The Development and Characterization of a Cotton–Chitosan Composite for Lead Removal from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Composite Development
2.2. Composites’ Characterization
2.3. Lead, Pb(II), Adsorption–Desorption Determination
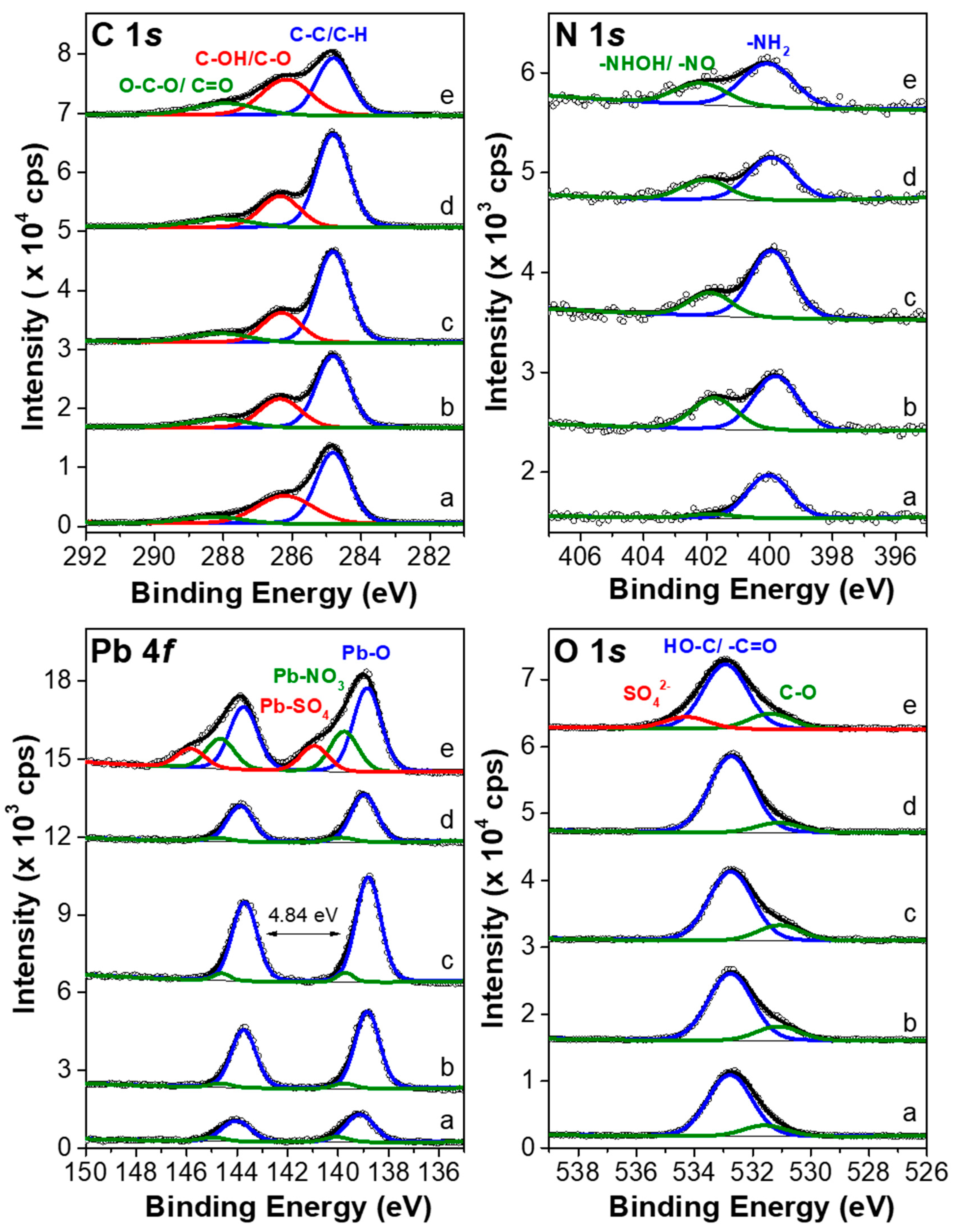
2.4. X-ray Photoelectron Spectroscopy (XPS)
3. Results
3.1. Composites’ Characterization
3.1.1. Elemental Analysis
3.1.2. Fourier Transform Infrared Spectra
3.1.3. Tensile Strength
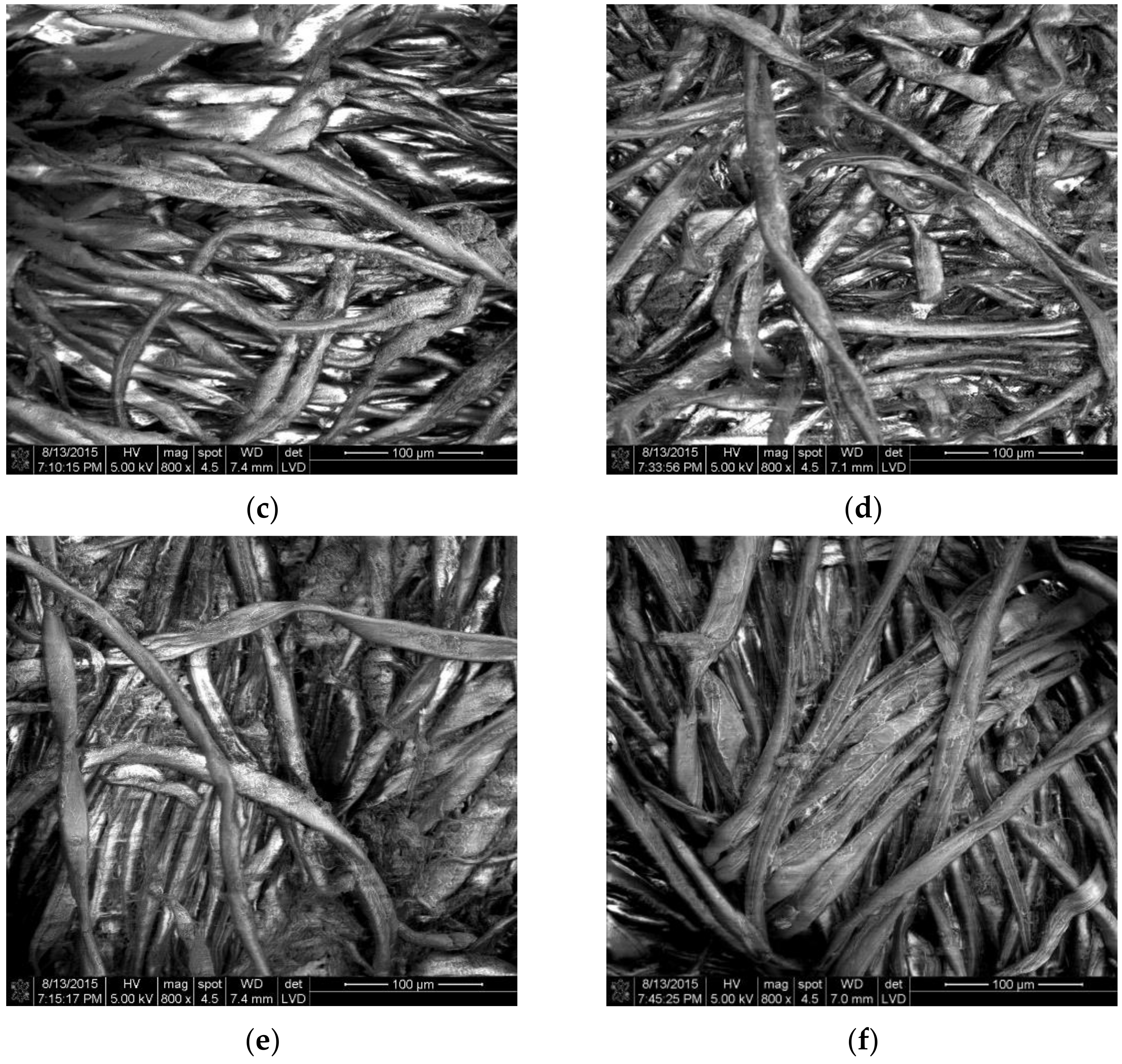
3.1.4. Atomic Force and Scanning Electron Microscopy
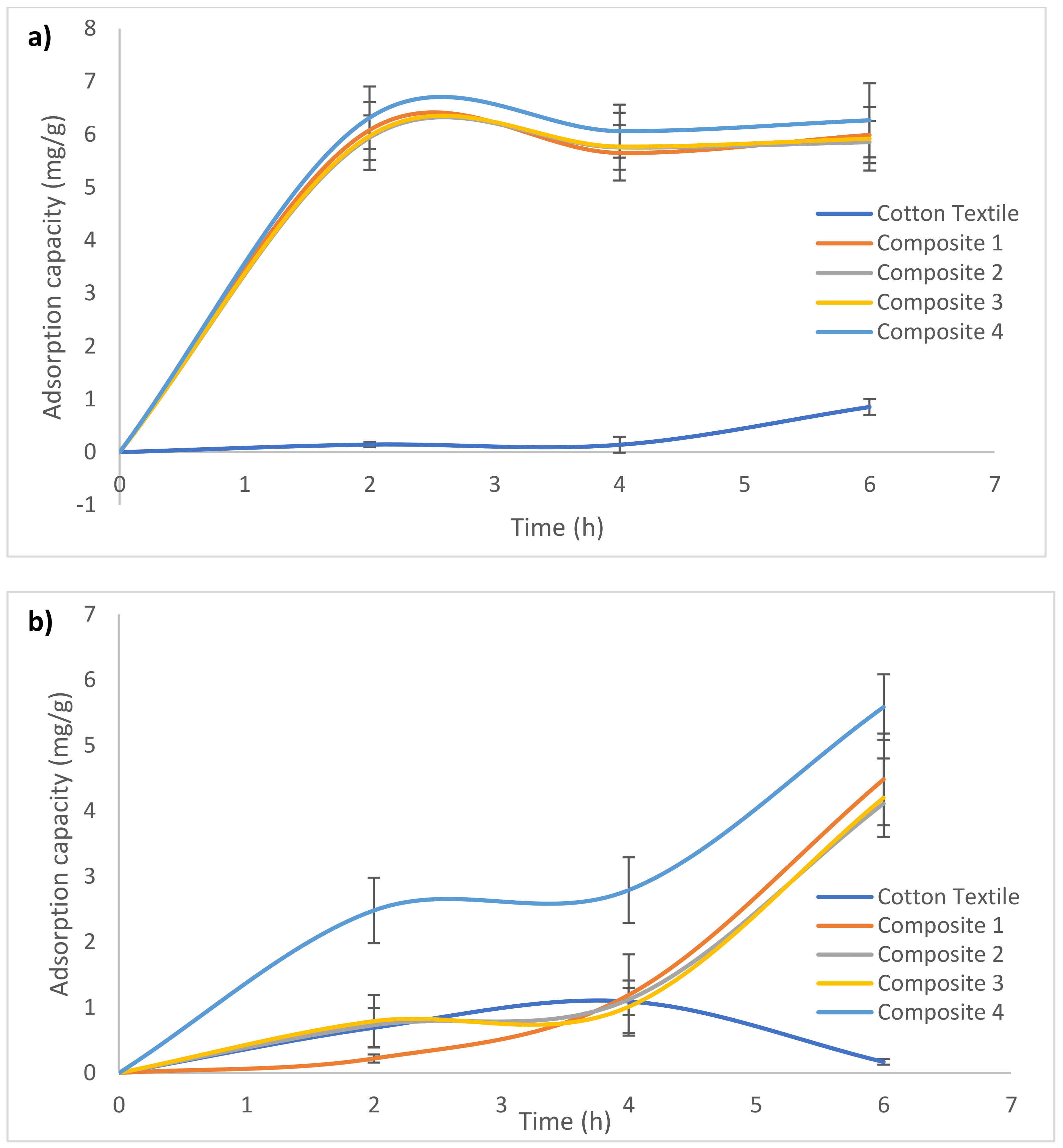
3.2. Lead Adsorption from Water
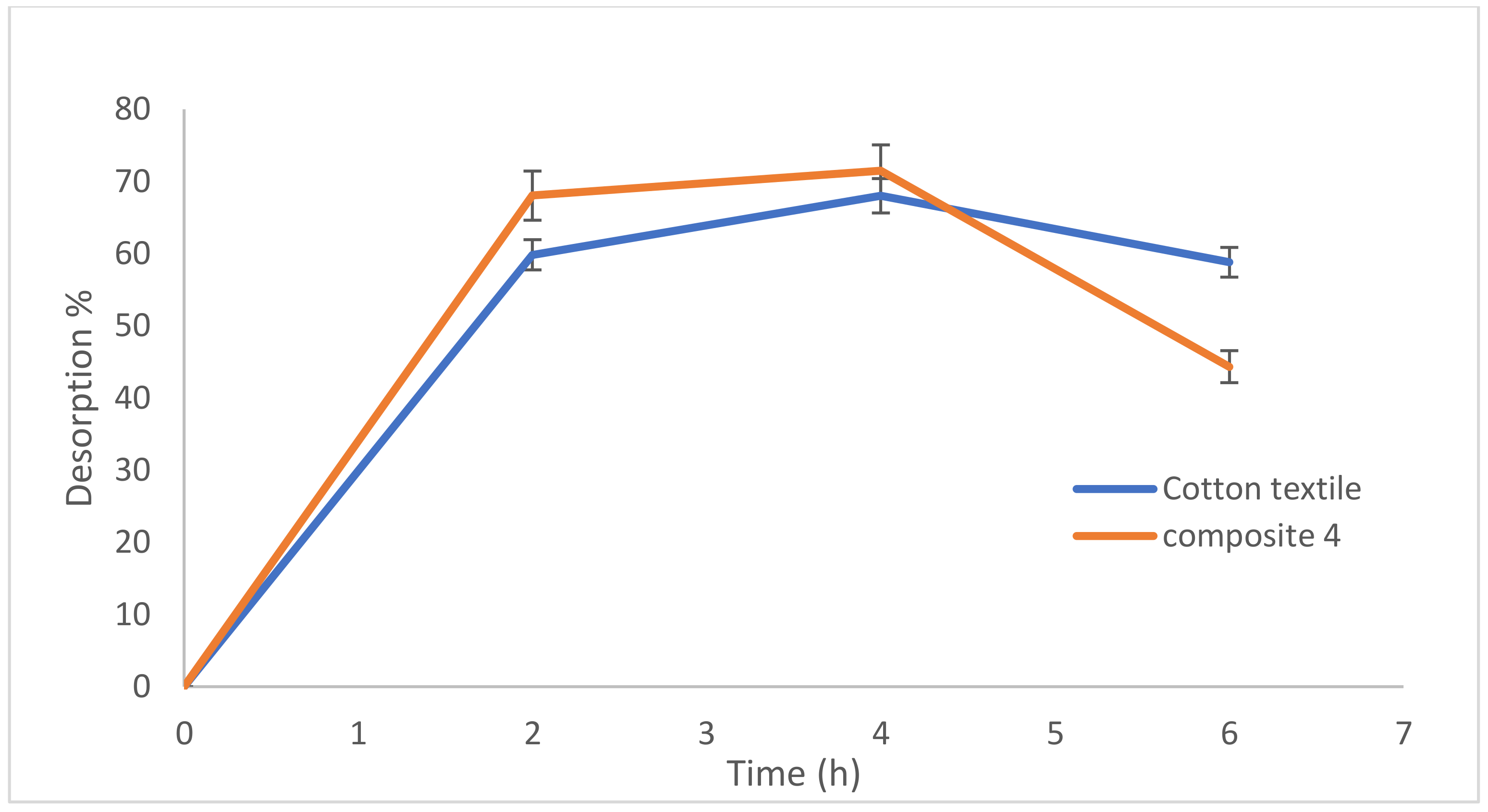
3.3. Lead Desorption from Composites
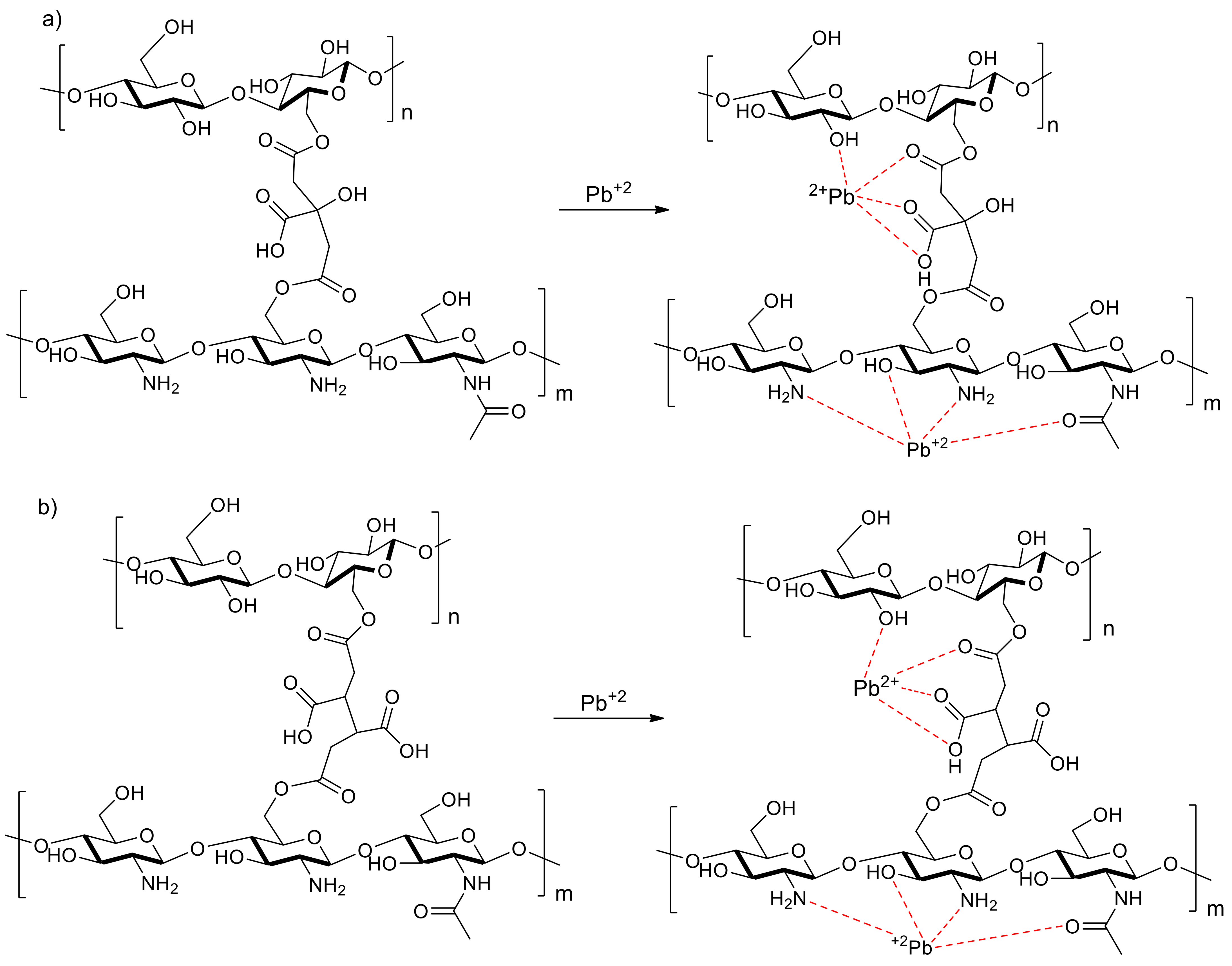
3.4. Surface Analysis by XPS
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahman, M.L.; Fui, C.J.; Ting, T.X.; Sarjadi, M.S.; Arshad, S.E.; Musta, B. Polymer Ligands Derived from Jute Fiber for Heavy Metal Removal from Electroplating Wastewater. Polymers 2020, 12, 2521. [Google Scholar] [CrossRef] [PubMed]
- Tighadouini, S.; Radi, S.; El Massaoudi, M.; Lakbaibi, Z.; Ferbinteanu, M.; Garcia, Y. Efficient and Environmental Friendly Adsorbent Based on β-Ketoenol-Pyrazole-Thiophene for Heavy-Metal Ion Removal from Aquatic Medium: A combined Experimental and Theoretical Study. ACS Omega 2020, 5, 17324–17336. [Google Scholar] [CrossRef] [PubMed]
