Elastin-Plasma Hybrid Hydrogels for Skin Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Elastin-Like Recombinamer (ELR) Biosynthesis and Purification
2.3. Synthesis of Azide/Cyclooctyne-Bearing ELRs
2.4. Preparation of Plasma-Derived Fibrin Hydrogels
2.5. Preparation of Elastin-Plasma Hydrogels
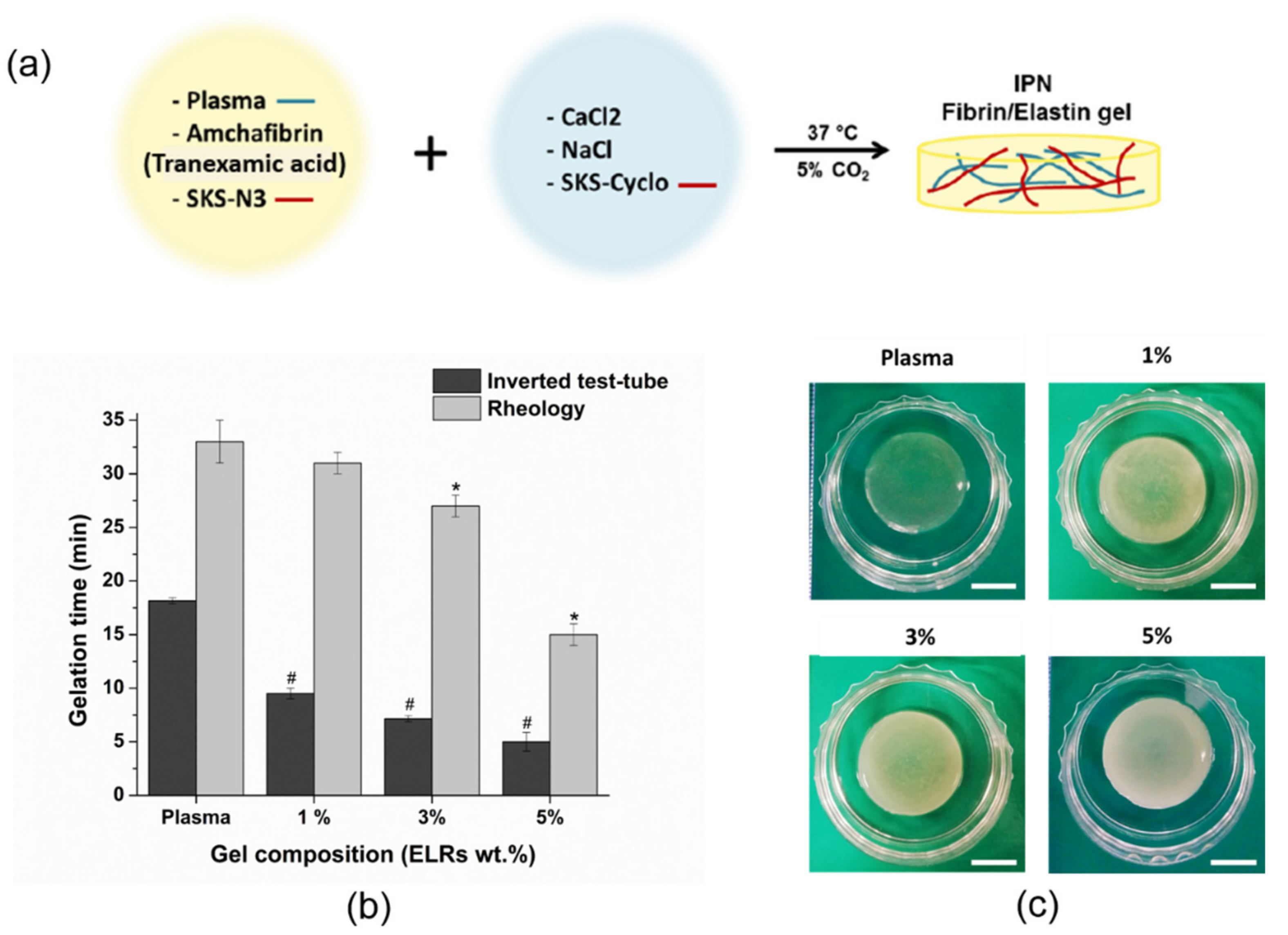
2.6. Gelation Time Determination
2.7. Physicochemical Properties of Hybrid Plasma-ELRs Hydrogels
2.7.1. Azide/Cyclooctyne-Bearing ELRs and Hybrid Plasma-ELRs Hydrogel Characterization
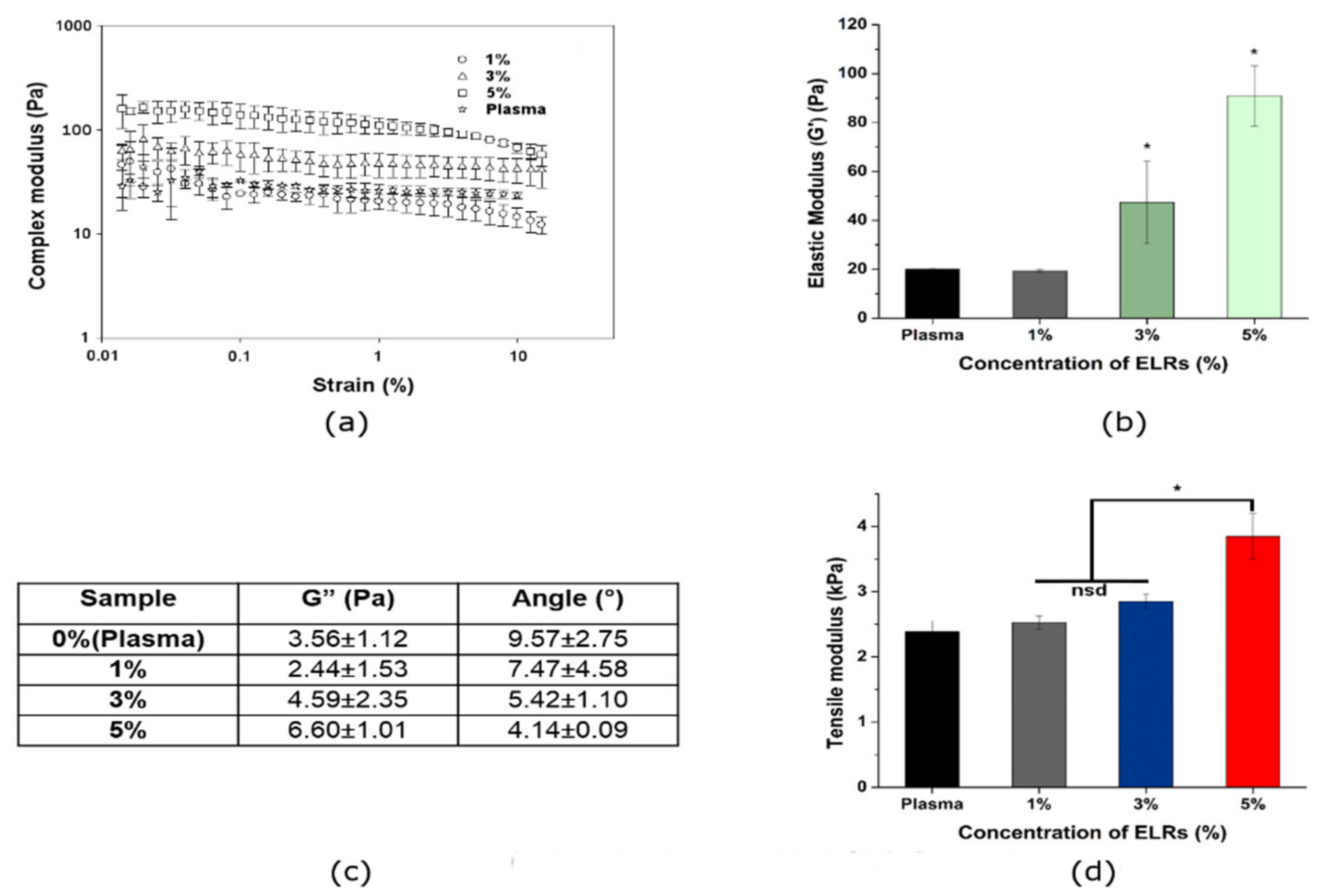
2.7.2. Rheological Measurements
2.7.3. Characterization of Hydrogel Structure
2.8. Contraction of Hydrogels in Phosphate-Buffered Saline (PBS)
2.9. Cell Studies
2.9.1. Primary Human Keratinocites (hKCs) and Human Fibroblasts (hFBs) Culture
2.9.2. hFB-Mediated Hydrogel Contraction
2.9.3. Cell Proliferation Assay of Encapsulated hFBs
2.9.4. Cell Viability of Human Keratinocytes (hKCs)
2.10. Statistical Analysis and Data Representation
3. Results and Discussion
3.1. Gelation Time Determination
3.2. Physicochemical Properties of Hybrid Plasma-ELR Hydrogels
3.3. Contraction of Hydrogels in Phosphate-Buffered Saline (PBS)
3.4. Cell Studies or Biological Evaluation
3.4.1. hFBs-Mediated Gel Contraction
3.4.2. Cell Proliferation of Encapsulated hFBs
3.4.3. Cell Viability of hKCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazlyzam, A.; Aminuddin, B.; Fuzina, N.; Norhayati, M.; Fauziah, O.; Isa, M.; Saim, L.; Ruszymah, B. Reconstruction of living bilayer human skin equivalent utilizing human fibrin as a scaffold. Burns 2007, 33, 355–363. [Google Scholar] [CrossRef]
- Llames, S.; Del Rio, M.; Larcher, F.; García, E.; García, M.; Escámez, M.J.; Jorcano, J.L.; Holguín, P.; Meana, A. Human plasma as a dermal scaffold for the generation of a completely autologous bioengineered skin. Transplantation 2004, 77, 350–355. [Google Scholar] [CrossRef]
- Meana, A.; Iglesias, J.; Del Rio, M.; Larcher, F.; Madrigal, B.; Fresno, M.; Martin, C.; Roman, F.S.; Tevar, F. Large surface of cultured human epithelium obtained on a dermal matrix based on live fibroblast-containing fibrin gels. Burns 1998, 24, 621–630. [Google Scholar] [CrossRef]
- Guerrero-Aspizua, S.; García, M.; Murillas, R.; Retamosa, L.; Illera, N.; Duarte, B.; Holguín, A.; Puig, S.; Hernández, M.I.; Meana, A.; et al. Development of a bioengineered skin-humanized mouse model for psoriasis: Dissecting epidermal-lymphocyte interacting pathways. Am. J. Pathol. 2010, 177, 3112–3124. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Santamaría, L.; Conti, C.J.; Llames, S.; García, E.; Retamosa, L.; Holguin, A.; Illera, N.; Duarte, B.; Camblor, L.; Llaneza, J.M.; et al. The regenerative potential of fibroblasts in a new diabetes-induced delayed humanised wound healing model. Exp. Dermatol. 2013, 22, 195–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cubo, N.; Garcia, M.; Del Cañizo, J.F.; Velasco, D.; Jorcano, J.L. 3D bioprinting of functional human skin: Production and in vivo analysis. Biofabrication 2016, 9, 015006. [Google Scholar] [CrossRef] [Green Version]
