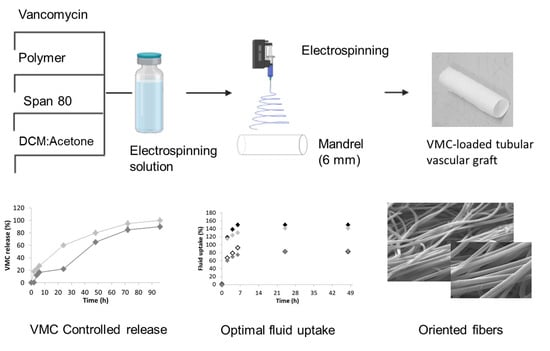
Tubular Electrospun Vancomycin-Loaded Vascular Grafts: Formulation Study and Physicochemical Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preformulation Study
Solubility of Vancomycin in Organic and Aqueous Phase
Stability of Vancomycin in Organic and Aqueous Phase
2.2.2. Method of Analysis
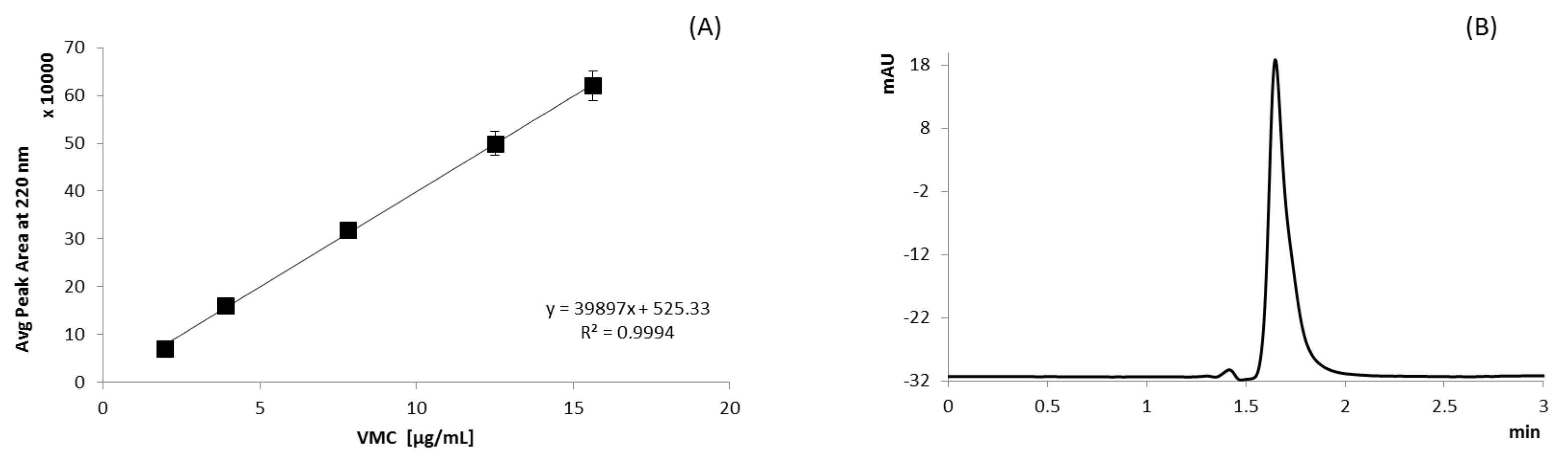
UV Method
HPLC Analysis
2.2.3. Polymer Solution Preparation and Conductimetric Analysis
2.2.4. Preparation of Tubular Vascular Grafts
2.2.5. Tubular Vascular Grafts Characterization
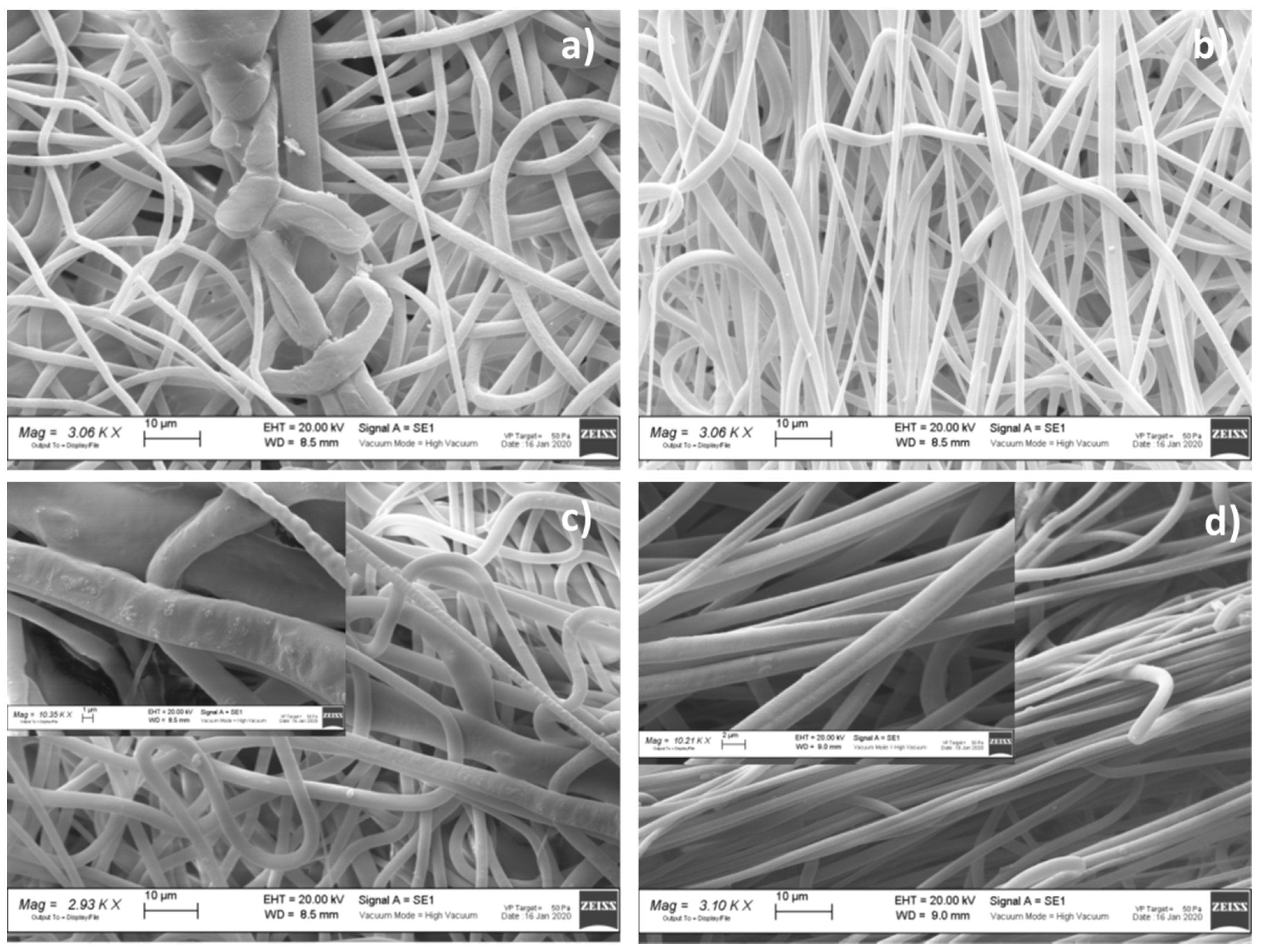
Morphometric Analysis
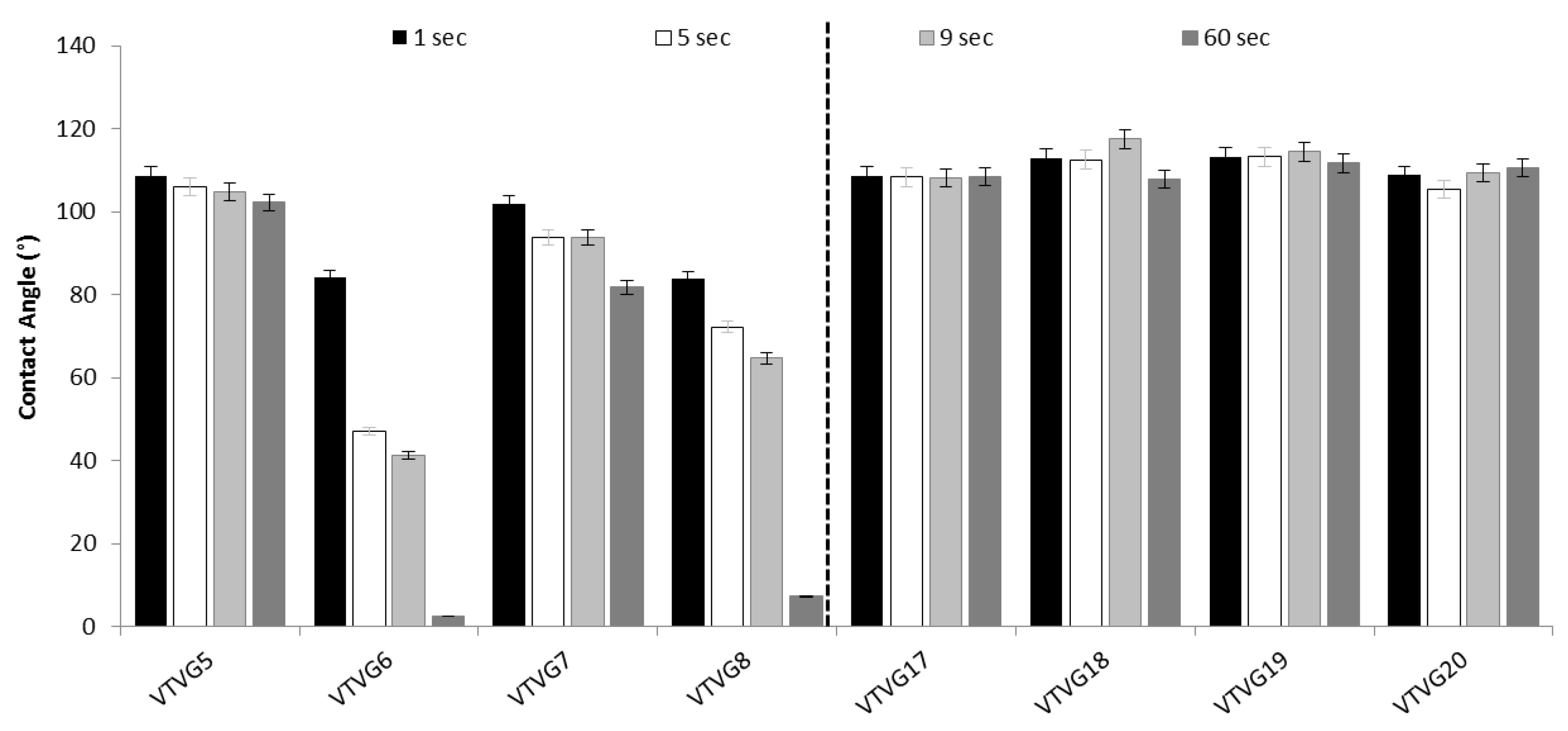
Wettability Evaluation
Fluid Uptake Capability
Drug Content and Encapsulation Efficiency Determination
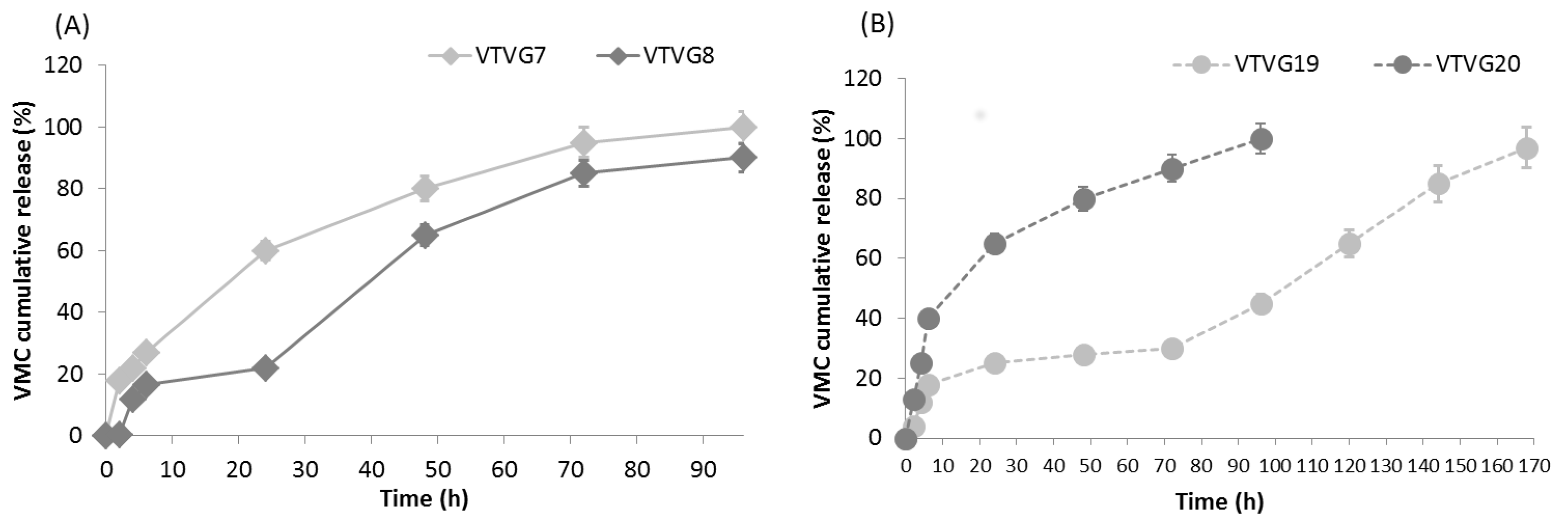
In Vitro Release Study
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gutowski, P.; Gage, S.; Guziewicz, M.; Ilzecki, M.; Kazimierczak, A.; Kirkton, R.D.; Niklason, L.E.; Pilgrim, A.; Prichard, H.L.; Przywara, S.; et al. Arterial reconstruction with human bioengineered acellular blood vessels in patients with peripheral arterial disease. J. Vasc. Surg. 2020, 72, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.S.; Lee, C.H.; Wang, Y.C.; Liu, S.J. Sustained release of vancomycin from novel biodegradable nano-fiber-loaded vascular prosthetic grafts: In vitro and in vivo study. Int. J. Nanomed. 2015, 10, 885–891. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Almasri, J.; Adusumalli, J.; Asi, N.; Lakis, S.; Alsawas, M.; Prokop, L.J.; Bradbury, A.; Kolh, P.; Conte, M.S.; Murad, M.H. A systematic review and meta-analysis of revascularization outcomes of infrainguinal chronic limb-threatening ischemia. J. Vasc. Surg. 2018, 68, 624–633. [Google Scholar] [CrossRef]
