An Overview on Collagen and Gelatin-Based Cryogels: Fabrication, Classification, Properties and Biomedical Applications
Abstract
:1. Introduction
2. Fabrication, Classifications, and Properties of CBCs and GBCs
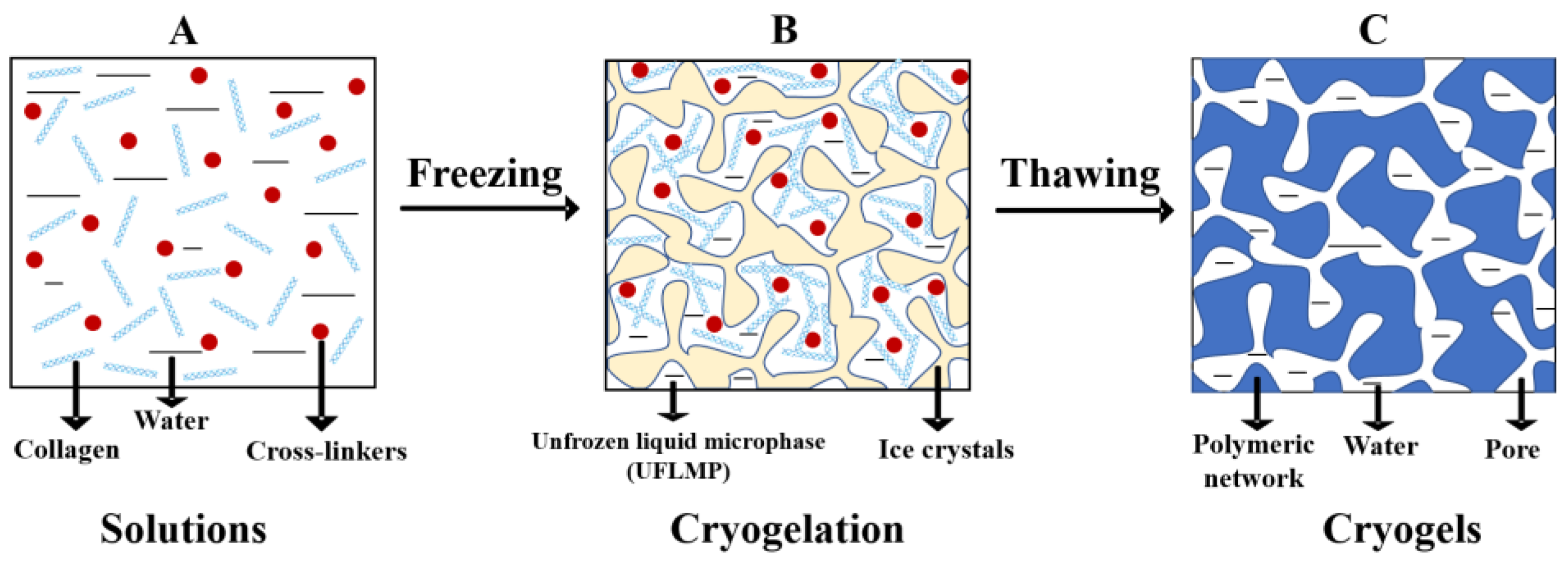
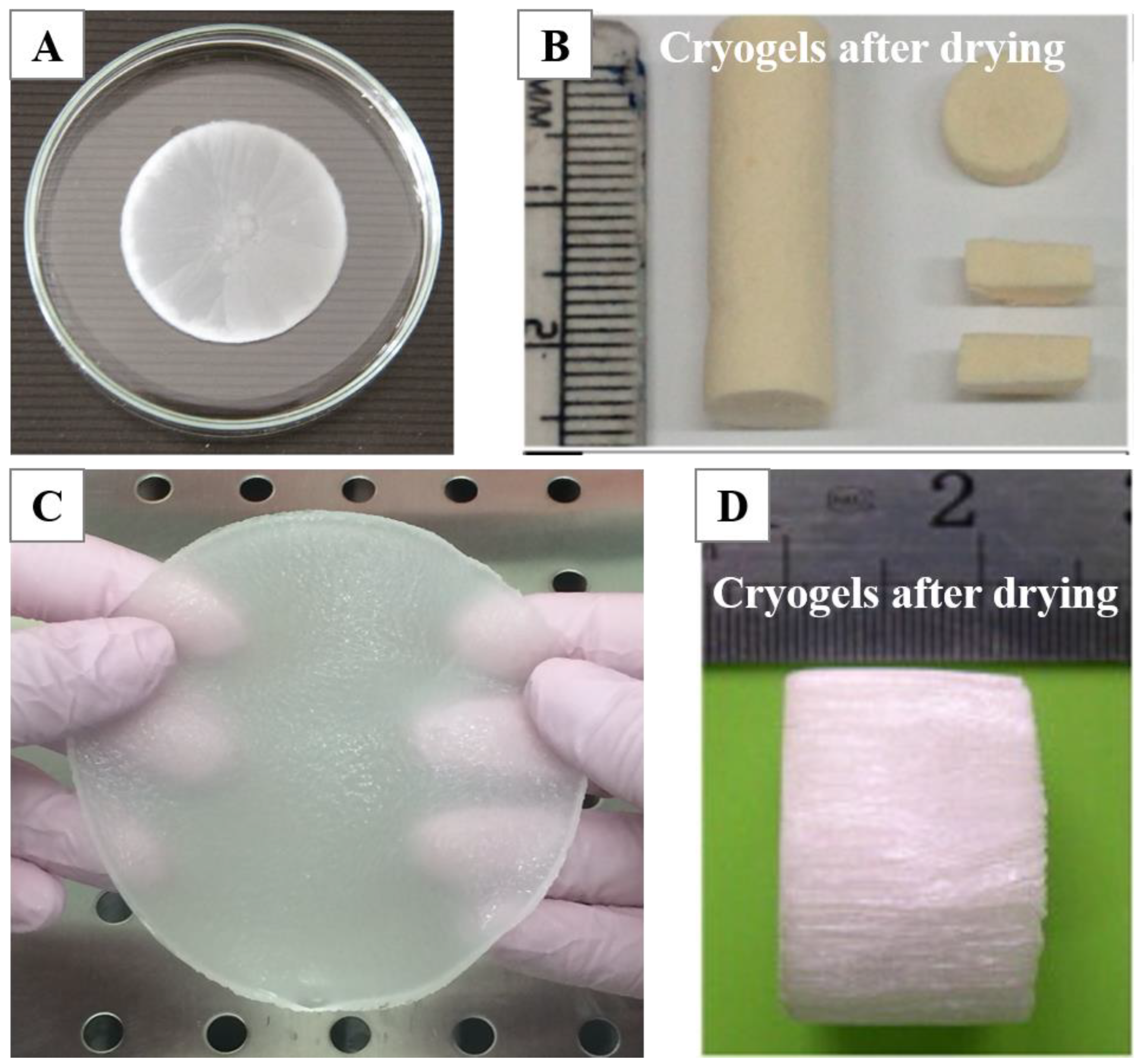
2.1. Fabrication
2.2. Classifications
2.2.1. Physical/Noncovalent Cross-Linking
2.2.2. Covalent/Chemical Cross-Linking
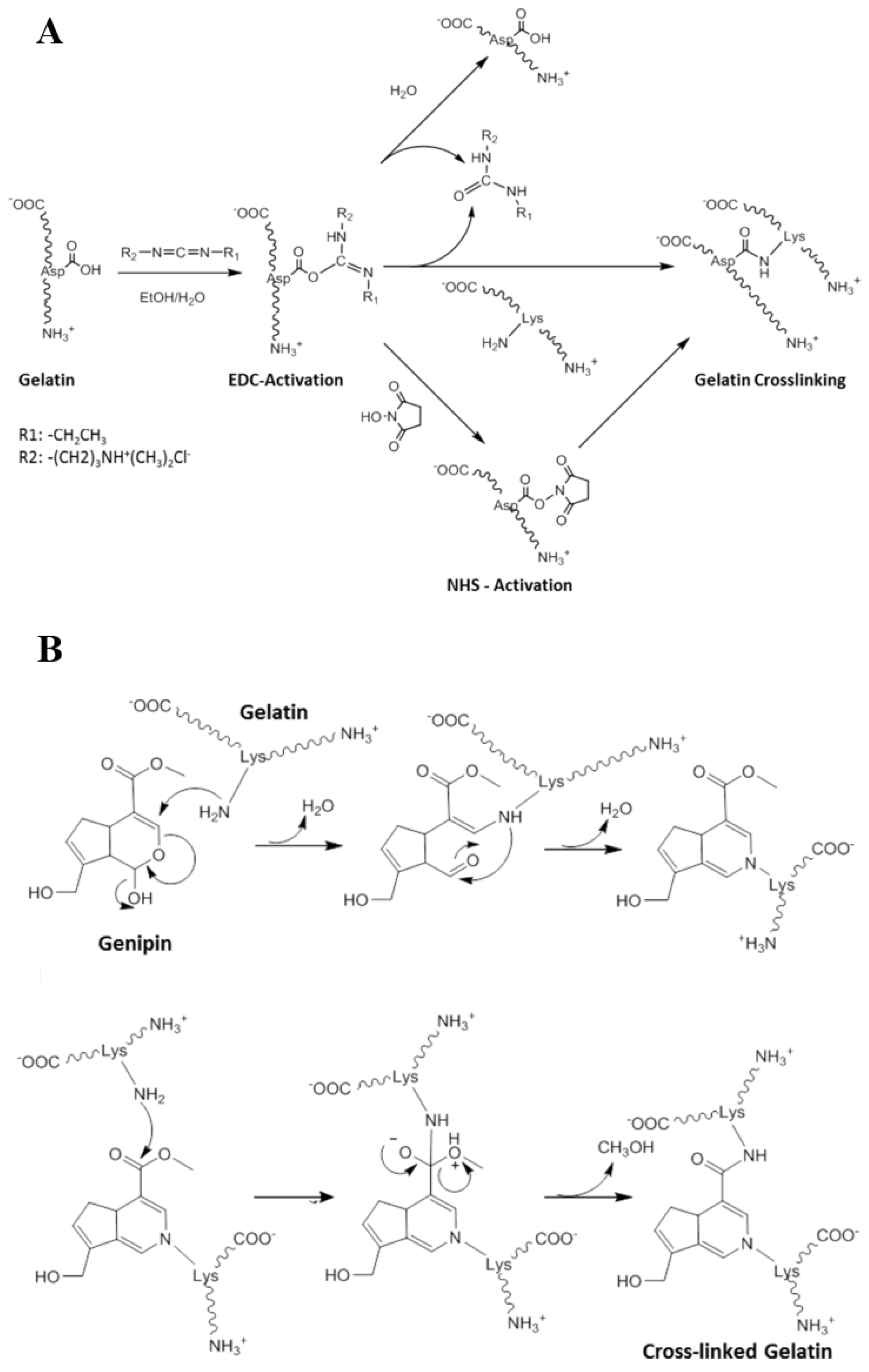
Cross-Linking via Small-Molecule Cross-Linkers
Enzymes-Mediated Cross-Linking
Cross-Linking via Macromolecular Cross-Linkers
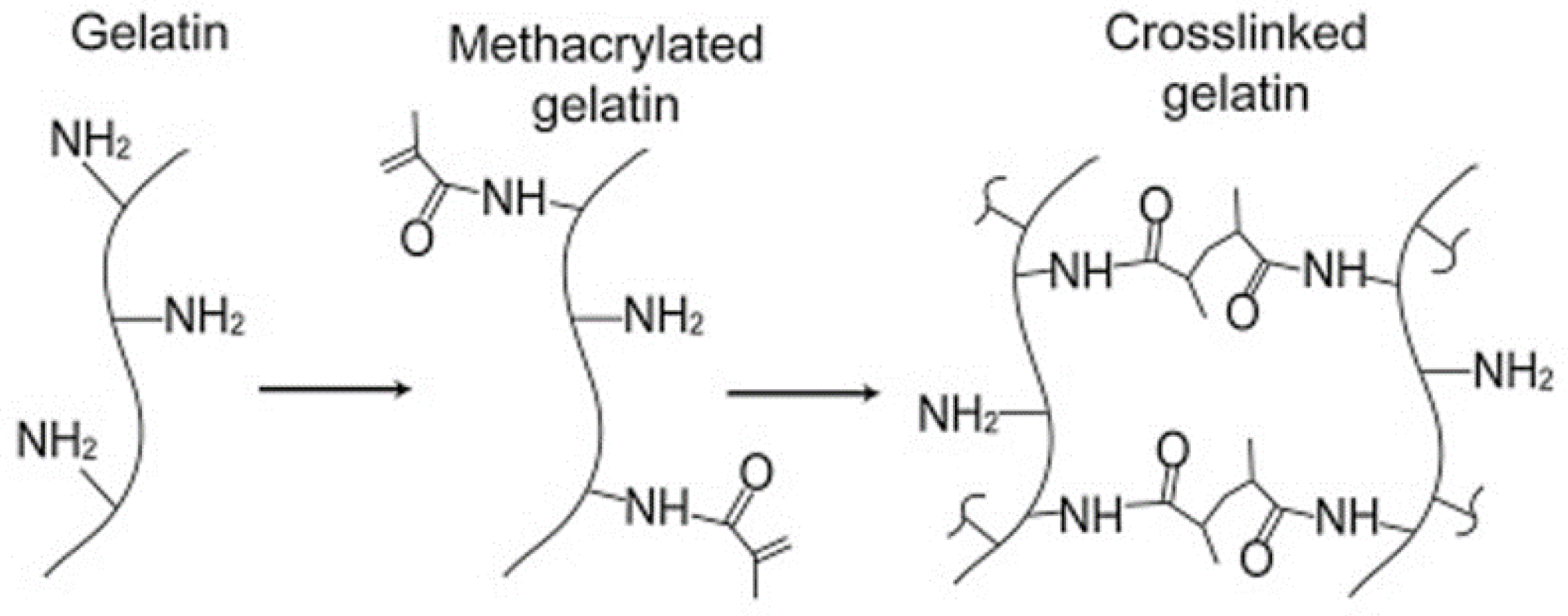
Free-Radical Cross-Linking Polymerization
2.2.3. Cryogels Based on Collagen/Gelatin
2.3. Properties of CBCs and GBCs
2.3.1. Pore Properties
2.3.2. Swelling Properties
3. Biomedical Applications of CBCs and GBCs
3.1. Tissue Engineering
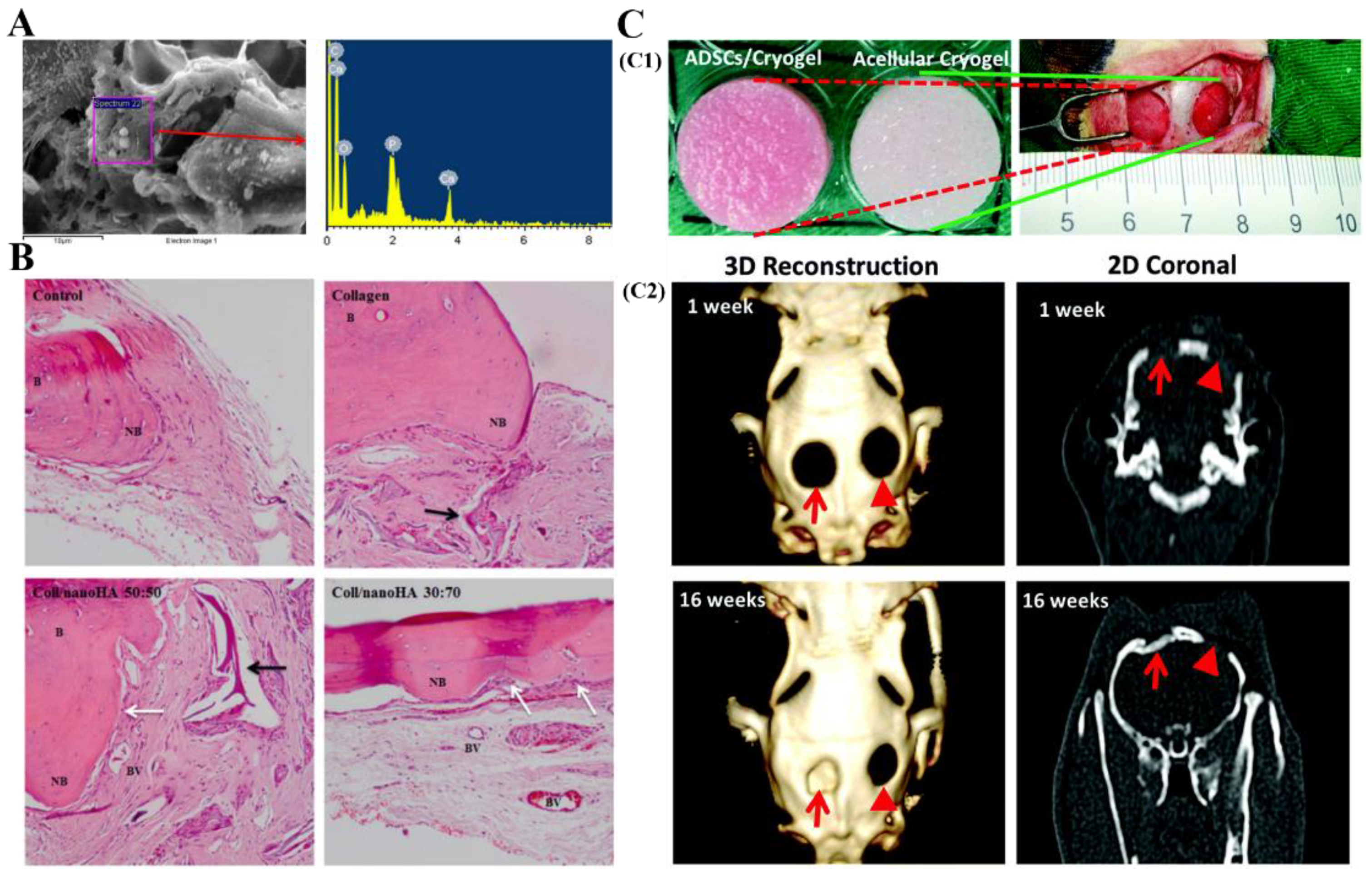
3.1.1. Bone Tissue Engineering
3.1.2. Cartilage Tissue Engineering
3.1.3. Skin Tissue Engineering/Wound Healing
3.1.4. Vascular Tissue Engineering
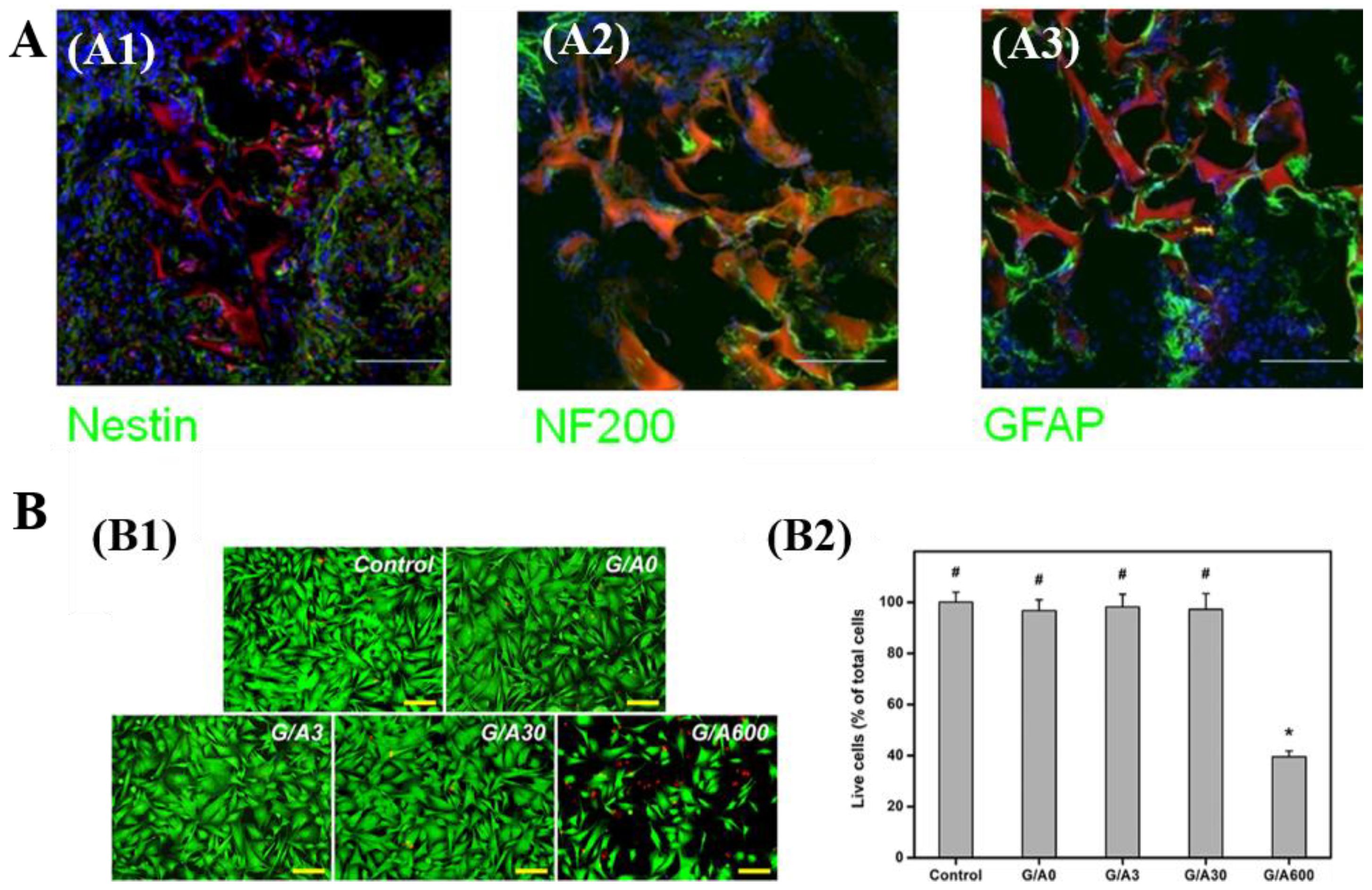
3.1.5. Neural Tissue Engineering and Other Tissue Engineering
3.2. Other Biomedical Applications
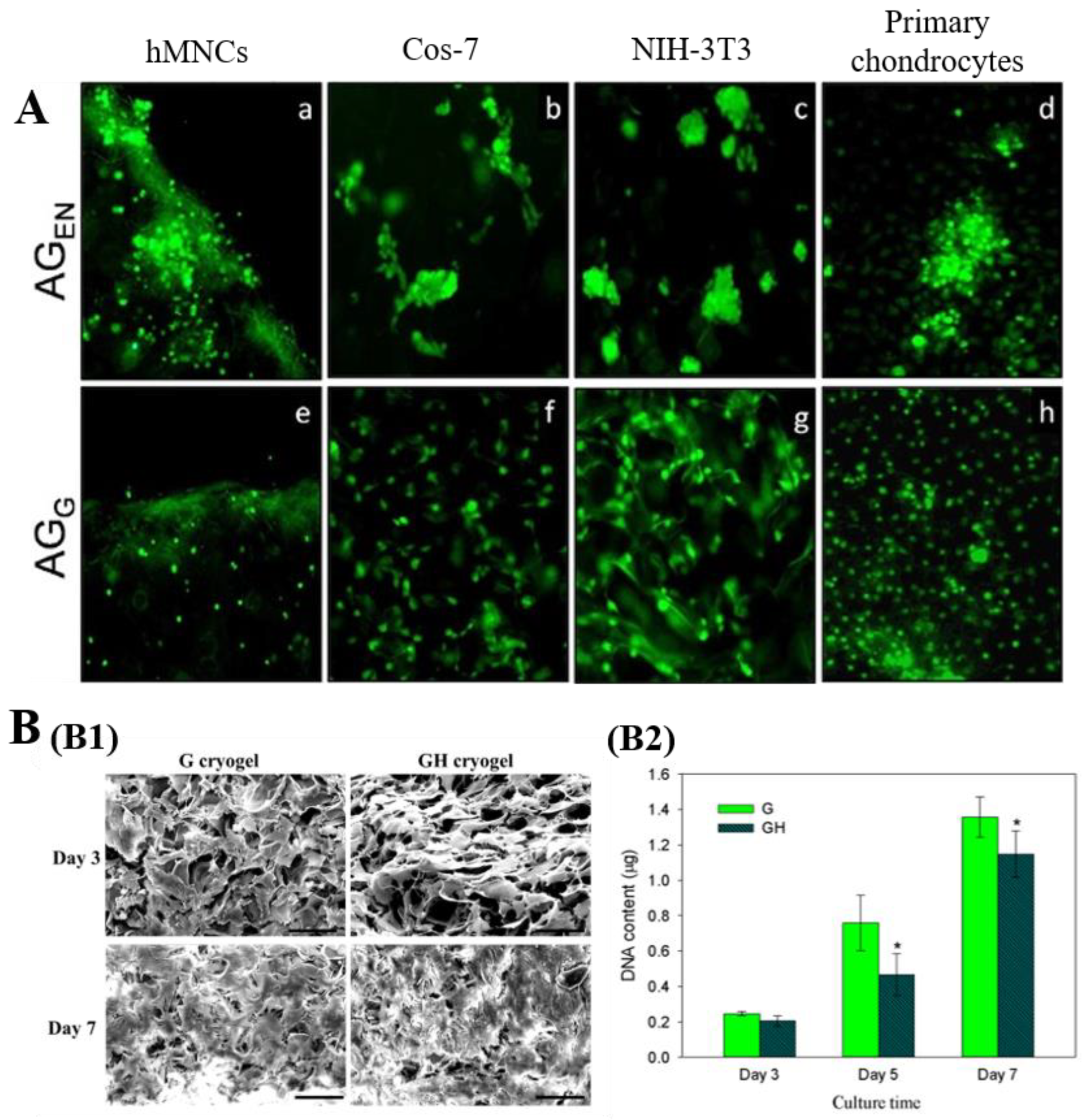
3.2.1. Cell Culture
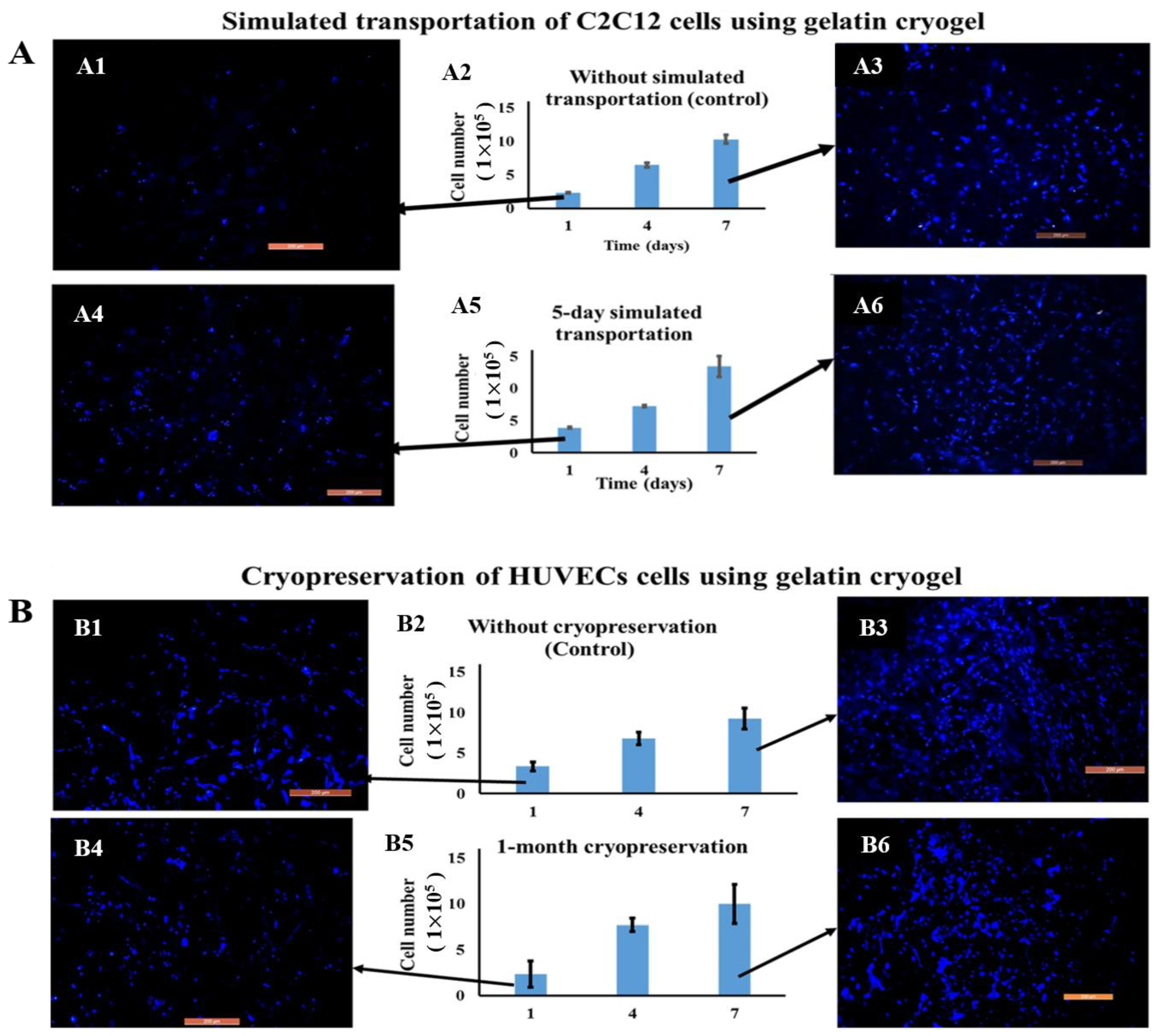
3.2.2. Cell Transportation and Cryopreservation
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lozinsky, V.I. A Brief History of Polymeric Cryogels. In Advances in Polymer Science; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2014; Volume 263, pp. 1–48. [Google Scholar]
- Lozinsky, V.I. Cryostructuring of polymeric systems. 50.† cryogels and cryotropic gel-formation: Terms and definitions. Gels 2018, 4, 77. [Google Scholar] [CrossRef] [Green Version]
- Lozinsky, V.I. Cryostructuring of Polymeric Systems. 55. Retrospective View on the More than 40 Years of Studies Performed in the AN Nesmeyanov Institute of Organoelement Compounds with Respect of the Cryostructuring Processes in Polymeric Systems. Gels 2020, 6, 29. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Galaev, I.Y.; Plieva, F.M.; Savina, I.; Jungvid, H.; Mattiasson, B. Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol. 2003, 21, 445–451. [Google Scholar] [CrossRef]
- Razavi, M.; Qiao, Y.; Thakor, A.S. Three-dimensional cryogels for biomedical applications. J. Biomed. Mater. Res. Part A 2019, 107, 2736–2755. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Plieva, F.M.; Galaev, I.Y.; Mattiasson, B. The potential of polymeric cryogels in bioseparation. Bioseparation 2001, 10, 163–188. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, D.; Zhu, S.; Liu, X.; Zhang, H. 3D cryogel composites as adsorbent for isolation of protein and small molecules. Talanta 2019, 191, 229–234. [Google Scholar] [CrossRef]
- Ertürk, G.; Mattiasson, B. Cryogels-versatile tools in bioseparation. J. Chromatogr. A 2014, 1357, 24–35. [Google Scholar] [CrossRef]
- Kumari, J.; Karande, A.A.; Kumar, A. Combined Effect of Cryogel Matrix and Temperature-Reversible Soluble–Insoluble Polymer for the Development of in Vitro Human Liver Tissue. ACS Appl. Mater. Interfaces 2015, 8, 264–277. [Google Scholar] [CrossRef]
- Zhou, D.; Shen, S.; Yun, J.; Yao, K.; Lin, D.-Q. Cryo-copolymerization preparation of dextran-hyaluronate based supermacroporous cryogel scaffolds for tissue engineering applications. Front. Chem. Sci. Eng. 2012, 6, 339–347. [Google Scholar] [CrossRef]
- Hixon, K.R.; Lu, T.; Sell, S.A. A comprehensive review of cryogels and their roles in tissue engineering applications. Acta Biomater. 2017, 62, 29–41. [Google Scholar] [CrossRef]
- Plieva, F.M.; Galaev, I.Y.; Mattiasson, B. Macroporous gels prepared at subzero temperatures as novel materials for chromatography of particulate-containing fluids and cell culture applications. J. Sep. Sci. 2007, 30, 1657–1671. [Google Scholar] [CrossRef]
- Raina, D.B.; Isaksson, H.; Teotia, A.; Lidgren, L.; Tägil, M.; Kumar, A. Biocomposite macroporous cryogels as potential carrier scaffolds for bone active agents augmenting bone regeneration. J. Control. Release 2016, 235, 365–378. [Google Scholar] [CrossRef]
- Kostova, B.; Momekova, D.; Petrov, P.; Momekov, G.; Toncheva-Moncheva, N.; Tsvetanov, C.B.; Lambov, N. Poly(ethoxytriethyleneglycol acrylate) cryogels as novel sustained drug release systems for oral application. Polymer 2011, 52, 1217–1222. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, C.; Luo, X.; Wang, X.; Jiang, H. Recent advances of collagen-based biomaterials: Multi-hierarchical structure, modification and biomedical applications. Mater. Sci. Eng. C 2019, 99, 1509–1522. [Google Scholar] [CrossRef]
- Lin, W.; Yan, L.; Mu, C.; Li, W.; Zhang, M.; Zhu, Q. Effect of pH on gelatin self-association investigated by laser light scattering and atomic force microscopy. Polym. Int. 2002, 51, 233–238. [Google Scholar] [CrossRef]
- Eastoe, J.E. The amino acid composition of mammalian collagen and gelatin. Biochem. J. 1955, 61, 589–600. [Google Scholar] [CrossRef] [Green Version]
- Yue, K.; Santiago, G.T.-D.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidenko, N.; Schuster, C.; Bax, D.; Raynal, N.; Farndale, R.; Best, S.; Cameron, R. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 2015, 25, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Rezaeeyazdi, M.; Colombani, T.; Memic, A.; Bencherif, S.A. Injectable Hyaluronic Acid-co-Gelatin Cryogels for Tissue-Engineering Applications. Materials 2018, 11, 1374. [Google Scholar] [CrossRef] [Green Version]