- Marck, R.E.; Middelkoop, E.; Breederveld, R.S. Considerations on the use of platelet-rich plasma, specifically for burn treatment. J. Burn. Care Res. 2014, 35, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Llames, S.; García, E.; García, V.; Del Rio, M.; Larcher, F.; Jorcano, J.L.; López, E.; Holguín, P.; Miralles, F.; Otero, J.; et al. Clinical results of an autologous engineered skin. Cell Tissue Bank. 2006, 7, 47–53. [Google Scholar] [CrossRef]
- Gómez, C.; Galán, J.; Torrero, V.; Ferreiro, I.; Pérez, D.; Palao, R.; Martínez, E.; Llames, S.; Meana, A.; Holguín, P. Use of an autologous bioengineered composite skin in extensive burns: Clinical and functional outcomes. A multicentric study. Burns 2011, 37, 580–589. [Google Scholar] [CrossRef]
- Burnouf, T.; Goubran, H.A.; Chen, T.-M.; Ou, K.-L.; El-Ekiaby, M.; Radosevic, M. Blood-derived biomaterials and platelet growth factors in regenerative medicine. Blood Rev. 2013, 27, 77–89. [Google Scholar] [CrossRef]
- Marx, R.E.; Carlson, E.R.; Eichstaedt, R.M.; Schimmele, S.R.; Strauss, J.E.; Georgeff, K.R. Platelet rich plasma, growth factor enhancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 85, 638–646. [Google Scholar] [CrossRef]
- Geer, D.J.; Swartz, D.D.; Andreadis, S.T. Fibrin promotes migration in a three dimensional in vitro model of wound regeneration. Tissue Eng. 2002, 8, 787–798. [Google Scholar] [CrossRef]
- Gil Park, Y.; Lee, I.H.; Park, E.S.; Kim, J.Y. Hydrogel and platelet-rich plasma combined treatment to accelerate wound healing in a nude mouse model. Arch. Plast. Surg. 2017, 44, 194–201. [Google Scholar] [CrossRef] [Green Version]
- Isola, G.; Polizzi, A.; Alibrandi, A.; Williams, R.C.; Giudice, A.L. Analysis of galectin-3 levels as a source of coronary heart disease risk during periodontitis. J. Periodontal Res. 2021, 56, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Polizzi, A.; Santonocito, S.; Alibrandi, A.; Williams, R.C. Periodontitis activates the NLRP3 inflammasome in serum and saliva. J. Periodontol. 2021. [Google Scholar] [CrossRef]
- Johnson, B.Z.; Stevenson, A.W.; Prêle, C.M.; Fear, M.W.; Wood, F.M. The Role of IL-6 in skin fibrosis and cutaneous wound healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef]
- McLeod, K.; Walker, J.T.; Hamilton, D.W. Galectin-3 regulation of wound healing and fibrotic processes: Insights for chronic skin wound therapeutics. J. Cell Commun. Signal. 2018, 12, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Montero, A.; Acosta, S.; Hernández, R.; Elvira, C.; Jorcano, J.L.; Velasco, D. Contraction of fibrin-derived matrices and its implications for in vitro human skin bioengineering. J. Biomed. Mater. Res. Part A 2021, 109, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ma, L.; Zhou, J.; Mao, Z.; Gao, C.; Shen, J. Fabrication and physical and biological properties of fibrin gel derived from human plasma. Biomed. Mater. 2007, 3, 015001. [Google Scholar] [CrossRef] [Green Version]
