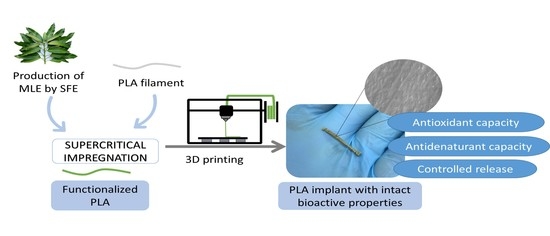
Supercritical Impregnation of PLA Filaments with Mango Leaf Extract to Manufacture Functionalized Biomedical Devices by 3D Printing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Raw Material
2.2. Extraction
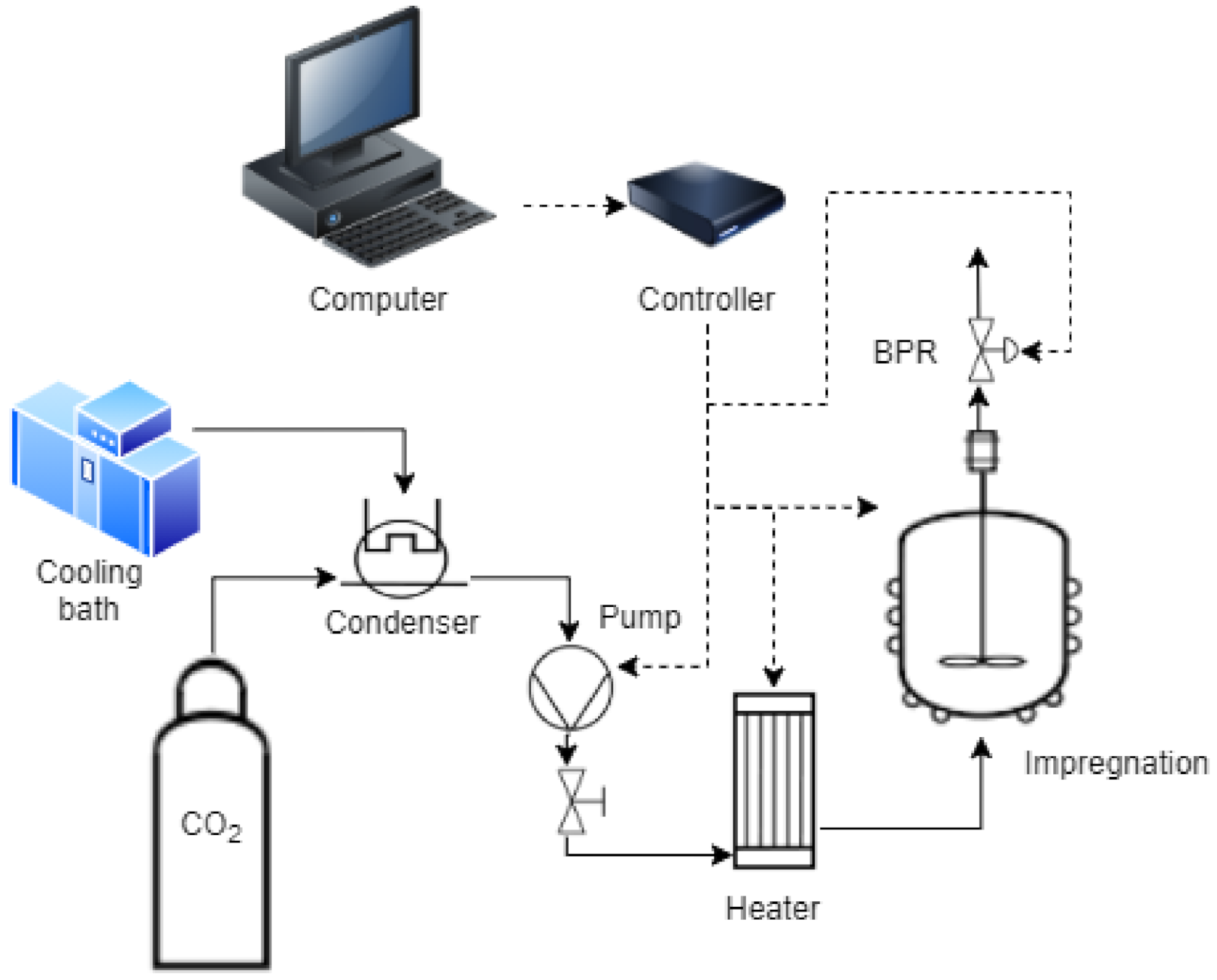
2.3. Supercritical Impregnation
2.4. Scanning Electron Microscopy (SEM)
2.5. 3D Printing
2.6. Foaming and Impregnation Loading
2.7. Impregnated Filaments Bioactivity
2.8. MLE Release and Evaluation of Long-Term Bioactivity
3. Results and Discussion
3.1. Impregnation of PLA
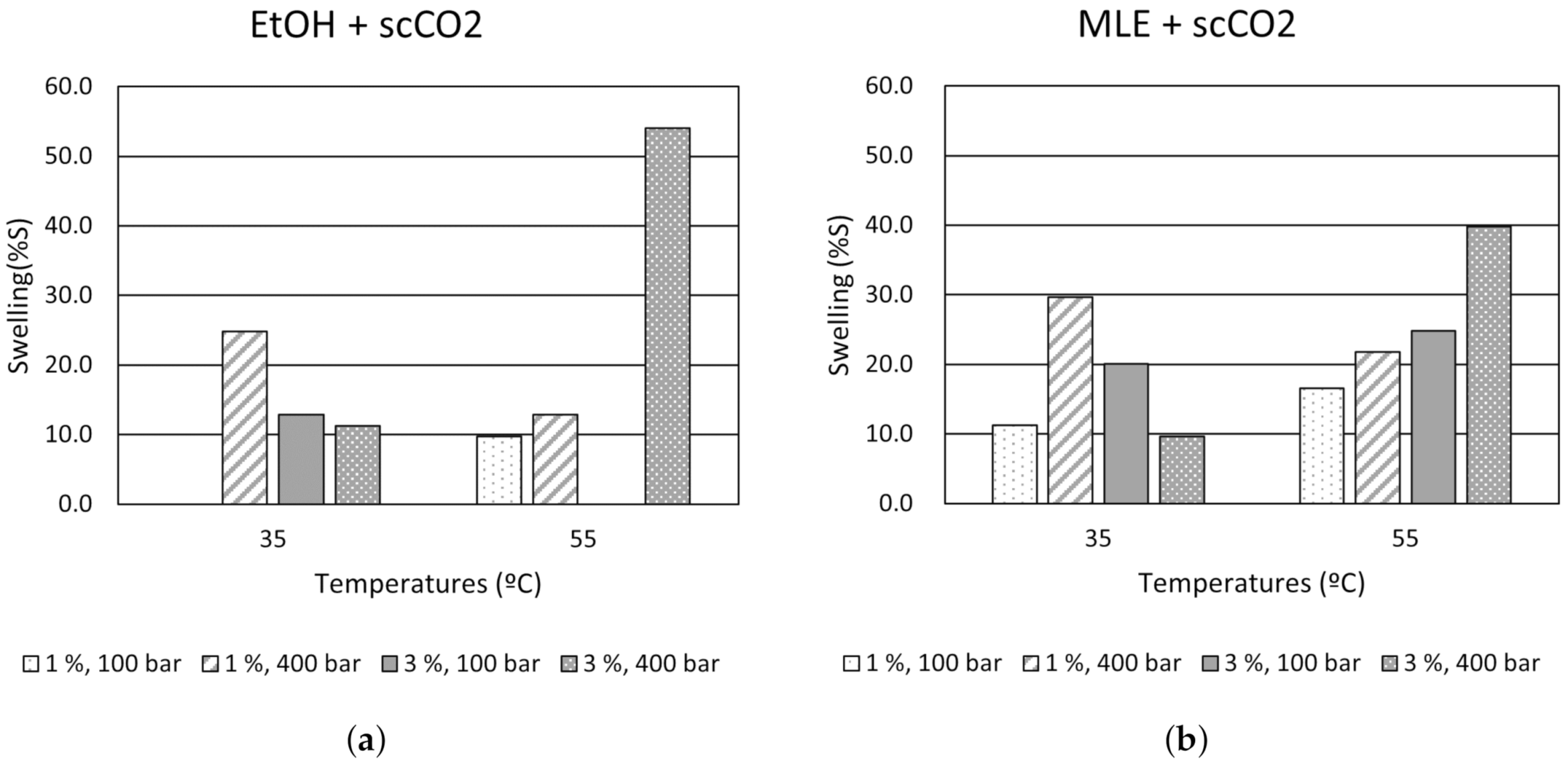
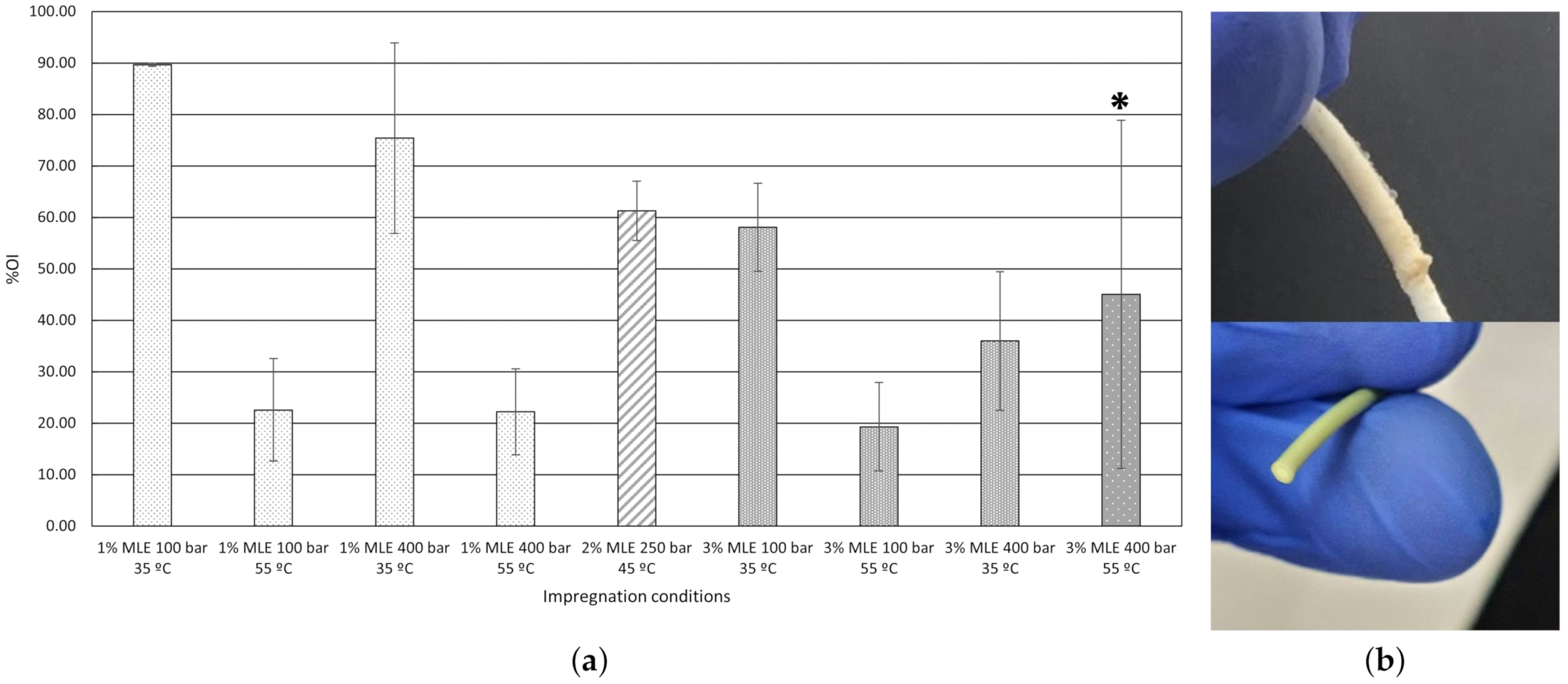
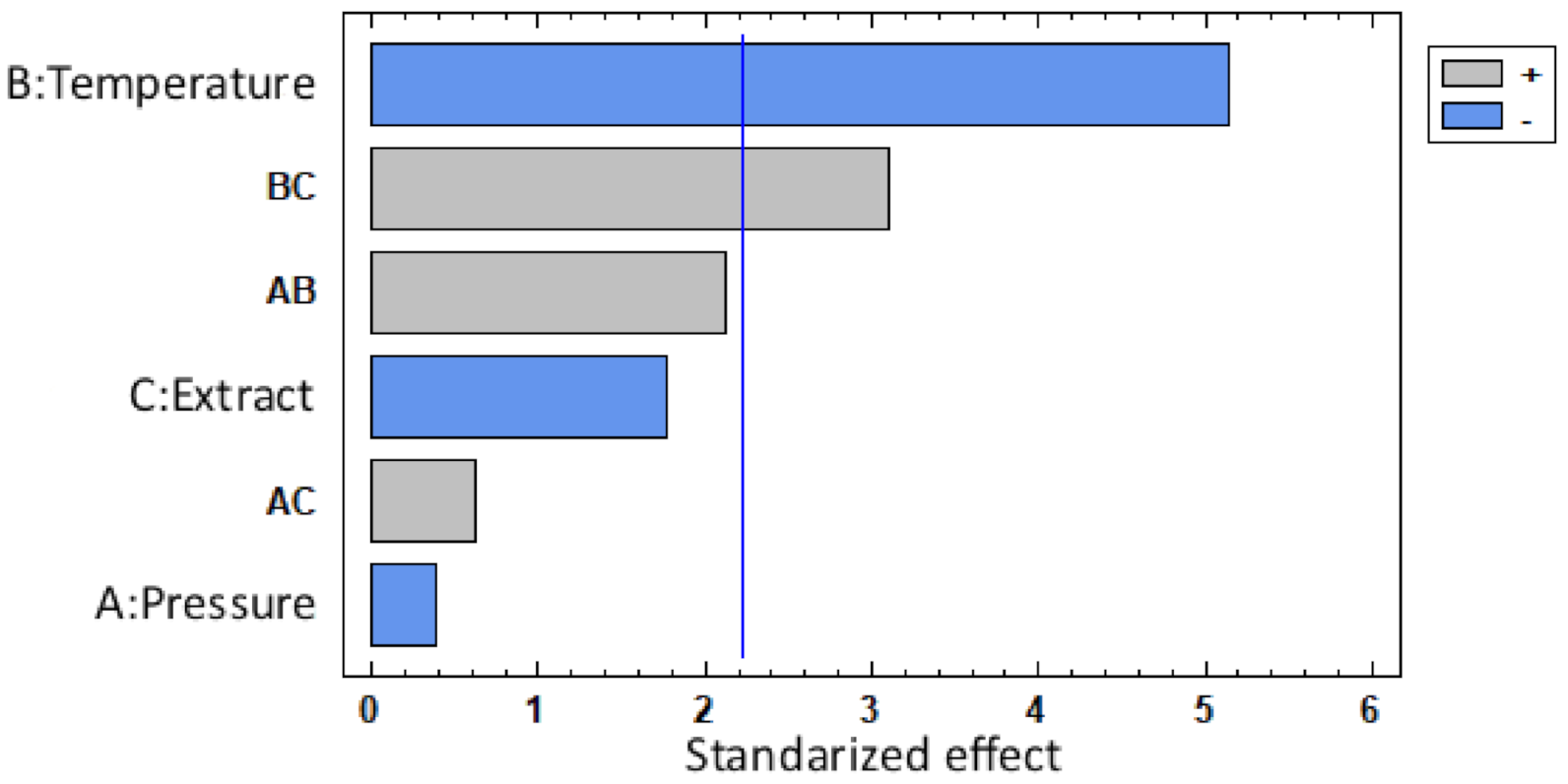
3.1.1. Swelling
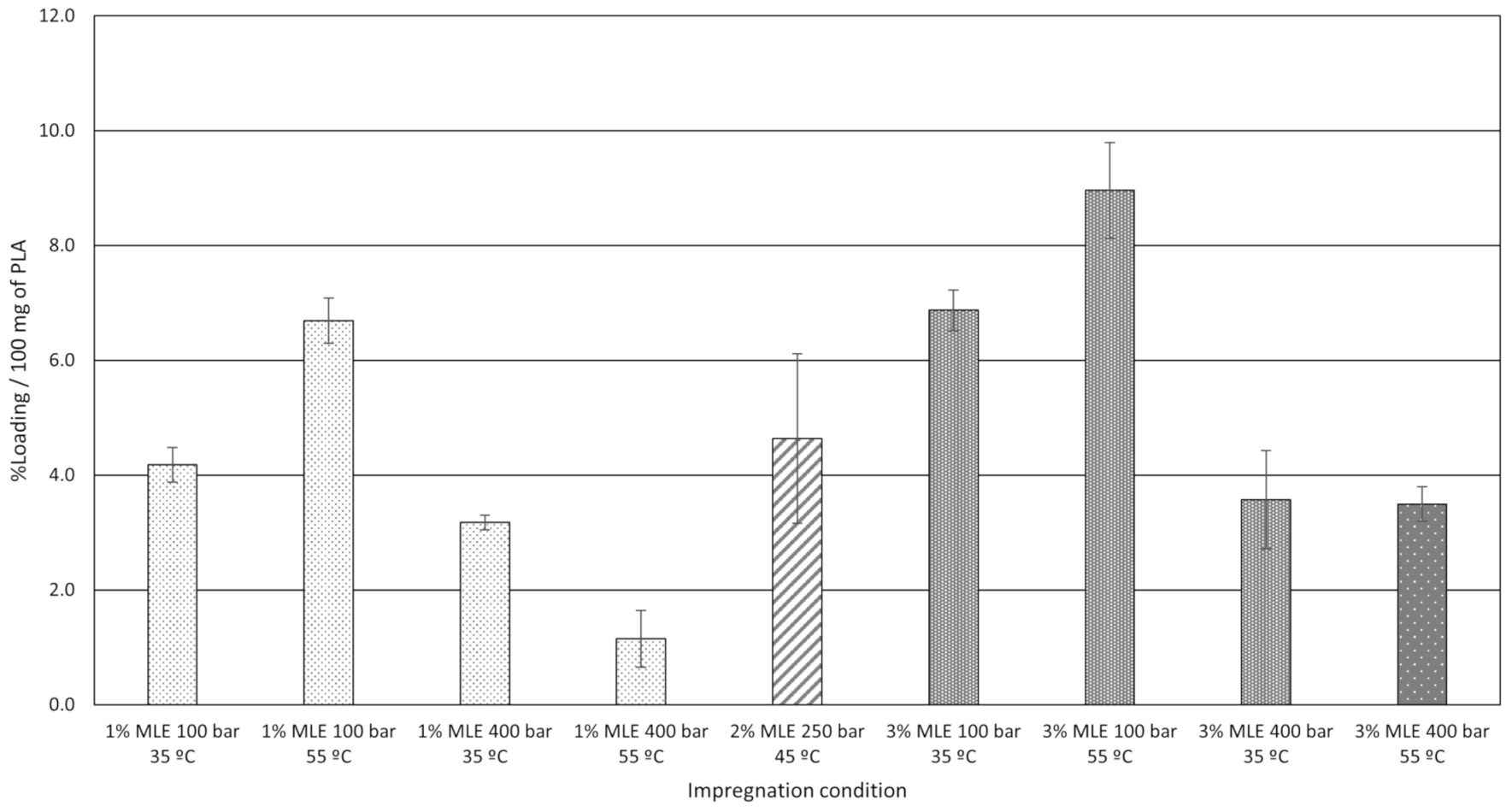
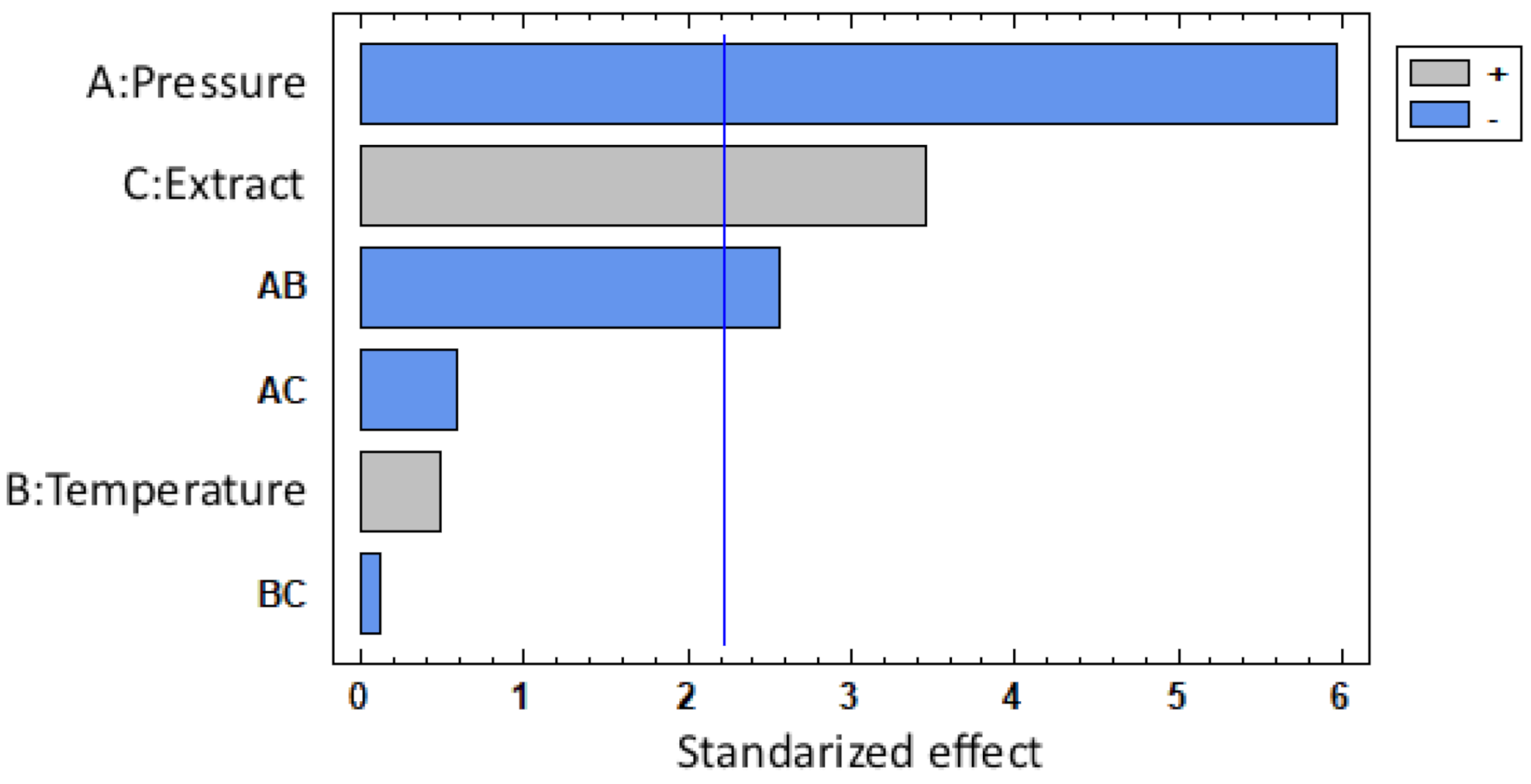
3.1.2. Impregnation Loading
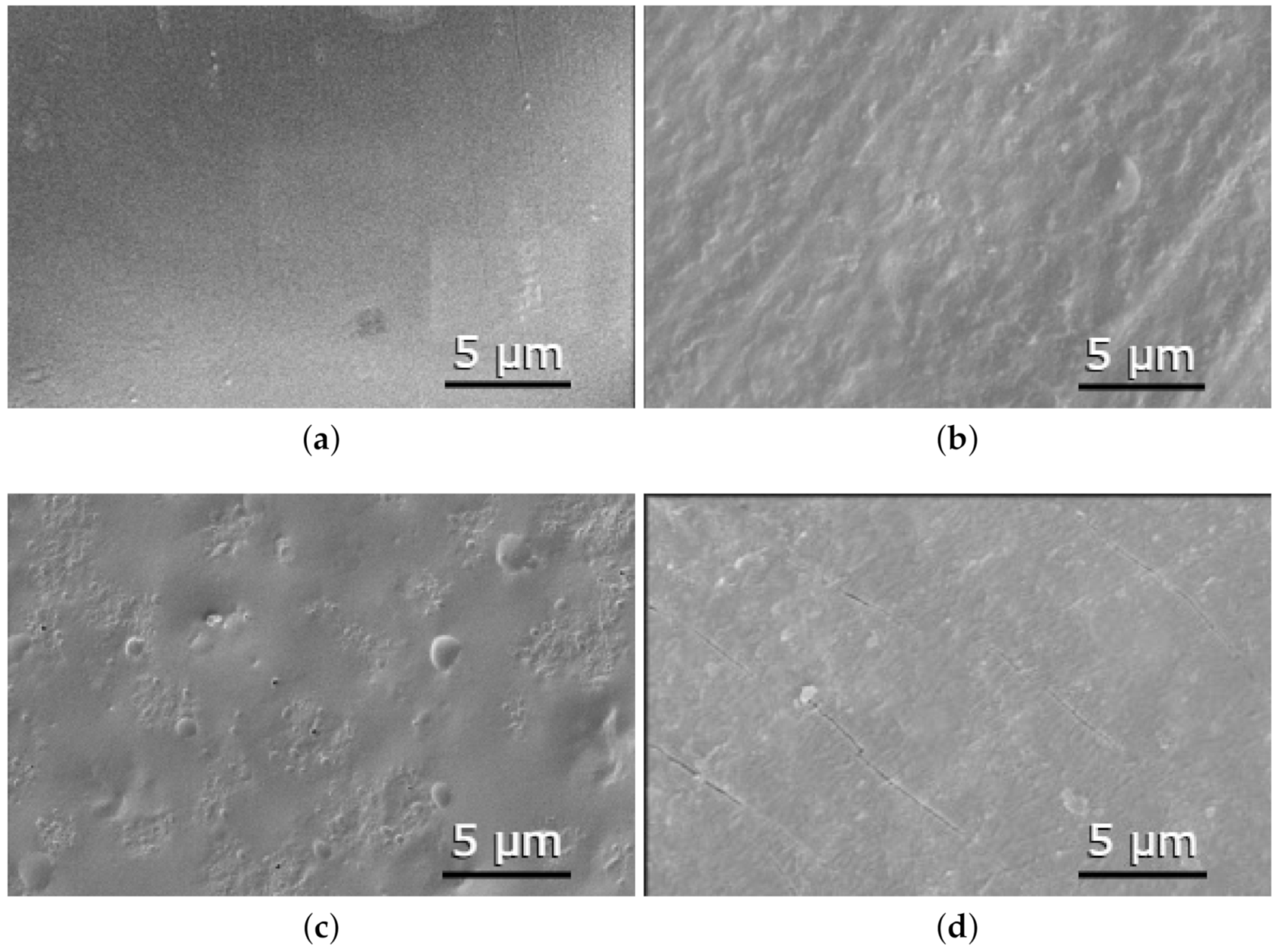
3.2. Scanning Electron Microscopy (SEM)
3.3. Bioactivity of the Impregnated PLA
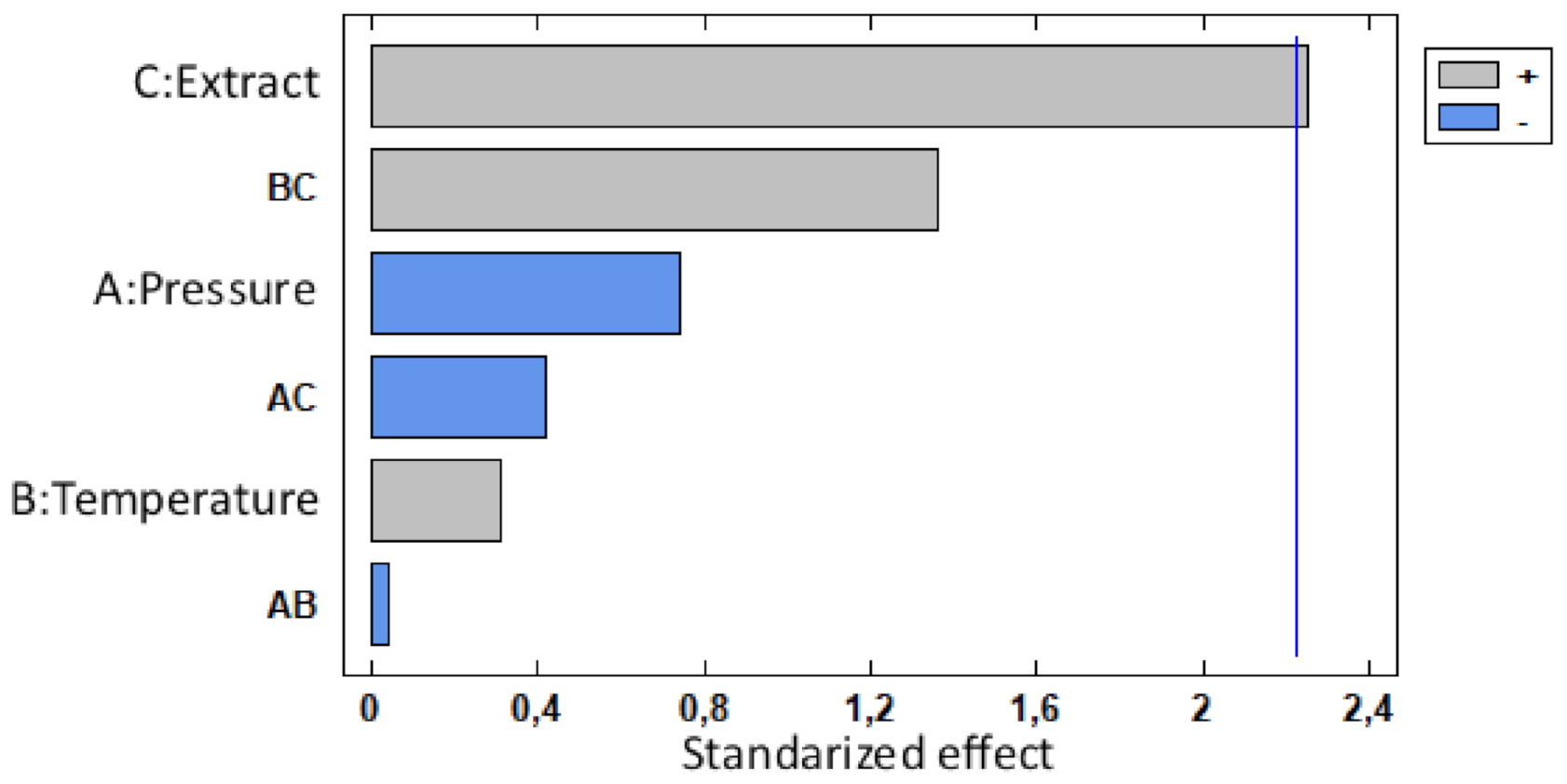
3.3.1. Antioxidant Activity
3.3.2. Antidenaturant Activity
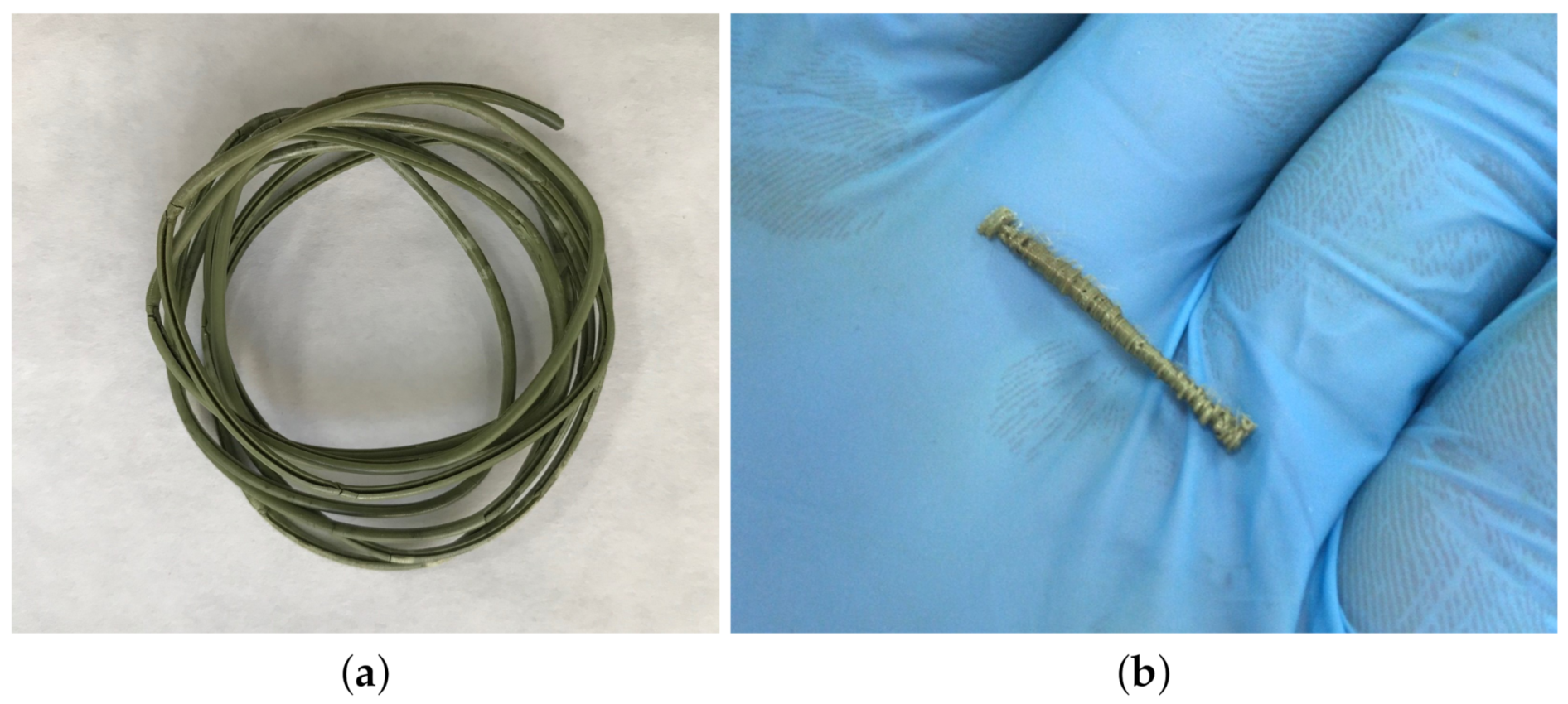
3.4. Filament Production for 3D Printing
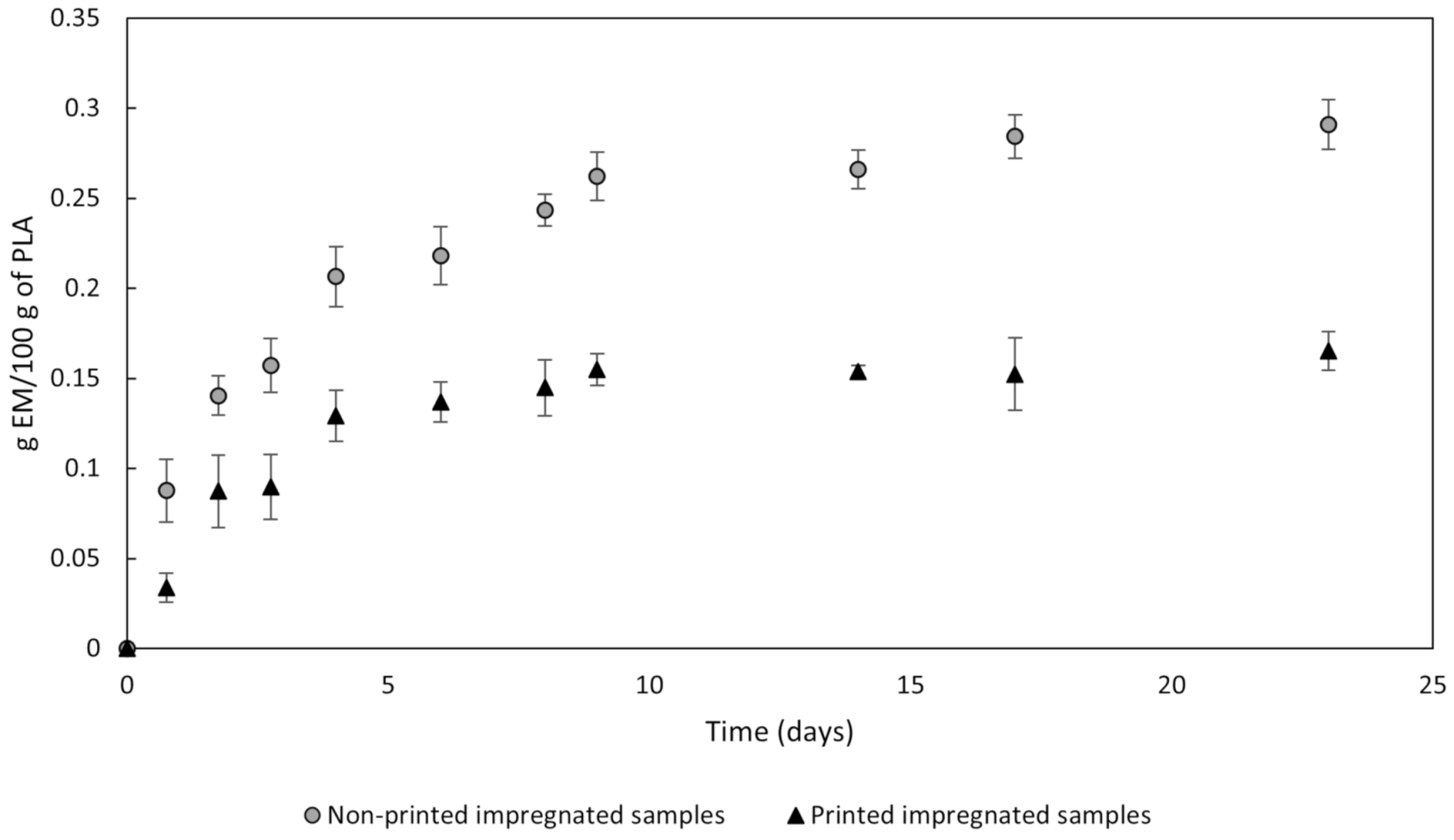
3.4.1. MLE Release Study of the Impregnated and Printed Filaments
3.4.2. Bioactivity of the Printed Bioactive PLA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ahangar, P.; Cooke, M.E.; Weber, M.H.; Rosenzweig, D.H. Current biomedical applications of 3D printing and additive manufacturing. Appl. Sci. 2019, 9, 1713. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Prakash, C.; Ramakrishna, S. 3D printing of polyether-ether-ketone for biomedical applications. Eur. Polym. J. 2019, 114, 234–248. [Google Scholar] [CrossRef]
- Placone, J.K.; Engler, A.J. Recent Advances in Extrusion-Based 3D Printing for Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, D.M.; Vaezi, M.; Yang, S.; Oreffo, R.O. Hope Versus Hype: What Can Additive Manufacturing Realistically Offer Trauma and Orthopedic Surgery? Regen Med. 2014, 9, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Ware, H.O.T.; Farsheed, A.C.; Akar, B.; Duan, C.; Chen, X.; Ameer, G.; Sun, C. High-speed on-demand 3D printed bioresorbable vascular scaffolds. Mater. Today Chem. 2018, 7, 25–34. [Google Scholar] [CrossRef]