- Shariatzadeh, F.J.; Solouk, A.; Khoulenjani, S.B.; Bonakdar, S.; Mirzadeh, H. Injectable and reversible preformed cryogels based on chemically crosslinked gelatin methacrylate (GelMA) and physically crosslinked hyaluronic acid (HA) for soft tissue engineering. Colloids Surf. B Biointerfaces 2021, 203, 111725. [Google Scholar] [CrossRef]
- Cecilia, A.; Baecker, A.; Hamann, E.; Rack, A.; van de Kamp, T.; Gruhl, F.; Hofmann, R.; Moosmann, J.; Hahn, S.; Kashef, J.; et al. Optimizing structural and mechanical properties of cryogel scaffolds for use in prostate cancer cell culturing. Mater. Sci. Eng. C 2017, 71, 465–472. [Google Scholar] [CrossRef]
- Carvalho, D.N.; López-Cebral, R.; Sousa, R.O.; Alves, A.L.; Reys, L.L.; Silva, S.S.; Oliveira, J.M.; Reis, R.L.; Silva, T.H. Marine collagen-chitosan-fucoidan cryogels as cell-laden biocomposites envisaging tissue engineering. Biomed. Mater. 2020, 15, 055030. [Google Scholar] [CrossRef]
- Agarwal, G.; Kumar, N.; Srivastava, A. Highly elastic, electroconductive, immunomodulatory graphene crosslinked collagen cryogel for spinal cord regeneration. Mater. Sci. Eng. C 2021, 118, 111518. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffold design and fabrication technologies for engineering tissues-state of the art and future perspectives. J. Biomater. Sci. Polym. Ed. 2001, 12, 107–124. [Google Scholar] [CrossRef]
- Shoichet, M.S. Polymer Scaffolds for Biomaterials Applications. Macromolecules 2009, 43, 581–591. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Zhang, L.; Xu, Z.; Dai, H.; Wu, W. Nanocellulose/Gelatin Composite Cryogels for Controlled Drug Release. ACS Sustain. Chem. Eng. 2019, 7, 6381–6389. [Google Scholar] [CrossRef]
- Gorgieva, S.; Kokol, V. Processing of gelatin-based cryogels with improved thermomechanical resistance, pore size gradient, and high potential for sustainable protein drug release. J. Biomed. Mater. Res. Part A 2014, 103, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Saraswathy, K.; Agarwal, G.; Srivastava, A. Hyaluronic acid microneedles-laden collagen cryogel plugs for ocular drug delivery. J. Appl. Polym. Sci. 2020, 137, 1–14. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, X.; Zhang, Z.; Liang, Y.; Yin, Z.; Chen, B.; Bai, L.; Han, Y.; Guo, B. Degradable Gelatin-Based IPN Cryogel Hemostat for Rapidly Stopping Deep Noncompressible Hemorrhage and Simultaneously Improving Wound Healing. Chem. Mater. 2020, 32, 6595–6610. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryogels on the basis of natural and synthetic polymers: Preparation, properties and areas of implementation. Russ. Chem. Rev. 2002, 71, 489–511. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Okay, O. Basic Principles of Cryotropic Gelation. In Advances in Polymer Science; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2014; Volume 263, pp. 49–101. [Google Scholar]
- Lozinsky, V.I.; Vainerman, E.S.; Rogozhin, S.V. Study of cryostructurization of polymer systems. II: The influence of freezing of reacting mass on the properties of products in the preparation of covalently cross-linked gels. Colloid Polym. Sci. 1982, 260, 776–780. [Google Scholar] [CrossRef]
- Mu, C.; Liu, F.; Cheng, Q.; Li, H.; Wu, B.; Zhang, G.; Lin, W. Collagen Cryogel Cross-Linked by Dialdehyde Starch. Macromol. Mater. Eng. 2010, 295, 100–107. [Google Scholar] [CrossRef]
- Podorozhko, E.; Kurskaya, E.; Kulakova, V.; Lozinsky, V. Cryotropic structuring of aqueous dispersions of fibrous collagen: Influence of the initial pH values. Food Hydrocoll. 2000, 14, 111–120. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Effect of Cryogenic Treatment on the Rheological Properties of Gelatin Hydrogels. J. Bioact. Compat. Polym. 2010, 25, 498–512. [Google Scholar] [CrossRef] [Green Version]
- Schoof, H.; Heschel, I. Control of pore structure and size in freeze-dried collagen sponges. J. Biomed. Mater. Res. 2001, 58, 352–357. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Li, X.; Wen, P.; Zhang, Y.; Long, Y.; Wang, X.; Guo, Y.; Xing, F.; Gao, J. Preparation of aligned porous gelatin scaffolds by unidirectional freeze-drying method. Acta Biomater. 2010, 6, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, Q.; Sun, J.; Zhou, Q. Preparation of poly(ethylene glycol) aligned porous cryogels using a unidirectional freezing technique. Soft Matter 2012, 8, 3620–3626. [Google Scholar] [CrossRef]
- Chau, M.; De France, K.; Kopera, B.; Machado, V.R.; Rosenfeldt, S.; Reyes, L.; Chan, K.J.W.; Förster, S.; Cranston, E.D.; Hoare, T.; et al. Composite Hydrogels with Tunable Anisotropic Morphologies and Mechanical Properties. Chem. Mater. 2016, 28, 3406–3415. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Kulakova, V.K.; Ivanov, R.V.; Petrenko, A.; Rogulska, O.; Petrenko, Y.A. Cryostructuring of polymer systems. 47. Preparation of wide porous gelatin-based cryostructurates in sterilizing organic media and assessment of the suitability of thus formed matrices as spongy scaffolds for 3D cell culturing. e-Polymers 2017, 18, 175–186. [Google Scholar] [CrossRef]
- Luong, D.; Yergeshov, A.; Zoughaib, M.; Sadykova, F.R.; Gareev, B.; Savina, I.; Abdullin, T.I. Transition metal-doped cryogels as bioactive materials for wound healing applications. Mater. Sci. Eng. C 2019, 103, 109759. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.W.; Huang, D.; Chang, W.H. Evaluation of Gelatin Hydrogel Crosslinked with Various Crosslinking Agents as Bioadhesives: In Vitro Study. J. Biomed. Mater. Res. Part A 1999, 46, 520–530. [Google Scholar] [CrossRef]