- Tuan, T.-L.; Song, A.; Chang, S.; Younai, S.; Nimni, M.E. In vitro fibroplasia: Matrix contraction, cell growth, and collagen production of fibroblasts cultured in fibrin gels. Exp. Cell Res. 1996, 223, 127–134. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, D.; Zhao, W.; Zhang, R.; Yu, B.; Ma, G.; Li, Y.; Hao, D.; Xu, F. Engineering platelet—Rich plasma based dual-network hydrogel as a bioactive wound dressing with potential clinical translational value. Adv. Funct. Mater. 2021, 31. [Google Scholar] [CrossRef]
- Burmeister, D.M.; Roy, D.C.; Becerra, S.C.; Natesan, S.; Christy, R.J. In situ delivery of fibrin-based hydrogels prevents contraction and reduces inflammation. J. Burn Care Res. 2017, 39, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, D.M.; Stone, R.; Wrice, N.L.; Becerra, S.C.; Natesan, S.; Christy, R.J. Fibrin hydrogels prevent contraction and deliver adipose stem cells to debrided deep partial thickness burns for accelerated angiogenesis. FASEB J. 2016, 30, 1300.7. [Google Scholar] [CrossRef]
- Natesan, S.; Stone, R.; Coronado, R.E.; Wrice, N.L.; Kowalczewski, A.C.; Zamora, D.O.; Christy, R.J. PEGylated platelet-free blood plasma-based hydrogels for full-thickness wound regeneration. Adv. Wound Care 2019, 8, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Ii, R.S.; Wall, J.T.; Natesan, S.; Christy, R.J.; Stone, R. PEG-plasma hydrogels increase epithelialization using a human ex vivo skin model. Int. J. Mol. Sci. 2018, 19, 3156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carriel, V.; Garzón, I.; Jiménez, J.-M.; Oliveira, C.-X.; Arias-Santiago, S.; Campos, A.; Sánchez-Quevedo, M.-C.; Alaminos, M. Epithelial and stromal developmental patterns in a novel substitute of the human skin generated with fibrin-agarose biomaterials. Cells Tissues Organs 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Haslik, W.; Kamolz, L.-P.; Manna, F.; Hladik, M.; Rath, T.; Frey, M. Management of full-thickness skin defects in the hand and wrist region: First long-term experiences with the dermal matrix Matriderm. J. Plast. Reconstr. Aesthetic. Surg. 2010, 63, 360–364. [Google Scholar] [CrossRef]
- Rnjak, J.; Wise, S.; Mithieux, S.; Weiss, A.S. Severe burn injuries and the role of elastin in the design of dermal substitutes. Tissue Eng. Part B Rev. 2011, 17, 81–91. [Google Scholar] [CrossRef]
- Vrhovski, B.; Weiss, A.S. Biochemistry of tropoelastin. JBIC J. Biol. Inorg. Chem. 1998, 258, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, F. Pathophysiology of the microfibril/elastic fiber system: Introduction. Matrix Biol. 2000, 19, 455–456. [Google Scholar] [CrossRef]
- Rodríguez-Cabello, J.C.; Prieto, S.; Reguera, J.; Arias, F.J.; Ribeiro, A. Biofunctional design of elastin-like polymers for advanced applications in nanobiotechnology. J. Biomater. Sci. Polym. Ed. 2007, 18, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.E.; Chilkot, A. Genetically encoded synthesis of protein-based polymers with precisely specified molecular weight and sequence by recursive directional ligation: Examples from the elastin-like polypeptide system. Biomacromolecules 2002, 3, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cabello, J.C.; Martín, L.; Alonso, M.; Arias, F.J.; Testera, A.M. “Recombinamers” as advanced materials for the post-oil age. Polymer 2009, 50, 5159–5169. [Google Scholar] [CrossRef] [Green Version]
