Carbon Fibers from High-Density Polyethylene Using a Hybrid Cross-Linking Technique
Abstract
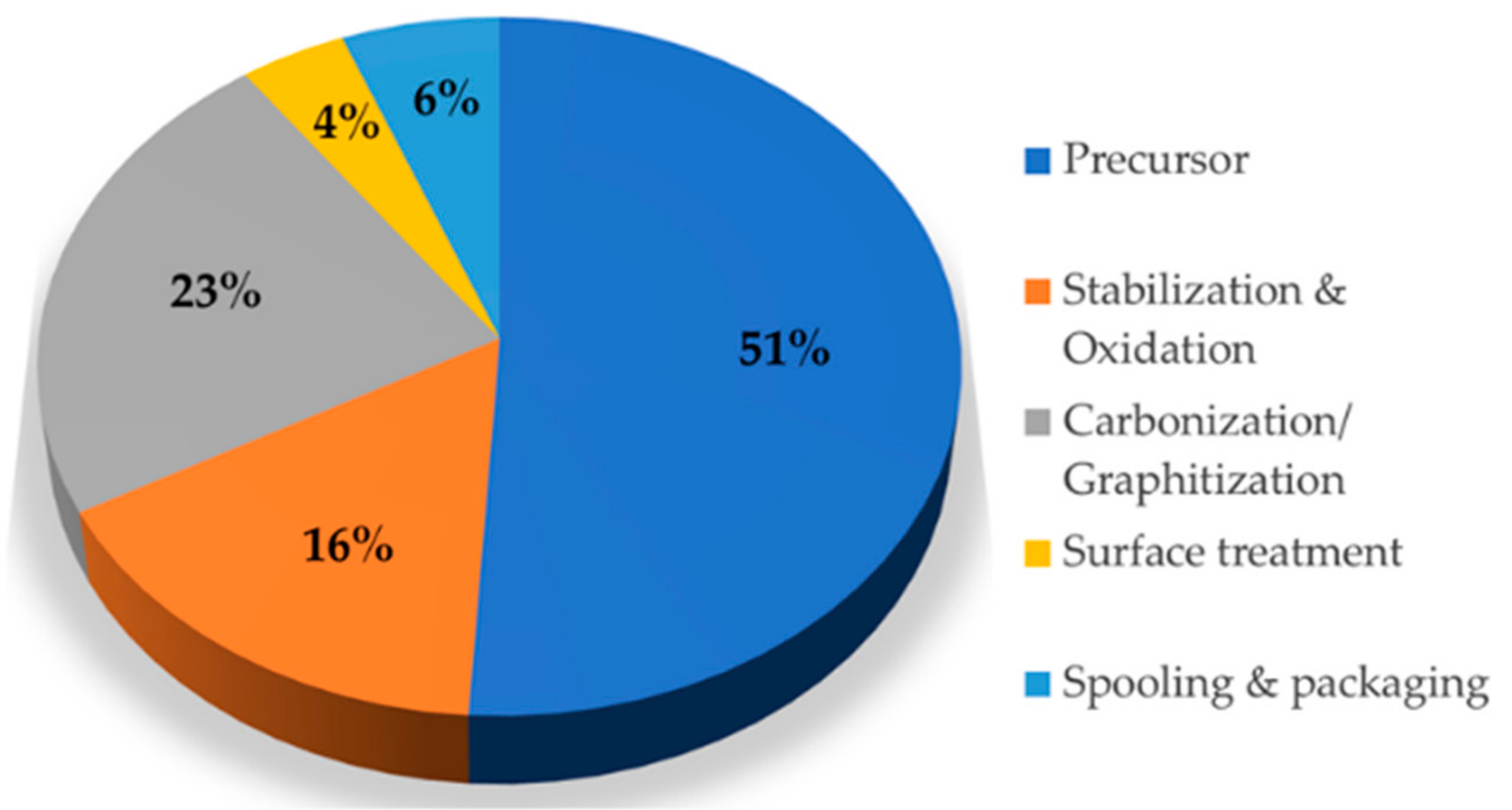
:1. Introduction
2. Experiment Details
2.1. Materials
2.2. Manufacturing the Stabilized Fiber Using Hybrid Cross-Linking
2.3. Carbon Fiber Manufacturing
2.4. Thermal Properties of the Stabilized Fiber
2.5. Morphology of the Stabilized Fiber and the HDPE-Based Carbon Fiber
2.6. Tensile Properties
3. Findings and Discussion
3.1. HDPE Fiber after Primary Cross-Linking
3.1.1. Calorimetry
3.1.2. X-ray Diffraction Analysis
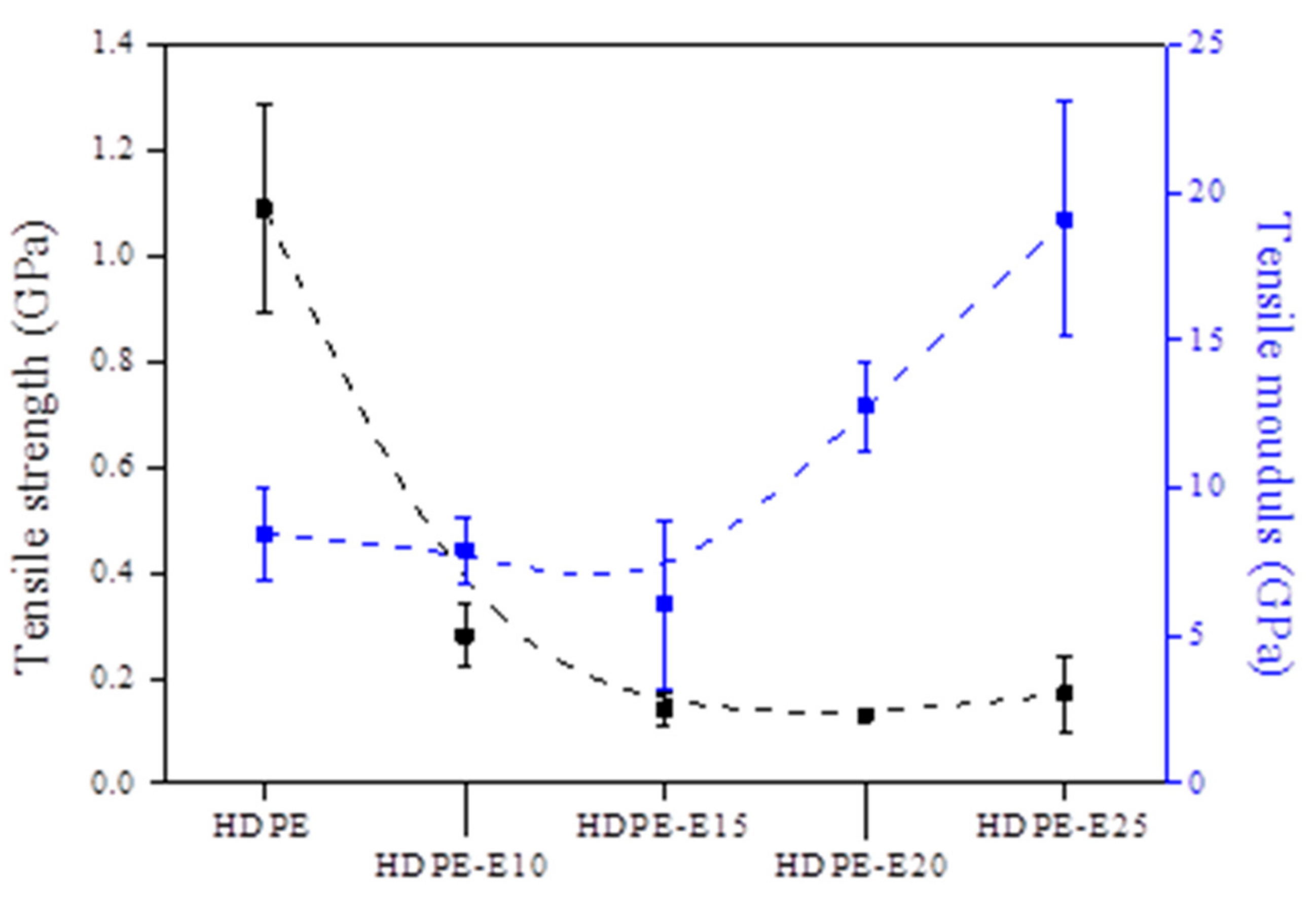
3.1.3. Tensile Properties
3.2. Hybrid Cross-Linked HDPE Fiber
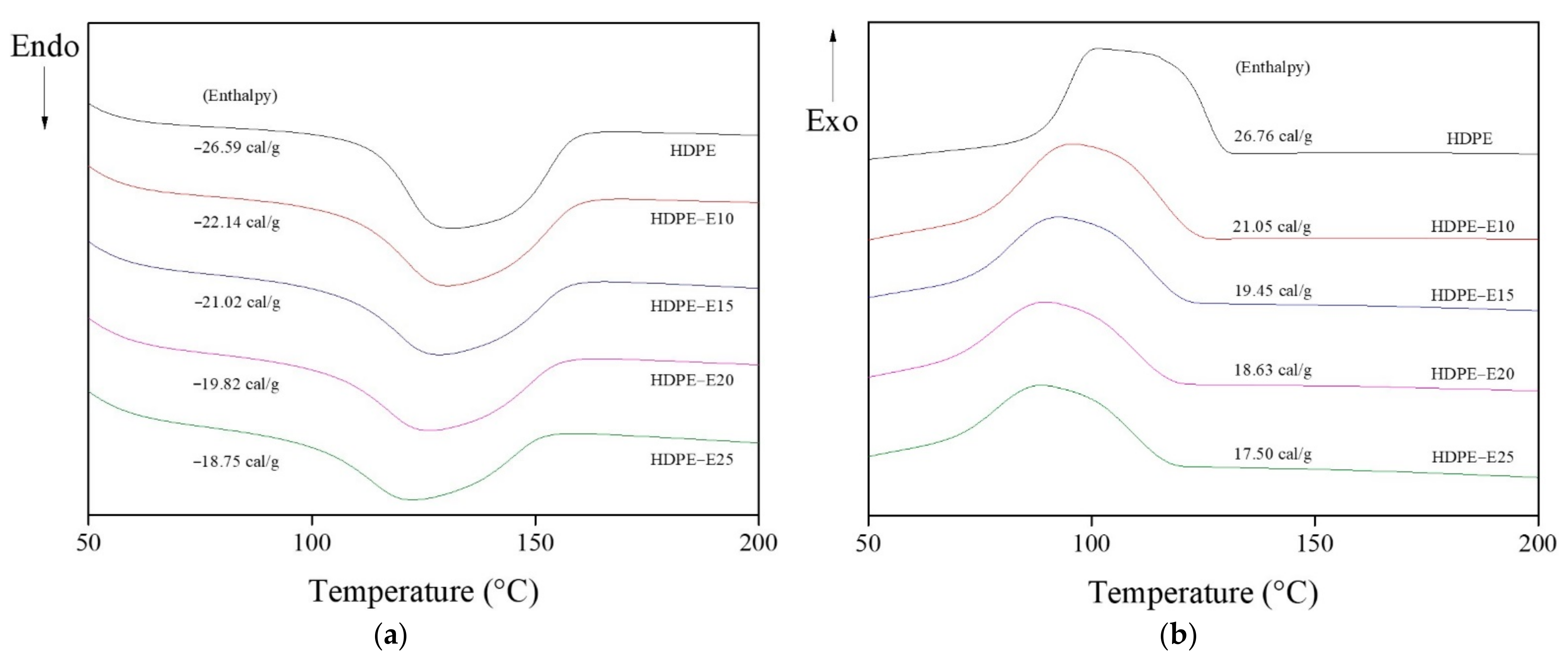
3.2.1. Thermal Properties
3.2.2. X-ray Diffraction Analysis
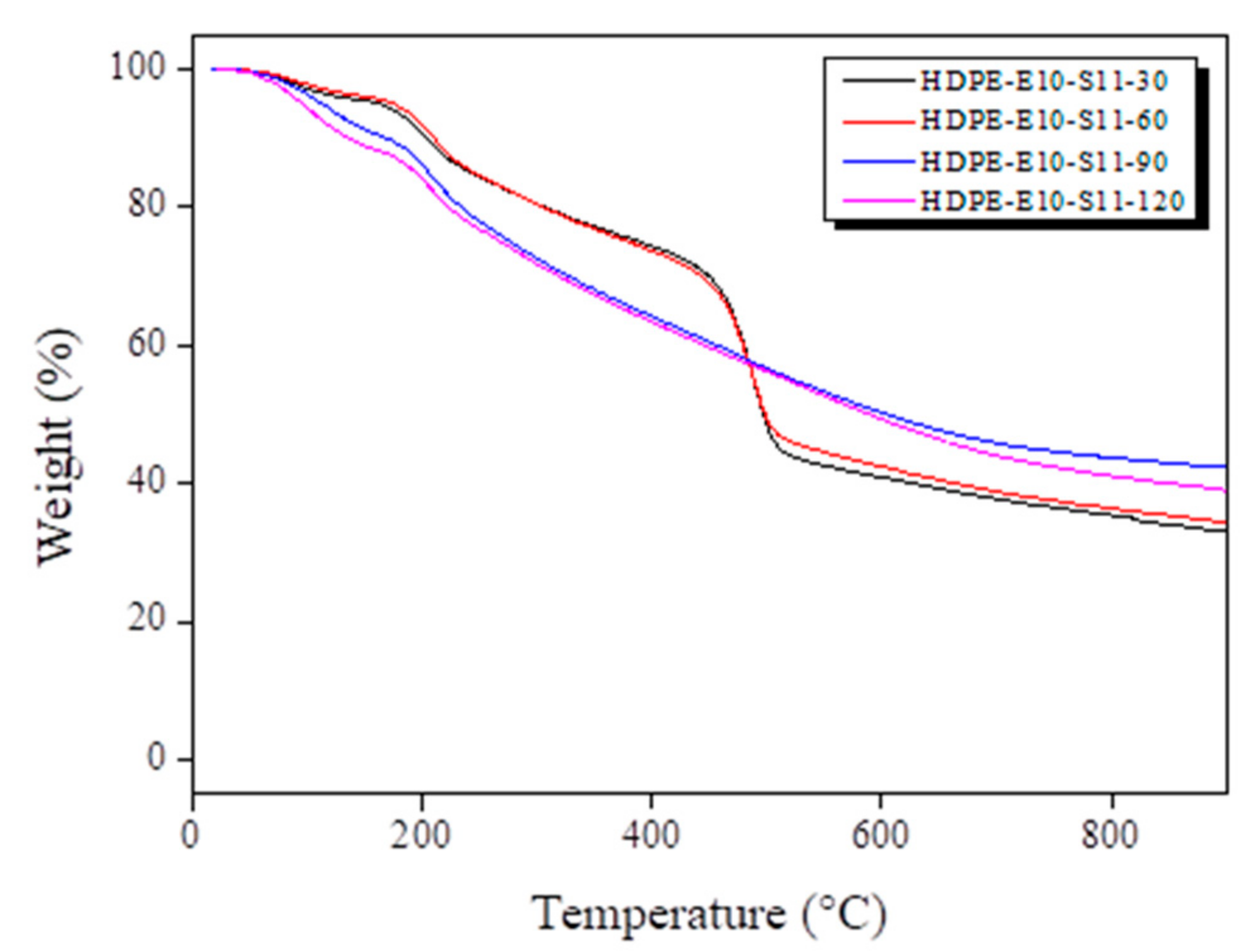
3.3. Carbonized Fiber
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cato, A.D.; Edie, D.D. Flow behavior of mesophase pitch. Carbon 2003, 41, 1411–1417. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, J.S. Preparation of carbon fibers from linear low density polyethylene. Carbon 2015, 94, 524–530. [Google Scholar] [CrossRef]
- Kim, K.W.; Jeong, J.S.; Chung, D.C.; An, K.H.; Kim, B.J. Effects of surface etching on microstructure and mechanical strength of carbon fibers. Carbon Lett. 2018, 28, 100–104. [Google Scholar]
