Physicochemical, Bacteriostatic, and Biological Properties of Starch/Chitosan Polymer Composites Modified by Graphene Oxide, Designed as New Bionanomaterials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Composites and Nanocomposites
- Control Composite (Composite C)
- Nanocomposite I
- Nanocomposite II
2.3. Dynamic Light Scattering (DLS) Measurements of Zeta Potential and Particle/Aggregate Sizes
2.4. Wettability and Free Surface Energy Determination
2.5. FTIR-ATR Spectrophotometry of Composites
2.6. Thermal Analysis of Composites
2.7. Surface Colour Measurements
2.8. Thickness Measurement
2.9. Transparency of Composites
2.10. UV–Vis Absorption Spectrophotometry
2.11. Scanning (SEM) and Transmission Electron Microscopy (TEM)
2.12. Water Content, Solubility, and Swelling Degree of Composites
2.13. Mechanical Properties of Composites
2.14. Cytotoxicity Analysis
2.14.1. Cell Culture Experiments
2.14.2. Cell Viability Assessment
2.15. Microbiology
- -
- Staphylococcus aureus ATCC 25923
- -
- Pseudomonas aeruginosa ATCC 27853
- -
- Enterococcus faecalis ATCC 29212
- -
- Escherichia coli ATCC 8739
- -
- Proteus mirabilis NCTC 11938
2.16. Statistical Analysis
3. Results and Discussion
3.1. Zeta Potential and the Particle Size of Composites
3.2. Contact Angle and Surface Free Energy
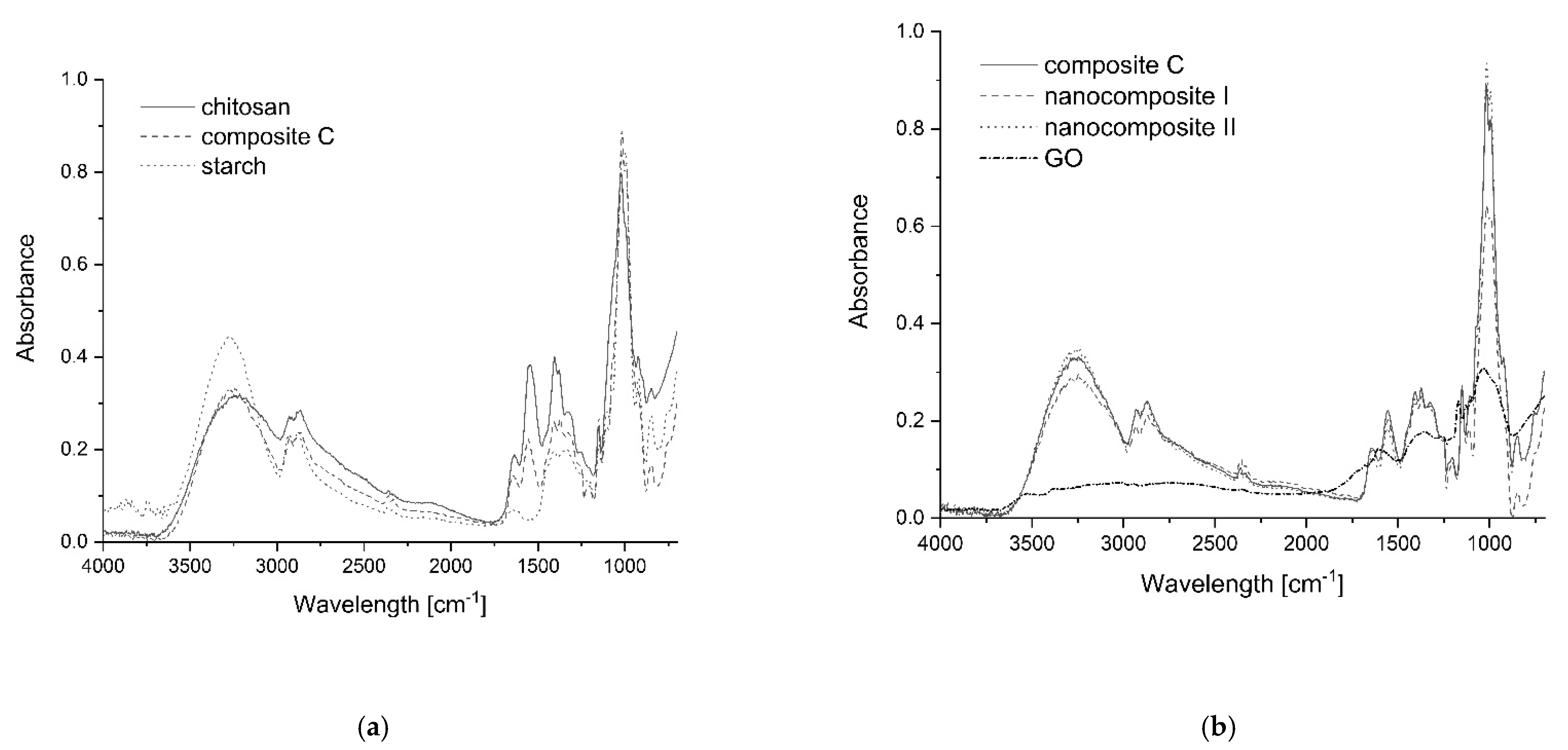
3.3. FTIR-ATR Spectrophotometry of Composites
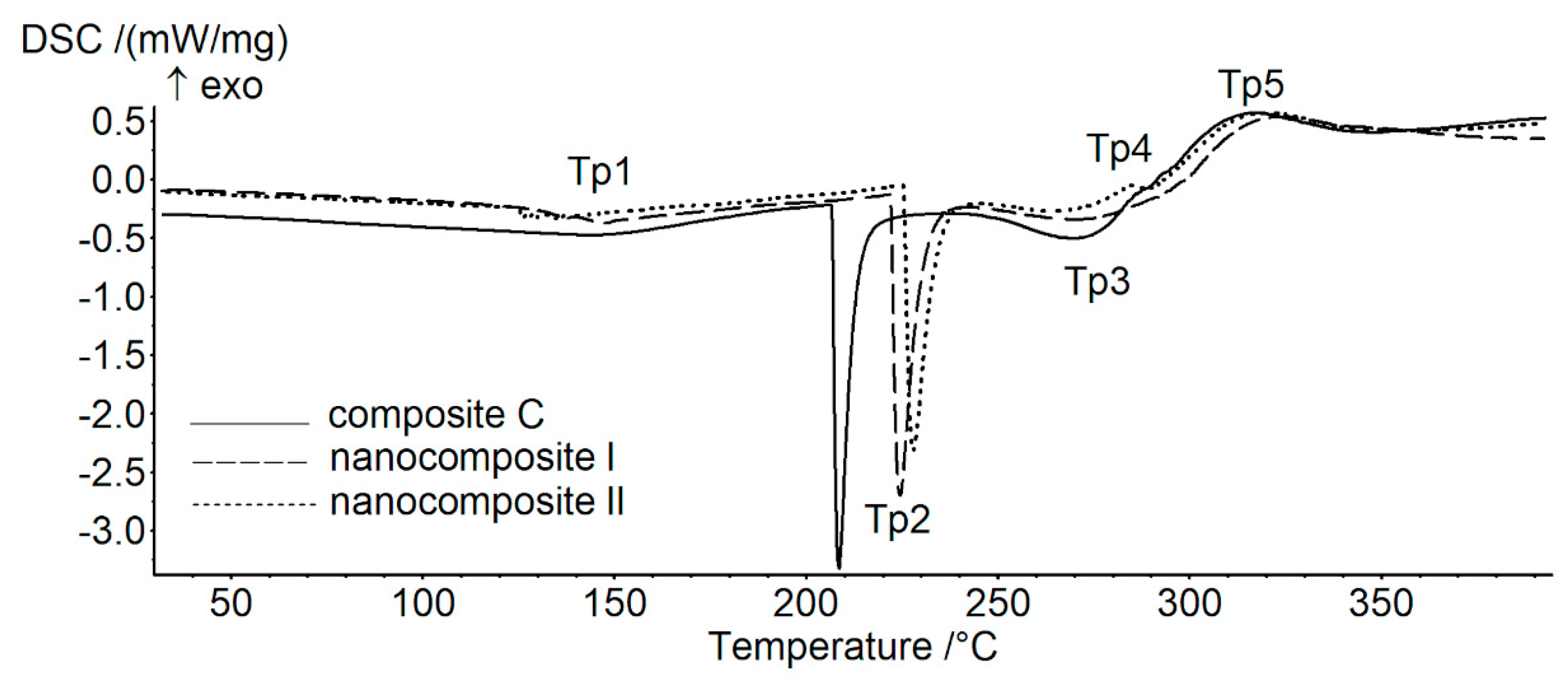
3.4. Thermal Analysis of Composites
3.5. Surface Colour and Transparency
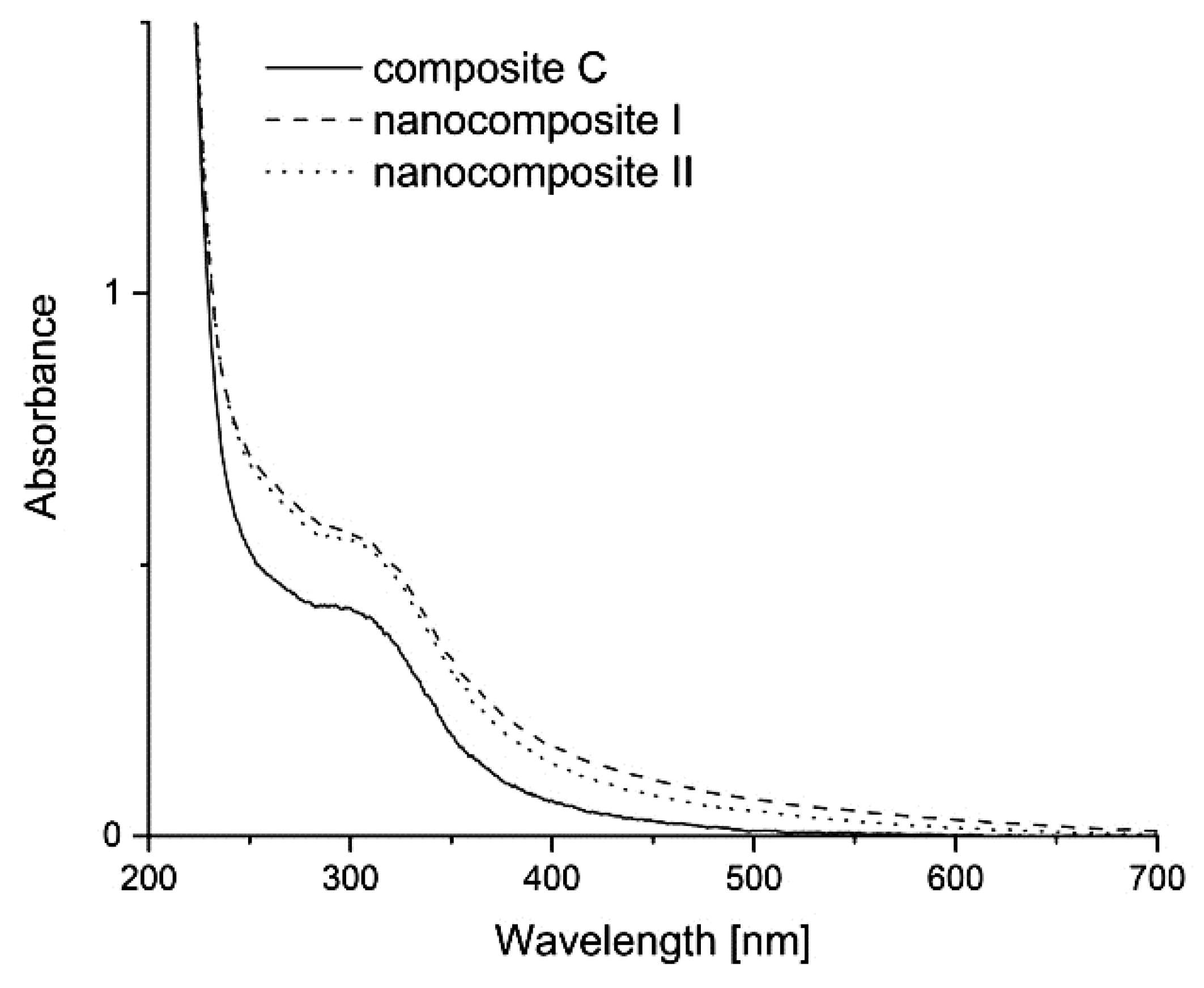
3.6. UV–Vis Absorption Spectra
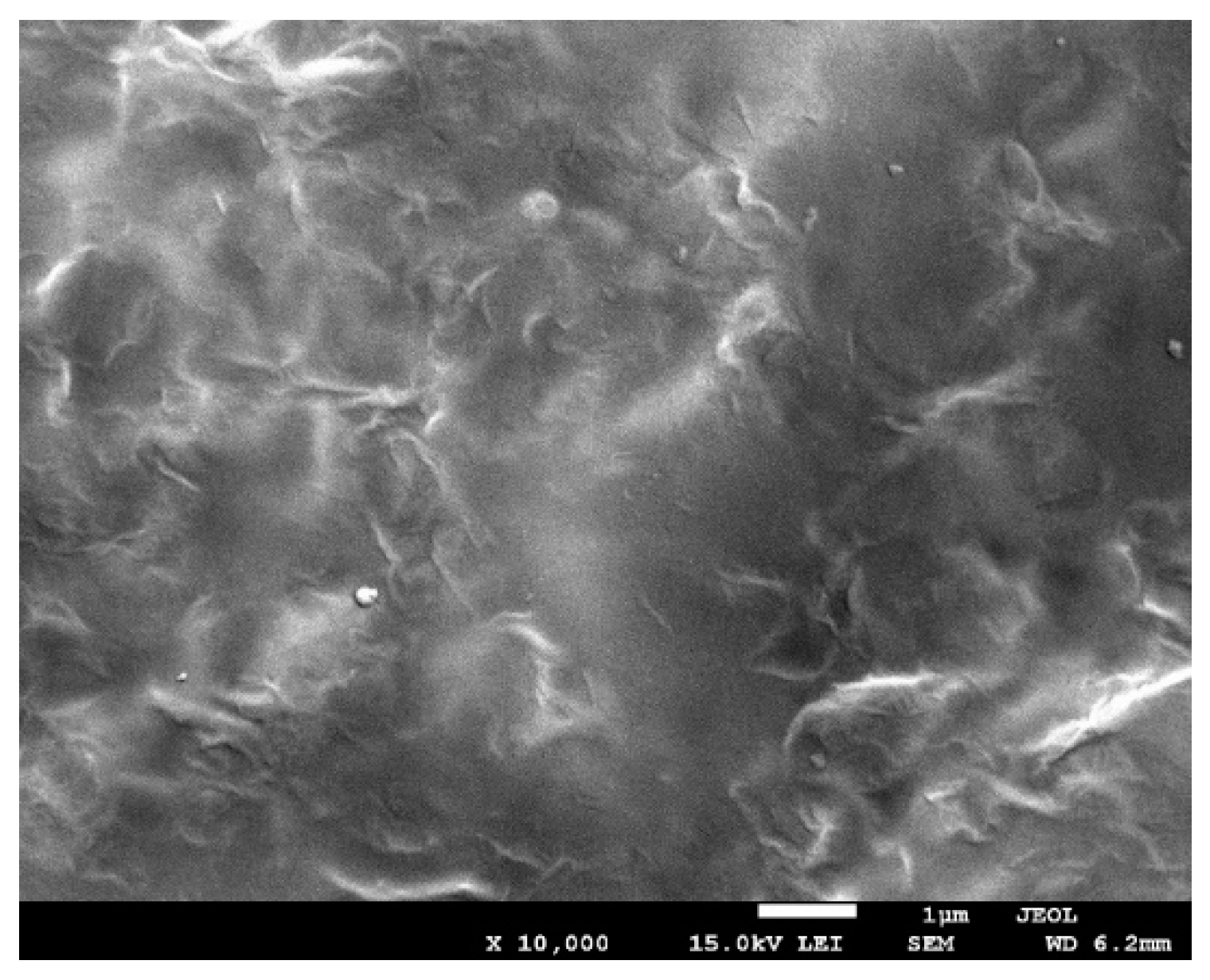