- Chow, D.; Nunalee, M.L.; Lim, D.W.; Simnick, A.J.; Chilkoti, A. Peptide-based biopolymers in biomedicine and biotechnology. Mater. Sci. Eng. R Rep. 2008, 62, 125–155. [Google Scholar] [CrossRef] [Green Version]
- MacEwan, S.; Chilkoti, A. Elastin-like polypeptides: Biomedical applications of tunable biopolymers. Biopolymers 2010, 94, 60–77. [Google Scholar] [CrossRef] [PubMed]
- Urry, D.W. Elastic molecular machines in metabolism and soft-tissue restoration. Trends Biotechnol. 1999, 17, 249–257. [Google Scholar] [CrossRef]
- Urry, D.W.; Pattanaik, A.; Xu, J.; Woods, T.C.; McPherson, D.T.; Parker, T.M. Elastic protein-based polymers in soft tissue augmentation and generation. J. Biomater. Sci. Polym. Ed. 1998, 9, 1015–1048. [Google Scholar] [CrossRef]
- Zhang, H.; Iwama, M.; Akaike, T.; Urry, D.W.; Pattanaik, A.; Parker, T.M.; Konishi, I.; Nikaido, T. Human amniotic cell sheet harvest using a novel temperature-responsive culture surface coated with protein-based polymer. Tissue Eng. 2006, 12, 391–401. [Google Scholar] [CrossRef]
- Urry, D.W. What Sustains Life? Consilient Mechanisms for Protein-Based Machines and Materials; Springer: New York, NY, USA, 2006. [Google Scholar]
- Ibáñez-Fonseca, A.; Ramos, T.L.; González de Torre, I.; Sánchez-Abarca, L.I.; Muntión, S.; Arias, F.J.; Del Cañizo, M.C.; Alonso, M.; Sánchez-Guijo, F.; Rodríguez-Cabello, J.C. Biocompatibility of two model elastin-like recombinamer-based hydrogels formed through physical or chemical cross-linking for various applications in tissue engineering and regenerative medicine. J Tissue Eng. Regen. Med. 2018, 12, e1450–e1460. [Google Scholar] [CrossRef] [Green Version]
- Changi, K.; Bosnjak, B.; Gonzalez-Obeso, C.; Kluger, R.; Rodríguez-Cabello, J.C.; Hoffmann, O.; Epstein, M.M. Biocompatibility and immunogenicity of elastin-like recombinamer biomaterials in mouse models. J. Biomed. Mater. Res. Part A 2018, 106, 924–934. [Google Scholar] [CrossRef]
- Urry, D.W.; Parker, T.M.; Reid, M.C.; Gowda, D.C. Biocompatibility of the Bioelastic Materials, Poly(GVGVP) and Its γ-irradiation cross-linked Matrix: Summary of generic biological test results. J. Bioact. Compat. Polym. 1991, 6, 263–282. [Google Scholar] [CrossRef]
- De Torre, I.G.; Wolf, F.; Santos, M.; Rongen, L.; Alonso, M.; Jockenhoevel, S.; Rodriguez-Cabello, J.C.; Mela, P. Elastin-like recombinamer-covered stents: Towards a fully biocompatible and non-thrombogenic device for cardiovascular diseases. Acta Biomater. 2015, 12, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Fernández, S.; Santos, M.; Alonso, M.; Quintanilla, L.; Rodríguez-Cabello, J.C. Genetically engineered elastin-like recombinamers with sequence-based molecular stabilization as advanced bioinks for 3D bioprinting. Appl. Mater. Today 2020, 18, 100500. [Google Scholar] [CrossRef]
- Buttafoco, L.; Engbers—Buijtenhuijs, P.; Poot, A.; Dijkstra, P.; Daamen, W.; Van Kuppevelt, T.; Vermes, I.; Feijen, J. First steps towards tissue engineering of small—Diameter blood vessels: Preparation of flat scaffolds of collagen and elastin by means of freeze drying. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 77, 357–368. [Google Scholar] [CrossRef]