- Guerra, A.J.; San, J.; Ciurana, J. Fabrication of PCL/PLA Composite Tube for Stent Manufacturing. Proc. CIRP 2017, 65, 231–235. [Google Scholar] [CrossRef]
- Guerra, A.J.; Cano, P.; Rabionet, M.; Puig, T.; Ciurana, J. 3D-printed PCL/PLA composite stents: Towards a new solution to cardiovascular problems. Materials 2018, 11, 1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Ramakrishna, S.; Singh, R. Material issues in additive manufacturing: A review. J. Manuf. Process. 2017, 25, 185–200. [Google Scholar] [CrossRef]
- Durán, I.R.; Vanslambrouck, S.; Chevallier, P.; Hoesli, C.A.; Laroche, G. Atmospheric pressure cold plasma versus wet-chemical surface treatments for carboxyl functionalization of polylactic acid: A first step toward covalent immobilization of bioactive molecules. Colloids Surf. B Biointerfaces 2020, 189, 110847. [Google Scholar] [CrossRef]
- Muenprasat, D.; Suttireungwong, S.; Tongpin, C. Functionalization of Poly (Lactic Acid) with Maleic Anhydride for Biomedical Application. J. Metals Mater. Miner. 2010, 20, 189–192. [Google Scholar]
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Progress Polym. Sci. 2012, 37, 237–280. [Google Scholar] [CrossRef]
- Pellis, A.; Silvestrini, L.; Scaini, D.; Coburn, J.M.; Gardossi, L.; Kaplan, D.L.; Herrero Acero, E.; Guebitz, G.M. Enzyme-catalyzed functionalization of poly(L-lactic acid) for drug delivery applications. Process Biochem. 2017, 59, 77–83. [Google Scholar] [CrossRef]
- Champeau, M.; Thomassin, J.M.; Tassaing, T.; Jerome, C. Drug Loading of Sutures by Supercritical CO2 Impregnation: Effect of Polymer/Drug Interactions and Thermal Transitions. Macromol. Mater. Eng. 2015, 300, 596–610. [Google Scholar] [CrossRef]
- Braeuer, A. Chapter 1—High Pressure: Fellow and Opponent of Spectroscopic Techniques. In Spectroscopic Techniques at High Pressure; Elsevier: Amsterdam, The Netherlands, 2015; Volume 7, pp. 1–40. [Google Scholar] [CrossRef]
