [Google Scholar] [CrossRef] [PubMed]
- Esokkiya, A.; Sudalaimani, S.; Kumar, K.S.; Sampathkumar, P.; Suresh, C.; Giribabu, K. Poly(methylene blue)-Based Electrochemical Platform for Label-Free Sensing of Acrylamide. ACS Omega 2021, 6, 9528–9536. [Google Scholar] [CrossRef] [PubMed]
- Marinho, M.I.C.; Cabral, M.F.; Mazo, L.H. Is the poly(methylene blue)-modified glassy carbon electrode an adequate electrode for the simple detection of thiols and amino acid-based molecules? J. Electroanal. Chem. 2012, 685, 8–14. [Google Scholar] [CrossRef]
- Manasa, G.; Mascarenhas, R.J.; Satpati, A.K.; D’Souza, O.J.; Dhason, A. Facile preparation of poly(methylene blue) modified carbon paste electrode for the detection and quantification of catechin. Mater. Sci. Eng. C 2017, 73, 552–561. [Google Scholar] [CrossRef]
- Tan, L.; Xie, Q.; Yao, S. Electrochemical and Spectroelectrochemical Studies on Pyridoxine Hydrochloride Using a Poly(methylene blue) Modified Electrode. Electroanalysis 2004, 16, 1592–1597. [Google Scholar] [CrossRef]
- Xiaoqin, L.; Zhang, P.; Li, M.; Guo, Z.; Zheng, X. Synthesis of Polymethylene Blue Nanoparticles and Their Application to Label-Free DNA Detection. Anal. Lett. 2016. [Google Scholar] [CrossRef]
- Brett, C.M.A.; Inzelt, G.; Kertesz, V. Poly(methylene blue) modifed electrode sensor for haemoglobin. Anal. Chim. Acta 1999, 385, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Ma, W.; Luo, Y.; Sun, D.; Shao, S. Simultaneous Determination of Uric Acid and Xanthine Using a Poly(Methylene Blue) and Electrochemically Reduced Graphene Oxide Composite Film Modified Electrode. J. Anal. Methods Chem. 2014, 984314. [Google Scholar] [CrossRef] [Green Version]
- Braun, W.A.; Horn, B.C.; Hoehne, L.; Stülp, S.R.; Marcelo, B.D.; Hilgemann, M. Poly(methylene blue)-modified electrode for indirect electrochemical sensing of OH radicals and radical scavengers. An. Acad. Bras. Cien. 2017, 89, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Xiao, S.J.; Su, C.W. Preparation of molecularly imprinted polymer for methylene blue and study on its molecular recognition mechanism. Colloid Polym. Sci. 2016, 294, 1305–1314. [Google Scholar] [CrossRef]
- Samyn, L.M.; Babu, R.S.; Devendiran, M.; de Barros, A.L.F. One-step electropolymerization of methylene blue films on highly flexible carbon fiber electrode as supercapacitors. Micro Nano Syst. Lett. 2021, 9, 3. [Google Scholar] [CrossRef]
- Hichem, H.; Djamila, A.; Hania, A. Optical, electrical and photoelectrochemical characterization of electropolymerized poly methylene blue on fluorine doped tin oxide conducting glass. Electrochim. Acta 2013, 106, 69–74. [Google Scholar] [CrossRef]
- Ajami, N.; Ehsani, A.; Babaei, F.; Safari, R. Electrochemical properties, optical modeling and electrocatalytic activity of pulse-electropolymerized ternary nanocomposite of poly(methylene blue) in aqueous solution. J. Mol. Liq. 2016, 215, 24–30. [Google Scholar] [CrossRef]
- Liu, J.; Mu, S. The electrochemical polymerization of methylene blue and properties of polymethylene blue. Synth. Met. 1999, 107, 159–165. [Google Scholar] [CrossRef]
- Kamiya, N.; Okawara, M. Photopolymerization Using Methylene Blue-Reducing Agent System and Its Application to Photoelectrochemical Cell. J. Soc. Chem. Ind. Jpn. 1969, 72, 2639–2644. [Google Scholar]
- Fouassier, J.-P.; Morlet-Savary, F.; Lalevée, J.; Allonas, X.; Ley, C. Dyes as Photoinitiators or Photosensitizers of Polymerization Reactions. Materials 2010, 3, 5130–5142. [Google Scholar] [CrossRef]
- Cenens, J.; Schoonheydt, R.A. Visible Spectroscopy of Methylene Blue on Hectorite, Laponite B, and Barasym in Aqueous Suspension. Clays Clay Miner. 1988, 36, 214–224. [Google Scholar] [CrossRef]