- Grover, C.N.; Cameron, R.E.; Best, S.M. Investigating the morphological, mechanical and degradation properties of scaffolds comprising collagen, gelatin and elastin for use in soft tissue engineering. J. Mech. Behav. Biomed. Mater. 2012, 10, 62–74. [Google Scholar] [CrossRef]
- Ryan, A.J.; O’Brien, F.J. Insoluble elastin reduces collagen scaffold stiffness, improves viscoelastic properties, and induces a contractile phenotype in smooth muscle cells. Biomaterials 2015, 73, 296–307. [Google Scholar] [CrossRef]
- Daamen, W.F.; Nillesen, S.T.; Wismans, R.G.; Reinhardt, D.P.; Hafmans, T.; Veerkamp, J.H.; Van Kuppevelt, T.H. A biomaterial composed of collagen and solubilized elastin enhances angiogenesis and elastic fiber formation without calcification. Tissue Eng. Part A 2008, 14, 349–360. [Google Scholar] [CrossRef] [Green Version]
- Daamen, W.; Nillesen, S.; Hafmans, T.; Veerkamp, J.; van Luyn, M.; van Kuppevelt, T. Tissue response of defined collagen–elastin scaffolds in young and adult rats with special attention to calcification. Biomaterials 2005, 26, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Mithieux, S.; Weiss, A.S.; Dehghani, F. The fabrication of elastin-based hydrogels using high pressure CO2. Biomaterials 2009, 30, 1–7. [Google Scholar] [CrossRef]
- Annabi, N.; Mithieux, S.; Boughton, E.A.; Ruys, A.J.; Weiss, A.S.; Dehghani, F. Synthesis of highly porous crosslinked elastin hydrogels and their interaction with fibroblasts in vitro. Biomaterials 2009, 30, 4550–4557. [Google Scholar] [CrossRef] [PubMed]
- Testera, A.M.; Girotti, A.; de Torre, I.G.; Quintanilla, L.; Santos, M.; Alonso, M.; Rodríguez-Cabello, J.C. Biocompatible elastin-like click gels: Design, synthesis and characterization. J. Mater. Sci. Mater. Med. 2015, 26, 1–13. [Google Scholar] [CrossRef]
- De Torre, I.G.; Weber, M.; Quintanilla, L.; Alonso, M.; Jockenhoevel, S.; Cabello, J.C.R.; Mela, P. Hybrid elastin-like recombinamer-fibrin gels: Physical characterization and in vitro evaluation for cardiovascular tissue engineering applications. Biomater. Sci. 2016, 4, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; de Torre, I.G.; Moreira, R.; Frese, J.; Oedekoven, C.A.; Alonso, M.; Cabello, C.J.R.; Jockenhoevel, S.; Mela, P. Multiple-step injection molding for fibrin-based tissue-engineered heart Valves. Tissue Eng. Part C Methods 2015, 21, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Staubli, S.M.; Cerino, G.; De Torre, I.G.; Alonso, M.; Oertli, D.; Eckstein, F.; Glatz, K.; Cabello, J.C.R.; Marsano, A. Control of angiogenesis and host response by modulating the cell adhesion properties of an Elastin-Like Recombinamer-based hydrogel. Biomaterials 2017, 135, 30–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AABB (American Association of Blood Banks). Technical Manual, 18th ed.; AABB: Bethesda, MD, USA, 2014. [Google Scholar]
- Brecher, M.E. Technical Manual, 15th ed.; AABB: Bethesda, MD, USA, 2005; p. 68. [Google Scholar]
- Rodríguez-Cabello, J.C.; Girroti, A.; Ribeiro, A.; Arias, F.J. Synthesis of genetically engineered protein polymers (recombinamers) as an example of advanced self-assembled smart materials. In Nanotechnology in Regenerative Medicine: Methods and Protocols; Navarro, M., Planell, J.A., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 17–38. [Google Scholar]
