A Critical Review for Synergic Kinetics and Strategies for Enhanced Photopolymerizations for 3D-Printing and Additive Manufacturing
Abstract
1. Introduction
- (1)
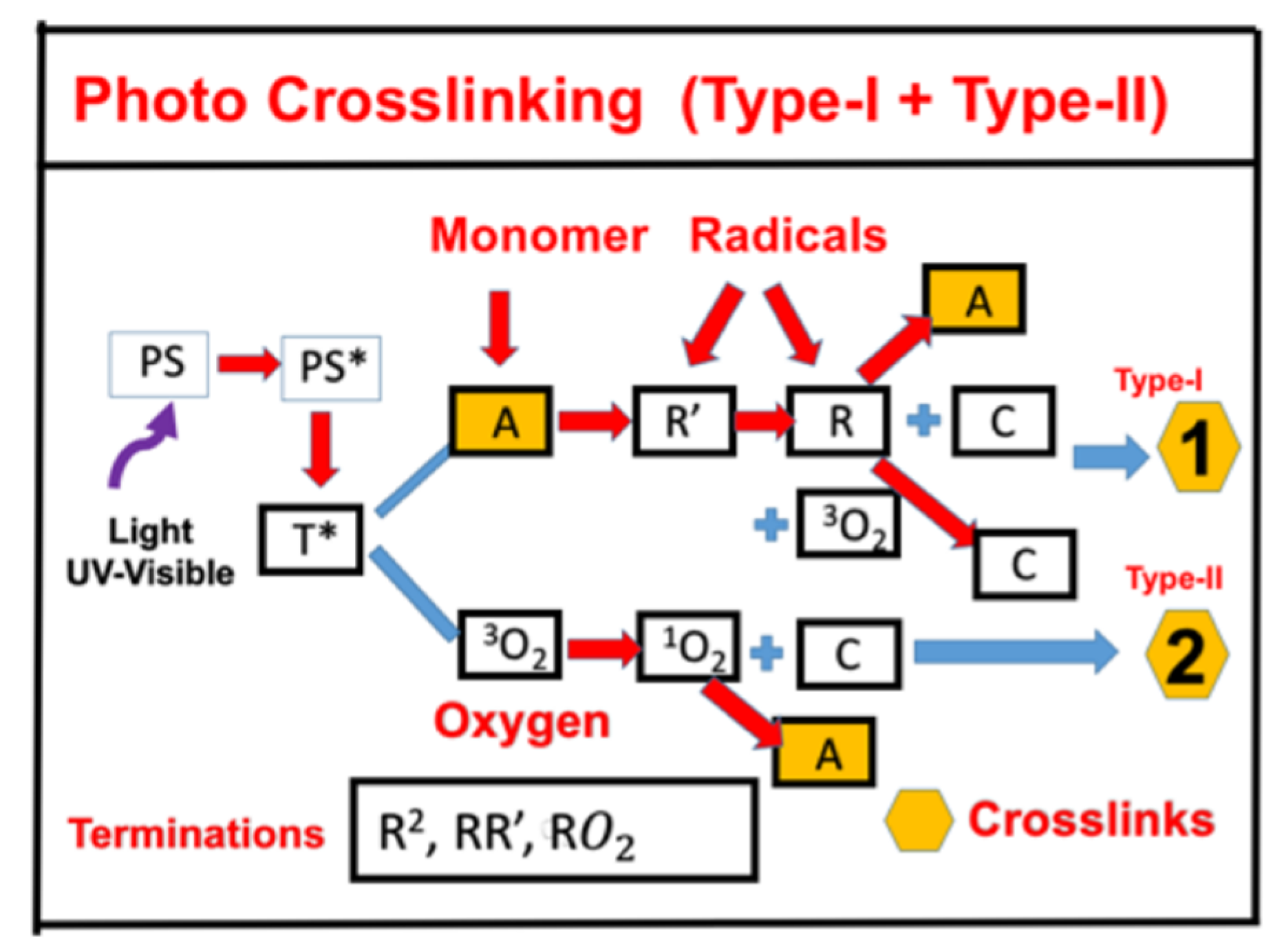
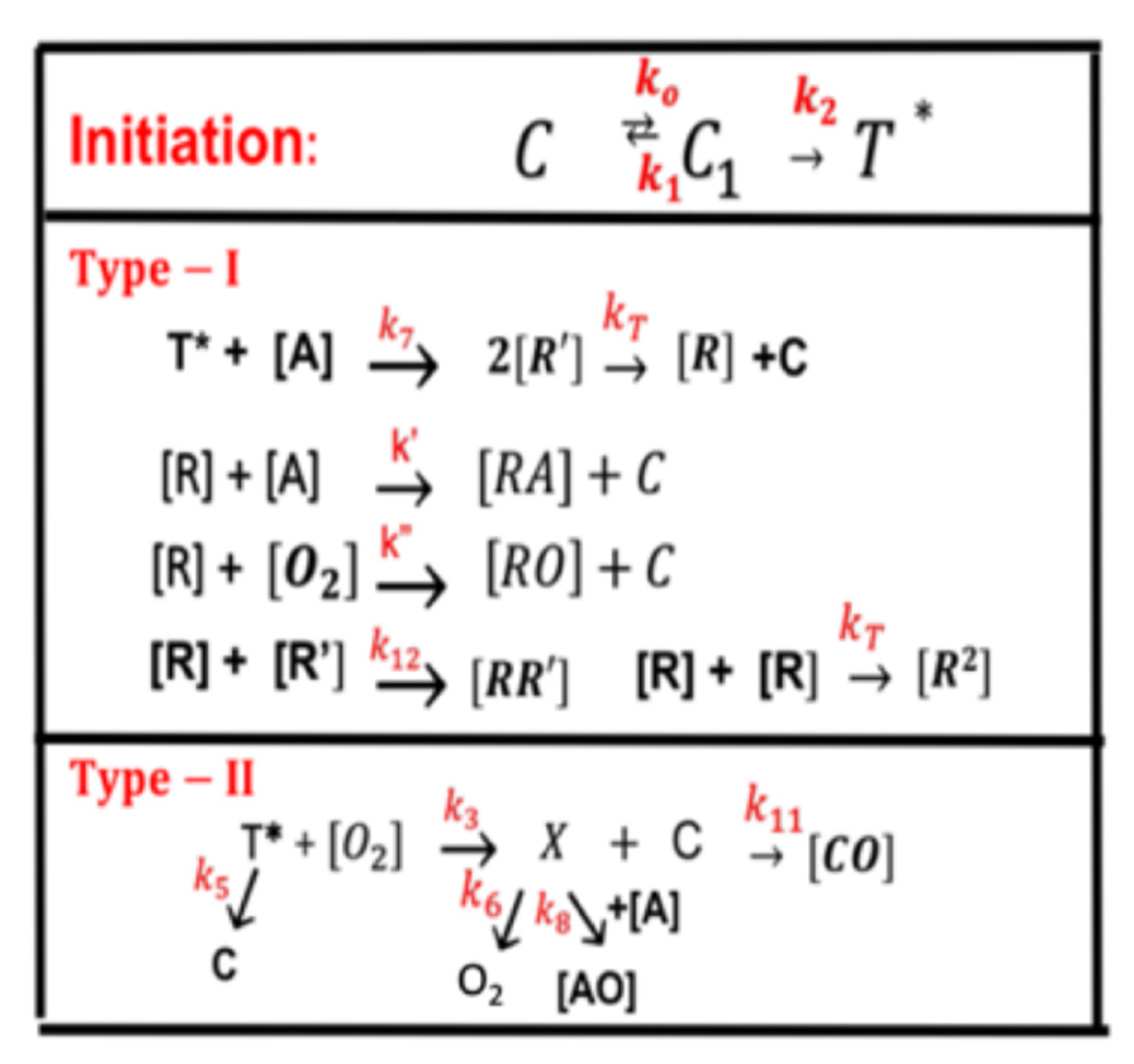
- Photo crosslinking of corneas for the treatment of corneal diseases using UVA-light (365 nm) light and riboflavin as the photosensitizer.
- (2)
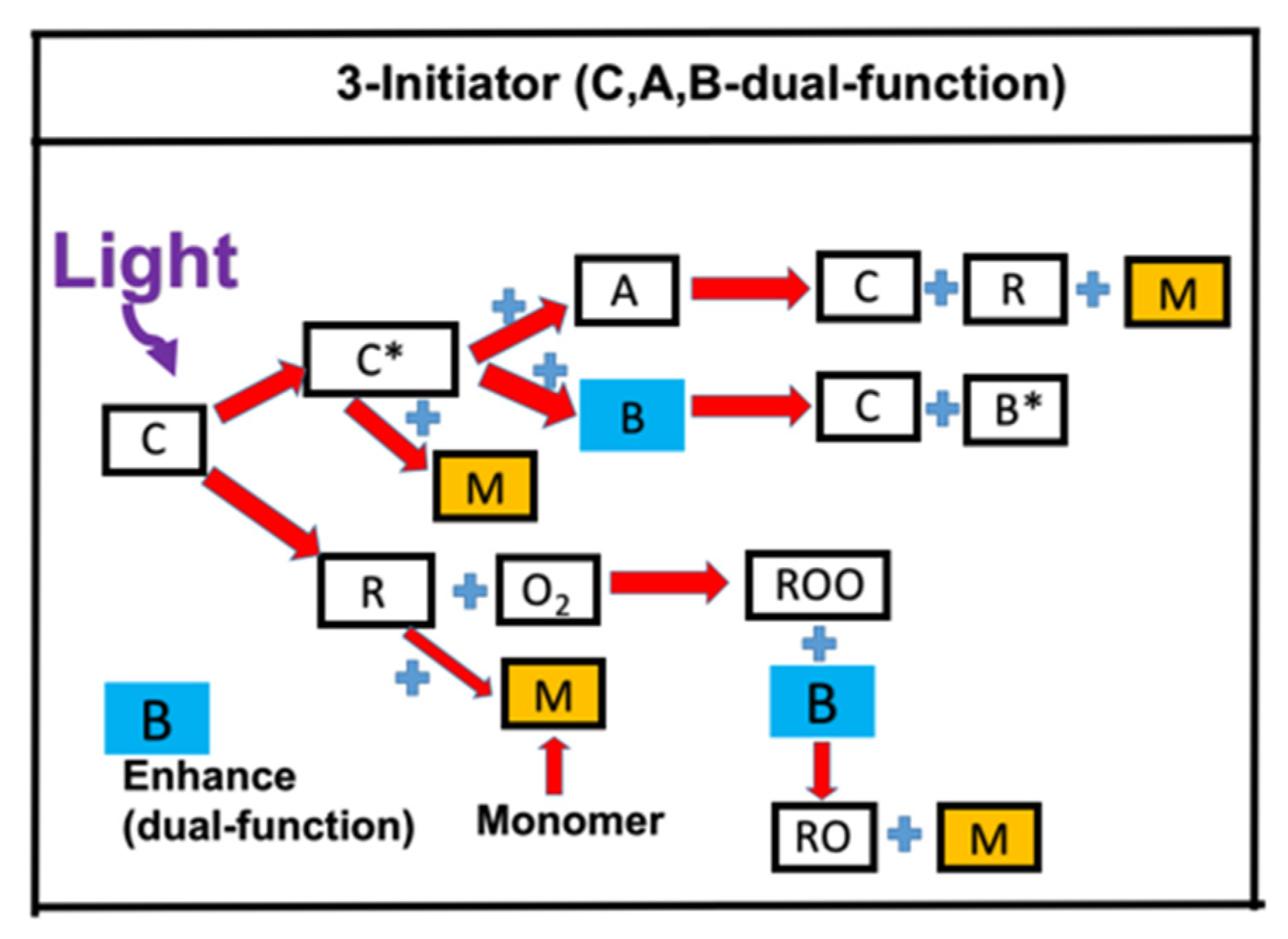
- Synergic effects by a dual-function enhancer in a three-initiator system (one-monomer).
