Effects of Repulsion Parameter and Chain Length of Homopolymers on Interfacial Properties of An/Ax/2BxAx/2/Bm Blends: A DPD Simulation Study
Abstract
:1. Introduction
2. Methods
2.1. Model
2.2. Simulation Details
3. Results and Discussion
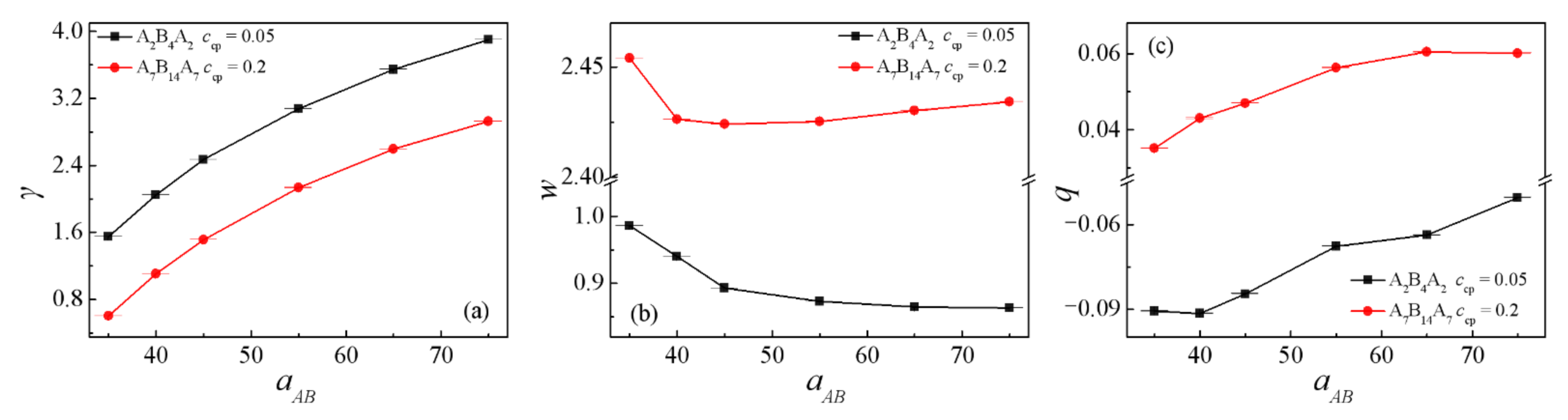
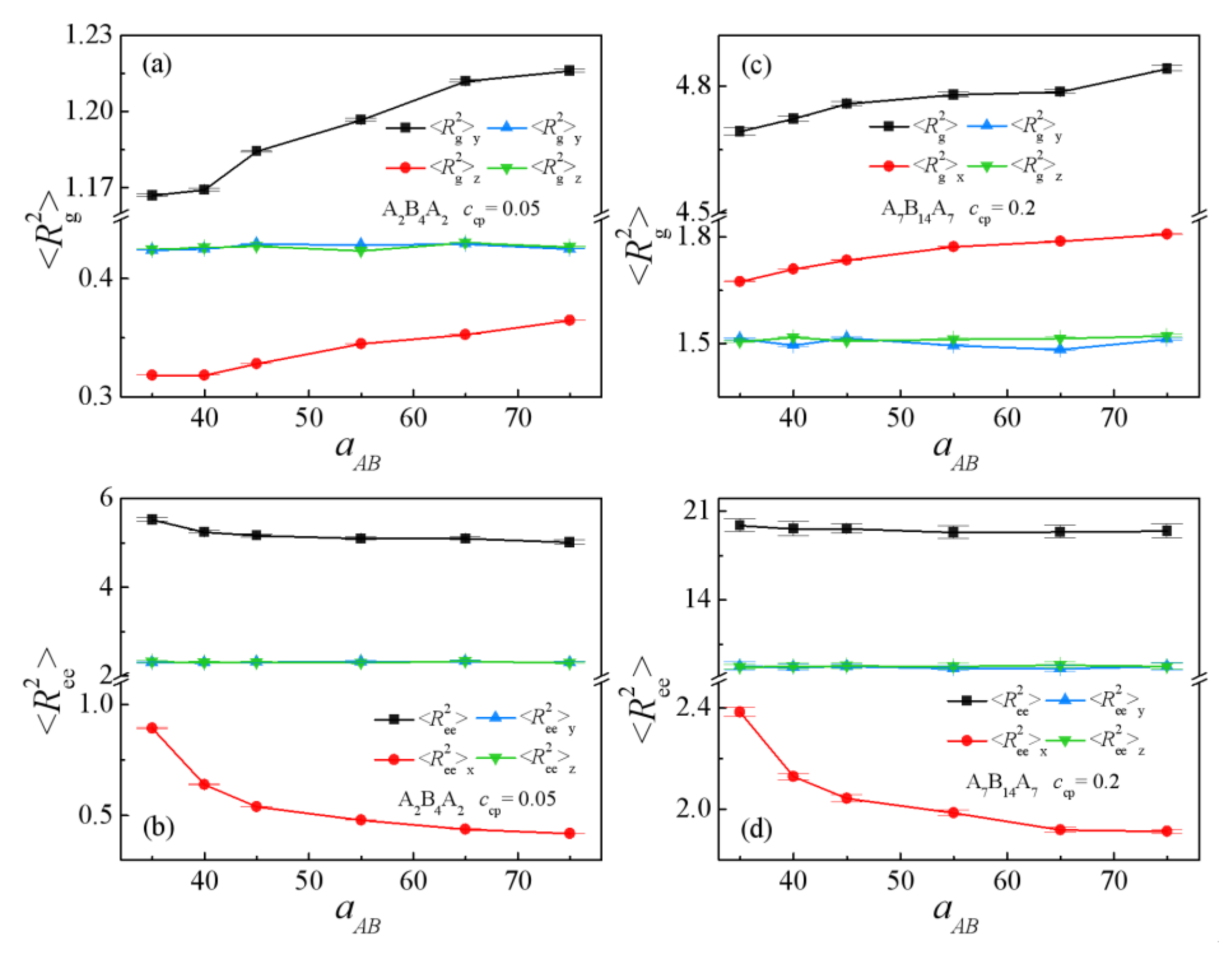
3.1. Effect of the Repulsion Parameters
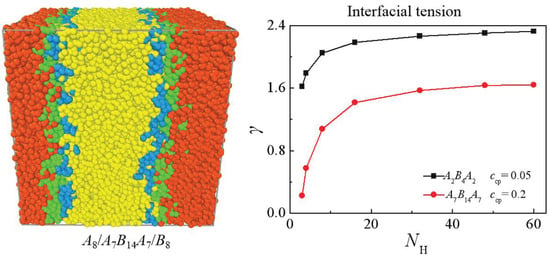
3.2. Effect of Chain Length of Homopolymers
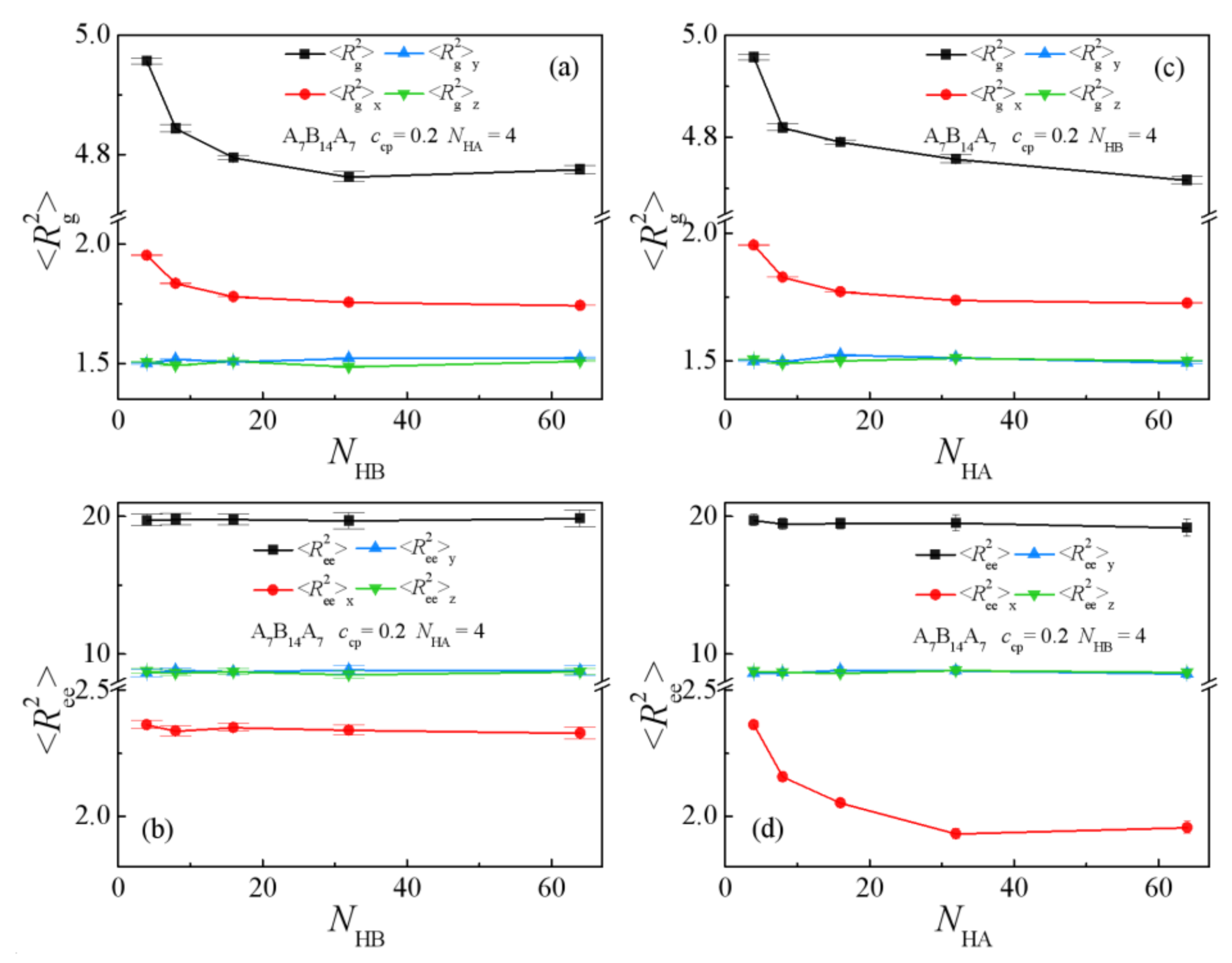
3.2.1. Symmetric Homopolymers with NHA = NHB
3.2.2. The Chain Length Effect of Single Homopolymer Component
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, H.X.; Cruz, M.O. A computer simulation study of the segregation of amphiphiles in binary immiscible matrices: Short asymmetric copolymers in short homopolymers. J. Chem. Phys. 2005, 123, 174903. [Google Scholar] [CrossRef]
- Anastasiadis, S.H. Interfacial tension in binary polymer blends and the effects of copolymers as emulsifying agents. Adv. Polym. Sci. 2011, 238, 179–269. [Google Scholar]
- Yu, Q.Y.; Zhang, C.L.; Gu, X.P.; Wang, J.J.; Feng, L.F. Compatibilizing efficiency of copolymer precursors for immiscible polymer blends. J. Appl. Poly. Sci. 2012, 124, 3392–3398. [Google Scholar] [CrossRef]
- Chang, K.; Macosko, C.W.; Morse, D.C. Ultralow interfacial tensions of polymer/polymer interfaces with diblock copolymer surfactants. Macromolecules 2007, 40, 3819–3830. [Google Scholar] [CrossRef]
- Retsos, H.; Margiolaki, A.; Anastasiadis, S.H. Interfacial tension in binary polymer blends in the presence of block copolymers: Effects of additive MW. Macromolecules 2001, 34, 5295–5305. [Google Scholar] [CrossRef]
- Retsos, H.; Anastasiadis, S.H.; Pispas, S.; Mays, J.W.; Hadjichristidis, N. Interfacial tension in binary polymer blends in the presence of block copolymers. 2. effects of additive architecture and composition. Macromolecules 2004, 37, 524–537. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Huang, M.X.; Lu, T.; Guo, H.X. Nanorods with different surface properties in directing the compatibilization behavior and the morphological transition of immiscible polymer blends in both shear and shear-free conditions. Macromolecules 2018, 51, 3135–3148. [Google Scholar] [CrossRef]