3.7. Scanning and Transmission Electron Microscopy
3.8. Water Content, Solubility, and Degree of Swelling of Composites
3.9. Mechanical Properties of Composites
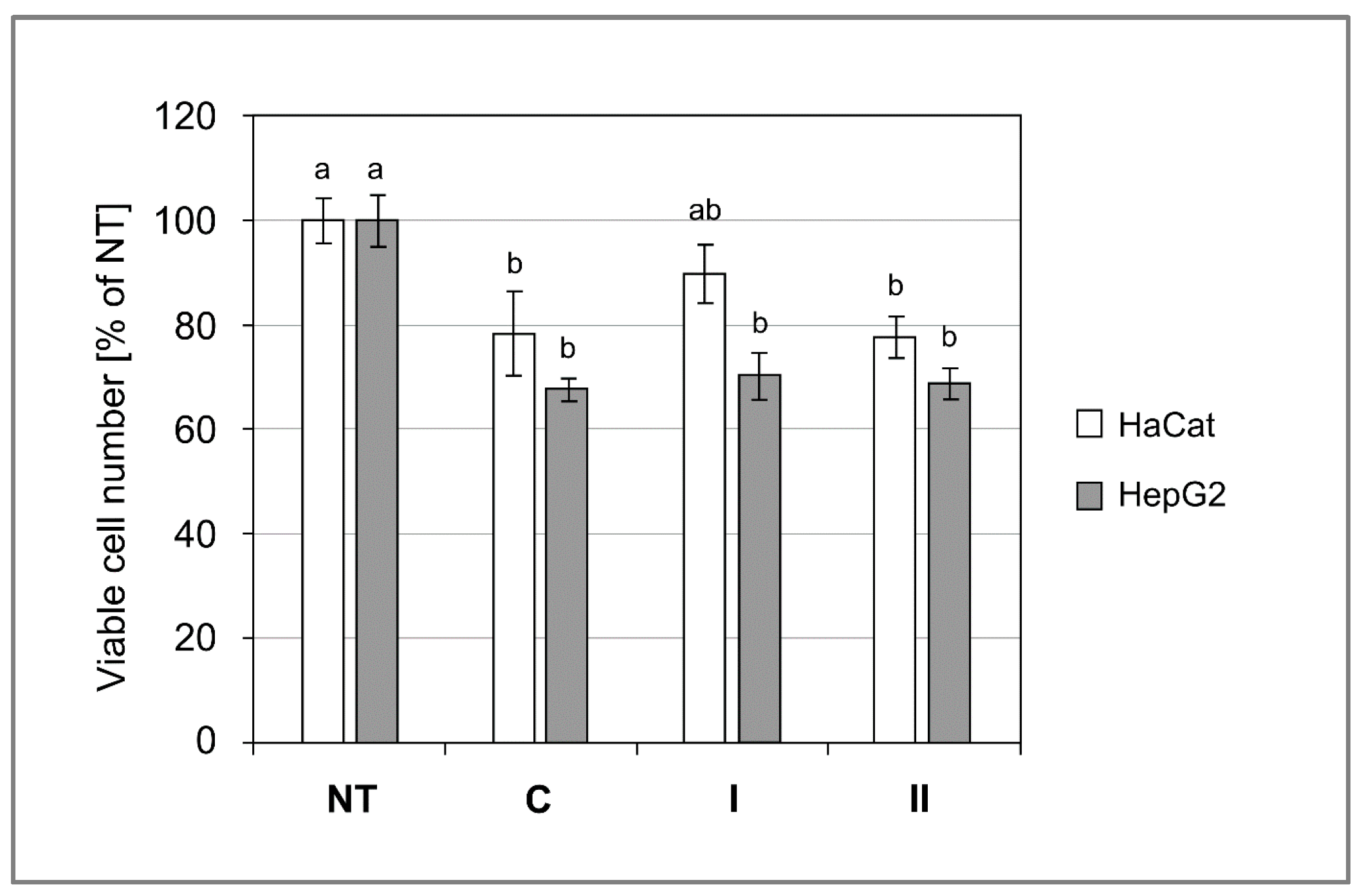
3.10. Cytotoxicity of Composites
3.11. Antimicrobial Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Parisi, C.; Vigani, M.; Rodríguez-Cerezo, E. Agricultural nanotechnologies: What are the current possibilities? Nano Today 2015, 10, 124–127. [Google Scholar] [CrossRef]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in food science: Applications, recent trends, and future perspectives. Nano-MicroLetters 2020, 12, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, A.P.A.; Conte Junior, C.A. Green strategies for active food packagings: A systematic review on active properties of graphene-based nanomaterials and biodegradable polymers. Trends Food Sci. Technol. 2020, 103, 130–143. [Google Scholar] [CrossRef]
- Arfin, T. Chitosan and its derivatives: Overview of commercial applications in diverse fields. In Chitosan: Derivatives, Composites and Applications; Shakeel Ahmed, A., Ikram, S., Eds.; Wiley-Scrivener: Hoboken, NJ, USA, 2017; pp. 115–146. [Google Scholar]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Khachatryan, G.; Khachatryan, K.; Krystyjan, M.; Pardus, L.; Bebak, E.; Grzyb, J. Formation and properties of chitosan/nanosilver bionanocomposite. In Proceedings of the 14th International Conference on Polysaccharides—Glycoscience, Prague, Czech Republic, 7–9 November 2018; Řápková, R., Hinková, A., Čopíková, J., Eds.; Czech Chemical Society: Prague, Czech Republic, 2018; pp. 191–195, ISBN 978-80-86238-80-7. [Google Scholar]