- Ong, C.S.; Zhou, X.; Huang, C.Y.; Fukunishi, T.; Zhang, H.; Hibino, N. Tissue engineered vascular grafts: Current state of the field. Expert Rev. Med. Devices 2017, 14, 383–392. [Google Scholar] [CrossRef]
- Pan, Y.; Zhou, X.; Wei, Y.; Zhang, Q.; Wang, T.; Zhu, M.; Li, W.; Huang, R.; Liu, R.; Chen, J.; et al. Small-diameter hybrid vascular grafts composed of polycaprolactone and polydioxanone fibers. Sci. Rep. 2017, 7, 3615. [Google Scholar] [CrossRef]
- Catto, V.; Farè, S.; Cattaneo, I.; Figliuzzi, M.; Alessandrino, A.; Freddi, G.; Remuzzi, A.; Tanzi, M.C. Small diameter elec-trospun silk fibroin vascular grafts: Mechanical properties, in vitro biodegradability, and in vivo biocompatibility. Mater. Sci. Eng. C 2015, 54, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Fukunishi, T.; Best, C.A.; Sugiura, T.; Shoji, T.; Yi, T.; Udelsman, B.; Ohst, D.; Ong, C.S.; Zhang, H.; Shinoka, T.; et al. Tissue-Engineered Small Diameter Arterial Vascular Grafts from Cell-Free Nanofiber PCL/Chitosan Scaffolds in a Sheep Model. PLoS ONE 2016, 11, e0158555. [Google Scholar] [CrossRef] [PubMed]
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The Tissue-Engineered Vascular Graft—Past, Present, and Future. Tissue Eng. Part B Rev. 2016, 22, 68–100. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, Y.; John, K.; Shoji, T.; Shinoka, T. The Evolution of Tissue Engineered Vascular Graft Technologies: From Preclinical Trials to Advancing Patient Care. Appl. Sci. 2019, 9, 1274. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, C.; Liu, Y.; Lu, T.; Wu, Z. Strategies in cell-free tissue-engineered vascular grafts. J. Biomed. Mater. Res. Part A 2020, 108, 426–445. [Google Scholar] [CrossRef]
