Ultraflexible and Mechanically Strong Polymer/Polyaniline Conductive Interpenetrating Nanocomposite via In Situ Polymerization of Vinyl Monomer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GPANI-PMB and GPANI/PMB Dispersions
2.3. Preparation of GPANI-PMB and GPANI/PMB Nanocomposite Films
2.4. Characterization
3. Results and Discussion
3.1. Fabrication of Conductive GPANI-PMB Interpenetrating Nanocomposite and GPANI/PMB Physical Blend
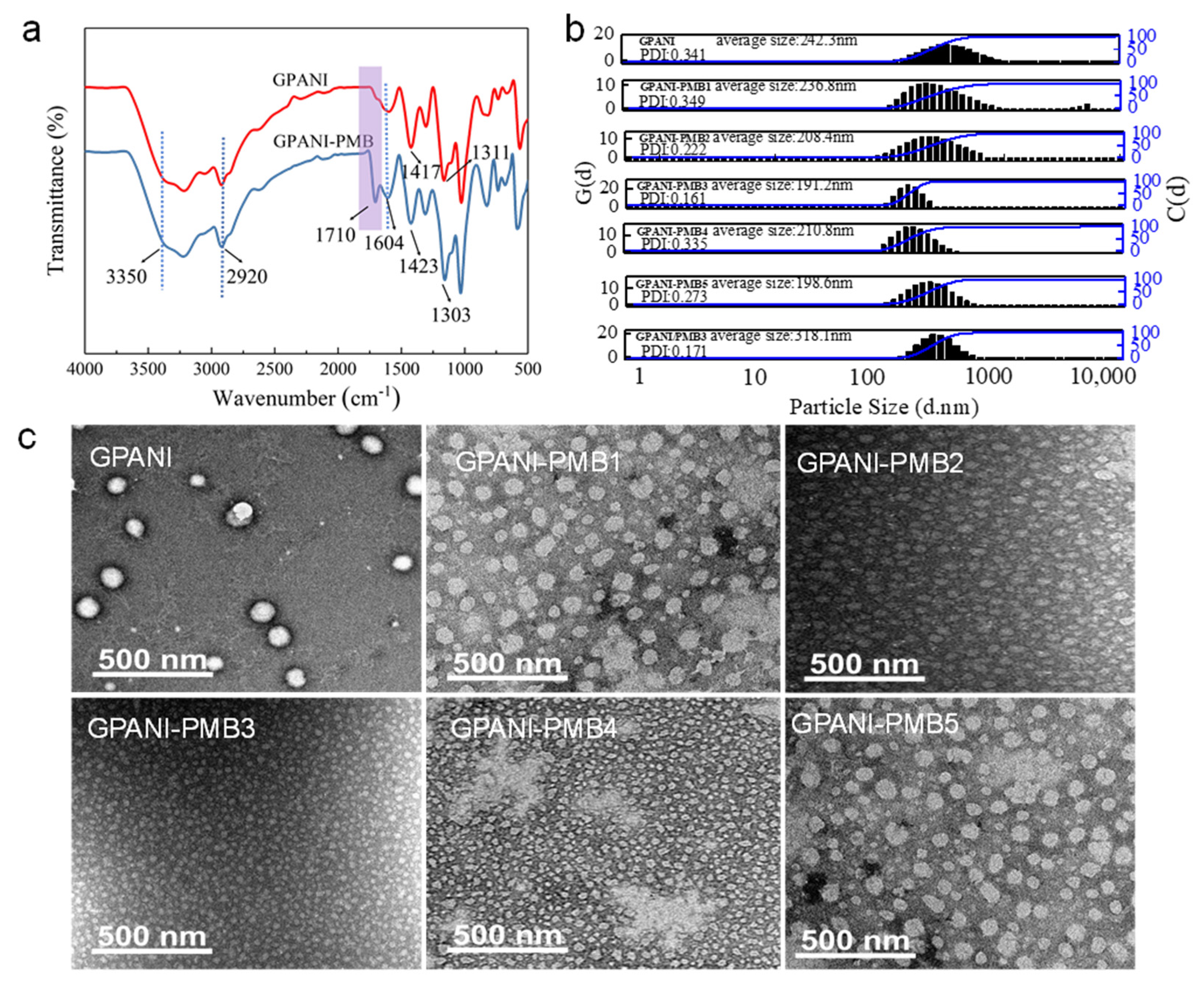
3.2. Structural and Morphological Analysis
3.3. SEM and Super Depth-of-Field Microscope Images of Nanocomposite Films
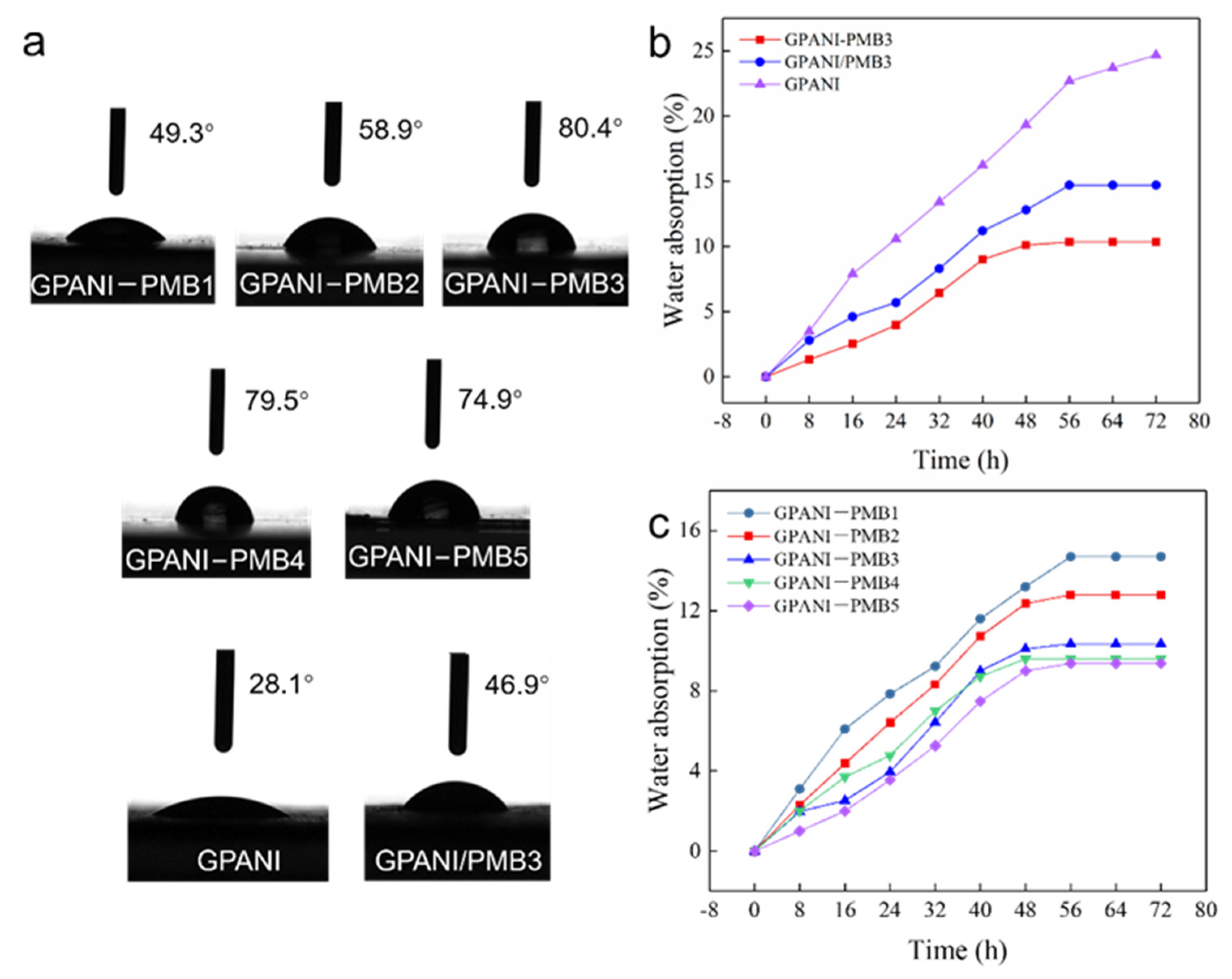
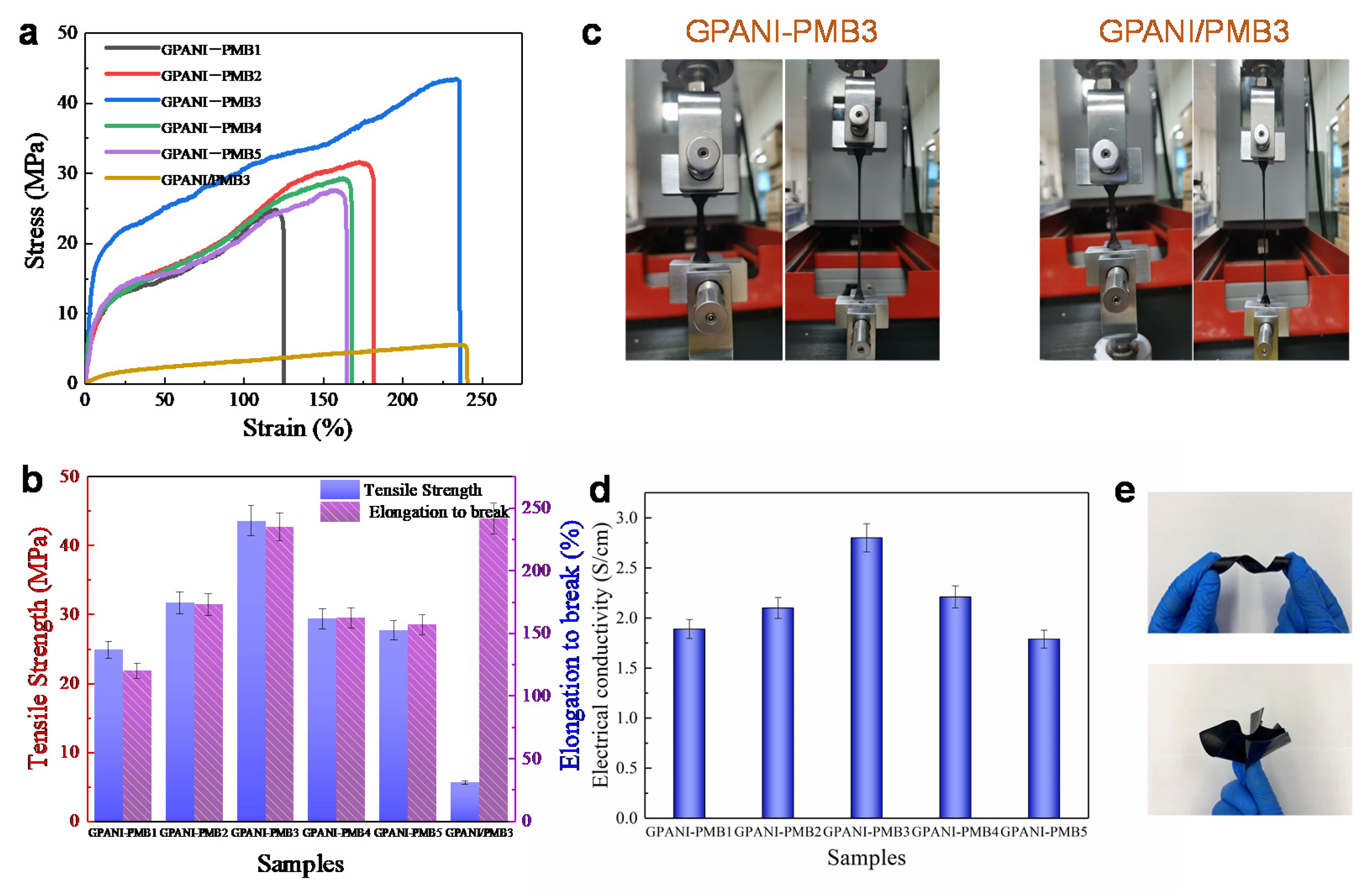
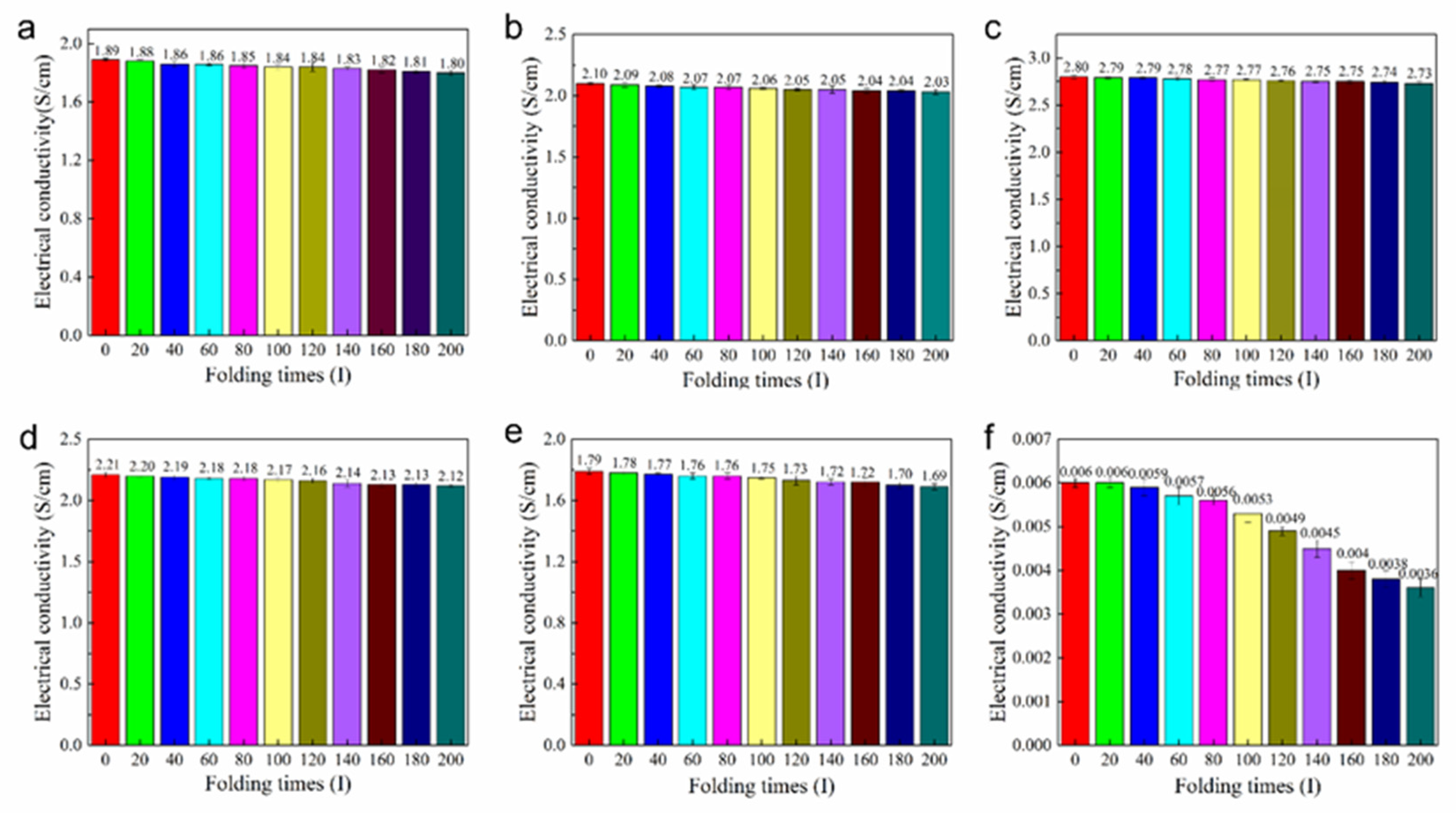
3.4. Water Resistance, Mechanical Properties and Electrical Conductivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qi, K.; Zhou, Y.; Ou, K.; Dai, Y.; You, X.; Wang, H.; He, J.; Qin, X.; Wang, R. Weavable and stretchable piezoresistive carbon nanotubes-embedded nanofiber sensing yarns for highly sensitive and multimodal wearable textile sensor. Carbon 2020, 170, 464–476. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, C.; Shi, L.; Zhang, Q.; Zhu, H. Highly stretchable, recyclable, notch-insensitive, and conductive polyacrylonitrile-derived organogel. J. Mater. Chem. A 2020, 8, 20346–20353. [Google Scholar] [CrossRef]