- Robards, K.; Haddad, P.R.; Jackson, P.E. Sample Handling in Chromatography. In Principles and Practice of Modern Chromatographic Methods; Robards, K., Haddad, P.R., Jackson, P.E., Eds.; Academic Press: Boston, MA, USA, 2004; Chapter 8; pp. 407–455. [Google Scholar] [CrossRef]
- Cabezas, L.I.; Fernández, V.; Mazarro, R.; Gracia, I.; De Lucas, A.; Rodríguez, J.F. Production of biodegradable porous scaffolds impregnated with indomethacin in supercritical CO2. J. Supercrit. Fluids 2012, 63, 155–160. [Google Scholar] [CrossRef]
- Dias, A.M.; Rey-Rico, A.; Oliveira, R.A.; Marceneiro, S.; Alvarez-Lorenzo, C.; Concheiro, A.; Júnior, R.N.; Braga, M.E.; De Sousa, H.C. Wound dressings loaded with an anti-inflammatory jucá (Libidibia ferrea) extract using supercritical carbon dioxide technology. J. Supercrit. Fluids 2013, 74, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Villegas, C.; Torres, A.; Rios, M.; Rojas, A.; Romero, J.; de Dicastillo, C.L.; Valenzuela, X.; Galotto, M.J.; Guarda, A. Supercritical impregnation of cinnamaldehyde into polylactic acid as a route to develop antibacterial food packaging materials. Food Res. Int. 2017, 99, 650–659. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Cran, M.J.; Casas Cardoso, L.; Mantell Serrano, C.; Martínez de la Ossa, E.J.; Bigger, S.W. Effect of supercritical CO2 and olive leaf extract on the structural, thermal and mechanical properties of an impregnated food packaging film. J. Supercrit. Fluids 2019, 145, 181–191. [Google Scholar] [CrossRef]
- Barreto, J.C.; Trevisan, M.T.; Hull, W.E.; Erben, G.; De Brito, E.S.; Pfundstein, B.; Würtele, G.; Spiegelhalder, B.; Owen, R.W. Characterization and quantitation of polyphenolic compounds in bark, kernel, leaves, and peel of mango (Mangifera indica L.). J. Agric. Food Chem. 2008, 56, 5599–5610. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; Sanchez-Sanchez, J.; Casas, L.; Mantell, C.; de la Ossa, E.J. Impregnation of mango leaf extract into a polyester textile using supercritical carbon dioxide. J. Supercrit. Fluids 2017, 128, 208–217. [Google Scholar] [CrossRef]