- Cordelle, J.; Mantero, S. Insight on the endothelialization of small silk-based tissue-engineered vascular grafts. Int. J. Artif. Organs 2020, 43, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Drews, J.D.; Pepper, V.K.; Best, C.A.; Szafron, J.M.; Cheatham, J.P.; Yates, A.R.; Hor, K.N.; Zbinden, J.C.; Chang, Y.-C.; Mirhaidari, G.J.M.; et al. Spontaneous reversal of stenosis in tissue-engineered vascular grafts. Sci. Transl. Med. 2020, 12, eaax6919. [Google Scholar] [CrossRef]
- Dreyfus, J.; Begier, E.; Yu, H.; Quintana, A.; Gayle, J.; Olsen, M.A. 1228. Incidence of Staphylococcus aureus Infection after Elective Surgeries Among Adults in US Hospitals. Open Forum Infect. Dis. 2018, 5, S372–S373. [Google Scholar] [CrossRef]
- Dorati, R.; DeTrizio, A.; Spalla, M.; Migliavacca, R.; Pagani, L.; Pisani, S.; Chiesa, E.; Conti, B.; Modena, T.; Genta, I. Gen-tamicin Sulfate PEG-PLGA/PLGA-H Nanoparticles: Screening Design and Antimicrobial Effect Evaluation toward Clinic Bacterial Isolates. Nanomaterials 2018, 8, 37. [Google Scholar] [CrossRef]
- Pisani, S.; Dorati, R.; Chiesa, E.; Genta, I.; Modena, T.; Bruni, G.; Grisoli, P.; Conti, B. Release Profile of Gentamicin Sulfate from Polylactide-co-Polycaprolactone Electrospun Nanofiber Matrices. Pharmaceutics 2019, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- Dorati, R.; De Trizio, A.; Genta, I.; Merelli, A.; Modena, T.; Conti, B. Gentamicin-Loaded Thermosetting Hydrogel and Moldable Composite Scaffold: Formulation Study and Biologic Evaluation. J. Pharm. Sci. 2017, 106, 1596–1607. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Qiu, L.J.; Mo, X.M.; Wang, S.; Xu, Y.F.; Peng, B.; Liu, M.; Huang, J.H.; Wang, G.C.; Zheng, J.H. Coaxial electro-spinning of P(LLA-CL)/heparin biodegradable polymer nanofibers: Potential vascular graft for substitution of femoral artery. J. Biomed. Mater. Res. Part B 2019, 107, 471–478. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, L.; Zhang, W.; Tang, Z.; Fu, W.; Hu, R.; Feng, B.; Hong, H.; Zhang, H. Shapeable large-pore electrospun polycaprolactam cotton facilitates the rapid formation of a functional tissue engineered vascular graft. Mater. Des. 2020, 191, 108631. [Google Scholar] [CrossRef]
- Huang, A.H.; Niklason, L.E. Engineering Biological-Based Vascular Grafts Using a Pulsatile Bioreactor. J. Vis. Exp. 2011, 52, e2646. [Google Scholar] [CrossRef]
- Hu, Q.; Su, C.; Zeng, Z.; Zhang, H.; Feng, R.; Feng, J.; Li, S. Fabrication of multilayer tubular scaffolds with aligned nan-ofibers to guide the growth of endothelial cells. J. Biomater. Appl. 2020, 35, 553–566. [Google Scholar] [CrossRef]
- Joy, J.; Aid-Launais, R.; Pereira, J.; Pavon-Djavid, G.; Ray, A.R.; Letourneur, D.; Meddahi-Pellé, A.; Gupta, B. Gela-tin-polytrimethylene carbonate blend based electrospun tubular construct as a potential vascular biomaterial. Mater. Sci. Eng. C 2020, 106, 110178. [Google Scholar] [CrossRef]
- Pisani, S.; Dorati, R.; Conti, B.; Modena, T.; Bruni, G.; Genta, I. Design of copolymer PLA-PCL electrospun matrix for biomedical applications. React. Funct. Polym. 2018, 124, 77–89. [Google Scholar] [CrossRef]
- Dorati, R.; Chiesa, E.; Pisani, S.; Genta, I.; Modena, T.; Bruni, G.; Brambilla, C.R.; Benazzo, M.; Conti, B. The Effect of Process Parameters on Alignment of Tubular Electrospun Nanofibers for Tissue Regeneration Purposes. J. Drug Deliv. Sci. Technol. 2020, 58, 101781. [Google Scholar] [CrossRef]