- de Torre, I.G.; Santos, M.; Quintanilla, L.; Testera, A.; Alonso, M.; Rodriguez-Cabello, J.C. Elastin-like recombinamer catalyst-free click gels: Characterization of poroelastic and intrinsic viscoelastic properties. Acta Biomater. 2014, 10, 2495–2505. [Google Scholar] [CrossRef]
- Vanderhooft, J.L.; Mann, B.K.; Prestwich, G.D. Synthesis and characterization of novel thiol-reactive poly(ethylene glycol) cross-linkers for extracellular-matrix-mimetic biomaterials. Biomacromolecules 2007, 8, 2883–2889. [Google Scholar] [CrossRef]
- Rheinwald, J.G.; Green, H. Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cells 1975, 6, 331–343. [Google Scholar] [CrossRef]
- Del Rio, M.; Larcher, F.; Serrano, F.; Meana, A.; Muñoz, M.; García, M.; Munoz, E.; Martin, C.; Bernad, A.; Jorcano, J.L. A Preclinical model for the analysis of genetically modified human skin in vivo. Hum. Gene Ther. 2002, 13, 959–968. [Google Scholar] [CrossRef] [Green Version]
- Chernysh, I.N.; Weisel, J.W. Dynamic imaging of fibrin network formation correlated with other measures of polymerization. Blood 2008, 111, 4854–4861. [Google Scholar] [CrossRef] [Green Version]
- Crescenzi, V.; Cornelio, L.; Di Meo, C.; Nardecchia, S.; Lamanna, R. Novel hydrogels via click chemistry: Synthesis and potential biomedical applications. Biomacromolecules 2007, 8, 1844–1850. [Google Scholar] [CrossRef]
- Wang, Q.; Chan, T.R.; Hilgraf, R.; Fokin, V.V.; Sharpless, K.B.; Finn, M.G. Bioconjugation by Copper(I)-Catalyzed Azide-Alkyne [3 + 2] Cycloaddition. J. Am. Chem. Soc. 2003, 125, 3192–3193. [Google Scholar] [CrossRef]
- Link, A.J.; Tirrell, D.A. Cell surface labeling of Escherichia coli via copper(I)-catalyzed [3+2] cycloaddition. J. Am. Chem. Soc. 2003, 125, 11164–11165. [Google Scholar] [CrossRef]
- Han, L.; Lu, X.; Liu, K.; Wang, K.; Fang, L.; Weng, L.-T.; Zhang, H.; Tang, Y.; Ren, F.; Zhao, C.; et al. Mussel-inspired adhesive and tough hydrogel based on nanoclay confined dopamine polymerization. ACS Nano 2017, 11, 2561–2574. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, C.; He, C.; Zhao, J.; Yang, X.; Wang, H. Synthesis of graphene peroxide and its application in fabricating super extensible and highly resilient nanocomposite hydrogels. ACS Nano 2012, 6, 8194–8202. [Google Scholar] [CrossRef]
- Lee, F.; Kurisawa, M. Formation and stability of interpenetrating polymer network hydrogels consisting of fibrin and hyaluronic acid for tissue engineering. Acta Biomater. 2013, 9, 5143–5152. [Google Scholar] [CrossRef] [PubMed]
- McHale, M.K.; Setton, L.A.; Chilkoti, A. Synthesis and in vitro evaluation of enzymatically cross-linked elastin-like polypeptide gels for cartilaginous tissue repair. Tissue Eng. 2005, 11, 1768–1779. [Google Scholar] [CrossRef]
- Ryan, E.A.; Mockros, L.F.; Weisel, J.W.; Lorand, L. Structural origins of fibrin clot rheology. Biophys. J. 1999, 77, 2813–2826. [Google Scholar] [CrossRef] [Green Version]