- (3)
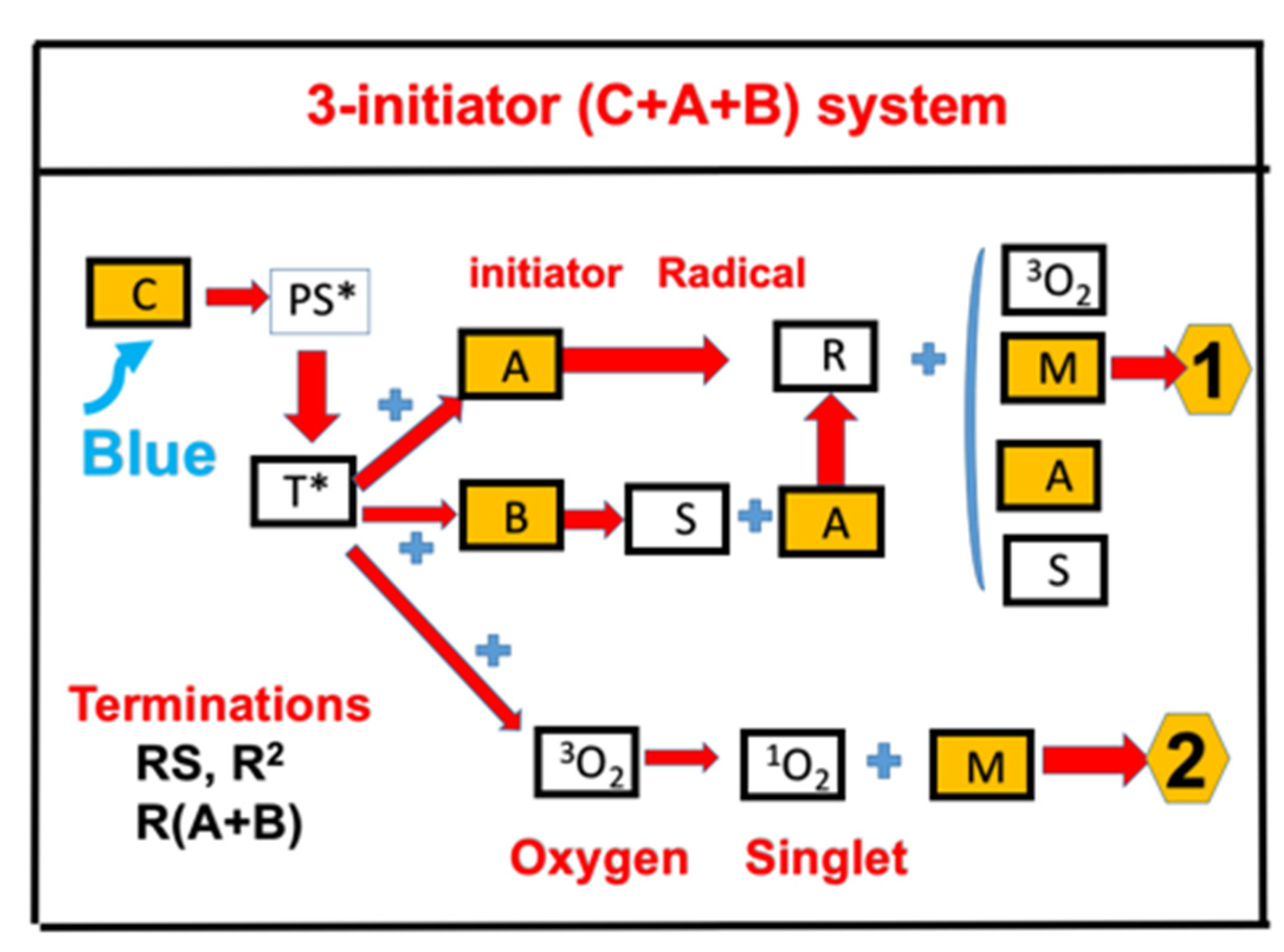
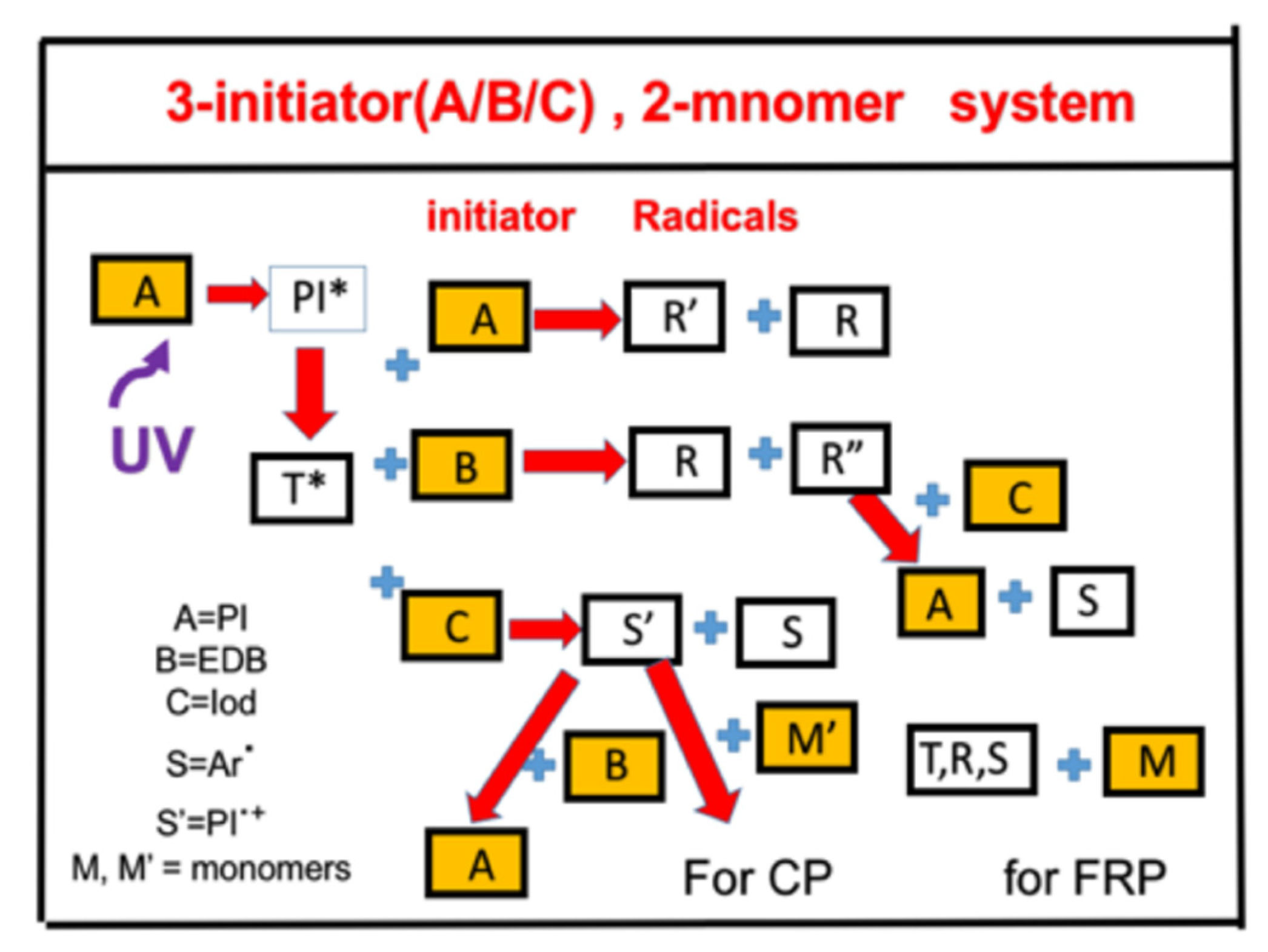
- Synergic effects by a three-initiator C/B/A system, with electron-transfer and oxygen-mediated energy-transfer pathways for free radical (FRP) and cationic photopolymerization (CP).
- (4)
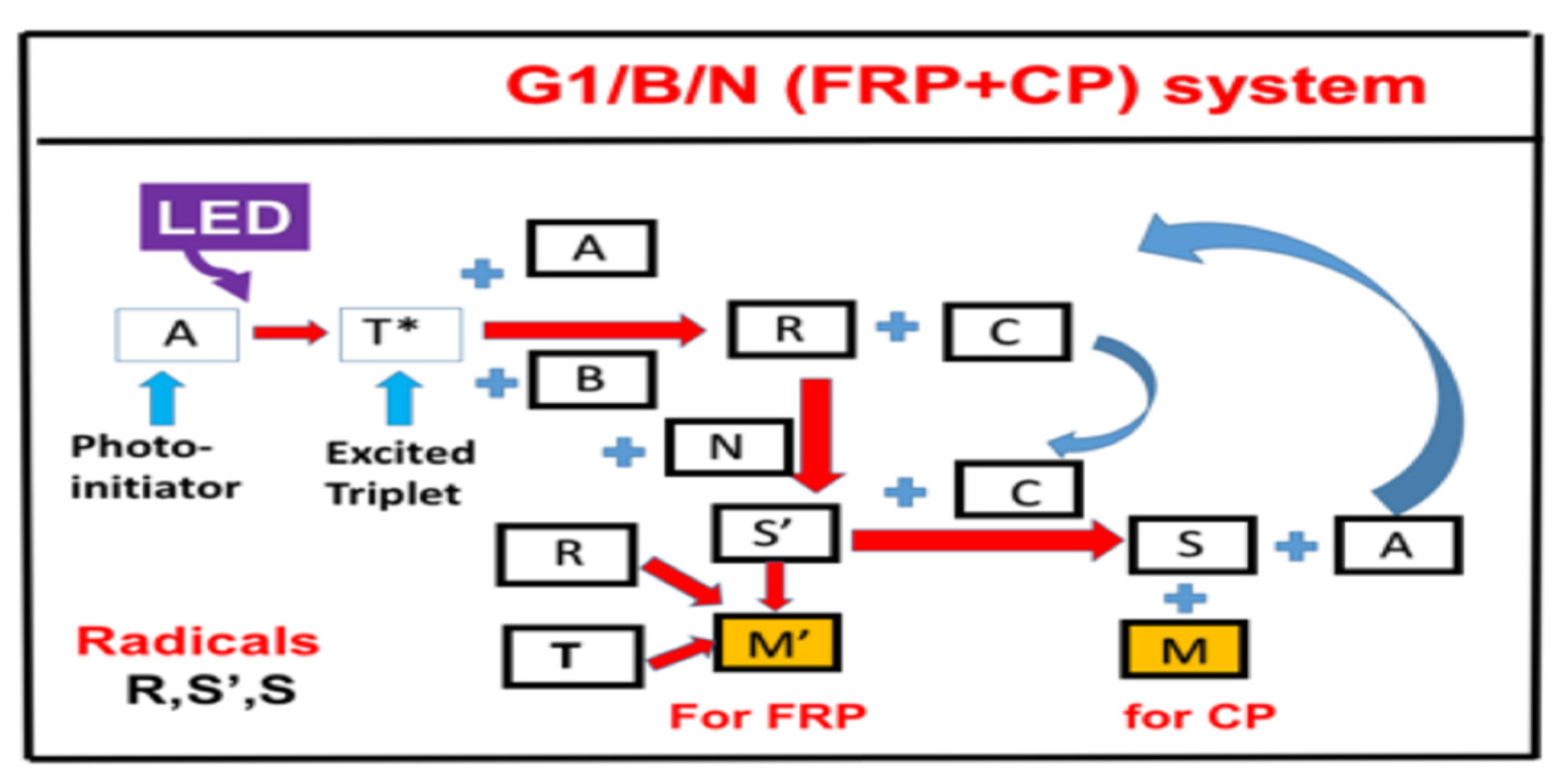
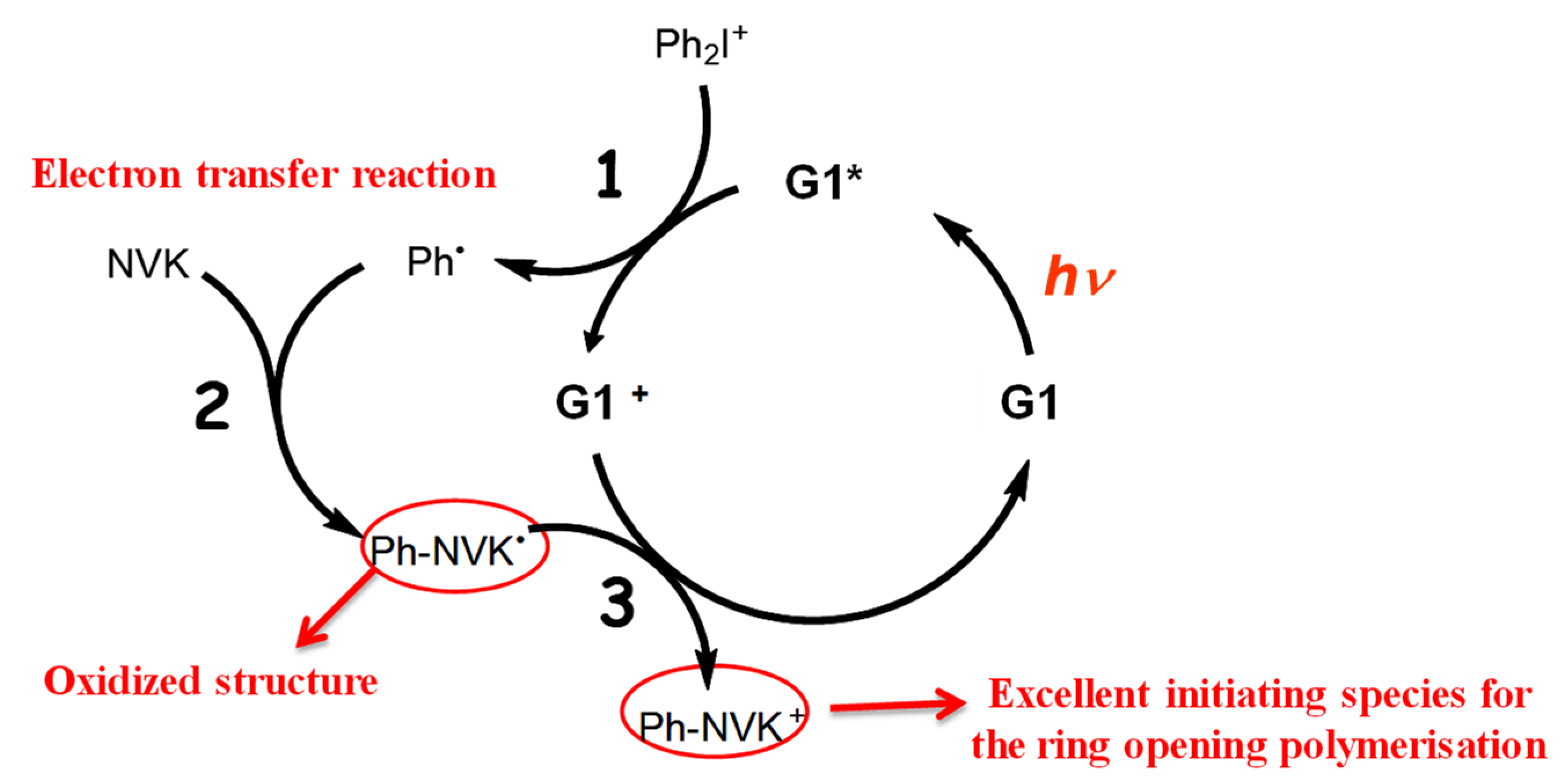
- Copper-complex (G1) photoredox catalyst in G1/Iod/NVK systems for FRP and CP.