- Abdullah, K.G.; Attiah, M.A.; Olsen, A.S.; Richardson, A.; Lucas, T.H. Reducing surgical site infections following cra-niotomy: Examination of the use of topical vancomycin. J. Neurosurg. 2015, 123, 1600–1604. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, E.; Keynan, Y. Vancomycin revisited-60 years later. Front. Public Health 2014, 2, 217. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, C.; Veve, M.P.; Walker, B.; Shorman, M.A. Long-term vancomycin use had low risk of ototoxicity. PLoS ONE 2019, 14, e0224561. [Google Scholar] [CrossRef] [PubMed]
- Zasowski, E.J.; Murray, K.P.; Trinh, T.D.; Finch, N.A.; Pogue, J.M.; Mynatt, R.P.; Rybak, M.J. Identification of Vancomycin Exposure-Toxicity Thresholds in Hospitalized Patients Receiving Intravenous Vancomycin. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Tseng, Y.Y.; Kao, Y.C.; Liao, J.Y.; Chen, W.A.; Liu, S.J. Biodegradable Drug-Eluting Poly [lactic-co-glycol acid] Nano-fibers for the Sustainable Delivery of Vancomycin to Brain Tissue: In Vitro and in Vivo Studies. ACS Chem. Neuro-Sci. 2013, 4, 1314–1321. [Google Scholar] [CrossRef]
- Jang, C.H.; Cho, Y.B.; Jang, Y.S.; Kim, M.S.; Kim, G.H. Antibacterial effect of electrospun polycaprolactone/polyethylene oxide/vancomycin nanofiber mat for prevention of periprosthetic infection and biofilm formation. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Valle, M.J.D.J.; López, F.G.; Navarro, A.S. Development and validation of an HPLC method for vancomycin and its application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2008, 48, 835–839. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.; Quinn, M. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press and American Pharmacists Association; Marcel-Dekker: New York, NY, USA, 2009; ISBN1 978 0 85369 792 3. (UK); ISBN2 978 1 58212 135 2. (USA). [Google Scholar]


| Parameters | Set Up | |
|---|---|---|
| Spindle | Nozzle–collector distance (mm) | 150 |