- Möller, H.; Grelier, S.; Pardon, P.; Coma, V. Antimicrobial and Physicochemical Properties of Chitosan−HPMC-Based Films. J. Agric. Food Chem. 2004, 52, 6585–6591. [Google Scholar] [CrossRef]
- Krystyjan, M.; Khachatryan, G.; Ciesielski, W.; Buksa, K.; Sikora, M. Preparation and characteristics of mechanical and functional properties of starch/Plantago psyllium seeds mucilage films. Starch/Staerke 2017, 69, 1700014. [Google Scholar] [CrossRef]
- Khachatryan, G.; Khachatryan, K. Starch based nanocomposites as sensors for heavy metals—Detection of Cu2+ and Pb2+ ions. Int. Agrophys. 2019, 33, 121–126. [Google Scholar] [CrossRef]
- Chen, P.; Xie, F.; Tang, F.; McNally, T. Graphene oxide enhanced ionic liquid plasticisation of chitosan/alginate bionanocomposites. Carbohydr. Polym. 2021, 253, 117231. [Google Scholar] [CrossRef]
- Hossain, K.; Rafatullah, M.; Abbas, S.Z.; Ahmad, A.; Ismail, N.; Maruthi, A.Y. Chapter 16—Antimicrobial activity of graphene-based nanomaterials: Current development and challenges. In Graphenebased Nanotechnologies for Energy and Environmental Applications; Jawaid, M., Ahmad, A., Lokhat, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 293–314. [Google Scholar] [CrossRef]
- Travlou, N.A.; Kyzas, G.Z.; Lazaridis, N.K.; Deliyanni, E.A. Graphite oxide/chitosan composite for reactive dye removal. Chem. Eng. J. 2013, 217, 256–265. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Deliyanni, E.A.; Matis, K.A. Graphene oxide and its application as an adsorbent for wastewater treatment. J. Chem. Technol. Biotechnol. 2014, 89, 196–205. [Google Scholar] [CrossRef]