- Pathan, S.; Suryasarathi, B. Arsenic removal using “Green” renewable feedstock-based hydrogels: Current and future perspectives. ACS Omega 2018, 3, 5910–5917. [Google Scholar] [CrossRef] [PubMed]
- D’Halluin, M.; Rull-Barrull, J.; Bretel, G.; Labrugère, C.; Le Grognec, E.; Felpin, F.X. Chemical Modified Cellulose Filter Paper for Heavy Metal Remediation in Water. ACS Sustain. Chem. Eng. 2017, 5, 1965–1973. [Google Scholar] [CrossRef]
- Ferrero, F.; Tonetti, C.; Periolatto, M. Adsorption of chromate and cupric ions onto chitosan-coated cotton gauze. Carbohyd. Polym 2014, 110, 367–373. [Google Scholar] [CrossRef]
- Gusmao, K.A.G.; Gurgel, L.V.A.; Melo, T.M.S.; Gil, L.F. Application of succinylated sugarcane bagasse as adsorbent to remove methylene blue and gentian violet from aqueous solutions—Kinetic and equilibrium studies. Dyes Pigments 2012, 92, 967–974. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Fu, K.; Yu, D.; Hristovski, K.D.; Westerhoff, P.; Crittenden, J.C. Review of Advances in Engineering Nanomaterial Adsorbents for Metal Removal and Recovery from Water: Synthesis and Microstructure Impacts. ACS ES&T Eng. 2021, 1, 623–661. [Google Scholar]
- Luo, J.; Yu, D.; Hristovski, K.D.; Fu, K.; Shen, Y.; Westerhoff, P.; Crittenden, J.C. Critical Review of Advances in Engineering Nanomaterial Adsorbents for Metal Removal and Recovery from Water: Mechanism Identification and Engineering Design. Environ. Sci. Technol. 2021, 55, 4287–4304. [Google Scholar] [CrossRef]