- (5)
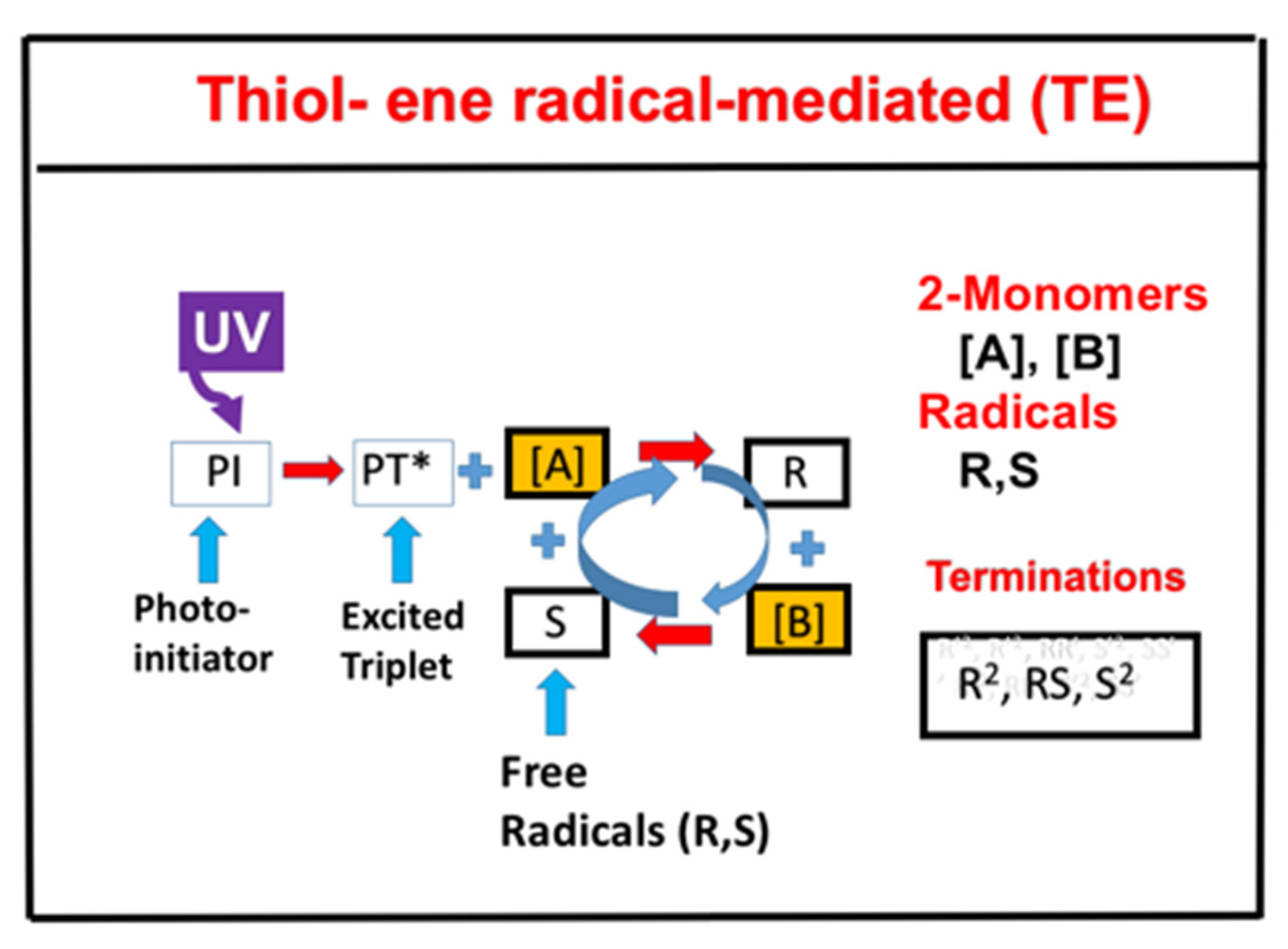
- Radical-mediated thiol-ene (TE) photopolymerizations.
- (6)
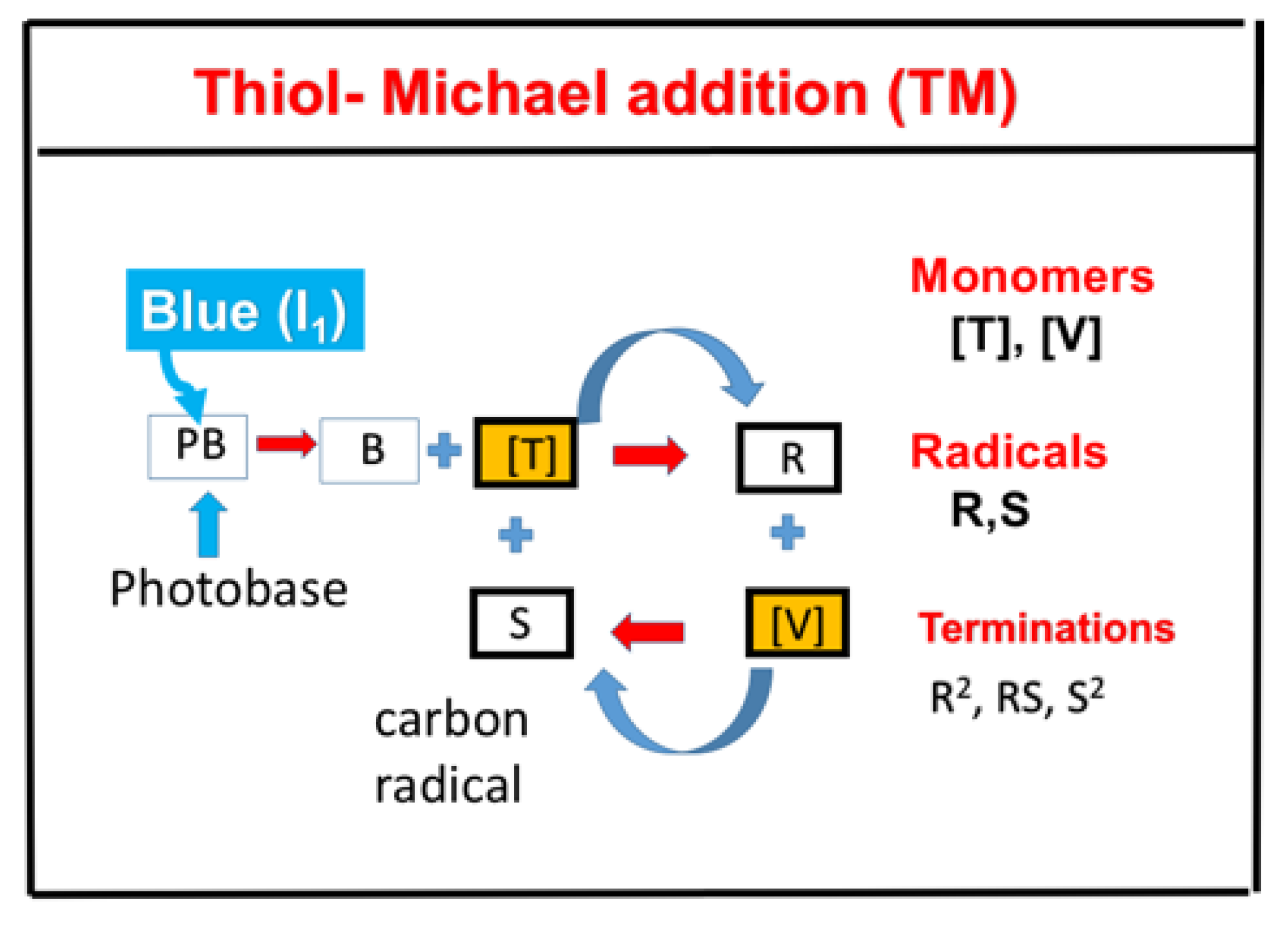
- Superbase photogenerator-based catalyzed thiol−acrylate Michael (TM) addition reaction and the combined system of TE and TM.