- Qian, H.J.; Lu, Z.Y.; Chen, L.J.; Li, Z.S.; Sun, C.C. Dissipative particle dynamics study on the interfaces in incompatible A/B homopolymer blends and with their block copolymers. J. Chem. Phys. 2005, 122, 187907. [Google Scholar] [CrossRef]
- Lemos, T.; Abreu, C.; Pinto, J.C. DPD simulations of homopolymer-copolymer-homopolymer mixtures: Effects of copolymer structure and concentration. Macromol. Theory Simul. 2020, 29, 2000014. [Google Scholar] [CrossRef]
- Balazs, A.C.; Siemasko, C.P.; Lantman, C.W. Monte-Carlo simulations for the behavior of multiblock copolymers at a penetrable interface. J. Chem. Phys. 1991, 94, 1653–1663. [Google Scholar] [CrossRef]
- Wang, Y.M.; Mattice, W.L. Simulation of the adsorption of symmetric diblock copolymers at the interface of the two monomeric homopolymers. J. Chem. Phys. 1993, 98, 9881–9887. [Google Scholar] [CrossRef]
- Wang, Y.M.; Mattice, W.L. Simulation of the adsorption of unsymmetric diblock copolymers at the interface between the two monomeric homopolymers. J. Chem. Phys. 1993, 99, 4068–4075. [Google Scholar] [CrossRef]
- Schmid, F.; Müller, M. Quantitative comparison of self-consistent-field theories for polymers near interfaces with monte-carlo simulations. Macromolecules 1995, 28, 8639–8645. [Google Scholar] [CrossRef]
- Müller, M.; Schick, M. Bulk and interfacial thermodynamics of a symmetric, ternary homopolymer-copolymer mixture: A Monte Carlo study. J. Chem. Phys. 1996, 105, 8885–8901. [Google Scholar] [CrossRef]
- Werner, A.; Schmid, F.; Binder, K.; Müller, M. Diblock copolymers at a homopolymer-homopolymer interface: A Monte Carlo simulation. Macromolecules 1996, 29, 8241–8248. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.M.; Duan, X.Z.; Shi, T.F.; Jiang, F.; Zhang, H.Z. Monte carlo simulation of effects of homopolymer chain length on interfacial properties of A/AB/B ternary polymer blends. Chem. J. Chin. Univ. Chin. 2015, 36, 2532–2539. [Google Scholar]