- Weisel, J.W.; Litvinov, R.I. Fibrin formation, structure and properties. Prokaryotic Cytoskelet. 2017, 82, 405–456. [Google Scholar]
- De Torre, I.G.; Quintanilla, L.; Pinedo-Martín, G.; Alonso, M.; Rodríguez-Cabello, J.C. Nanogel formation from dilute solutions of clickable elastin-like recombinamers and its dependence on temperature: Two fractal gelation modes. ACS Appl. Mater. Interfaces 2014, 6, 14509–14515. [Google Scholar] [CrossRef] [Green Version]
- Weise, J.W.; Litvinov, R.I. Mechanisms of fibrin polymerization and clinical implications. Blood 2013, 121, 1712–1719. [Google Scholar]
- Li, W.; Sigley, J.; Pieters, M.; Helms, C.C.; Nagaswami, C.; Weisel, J.W.; Guthold, M. Fibrin fiber stiffness is strongly affected by fiber diameter, but not by fibrinogen glycation. Biophys. J. 2016, 110, 1400–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, S.L.; Lee, S.; Stegemann, J.P. Influence of thrombin concentration on the mechanical and morphological properties of cell-seeded fibrin hydrogels. Acta Biomater. 2007, 3, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Weisel, J.; Nagaswami, C. Computer modeling of fibrin polymerization kinetics correlated with electron microscope and turbidity observations: Clot structure and assembly are kinetically controlled. Biophys. J. 1992, 63, 111–128. [Google Scholar] [CrossRef] [Green Version]
- Kurniawan, N.N.; Grimbergen, J.; Koopman, J.; Koenderink, G.H. Factor XIII stiffens fibrin clots by causing fiber compaction. J. Thromb. Haemost. 2014, 12, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.; Miller, S.; Case, E.; Leach, J. Supplementation of fibrin gels with sodium chloride enhances physical properties and ensuing osteogenic response. Acta Biomater. 2011, 7, 691–699. [Google Scholar] [CrossRef]
- Lai, V.K.; Frey, C.R.; Kerandi, A.M.; Lake, S.; Tranquillo, R.T.; Barocas, V.H. Microstructural and mechanical differences between digested collagen–fibrin co-gels and pure collagen and fibrin gels. Acta Biomater. 2012, 8, 4031–4042. [Google Scholar] [CrossRef] [Green Version]
- Ruszymah, B.; Chua, K.; Mazlyzam, A.; Aminuddin, B. Formation of tissue engineered composite construct of cartilage and skin using high density polyethylene as inner scaffold in the shape of human helix. Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 805–810. [Google Scholar] [CrossRef]
- Ronfard, V.; Rives, J.M.; Neveux, Y.; Carsin, H.; Barrandon, Y. Long-term regeneration of human epidermis on third degree burns transplanted with autologous cultured epithelium grown on a fibrin matrix1,2. Transplantation 2000, 70, 1588–1598. [Google Scholar] [CrossRef] [PubMed]
- Grinnell, F.; Lamke, C. Reorganization of hydrated collagen lattices by human skin fibroblasts. J. Cell Sci. 1984, 66, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Yamato, M.; Adachi, E.; Yamamoto, K.; Hayashi, T. Condensation of collagen fibrils to the direct vicinity of fibroblasts as a cause of gel Contraction1. J. Biochem. 1995, 117, 940–946. [Google Scholar] [CrossRef]