- (7)
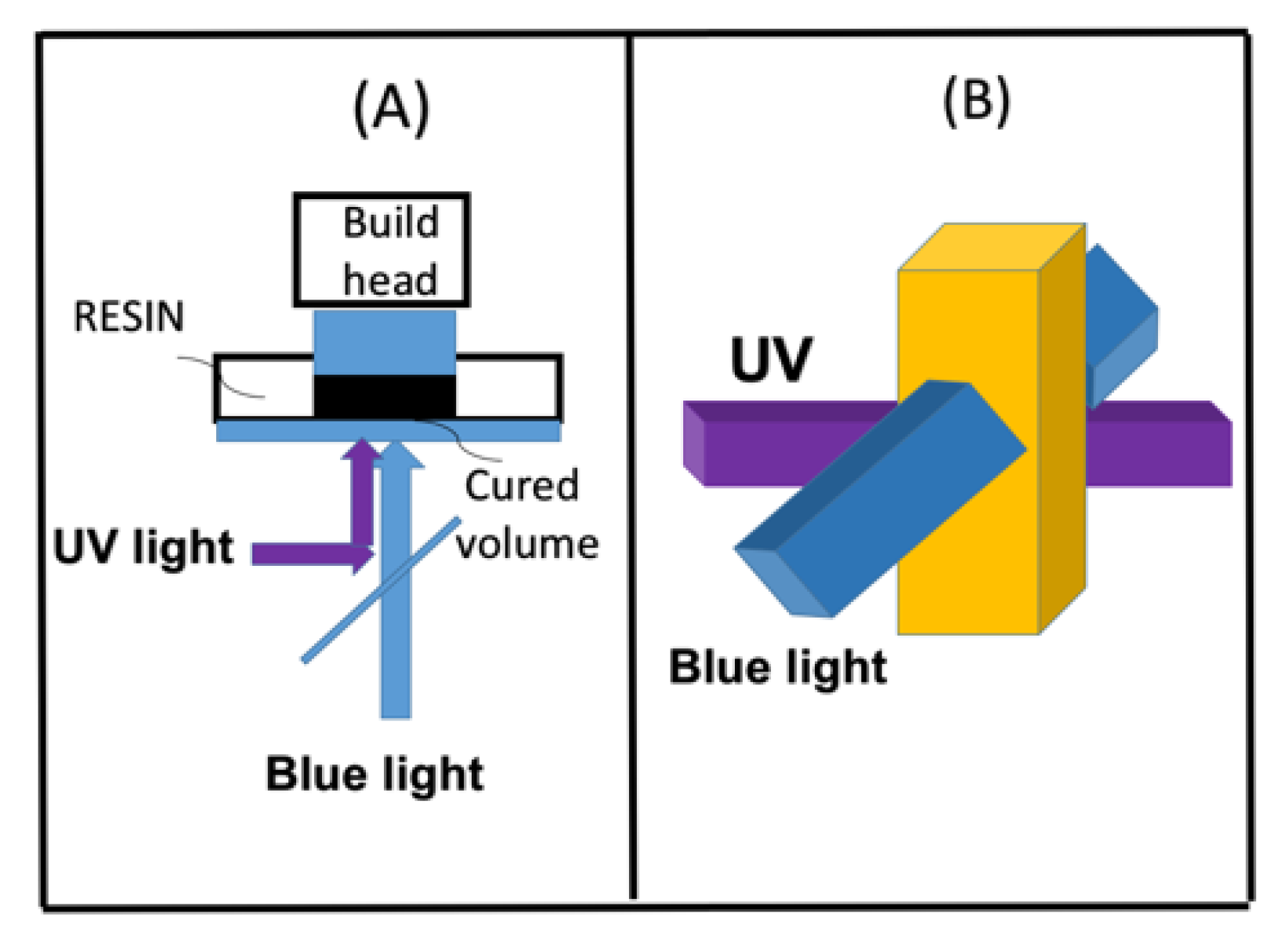
- Dual-wavelength (UV and blue) controlled photopolymerization confinement (PC)
- (8)
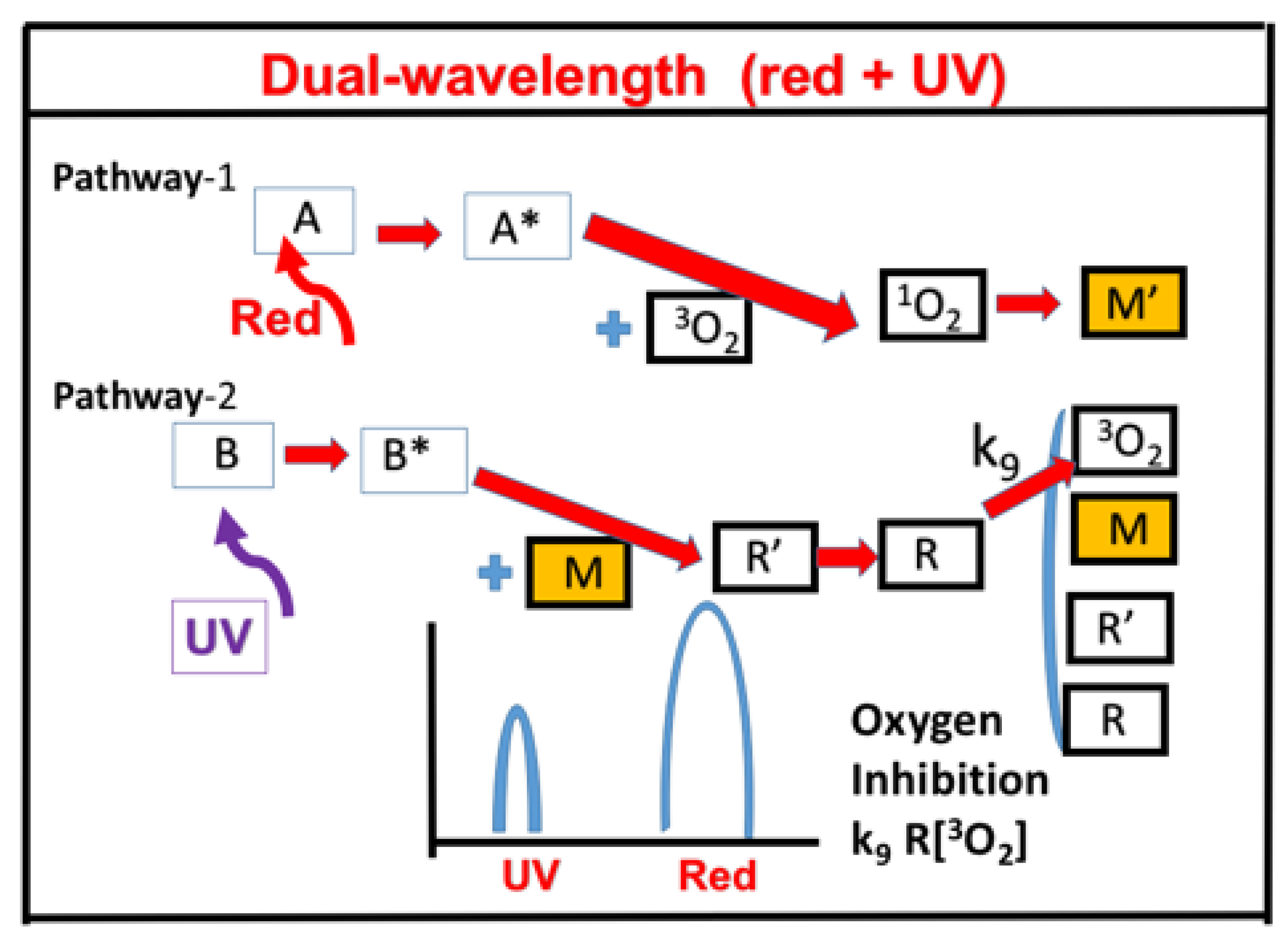
- Dual-wavelength (UV and red) selectively controlled 3D printing.
- (9)
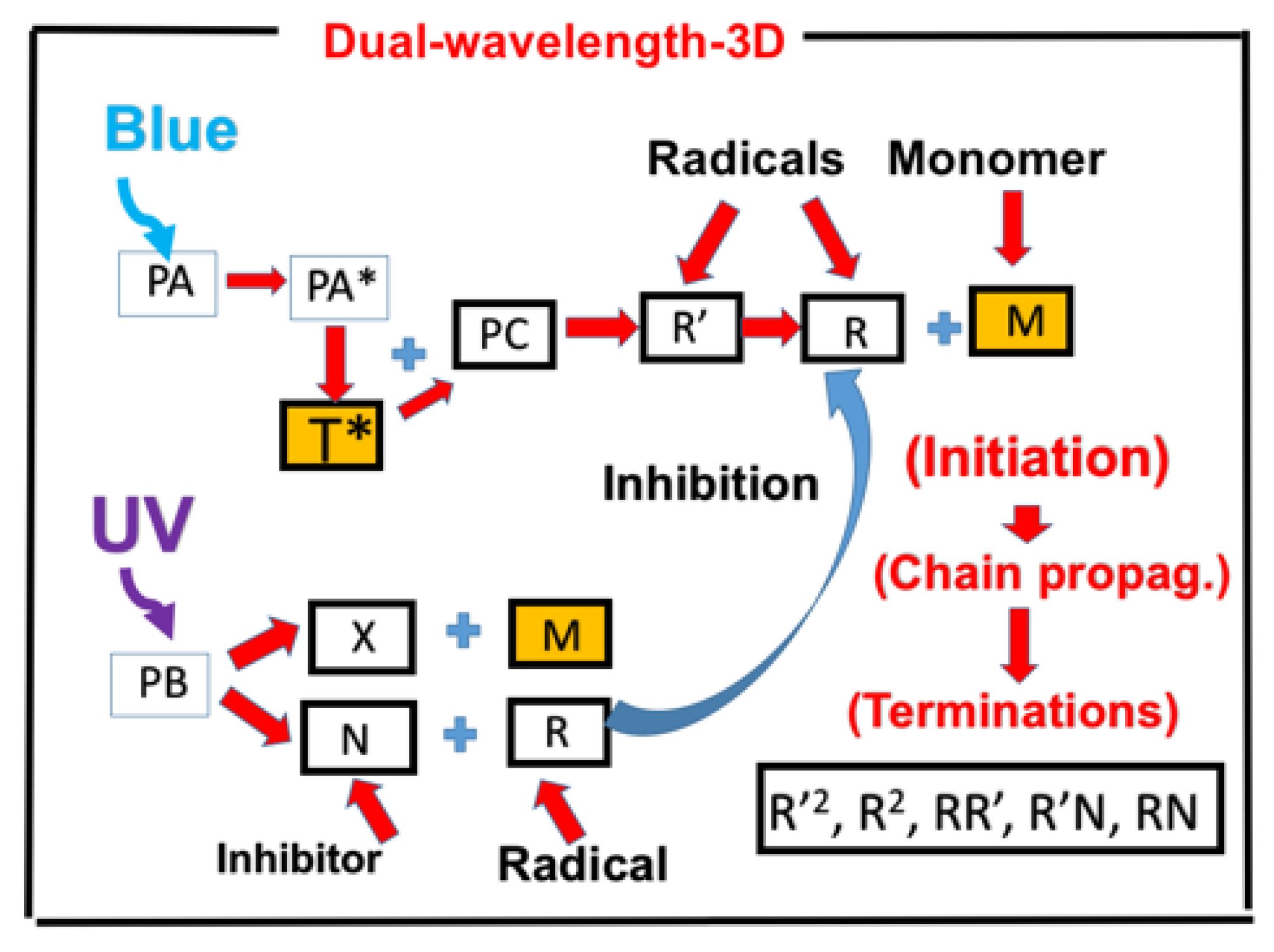
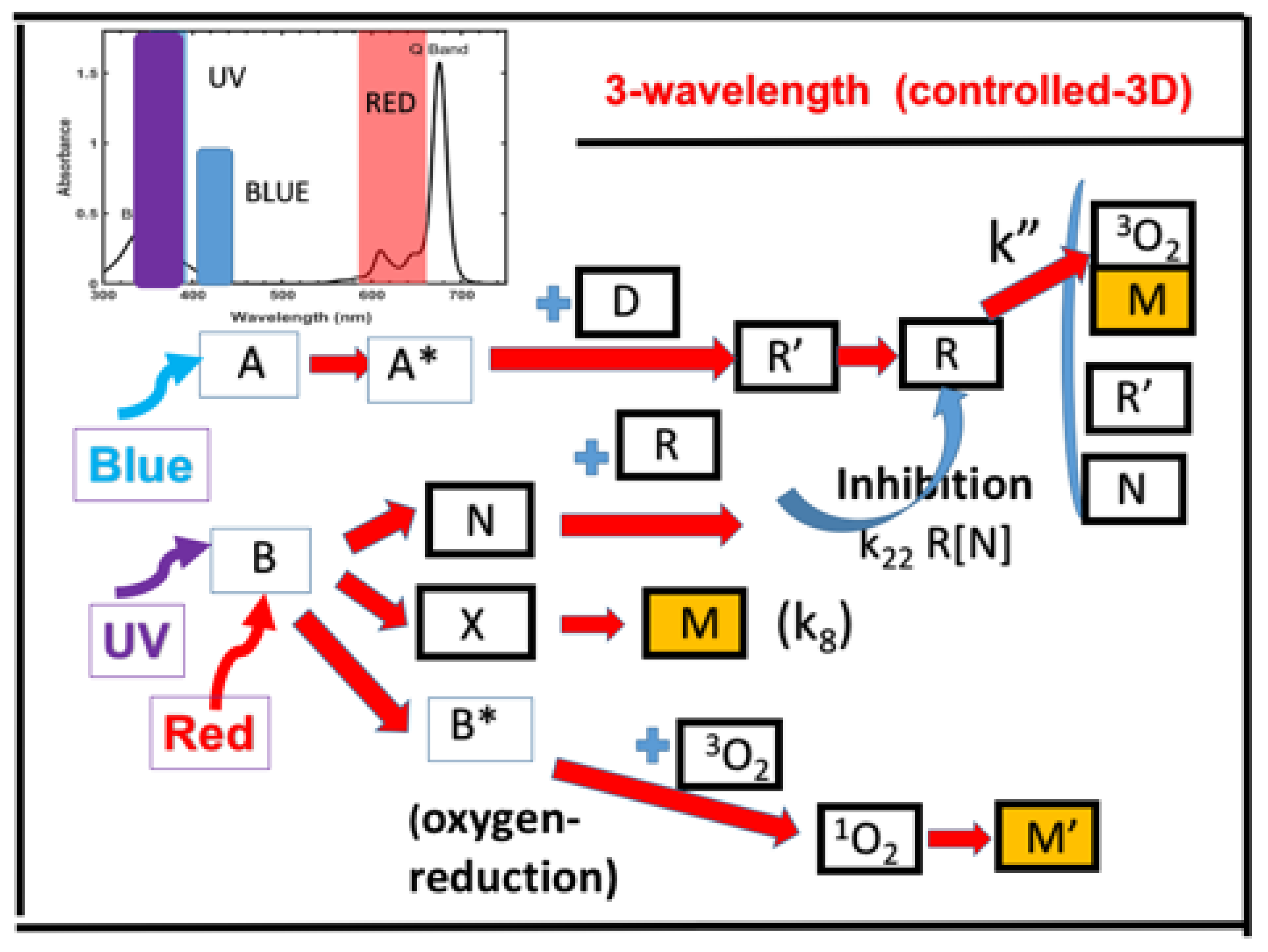
- Three-wavelength selectively controlled in 3D printing and additive manufacturing (AM).