- Nair, V.; Panigrahy, A.; Vinu, R. Development of novel chitosan-lignin composites for adsorption of dyes and metal ions from wastewater. Chem. Eng. J. 2014, 245, 491–502. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, A. Adsorption of Pb (II) from aqueous solution by Chitosan-g-poly (acrylic acid)/Attapulgite/sodium humate composite hydrogels. J. Chem. Eng. D 2010, 55, 2379–2384. [Google Scholar] [CrossRef]
- Qu, R.; Sun, C.; Wang, M.; Ji, C.; Xu, Q.; Zhang, Y.; Wang, C.; Chen, H.; Yin, P. Adsorption of Au (III) from aqueous solution using cotton fiber/chitosan composite adsorbents. Hydrometallurgy 2009, 100, 65–71. [Google Scholar] [CrossRef]
- Azizkhani, S.; Mahmoudi, E.; Abdullah, N.; Ismail, M.H.S.; Mohammad, A.W.; Hussain, S.A. Synthesis and Characterisation of Graphene Oxide-Silica-Chitosan for eliminating the Pb(II) from Aqueous Solution. Polymers 2020, 12, 1922. [Google Scholar] [CrossRef]
- Yu, D.; Wang, Y.; Wu, M.; Zhang, L.; Wang, L.; Ni, H. Surface functionalization of cellulose with hyperbranched polyamide for efficient adsorption of organic dyes and heavy metals. J. Clean. Prod. 2019, 232, 774–783. [Google Scholar] [CrossRef]
- Hashem, A.; Fletcher, A.J.; Younis, H.; Mauof, H.; Abou-Okeil, A. Adsorption of Pb(II) ions from contaminated water by 1,2,3,4-butanetetracarboxylic acid-modified microcrystalline cellulose: Isotherms, kinetics, and thermodynamic studies. Int. J. Biol. Macromol. 2019, 164, 3193–3203. [Google Scholar] [CrossRef]