- Lai, J.-Y. Biocompatibility of chemically cross-linked gelatin hydrogels for ophthalmic use. J. Mater. Sci. Mater. Med. 2010, 21, 1899–1911. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Roveri, N.; Rubini, K. Stabilization of gelatin films by crosslinking with genipin. Biomaterials 2002, 23, 4827–4832. [Google Scholar] [CrossRef]
- Elowsson, L.; Kirsebom, H.; Carmignac, V.; Durbeej, M.; Mattiasson, B. Porous protein-based scaffolds prepared through freezing as potential scaffolds for tissue engineering. J. Mater. Sci. Mater. Electron. 2012, 23, 2489–2498. [Google Scholar] [CrossRef] [PubMed]
- Aigner, T.B.; Haynl, C.; Salehi, S.; O’Connor, A.; Scheibel, T. Nerve guidance conduit design based on self-rolling tubes. Mater. Today Bio 2020, 5, 100042. [Google Scholar] [CrossRef] [PubMed]
- Henderson, T.M.A.; Ladewig, K.; Haylock, D.N.; McLean, K.M.; O’Connor, A.J. Cryogels for biomedical applications. J. Mater. Chem. B 2013, 1, 2682–2695. [Google Scholar] [CrossRef] [PubMed]
- Dainiak, M.B.; Allan, I.U.; Savina, I.; Cornelio, L.; James, E.S.; James, S.L.; Mikhalovsky, S.; Jungvid, H.; Galaev, I.Y. Gelatin–fibrinogen cryogel dermal matrices for wound repair: Preparation, optimisation and in vitro study. Biomaterials 2010, 31, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Kathuria, N.; Tripathi, A.; Kar, K.K.; Kumar, A. Synthesis and characterization of elastic and macroporous chitosan–gelatin cryogels for tissue engineering. Acta Biomater. 2009, 5, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, S.; Göktürk, D.; Bölgen, N. Effect of crosslinking methods on the structure and biocompatibility of polyvinyl alcohol/gelatin cryogels. Bio-Med. Mater. Eng. 2016, 27, 327–340. [Google Scholar] [CrossRef]
- Van Vlierberghe, S. Crosslinking strategies for porous gelatin scaffolds. J. Mater. Sci. 2016, 51, 4349–4357. [Google Scholar] [CrossRef]
- Rose, J.B.; Pacelli, S.; El Haj, A.J.; Dua, H.S.; Hopkinson, A.; White, L.J.; Rose, F.R.A.J. Gelatin-Based Materials in Ocular Tissue Engineering. Materials 2014, 7, 3106–3135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, H.C.; Chang, W.H.; Liang, H.F.; Lee, M.H.; Sung, H.W. Crosslinking structures of gelatin hydrogels crosslinked with genipin or a water-soluble carbodiimide. J. Appl. Polym. Sci. 2004, 91, 4017–4026. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Huang, R.N.; Sung, H.-W.; Liang, H.C. In vitro evaluation of the genotoxicity of a naturally occurring crosslinking agent (genipin) for biologic tissue fixation. J. Biomed. Mater. Res. 2000, 52, 58–65. [Google Scholar] [CrossRef]
- Poursamar, S.A.; Lehner, A.N.; Azami, M.; Ebrahimibarough, S.; Samadikuchaksaraei, A.; Antunes, A.P.M. The effects of crosslinkers on physical, mechanical, and cytotoxic properties of gelatin sponge prepared via in-situ gas foaming method as a tissue engineering scaffold. Mater. Sci. Eng. C 2016, 63, 1–9. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Long, H.; Ma, K.; Zhang, J.; Ren, X.; Zhang, J. Assessment of the characteristics and biocompatibility of gelatin sponge scaffolds prepared by various crosslinking methods. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Gu, L.; Shan, T.; Ma, Y.-X.; Tay, F.R.; Niu, L. Novel Biomedical Applications of Crosslinked Collagen. Trends Biotechnol. 2019, 37, 464–491. [Google Scholar] [CrossRef]
- Yung, C.; Wu, L.; Tullman, J.; Payne, G.; Bentley, W.; Barbari, T. Transglutaminase crosslinked gelatin as a tissue engineering scaffold. J. Biomed. Mater. Res. Part A 2007, 83, 1039–1046. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Ren, X.; Long, H.; Qian, H.; Ma, K.; Guo, Y. Enzymatically crosslinked gelatin hydrogel promotes the proliferation of adipose tissue-derived stromal cells. PeerJ 2016, 4, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Kirsebom, H.; Elowsson, L.; Berillo, D.; Cozzi, S.; Inci, I.; Pişkin, E.; Galaev, I.Y.; Mattiasson, B. Enzyme-Catalyzed Crosslinking in a Partly Frozen State: A New Way to Produce Supermacroporous Protein Structures. Macromol. Biosci. 2012, 13, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Embree, H.D.; Brown, E.M.; Taylor, M.M.; Payne, G.F. Enzyme-catalyzed gel formation of gelatin and chitosan: Potential for in situ applications. Biomaterials 2003, 24, 2831–2841. [Google Scholar] [CrossRef]
- Tan, H.; Wu, B.; Li, C.; Mu, C.; Li, H.; Lin, W. Collagen cryogel cross-linked by naturally derived dialdehyde carboxymethyl cellulose. Carbohydr. Polym. 2015, 129, 17–24. [Google Scholar] [CrossRef]
- Inci, I.; Kirsebom, H.; Galaev, I.Y.; Mattiasson, B.; Pişkin, E. Gelatin cryogels crosslinked with oxidized dextran and containing freshly formed hydroxyapatite as potential bone tissue-engineering scaffolds. J. Tissue Eng. Regen. Med. 2012, 7, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Berillo, D.; Volkova, N. Preparation and physicochemical characteristics of cryogel based on gelatin and oxidised dextran. J. Mater. Sci. 2014, 49, 4855–4868. [Google Scholar] [CrossRef]
