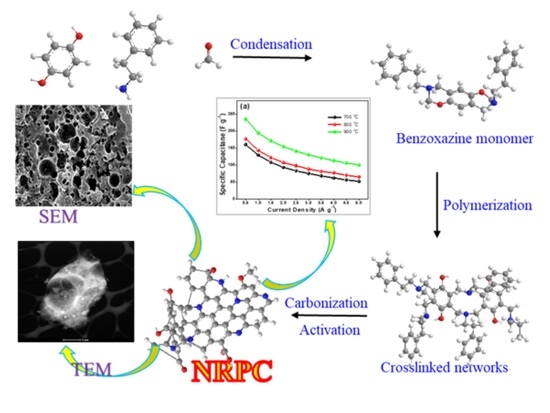
N-Doped Mesoporous Carbon Prepared from a Polybenzoxazine Precursor for High Performance Supercapacitors
Abstract
:1. Introduction
2. Synthesis of Benzoxazine Monomer and NRPCs
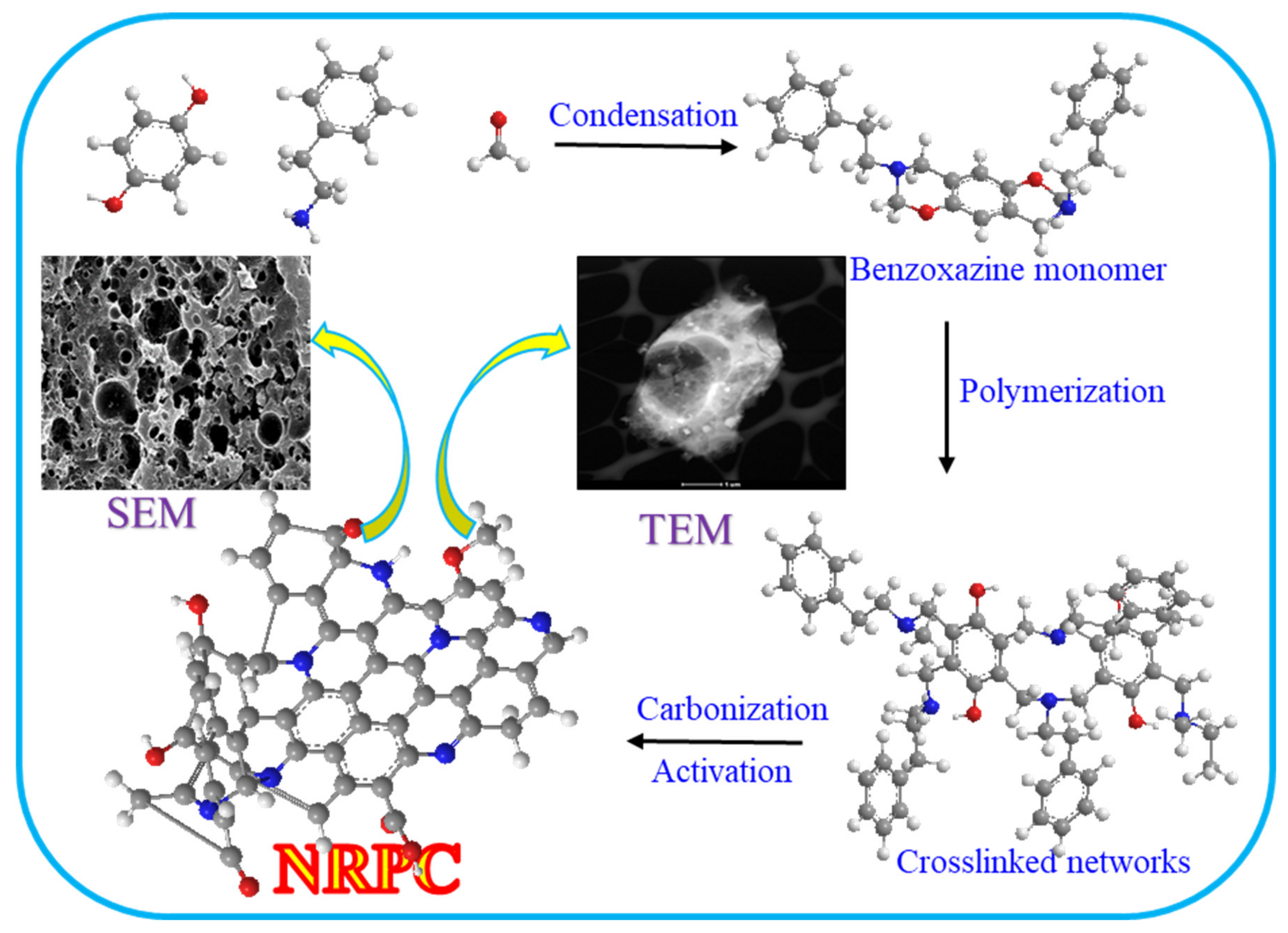