- Yang, H.; Sheikhi, A.; van de Ven, T.G.M. Reusable green aerogels from crosslinked hair nanocrystalline cellulose and modified chitosan for dye removal. Langmuir 2016, 32, 11771–11779. [Google Scholar] [CrossRef]
- Schiffman, J.F.; Schauer, C.L. Cross-linking chitosan fibers. Biomacromolecules 2007, 8, 549–601. [Google Scholar]
- Zhang, G.; Qu, R.; Changmei, S.; Ji, C.; Chen, H. Adsorption for Metal Ions of Chitosan Coated Cotton Fiber. J. Appl. Polym. Sci. 2008, 110, 2321–2327. [Google Scholar] [CrossRef]
- Madala, S.; Nadavala, S.K.; Vudagandla, S.; Boddu, V.M.; Abburi, K. Equilibrium, kinetics and thermodynamics of Cadmium (II) biosorption on to composite chitosan biosorbent. Arab. J. Chem. 2017, 10, S1883–S1893. [Google Scholar] [CrossRef] [Green Version]
- Dhanapal, V.; Subramanian, K. Modified chitosan for the collection of reactive blue 4, arsenic and mercury from aqueous media. Carbohyd. Polym. 2015, 117, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Nazari, A.; Montazer, M.; Rashidi, A.; Yazdanshenas, M.; Moghadam, M.B. Optimization of cotton crosslinking with polycarboxylic acids and Nano TiO2 using central composite design. J. Appl. Polym. Sci. 2010, 117, 2740–2748. [Google Scholar] [CrossRef]
- Alonso, D.; Gimeno, M.; Olayo, R.; Vázquez-Torres, H.; Sepúlveda-Sánchez, J.D.; Shirai, K. Cross-linking chitosan into UV-irradiated cellulose fibers for the preparation of antimicrobial-finished textiles. Carbohyd. Polym. 2009, 77, 536–546. [Google Scholar] [CrossRef]