| Rotating mandrel diameter (mm) | 6 | |
| Mandrel rotation speed (rpm) | 2500 | |
| Spinneret | Speed (mm/s) | 50 |
| Width (mm) | 50 | |
| Cleaning | Frequency (s) | 60 |
| Time (s) | 1 | |
| Voltage (kV) | 30 | |
| Syringe | Flow rate (mL/h) | 5 |
| Nozzle diameter (gauge) | 18 and 22 | |
| Electrospinning time | min | 7 |
| Formulation No. | Composition | Conductivity (mS/cm) | Electrospun Fiber Morphology * | ||
|---|---|---|---|---|---|
| VMC (w/v %) | Surfactant (v/v %) | DCM/Acetone Ratio (v/v) | |||
| PLA-PCL 15% w/v | |||||
| VTVG1 | - | - | 80:20 | 0.095 | - |
| VTVG2 | - | 0.05 | 80:20 | 0.128 | +/− |
| VTVG3 | 5 | - | 80:20 | 0.568 | +/−,^ |
| VTVG4 | 5 | 0.05 | 80:20 | 0.174 | +/− |
| VTVG5 | - | - | 70:30 | 1.892 | - |
| VTVG6 | - | 0.05 | 70:30 | 1.784 | + |
| VTVG7 | 5 | - | 70:30 | 3.274 | +^ |
| VTVG8 | 5 | 0.05 | 70:30 | 2.220 | + |
| VTVG9 | - | - | 60:40 | 0.500 | - |
| VTVG10 | - | 0.05 | 60:40 | 1.076 | +/− |
| VTVG11 | 5 | - | 60:40 | 0.844 | +/−,^ |
| VTVG12 | 5 | 0.05 | 60:40 | 2.855 | +/− |
| PLGA 15% w/v | |||||
| VTVG13 | - | - | 80:20 | 0.033 | +/− |
| VTVG14 | - | 0.05 | 80:20 | 0.045 | +/− |
| VTVG15 | 5 | - | 80:20 | 0.086 | +/−,^ |
| VTVG16 | 5 | 0.05 | 80:20 | 0.174 | +/− |
| VTVG17 | - | - | 70:30 | 0.595 | +/− |
| VTVG18 | - | 0.05 | 70:30 | 0.225 | + |
| VTVG19 | 5 | - | 70:30 | 0.770 | +/−,^ |
| VTVG20 | 5 | 0.05 | 70:30 | 0.659 | + |
| VTVG21 | - | - | 60:40 | 0.308 | +/− |
| VTVG22 | - | 0.05 | 60:40 | 0.176 | +/− |
| VTVG23 | 5 | - | 60:40 | 0.032 | +/−,^ |
| VTVG24 | 5 | 0.05 | 60:40 | 0.446 | + |
| Formulation No. | Fiber Diameter Range (μm) | Nano-Sized Fiber (%) | High-Frequency Orientation (°) | Porosity (% ± SD) | Number of Pores | Pore Area Range (μm2) |
|---|---|---|---|---|---|---|