- Jiang, T.; Han, F.; Gao, G.; Liu, M. Mangiferin exert cardioprotective and anti-apoptotic effects in heart failure induced rats. Life Sci. 2020, 249, 117476. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Major Tropical Fruits—Preliminary Market Results 2019; FAO: Rome, Italy, 2020; pp. 3–4. [Google Scholar]
- López-Ríos, L.; Wiebe, J.C.; Vega-Morales, T.; Gericke, N. Central nervous system activities of extract Mangifera indica L. J. Ethnopharmacol. 2020, 260, 112996. [Google Scholar] [CrossRef] [PubMed]
- Severi, J.A.; Lima, Z.P.; Kushima, H.; Brito, A.R.M.S.; Santos, L.C.D.; Vilegas, W.; Hiruma-Lima, C.A. Polyphenols with antiulcerogenic action from aqueous decoction of mango leaves (Mangifera indica L.). Molecules 2009, 14, 1098–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wightman, E.L.; Jackson, P.A.; Forster, J.; Khan, J.; Wiebe, J.C.; Gericke, N.; Kennedy, D.O. Acute Effects of a Polyphenol-Rich Leaf Extract of Mangifera indica L. (Zynamite) on Cognitive Function in Healthy Adults: A Double-Blind, Placebo-Controlled Crossover Study. Nutrients 2020, 12, 2194. [Google Scholar] [CrossRef] [PubMed]
- Kuganesan, A.; Thiripuranathar, G.; Navaratne, A.N.; Paranagama, P.A. Antioxidant and Anti-Inflammatory Activities of Peels, Pulps and Seed Kernels of Three Common Mango (Mangifera Indical L.) Varieties in Sri Lanka. Int. J. Pharmaceut. Sci. Res. 2017, 8, 70–78. [Google Scholar] [CrossRef]
- Swaroop, A.; Stohs, S.J.; Bagchi, M.; Moriyama, H.; Bagchi, D. Mango (Mangifera indica Linn) and Anti-Inflammatory Benefits: Versatile Roles in Mitochondrial Bio-Energetics and Exercise Physiology. Funct. Foods Health Dis. 2018, 8, 267. [Google Scholar] [CrossRef]
- Huang, C.Y.; Kuo, C.H.; Wu, C.H.; Kuan, A.W.; Guo, H.R.; Lin, Y.H.; Wang, P.K. Free Radical-Scavenging, Anti-Inflammatory, and Antibacterial Activities of Water and Ethanol Extracts Prepared from Compressional-Puffing Pretreated Mango (Mangifera indica L.) Peels. J. Food Quality 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Khumpook, T.; Saenphet, S.; Tragoolpua, Y.; Saenphet, K. Anti-inflammatory and antioxidant activity of Thai mango (Mangifera indica Linn.) leaf extracts. Comp. Clin. Pathol. 2019, 28, 157–164. [Google Scholar] [CrossRef]