- Ghosh, D.T.; Netravali, A. Cross-linked waxy maize starch-based “green” composites. ACS Sustain. Chem. Eng. 2013, 1, 1537–1544. [Google Scholar] [CrossRef]
- Alonso, D.; Gimeno, M.; Sepúlveda-Sánchez, J.D.; Shirai, K. Chitosan-based microcapsules containing grapefruit seed extract grafted onto cellulose fibers by non-toxic procedure. Carbohyd. Res. 2010, 354, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wen, Y.; Kang, X.; Liu, W. H2O2-induced surface modification: A facile, effective and environmentally friendly pretreatment of chitosan for dyes removal. Chem. Eng. J. 2011, 166, 474–482. [Google Scholar] [CrossRef]
- Wu, S.J.; Cai, R.Z.; Sun, Y.Y. Degradation of curdlan using hydrogen peroxide. Food Chem. 2012, 135, 2436–2438. [Google Scholar] [CrossRef]
- Razak, N.I.A.; Ibrahim, N.A.; Zainuddin, N.; Rayung, M.; Saad, W.Z. The influence of chemical surface modification of kenaf fiber using hydrogen peroxide on the mechanical properties of biodegradable kenaf fiber/poly (lactic acid) composites. Molecules 2014, 19, 2957–2968. [Google Scholar] [CrossRef] [Green Version]
- Pearson, R.G. Hard and Soft Acids and Bases, HASB, Part 1: Fundamental Principles. J. Chem. Educ. 1986, 45, 581–587. [Google Scholar] [CrossRef]
- Xu, X.; Yu, J.; Liu, C.; Yang, G.; Shi, L.; Zhuang, X. Xanthated chitosan/cellulose sponges for the efficient removal of anionic and cationic dyes. React. Funct. Polym. 2021, 160, 104840. [Google Scholar] [CrossRef]