- Serkov, A.T.; Budnitskii, G.A.; Radishevskii, M.B.; Medvedev, V.A.; Zlatoustova, L.A. Improving carbon fiber production technology. Fibre Chem. 2003, 35, 117–121. [Google Scholar] [CrossRef]
- Kim, J.D.; Roh, J.S.; Kim, M.S. Effect of carbonization temperature on crystalline structure and properties of isotropic pitch-based carbon fiber. Carbon Lett. 2017, 21, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Fitzer, E. Pan-based carbon fibers-present state and trend of the technology from the viewpoint of possibilities and limits to influence and to control the fiber properties by the process parameters. Carbon 1989, 27, 621–645. [Google Scholar] [CrossRef]
- Yusof, N.; Ismail, A.F. Post spinning and pyrolysis processes of poly-acrylonitrile (PAN)-based carbon fiber and activated carbon fiber: A review. J. Anal. Appl. Pyrol. 2012, 93, 1–13. [Google Scholar] [CrossRef]
- Jiang, X.; Quyang, Q.; Liu, D.; Huang, J.; Ma, H.; Chen, Y.; Wang, X.; Sun, W. Preparation of low-cost carbon fiber precursors from blends of wheat straw lignin and commercial textile-grade polyacrylonitrile (PAN). Holzforschung 2018, 72, 727–734. [Google Scholar] [CrossRef]