- Stohs, S.; Swaroop, A.; Moriyama, H.; Bagchi, M.; Ahmad, T.; Bagchi, D. A Review on Antioxidant, Anti-Inflammatory and Gastroprotective Abilities of Mango (Magnifera indica) Leaf Extract and Mangiferin. J. Nutr. Health Sci. 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Dinarello, C.A. Blocking IL-1 in systemic inflammation. J. Exp. Med. 2005, 201, 1355–1359. [Google Scholar] [CrossRef]
- Gabay, C.; Lamacchia, C.; Palmer, G. IL-1 pathways in inflammation and human diseases. Nat. Rev. Rheumatol. 2010, 6, 232–241. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Dinarello, C.A. The IL-1 receptor/toll-like receptor superfamily: Crucial receptors for inflammation and host defense. Immunol. Today 2000, 21, 206–209. [Google Scholar] [CrossRef]
- González-Flores, D.; Rodríguez, A.B.; Pariente, J.A. TNFα-induced apoptosis in human myeloid cell lines HL-60 and K562 is dependent of intracellular ROS generation. Mol. Cell. Biochem. 2014, 390, 281–287. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; De La Ossa, E.M. Use of high pressure techniques to produce Mangifera indica L. leaf extracts enriched in potent antioxidant phenolic compounds. Innov. Food Sci. Emerg. Technol. 2015, 29, 94–106. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; Parjikolaei, B.R.; Lari, H.N.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.J. Pilot-plant scale extraction of phenolic compounds from mango leaves using different green techniques: Kinetic and scale up study. Chem. Eng. J. 2016, 299, 420–430. [Google Scholar] [CrossRef]
- Champeau, M.; Thomassin, J.M.; Tassaing, T.; Jérôme, C. Drug loading of polymer implants by supercritical CO2 assisted impregnation: A review. J. Control. Release 2015, 209, 248–259. [Google Scholar] [CrossRef]
- Jacobs, L.J.; Kemmere, M.F.; Keurentjes, J.T. Sustainable polymer foaming using high pressure carbon dioxide: A review on fundamentals, processes and applications. Green Chem. 2008, 10, 731–773. [Google Scholar] [CrossRef]
