Effects of Recycled Fe2O3 Nanofiller on the Structural, Thermal, Mechanical, Dielectric, and Magnetic Properties of PTFE Matrix
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
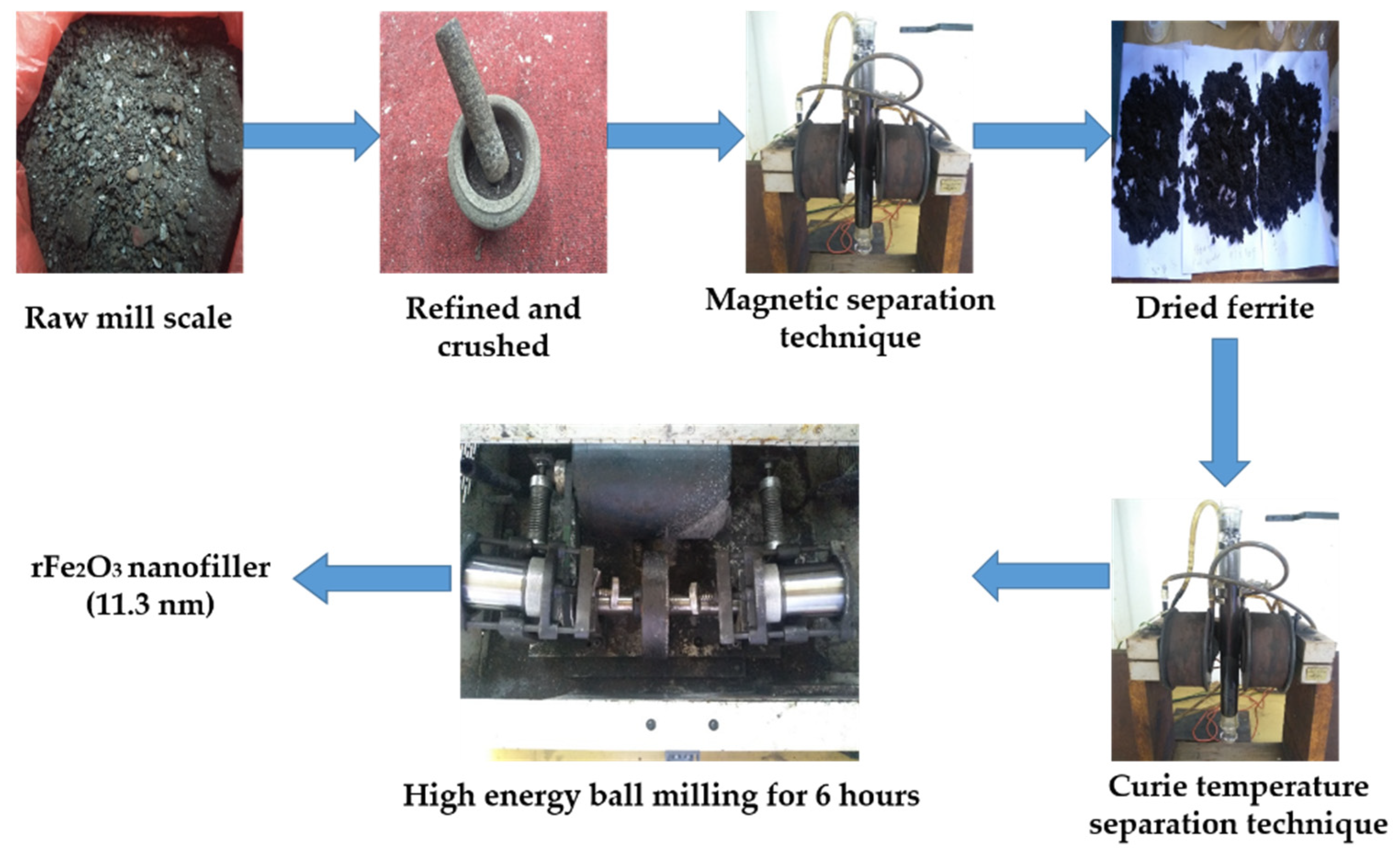
2.2. Synthesis of rFe2O3 Nanopartilces from Mill Scale
2.3. Fabrication of rFe2O3–PTFE Nanocomposites
2.4. Characterization
2.4.1. X-ray Diffraction (XRD)
2.4.2. High-Resolution Transmission Electron Microscopy (HRTEM)
2.4.3. Field Emission Scanning Electron Microscopy (FESEM)