- Yan, C.; Wang, J.; Kang, W.; Cui, M.; Wang, X.; Foo, C.Y.; Chee, K.J.; Lee, P.S. Highly stretchable piezoresistive graphene-nanocellulose nanopaper for strain sensors. Adv. Mater. 2014, 26, 2022–2027. [Google Scholar] [CrossRef]
- Ma, R.; Lee, J.; Choi, D.; Moon, H.; Baik, S. Knitted fabrics made from highly conductive stretchable fibers. Nano Lett. 2014, 14, 1944–1951. [Google Scholar] [CrossRef]
- Hong, S.; Lee, H.; Lee, J.; Kwon, J.; Han, S.; Suh, Y.D.; Cho, H.; Shin, J.; Yeo, J.; Ko, S.H. Highly stretchable and transparent metal nanowire heater for wearable electronics applications. Adv. Mater. 2015, 27, 4744–4751. [Google Scholar] [CrossRef]
- Chabot, V.; Higgins, D.; Yu, A.; Xiao, X.; Chen, Z.; Zhang, J. A review of graphene and graphene oxide sponge: Material synthesis and applications to energy and the environment. Energy Environ. Sci. 2014, 7, 1564–1596. [Google Scholar] [CrossRef]
- Dickey, M.D. Stretchable and soft electronics using liquid metals. Adv. Mater. 2017, 29, 1606425. [Google Scholar] [CrossRef]
- Chun, K.-Y.; Oh, Y.; Rho, J.; Ahn, J.-H.; Kim, Y.-J.; Choi, H.R.; Baik, S. Highly conductive, printable and stretchable composite films of carbon nanotubes and silver. Nat. Nanotechnol. 2010, 5, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Kim, S.; Baik, H.-K.; Jeong, U. Conducting polymer dough for deformable electronics. Adv. Mater. 2016, 28, 4455–4461. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.; Liu, Q.; Cheng, W.; Wang, X.; Pan, L.; Xu, B.; Xu, H. A self-healable, highly stretchable, and solution processable conductive polymer composite for ultrasensitive strain and pressure sensing. Adv. Funct. Mater. 