2. Kinetic Systems and Discussions
2.1. Photo Crosslinking of Corneas
2.2. Synergic Effects of a Dual-Function Enhancer (3-Initiator System)
2.3. Synergic Effects of a 3-Initiator Enhanced C/B/A System
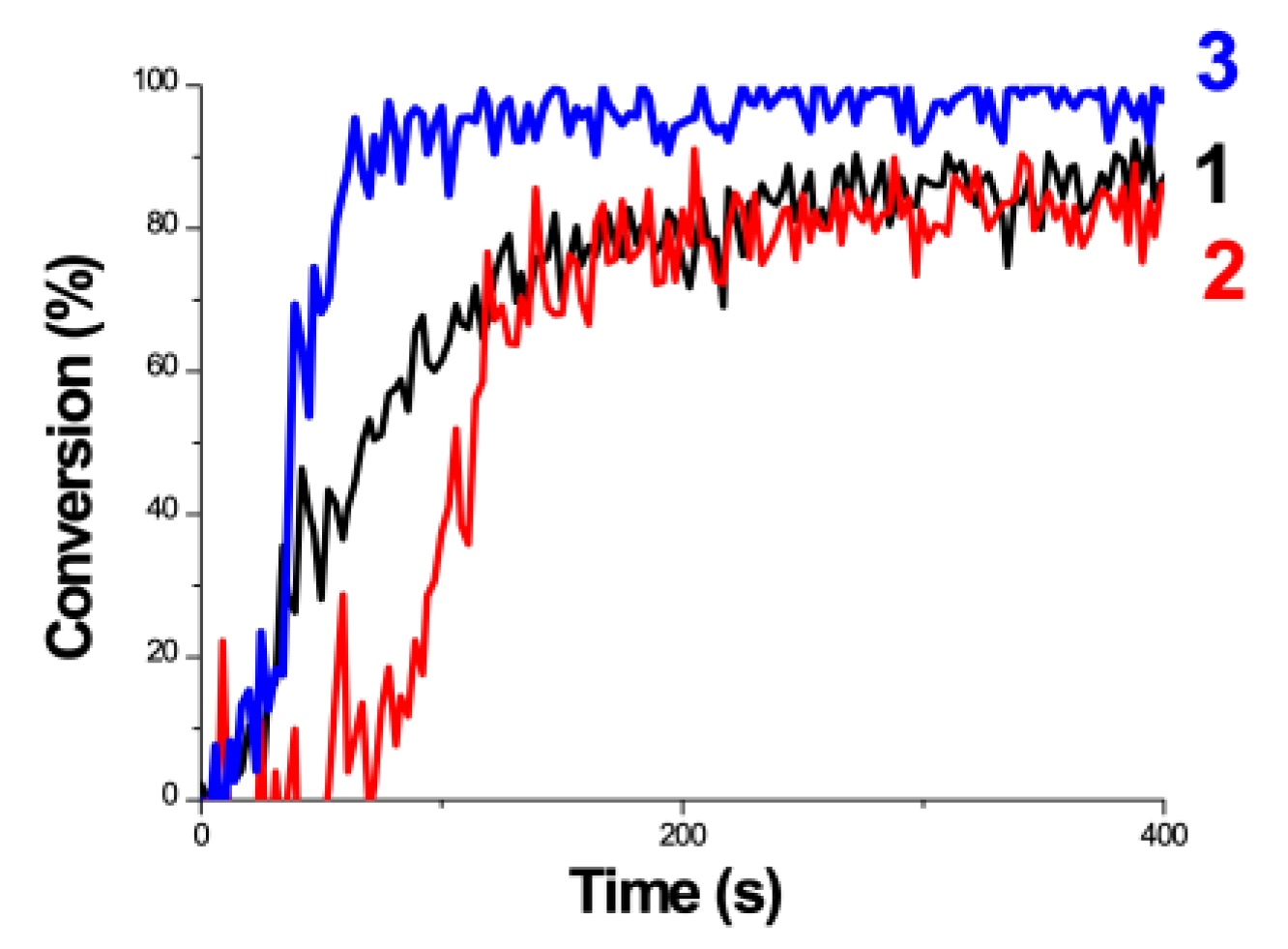
2.4. Synergic Effects in a 3-Initiator (A/B/C) System for FRP and CP
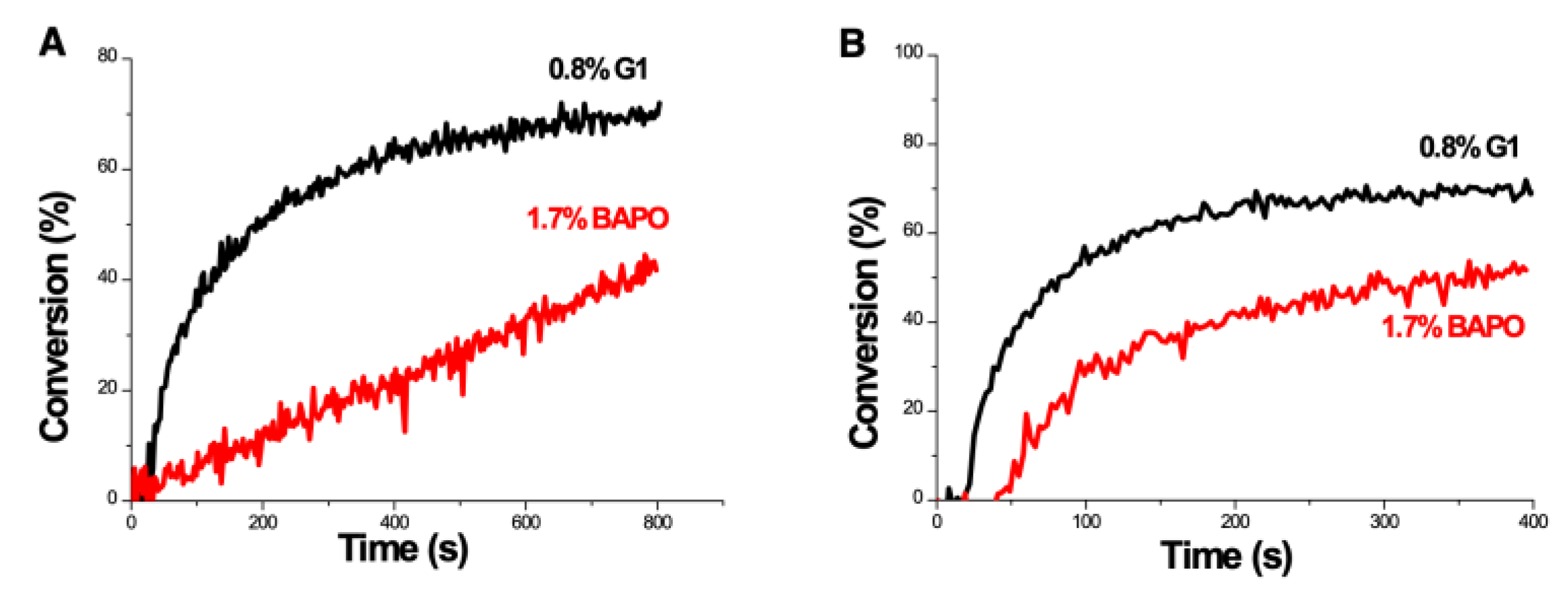
2.5. Copper-Complex (G1) Photoredox Catalyst in G1/Iod/NVK Systems
2.6. Radical-Mediated Thiol-Ene (TE) Photopolymerizations
- (i)
- Without the viscosity or homopolymerization (or kCV = 0) effects, [A] and [B] have an equal overall polymerization rate (RP); CV (CT) is an increasing (decreasing) function of the ratio RC = [A]0/[B]0. For RK = 1 (or kp = k) CT, CV, and CT have the same temporal profiles, but have a reversed dependence on RC.
- (ii)
- For RK > >1, [A] and CT are almost independent of RC, but the second-order correction is inversely proportional to R2, an opposite trend in comparing with CV. As predicted by analytic formulas.
- (iii)
- With the presence of the viscosity effect, the free volume is reduced when crosslink efficacy increases. The reduction factor only affects the propagation rate constant; therefore, the viscosity effect does not affect the efficacy for the case of RK > > 1 and affects the efficacy for other ratios of kp and k CT where the viscosity effect reduces the efficacy of [B].
- (iv)
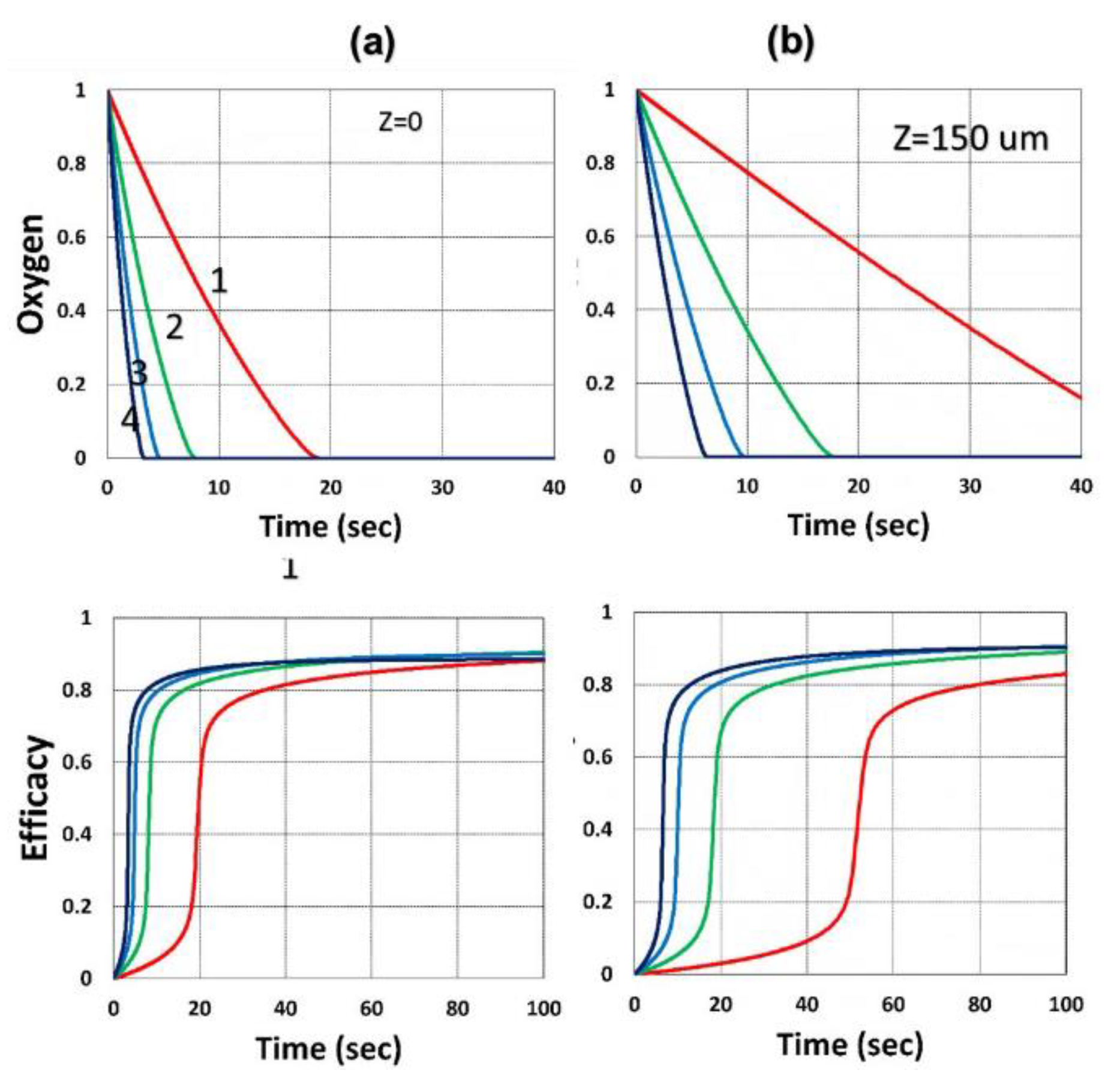
- For an optically thick polymer, the influence of dynamic light intensity is due to PI depletion. In most previous modeling with constant light intensity, the assumption suffers an error of 5% to 20% (underestimated) for a crosslink depth (ZC) ranging 300 to 500 um.