- Khiari, R.; Rol, F.; Brochier Salon, M.C.; Bras, J.; Belgacem, M.N. Efficiency of Cellulose Carbonates to Produce Cellulose Nanofibers. ACS Sustain. Chem. Eng. 2019, 7, 8155–8167. [Google Scholar] [CrossRef]
- Shi, B.; Zhao, C.; Ji, Y.; Shi, J.; Yang, H. Promotion effect of PANI on Fe-PANI/Zeoliteas an active and recyclable Fenton-like catalyst under near-neutral condition. Appl. Surf. Sci. 2020, 508, 145298. [Google Scholar] [CrossRef]
- Fras, L.; Johansson, L.S.; Stenius, P.; Laine, J.; Stana-Kleinschek, K.; Ribitsch, V. Analysis of the oxidation of cellulose fibres by titration and XPS. Colloids Surf. A Physicochem. Eng. Asp. 2005, 260, 101–108. [Google Scholar] [CrossRef]
- Rouxhet, P.G.; Genet, M.J. XPS analysis of bio-organic systems. Surf. Interface Analysis 2011, 43, 1453–1470. [Google Scholar] [CrossRef]
- Zhuang, S.; Zhu, K.; Wang, J. Fibrous chitosan/cellulose composite as an efficient adsorbent for Co(II) removal. J. Clean. Prod. 2021, 285, 124911. [Google Scholar] [CrossRef]
- Maachou, H.; Genet, M.J.; Aliouche, D.; Dupont-Gillain, C.C.; Rouxhet, P.G. XPS analysis of chitosan–hydroxyapatite biomaterials: From elements to compounds. Surf. Interface Anal. 2013, 45, 1088–1097. [Google Scholar] [CrossRef]