2018, 28, 1705551. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Lu, H.; Wang, Y.; Xu, J.; Zhu, J.; Zhang, C.; Liu, T. Cryopolymerization enables anisotropic polyaniline hybrid hydrogels with superelasticity and highly deformation-tolerant electrochemical energy storage. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wen, H.; Hu, B.; Fei, G.; Shen, Y.; Sun, L.; Yang, D. Facile approach to fabricate waterborne polyaniline nanocomposites with environmental benignity and high physical properties. Sci. Rep. 2017, 7, 43694. [Google Scholar] [CrossRef] [Green Version]
- Farooq, S.; Tahir, A.A.; Krewer, U.; Shah, A.U.H.A.; Bilal, S. Efficient Photocatalysis Through Conductive Polymer Coated FTO Counter Electrode in Platinum Free Dye Sensitized Solar Cells. Electrochim. Acta 2019, 320, 134544. [Google Scholar] [CrossRef]
- Rahman, S.U.; Bilal, S.; Shah, A.U.H.A. Synthesis and Characterization of Polyaniline-Chitosan Patches with Enhanced Stability in Physiological Conditions. Polymers 2020, 12, 2870. [Google Scholar] [CrossRef]
- Tarawneh, M.A.; Saraireh, S.A.; Chen, R.S.; Ahmad, S.H.; Yu, L.J. Mechanical, thermal, and conductivity performances of novel thermoplastic natural rubber/graphene nanoplates/polyaniline composites. J. Appl. Polym. Sci. 2019, 137, 2705. [Google Scholar] [CrossRef]