2.4.4. Electronic Densitometer
2.4.5. Dilatometer Linseis L75 Platinum
2.4.6. Extensometer (Shimadzu AGS-X 100kN)
2.4.7. Rectangular Waveguide (RWG)
3. Results and Discussion
3.1. Microstructural Characterization
3.2. Coefficients of Linear Thermal Expansion (CTEs)
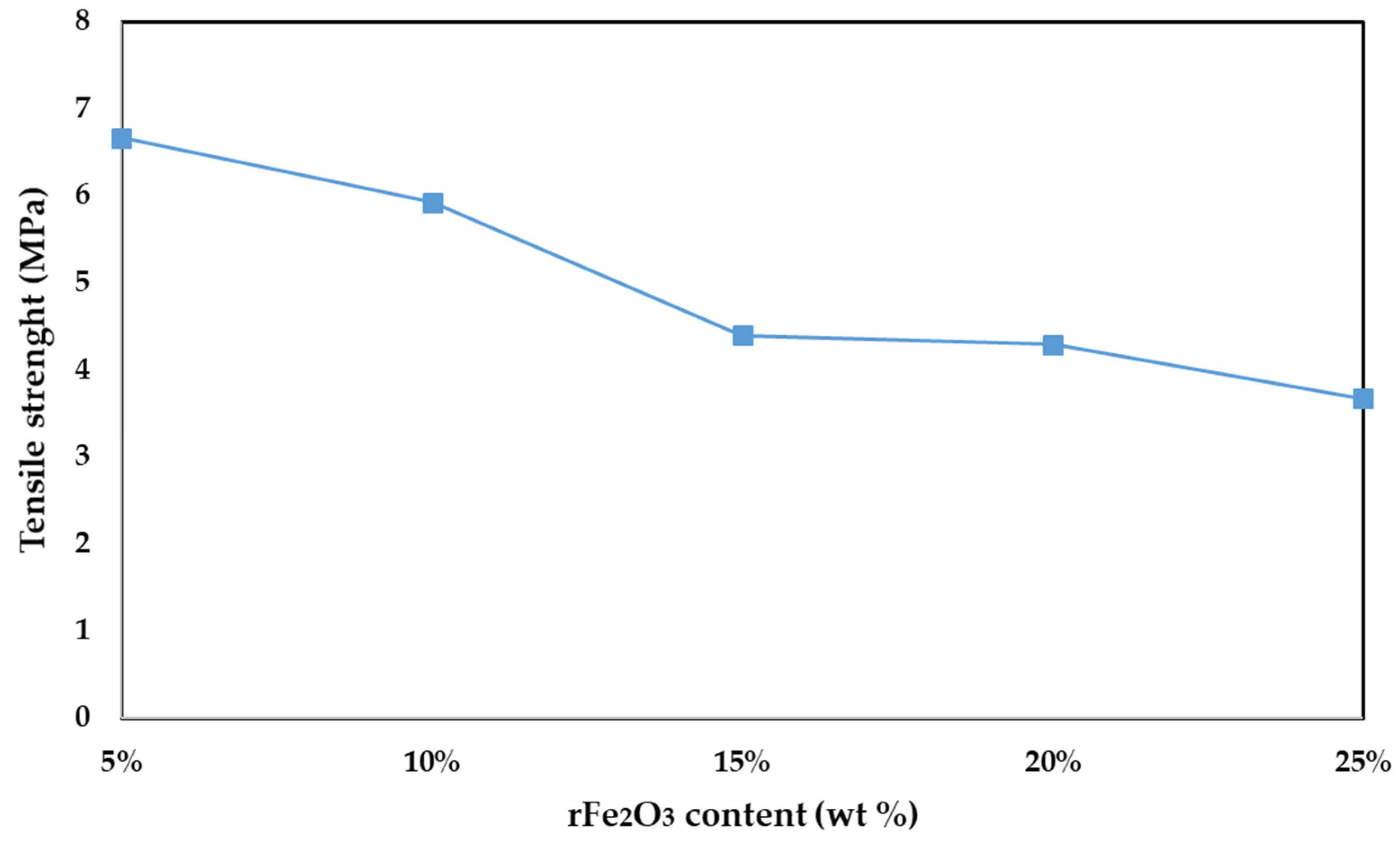
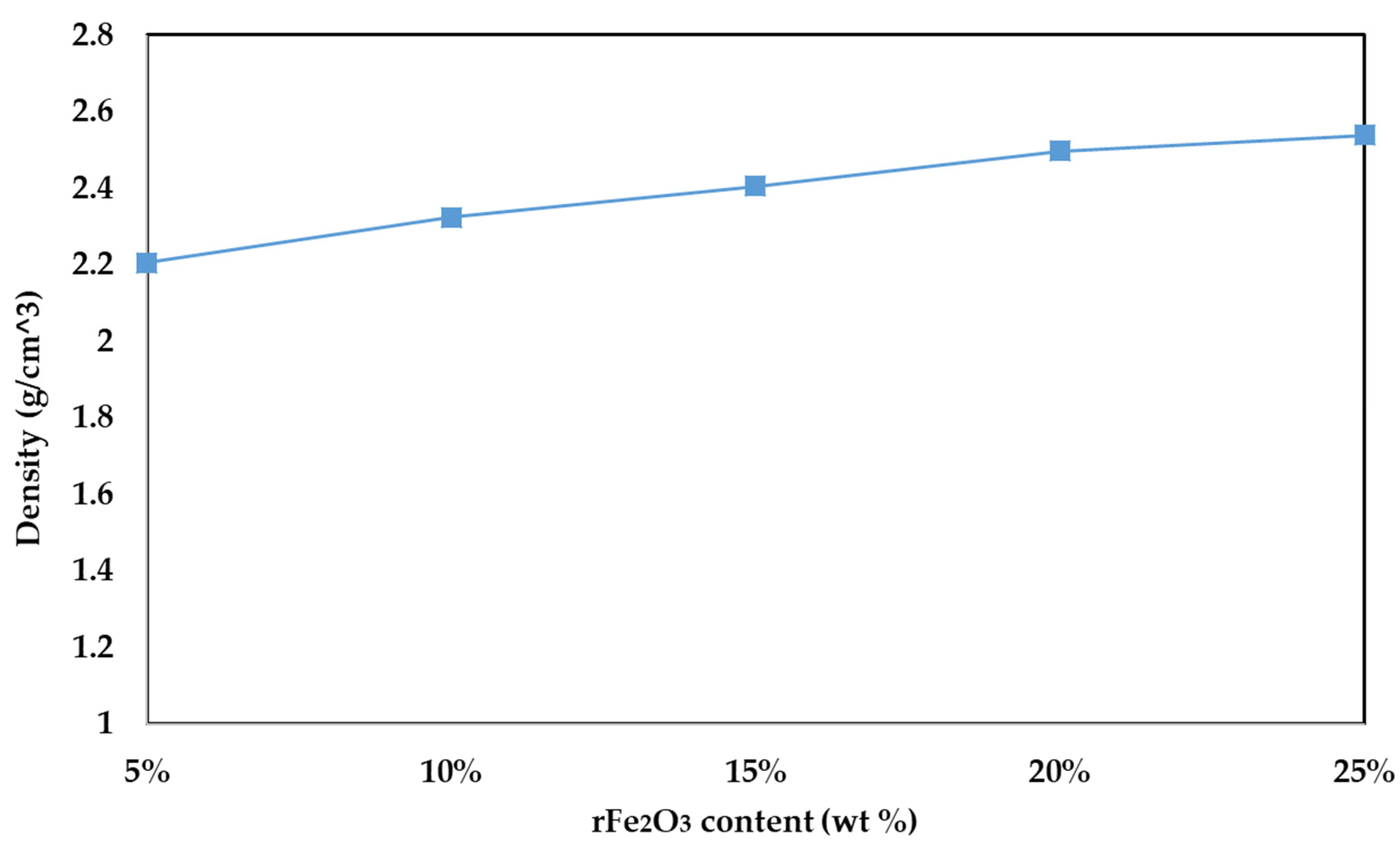
3.3. Tensile Strength and Density of rFe2O3–PTFE Nanocomposites
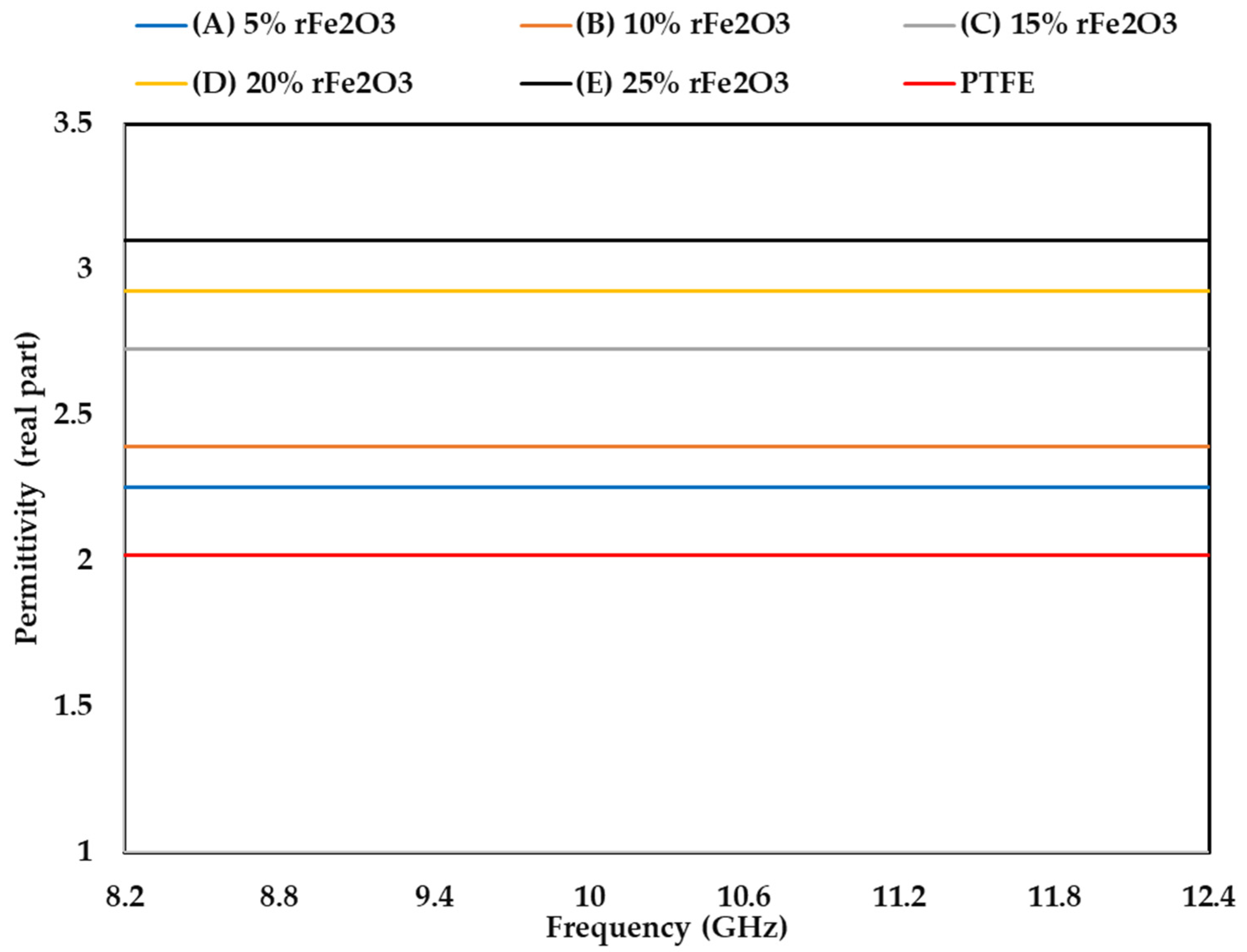