| VTVG5 | 0.22–9.08 ± 0.59 | 10.4 | +45° | 55 ± 2.1 | 120.33 | 0.15–504.06 |
| VTVG6 | 0.23–5.62 ± 0.99 | 4.0 | +1° | 49 ± 3.0 | 75.00 | 0.19–218.80 |
| VTVG7 | 0.23–5.23 ± 0.72 | 7.2 | +44° | 56 ± 3.5 | 161.34 | 0.16–251.52 |
| VTVG8 | 0.21–7.37 ± 0.90 | 5.2 | +4° | 40 ± 1.3 | 86.33 | 0.23–240.68 |
| VTVG17 | 0.21–7.59 ± 0.82 | 4.7 | −87° | 50 ± 2.1 | 82.33 | 0.22–228.76 |
| VTVG18 | 0.21–6.32 ± 0.88 | 6.3 | +88° | 49 ± 2.3 | 100.33 | 0.16–279.28 |
| VTVG19 | 0.23–8.70 ± 1.14 | 4.8 | −9° | 46 ± 2.6 | 89.67 | 0.18–178.91 |
| VTVG20 | 0.21–7.79 ± 1.89 | 4.4 | −89° | 39 ± 1.5 | 49.33 | 0.15–183.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorati, R.; Chiesa, E.; Rosalia, M.; Pisani, S.; Genta, I.; Bruni, G.; Modena, T.; Conti, B. Tubular Electrospun Vancomycin-Loaded Vascular Grafts: Formulation Study and Physicochemical Characterization. Polymers 2021, 13, 2073. https://doi.org/10.3390/polym13132073
Dorati R, Chiesa E, Rosalia M, Pisani S, Genta I, Bruni G, Modena T, Conti B. Tubular Electrospun Vancomycin-Loaded Vascular Grafts: Formulation Study and Physicochemical Characterization. Polymers. 2021; 13(13):2073. https://doi.org/10.3390/polym13132073
Chicago/Turabian StyleDorati, Rossella, Enrica Chiesa, Mariella Rosalia, Silvia Pisani, Ida Genta, Giovanna Bruni, Tiziana Modena, and Bice Conti. 2021. "Tubular Electrospun Vancomycin-Loaded Vascular Grafts: Formulation Study and Physicochemical Characterization" Polymers 13, no. 13: 2073. https://doi.org/10.3390/polym13132073
APA StyleDorati, R., Chiesa, E., Rosalia, M., Pisani, S., Genta, I., Bruni, G., Modena, T., & Conti, B. (2021). Tubular Electrospun Vancomycin-Loaded Vascular Grafts: Formulation Study and Physicochemical Characterization. Polymers, 13(13), 2073. https://doi.org/10.3390/polym13132073