- Liu, D.M.; Dai, L.J.; Duan, X.Z.; Shi, T.F.; Zhang, H.Z. Monte Carlo Simulation of Interfacial Properties in Homopolymer/Diblock Copolymer/Homopolymer Ternary Polymer Blends. Chem. J. chin. Univ. Chin. 2015, 36, 1752–1758. [Google Scholar]
- Wanakule, N.S.; Nedoma, A.J.; Robertson, M.L.; Fang, Z.; Jackson, A.; Garetz, B.A.; Balsara, N.P. Characterization of micron-sized periodic structures in multicomponent polymer blends by ultra-small-angle neutron scattering and optical microscopo. Macromolecules 2008, 41, 471–477. [Google Scholar] [CrossRef]
- Garnier, S.; Laschewsky, A. New amphiphilic diblock copolymers: Surfactant properties and solubilization in their micelles. Langmuir 2006, 22, 4044–4053. [Google Scholar] [CrossRef]
- Fortelny, L.; Juza, J. Analysis of the effect of block copolymers on interfacial tension in immiscible polymer blends. Polymer 2018, 150, 380–390. [Google Scholar] [CrossRef]
- Goodson, A.D.; Liu, G.L.; Rick, M.S.; Raymond, A.W.; Uddin, M.F.; Ashbaugh, H.S.; Albert, J.N.L. Nanostructure stability and swelling of ternary block copolymer/homopolymer blends: A direct comparison between dissipative particle dynamics and experiment. J. Polym. Sci. Pt. B Polym. Phys. 2019, 57, 794–803. [Google Scholar] [CrossRef]
- Russell, T.P.; Mayes, A.M.; Deline, V.R.; Chung, T.C. Hairpin configurations of triblock copolymers at homopolymer interfaces. Macromolecules 1992, 25, 5783–5789. [Google Scholar] [CrossRef]
- Dai, K.D.; Washiyama, J.; Kramer, E.J. Segregation study of a BAB triblock copolymer at the A/B homopolymer interface. Macromolecules 1994, 27, 4544–4553. [Google Scholar] [CrossRef]
- Wagner, M.; Wolf, B.A. Effect of block copolymers on the interfacial-tension between 2. Immiscible/homopolymers. Polymer 1993, 34, 1460–1464. [Google Scholar] [CrossRef]