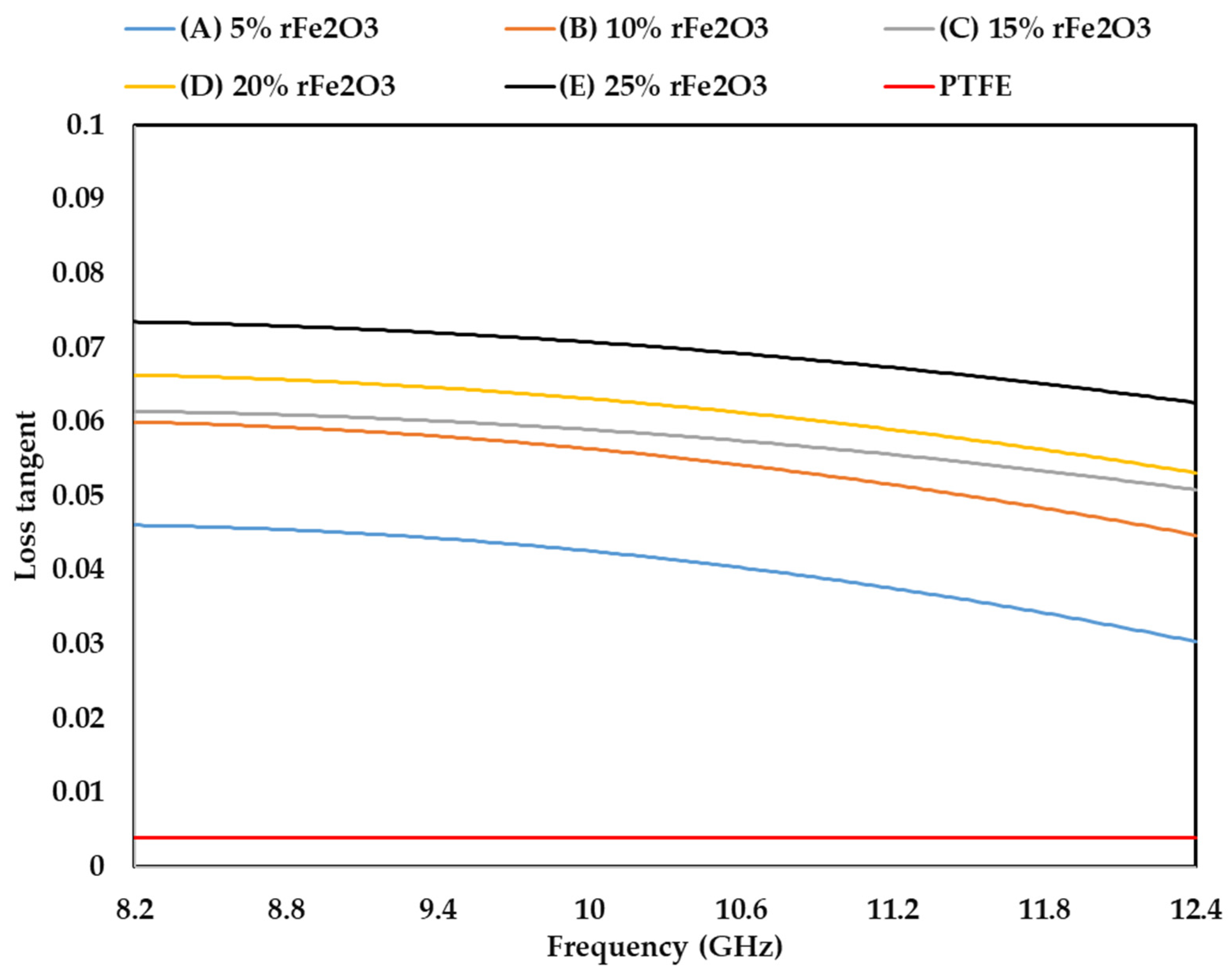
3.4. Complex Permittivity of rFe2O3–PTFE Nanocomposites
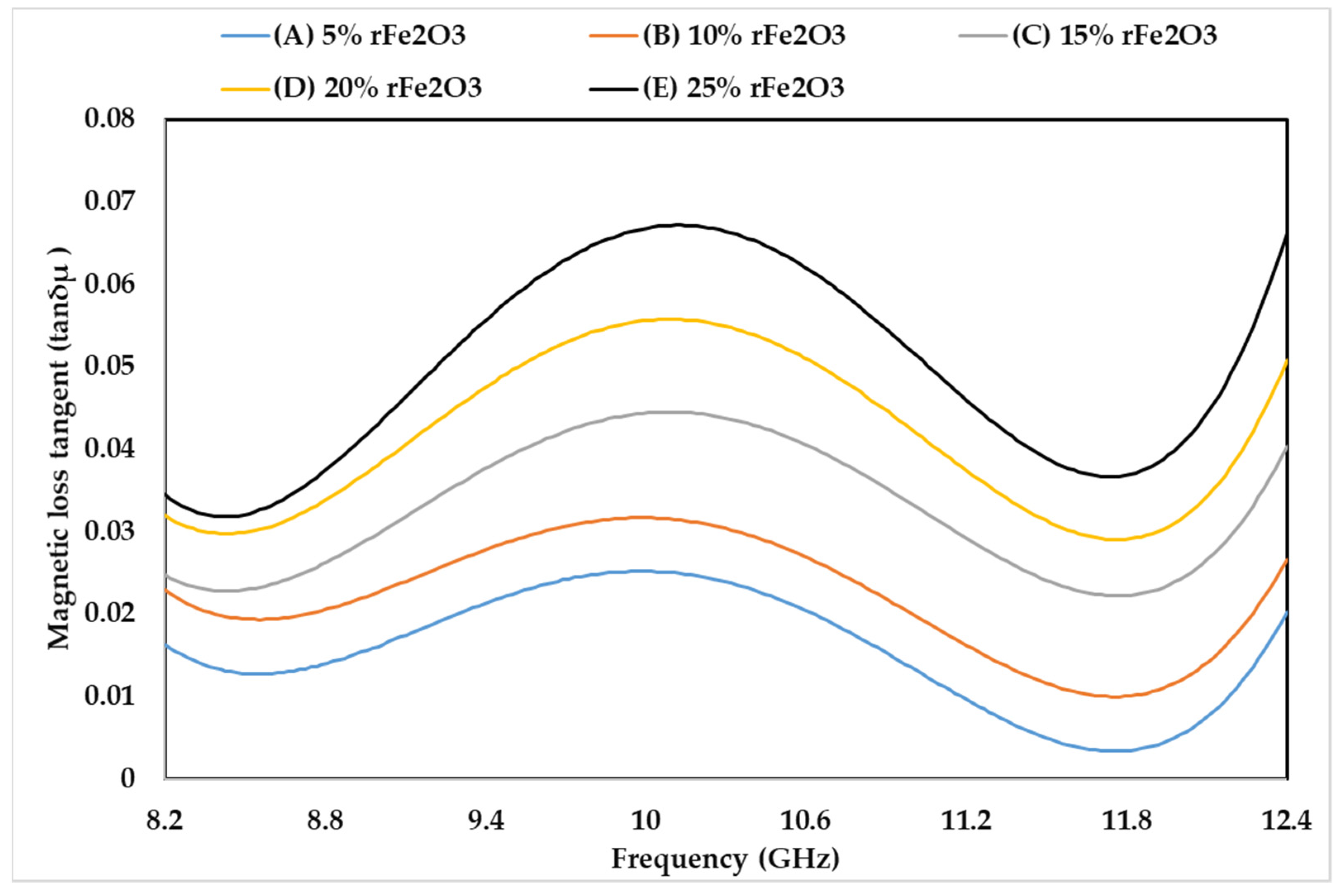
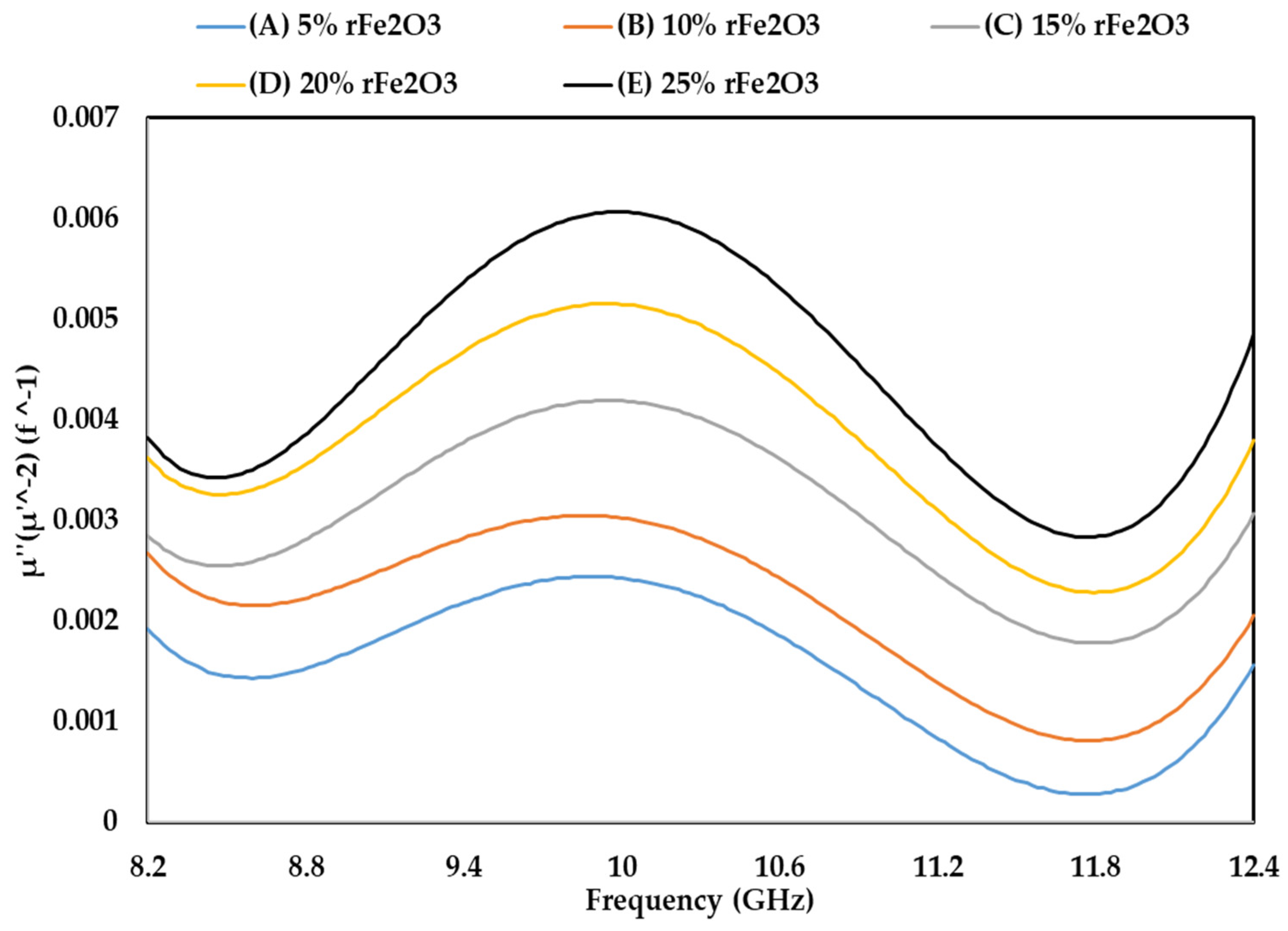
3.5. Complex Permeability of rFe2O3–PTFE Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, B.; Zhang, L.; Zhang, Y.; Han, G.; Zhang, B. Innovative methodology for co-treatment of mill scale scrap and manganese ore via oxidization roasting-magnetic separation for preparation of ferrite materials. Ceram. Int. 2021, 47, 6139–6153. [Google Scholar] [CrossRef]
- Mensah, E.E.; Abbas, Z.; Azis, R.S.; Ibrahim, N.A.; Khamis, A.M. Complex Permittivity and Microwave Absorption Properties of OPEFB Fiber–Polycaprolactone Composites Filled with Recycled Hematite (α-Fe2O3) Nanoparticles. Polymers 2019, 11, 918. [Google Scholar] [CrossRef] [PubMed]
- Eid, C.; Brioude, A.; Salles, V.; Plenet, J.-C.; Asmar, R.; Monteil, Y.; Khoury, R.; Khoury, A.; Miele, P. Iron-based 1D nanostructures by electrospinning process. Nanotechnology 2010, 21, 125701. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El-Leathy, A.; Danish, S.N.; Al-Ansary, H.; Jeter, S.; Al-Suhaibani, Z. Experimental study of compatibility of reduced metal oxides with thermal energy storage lining materials. In Proceedings of the AIP Conference Proceedings, Cape Town, South Africa, 13–16 October 2015; p. 050013. Available online: https://aip.scitation.org/toc/apc/1734/1?size=all (accessed on 31 May 2016).