- Ramanathan, T.; Adbala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.; Ruoff, R.S.; et al. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef]
- Frindy, S.; Primo, A.; Ennajih, H.; Qaiss, A.; Bouhfid, R.; Lahcini, M.; Essassi, E.; Garcia, H.; Kadib, A. Chitosan–graphene oxide films and CO2-dried porous aerogel microspheres: Interfacial interplay and stability. Carbohydr. Polym. 2017, 167, 297–305. [Google Scholar] [CrossRef]
- Yousefi, M.; Dadashpour, M.; Hejazi, M.; Hasanzadeh, M.; Behnam, B.; de la Guardia, M. Antibacterial activity of graphene oxide as a new weapon nanomaterial to combat multidrug-resistance bacteria. Mater. Sci. Eng. C 2017, 74, 568–581. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Wang, W.; Wang, W.; Chen, X.; Wu, Y.; Dong, L. Synthesis and characterization of Ag/graphene nano-composite. Rare Met. Mater. Eng. 2015, 44, 2138–2142. [Google Scholar] [CrossRef]
- Nowak, N.; Grzebieniarz, W.; Khachatryan, G.; Khachatryan, K.; Konieczna-Molenda, A.; Krzan, M.; Grzyb, J. Synthesis of Silver and Gold Nanoparticles in Sodium Alginate Matrix Enriched with Graphene Oxide and Investigation of Properties of the Obtained Thin Films. Appl. Sci. 2021, 11, 3857. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Jafari, S.M. Bio-nanocomposites of graphene with biopolymers; fabrication, properties, and applications. Adv. Colloid Interface Sci. 2021, 292, 102416. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, E.; Khachatryan, G.; Kopel, P.; Juszczak, L.; Kawecka, A.; Krzyściak Kucharek, M.; Bębenek, Z.; Zimowska, M. Furcellaran nanocomposite films: The effect of nanofillers on the structural, thermal, mechanical and antimicrobial properties of biopolymer films. Carb. Polym. 