- Samad, H.A.; Watson, P.R. An XPS study of the adsorption of lead on goethitea-FeOOH. Appl. Surf. Sci. 1998, 136, 46–54. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Ning, J.L.; Xie, H.; Lv, J.F.; Hu, P.J.; Pang, J. Study on surface modification of cerussite by thermochemical processing with pyrite. Physicochem. Probl. Miner. Process. 2021, 57, 156–167. [Google Scholar] [CrossRef]
- Wagner, C.D.; Zatko, D.A.; Raymond, R.H. Use of the Oxygen KLL Auger Lines in Identification of Surface Chemical States by Electron Spectroscopy for Chemical Analysis. Anal. Chem. 1980, 52, 1445–1451. [Google Scholar] [CrossRef]
- Marchon, B.; Carrazza, J.; Heinemann, H.; Somorjai, G.A. TPD and XPS studies of O2, CO2, and H2O adsorption on clean polycrystalline graphite. Carbon 1998, 26, 507–514. [Google Scholar] [CrossRef] [Green Version]






| Composite | H₂O₂ (35% v/v) | CA | BTCA | NaH₂PO₄ |
|---|---|---|---|---|
| 1 | 40 min | 4% w/v | - | - |
| 2 | 40 min | - | 4% w/v | - |
| 3 | 40 min | 4% w/v | - | 2.3% w/v |
| 4 | 40 min | - | 4% w/v | 2.3% w/v |
| Composite | mgchitosan to gcomposite * |
|---|---|
| 1 | 13.56 ± 2.00 |
| 2 | 22.60 ± 1.81 |
| 3 | 20.13 ± 0.87 |
| 4 | 27.62 ± 5.03 |
| Orbital | C 1s | N 1s | O 1s | Pb 4f | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Composite | C–C/C–H | C–OH/C–O | O–C–O/C=O | NH2 | –NHOH/–NO | O–C | OH–/O=C– | SO42− | Pb–O | Pb–NO3 | Pb–SO4 |
| Cotton textile | 43.6 | 27.7 | 7.1 | 1.2 | 0.0 | 16.9 | 3.0 | 0.0 | 0.3 | 0.1 | 0.0 |
| 1 | 43.7 | 21.4 | 8.8 | 1.5 | 0.9 | 18.8 | 4.0 | 0.0 | 0.8 | 0.1 | 0.0 |