- Palmenaer, A.D.; Wortberg, G.; Drissen, F.; Seide, G.; Gries, T. Production of polyethylene based carbon fibres. Chem. Eng. Trans. 2015, 43, 1699–1704. [Google Scholar]
- Warren, C.D.D. Low Cost Carbon Fiber Overview; U.S. Department of Energy: Washington, DC, USA, 2011. Available online: https://www.energy.gov/sites/prod/files/2014/03/f11/lm002_warren_2011_o.pdf (accessed on 29 June 2021).
- Wortberg, G.; Plamenaer, A.D.; Beckers, M.; Seide, G.; Gries, T. Polyethylene-based carbon fibers by the use of sulphonation for stabilization. Fibers 2015, 3, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Qin, W.; Kadla, J.F. Carbon fibers based on prolytic lignin. J. Appl. Polym. Sci. 2012, 126, 204–213. [Google Scholar] [CrossRef]
- Benli, H.; Karacan, I. The effect of sulfonation treatment on the structure and properties of isotactic polypropylene fibers prior to the carbonization stage. J. Appl. Polym. Sci. 2012, 123, 3375–3389. [Google Scholar]
- Zhang, D.; Bhat, G.S. Carbon fibers from polyethylene-based precursors. Mater. Manuf. Process. 1994, 9, 221–235. [Google Scholar] [CrossRef]
- Chang, H.; Luo, J.; Liu, H.C.; Zhang, S.; Park, J.G.; Liang, R.; Kumar, S. Carbon fibers from polyacrylonitrile/cellulose nanocrystal nanocomposite fibers. Carbon 2019, 145, 764–771. [Google Scholar] [CrossRef]