- Koshy, S.; Ferrante, T.C.; Lewin, S.A.; Mooney, D.J. Injectable, porous, and cell-responsive gelatin cryogels. Biomaterials 2014, 35, 2477–2487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and Rheological Properties of Methacrylamide Modified Gelatin Hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Wang, C.; Yang, L.; He, Y.; Xiao, H.; Lin, W. Microsphere-structured hydrogel crosslinked by polymerizable protein-based nanospheres. Polymer 2020, 211, 123114. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 26, 1211–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrov, P.D.; Tsvetanov, C.B. Cryogels via UV Irradiation. In Advances in Polymer Science; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2014; Volume 263, pp. 199–222. [Google Scholar]
- Kwon, S.; Lee, S.S.; Sivashanmugam, A.; Kwon, J.; Kim, S.H.L.; Noh, M.Y.; Kwon, S.K.; Jayakumar, R.; Hwang, N.S. Bioglass-Incorporated Methacrylated Gelatin Cryogel for Regeneration of Bone Defects. Polymer 2018, 10, 914. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kwon, S.; Hwang, N.S.; Kang, B.J. Clinical Application of Bone Morphogenetic Protein-2 Microcarriers Fabricated by the Cryopolymerization of Gelatin Methacrylate for the Treatment of Radial Fracture in Two Dogs. In Vivo 2018, 32, 575–581. [Google Scholar] [CrossRef]
- Han, M.-E.; Kang, B.J.; Kim, S.-H.; Kim, H.D.; Hwang, N.S. Gelatin-based extracellular matrix cryogels for cartilage tissue engineering. J. Ind. Eng. Chem. 2017, 45, 421–429. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Cnudde, V.; Dubruel, P.; Masschaele, B.; Cosijns, A.; De Paepe, I.; Jacobs, P.J.S.; Van Hoorebeke, L.; Remon, J.P.; Schacht, E. Porous Gelatin Hydrogels: 1. Cryogenic Formation and Structure Analysis. Biomacromolecules 2007, 8, 331–337. [Google Scholar] [CrossRef]
- Hoch, E.; Schuh, C.; Hirth, T.; Tovar, G.E.M.; Borchers, K. Stiff gelatin hydrogels can be photo-chemically synthesized from low viscous gelatin solutions using molecularly functionalized gelatin with a high degree of methacrylation. J. Mater. Sci. Mater. Med. 2012, 23, 2607–2617. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Saini, R. Preparation and characterization of biocompatible spongy cryogels of poly(vinyl alcohol)-gelatin and study of water sorption behaviour. Polym. Int. 2005, 54, 1233–1242. [Google Scholar] [CrossRef]
- Choi, S.M.; Singh, D.; Kumar, A.; Oh, T.H.; Cho, Y.W.; Han, S.S. Porous Three-Dimensional PVA/Gelatin Sponge for Skin Tissue Engineering. Int. J. Polym. Mater. 2013, 62, 384–389. [Google Scholar] [CrossRef]
- Jain, E.; Srivastava, A.; Kumar, A. Macroporous interpenetrating cryogel network of poly(acrylonitrile) and gelatin for biomedical applications. J. Mater. Sci. Mater. Electron. 2009, 20, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Milakin, K.A.; Capáková, Z.; Acharya, U.; Vajďák, J.; Morávková, Z.; Hodan, J.; Humpolíček, P.; Bober, P. Biocompatible and antibacterial gelatin-based polypyrrole cryogels. Polymer 2020, 197, 122491. [Google Scholar] [CrossRef]
- Sarkar, J.; Kumar, A. Thermo-responsive polymer aided spheroid culture in cryogel based platform for high throughput drug screening. Analyst 2016, 141, 2553–2567. [Google Scholar] [CrossRef]
- Tripathi, A.; Kathuria, N.; Kumar, A. Elastic and macroporous agarose-gelatin cryogels with isotropic and anisotropic porosity for tissue engineering. J. Biomed. Mater. Res. Part A 2009, 90, 680–694. [Google Scholar] [CrossRef]
- Huang, Y.; Onyeri, S.; Siewe, M.; Moshfeghian, A.; Madihally, S.V. In vitro characterization of chitosan–gelatin scaffolds for tissue engineering. Biomaterials 2005, 26, 7616–7627. [Google Scholar] [CrossRef] [PubMed]
- Berillo, D.; Elowsson, L.; Kirsebom, H. Oxidized Dextran as Crosslinker for Chitosan Cryogel Scaffolds and Formation of Polyelectrolyte Complexes between Chitosan and Gelatin. Macromol. Biosci. 2012, 12, 1090–1099. [Google Scholar] [CrossRef]
- Han, F.; Dong, Y.; Su, Z.; Yin, R.; Song, A.; Li, S. Preparation, characteristics and assessment of a novel gelatin–chitosan sponge scaffold as skin tissue engineering material. Int. J. Pharm. 2014, 476, 124–133. [Google Scholar] [CrossRef]
- Nguyen, V.C.; Nguyen, V.B.; Hsieh, M.F. Curcumin-Loaded Chitosan/Gelatin Composite Sponge for Wound Healing Application. Int. J. Polym. Sci. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Bhat, S.; Vishnoi, T.; Nayak, V.; Kumar, A. Three-Dimensional Supermacroporous Carrageenan-Gelatin Cryogel Matrix for Tissue Engineering Applications. BioMed Res. Int. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, Y.-M.; Gwon, H.-J.; Choi, J.-H.; Shin, J.; Nho, Y.-C.; Jeong, S.I.; Chong, M.S.; Lee, Y.M.; Kwon, I.K.; Kim, S.E. Preparation and biocompatibility study of gelatin/kappa-carrageenan scaffolds. Macromol. Res. 2010, 18, 29–34. [Google Scholar] [CrossRef]
- Allan, I.U.; Tolhurst, B.; Shevchenko, R.V.; Dainiak, M.B.; Illsley, M.; Ivanov, A.; Jungvid, H.; Galaev, I.Y.; James, S.L.; Mikhalovsky, S.V.; et al. An in vitro evaluation of fibrinogen and gelatin containing cryogels as dermal regeneration scaffolds. Biomater. Sci. 2016, 4, 1007–1014. [Google Scholar] [CrossRef] [Green Version]
- Gorgieva, S.; Girandon, L.; Kokol, V. Mineralization potential of cellulose-nanofibrils reinforced gelatine scaffolds for promoted calcium deposition by mesenchymal stem cells. Mater. Sci. Eng. C 2017, 73, 478–489. [Google Scholar] [CrossRef]
- Kim, I.; Lee, S.S.; Bae, S.; Lee, H.; Hwang, N.S. Heparin Functionalized Injectable Cryogel with Rapid Shape-Recovery Property for Neovascularization. Biomacromolecules 2018, 19, 2257–2269. [Google Scholar] [CrossRef]