- Jorzik, U.; Wagner, M.; Wolf, B.A. Effect of block copolymer architecture on the interfacial tension between immiscible polymers. Prog. Colloid. Polym. Sci. 1996, 101, 170–171. [Google Scholar]
- Xu, Y.; Thurber, C.M.; Macosko, C.W.; Lodge, T.P.; Hillmyer, M.A. Poly(methyl methacrylate) -block -polyethylene -block -poly(methyl methacrylate) Triblock Copolymers as Compatibilizers for Polyethylene/Poly(methyl methacrylate) Blends. Ind. Eng. Chem. Res. 2014, 53, 4718–4725. [Google Scholar] [CrossRef]
- Sun, Z.Q.; Zhang, B.; Bian, X.C. Synergistic effect of PLA-PBAT-PLA tri-block copolymers with two molecular weights as compatibilizers on the mechanical and rheological properties of PLA/PBAT blends. RSC Adv. 2015, 5, 73842–73849. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Huang, Y.J.; Kong, M.Q.; Li, G.X. Assessment of compatibilization efficiency of SEBS in the PP/PS blend. J. Appl. Polym. Sci. 2018, 135, 46244. [Google Scholar] [CrossRef]
- Liu, D.M.; Gong, K.; Lin, Y.; Liu, T.; Liu, Y.; Duan, X.Z. Dissipative Particle Dynamics Study on Interfacial Properties of Symmetric Ternary Polymeric Blends. Polymers. 2021, 13, 1516. [Google Scholar] [CrossRef]
- Zhu, P.F.; Li, Y.; Li, Q.W. Mesoscopic simulation of the interfacial behavior of biosurfactant rhamnolipids and the synergistic systems. Acta. Chim. Sin. 2011, 69, 2420–2426. [Google Scholar]
- Catarino, C.R.; Bustamante-Rendon, R.A.; Hernandez–Fragoso, J.S. Surfactant chain length and concentration influence on the interfacial tension of two immiscible model liquids: A coarse-grained approach. J. Mol. Model. 2017, 23, 306. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Xu, J.B.; He, X.F. Effect of surfactants on the deformation of single droplet in shear flow studied by dissipative particle dynamics. Mol. Phys. 2018, 116, 1851–1861. [Google Scholar] [CrossRef]
- Liang, X.P.; Wu, J.Q.; Yang, X.G. Investigation of oil-in-water emulsion stability with relevant interfacial characteristics simulated by dissipative particle dynamics. Colloid surf. A-Physicochem. Eng. Asp. 2018, 546, 107–114. [Google Scholar] [CrossRef]