2020, 240, 116244. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Mahajan, R.L. A facile method for coal to graphene oxide and its application to a biosensor. Carbon 2021, 181, 408–420. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Liu, X.; Wu, J.; Zhu, X.; Bai, Z.; Yu, Z. Preparation of β-cyclodextrin/graphene oxide and its adsorption properties for methylene blue. Colloids Surfaces B Biointerfaces 2021, 200, 111605. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, X.; Wang, J.; Zhang, J.; Yin, C.; Fan, L.; Zhang, D. Graphene oxide–cerium oxide hybrids for enhancement of mechanical properties and corrosion resistance of epoxy coatings. J. Mater. Sci. 2021, 56, 10108–10123. [Google Scholar] [CrossRef]
- Lyn, F.H.; Tan, C.P.; Zawawi, R.M.; Hanani, Z.N. Physicochemical properties of chitosan/graphene oxide composite films and their effects on storage stability of palm-oil based margarine. Food Hydrocoll. 2021, 117, 106707. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Park, G.T.; Han, S.S. Antibacterial properties of starch-reduced graphene oxide–polyiodide nanocomposite. Food Chem. 2021, 342, 128385. [Google Scholar] [CrossRef] [PubMed]
- REGULATION (EC) No 1935/2004 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 27 October 2004 on Materials and Articles Intended to Come into Contact with Food and Repealing Directives 80/590/EEC and 89/109/EEC. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32004R1935 (accessed on 25 May 2021).