- Milovanović, S.L.; Kuska, R.M.; Škorić-Lučić, M.L.; Kalagasidis-Krušić, M.T.; Frerich, S.; Žižović, I.T.; Ivanović, J.Z. Swelling kinetics and impregnation of PLA with thymol under supercritical CO2 conditions. Tehnika 2016, 71, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Microflown E-Book 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Rodríguez, M.; Martínez De La Ossa, E. Extraction of antioxidant compounds from different varieties of Mangifera indica leaves using green technologies. J. Supercrit. Fluids 2012, 72, 168–175. [Google Scholar] [CrossRef]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Rahman, H.; Eswaraiah, M.C.; Dutta, A.M. In-vitro Anti-inflammatory and Anti-arthritic Activity of Oryza sativa Var. Joha Rice (An Aromatic Indigenous Rice of Assam). Am. Eurasian J. Agric. Environ. Sci. 2015, 15, 115–121. [Google Scholar] [CrossRef]
- Pöhler, H.; Kiran, E. Volumetric properties of carbon dioxide + ethanol at high pressures. J. Chem. Eng. Data 1997, 42, 384–388. [Google Scholar] [CrossRef]
- Rojas, A.; Torres, A.; José Galotto, M.; Guarda, A.; Julio, R. Supercritical impregnation for food applications: A review of the effect of the operational variables on the active compound loading. Crit. Rev. Food Sci. Nutr. 2020, 60, 1290–1301. [Google Scholar] [CrossRef]
- Milovanovic, S.; Markovic, D.; Mrakovic, A.; Kuska, R.; Zizovic, I.; Frerich, S.; Ivanovic, J. Supercritical CO2—assisted production of PLA and PLGA foams for controlled thymol release. Mater. Sci. Eng. C 2019, 99, 394–404. [Google Scholar] [CrossRef]
- Sugiura, K.; Ogawa, S.; Tabata, I.; Hori, T. Impregnation of tranilast to the poly(lactic acid) fiber with supercritical carbon dioxide and the release behavior of tranilast. J. Fiber Sci. Technol. 2005, 61, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Goñi, M.L.; Gañán, N.A.; Strumia, M.C.; Martini, R.E. Eugenol-loaded LLDPE films with antioxidant activity by supercritical carbon dioxide impregnation. J. Supercrit. Fluids 2016, 111, 28–35. [Google Scholar] [CrossRef]