- Müller, M.; Villalba, J.C.; Mariani, F.Q.; Dalpasquale, M.; Lemos, M.Z.; Huila, M.F.G.; Anaissi, F.J. Synthesis and characterization of iron oxide pigments through the method of the forced hydrolysis of inorganic salts. Dye. Pigment. 2015, 120, 271–278. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, W.; Ma, D.; Ma, Q.; Bridges, D.; Ma, Y.; Hu, A. Electrospinning of nanofibers and their applications for energy devices. J. Nanomater. 2015, 2015, 122. [Google Scholar] [CrossRef]
- Bai, H.; Yin, P.; Lu, X.; Zhang, L.; Wu, W.; Feng, X.; Wang, J.; Dai, J. Recent advances of magnetism-based microwave absorbing composites: An insight from perspective of typical morphologies. J. Mater. Sci. Mater. Electron. 2020, 1–26. [Google Scholar] [CrossRef]
- Raveendran, A.; Sebastian, M.T.; Raman, S. Applications of microwave materials: A review. J. Electron. Mater. 2019, 48, 2601–2634. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Das, C.K. In situ synthesis and characterization of CuFe10Al2O19/MWCNT nanocomposites for supercapacitor and microwave-absorbing applications. Ind. Eng. Chem. Res. 2013, 52, 9594–9606. [Google Scholar] [CrossRef]
- Oladele, I.O.; Omotosho, T.F.; Adediran, A.A. Polymer-Based Composites: An Indispensable Material for Present and Future Applications. Int. J. Polym. Sci. 2020, 2020, 8834518. [Google Scholar] [CrossRef]
- Chandra, R.J.; Shivamurthy, B.; Kulkarni, S.D.; Kumar, M.S. Hybrid polymer composites for EMI shielding application—A review. Mater. Res. Express 2019, 6, 082008. [Google Scholar]
- Wu, K.-T.; Yuan, Y.; Zhang, S.-R.; Yan, X.-Y.; Cui, Y.-R. ZrTi2O6 filled PTFE composites for microwave substrate applications. J. Polym. Res. 2013, 20, 223. [Google Scholar] [CrossRef]
- Murali, K.; Rajesh, S.; Prakash, O.; Kulkarni, A.; Ratheesh, R. Preparation and properties of silica filled PTFE flexible laminates for microwave circuit applications. Compos. Part A Appl. Sci. Manuf. 2009, 40, 1179–1185. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lin, H.-C.; Lee, Y.-D. The effects of filler content and size on the properties of PTFE/SiO2 composites. J. Polym. Res. 2003, 10, 247–258. [Google Scholar] [CrossRef]
- Xie, C.; Liang, F.; Ma, M.; Chen, X.; Lu, W.; Jia, Y. Microstructure and dielectric properties of PTFE-based composites filled by micron/submicron-blended CCTO. Crystals 2017, 7, 126. [Google Scholar]
- Madusanka, H.; Samarasekara, P.; Fernando, C. Polytetrafluoroethylene bindered hematite (α—Fe2O3) nanostructure for ammonia gas detection at room temperature. Mater. Res. Express 2018, 5, 125002. [Google Scholar] [CrossRef]
- Kang, W.; Li, F.; Zhao, Y.; Qiao, C.; Ju, J.; Cheng, B. Fabrication of porous Fe2O3/PTFE nanofiber membranes and their application as a catalyst for dye degradation. RSC Adv. 2016, 6, 32646–32652. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Fang, X.; Li, Y.-C.; Feng, B.; Wang, H.-X.; Du, K. The mechanical and reaction behavior of PTFE/Al/Fe2O3 under impact and quasi-static compression. Adv. Mater. Sci. Eng. 2017, 2017, 3540320. [Google Scholar] [CrossRef]
- Ai, Z.; Mei, T.; Liu, J.; Li, J.; Jia, F.; Zhang, L.; Qiu, J. Fe@ Fe2O3 core-shell nanowires as an iron reagent. 3. Their combination with CNTs as an effective oxygen-fed gas diffusion electrode in a neutral electro-Fenton system. J. Phys. Chem. C 2007, 111, 14799–14803. [Google Scholar] [CrossRef]
- Singh, K.; Ohlan, A.; Kotnala, R.; Bakhshi, A.; Dhawan, S. Dielectric and magnetic properties of conducting ferromagnetic composite of polyaniline with γ-Fe2O3 nanoparticles. Mater. Chem. Phys. 2008, 112, 651–658. [Google Scholar] [CrossRef]
- Reda, S. electric and dielectric properties of Fe2O3/silica nanocomposites. Int. J. Nano Sci. Technol. 2013, 1, 17–28. [Google Scholar]
- Ul-Haq, Y.; Murtaza, I.; Mazhar, S.; Ullah, R.; Iqbal, M.; Qarni, A.A.; Amin, S. Dielectric, thermal and mechanical properties of hybrid PMMA/RGO/Fe2O3 nanocomposites fabricated by in-situ polymerization. Ceram. Int. 2020, 46, 5828–5840. [Google Scholar] [CrossRef]
- Mensah, E.E.; Abbas, Z.; Ibrahim, N.A.; Khamis, A.M.; Abdalhadi, D.M. Complex permittivity and power loss characteristics of α-Fe2O3/polycaprolactone (PCL) nanocomposites: Effect of recycled α-Fe2O3 nanofiller. Heliyon 2020, 6, e05595. [Google Scholar] [CrossRef]
- Paula, A.L.D.; Rezende, M.C.; Barroso, J.J. Experimental measurements and numerical simulation of permittivity and permeability of Teflon in X band. J. Aerosp. Technol. Manag. 2011, 3, 59–64. [Google Scholar] [CrossRef]
- Mensah, E.E.; Abbas, Z.; Azis, R.a.S.; Khamis, A.M. Enhancement of complex permittivity and attenuation properties of recycled hematite (α-Fe2O3) using nanoparticles prepared via ball milling technique. Materials 2019, 12, 1696. [Google Scholar] [CrossRef]