- Khachatryan, G.; Khachatryan, K.; Krystyjan, M.; Krzan, M.; Khachatryan, L. Functional properties of composites containing silver nanoparticles embedded in hyaluronan and hyaluronan-lecithin matrix. Int. J. Biol. Macromol. 2020, 149, 417–423. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1963, 13, 1741–1747. [Google Scholar] [CrossRef]
- Rudawska, A.; Jacniacka, E. Analysis for determining surface free energy uncertainty by the Owen-Wendt method. Int. J. Adhes. Adhes. 2009, 29, 451–457. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes AASFernandes, F.M.B. Physical properties of chitosan films incorporated with natural antioxidants. Ind. Crops Prod. 2017, 107, 565–572. [Google Scholar] [CrossRef]
- ISO 527-1:2019 Determination of Tensile Properties–Part 1: General Principles. Available online: https://www.iso.org/standard/75824.html (accessed on 25 May 2021).
- Bourtoom, T.; Chinnan, M.S. Preparation and properties of rice starch–chitosan blend biodegradable film. LWT-Food Sci. Technol. 2008, 41, 1633–1641. [Google Scholar] [CrossRef]
- Chaiyakun, S.; Witit-Anun, N.; Nuntawong, N.; Chindaudom, P.; Oaew, S.; Kedkeaw, C.; Limsuwan, P. Preparation and characterization of graphene oxide nanosheets. Procedia Eng. 2012, 32, 759–764. [Google Scholar]
- Ren, L.; Yan, X.; Zhhou, J.; Tong, J.; Su, X. Influence of chitosan concentration on mechanical and barrier properties of corn starch/chitosan films. Int. J. Biol. Macromol. 2017, 105, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Shiku, Y.; Hamaguchi, P.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Effect of surimi quality on properties of edible films based on Alaska pollack. Food Chem. 2004, 86, 493–499. [Google Scholar] [CrossRef]
- Kumar, S.; Koh, J. Physiochemical and optical properties of chitosan based graphene oxide bionanocomposite. Int. J. Biol. Macromol. 2014, 70, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, X.; Wang, Y.; Zhang, X.; Tong, Z. Comparison of chitosan/starch composite film properties before and after cross-linking. Int. J. Biol. Macromol. 2013, 52, 275–279. [Google Scholar] [CrossRef]
- Han, J.T.; Jang, J.I.; Kim, S.H.; Jeong, S.Y.; Jeong, H.J.; Lee, G.W. Size sorting of chemically modified graphene nanoplatelets. Carbon Lett. 2013, 14, 89–93. [Google Scholar] [CrossRef]
- Hong, J.T.; Lee, K.M.; Son, B.H.; Park, S.J.; Park, D.J.; Park, J.Y.; Lee, S.; Ahn, Y.H. Terahertz conductivity of reduced graphene oxide films. Opt. Express 2013, 21, 7633–7640. [Google Scholar] [CrossRef]
- Tian, Q.; Liu, S.; Sun, X.; Sun, H.; Xue, Z.; Mu, T. Theoretical studies on the dissolution of chitosan in 1-butyl-3-methylimidazolium acetate ionic liquid. Carbohydr. Res. 2015, 408, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Cobos, M.; González, B.; Fernández, M.J.; Fernández, M.D. Chitosan–graphene oxide nanocomposites: Effect of graphene oxidenanosheets and glycerol plasticizer on thermal and mechanicalproperties. J. Appl. Polym. Sci. 2017, 45092. [Google Scholar] [CrossRef]
- Khachatryan, G.; Khachatryan, K.; Krystyjan, M.; Grzyb, J.; Stobiński, L. Polysaccharides-modified graphene oxide as new packing materials. In Proceedings of the International Conference on Polysaccharides—Glycoscience, Prague, Czech Republic, 8–10 November 2017; Řápková, R., Hinková, A., Čopíková, J., Eds.; Czech Chemical Society: Prague, Czech Republic, 2017; pp. 156–160. ISBN 978-80-86238-74-6. [Google Scholar]