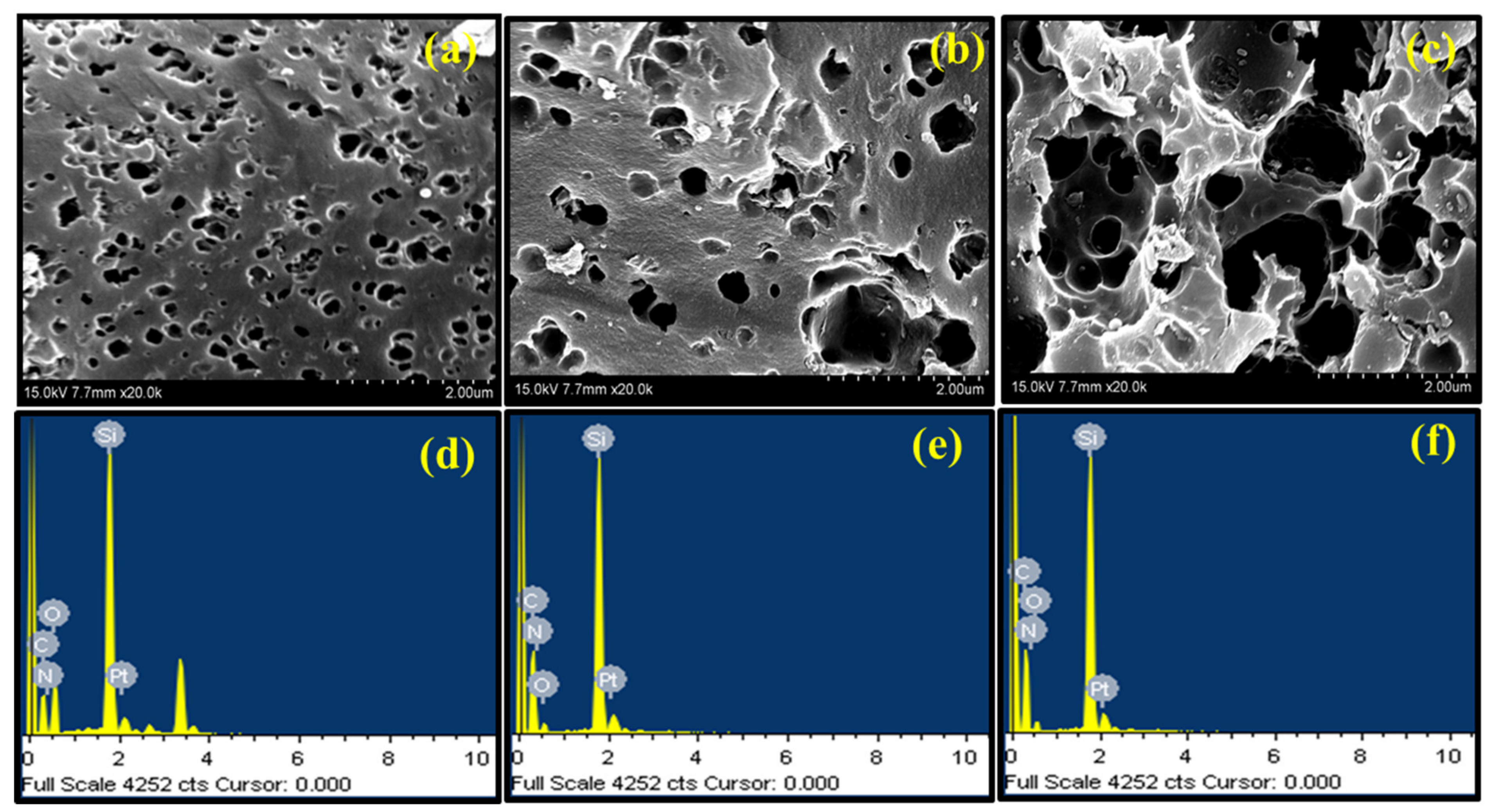
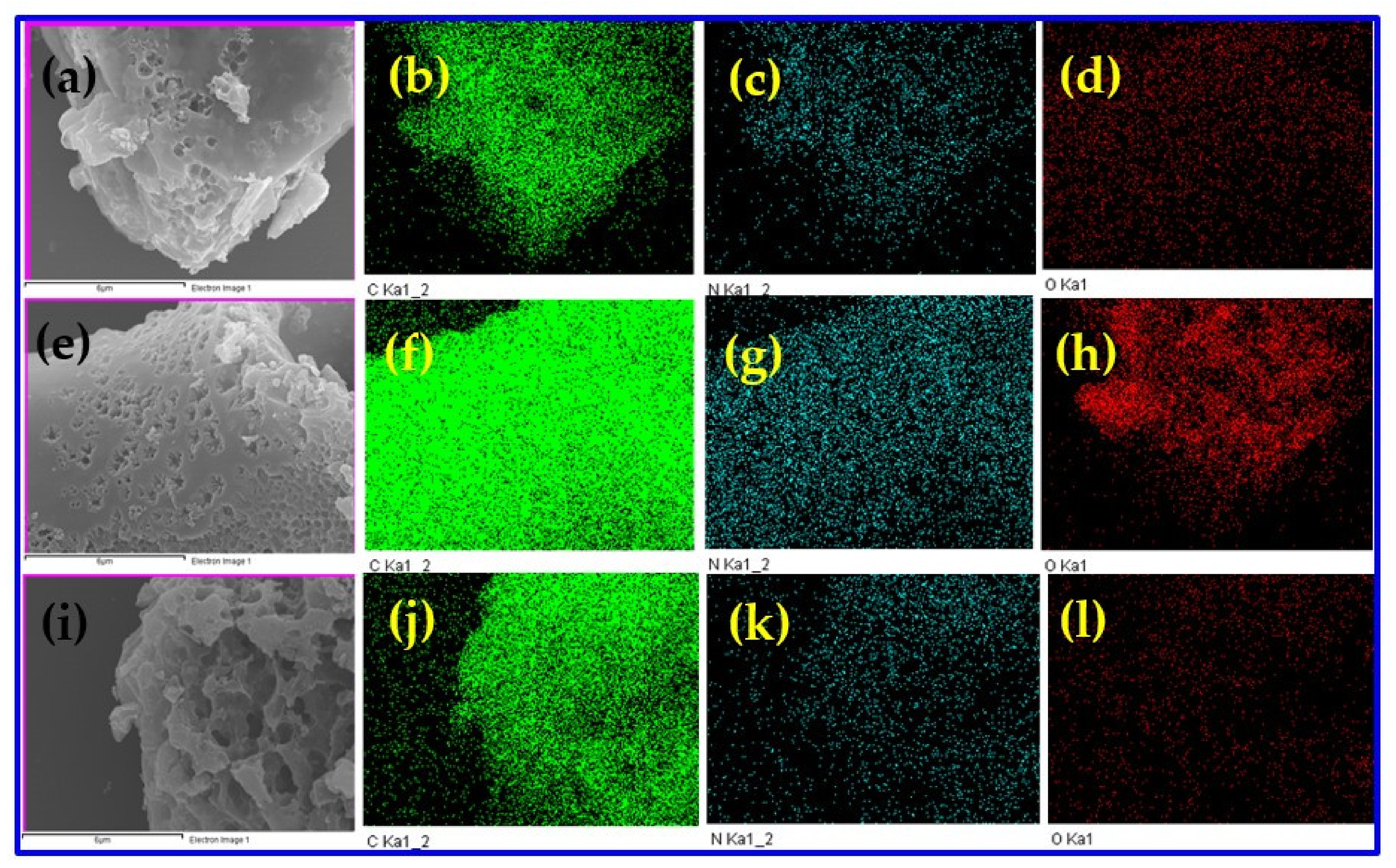
3. Results and Discussion