- Rahman, S.U.; Rose, P.; Surati, M.; Shah, A.; Krewer, U.; Bilal, S. 3D Polyaniline Nanofibers Anchored on Carbon Paper for High-Performance and Light-Weight Supercapacitors. Polymers 2020, 12, 2705. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Noh, J.-S.; Kim, S.H. Facile control of stretchability and electrical resistance of elastomer/polyaniline composites for stretchable conductors. Mater. Chem. Phys. 2017, 190, 68–73. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Jagminas, A.; Ramanavicius, A. Advances in Molecularly Imprinted Polymers Based Affinity Sensors. Polymers 2021, 13, 974. [Google Scholar] [CrossRef]
- German, N.; Ramanaviciene, A.; Ramanavicius, A. Formation and Electrochemical Evaluation of Polyaniline and Polypyrrole Nanocomposites Based on Glucose Oxidase and Gold Nanostructures. Polymers 2020, 12, 3026. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Conducting Polymers in the Design of Biosensors and Biofuel Cells. Polymers 2021, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.; Brasiunas, B.; Damaskaite, A.; Plikusiene, I.; Ramanavicius, A.; Ramanaviciene, A. Electrodeposited Gold Nanostructures for the Enhancement of Electrochromic Properties of PANI-PEDOT Film Deposited on Transparent Electrode. Polymers 2020, 12, 2278. [Google Scholar]
- Meng, C.; Liu, C.; Fan, S. Flexible carbon nanotube/polyaniline paper-like films and their enhanced electrochemical properties. Electrochem. Commun. 2009, 11, 186–189. [Google Scholar] [CrossRef]