- Liu, H.C.; Chien, A.T.; Newcomb, B.A.; Liu, Y.; Kumar, S. Processing, Structure, and properties of lignin- and CNT-incorporated polyacrylonitrile-based carbon fibers. ACS Sustain. Chem. Eng. 2015, 3, 1943–1954. [Google Scholar] [CrossRef]
- Khonakdar, H.A.; Morshedian, J.; Wagenknecht, U.; Jafari, S.H. An investigation of chemical crosslinking effect on properties of high-density polyethylene. Polymer 2003, 44, 4301–4309. [Google Scholar] [CrossRef]
- Tamboli, S.M.; Mhaske, S.T.; Kale, D.D. Crosslinked polyethylene. Indian. J. Chem. Technol. 2004, 11, 853–864. [Google Scholar]
- Morshedian, J.; Hoseinpour, P.M. Polyethylene cross-linking by two-step silane method: A review. Iran. Polym. J. 2009, 18, 103–128. [Google Scholar]
- Kim, K.W.; Lee, H.M.; Kim, B.J.; Kim, B.S.; Hwang, S.H.; Kwac, L.K.; An, K.H.; Kim, B.J. Preparation and thermal properties of polyethylene-based carbonized fibers. Carbon Lett. 2015, 16, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Shieh, Y.T.; Tsai, T.H. Silane grafting reactions of low-density polyethylene. J. Appl. Polym. Sci. 1998, 69, 255–261. [Google Scholar] [CrossRef]
