Research on the Anticorrosion Properties of CeO2-GO/EP Nanocomposite Coating in Simulated Sea Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of Simulated Seawater of Different Concentrations
2.3. Preparation of CeO2-GO(4:1)/EP Coationgs
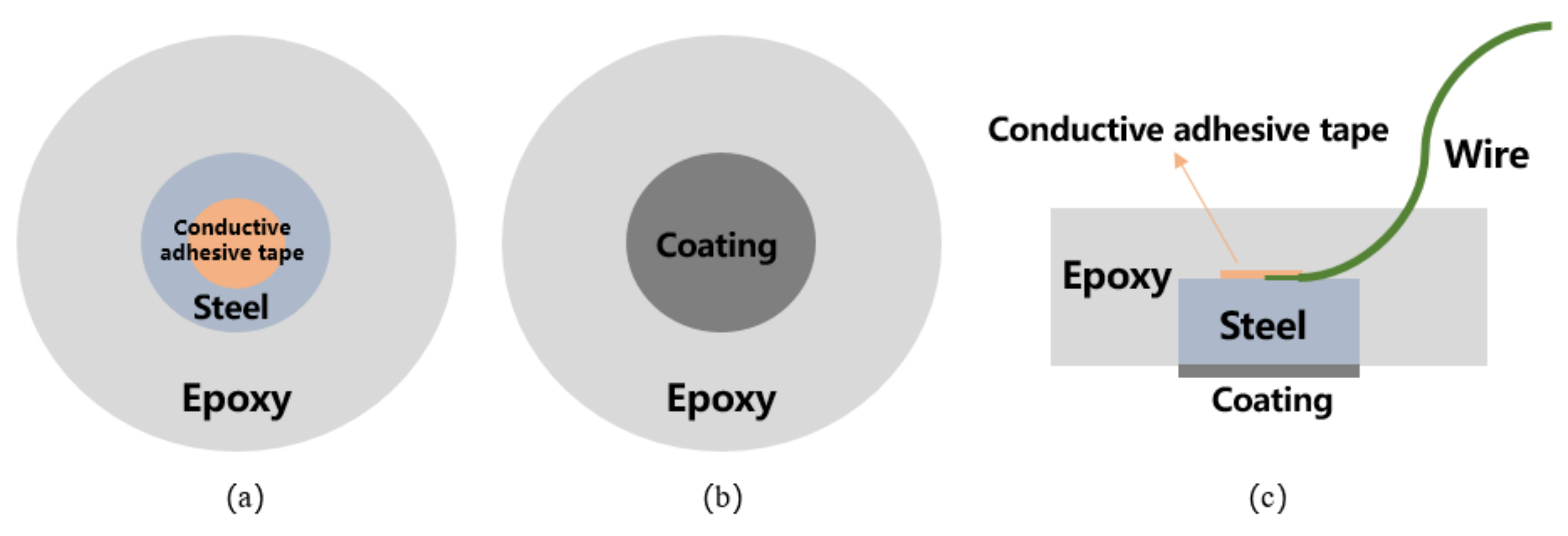
2.4. Testing Procedures
2.4.1. Micromorphological Characterization
2.4.2. Electrochemical Testing
3. Anti-Corrosion Performance
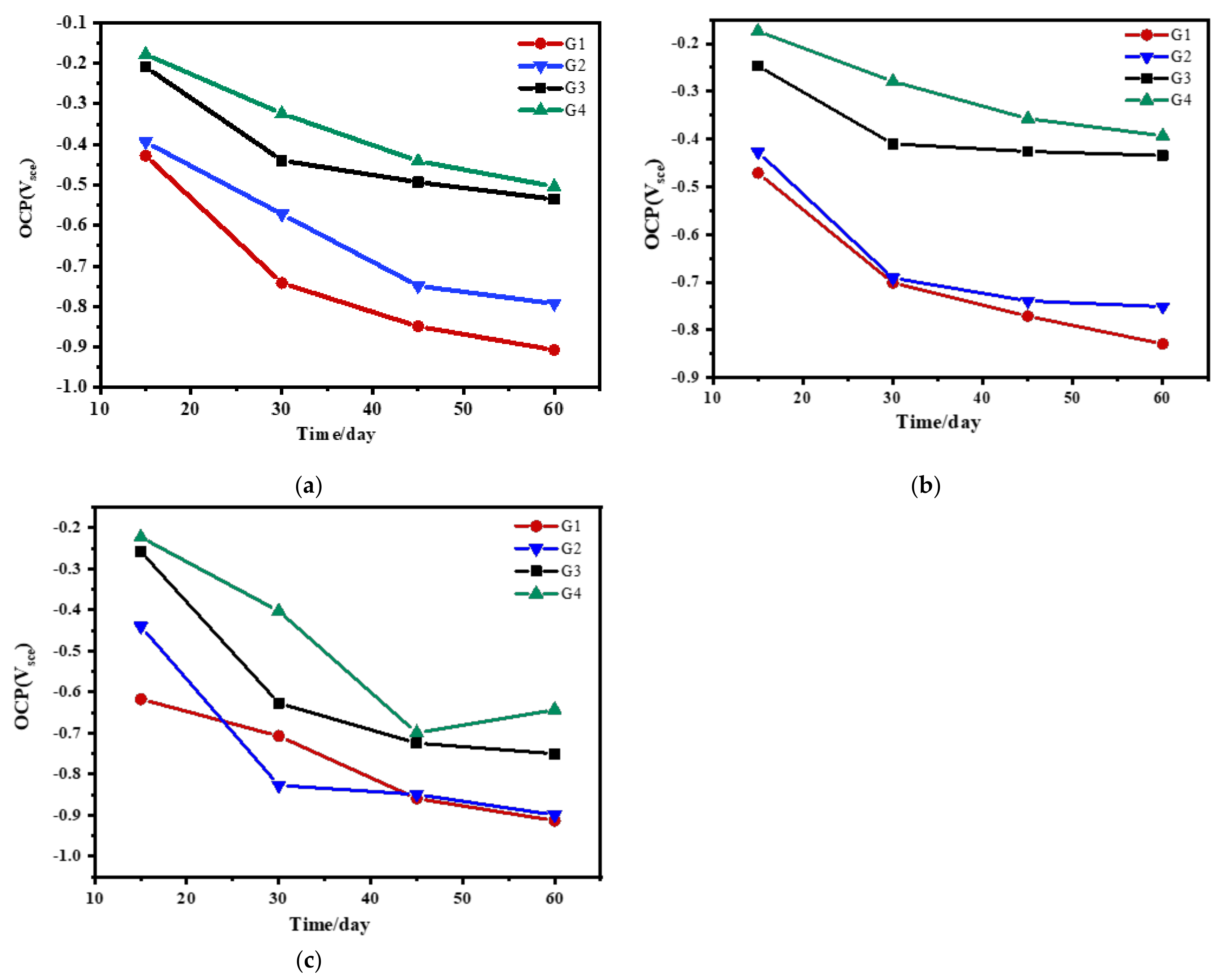
3.1. Open Circuit Potential(OCP) Analysis
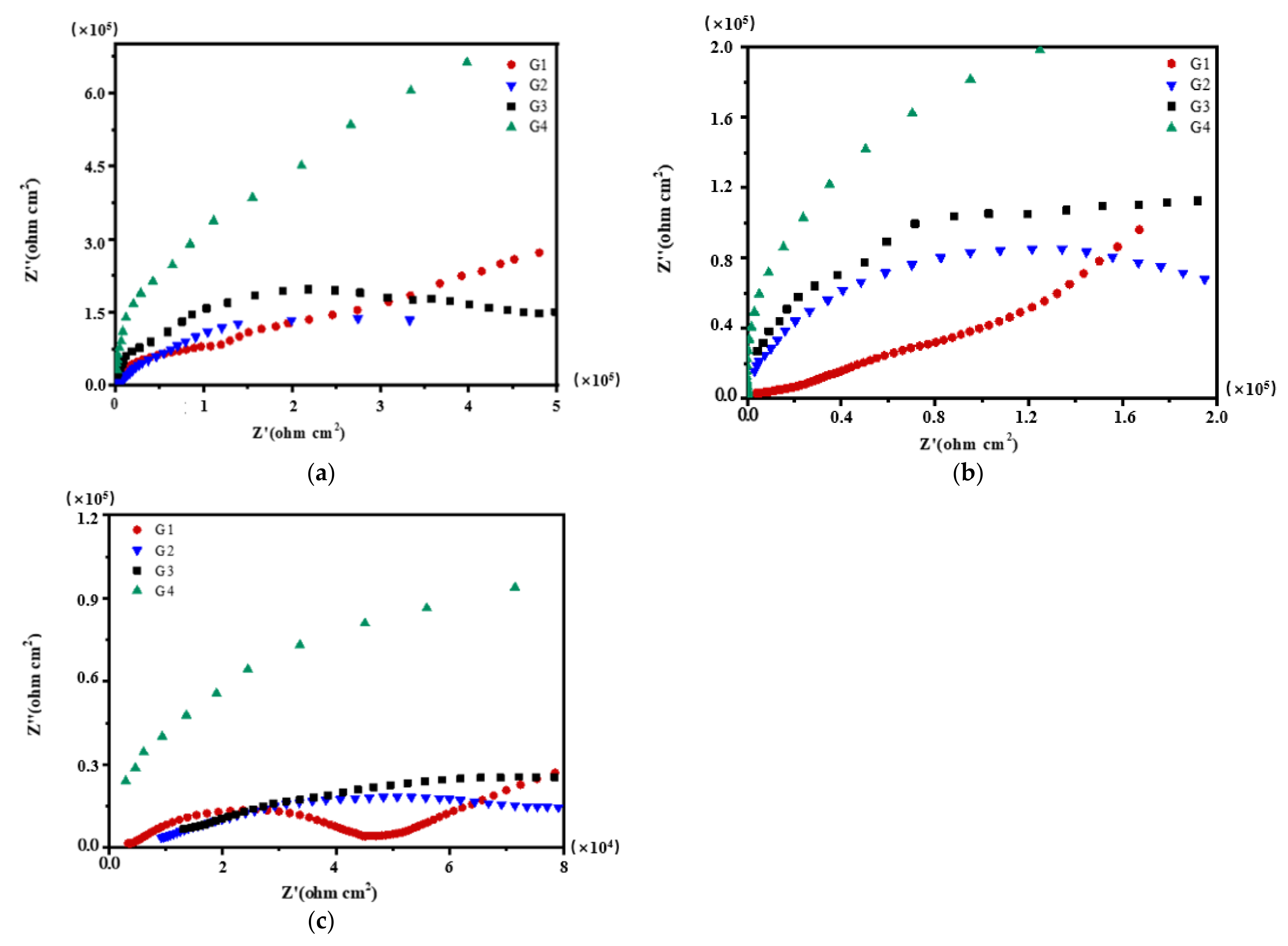
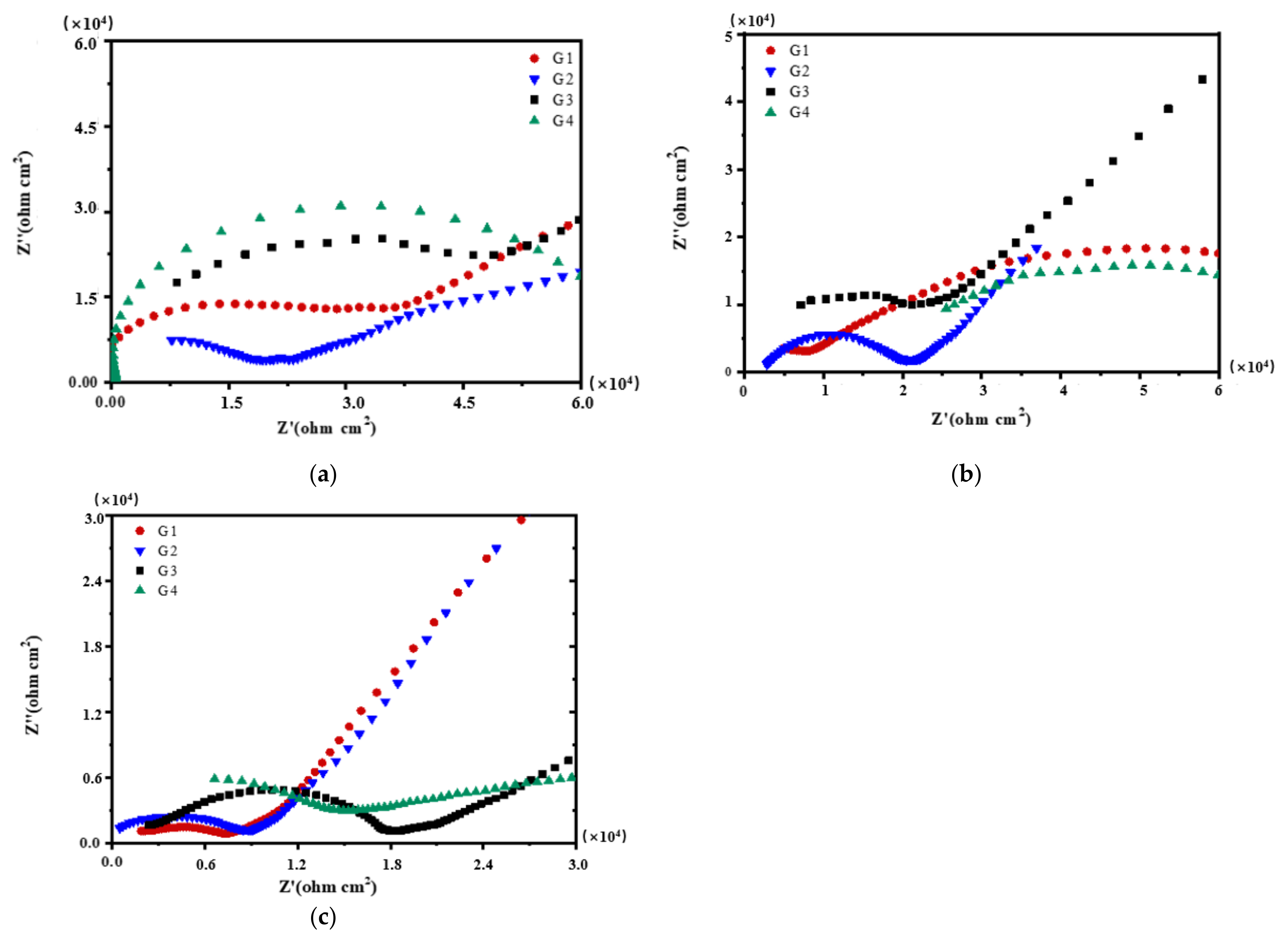
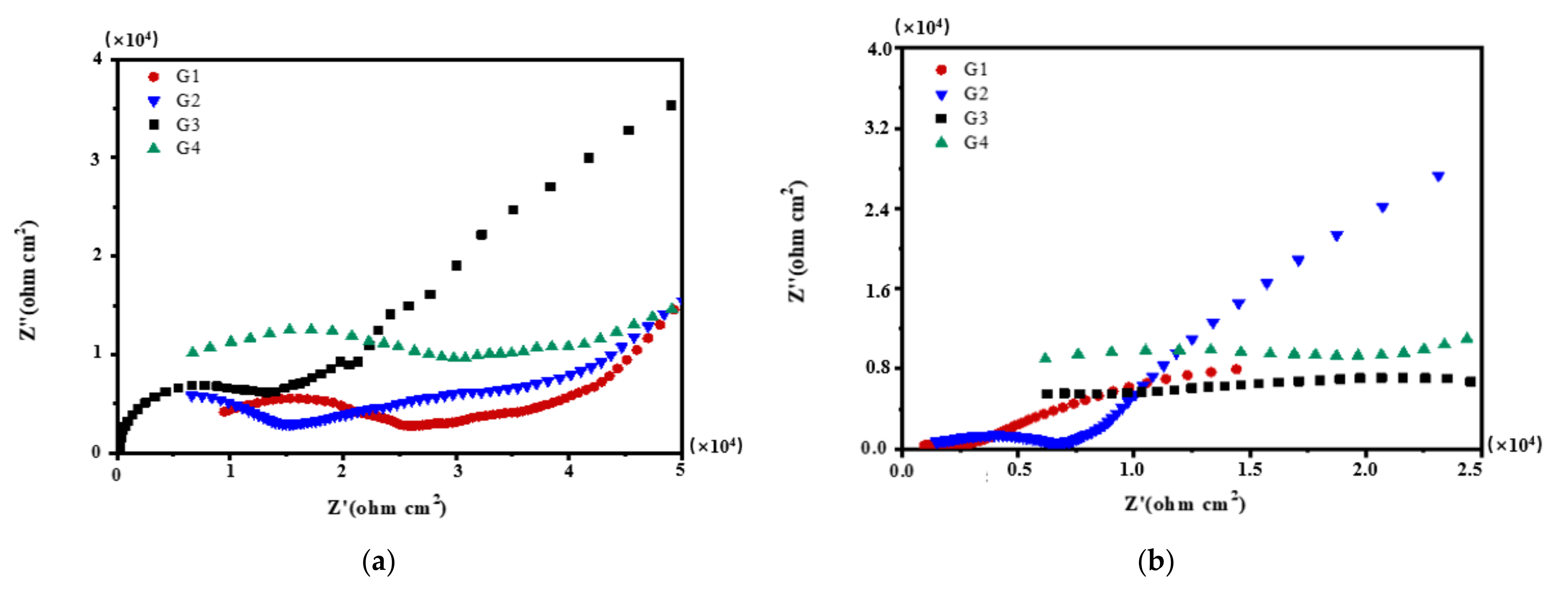
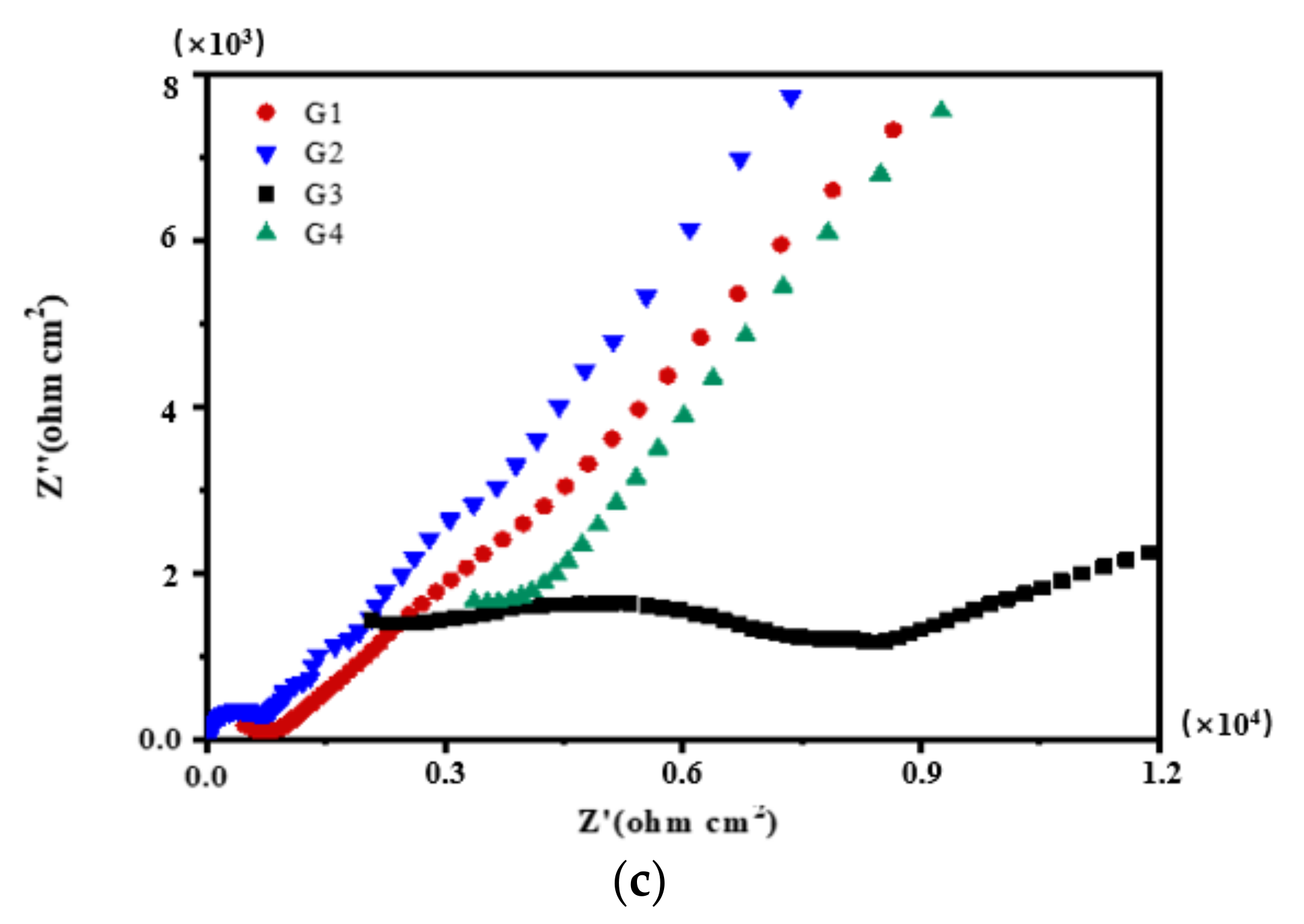
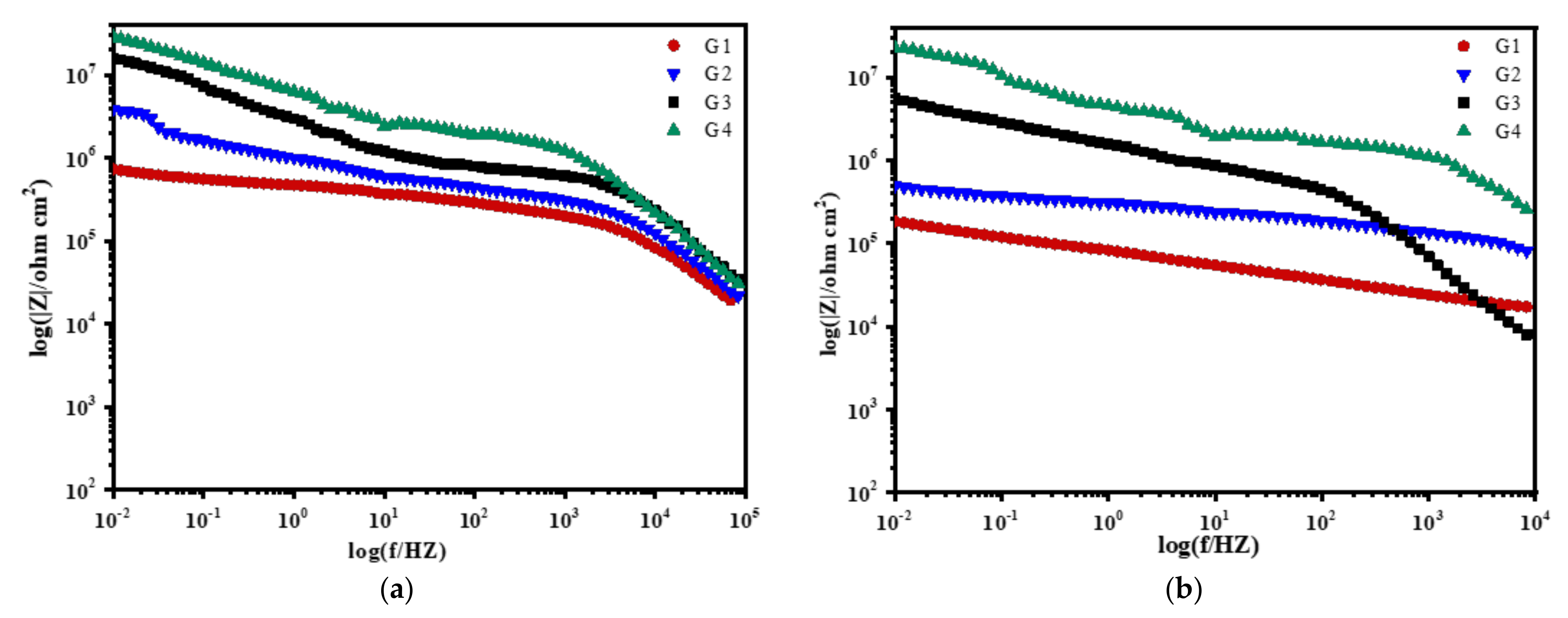
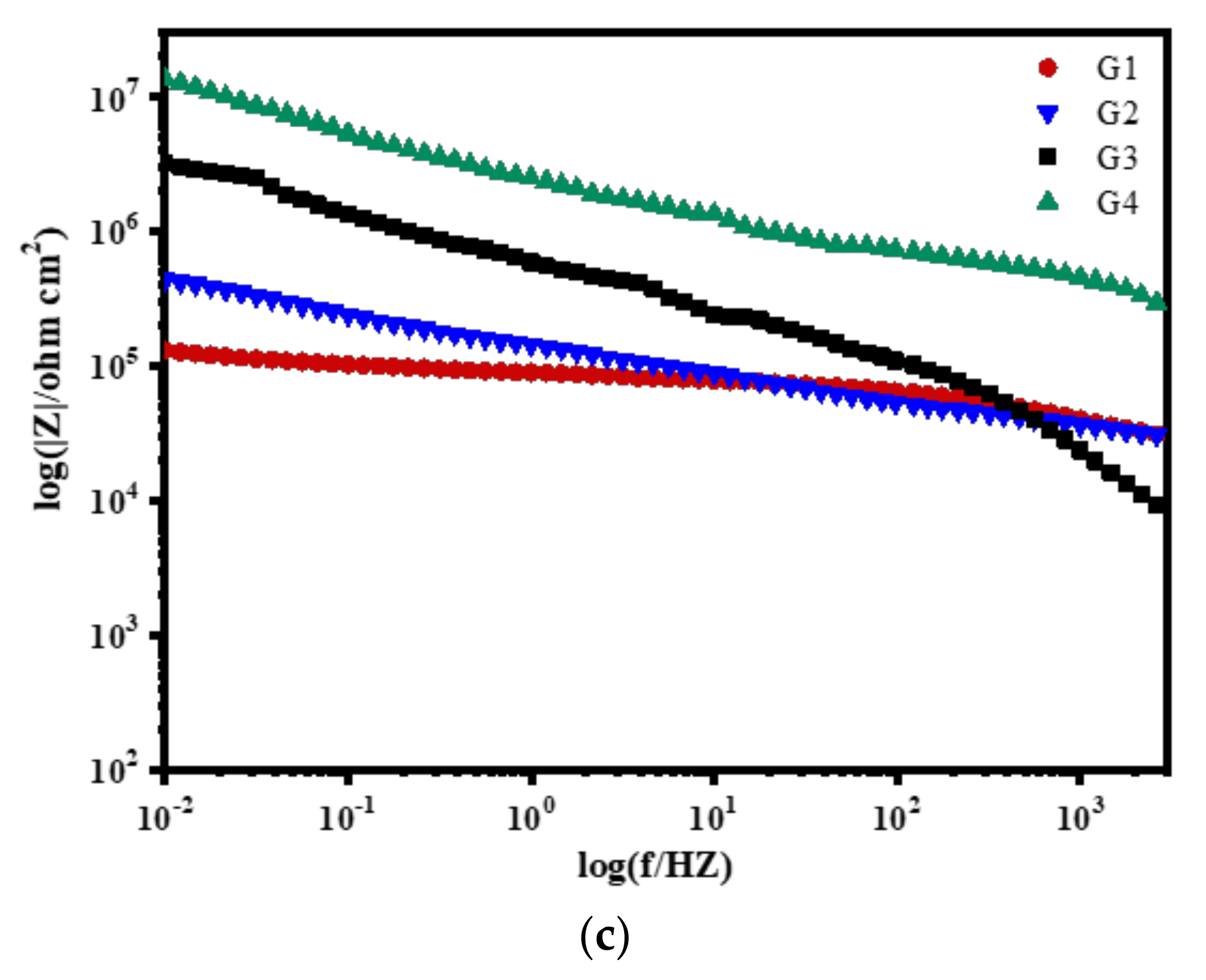
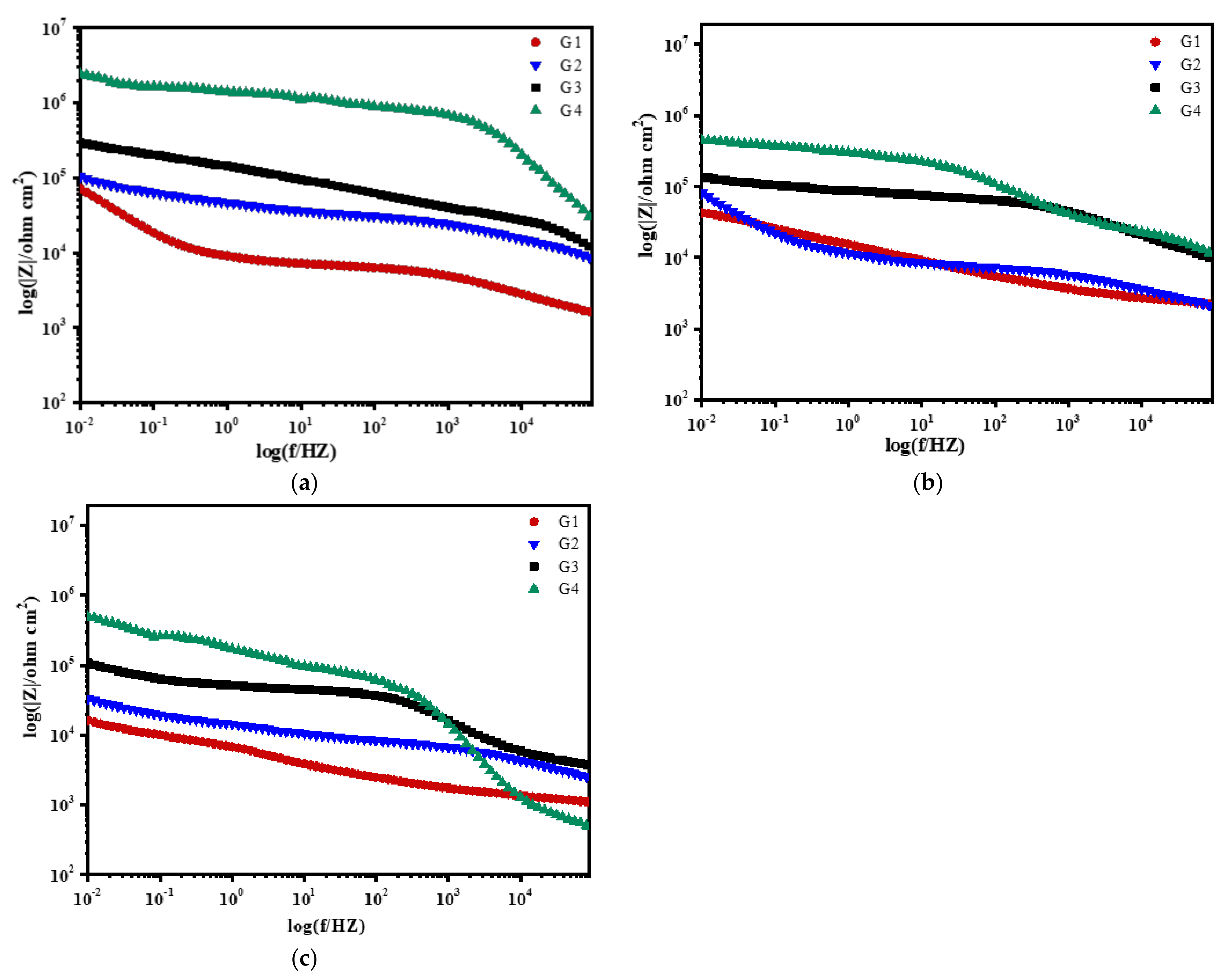
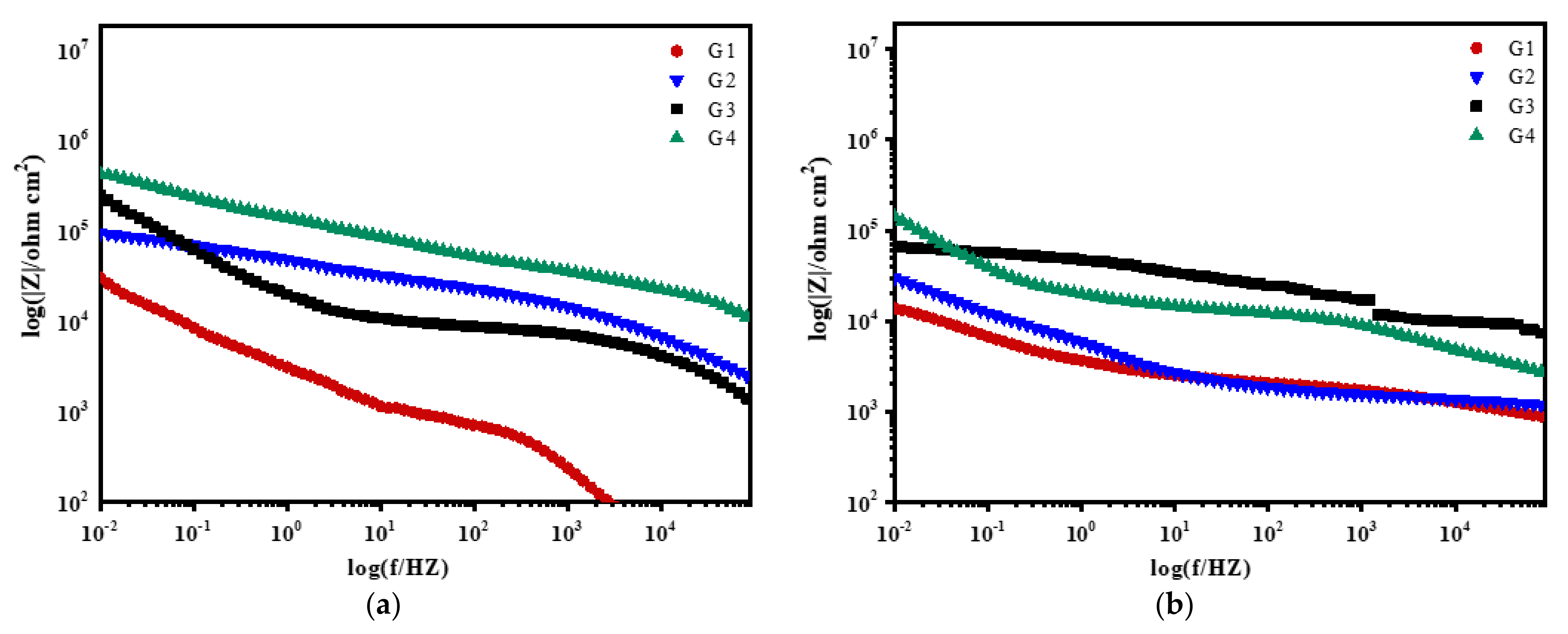
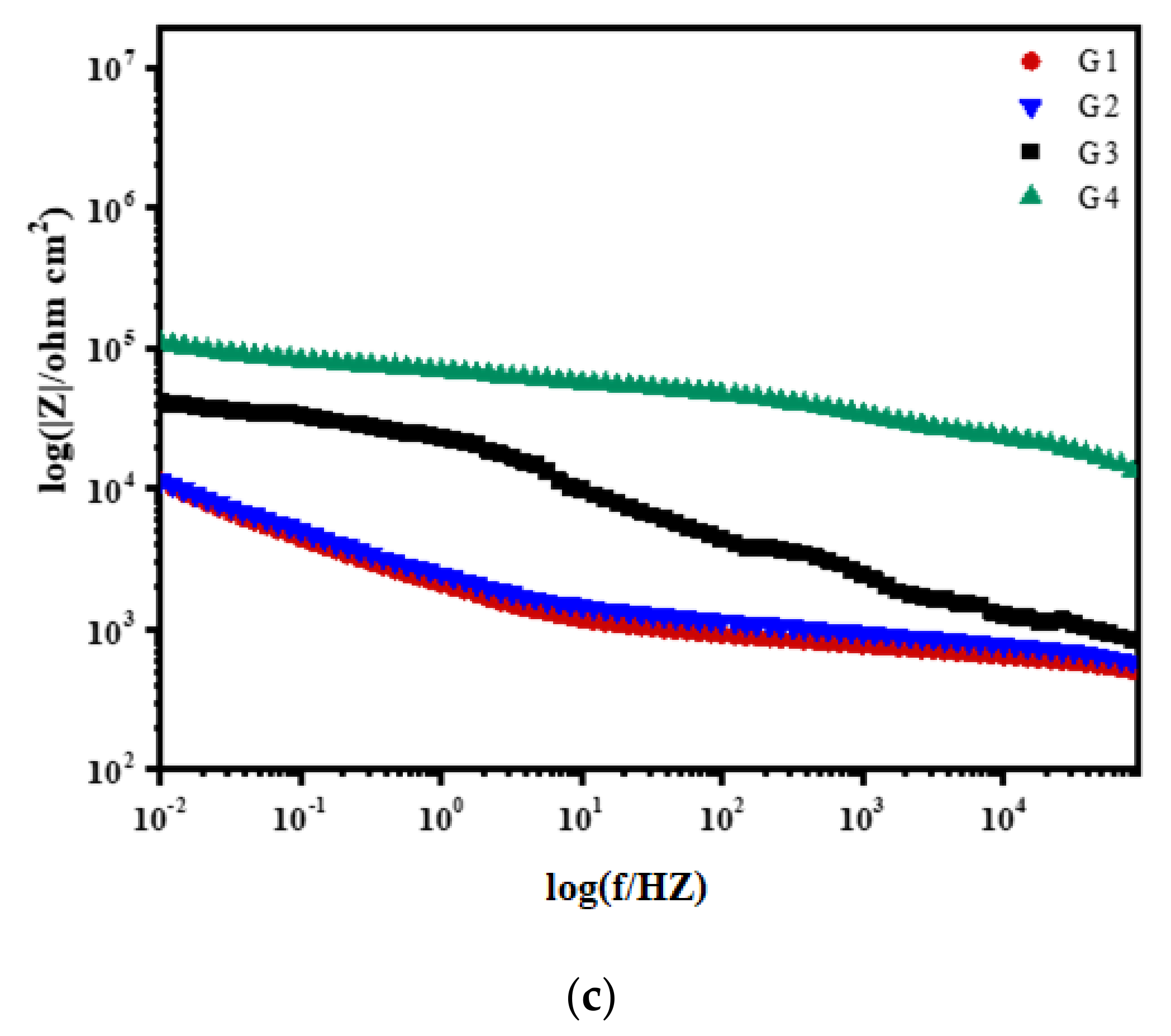
3.2. Electrochemical Alternating Current Impedance Spectroscopy (EIS) Analysis
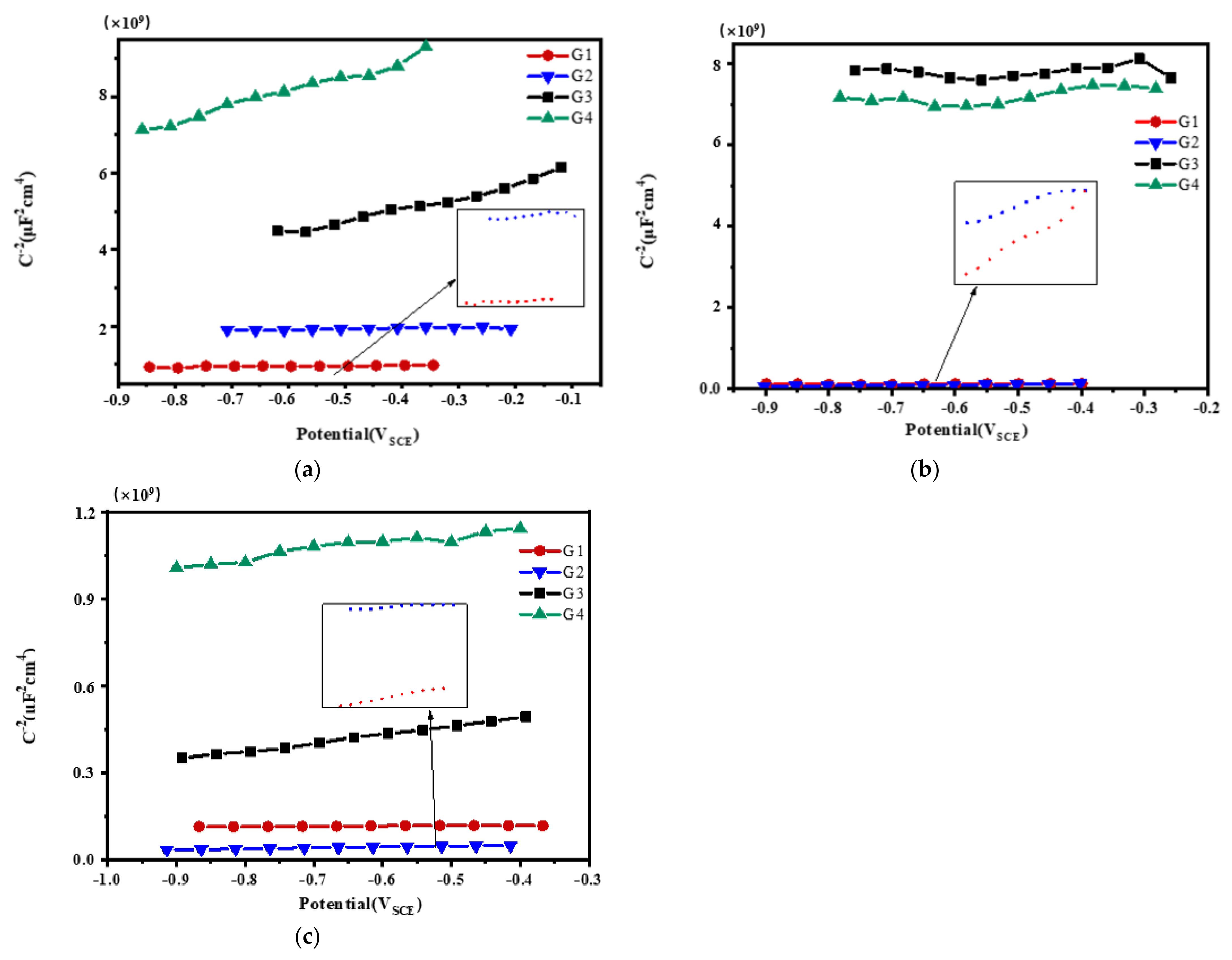
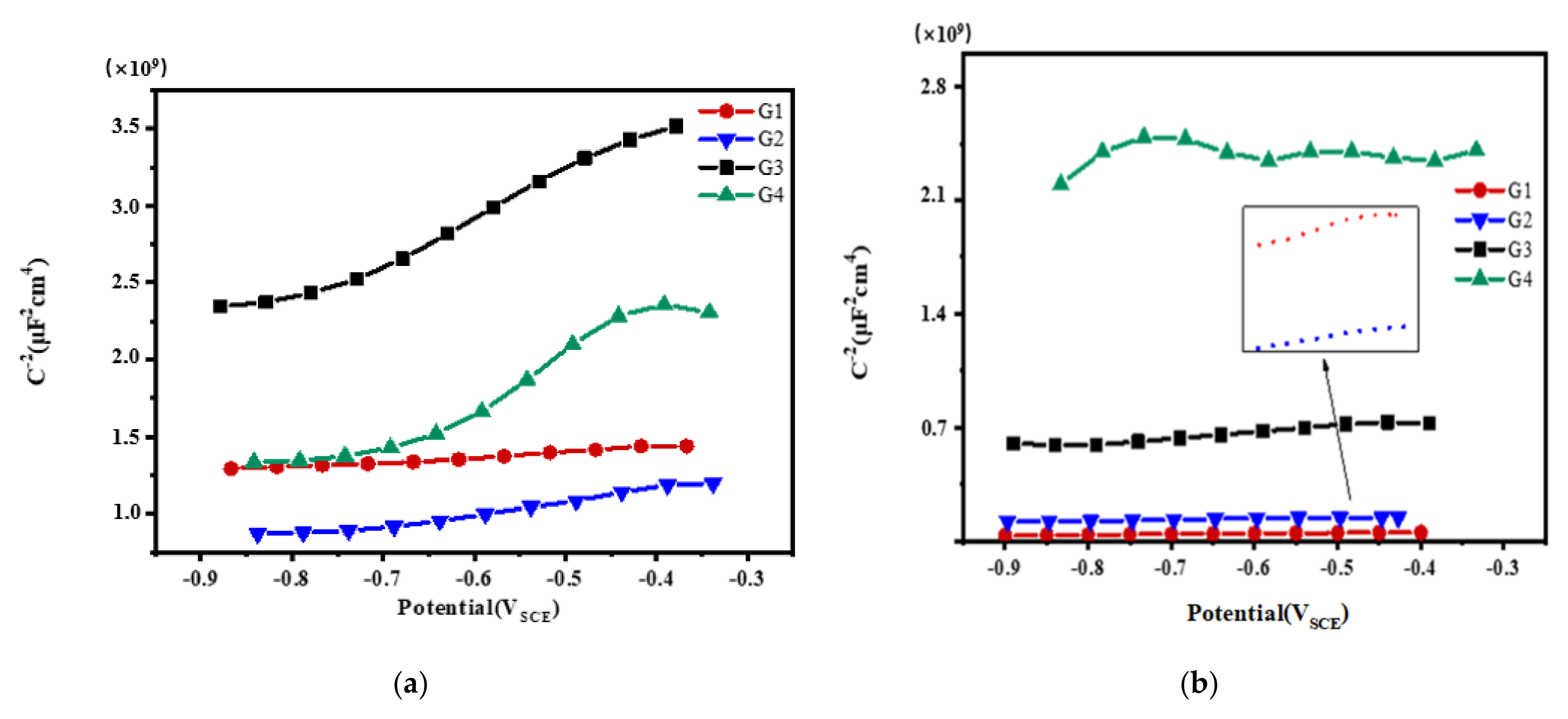
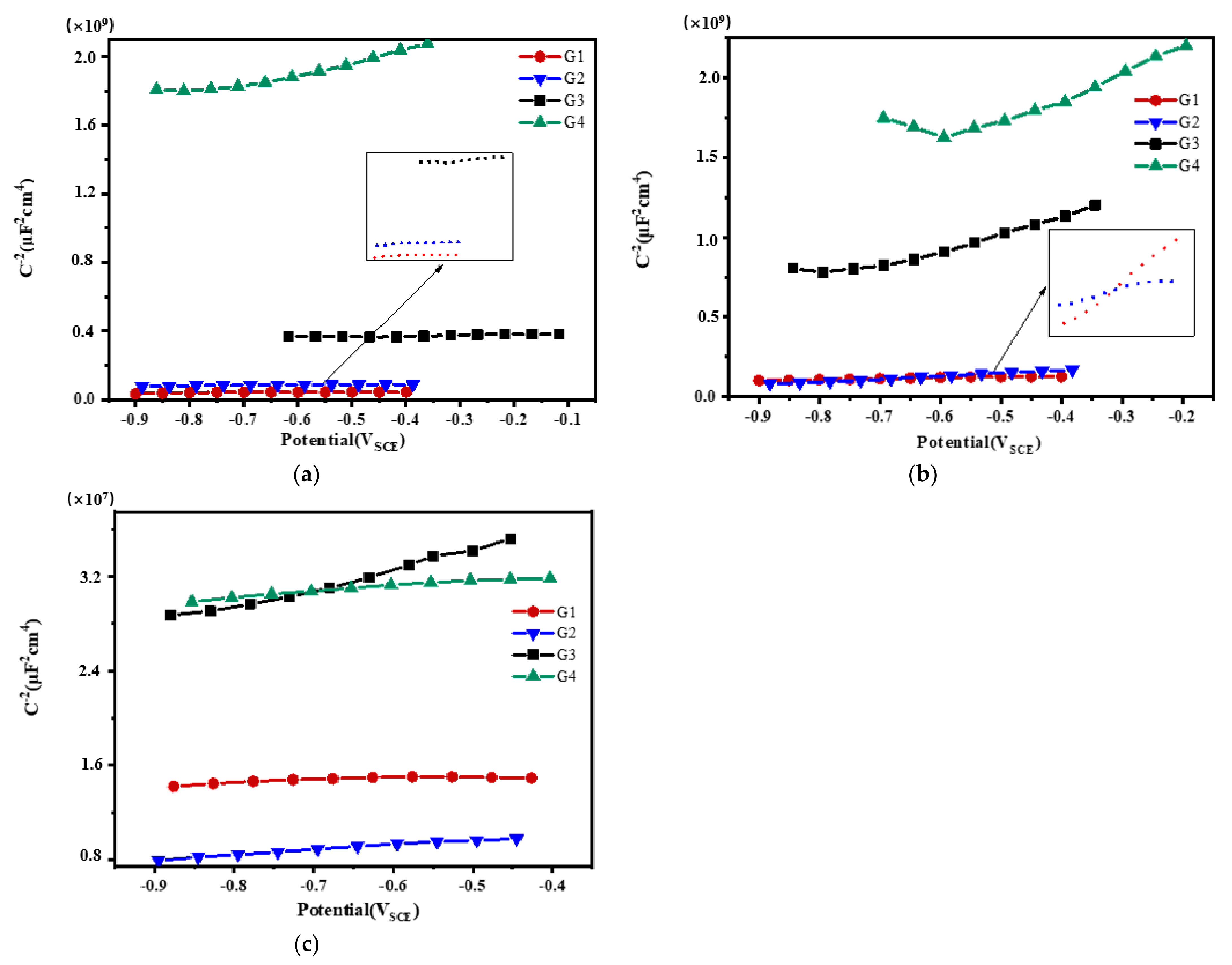
3.3. Mott–Schottky Curve Analysis
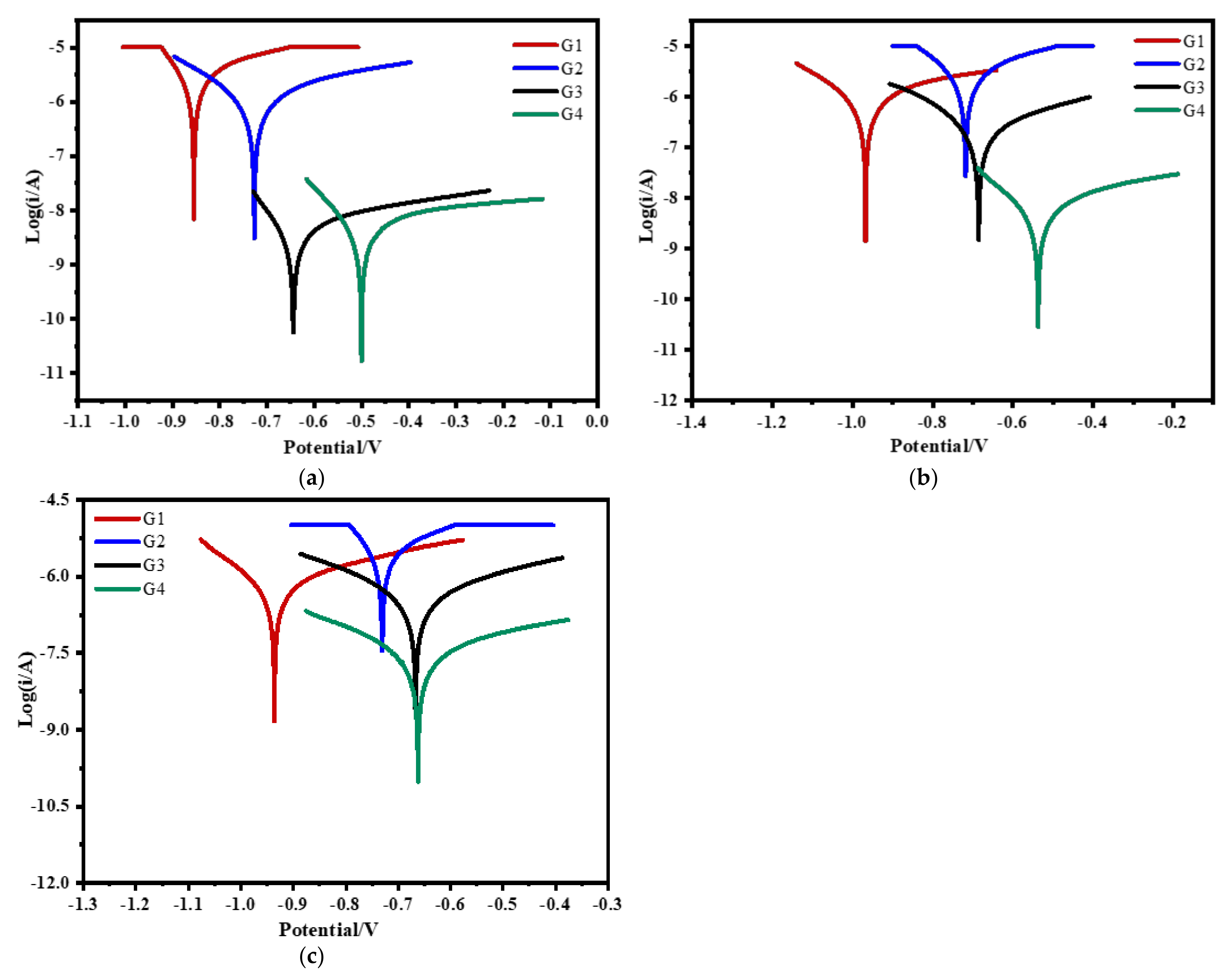
3.4. Tafel Curve Analysis
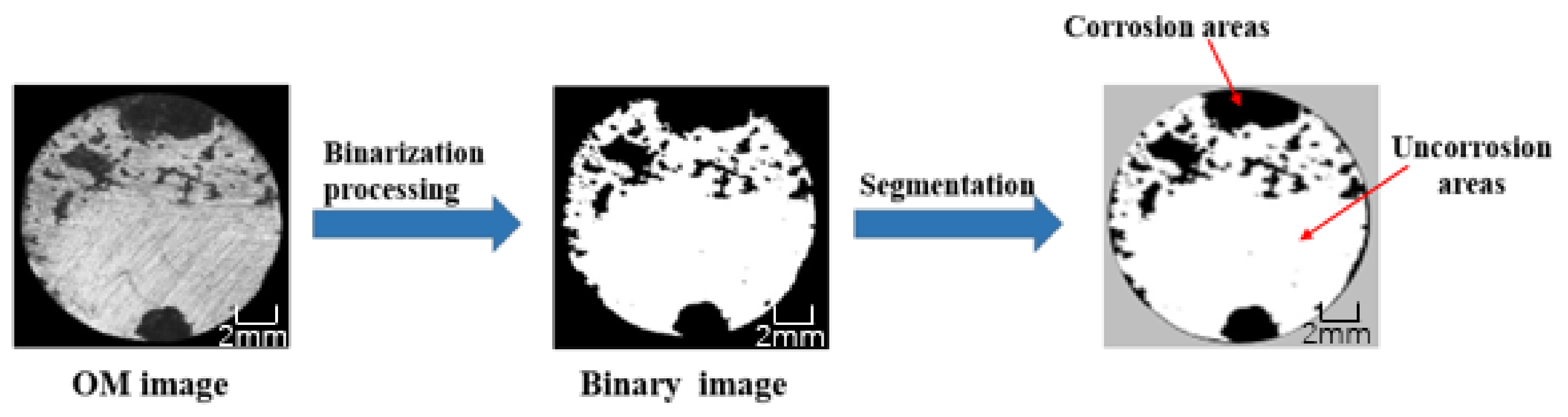
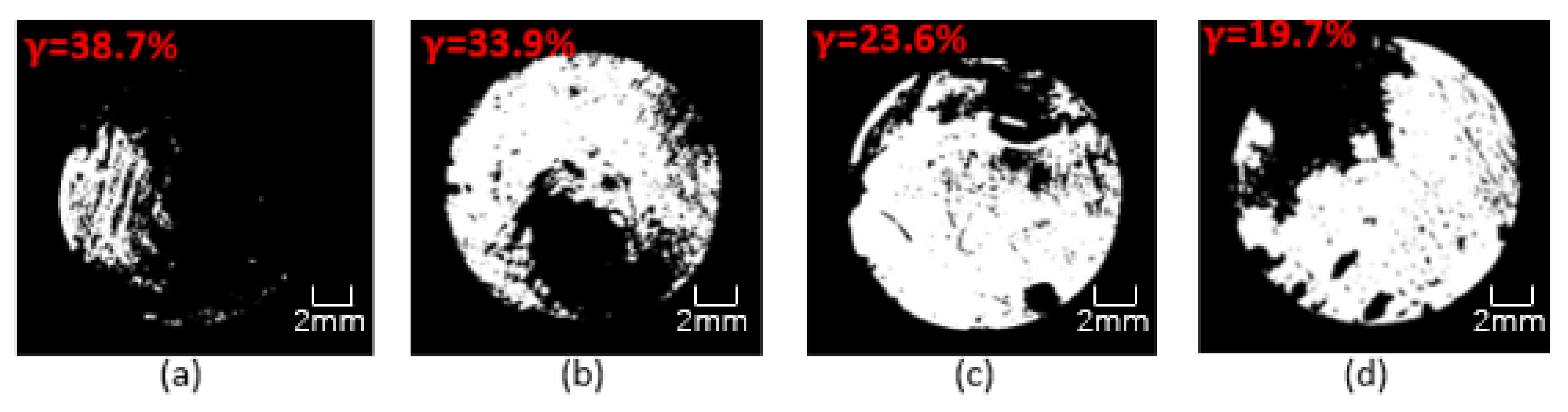
4. Corrosion Morphology Comparison