- Wang, S.Y.; Yang, S.W.; Wang, R.C. Dissipative particle dynamics study on the temperature dependent interfacial tension in surfactant-oil-water mixtures. J. Pet. Sci. Eng. 2018, 169, 81–95. [Google Scholar] [CrossRef]
- Goodarzi, F.; Zendehboudi, S. Effects of salt and surfactant on interfacial characteristics of water/oil systems: Molecular dynamic simulations and dissipative particle dynamics. Ind. Eng. Chem. Res. 2019, 58, 8817–8834. [Google Scholar] [CrossRef] [Green Version]
- Goodarzi, F.; Kondori, J.; Rezaei, N. Meso- and molecular-scale modeling to provide new insights into interfacial and structural properties of hydrocarbon/water/surfactant systems. J. Mol. Liq. 2019, 295, 111357. [Google Scholar] [CrossRef]
- Li, B.Y.; Zhao, L.; Lu, Z.Y. Microscopic characteristics of janus nanoparticles prepared via a grafting-from reaction at the immiscible liquid interface. Phys. Chem. Chem. Phys. 2020, 22, 5347–5354. [Google Scholar] [CrossRef]
- Schlijper, A.G.; Hoogerbrugge, P.J.; Manke, C.W. Computer-simulation of dilute polymer-solutions with the dissipative particle dynamics method. J. Rheol. 1995, 39, 567–579. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Boker, A.; He, J.; Sill, K.; Xiang, H.; Abetz, C.; Li, X.; Wang, J.; Emrick, T.; Long, S.; et al. Self-directed self-assembly of nanoparticle/copolymer mixtures. Nature 2005, 434, 55–59. [Google Scholar] [CrossRef]
- Hong, Z.H.; Xiao, N.; Li, L.; Xie, X.N. Investigation of nanoemulsion interfacial properties: A mesoscopic simulation. J. Food Eng. 2020, 276, 109877. [Google Scholar] [CrossRef]
- Zhang, J.W.; Chen, L.; Wang, A.L.; Yan, Z.C. Dissipative particle dynamics simulation of ionic liquid-based microemulsion: Quantitative properties and emulsification mechanism. Ind. Eng. Chem. Res. 2020, 59, 763–773. [Google Scholar] [CrossRef]
- Groot, R.D.; Warren, P.B. Dissipative particle dynamics: Bridging the gap between atomistic and mesoscopic simulation. J. Chem. Phys. 1997, 107, 4423–4435. [Google Scholar] [CrossRef]