- Narkis, M.; Raiter, I.; Shkolnik, S.; Siegmannz, A.; Eyerer, P. Structure and tensile behavior of irradiation-and peroxide crosslinked polyethylenes. J. Macromol. Sci. Phys. 1987, 26, 37–58. [Google Scholar] [CrossRef]
- Grubb, D.T.; Prasad, K. High-modulus polyethylene fiber structure as shown by X-ray diffraction. Macromolecules 1992, 25, 4575–4582. [Google Scholar] [CrossRef]
- Wang, H.T.; Jiang, H.Q.; Shen, R.F.; Ding, X.J.; Zhang, C.; Li, L.F.; Li, J.Y. Electron-beam radiation effects on the structure and properties of polypropylene at low dose rates. Nucl. Sci. Tech. 2018, 29, 87–95. [Google Scholar] [CrossRef]





| Cross-Linking Conditions | |||
|---|---|---|---|
| Sample | Electron Beam | Sulfuric Acid | |
| Irradiation (kGy) | Temp (°C) | Time (min) | |
| HDPE | As-received | ||
| HDPE-E10 a | 1000 | - | - |
| HDPE-E10-S8-60 b | 1000 | 80 | 60 |
| Sample | Onset Tm2 (°C) | Peak Tm2 (°C) | Onset Tc2 (°C) | Peak Tc2 (°C) |
|---|---|---|---|---|
| HDPE | 114.74 | 131.45 | 129.18 | 101.60 |
| HDPE-E10 | 113.08 | 132.27 | 121.16 | 94.71 |
| HDPE-E15 | 109.74 | 128.54 | 119.43 | 92.45 |
| HDPE-E20 | 107.63 | 126.25 | 116.61 | 89.64 |
| HDPE-E25 | 103.48 | 122.51 | 116.18 | 88.67 |
| Sample | 110 Peak | 200 Peak | ||||||
|---|---|---|---|---|---|---|---|---|