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Yan, F.; Xue, Q. Tribological behavior of PTFE sliding against steel in sea water. Wear 2009, 267, 1634–1641. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Hou, B.; Zhang, J. Study on rust layer of low alloy steel in Marine corrosion zone. Ocean. Lakes 1998, 6, 651–655. [Google Scholar]
- Wang, Z.-X.; Zhao, J.-H.; Jia, X.-H.; Fang, J.-G.; Zhang, B.; Ding, G.-Q. Influence of Inactivation Treatment of Ship’s Ballast Water on Corrosion Resistance of Ship Steel Plate. J. Iron Steel Res. Int. 2013, 20, 85–89. [Google Scholar] [CrossRef]
- Su, X. Corrosion Behavior of Zn-Al Coating in Typical Simulated Marine Environment. Master’s Thesis, Wuhan University of Technology, Wuhan, China, 2015. [Google Scholar]
- Xiao, L.; Yu-wei, L.; Hong-tao, Z.; Chen, P.; Zhen-yao, W. Corrosion behavior of copper in extremely harsh marine atmosphere in Nansha Islands, China. Trans. Nonferr. Met. Soc. China 2021, 31, 703–714. [Google Scholar]
- Ding, Y. Preparation and Properties of High Corrosion Resistance Water-Based Resins under Marine Climate Conditions. Master’s Thesis, Hefei University of Technology, Hefei, China, 2015. [Google Scholar]
- Ma, S. Marine corrosion and protection. Mar. Sci. 1978, 2, 53–56. [Google Scholar]
- Gong, B. Study on the Mechanism of Corrosion Action on Steel Property Deterioration in Offshore Engineering Environment. Master’s Thesis, China University of Mining and Technology, Beijing, China, 2018. [Google Scholar]