- Kim, I.; Lee, S.S.; Kim, S.H.L.; Bae, S.; Lee, H.; Hwang, N.S. Osteogenic Effects of VEGF-Overexpressed Human Adipose-Derived Stem Cells with Whitlockite Reinforced Cryogel for Bone Regeneration. Macromol. Biosci. 2019, 19, e1800460. [Google Scholar] [CrossRef]
- Tyeb, S.; Shiekh, P.A.; Verma, V.; Kumar, A. Adipose-Derived Stem Cells (ADSCs) Loaded Gelatin-Sericin-Laminin Cryogels for Tissue Regeneration in Diabetic Wounds. Biomacromolecules 2019, 21, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-T.; Shalumon, K.T.; Chang, K.-H.; Sheu, C.; Chen, J.-P. Investigation of synergistic effects of inductive and conductive factors in gelatin-based cryogels for bone tissue engineering. J. Mater. Chem. B 2016, 4, 1827–1841. [Google Scholar] [CrossRef] [PubMed]
- Kemence, N.; Bolgen, N. Gelatin-and hydroxyapatite-based cryogels for bone tissue engineering: Synthesis, characterization, in vitro and in vivo biocompatibility. J. Tissue Eng. Regen. Med. 2017, 11, 20–33. [Google Scholar] [CrossRef]
- Ozturk, B.Y.; Eğri, S.; Ozturk, A.M.; Yetkin, H.; Göktas, G.; Elmas, C.; Pişkin, E.; Erdogan, D.; Inci, I. The treatment of segmental bone defects in rabbit tibiae with vascular endothelial growth factor (VEGF)-loaded gelatin/hydroxyapatite “cryogel” scaffold. Eur. J. Orthop. Surg. Traumatol. 2012, 23, 767–774. [Google Scholar] [CrossRef]
- Bhat, S.; Tripathi, A.; Kumar, A. Supermacroprous chitosan–agarose–gelatin cryogels: In vitro characterization and in vivo assessment for cartilage tissue engineering. J. R. Soc. Interface 2010, 8, 540–554. [Google Scholar] [CrossRef] [Green Version]
- Bhat, S.; Kumar, A. Cell proliferation on three-dimensional chitosan–agarose–gelatin cryogel scaffolds for tissue engineering applications. J. Biosci. Bioeng. 2012, 114, 663–670. [Google Scholar] [CrossRef]
- Gupta, A.; Bhat, S.; Jagdale, P.R.; Chaudhari, B.P.; Lidgren, L.; Gupta, K.C.; Kumar, A. Evaluation of Three-Dimensional Chitosan-Agarose-Gelatin Cryogel Scaffold for the Repair of Subchondral Cartilage Defects: AnIn VivoStudy in a Rabbit Model. Tissue Eng. Part A 2014, 20, 3101–3111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, K.-H.; Liao, H.-T.; Chen, J.-P. Preparation and characterization of gelatin/hyaluronic acid cryogels for adipose tissue engineering: In vitro and in vivo studies. Acta Biomater. 2013, 9, 9012–9026. [Google Scholar] [CrossRef] [PubMed]
- Katsen-Globa, A.; Meiser, I.; Petrenko, Y.A.; Ivanov, R.V.; Lozinsky, V.I.; Zimmermann, H.; Petrenko, A. Towards ready-to-use 3-D scaffolds for regenerative medicine: Adhesion-based cryopreservation of human mesenchymal stem cells attached and spread within alginate–gelatin cryogel scaffolds. J. Mater. Sci. Mater. Med. 2014, 25, 857–871. [Google Scholar] [CrossRef]
- Luo, L.-J.; Lai, J.-Y.; Chou, S.-F.; Hsueh, Y.-J.; Ma, D.H.-K. Development of gelatin/ascorbic acid cryogels for potential use in corneal stromal tissue engineering. Acta Biomater. 2018, 65, 123–136. [Google Scholar] [CrossRef]
- Razavi, M.; Hu, S.; Thakor, A.S. A collagen based cryogel bioscaffold coated with nanostructured polydopamine as a platform for mesenchymal stem cell therapy. J. Biomed. Mater. Res. Part A 2018, 106, 2213–2228. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Primavera, R.; Kevadiya, B.D.; Wang, J.; Thakor, A.S. A Collagen Based Cryogel Bioscaffold that Generates Oxygen for Islet Transplantation. Adv. Funct. Mater. 2020, 30, 1902463. [Google Scholar] [CrossRef] [PubMed]
- Salgado, C.L.; Grenho, L.; Fernandes, M.H.; Colaço, B.J.; Monteiro, F.J. Biodegradation, biocompatibility, and osteoconduction evaluation of collagen-nanohydroxyapatite cryogels for bone tissue regeneration. J. Biomed. Mater. Res. Part A 2015, 104, 57–70. [Google Scholar] [CrossRef]
- Rodrigues, S.; Salgado, C.; Sahu, A.; Garcia, M.; Fernandes, M.H.; Monteiro, F. Preparation and characterization of collagen-nanohydroxyapatite biocomposite scaffolds by cryogelation method for bone tissue engineering applications. J. Biomed. Mater. Res. Part A 2012, 101, 1080–1094. [Google Scholar] [CrossRef]
- Van Rie, J.; Declercq, H.; Van Hoorick, J.; Dierick, M.; Van Hoorebeke, L.; Cornelissen, R.; Thienpont, H.; Dubruel, P.; Van Vlierberghe, S. Cryogel-PCL combination scaffolds for bone tissue repair. J. Mater. Sci. Mater. Electron. 2015, 26, 1–7. [Google Scholar] [CrossRef]
- O’Brien, F.; Harley, B.; Yannas, I.; Gibson, L. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 2005, 26, 433–441. [Google Scholar] [CrossRef]
- Lien, S.-M.; Ko, L.-Y.; Huang, T.-J. Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering. Acta Biomater. 2009, 5, 670–679. [Google Scholar] [CrossRef]
- Savina, I.; Gun’Ko, V.; Turov, V.V.; Dainiak, M.; Phillips, G.J.; Galaev, I.Y.; Mikhalovsky, S. Porous structure and water state in cross-linked polymer and protein cryo-hydrogels. Soft Matter 2011, 7, 4276–4283. [Google Scholar] [CrossRef]
- Sharma, A.; Bhat, S.; Nayak, V.; Kumar, A. Efficacy of supermacroporous poly(ethylene glycol)–gelatin cryogel matrix for soft tissue engineering applications. Mater. Sci. Eng. C 2015, 47, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, P.; Bhat, S.; Nayak, V.; Kumar, A. Study of Different Delivery Modes of Chondroitin Sulfate Using Microspheres and Cryogel Scaffold for Application in Cartilage Tissue Engineering. Int. J. Polym. Mater. 2014, 63, 859–872. [Google Scholar] [CrossRef]