- Wu, L.; Ge, Y.; Zhang, L.; Yu, D.; Wu, M.; Ni, H. Enhanced electrical conductivity and competent mechanical properties of polyaniline/polyacrylate(pani/pa) composites for antistatic finishing prepared at the aid of polymeric stabilizer. Prog. Org. Coat. 2018, 125, 99–108. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, T.; Yang, Z. Flexible polyethylene terephthalate/polyaniline composite paper with bending durability and effective electromagnetic shielding performance. Chem. Eng. J. 2020, 389, 124433. [Google Scholar] [CrossRef]
- Guo, R.; Wang, J.; Wang, H.; Fei, G.; Wang, C.; Sun, L. Engineering the poly(vinyl alcohol)-polyaniline colloids for high-performance waterborne alkyd anticorrosion coating. Appl. Surf. Sci. 2019, 481, 960–971. [Google Scholar] [CrossRef]
- Han, M.G.; Sperry, J.; Gupta, A.; Huebner, C.F.; Foulger, S.H. Polyaniline coated poly(butyl methacrylate) core-shell particles: Roll-to-roll printing of templated electrically conductive structures. Mater. Chem. 2007, 17, 1347–1352. [Google Scholar] [CrossRef]
- Contu, F.; Fenzy, L.; Taylor, S.R. An FT-IR investigation of epoxy coatings as a function of electrolyte composition. Prog. Org. Coat. 2012, 75, 92–96. [Google Scholar] [CrossRef]
- Hino, T.; Namiki, T.; Kuramoto, N. Synthesis and characterization of novel conducting composites of polyaniline prepared in the presence of sodium dodecylsulfonate and several water soluble polymers. Synth. Met. 2006, 156, 1327–1332. [Google Scholar] [CrossRef]