- Ruohan, Y.; Fuyong, C.; Chen, Z.; Jinghua, Y.; Jingjing, W.; Ziming, W.; Zhenwen, Z.; Dajiang, Z.; Jinling, C.; Ling, S.G. The marine atmospheric corrosion of pure Mg and Mg alloys in field exposure and lab simulation. Corros. Eng. Sci. Technol. 2020, 55, 609–621. [Google Scholar]
- Xuexu, X.; Tianyi, Z.; Wei, W.; Shan, J.; Jianwei, Y.; Zhiyong, L. Optimizing the resistance of Ni-advanced weathering steel to marine atmospheric corrosion with the addition of Al or Mo. Constr. Build. Mater. 2021, 279, 122341. [Google Scholar] [CrossRef]
- Chuang, Q.; Mingna, W.; Long, H.; Xiahe, L.; Xiaolin, J.; Xizhong, A.; Duanyang, L. Temperature and NaCl deposition dependent corrosion of SAC305 solder alloy in simulated marine atmosphere. J. Mater. Sci. Technol. 2021, 75, 252–264. [Google Scholar]
- Gao, Y.; Wang, X.; Wang, J.; Gao, L.; Li, Z. Durability of epoxy coated reinforced concrete specimens under fatigue load and environmental corrosion. Build. Sci. Res. Sichuan Prov. 2015, 41, 24–30. [Google Scholar]
- Huang, W.; Ma, H.; Li, Z. Effect of polyurethane and polyurea coatings on chloride ion permeability of concrete. Concrete 2011, 6, 94–96. [Google Scholar]
- Pang, Y. Study on Corrosion Resistance of Polyurea Coating in Marine. Int. Core J. Eng. 2021, 7, 5–11. [Google Scholar]