- Yusoff, A.; Abdullah, M. Microwave electromagnetic and absorption properties of some LiZn ferrites. J. Magn. Magn. Mater. 2004, 269, 271–280. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Wu, H. Enhanced microwave absorption properties of α-Fe2O3-filled ordered mesoporous carbon nanorods. Materials 2013, 6, 1520–1529. [Google Scholar] [CrossRef]
- Hong, Y.-K.; Lee, J. Ferrites for RF passive devices. Solid State Phys. 2013, 64, 237–329. [Google Scholar]
- Ismail, N.Q.A.; Saat, N.K.; Zaid, M.H.M. Effect of soda lime silica glass doping on ZnO varistor ceramics: Dry milling method. J. Asian Ceram. Soc. 2020, 8, 909–914. [Google Scholar] [CrossRef]
- James, J.; Spittle, J.; Brown, S.; Evans, R. A review of measurement techniques for the thermal expansion coefficient of metals and alloys at elevated temperatures. Meas. Sci. Technol. 2001, 12, R1. [Google Scholar] [CrossRef]
- Wool, R. Bio-Based Composites from Soybean Oil and Chicken Feathers; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Shabana, R.; Sarojini, J.; Vikram, K.A.; Lakshmi, V. Evaluating the mechanical properties of commonly used 3D printed ABS and PLA polymers with multi layered polymers. Int. J. Eng. Adv. Technol. 2019, 8, 2351–2356. [Google Scholar]
- Khamis, A.; Abbas, Z.; Ahmad, A.; Azis, R.S.; Abdalhadi, D.; Mensah, E.E. Experimental and Computational Study on Epoxy Resin Reinforced with Micro-Sized OPEFB Using Rectangular Waveguide and Finite Element Method. IET Microw. Antennas Propag. 2020, 14, 752–758. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Kido, H.; Ukita, Y.; Kishihara, M.; Utsumi, Y. Anisotropic pyrochemical microetching of poly (tetrafluoroethylene) initiated by synchrotron radiation-induced scission of molecule bonds. Appl. Phys. Lett. 2016, 108, 051610. [Google Scholar] [CrossRef]
- Wen, B.; Li, J.; Lin, Y.; Liu, X.; Fu, J.; Miao, H.; Zhang, Q. A novel preparation method for γ-Fe2O3 nanoparticles and their characterization. Mater. Chem. Phys. 2011, 128, 35–38. [Google Scholar] [CrossRef]
- Ang, B.; Yaacob, I.; Nurdin, I. Investigation of Fe2O3/SiO2 nanocomposite by FESEM and TEM. J. Nanomater. 2013, 2013, 980390. [Google Scholar] [CrossRef]
- Glaris, P.; Coulon, J.-F.; Dorget, M.; Poncin-Epaillard, F. Thermal annealing as a new simple method for PTFE texturing. Polymer 2013, 54, 5858–5864. [Google Scholar] [CrossRef]
- Abusafieh, A.A.; Federico, D.R.; Connell, S.J.; Cohen, E.J.; Willis, P.B. Dimensional stability of CFRP composites for space-based reflectors. In Proceedings of the Optomechanical Design and Engineering 2001, San Diego, CA, USA, 29 July–3 August 2001; pp. 9–16. [Google Scholar]
- Jahan, M.; Inakpenu, R.O.; Li, K.; Zhao, G. Enhancing the mechanical strength for a microwave absorption composite based on graphene nanoplatelet/epoxy with carbon fibers. Open J. Compos. Mater. 2019, 9, 230. [Google Scholar] [CrossRef][Green Version]
- Jiang, Z.; Yuan, Y. Effects of particle size distribution of silica on properties of PTFE/SiO2 composites. Mater. Res. Express 2018, 5, 066306. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Nemati, A.; Shahraki, A.; Farooghi, M. Densification and properties of Fe2O3 nanoparticles added CaO refractories. Ceram. Int. 2016, 42, 12270–12275. [Google Scholar] [CrossRef]
- Jedrzejewska, A.; Sibera, D.; Narkiewicz, U.; Pełech, R.; Jedrzejewski, R. Effect of Synthesis Parameters of Graphene/Fe2O3 Nanocomposites on Their Structural and Electrical Conductivity Properties. Acta Phys. Pol. A 2017, 132, 1424–1429. [Google Scholar] [CrossRef]
- Gorshkov, N.; Goffman, V.; Vikulova, M.; Burmistrov, I.; Kovnev, A.; Gorokhovsky, A. Dielectric properties of the polymer–matrix composites based on the system of Co-modified potassium titanate–polytetrafluorethylene. J. Compos. Mater. 2018, 52, 135–144. [Google Scholar] [CrossRef]
- Lanza, V.; Herrmann, D. The density dependence of the dielectric constant of polyethylene. J. Polym. Sci. 1958, 28, 622–625. [Google Scholar] [CrossRef]
- Leyland, R.; Maharaj, A. Dielectric constant as a means of assessing the properties of road construction materials. SATC 2010, 2010, 487–498. [Google Scholar]
- Fahad Ahmad, A.; Aziz, S.H.A.; Abbas, Z.; Mohammad Abdalhadi, D.; Khamis, A.M.; Aliyu, U.S.A. Computational and Experimental Approaches for Determining Scattering Parameters of OPEFB/PLA Composites to Calculate the Absorption and Attenuation Values at Microwave Frequencies. Polymers 2020, 12, 1919. [Google Scholar] [CrossRef] [PubMed]
- Przybyszewska, M.; Zaborski, M. The effect of zinc oxide nanoparticle morphology on activity in crosslinking of carboxylated nitrile elastomer. Express Polym. Lett 2009, 3, 542–552. [Google Scholar] [CrossRef]