- Kumar, S.; Mitra, A.; Halder, D. Centella asiatica leaf mediated synthesis of silver nanocolloid and its application as filler in gelatin based antimicrobial nanocomposite film. LWT 2017, 75, 293–300. [Google Scholar] [CrossRef]
- Kumar, S.; Shukla, A.; Baul, P.P.; Mitra, A.; Halder, D. Biodegradable hybrid nanocomposites of chitosan/gelatin and silver nanoparticles for active food packaging applications. Food Packag. Shelf Life 2018, 16, 178–184. [Google Scholar] [CrossRef]
- Raslan, A.; Saenz del Burgo, L.; Ciriza, J.; Pedraz, J.L. Graphene oxide and reduced graphene oxide-based scaffolds in regenerative medicine. Int. J. Pharm. 2020, 580, 119226. [Google Scholar] [CrossRef]
- Sun, C.; Holowka, D.A.; Baird, B.A.; Dichtel, W.R. Beyond Media Composition: Cell Plasma Membrane Disruptions by Graphene Oxide. Chem 2017, 2, 324–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Wei, P.; Zhou, Z.; Wei, T. Interactions of graphene with mammalian cells: Molecular mechanisms and biomedical insights. Adv. Drug Deliv. Rev. 2016, 105, 145–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gies, V.; Lopinski, G.; Augustine, J.; Cheung, T.; Kodrac, O.; Zou, S. The impact of processing on the cytotoxicity of graphene oxide. Nanoscale Adv. 2019, 1, 817–826. [Google Scholar] [CrossRef] [Green Version]
- Gould, S.B. Membranes and evolution. Curr. Biol. 2018, 28, R381–R385. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Sample | Particle Size (nm) | Zeta Potential (mV) | |
|---|---|---|---|
| composite C | thin | 2545 | 50.5 |
| nanocomposite I | 1935 | 36.5 | |
| nanocomposite II | 2140 | 40.0 | |
| composite C | thick | 620 | 56.2 |
| nanocomposite I | 880 | 28.3 | |
| nanocomposite II | 590 | 51.8 | |
| Sample | Contact Angle | Surface Free Energy | ||||
|---|---|---|---|---|---|---|
| Water | Diiodomethane | Polar (mJ/m2) | Dispersive (mJ/m2) | Total Free Energy (mJ/m2) | ||
| composite C | thin | 70.0° | 45.9° | 9.140 | 34.14 | 43.28 |
| nanocomposite I | 45.7° | 53.5° | 29.96 | 23.76 | 53.72 | |
| nanocomposite II | 47.7° | 33.8° | 21.31 | 35.86 | 57.17 | |
| composite C | thick | 53.8° | 43.6° | 19.65 | 31.57 | 51.23 |
| nanocomposite I | 80.2° | 40.0° | 3.15 | 41.08 | 44.23 | |
| nanocomposite II | 64.1° | 39.1° | 11.25 | 36.91 | 48.16 | |
| Sample | Tp1 | Tp2 | Tp3 | Tp4 | Tp5 |
|---|---|---|---|---|---|
| °C | °C | °C | °C | °C | |
| composite C | 142.2 ± 2.3 b | 215.5 ± 6.4 a | 265.2 ± 5.2 | nd | 318.6 ± 1.7 |
| nanocomposite I | 123.2 ± 3.2 a | 226.4 ± 1.5 b | 267.8 ± 6.3 | 284.9 ± 0.8 | 320.8 ± 1.1 |
| nanocomposite II | 141.2 ± 9.1 b | 223.9 ± 0.7 b | 273.8 ± 7.1 | nd | 322.6 ± 2.1 |
| One-way ANOVA - p | 0.011 | 0.029 | 0.297 | - | 0.068 |
| Sample | Ton | Tp | Tend | -ΔH |
|---|---|---|---|---|
| °C | °C | °C | J·g−1 | |
| composite C | 213.0 ± 5.6 a | 215.5 ± 6.4 a | 222.6 ± 8.3 | 88.1 ± 1.1 b |
| nanocomposite I | 224.0 ± 1.6 b | 226.4 ± 1.5 b | 232.6 ± 1.5 | 81.0 ± 3.2 a |
| nanocomposite II | 221.7 ± 0.5 b | 223.9 ± 0.7 b | 229.7 ± 1.2 | 87.5 ± 2.4 b |
| One-way ANOVA - p | 0.015 | 0.029 | 0.109 | 0.020 |
| Sample | L* (D65) | a* (D65) | b* (D65) | T (A·mm−1) | |
|---|---|---|---|---|---|
| composite C | thin | 97.68 ± 0.03 a | −0.91 ± 0.01 e | 6.63 ± 0.08 f | 1.21 ± 0.10 b |
| nanocomposite I | 83.31 ± 0.87 d | 1.13 ± 0.13 c | 12.66 ± 0.34 d | 1.54 ± 0.07 a | |
| nanocomposite II | 88.49 ± 0.25 c | 0.27 ± 0.01 d | 11.76 ± 0.22 e | 1.49 ± 0.05 a | |
| composite C | thick | 95.35 ± 0.16 b | −2.03 ± 0.02 f | 16.47 ± 0.37 c | 0.57 ± 0.01 c |