- Tsai, P.-Y.; Chen, T.-E.; Lee, Y.-L. Development and Characterization of Anticorrosion and Antifriction Properties for High Performance Polyurethane/Graphene Composite Coatings. Coatings 2018, 8, 250. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Zhao, W.; Wu, Y.; Wang, D.; Feng, R. Tribological properties of epoxy composite coatings reinforced with functionalized C-BN and H-BN nanofillers. Appl. Surf. Sci. 2018, 434, 1311–1320. [Google Scholar] [CrossRef]
- Arash, H.S.; Ahmad, R.S.A.; Mohammad, M.; Mohammad, A. Epoxy nanocomposite coatings with enhanced dual active/barrier behavior containing graphene-based carbon hollow spheres as corrosion inhibitor nanoreservoirs. Corros. Sci. 2021, 185, 109428. [Google Scholar]
- Zhu, Q.; Li, E.; Liu, X.; Song, W.; Li, Y.; Wang, X.; Liu, C. Epoxy coating with in-situ synthesis of polypyrrole functionalized graphene oxide for enhanced anticorrosive performance. Prog. Org. Coat. 2020, 140, 105488. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Bahlakeh, G.; Ramezanzadeh, M. Polyaniline-cerium oxide (PAni-CeO2) coated graphene oxide for enhancement of epoxy coating corrosion protection performance on mild steel. Corros. Sci. 2018, 137, 111–126. [Google Scholar] [CrossRef]