- Mandal, S.; Singh, S.; Dey, P.; Roy, J.; Mandal, P.; Nath, T. Frequency and temperature dependence of dielectric and electrical properties of TFe2O4 (T=Ni, Zn, Zn0.5Ni0.5) ferrite nanocrystals. J. Alloys Compd. 2016, 656, 887–896. [Google Scholar] [CrossRef]
- Ahmad, A.F.; Ab Aziz, S.; Abbas, Z.; Obaiys, S.J.; Khamis, A.M.; Hussain, I.R.; Zaid, M.H.M. Preparation of a chemically reduced graphene oxide reinforced epoxy resin polymer as a composite for electromagnetic interference shielding and microwave-absorbing applications. Polymers 2018, 10, 1180. [Google Scholar] [CrossRef]
- Giacometti, J.A.; Wisniewski, C.; Ribeiro, P.A.; Moura, W.A. Electric measurements with constant current: A practical method for characterizing dielectric films. Rev. Sci. Instrum. 2001, 72, 4223–4227. [Google Scholar] [CrossRef][Green Version]
- Huashen, W.; Shan, J.; Guodong, W.; Ke, X. Electromagnetic parameters test system based on a refined NRW transmission/reflection algorithm. In Proceedings of the 2007 International Symposium on Microwave, Antenna, Propagation and EMC Technologies for Wireless Communications, Hangzhou, China, 16–17 August 2007; pp. 1276–1280. [Google Scholar]
- Abdalhadi, D.M.; Abbas, Z.; Ahmad, A.F.; Matori, K.A.; Esa, F. Controlling the properties of OPEFB/PLA polymer composite by using Fe2O3 for microwave applications. Fibers Polym. 2018, 19, 1513–1521. [Google Scholar] [CrossRef]
- Ahmad, A.F.; Abbas, Z.; Obaiys, S.J.; Abdalhadi, D.M. Improvement of dielectric, magnetic and thermal properties of OPEFB fibre–polycaprolactone composite by adding Ni–Zn ferrite. Polymers 2017, 9, 12. [Google Scholar] [CrossRef]
- Barba, A.; Clausell, C.; Jarque, J.; Nuño, L. Magnetic complex permeability (imaginary part) dependence on the microstructure of a Cu-doped Ni–Zn-polycrystalline sintered ferrite. Ceram. Int. 2020, 46, 14558–14566. [Google Scholar] [CrossRef]
- Luo, J.; Shen, P.; Yao, W.; Jiang, C.; Xu, J. Synthesis, characterization, and microwave absorption properties of reduced graphene oxide/strontium ferrite/polyaniline nanocomposites. Nanoscale Res. Lett. 2016, 11, 141. [Google Scholar] [CrossRef]
- Li, W.; Qiu, T.; Wang, L.; Ren, S.; Zhang, J.; He, L.; Li, X. Preparation and electromagnetic properties of core/shell polystyrene@ polypyrrole@ nickel composite microspheres. ACS Appl. Mater. Interfaces 2013, 5, 883–891. [Google Scholar] [CrossRef]
- Liu, P.; Huang, Y.; Yan, J.; Zhao, Y. Magnetic graphene@ PANI@ porous TiO2 ternary composites for high-performance electromagnetic wave absorption. J. Mater. Chem. C 2016, 4, 6362–6370. [Google Scholar] [CrossRef]
- Li, C.; Ji, S.; Jiang, X.; Waterhouse, G.I.; Zhang, Z.; Yu, L. Microwave absorption by watermelon-like microspheres composed of γ-Fe2O3, microporous silica and polypyrrole. J. Mater. Sci. 2018, 53, 9635–9649. [Google Scholar] [CrossRef]
- Liang, K.; Qiao, X.-J.; Sun, Z.-G.; Guo, X.-D.; Wei, L.; Qu, Y. Preparation and microwave absorbing properties of graphene oxides/ferrite composites. Appl. Phys. A 2017, 123, 445. [Google Scholar] [CrossRef]










| Composite Code | Recycled Fe2O3 Nano-Powder | PTFE | Total Mass (g) | ||
|---|---|---|---|---|---|
| Percentage % | Mass (g) | Percentage % | Mass (g) | ||
| Control | 0 | 0 | 100 | 60 | 60 |
| A | 5 | 3 | 95 | 57 | 60 |
| B | 10 | 6 | 90 | 54 | 60 |
| C | 15 | 9 | 85 | 51 | 60 |
| D | 20 | 12 | 80 | 48 | 60 |
| E | 25 | 15 | 75 | 45 | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khamis, A.M.; Abbas, Z.; Azis, R.S.; Mensah, E.E.; Alhaji, I.A. Effects of Recycled Fe2O3 Nanofiller on the Structural, Thermal, Mechanical, Dielectric, and Magnetic Properties of PTFE Matrix. Polymers 2021, 13, 2332. https://doi.org/10.3390/polym13142332
Khamis AM, Abbas Z, Azis RS, Mensah EE, Alhaji IA. Effects of Recycled Fe2O3 Nanofiller on the Structural, Thermal, Mechanical, Dielectric, and Magnetic Properties of PTFE Matrix. Polymers. 2021; 13(14):2332. https://doi.org/10.3390/polym13142332
Chicago/Turabian StyleKhamis, Ahmad Mamoun, Zulkifly Abbas, Raba’ah Syahidah Azis, Ebenezer Ekow Mensah, and Ibrahim Abubakar Alhaji. 2021. "Effects of Recycled Fe2O3 Nanofiller on the Structural, Thermal, Mechanical, Dielectric, and Magnetic Properties of PTFE Matrix" Polymers 13, no. 14: 2332. https://doi.org/10.3390/polym13142332
APA StyleKhamis, A. M., Abbas, Z., Azis, R. S., Mensah, E. E., & Alhaji, I. A. (2021). Effects of Recycled Fe2O3 Nanofiller on the Structural, Thermal, Mechanical, Dielectric, and Magnetic Properties of PTFE Matrix. Polymers, 13(14), 2332. https://doi.org/10.3390/polym13142332