- Franco, P.; Incarnato, L.; De Marco, I. Supercritical CO2 impregnation of α-tocopherol into PET/PP films for active packaging applications. J. CO2 Util. 2019, 34, 266–273. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Casas Cardoso, L.; Fernández Ponce, M.T.; Mantell Serrano, C.; Martínez de la Ossa-Fernández, E.J. Characterization of olive leaf extract polyphenols loaded by supercritical solvent impregnation into PET/PP food packaging films. J. Supercrit. Fluids 2018, 140, 196–206. [Google Scholar] [CrossRef]
- Bastante, C.C.; Silva, N.H.; Cardoso, L.C.; Serrano, C.M.; Martínez de la Ossa, E.J.; Freire, C.S.; Vilela, C. Biobased films of nanocellulose and mango leaf extract for active food packaging: Supercritical impregnation versus solvent casting. Food Hydrocoll. 2021, 117, 106709. [Google Scholar] [CrossRef]
- Chandra, S.; Chatterjee, P.; Dey, P.; Bhattacharya, S. Evaluation of in vitro anti-inflammatory activity of coffee against the denaturation of protein. Asian Pacific J. Trop. Biomed. 2012, 2, S178–S180. [Google Scholar] [CrossRef]
- Gambhire Manoj, N.; Juvekar Archana, R.; Sakat Sachin, S. Evaluation of anti-inflammatory activity of methanol extract of Murraya Koenigi leaves by in vivo and in vitro methods. Pharmacologyonline 2009, 1, 1072–1094. [Google Scholar]
- Pop, M.A.; Croitoru, C.; Bedő, T.; Geaman, V.; Radomir, I.; Coșnița, M.; Zaharia, S.M.; Chicoș, L.A.; Miloșan, I. Structural changes during 3D printing of bioderived and synthetic thermoplastic materials. J. Appl. Polym. Sci. 2019, 136, 1–11. [Google Scholar] [CrossRef]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Kim, K.H.; Park, J.W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
| Property | Value (°C) | Standard Test |
|---|---|---|
| Heat distrosion temperature | 56 | ISO 75/B |
| Melting temperature | 145–160 | ASTM D3418 |
| Glass transition temperature | 56–64 | ASTM D3418 |
| Variable | Value |
|---|---|
| Temperature (°C) | 35, 45 *, 55 |
| Pressure (atm) | 100, 250 *, 400 |
| Amount of MLE (% vessel volume) | 1, 2 *, 3 |
| Impregnation Condition | %ADC | [MLE] (g/mL) | %MLE Released |
|---|---|---|---|
| 1% MLE, 100 bar, 35 °C | 5.90 ± 4.81 | 10.02 ± 2.55 | 1.36 ± 0.54 |
| 1% MLE, 100 bar, 55 °C | 13.33 ± 0.00 | 14.68 ± 0.00 | 1.23 ± 0.10 |