- Da Silva, M.F.P.; de Jesus Fraga da Costa, H.C.; Triboni, E.R.; Politi, M.J.; Isolani, P.C. Synthesis and characterization of CeO2–graphene composite. J. Therm. Anal. Calorim. 2012, 107, 257–263. [Google Scholar] [CrossRef]
- Liu, X.; Jie, H.; Liu, R.; Liu, Y.; Li, T.; Lyu, K. Research on the Preparation and Anticorrosion Properties of EP/CeO2-GO Nanocomposite Coating. Polymers 2021, 13, 183. [Google Scholar] [CrossRef]
- Rani, B.E.A.; Basu, B.B.J.; El-Etre, A.Y. Green Inhibitors for Corrosion Protection of Metals and Alloys: An Overview. Int. J. Corros. 2011, 2012. [Google Scholar] [CrossRef]
- Kesavan, D.G.M.; Sulochana, N. Green inhibitors for corrosion of metals:a review. Chem. Sci. Rev. Lett. 2012, 1, 1–8. [Google Scholar]
- Okafor, P.C.; Liu, C.B.; Liu, X.; Zheng, Y.G.; Wang, F.; Liu, C.Y.; Wang, F. Corrosion inhibition and adsorption behavior of imidazoline salt on N80 carbon steel in CO2-saturated solutions and its synergism with thiourea. J. Solid State Electrochem. 2010, 14, 1367–1376. [Google Scholar] [CrossRef]
- Desimone, M.P.; Grundmeier, G.; Gordillo, G.; Simison, S.N. Amphiphilic amido-amine as an effective corrosion inhibitor for mild steel exposed to CO2 saturated solution: Polarization, EIS and PM-IRRAS studies. Electrochim. Acta 2011, 56, 2990–2998. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, T.; Yu, X.; Chen, D. Corrosion inhibition efficiency of triethanolammonium dodecylbenzene sulfonate on Q235 carbon steel in simulated concrete pore solution. Corros. Sci. 2019, 158, 108097. [Google Scholar] [CrossRef]