- Zhou, Y.; Long, X.P.; Zeng, Q.X. Simulation studies of the interfaces of incompatible glycidyl azide polymer/hydroxyl-terminated polybutadiene blends by dissipative particle dynamics. I. The effect of block copolymers and plasticizers. J. Appl. Poly. Sci. 2012, 125, 1530–1537. [Google Scholar] [CrossRef]
- Irving, J.H.; Kirkwood, J.G. The statistical mechanical theory of transport processes.4. the equations of hydrodynamics. J. Chem. Phys. 1950, 18, 817–829. [Google Scholar] [CrossRef]
- Helfand, E.; Tagami, Y. Theory of the Interface Between Immiscible Polymers. J. Chem. Phys. 1972, 57, 3592–3601. [Google Scholar] [CrossRef]
- Liu, B.L.; Hu, B.; Du, J.; Cheng, D.M.; Zang, H.Y.; Ge, X.; Tan, H.Q.; Wang, Y.H.; Duan, X.Z.; Jin, Z.; et al. Precise Molecular-Level Modification of Nafion with Bismuth Oxide Clusters for High-performance Proton-Exchange Membranes. Angew. Chem. Int. Edit. 2021, 60, 6076–6085. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Gong, K.; Lin, Y.; Bo, H.; Liu, T.; Duan, X. Effects of Repulsion Parameter and Chain Length of Homopolymers on Interfacial Properties of An/Ax/2BxAx/2/Bm Blends: A DPD Simulation Study. Polymers 2021, 13, 2333. https://doi.org/10.3390/polym13142333
Liu D, Gong K, Lin Y, Bo H, Liu T, Duan X. Effects of Repulsion Parameter and Chain Length of Homopolymers on Interfacial Properties of An/Ax/2BxAx/2/Bm Blends: A DPD Simulation Study. Polymers. 2021; 13(14):2333. https://doi.org/10.3390/polym13142333
Chicago/Turabian StyleLiu, Dongmei, Kai Gong, Ye Lin, Huifeng Bo, Tao Liu, and Xiaozheng Duan. 2021. "Effects of Repulsion Parameter and Chain Length of Homopolymers on Interfacial Properties of An/Ax/2BxAx/2/Bm Blends: A DPD Simulation Study" Polymers 13, no. 14: 2333. https://doi.org/10.3390/polym13142333
APA StyleLiu, D., Gong, K., Lin, Y., Bo, H., Liu, T., & Duan, X. (2021). Effects of Repulsion Parameter and Chain Length of Homopolymers on Interfacial Properties of An/Ax/2BxAx/2/Bm Blends: A DPD Simulation Study. Polymers, 13(14), 2333. https://doi.org/10.3390/polym13142333