| nanocomposite I | 71.98 ± 1.24 f | 2.41 ± 0.26 a | 21.22 ±1.01 b | 1.12 ± 0.04 b | |
| nanocomposite II | 77.46 ± 0.45 e | 1.51 ± 0.08 b | 22.10 ± 0.53 a | 0.95 ± 0.06 c | |
| Sample | Water Content (%) | Solubility (%) | Swelling Degree (%) | |
|---|---|---|---|---|
| composite C | thin | 10.92 ± 1.30 a | 27.80 ± 1.08 c | 95.18 ± 17.88 c |
| nanocomposite I | 8.81 ± 0.42 c | 30.52 ± 2.05 b | 107.57 ± 7.79 bc | |
| nanocomposite II | 9.79 ± 0.75 bc | 29.65 ± 0.32 bc | 102.49 ± 7.08 c | |
| composite C | thick | 10.16 ± 0.22 ab | 36.82 ± 0.38 a | 127.66 ± 8.31 a |
| nanocomposite I | 9.90 ± 0.59 ab | 37.42 ± 3.07 a | 121.94 ± 11.17 ab | |
| nanocomposite II | 9.43 ± 0.54 bc | 36.29 ± 1.01 a | 125.15 ± 13.85 a | |
| Sample | Thickness (mm) | TS (MPa) | EAB (%) | |
|---|---|---|---|---|
| composite C | thin | 0.095± 0.008 d | 21.22 ± 4.34 a | 37.75 ± 6.12 c |
| nanocomposite I | 0.102 ± 0.005 c | 20.98 ± 1.97 a | 60.14 ± 5.27 ab | |
| nanocomposite II | 0.107 ± 0.008 c | 20.49 ± 3.22 a | 59.23 ± 8.01 ab | |
| composite C | thick | 0.205 ± 0.012 b | 8.43 ± 1.79 b | 52.50 ± 6.37 b |
| nanocomposite I | 0.216 ± 0.024 a | 8.15 ± 1.32 b | 66.52 ± 9.98 a | |
| nanocomposite II | 0.219 ± 0.009 a | 8.10 ± 1.85 b | 64.29 ± 8.04 a | |
| Species of Bacteria | Samples | Average ± Standard Deviation (mm) | Range (mm) |
|---|---|---|---|
| Escherichia coli (EC) | composite C | 0.0 ± 0.0 | 0.0–0.0 |
| nanocomposite I | 5.0 ± 0.0 | 5.0–5.0 | |
| nanocomposite II | 5.0 ± 0.0 | 5.0–5.0 | |
| Proteus mirabilis (PM) | composite C | 0.0 ± 0.0 | 0.0–0.0 |
| nanocomposite I | 5.0 ± 0.0 | 5.0–5.0 | |
| nanocomposite II | 5.0 ± 0.0 | 5.0–5.0 | |
| Staphylococcus aureus (SA) | composite C | 0.0 ± 0.0 | 0.0–0.0 |
| nanocomposite I | 5.0 ± 0.0 | 5.0–5.0 | |
| nanocomposite II | 5.0 ± 0.0 | 5.0–5.0 | |
| Enterococcus faecalis (EF) | composite C | 9.7 ± 0.6 | 9.1–10.3 |
| nanocomposite I | 9.0 ± 1.0 | 8.0–10.0 | |
| nanocomposite II | 10.0 ± 1.0 | 9.0–11.0 | |
| Pseudomonas aeruginosa (PA) | composite C | 11.0 ± 1.0 | 10.0–12.0 |
| nanocomposite I | 10.0 ± 0.0 | 10.0–10.0 | |
| nanocomposite II | 5.0 ± 0.0 | 5.0–5.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krystyjan, M.; Khachatryan, G.; Grabacka, M.; Krzan, M.; Witczak, M.; Grzyb, J.; Woszczak, L. Physicochemical, Bacteriostatic, and Biological Properties of Starch/Chitosan Polymer Composites Modified by Graphene Oxide, Designed as New Bionanomaterials. Polymers 2021, 13, 2327. https://doi.org/10.3390/polym13142327
Krystyjan M, Khachatryan G, Grabacka M, Krzan M, Witczak M, Grzyb J, Woszczak L. Physicochemical, Bacteriostatic, and Biological Properties of Starch/Chitosan Polymer Composites Modified by Graphene Oxide, Designed as New Bionanomaterials. Polymers. 2021; 13(14):2327. https://doi.org/10.3390/polym13142327
Chicago/Turabian StyleKrystyjan, Magdalena, Gohar Khachatryan, Maja Grabacka, Marcel Krzan, Mariusz Witczak, Jacek Grzyb, and Liliana Woszczak. 2021. "Physicochemical, Bacteriostatic, and Biological Properties of Starch/Chitosan Polymer Composites Modified by Graphene Oxide, Designed as New Bionanomaterials" Polymers 13, no. 14: 2327. https://doi.org/10.3390/polym13142327
APA StyleKrystyjan, M., Khachatryan, G., Grabacka, M., Krzan, M., Witczak, M., Grzyb, J., & Woszczak, L. (2021). Physicochemical, Bacteriostatic, and Biological Properties of Starch/Chitosan Polymer Composites Modified by Graphene Oxide, Designed as New Bionanomaterials. Polymers, 13(14), 2327. https://doi.org/10.3390/polym13142327