- Flores, J.M.; Cywiak, M.; Servin, M.; Juarez, L. Bode plots applied to microscopic interferometry. Rev. Mex. Fis. 2009, 55, 347–353. [Google Scholar]
- Ruan, Q.; Miao, T.; Wang, H.; Tang, J. Insight on Shallow Trap States-Introduced Photocathodic Performance in n-Type Polymer Photocatalysts. J. Am. Chem. Soc. 2020, 142, 2795–2802. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Soulte Quality/g | 20% Seawater | 40% Seawater | 60% Seawater |
|---|---|---|---|
| SrCl2∙6H2O | 0.43 | 1.14 | 2.58 |
| MgCl∙6H2O | 112.8 | 300.81 | 676.83 |
| CaCl2 | 11.75 | 31.37 | 70.59 |
| NaF | 0.05 | 0.14 | 0.31 |
| KCl | 11.84 | 31.5 | 71.06 |
| NaHCO3 | 3.43 | 9.14 | 20.56 |
| KBr | 1.73 | 4.57 | 10.29 |
| H3BO3 | 0.46 | 1.23 | 2.78 |
| NaCL | 24.54 | 24.54 | 24.54 |
| Na2SO4 | 4.09 | 4.09 | 4.090 |
| Coating | Constitute |
|---|---|
| G1 | EP coating |
| G2 | EP coating + corrosion inhibitor |
| G3 | CeO2-GO(4:1)/EP coating |
| G4 | CeO2-GO(4:1)/EP coating + corrosion inhibitor |
| Coating | 15 d ND (cm−3) | 30 d ND (cm−3) | 45 d ND (cm−3) | 60 d ND (cm−3) |
|---|---|---|---|---|
| G1 | 4.681 × 1013 | 2.235 × 1014 | 3.973 × 1013 | 7.123 × 1014 |
| G2 | 1.540 × 1013 | 1.837 × 1014 | 3.620 × 1013 | 2.008 × 1014 |
| G3 | 2.645 × 1013 | 1.708 × 1012 | 9.865 × 1012 | 5.399 × 1012 |