- (v)
- Scaling law for the functional group concentration of thiol, [A], and ene, [B], given by [A]m[B]n. For RK > >1, the polymerization rates are first order in the ene concentration (or n = 1.0) and nearly independent of the thiol concentration (or m = 0); in contrast, m = 1.0 and n = 0 for RK < <1. For RK values near unity, polymerization rates are approximately 0.5 order in both thiol and ene functional group concentrations (m = n = 0.5). However, a scaling law of m = 0.4 and n = 0.6 was found in an acrylate system (with RK = 13), due to contributions from homopolymerization [14].
2.7. Superbase Thiol−Acrylate Michael (TM) Addition and TE/TM Systems
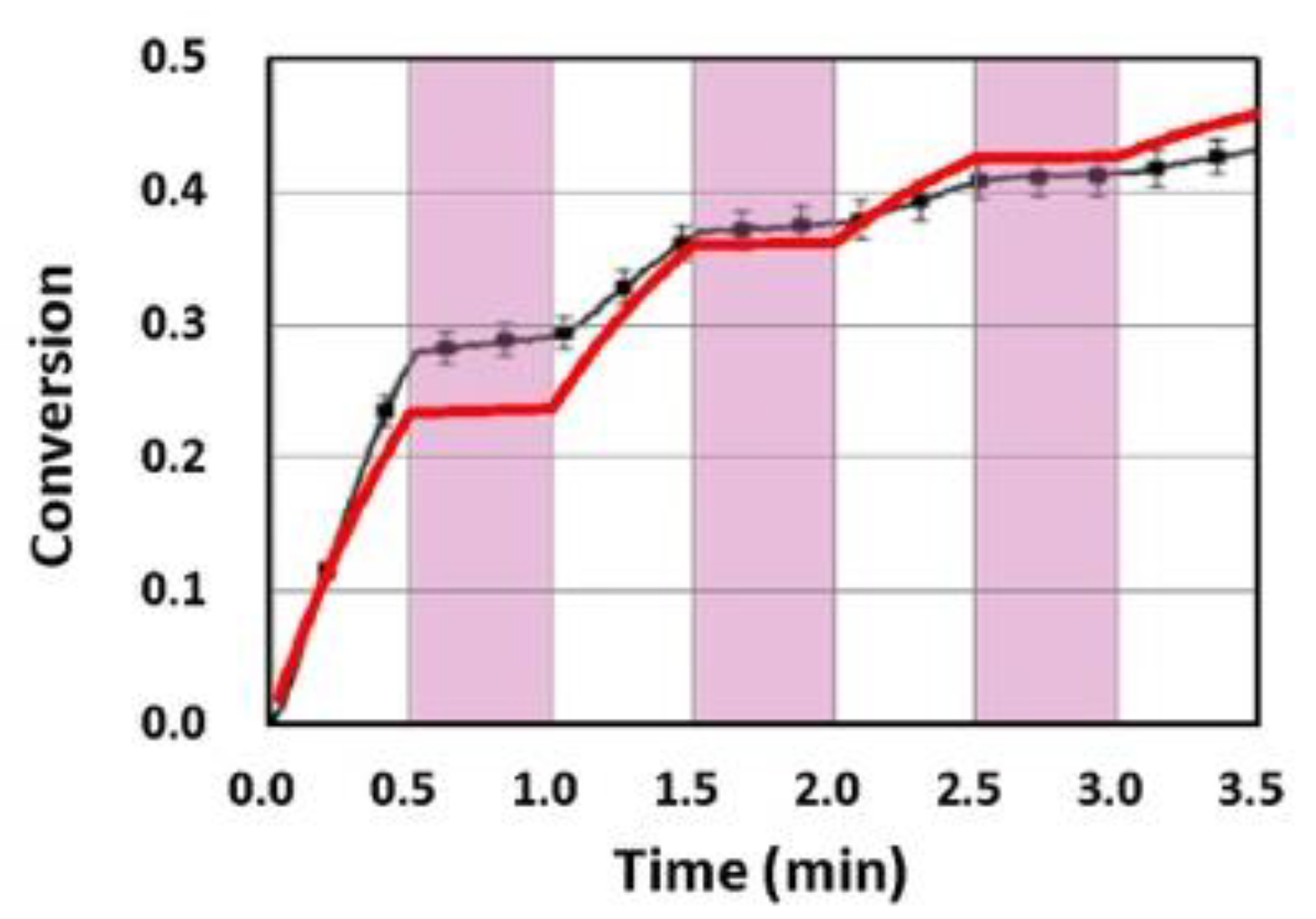
2.8. Dual-Wavelength (UV and Blue) Controlled Photopolymerization Confinement (PC)
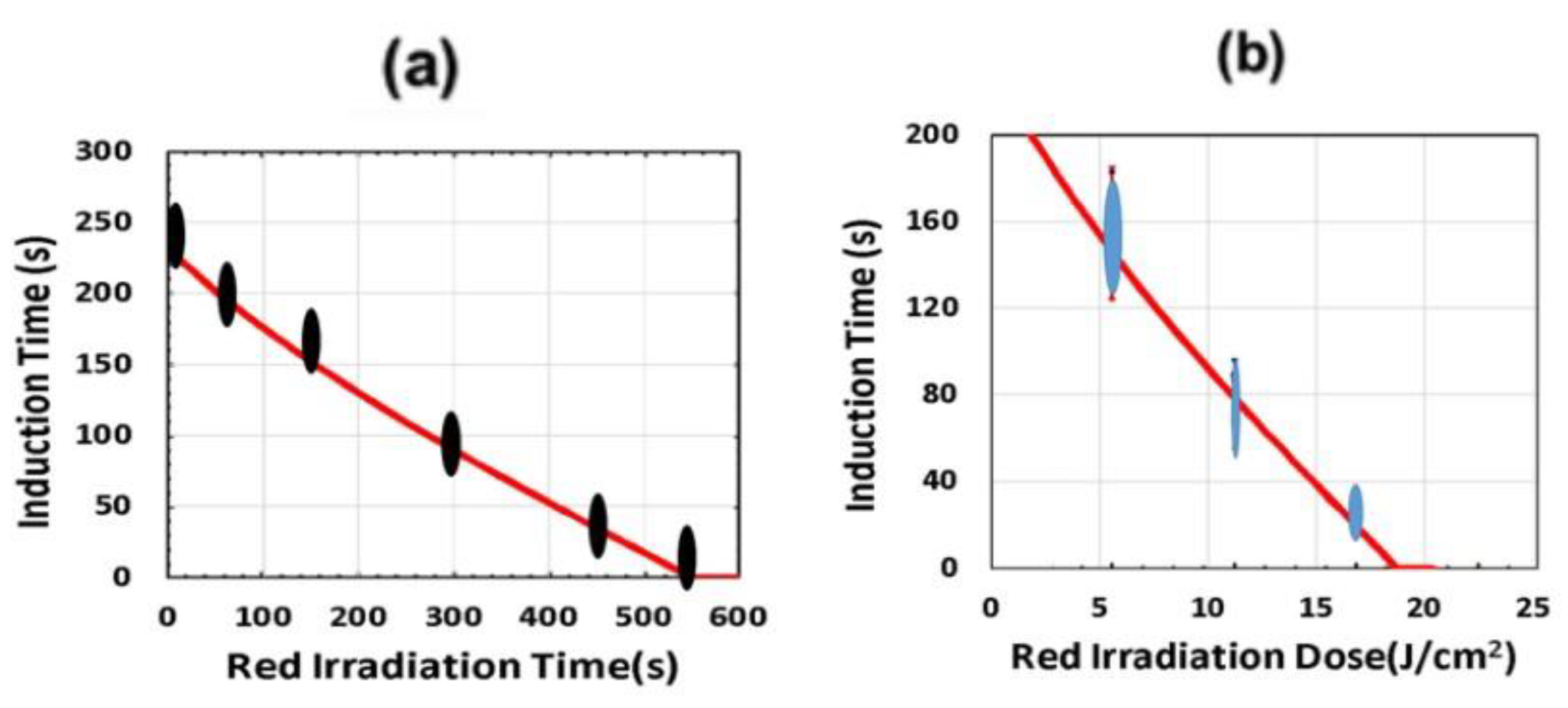
2.9. Dual-Wavelength (UV and Red) Controlled 3D Printing
2.10. Three-Wavelength Controlled in 3D Printing and Additive Manufacturing (AM)
3. Kinetics and Efficacy Formulas
3.1. The Kinetic Equations for General Systems
3.2. Basic Formulas for Conversion and Rate Functions
3.3. Basic Formulas for 3D Printing
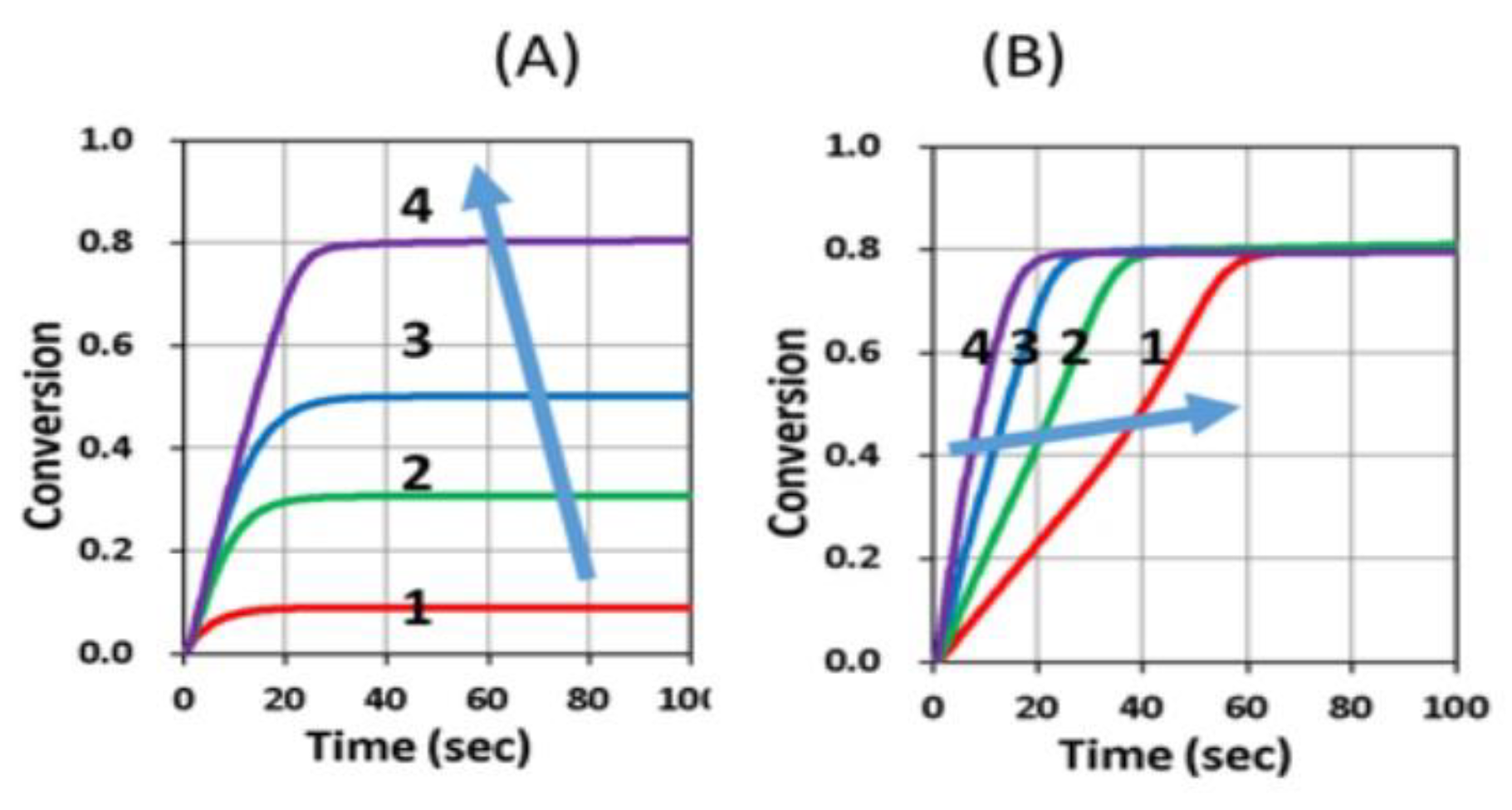
3.4. Conversion Profiles
4. Conclusions
- (i)
- CXL using UVA (365 nm) and a riboflavin solution as the initiator (photosensitizer) has type-I and type-II FRP pathways. Oxygen plays an important role, especially for type-II, in which the oxygen singlet radical has been used to kill cancer cells.