- Zhu, S.; Yuan, Q.; Yin, T.; You, J.; Gu, Z.; Xiong, S.; Hu, Y. Self-assembly of collagen-based biomaterials: Preparation, characterizations and biomedical applications. J. Mater. Chem. B 2018, 6, 2650–2676. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Panigrahi, S.; Zhang, C. Collagen as Biomaterial for Medical Application--Drug Delivery and Scaffolds for Tissue Regeneration: A Review. Biol. Eng. 2010, 2, 63–88. [Google Scholar] [CrossRef]
- Chang, H.C.; Yang, C.; Feng, F.; Lin, F.H.; Wang, C.H. Bone morphogenetic protein-2 loaded poly(D,L-lactide-co-glycolide) microspheres enhance osteogenic potential of gelatin/hydroxyapatite/beta-tricalcium phosphate cryogel composite for alveolar ridge augmentation. J. Formos. Med. Assoc. 2017, 116, 973–981. [Google Scholar] [CrossRef]
- Dubruel, P.; Unger, R.; Van Vlierberghe, S. Porous gelatin hydrogels: 2. In vitro cell interaction study. Biomacromolecules 2007, 8, 338–344. [Google Scholar] [CrossRef]
- Priya, S.G.; Gupta, A.; Jain, E.; Sarkar, J.; Damania, A.; Jagdale, P.R.; Chaudhari, B.P.; Gupta, K.C.; Kumar, A. Bilayer Cryogel Wound Dressing and Skin Regeneration Grafts for the Treatment of Acute Skin Wounds. ACS Appl. Mater. Interfaces 2016, 8, 15145–15159. [Google Scholar] [CrossRef]
- Wang, H.M.; Chou, Y.T.; Wen, Z.H.; Wang, C.Z.; Chen, C.H.; Ho, M.L. Novel biodegradable porous scaffold applied to skin regeneration. PLoS ONE 2013, 8, 56330. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhu, L.; Han, Q.; Liu, W.; Mao, X.; Li, Y.; Yu, N.; Feng, S.; Fu, Q.; Wang, X.; et al. Preformed gelatin microcryogels as injectable cell carriers for enhanced skin wound healing. Acta Biomater. 2015, 25, 291–303. [Google Scholar] [CrossRef]
- Choi, Y.S.; Lee, S.B.; Hong, S.R.; Lee, Y.M.; Song, K.W.; Park, M.H. Studies on gelatin-based sponges. Part III: A comparative study of cross-linked gelatin/alginate, gelatin/hyaluronate and chitosan/hyaluronate sponges and their application as a wound dressing in full-thickness skin defect of rat. J. Mater. Sci. Mater. Med. 2001, 12, 67–73. [Google Scholar] [CrossRef]
- Vrana, N.E.; Cahill, P.A.; McGuinness, G.B. Endothelialization of PVA/gelatin cryogels for vascular tissue engineering: Effect of disturbed shear stress conditions. J. Biomed. Mater. Res. Part A 2010, 94, 1080–1090. [Google Scholar] [CrossRef]
- Liu, Y.; Vrana, N.E.; Cahill, P.; McGuinness, G.B. Physically crosslinked composite hydrogels of PVA with natural macromolecules: Structure, mechanical properties, and endothelial cell compatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90, 492–502. [Google Scholar] [CrossRef]
- Hixon, K.R.; Eberlin, C.T.; Kadakia, P.U.; McBride-Gagyi, S.H.; Jain, E.; Sell, S.A. A comparison of cryogel scaffolds to identify an appropriate structure for promoting bone regeneration. Biomed. Phys. Eng. Express 2016, 2, 035014. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, Y.; Zhang, L.; Huang, Y.; Zuo, D.; Cai, Q.; Yang, X. Comparative study of gelatin cryogels reinforced with hydroxyapatites with different morphologies and interfacial bonding. Biomed. Mater. 2020, 15, 035012. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, J.; Li, L.; Du, Z.; Cai, Q.; Yang, X. Hydroxyapatite nanowire composited gelatin cryogel with improved mechanical properties and cell migration for bone regeneration. Biomed. Mater. 2019, 14, 045001. [Google Scholar] [CrossRef]
- Teotia, A.; Qayoom, I.; Kumar, A. Endogenous Platelet-Rich Plasma Supplements/Augments Growth Factors Delivered via Porous Collagen-Nanohydroxyapatite Bone Substitute for Enhanced Bone Formation. ACS Biomater. Sci. Eng. 2018, 5, 56–69. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Chen, C.-H.; Hsiao, C.-Y.; Chen, J.-P. Incorporation of chitosan in biomimetic gelatin/chondroitin-6-sulfate/hyaluronan cryogel for cartilage tissue engineering. Carbohydr. Polym. 2015, 117, 722–730. [Google Scholar] [CrossRef]
- Chen, C.-H.; Kuo, C.-Y.; Wang, Y.-J.; Chen, J.-P. Dual Function of Glucosamine in Gelatin/Hyaluronic Acid Cryogel to Modulate Scaffold Mechanical Properties and to Maintain Chondrogenic Phenotype for Cartilage Tissue Engineering. Int. J. Mol. Sci. 2016, 17, 1957. [Google Scholar] [CrossRef]
- Jurga, M.; Dainiak, M.B.; Sarnowska, A.; Jablonska, A.; Tripathi, A.; Plieva, F.M.; Savina, I.; Strojek, L.; Jungvid, H.; Kumar, A. The performance of laminin-containing cryogel scaffolds in neural tissue regeneration. Biomaterials 2011, 32, 3423–3434. [Google Scholar] [CrossRef]
- Wu, S.; Kuss, M.A.; Qi, D.; Hong, J.; Wang, H.-J.; Zhang, W.; Chen, S.; Ni, S.; Duan, B. Development of Cryogel-Based Guidance Conduit for Peripheral Nerve Regeneration. ACS Appl. Bio Mater. 2019, 2, 4864–4871. [Google Scholar] [CrossRef]
- Singh, A.; Shiekh, P.A.; Das, M.; Seppälä, J.V.; Kumar, A. Aligned Chitosan-Gelatin Cryogel-Filled Polyurethane Nerve Guidance Channel for Neural Tissue Engineering: Fabrication, Characterization, and In Vitro Evaluation. Biomacromolecules 2019, 20, 662–673. [Google Scholar] [CrossRef]
- Vishnoi, T.; Singh, A.; Teotia, A.; Kumar, A. Chitosan-Gelatin-Polypyrrole Cryogel Matrix for Stem Cell Differentiation into Neural Lineage and Sciatic Nerve Regeneration in Peripheral Nerve Injury Model. ACS Biomater. Sci. Eng. 2019, 5, 3007–3021. [Google Scholar] [CrossRef]
- Tao, J.; Hu, Y.; Wang, S.; Zhang, J.; Liu, X.; Gou, Z.; Cheng, H.; Liu, Q.; Zhang, Q.; You, S.; et al. A 3D-engineered porous conduit for peripheral nerve repair. Sci. Rep. 2017, 7, 46038. [Google Scholar] [CrossRef] [Green Version]