| 3 | 48.8 | 18.5 | 8.4 | 1.6 | 0.6 | 17.2 | 3.9 | 0.0 | 1.0 | 0.1 | 0.0 |
| 2 | 49.0 | 19.5 | 8.3 | 1.1 | 0.5 | 18.6 | 2.4 | 0.0 | 0.5 | 0.0 | 0.0 |
| 4 | 31.4 | 29.2 | 12.1 | 1.3 | 0.7 | 16.6 | 4.0 | 3.0 | 0.9 | 0.4 | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso-Segura, D.; Hernández-García, L.; Menchaca-Arredondo, J.; Sánchez, M.; Chamorro-Garza, B.; Garza-Hernández, R. The Development and Characterization of a Cotton–Chitosan Composite for Lead Removal from Water. Polymers 2021, 13, 2066. https://doi.org/10.3390/polym13132066
Alonso-Segura D, Hernández-García L, Menchaca-Arredondo J, Sánchez M, Chamorro-Garza B, Garza-Hernández R. The Development and Characterization of a Cotton–Chitosan Composite for Lead Removal from Water. Polymers. 2021; 13(13):2066. https://doi.org/10.3390/polym13132066
Chicago/Turabian StyleAlonso-Segura, Diana, Luis Hernández-García, Jorge Menchaca-Arredondo, Mario Sánchez, Belén Chamorro-Garza, and Raquel Garza-Hernández. 2021. "The Development and Characterization of a Cotton–Chitosan Composite for Lead Removal from Water" Polymers 13, no. 13: 2066. https://doi.org/10.3390/polym13132066
APA StyleAlonso-Segura, D., Hernández-García, L., Menchaca-Arredondo, J., Sánchez, M., Chamorro-Garza, B., & Garza-Hernández, R. (2021). The Development and Characterization of a Cotton–Chitosan Composite for Lead Removal from Water. Polymers, 13(13), 2066. https://doi.org/10.3390/polym13132066