| G4 | 2.163 × 1011 | 1.101 × 1012 | 6.374 × 1012 | 2.202 × 1012 |
| Coating | 15 d ND (cm−3) | 30 d ND (cm−3) | 45 d ND (cm−3) | 60 d ND (cm−3) |
|---|---|---|---|---|
| G1 | 7.331 × 1014 | 2.790 × 1015 | 2.871 × 1015 | 9.372 × 1015 |
| G2 | 4.542 × 1014 | 8.691 × 1014 | 6.042 × 1014 | 2.362 × 1015 |
| G3 | 3.101 × 1012 | 2.646 × 1013 | 2.115 × 1013 | 1.997 × 1014 |
| G4 | 6.937 × 1011 | 3.207 × 1012 | 3.417 × 1012 | 4.112 × 1012 |
| Coating | 15 d ND (cm−3) | 30 d ND (cm−3) | 45 d ND (cm−3) | 60 d ND (cm−3) |
|---|---|---|---|---|
| G1 | 4.091 × 1015 | 3.960 × 1016 | 2.470 × 1016 | 2.563 × 1017 |
| G2 | 3.340 × 1015 | 2.564 × 1016 | 2.280 × 1016 | 1.303 × 1017 |
| G3 | 3.715 × 1013 | 6.830 × 1014 | 8.861 × 1015 | 3.483 × 1016 |
| G4 | 2.663 × 1013 | 5.728 × 1014 | 1.266 × 1013 | 1.040 × 1016 |
| Coating | Ecorr (V) | Icorr (A/cm2) | η (%) | Rp (ohm) |
|---|---|---|---|---|
| G1 | −0.866 | 2.473 × 10−6 | / | 4.395 × 105 |
| G2 | −0.723 | 4.781 × 10−7 | 80.67 | 2.564 × 106 |
| G3 | −0.627 | 2.804 × 10−8 | 98.87 | 4.214 × 107 |
| G4 | −0.487 | 1.543 × 10−9 | 99.93 | 7.170 × 108 |
| Coating | Ecorr (V) | Icorr (A/cm2) | η (%) | Rp (ohm) |
|---|---|---|---|---|
| G1 | −0.981 | 3.963 × 10−6 | / | 2.858 × 105 |
| G2 | −0.731 | 1.003 × 10−6 | 72.77 | 4.650 × 105 |
| G3 | −0.661 | 1.591 × 10−7 | 95.69 | 4.370 × 106 |