- (ii)
- Synergic effects are achieved by a dual-function enhancer, in which the FRP is improved by the reduction of oxygen inhibition effects. The reported measurement system [17] is a three-component system of C/B/A, in which [C] = IR-140 borate, [B] = 4-(Diphenylphosphino) benzoic acid (4-dppba), and [A] = iodonium salt Ar2I+PF6−, with an initial concentration of [0.1/2.0/3.0] wt%, mixed in a monomer [M] = methacrylate.
- (iii)
- Synergic effects are achieved by a three-initiator system, with two pathways of electron-transfer and oxygen-mediated energy-transfer, in which the presence of amine produces additional initiating radicals and hence improves the FRP. The reported measurement system is a (CQ)/amine/AY system of Kirschner et al. [15], in which higher AY (from 0% to 0.75%) leads to a higher conversion (from about 40% to 60%).
- (iv)
- The reported measurement system [29] was the copper-complex (G1) photoredox catalyst in G1/Iod/NVK systems for FRP and CP, in which the co-initiators/additives Iod and NVK have dual functions of (i) the regeneration of the photoinitiator and (ii) the generation of extra radicals. The synergic effects lead to higher conversion of FRP and CP.
- (v)
- Radical-mediated thiol-ene (TE) photopolymerizations. It offers the advantages of being rapid and optically clear, exhibits delayed gelation, and is relatively uninhibited by oxygen for efficient FRP.
- (vi)
- Superbase photogenerator-based catalyzed thiol−acrylate Michael (TM) addition reaction. It has the unique potential for long-term dark-cure capability and insensitivity to oxygen-inhibition effects. A dual-wavelength combined system of TE and TM could offer very efficient conversion with controlled profiles.
- (vii)
- A dual-wavelength (UV and blue) system was reported for controlled photopolymerization confinement (PC) for volumetric 3D printing and AM using parallel lights and orthogonal lights patterns [11].
- (viii)
- (ix)
- A three-wavelength system is proposed for controlled 3D printing and AM, in which the two competing factors, N-inhibition and S-inhibition, could be independently and selectively tailored to achieve: (i) High conversion of blue-light (without UV-light), enhanced by red-light pre-irradiation for minimal S-inhibition; and (ii) efficient PC initiated by UV-light-produced N-inhibition for reduced confinement thickness and for high print speed.
- (x)
- For dual-wavelength (UV and blue) controlled photopolymerization confinement (PC), the maximum print speed (Smax) is proportional to the dose difference of blue light and UV light, shown by Eq. (9). Curing depth (ZC) is proportional to the light dose, as shown by Eq. (10), which also defines pillar height measured in AM.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fouassier, J.P.; Lalevée, J. Photoinitiators for Polymer Synthesis-Scope, Reactivity and Efficiency; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated polymerization: Advances, challenges and opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mulhaupt, R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Takagishi, K.; Umezu, S. Development of the improving process for the 3D printed structure. Sci. Rep. 2017, 7, 39852. [Google Scholar] [CrossRef]
- Shusteff, M.; Browar, A.E.M.; Kelly, B.E.; Henriksson, J.; Weisgraber, T.H.; Panas, R.M.; Fang, N.; Spadaccini, C.M. One-step Volumetric Additive Manufacturing of Complex Polymer Structures. Sci. Adv. 2017, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Janusziewicz, R.; Tumbleston, J.R.; Quintanilla, A.L.; Mecham, S.J.; DeSimone, M. Layerless fabrication with Continuous Liquid Interface Production. Proc. Natl. Acad. Sci. USA 2016, 113, 11703–11708. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.E.; Bhattacharya, I.; Heidari, H.; Shsteff, C.M.; Taylor, H.K. Volumetric Additive Manufacturing via Tomographic Reconstruction. Science 2019, 363, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- De Beer, M.P.; van der Laan, H.L.; Cole, M.A.; Whelan, R.J. Rapid, Continuous Additive Manufacturing by Volumetric Polymerization Inhibition Patterning. Sci. Adv. 2019, 5, 8. [Google Scholar] [CrossRef]
- Van der Laan, H.L.; Burns, M.A.; Scott, T.F. Volumetric Photopolymerization Confinement through Dual-Wavelength Photoinitiation and Photoinhibition. ACS Macro Lett. 2019, 8, 899–904. [Google Scholar] [CrossRef]
- Childress, K.K.; Kim, K.; Glugla, D.J.; Musgrave, C.B.; Bowman, C.N.; Stansbuery, J.W. Independent control of singlet oxygen and radical generation via irradiation of a two-color photosensitive molecule. Macromolecules 2019, 52, 4968–4978. [Google Scholar] [CrossRef]
- Scott, T.F.; Kowalski, B.A.; Sullivan, A.C. Two-Color Single-Photon Photoinitiation and Photoinhibition for Subdiffraction Photolithography. Science 2009, 324, 913–917. [Google Scholar] [CrossRef]
- Claudino, M.; Zhang, X.; Alim, M.D.; Bowman, C.N. Mechanistic Kinetic Modeling of Thiol-Michael Addition Photopolymerizations via Photocaged “superbase” Generators: An Analytical Approach. Macromolecules 2016, 49, 8061–8074. [Google Scholar] [CrossRef]
- Huang, S.; Sinha, J.; Podgorski, M.; Bowman, C.N. Mechanistic modeling of the Thiol−Michael addition polymerization kinetics: Structural effects of the Thiol and Vinyl monomers. Macromolecules 2018, 51, 5979–5988. [Google Scholar] [CrossRef]
- Chen, K.T.; Cheng, D.C.; Lin, J.T.; Liu, H.W. Thiol-Ene photopolymerization in thick polymers: Kinetics and analytic formulas for the efficacy and crosslink depth. Polymers 2019, 11, 1640. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, J.; Paillard, J.; Bouzrati-Zerell, M.; Becht, J.; Klee, J.E.; Chelli, S.; Lakhdar, S.; Lalevee, J. Aryliodonium ylides as novel and efficient additives for radical chemistry: Example in camphorquinone (CQ)/Amine based photoinitiating systems. Molecules 2019, 24, 2913. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; Halbhuber, A.; Keil, D.; Strehmel, B. NIR-sensitized photoinitiated radical polymerization and proton generation with cyanines and LED arrays. Prog. Org. Coat. 2016, 100, 32–46. [Google Scholar] [CrossRef]
- Bonardi, A.H.; Dumur, F.; Grant, T.M.; Noribent, G.; Gigmes, D.; Lessard, B.H.; Fouassier, J.P.; Lalevee, L. High performance near-Infrared (NIR) photoinitiating systems operating under low light intensity and in the presence of oxygen. Macromolecules 2018, 51, 1314–1324. [Google Scholar] [CrossRef]
- Chiu, Y.C.; Cheng, D.C.; Lin, J.T.; Chen, K.T.; Liu, H.W. Dual-function enhancer for near-infrared photopolymerization: Kinetic modeling for improved efficacy by suppressed oxygen inhibition. IEEE Access 2020, 8, 83465–83471. [Google Scholar] [CrossRef]
- Lin, J.T.; Liu HWChen, K.T.; Chiu, Y.C.; Cheng, D.C. Enhancing UV photopolymerization by a red-light pre-irradiation: Kinetics and modeling strategies for reduced oxygen-inhibition. J. Polymer. Sci. 2020, 58, 683–691. [Google Scholar] [CrossRef]
- Lin, J.T.; Chen, K.T.; Chang, P.K.; Cheng, D.C. Enhancing blue-light-initiated photopolymerization in a three-component system: Kinetic and modeling of conversion strategies. J. Polymer. Res. 2021, 28, 2. [Google Scholar] [CrossRef]
- Lin, J.T.; Chen, K.T.; Cheng, D.C.; Liu, H.W. Dual-wavelength (UV and blue) controlled photopolymerization confinement for 3D-printing: Modeling and analysis of measurements. Polymers 2019, 11, 1819. [Google Scholar] [CrossRef]
- Lin, J.T.; Liu, H.W.; Chen, K.T.; Cheng, D.C. 3-wavelength (UV, blue, red) controlled photopolymerization: Improved conversion and confinement in 3D-printing. IEEE Access 2020, 8, 49353–49362. [Google Scholar] [CrossRef]
- Liu, S.; Chen, H.; Zhang, Y.; Sun, K.; Xu, Y.; Moriet-Savary, F.; Graff, B.; Xiao, P.; Dumur, F.; Lalevee, J. Monocomponent photoinitiators based on Benzophenone-carbazole structure for LED photoinitiating systems and application on 3D printing. Polymers 2020, 12, 1394. [Google Scholar] [CrossRef]
- Chen, K.T.; Lin, J.T.; Liu, H.W. Enhancing Radical-mediated Photopylomerization Efficacy and Crosslink Depth: Kinetic and Model of a Two-monomer System. Res Med. Eng. Sci. 2019, 8, 853–860. [Google Scholar]
- Abdallah, M.; Dumur, F.; Graff, G.; Hijazi, A.; Lalevee, L. High performance dyes based on triphenylamine, cinnamaldehyde and indane-1,3-dione derivatives for blue light induced polymerization for 3D printing and photocomposites. Dyes Pigments 2020, 182, 108580. [Google Scholar] [CrossRef]
- Abdallah, A.; Hijazi, A.; Lin, J.T.; Graff, B.; Dumur, F.; Lalevee, J. Coumarin Derivatives as Photoinitiators in Photo-Oxidation and Photo-Reduction Processes and a Kinetic Model for Simulations of the Associated Polymerization Profiles. ACS Appl. Polym. Mater. 2020, 2, 2769–2780. [Google Scholar] [CrossRef]
- Sun, K.; Liu, S.; Pigot, C.; Brunel, D.; Graff, B.; Nechab, M.; Gigmes, D.; Moriet-Savary, F.; Zhang, Y.; Lalevee, L. Novel Push–Pull Dyes Derived from 1H-cyclopenta[b]naphthalene-1,3(2H)-dione as Versatile Photoinitiators for Photopolymerization and Their Related Applications: 3D Printing and Fabrication of Photocomposites. Catalysts 2020, 10, 1196. [Google Scholar] [CrossRef]
- Mokbel, H.; Anderson, D.; Plenderleith, R.; Dietlin, C.; Gigmes, D.; Moriet-Savary, F.; Dumur, F.; Fouassier, J.P.; Lalevee, L. Copper photoredox catalyst “G1”: A new high performance photoinitiator for near-UV and visible LEDs. J. Polym. Chem. 2017, 8, 5580–5592. [Google Scholar] [CrossRef]
- Mokbel, H.; Anderson, D.; Plenderleith, R.; Dietlin, C.; Gigmes, D.; Moriet-Savary, F.; Dumur, F.; Fouassier, J.P.; Lalevee, L. Simultaneous initiation of radical and cationic polymerization reactions using the “G1” copper complex as photoredox catalyst: Applications of free radical/cationic hybrid photopolymerization in the composites and 3D printingfields. Prog. Org. Coat. 2019, 132, 50–61. [Google Scholar] [CrossRef]
- Lin, J.T.; Lalevee, J.; Cheng, D.C. Kinetics analysis of copper complex photoredox catalyst: Roles of oxygen, thickness, and optimal concentration for radical/cationic hybrid photopolymerization. Polymers 2021, in press. [Google Scholar]