- Mezhuev, Y.O.; Varankin, A.V.; Luss, A.L.; Dyatlov, V.A.; Tsatsakis, A.M.; Stratidakis, A.K.; Korshak, Y.V. Abnormally slow reaction of oppositely charged ions: The kinetics of dopamine hydrochloride oxidation by ammonium peroxydisulfate. Int. J. Chem. Kinet. 2020, 52, 520–525. [Google Scholar] [CrossRef]
- Erickson, P.R.; Walpen, N.; Guerard, J.J.; Eustis, S.N.; Arey, J.S.; McNeill, K. Controlling Factors in the Rates of Oxidation of Anilines and Phenols by Triplet Methylene Blue in Aqueous Solution. J. Phys. Chem. A 2015, 119, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Kayser, R.H.; Young, R.H. The Photoreduction of Methylene Blue by Amines-I. A Flash Photolysis Study of The Reaction Between Triplet Methylene Blue and Amines. Photochem. Photobiol. 1976, 24, 395–401. [Google Scholar] [CrossRef]
- Murthy, A.S.N.; Bhardwaj, A.P. Electronic absorption spectroscopic studies on the red form of methylene blue ion. Proc. Indian Acad. Sci. Chem. Sci. 1980, 89, 463–468. [Google Scholar]
- Junqueira, H.C.; Severino, D.; Dias, L.G.; Gugliotti, M.S.; Baptista, M.S. Modulation of methylene blue photochemical properties based on adsorption at aqueous micelle interfaces. Phys. Chem. Chem. Phys. 2002, 4, 2320–2328. [Google Scholar] [CrossRef]
- Mohammad, N.; Atassi, Y. Adsorption of methylene blue onto electrospun nanofibrous membranes of polylactic acid and polyacrylonitrile coated with chloride doped polyaniline. Sci. Rep. 2020, 10, 13412. [Google Scholar] [CrossRef]
- Ayad, M.M.; El-Nasr, A.A. Adsorption of Cationic Dye (Methylene Blue) from Water Using Polyaniline Nanotubes Base. J. Phys. Chem. C 2010, 114, 14377–14383. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Kumar, V.B.; Luong, J.H.T.; Gedanken, A. Kinetics, Isotherm, and Thermodynamic Studies of Methylene Blue Adsorption on Polyaniline and Polypyrrole Macro-Nanoparticles Synthesized by C-Dot-Initiated Polymerization. ACS Omega 2018, 3, 7196–7203. [Google Scholar] [CrossRef]
- Ayad, M.M.; Amer, W.A.; Zaghlol, S.; Minisy, I.M.; Bober, P.; Stejskal, J. Polypyrrole-coated cotton textile as adsorbent of methylene blue dye. Chem. Pap. 2018, 72, 1605–1618. [Google Scholar] [CrossRef]
- Kirk-Othmer Encyclopedia of Chemical Technology, 3rd ed.; John Wiley and Sons: New York, NY, USA, 1978; Volume 3, p. 379.





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezhuev, Y.O.; Vorobev, I.Y.; Plyushchii, I.V.; Krivoborodov, E.G.; Artyukhov, A.A.; Motyakin, M.V.; Luss, A.L.; Ionova, I.S.; Kovarskii, A.L.; Derevnin, I.A.; et al. Chemical Oxidative Polymerization of Methylene Blue: Reaction Mechanism and Aspects of Chain Structure. Polymers 2021, 13, 2188. https://doi.org/10.3390/polym13132188
Mezhuev YO, Vorobev IY, Plyushchii IV, Krivoborodov EG, Artyukhov AA, Motyakin MV, Luss AL, Ionova IS, Kovarskii AL, Derevnin IA, et al. Chemical Oxidative Polymerization of Methylene Blue: Reaction Mechanism and Aspects of Chain Structure. Polymers. 2021; 13(13):2188. https://doi.org/10.3390/polym13132188
Chicago/Turabian StyleMezhuev, Yaroslav O., Igor Y. Vorobev, Ivan V. Plyushchii, Efrem G. Krivoborodov, Alexander A. Artyukhov, Mikhail V. Motyakin, Anna L. Luss, Irina S. Ionova, Alexander L. Kovarskii, Igor A. Derevnin, and et al. 2021. "Chemical Oxidative Polymerization of Methylene Blue: Reaction Mechanism and Aspects of Chain Structure" Polymers 13, no. 13: 2188. https://doi.org/10.3390/polym13132188
APA StyleMezhuev, Y. O., Vorobev, I. Y., Plyushchii, I. V., Krivoborodov, E. G., Artyukhov, A. A., Motyakin, M. V., Luss, A. L., Ionova, I. S., Kovarskii, A. L., Derevnin, I. A., Dyatlov, V. A., Alekperov, R. A., Toropygin, I. Y., Volkov, M. A., Shtilman, M. I., & Korshak, Y. V. (2021). Chemical Oxidative Polymerization of Methylene Blue: Reaction Mechanism and Aspects of Chain Structure. Polymers, 13(13), 2188. https://doi.org/10.3390/polym13132188