| G4 | −0.526 | 8.249 × 10−8 | 97.77 | 6.733 × 106 |
| Coating | Ecorr (V) | Icorr (A/cm2) | η (%) | Rp (ohm) |
|---|---|---|---|---|
| G1 | −0.916 | 4.274 × 10−6 | / | 2.687 × 105 |
| G2 | −0.782 | 1.417 × 10−6 | 66.81 | 3.755 × 105 |
| G3 | −0.695 | 7.903 × 10−6 | 86.75 | 7.918 × 105 |
| G4 | −0.658 | 3.581 × 10−7 | 91.62 | 1.162 × 106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Liu, R.; Li, T.; Liu, Y.; Liu, L.; Lyu, K.; Shah, S.P. Research on the Anticorrosion Properties of CeO2-GO/EP Nanocomposite Coating in Simulated Sea Water. Polymers 2021, 13, 2072. https://doi.org/10.3390/polym13132072
Liu X, Liu R, Li T, Liu Y, Liu L, Lyu K, Shah SP. Research on the Anticorrosion Properties of CeO2-GO/EP Nanocomposite Coating in Simulated Sea Water. Polymers. 2021; 13(13):2072. https://doi.org/10.3390/polym13132072
Chicago/Turabian StyleLiu, Xiaoyan, Ruidan Liu, Tianyu Li, Yanqi Liu, Li Liu, Kai Lyu, and Surendra P. Shah. 2021. "Research on the Anticorrosion Properties of CeO2-GO/EP Nanocomposite Coating in Simulated Sea Water" Polymers 13, no. 13: 2072. https://doi.org/10.3390/polym13132072
APA StyleLiu, X., Liu, R., Li, T., Liu, Y., Liu, L., Lyu, K., & Shah, S. P. (2021). Research on the Anticorrosion Properties of CeO2-GO/EP Nanocomposite Coating in Simulated Sea Water. Polymers, 13(13), 2072. https://doi.org/10.3390/polym13132072