- Tsung, L.H.; Chang, K.-H.; Chen, J.-P. Osteogenesis of Adipose-Derived Stem Cells on Three-Dimensional, Macroporous Gelatin–Hyaluronic Acid Cryogels. Biomed. Eng. Appl. Basis Commun. 2011, 23, 127–133. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, J.; Hua, W.; Darabi, A.; Song, X.; Song, C.; Zhong, W.; Xing, M.M.Q.; Qiu, X. Mussel-Inspired Conductive Cryogel as Cardiac Tissue Patch to Repair Myocardial Infarction by Migration of Conductive Nanoparticles. Adv. Funct. Mater. 2016, 26, 4293–4305. [Google Scholar] [CrossRef]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Memic, A.; Colombani, T.; Eggermont, L.; Rezaeeyazdi, M.; Steingold, J.; Rogers, Z.J.; Joshi-Navare, K.; Mohammed, H.S.; Bencherif, S.A. Latest Advances in Cryogel Technology for Biomedical Applications. Adv. Ther. 2019, 2, 1800114. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Li, X.; Xie, X.; Lei, J.; Ge, L.; Yuan, L.; Li, D.; Mu, C. Controlling the Pore Structure of Collagen Sponge by Adjusting the Cross-Linking Degree for Construction of Heterogeneous Double-Layer Bone Barrier Membranes. ACS Appl. Bio Mater. 2020, 3, 2058–2067. [Google Scholar] [CrossRef]
- Zhang, Y.; Leng, H.; Du, Z.; Huang, Y.; Liu, X.; Zhao, Z.; Zhang, X.; Cai, Q.; Yang, X. Efficient regeneration of rat calvarial defect with gelatin-hydroxyapatite composite cryogel. Biomed. Mater. 2020, 15, 065005. [Google Scholar] [CrossRef]
- Hixon, K.R.; Eberlin, C.T.; Lu, T.; Neal, S.M.; Case, N.D.; McBride-Gagyi, S.H.; Sell, S.A. The calcification potential of cryogel scaffolds incorporated with various forms of hydroxyapatite for bone regeneration. Biomed. Mater. 2017, 12, 025005. [Google Scholar] [CrossRef]
- Yang, S.-H.; Hsu, C.-K.; Wang, K.-C.; Hou, S.-M.; Lin, F.-H. Tricalcium phosphate and glutaraldehyde crosslinked gelatin incorporating bone morphogenetic protein—A viable scaffold for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 74, 468–475. [Google Scholar] [CrossRef]
- Farokhi, M.; Jonidi Shariatzadeh, F.; Solouk, A.; Mirzadeh, H. Alginate Based Scaffolds for Cartilage Tissue Engineering: A Review. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 230–247. [Google Scholar] [CrossRef]
- Irawan, V.; Sung, T.-C.; Higuchi, A.; Ikoma, T. Collagen Scaffolds in Cartilage Tissue Engineering and Relevant Approaches for Future Development. Tissue Eng. Regen. Med. 2018, 15, 673–697. [Google Scholar] [CrossRef]
- Yuan, T.; Zhang, L.; Li, K.; Fan, H.; Fan, Y.; Liang, J.; Zhang, X. Collagen hydrogel as an immunomodulatory scaffold in cartilage tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 102, 337–344. [Google Scholar] [CrossRef]
- Armiento, A.; Stoddart, M.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef]
- Chang, C.-H.; Liu, H.-C.; Lin, C.-C.; Chou, C.-H.; Lin, F.-H. Gelatin-chondroitin-hyaluronan tri-copolymer scaffold for cartilage tissue engineering. Biomaterials 2003, 24, 4853–4858. [Google Scholar] [CrossRef]
- Gelse, K.; Poschl, E.; Aigner, T. Collagens--structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Bhat, S.; Chaudhari, B.P.; Gupta, K.C.; Tägil, M.; Zheng, M.H.; Kumar, A.; Lidgren, L. Cell factory-derived bioactive molecules with polymeric cryogel scaffold enhance the repair of subchondral cartilage defect in rabbits. J. Tissue Eng. Regen. Med. 2015, 11, 1689–1700. [Google Scholar] [CrossRef]
- Zhong, S.P.; Zhang, Y.; Lim, C.T. Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 510–525. [Google Scholar] [CrossRef]
- Groeber, F.; Holeiter, M.; Hampel, M.; Hinderer, S.; Schenke-Layland, K. Skin tissue engineering—In vivo and in vitro applications. Adv. Drug Deliv. Rev. 2011, 63, 352–366. [Google Scholar] [CrossRef]
- Shevchenko, R.V.; Eeman, M.; Rowshanravan, B.; Allan, I.U.; Savina, I.; Illsley, M.; Salmon, M.; James, S.E.; Mikhalovsky, S. The in vitro characterization of a gelatin scaffold, prepared by cryogelation and assessed in vivo as a dermal replacement in wound repair. Acta Biomater. 2014, 10, 3156–3166. [Google Scholar] [CrossRef]
- Sensharma, P.; Madhumathi, G.; Jayant, R.D.; Jaiswal, A.K. Biomaterials and cells for neural tissue engineering: Current choices. Mater. Sci. Eng. C 2017, 77, 1302–1315. [Google Scholar] [CrossRef]
- Gu, X. Progress and perspectives of neural tissue engineering. Front. Med. 2015, 9, 401–411. [Google Scholar] [CrossRef]
- Sazwi, N.N.; Nalina, T.; Rahim, Z.H.A. Antioxidant and cytoprotective activities of Piper betle, Areca catechu, Uncaria gambir and betel quid with and without calcium hydroxide. BMC Complement. Altern. Med. 2013, 13, 351. [Google Scholar] [CrossRef] [Green Version]
- Chularojmontri, L.; Suwatronnakorn, M.; Wattanapitayakul, S.K. Phyllanthus emblicaL. Enhances Human Umbilical Vein Endothelial Wound Healing and Sprouting. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The Use of Natural Polymers in Tissue Engineering: A Focus on Electrospun Extracellular Matrix Analogues. Polymer 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Gandomani, M.G.; Lotfi, A.S.; Tamandani, D.K.; Arjmand, S.; Alizadeh, S. The enhancement of differentiating adipose derived mesenchymal stem cells toward hepatocyte like cells using gelatin cryogel scaffold. Biochem. Biophys. Res. Commun. 2017, 491, 1000–1006. [Google Scholar] [CrossRef]