- Ribeiro, A.; Arias, F.J.; Reguera, J.; Alonso, M.; Rodríguez-Cabello, J.C. Influence of the amino-acid sequence on the inverse temperature transition of elastin-like polymers. Biophys. J. 2009, 97, 312–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nien, Y.-D.; Han, Y.-P.; Tawil, B.; Chan, L.S.; Tuan, T.-L.; Garner, W.L. Fibrinogen inhibits fibroblast-mediated contraction of collagen. Wound Repair Regen. 2003, 11, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.P.; Wyler, D.J. Fibroblast contraction of collagen lattices in vitro: Inhibition by chronic inflammatory cell mediators. J. Cell. Physiol. 1983, 116, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Rnjak, J.; Li, Z.; Maitz, P.K.; Wise, S.; Weiss, A.S. Primary human dermal fibroblast interactions with open weave three-dimensional scaffolds prepared from synthetic human elastin. Biomaterials 2009, 30, 6469–6477. [Google Scholar] [CrossRef]
- Lee, H.-J.; Sen, A.; Bae, S.; Lee, J.S.; Webb, K. Poly(ethylene glycol) diacrylate/hyaluronic acid semi-interpenetrating network compositions for 3-D cell spreading and migration. Acta Biomater. 2015, 14, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Thibeault, S.L. Biocompatibility of a synthetic extracellular matrix on immortalized vocal fold fibroblasts in 3-D culture. Acta Biomater. 2010, 6, 2940–2948. [Google Scholar] [CrossRef] [Green Version]
- Zeltinger, J.; Sherwood, J.K.; Graham, D.A.; Müeller, R.; Griffith, L. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng. 2001, 7, 557–572. [Google Scholar] [CrossRef]
- Mazzucotelli, J.-P.; Klein-Soyer, C.; Beretz, A.; Brisson, C.; Archipoff, G.; Cazenave, J.-P. Endothelial cell seeding: Coating dacron and expanded polytetrafluoroethylene vascular grafts with a biological glue allows adhesion and growth of human saphenous vein endothelial cells. Int. J. Artif. Organs 1991, 14, 482–490. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Luo, X.; Qiu, J.; Tang, C. Substrate stiffness regulates the proliferation, migration, and differentiation of epidermal cells. Burns 2012, 38, 414–420. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojic, M.; Ródenas-Rochina, J.; López-Donaire, M.L.; González de Torre, I.; González Pérez, M.; Rodríguez-Cabello, J.C.; Vojtová, L.; Jorcano, J.L.; Velasco, D. Elastin-Plasma Hybrid Hydrogels for Skin Tissue Engineering. Polymers 2021, 13, 2114. https://doi.org/10.3390/polym13132114
Stojic M, Ródenas-Rochina J, López-Donaire ML, González de Torre I, González Pérez M, Rodríguez-Cabello JC, Vojtová L, Jorcano JL, Velasco D. Elastin-Plasma Hybrid Hydrogels for Skin Tissue Engineering. Polymers. 2021; 13(13):2114. https://doi.org/10.3390/polym13132114
Chicago/Turabian StyleStojic, Marija, Joaquín Ródenas-Rochina, María Luisa López-Donaire, Israel González de Torre, Miguel González Pérez, José Carlos Rodríguez-Cabello, Lucy Vojtová, José Luis Jorcano, and Diego Velasco. 2021. "Elastin-Plasma Hybrid Hydrogels for Skin Tissue Engineering" Polymers 13, no. 13: 2114. https://doi.org/10.3390/polym13132114
APA StyleStojic, M., Ródenas-Rochina, J., López-Donaire, M. L., González de Torre, I., González Pérez, M., Rodríguez-Cabello, J. C., Vojtová, L., Jorcano, J. L., & Velasco, D. (2021). Elastin-Plasma Hybrid Hydrogels for Skin Tissue Engineering. Polymers, 13(13), 2114. https://doi.org/10.3390/polym13132114