| 2θ | FWHM (2θ) | d110 (Å) | La (Å) | 2θ | FWHM (2θ) | d200 (Å) | La (Å) | |
| HDPE | 21.41 | 0.73 | 4.15 | 111.11 | 23.58 | 0.71 | 3.77 | 232.98 |
| HDPE-E10 | 21.38 | 0.84 | 4.15 | 96.35 | 23.70 | 0.85 | 3.75 | 194.96 |
| HDPE-E15 | 21.42 | 0.77 | 4.14 | 105.47 | 23.72 | 0.80 | 3.75 | 207.18 |
| HDPE-E20 | 21.26 | 0.67 | 4.18 | 119.82 | 23.54 | 0.69 | 3.78 | 239.30 |
| HDPE-E25 | 21.32 | 0.68 | 4.16 | 118.82 | 23.58 | 0.71 | 3.77 | 232.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.-H.; Kim, K.-W.; Kim, B.-J. Carbon Fibers from High-Density Polyethylene Using a Hybrid Cross-Linking Technique. Polymers 2021, 13, 2157. https://doi.org/10.3390/polym13132157
Kang S-H, Kim K-W, Kim B-J. Carbon Fibers from High-Density Polyethylene Using a Hybrid Cross-Linking Technique. Polymers. 2021; 13(13):2157. https://doi.org/10.3390/polym13132157
Chicago/Turabian StyleKang, Seong-Hyun, Kwan-Woo Kim, and Byung-Joo Kim. 2021. "Carbon Fibers from High-Density Polyethylene Using a Hybrid Cross-Linking Technique" Polymers 13, no. 13: 2157. https://doi.org/10.3390/polym13132157
APA StyleKang, S.-H., Kim, K.-W., & Kim, B.-J. (2021). Carbon Fibers from High-Density Polyethylene Using a Hybrid Cross-Linking Technique. Polymers, 13(13), 2157. https://doi.org/10.3390/polym13132157