- Mahmud, M.S.; Buys, Y.F.; Anuar, H.; Sopyan, I. Miscibility, morphology and mechanical properties of compatibilized polylactic acid/thermoplastic polyurethane blends. Mater. Today Proc. 2019, 17, 778–786. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wu, X.; Qin, X.; Fei, G.; Sun, L.; Li, Y.; Wang, M. Ultraflexible and Mechanically Strong Polymer/Polyaniline Conductive Interpenetrating Nanocomposite via In Situ Polymerization of Vinyl Monomer. Polymers 2021, 13, 2159. https://doi.org/10.3390/polym13132159
Wang H, Wu X, Qin X, Fei G, Sun L, Li Y, Wang M. Ultraflexible and Mechanically Strong Polymer/Polyaniline Conductive Interpenetrating Nanocomposite via In Situ Polymerization of Vinyl Monomer. Polymers. 2021; 13(13):2159. https://doi.org/10.3390/polym13132159
Chicago/Turabian StyleWang, Haihua, Xiaojing Wu, Xuan Qin, Guiqiang Fei, Liyu Sun, Yanyu Li, and Mengxi Wang. 2021. "Ultraflexible and Mechanically Strong Polymer/Polyaniline Conductive Interpenetrating Nanocomposite via In Situ Polymerization of Vinyl Monomer" Polymers 13, no. 13: 2159. https://doi.org/10.3390/polym13132159
APA StyleWang, H., Wu, X., Qin, X., Fei, G., Sun, L., Li, Y., & Wang, M. (2021). Ultraflexible and Mechanically Strong Polymer/Polyaniline Conductive Interpenetrating Nanocomposite via In Situ Polymerization of Vinyl Monomer. Polymers, 13(13), 2159. https://doi.org/10.3390/polym13132159