| 1% MLE, 400 bar, 35 °C | 15.93 ± 4.43 | 17.10 ± 3.99 | 2.81 ± 0.53 |
| 1% MLE, 400 bar, 55 °C | n.d. | 7.19 ± 0.00 | 10.04 ± 11.21 |
| 2% MLE, 250 bar, 45 °C | 4.30 ± 2.94 | 9.11 ± 1.43 | 1.27 ± 0.25 |
| 3% MLE, 100 bar, 35 °C | 22.78 ± 1.92 | 24.40 ± 2.50 | 1.93 ± 0.26 |
| 3% MLE, 100 bar, 55 °C | 18.86 ± 3.10 | 19.87 ± 3.28 | 1.54 ± 0.31 |
| 3% MLE, 400 bar, 35 °C | 6.67 ± 0.00 | 10.27 ± 0.00 | 1.39 ± 0.24 |
| 3% MLE, 400 bar, 55 °C | 25.00 ± 0.00 | 27.41 ± 0.00 | 4.66 ± 0.33 |
| Factor | Low | High | Optimum |
|---|---|---|---|
| Pressure | 100.0 | 400.0 | 100.0 |
| Temperature | 35.0 | 55.0 | 38.7 |
| Extract | 1.0 | 3.0 | 3.0 |
| %ADCnon−inc | %AOCinc | %AOCnon−inc | %AOCinc | |
|---|---|---|---|---|
| Non-printed PLA | 11.1 ± 2.8 | 30.1 ± 6.5 | 88.1 ± 1.9 | 9.9 ± 1.1 |
| Printed PLA | n.d. | 23.5 ± 2.2 | 79.1 ± 16.2 | 2.5 ± 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosales, J.M.; Cejudo, C.; Verano, L.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.J. Supercritical Impregnation of PLA Filaments with Mango Leaf Extract to Manufacture Functionalized Biomedical Devices by 3D Printing. Polymers 2021, 13, 2125. https://doi.org/10.3390/polym13132125
Rosales JM, Cejudo C, Verano L, Casas L, Mantell C, Martínez de la Ossa EJ. Supercritical Impregnation of PLA Filaments with Mango Leaf Extract to Manufacture Functionalized Biomedical Devices by 3D Printing. Polymers. 2021; 13(13):2125. https://doi.org/10.3390/polym13132125
Chicago/Turabian StyleRosales, José María, Cristina Cejudo, Lidia Verano, Lourdes Casas, Casimiro Mantell, and Enrique José Martínez de la Ossa. 2021. "Supercritical Impregnation of PLA Filaments with Mango Leaf Extract to Manufacture Functionalized Biomedical Devices by 3D Printing" Polymers 13, no. 13: 2125. https://doi.org/10.3390/polym13132125
APA StyleRosales, J. M., Cejudo, C., Verano, L., Casas, L., Mantell, C., & Martínez de la Ossa, E. J. (2021). Supercritical Impregnation of PLA Filaments with Mango Leaf Extract to Manufacture Functionalized Biomedical Devices by 3D Printing. Polymers, 13(13), 2125. https://doi.org/10.3390/polym13132125