- Wertheimer, C.M.; Elhardt, C.; Kaminsky, S.M.; Pharm, L.; Pei, Q.; Bryan, M.; Afshar, S.; Kochevar, I.E. Enhancing rose Bengal photosensitized protein crosslinking in the cornea. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1845–1852. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.T.; Cheng, D.C. Modeling the efficacy profiles of UV-light activated corneal collagen crosslinking. PLoS ONE 2017, 12, e0175002. [Google Scholar] [CrossRef]
- Lin, J.T. Photochemical Kinetic modeling for oxygen-enhanced UV-light-activated corneal collagen crosslinking. Ophthalmol. Res. 2017, 7, 1–8. [Google Scholar] [CrossRef][Green Version]
- Lin, J.T. Efficacy S-formula and kinetics of oxygen-mediated (type-II) and non-oxygen-mediated (type-I) corneal cross-linking. Ophthalmol. Res. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Lin, J.T.; Chiang, S.; Lin, G.H.; Liu, H.W. In vitro photothermal destruction of cancer cells using gold nanorods and pulsed-train near-infrared lase. J. Nanomaterials. 2012, 861385. [Google Scholar] [CrossRef]
- Lin, J.T.; Chen, K.T.; Liu, H.W. Analysis of kinetics and efficacy of anti-cancer via oxygen-enhanced photodynamic therapy. J. Cancer Res. Update 2018, 7, 21–23. [Google Scholar] [CrossRef]
- Zhu, T.C.; Finlay, J.C.; Zhou, X.; Li, J. Macroscopic modeling of the singlet oxygen production during PDT. Proc. SPIE 2007, 6427, 6427O81–6427O812. [Google Scholar]
- Lin, J.T. Advances of Cancer Synergic Photo-Therapy: Kinetics and Efficacy. Nov. Appr. Cancer Study 2018, 2, NACS.000529. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, W.; Kim, M.; Tae, G. Tumor-targeting nanogel that can function independently for both photodynamic and photothermal therapy and its synergy from the procedure of PDT followed by PTT. J. Controll. Release 2013, 171, 113–121. [Google Scholar] [CrossRef]
- Wang, Y.H.; Chen, S.P.; Liao, A.H.; Yang, Y.C.; Lee, C.R.; Wu, C.H.; Wu, P.C.; Liu, T.M.; Wang, C.R.C.; Li, P.C. Synergic delivery of gold nanorods using multifunctional microsbubbles for enhanced plasmonic photothermal therapy. Sci. Rep. 2014, 5685. [Google Scholar] [CrossRef]
- Lin, J.T.; Chen, K.T.; Cheng, D.C.; Liu, H.W. Modeling the efficacy of radical-mediated photopolymerization: The role of oxygen inhibition, viscosity and induction time. Front. Chem. 2019, 7, 760. [Google Scholar] [CrossRef]
- Ley, C.; Ibrahim, A.; Allonas, X. Isomerization controlled photopolymerization: Effect of dye photophysics on photoinitiation efficiency. Photochem. Photobiol. Sci. 2016, 15, 1054–1060. [Google Scholar] [CrossRef]
- Fouassier, J.-P.; Ruhlmann, D.; Takimoto, Y.; Harada, M.; Kawabata, M. New three-component initiation systems in UV curing: A time-resolved laser-spectroscopy investigation. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 2245–2248. [Google Scholar] [CrossRef]
- Fouassier, J.-.P.; Erddalane, A.; Morlet-Savary, F.; Harada, M.; Kawabata, M. Photoinitiation processes of radical polymerization in the presence of a three- component system based on ketone-amine-bromo compound. Macromolecules 1994, 27, 3349–3356. [Google Scholar] [CrossRef]
- Costela, A.; Dabrio, J.; Figuera, J.M.; Garcia-Morno, I.; Sastre, R. Photochemistry of the photoinitiator 4-[2’-N-N,-(diethylaminoeethoxy]-benzophe- none. Spectroscopy, radical generation and quenching. J. Photochem. Photobiol. 1995, A 92, 213–221. [Google Scholar]
- Catalina, F.; Tercero, J.M.; Peinado, C.; Sastre, R.; Allen, M.N. Photochemistry and photopolymerization study on 2-acetoxy and methyl-2-acetoxy derivatives of thioxanthone as photoinitiators. J. Photochem. Photobiol. 1989, A 50, 249–258. [Google Scholar] [CrossRef]
- Kayaman, N.; Önen, A.; Yagci, Y. Photosensitized free radical polymerization using pyridinium salts. Polym. Bull. 1994, 32, 589–596. [Google Scholar] [CrossRef]
- Allonas, X.; Ley, C.; Bibaut, C.; Fouassier, J.P. Investigation of the triplet quantum yield of thioxanthone by time-resolved thermal lens spectroscopy: Solvent and population lens effects. Chem. Phys. Lett. 2000, 322, 483–490. [Google Scholar] [CrossRef]
- Allonas, X.; Fouassier, J.P.; Angiolini, L.; Care, J.D. Excited-state properties of camphorquinone based monomeric and polymeric photoinitiators. Helv. Chim. Acta 2001, 84, 2577–2588. [Google Scholar] [CrossRef]
- Kucybaa, Z.; Pietrzak, M.; Paczkowski, J.; Linden, L.A.; Rabek, J.F. Kinetic studies of a new photoinitiator hybrid system based on camphorquinone-N-phenylglicyne derivatives for laser polymerization of dental restorative and stereolithographic (3D) formulations. Polymer 1996, 37, 4585–4591. [Google Scholar] [CrossRef]
- Jakubiak, J.; Allonas, X.; Fouassier, J.P.; Sionkowska, A. Camphorquinone-amines photoinitating systems for the initiation of free radical polymerization. Polymer 2003, 44, 5219–5226. [Google Scholar] [CrossRef]
- Allen, N.S.; Pullen, G.; Edge, M. Photoinitiator properties of 2-substituted amido and acryloxyanthraquinones. Eur. Polym. J. 1995, 31, 15–21. [Google Scholar] [CrossRef]
- Fouassier, J.P. Recent advances on visible light photoinitiators of polymerization based on Indane-1,3-dione and related derivatives. Eur. Polym. J. 2021, 143, 110178. [Google Scholar]
- Mallavia, R.; Fimia, A.; García, C.; Sastre, R. Twodyesforholographicrecording material: Panchromatic ion pair from Rose Bengal and methylene blue. J. Mod. Opt. 2001, 48, 941–945. [Google Scholar] [CrossRef]
- Mauguière-Guyonnet, F.; Burget, D.; Fouassier, J.P. Theroleofthephotoinitiating systems towards the inhibiting e ect of phenolic compounds in the photopolymeriza- tion of acrylates. Prog. Org. Coat. 2007, 59, 37–45. [Google Scholar] [CrossRef]
- Mallavia, R.; Amat-Guerri, F.; Fimia, A.; Sastre, R. Synthesisandevaluationasa visible-light polymerization photoinitiator of a New Eosin Ester with an O-Benzo- yl-.alpha.-oxooxime group. Macromolecules 1994, 27, 2643–2646. [Google Scholar] [CrossRef]
- Ibrahim, A.; Ley, C.; Tarzi, O.I.; Ines, O.; Fouassier, J.P. Visible light photoinitiating systems: Toward a good control of the photopolymerization efficiency. J. Photopolym. Sci. Technol. 2010, 23, 101–108. [Google Scholar] [CrossRef]
- Tarzi, O.I.; Allonas, X.; Ley, C.; Fouassier, J.P. Pyrromethenederivativesinthree- component photoinitiating systems for free radical photopolymerization. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2594–2603. [Google Scholar] [CrossRef]
- Costela, A.; García, O.; Garcia-Moreno, I.; Sastre, R. Pyrromethene 567 dye as visible light photoinitiator for free radical polymerization. Macromol. Chem. Phys. 2004, 204, 2233–2239. [Google Scholar]
- Cook, W.D. Photopolymerization kinetics of dimethacrylates using the cam- phorquinone/amine initiator system. Polymer 1992, 33, 600–609. [Google Scholar] [CrossRef]
- Mateo, J.L.; Bosch, P.; Lozano, A.E. Reactivityofradicalsderivedfromdimethy- lanilines in acrylic photopolymerization. Macromolecules 1994, 27, 7794–7799. [Google Scholar] [CrossRef]
- Lalevée, J.; Morlet-Savary, F.; Roz, M.E.; Allons, X.; El Ros, M.; Fouassier, J.P. Thiylradical generation in thiol or disul de containing photosensitive systems. Macromol. Chem. Phys. 2009, 210, 311–319. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiolene click chemistry. Angew. Chem. Int. 2010, 49, 1540–1573. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.T. Unified kinetic theory for hybrid-photocatalysts: A review on new strategies for synergic conversion efficacy and photoredox cycle. J. Polymer. Res. 2021, in press. [Google Scholar]
- Semchishen, J.; Mrochen, A.; Semchishen, V. Model for optimization of the UV-A/Riboflavin strengthening (cross-linking) of the cornea: Percolation threshold. Photochem. Photobiol. 2015, 91, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-mediated polymerization. Prog. Polym. Sci. 2013, 38, 63. [Google Scholar] [CrossRef]
- Krys, P.; Matyjaszewski, K. Kinetics of atom transfer radical polymerization. Eur. Polym. J. 2017, 89, 482. [Google Scholar] [CrossRef]
- Corrigan, N.; Jung, K.; Moad, G.; Hawker, C.J.; Matyjaszewiski, K.; Boyer, C. Reversible-deactivation radical polymerization (Controlled/living radical polymerization): From discovery to materials design and applications. Prog. Polym. Sci. 2020, 111, 101311. [Google Scholar] [CrossRef]
- Bagheri, A.; Fellows, C.M.; Boyer, C. Reversible deactivation radical polymerization: From polymer network synthesis to 3D printing. Adv. Sci. 2021, 8, 2003701. [Google Scholar] [CrossRef]



| System | Light | Enhancer | References |
|---|---|---|---|
| One-component | co-initiators | ||
| blue (477 nm) | CQ/EDB/AY | Kirschner et al. [15] | |
| UV (365 nm) | BP/EDB/Iod | Liu et al. [23] | |
| green (532 nm) | CQ/rose-Bengal | Wertheimer et al. [31] | |
| NIR (785 nm) | phosphine/Iod | Bonardi et al. [17]; Chiu et al. [18] | |
| two-component | co-monomers | ||
| UV (365 nm) | thiol–Vinyl (Michael) - | Claudino et al. [12] | |
| thiol–Ene | Chen et al. [14] | ||
| dual (365 + 660 nm) | -DEGEEA/ZnTTP | van der Laan et al. [9] | |
| Childress [10] | |||
| Lin et al. [19] | |||
| Dual (365 + 430 nm) | -DEGEEA/ZnTTP | Scott et al. [11] | |
| - | Lin et al. [20,21] | ||
| 3-wave (365,430, 660) | DEGEEA/ZnTTP | Lin et al. [20,21 | |
| three-component | UV (365 nm) | Thiol BMP/EVS/BA | Huang et al. [13] |
| UV (365 nm) | PI/EDB/Iod | Liu et al. [24] | |
| UV (405 nm) | Meth/Iod/NPG | Abdallah et al. [26,27] | |
| UV (405 nm) | G1/Iod/NVK | Mokbel et al. [29,30] | |
| 5.9 | 352.7 | Lin et al. [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.-T.; Lalevee, J.; Cheng, D.-C. A Critical Review for Synergic Kinetics and Strategies for Enhanced Photopolymerizations for 3D-Printing and Additive Manufacturing. Polymers 2021, 13, 2325. https://doi.org/10.3390/polym13142325
Lin J-T, Lalevee J, Cheng D-C. A Critical Review for Synergic Kinetics and Strategies for Enhanced Photopolymerizations for 3D-Printing and Additive Manufacturing. Polymers. 2021; 13(14):2325. https://doi.org/10.3390/polym13142325
Chicago/Turabian StyleLin, Jui-Teng, Jacques Lalevee, and Da-Chun Cheng. 2021. "A Critical Review for Synergic Kinetics and Strategies for Enhanced Photopolymerizations for 3D-Printing and Additive Manufacturing" Polymers 13, no. 14: 2325. https://doi.org/10.3390/polym13142325
APA StyleLin, J.-T., Lalevee, J., & Cheng, D.-C. (2021). A Critical Review for Synergic Kinetics and Strategies for Enhanced Photopolymerizations for 3D-Printing and Additive Manufacturing. Polymers, 13(14), 2325. https://doi.org/10.3390/polym13142325