- Fassina, L.; Saino, E.; Visai, L.; Avanzini, M.A.; De Angelis, M.G.C.; Benazzo, F.; Van Vlierberghe, S.; Dubruel, P.; Magenes, G. Use of a Gelatin Cryogel as Biomaterial Scaffold in the Differentiation Process of Human Bone Marrow Stromal Cells. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; Volume 2010, pp. 247–250. [Google Scholar]
- Tripathi, A.; Vishnoi, T.; Singh, D.; Kumar, A. Modulated Crosslinking of Macroporous Polymeric Cryogel Affects In Vitro Cell Adhesion and Growth. Macromol. Biosci. 2013, 13, 838–850. [Google Scholar] [CrossRef]
- Kao, H.-H.; Kuo, C.-Y.; Chen, K.-S.; Chen, J.-P. Preparation of Gelatin and Gelatin/Hyaluronic Acid Cryogel Scaffolds for the 3D Culture of Mesothelial Cells and Mesothelium Tissue Regeneration. Int. J. Mol. Sci. 2019, 20, 4527. [Google Scholar] [CrossRef] [Green Version]
- Vrana, N.E.; Matsumura, K.; Hyon, S.H.; Geever, L.M.; Kennedy, J.E.; Lyons, J.G.; Higginbotham, C.L.; Cahill, P.A.; McGuinness, G.B. Cell encapsulation and cryostorage in PVA-gelatin cryogels: Incorporation of carboxylated epsilon-poly-l-lysine as cryoprotectant. J. Tissue Eng. Regen. Med. 2012, 6, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Kumari, J.; Kumar, A. Development of polymer based cryogel matrix for transportation and storage of mammalian cells. Sci. Rep. 2017, 7, srep41551. [Google Scholar] [CrossRef] [PubMed]





| Polymers Added to Collagen/Gelatin | Crosslinker/Initiator | Temperature of Cryotropic Gelation (°C) | References |
|---|---|---|---|
| Gelatin/PVA | None/Glutaraldehyde | −12 °C/−16 °C | [51,76,77] |
| Gelatin/PAN | Glutaraldehyde | −12 °C | [78] |
| GelMA/PPY | None | −24 °C | [79] |
| Gelatin/PNIPAAm | Glutaraldehyde | −12 °C | [80] |
| Gelatin/Agarose | Glutaraldehyde | −12 °C | [81] |
| Gelatin/Chitosan | Glutaraldehyde/Oxidized dextran | −20 °C/−12 °C | [50,82,83,84,85] |
| Gelatin/Carrageenan | EDC/Glutaraldehyde | −12 °C | [86,87] |
| Gelatin/Fibrinogen | Glutaraldehyde | −12 °C | [49,88] |
| Gelatin/Nanocellulose | Dialdehyde starch | 4 °C | [27,89] |
| Gelatin/Heparin | EDC/NHS | −20 °C | [90,91] |
| Gelatin/Sericin | Glutaraldehyde | −12 °C | [92] |
| Gelatin/Hydroxyapatite | EDC/Glutaraldehyde/Oxidized dextran | −20 °C | [64,93,94,95] |
| Gelatin/Chitosan/Agarose | Glutaraldehyde | −12 °C | [96,97,98] |
| Gelatin/Hyaluronic acid | EDC | −20 °C | [99] |
| Gelatin/Alginate | No | −15 °C | [100] |
| Gelatin/Ascorbic acid | EDC | −20 °C | [101] |
| Collagen/polydopamine | EDC | −20 °C | [102] |
| Collagen/calcium peroxide | EDC | −20 °C | [103] |
| Collagen/graphene | EDC/NHS | −12 °C | [24] |
| Collagen/hydroxyapatite | EDC | −18 °C | [104,105] |
| Polymers | Cell Type | Tissue | Reference |
|---|---|---|---|
| Gelatin + Fibrinogen | Human dermal fibroblasts | Skin | [49,88] |
| Gelatin + Sericin + Laminin | Adipose-derived stem cell | [92] | |
| Gelatin + Polyvinylpyrrolidone-iodine | Fibroblasts, Keratinocytes | [116] | |
| Gelatin + Collagen + Hyaluronic acid | Human skin cells | [117] | |
| Gelatin + Poly (vinyl alcohol) | Fibroblasts | [77] | |
| Gelatin + Polymethyl methacrylate | Adipose-derived stem cell | [118] | |
| Gelatin + Alginate/Hyaluronic acid | None | [119] | |
| Gelatin + Pectin + Transition metal | Fibroblasts | [42] | |
| Gelatin + Poly (vinyl alcohol) | Endothelial cells | Vascular | [120,121] |
| Gelatin + Heparin | Human umbilical vein endothelial cells | Bone | [90] |
| Gelatin + Chitosan | Bone osteosarcoma-derived cells | [122] | |
| GelMA + Hydroxyapatites | Bone marrow mesenchymal stromal cells | [123,124] | |
| Gelatin + Hydroxyapatite + Vascular endothelial growth factor | None | [95] | |
| GelMA + Bioglass | Human tonsil-derived mesenchymal stem cells | [71] | |
| Collagen/nanohydroxyapatite | Human bone marrow stromal cells | [104,105,125] | |
| Gelatin + Chondroitin-6-suifate + hyalueonan | Chondrocytes | Cartilage | [126] |
| GelMA + Mecs | Chondrocytes | [73] | |
| Gelatin + Hyaluronic acid | Chondrocytes | [127] | |
| Gelatin + Laminin | Human cord blood-derived stem cells | Neural | [128] |
| GelMA + methacrylated hyaluronic acid | rabbit Schwann cells | [129] | |
| Gelatin + Chitosan | Neuro 2a cells bone, Bone marrow stem cells | [130] | |
| Gelatin + Chitosan + Polypyrrole | Bone marrow stem cells | [131] | |
| Gelatin | NIH-3T3 cells | [132] | |
| Gelatin + Haluronic acid | Adipose-derived stem cells | Adipose | [99,133] |
| Gelatin + Ascorbic acid | Corneal keratocytes | Corneal | [101] |
| GelMA + poly(ethylene glycol) diacrylate | None | Cardiac | [134] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Wang, C.; Wang, C.; Xiao, Y.; Lin, W. An Overview on Collagen and Gelatin-Based Cryogels: Fabrication, Classification, Properties and Biomedical Applications. Polymers 2021, 13, 2299. https://doi.org/10.3390/polym13142299
He Y, Wang C, Wang C, Xiao Y, Lin W. An Overview on Collagen and Gelatin-Based Cryogels: Fabrication, Classification, Properties and Biomedical Applications. Polymers. 2021; 13(14):2299. https://doi.org/10.3390/polym13142299
Chicago/Turabian StyleHe, Yujing, Chunhua Wang, Chenzhi Wang, Yuanhang Xiao, and Wei Lin. 2021. "An Overview on Collagen and Gelatin-Based Cryogels: Fabrication, Classification, Properties and Biomedical Applications" Polymers 13, no. 14: 2299. https://doi.org/10.3390/polym13142299
APA StyleHe, Y., Wang, C., Wang, C., Xiao, Y., & Lin, W. (2021). An Overview on Collagen and Gelatin-Based Cryogels: Fabrication, Classification, Properties and Biomedical Applications. Polymers, 13(14), 2299. https://doi.org/10.3390/polym13142299