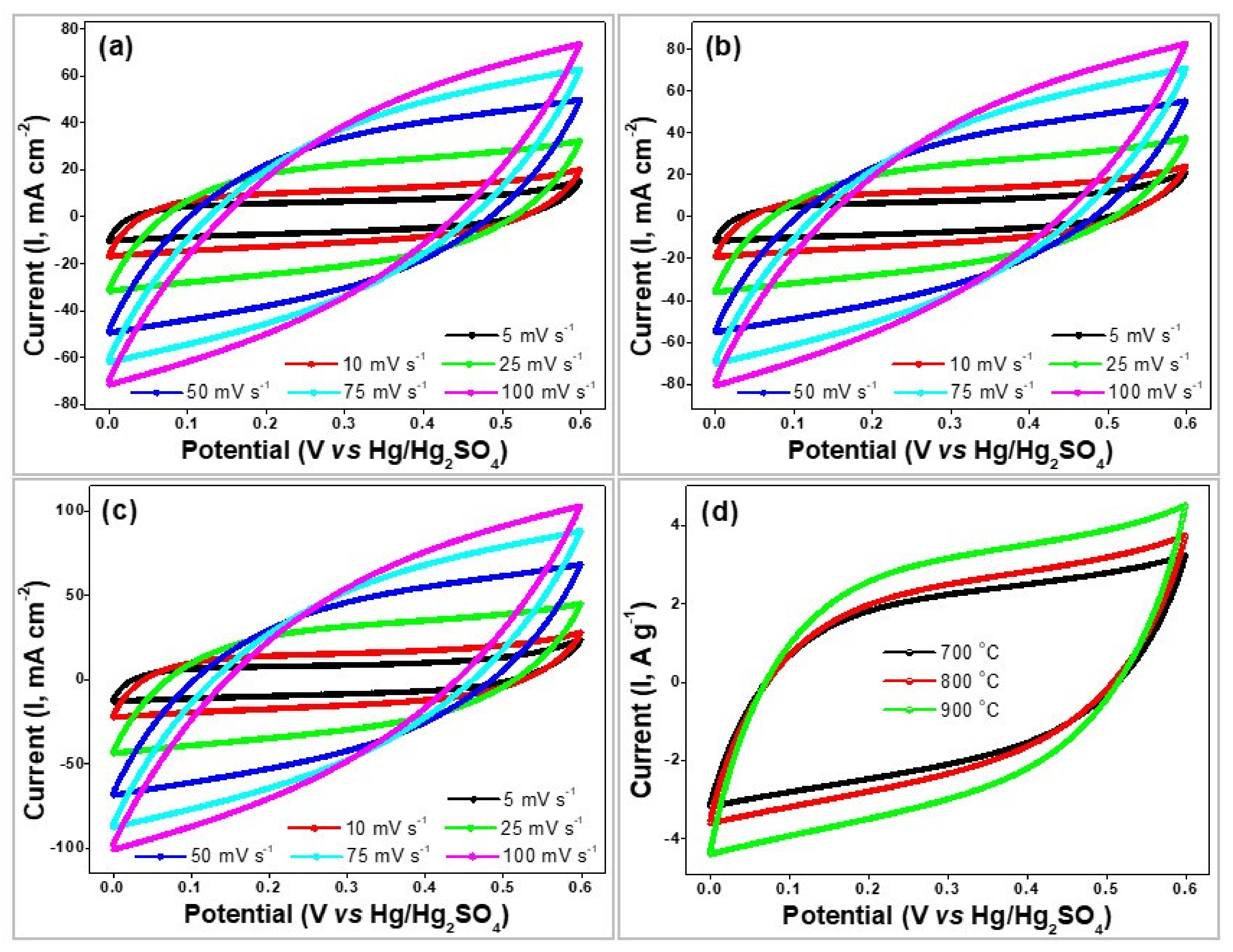
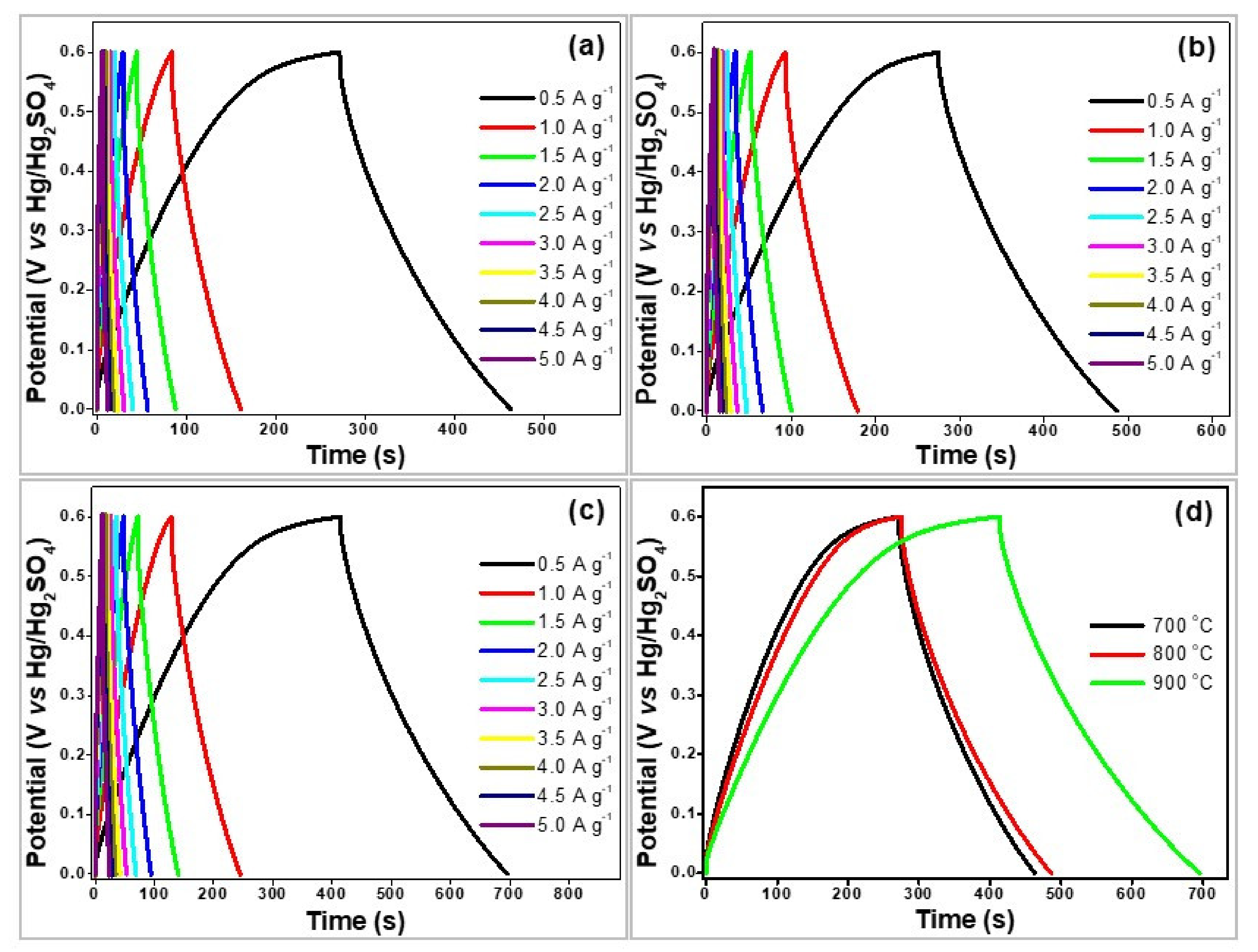
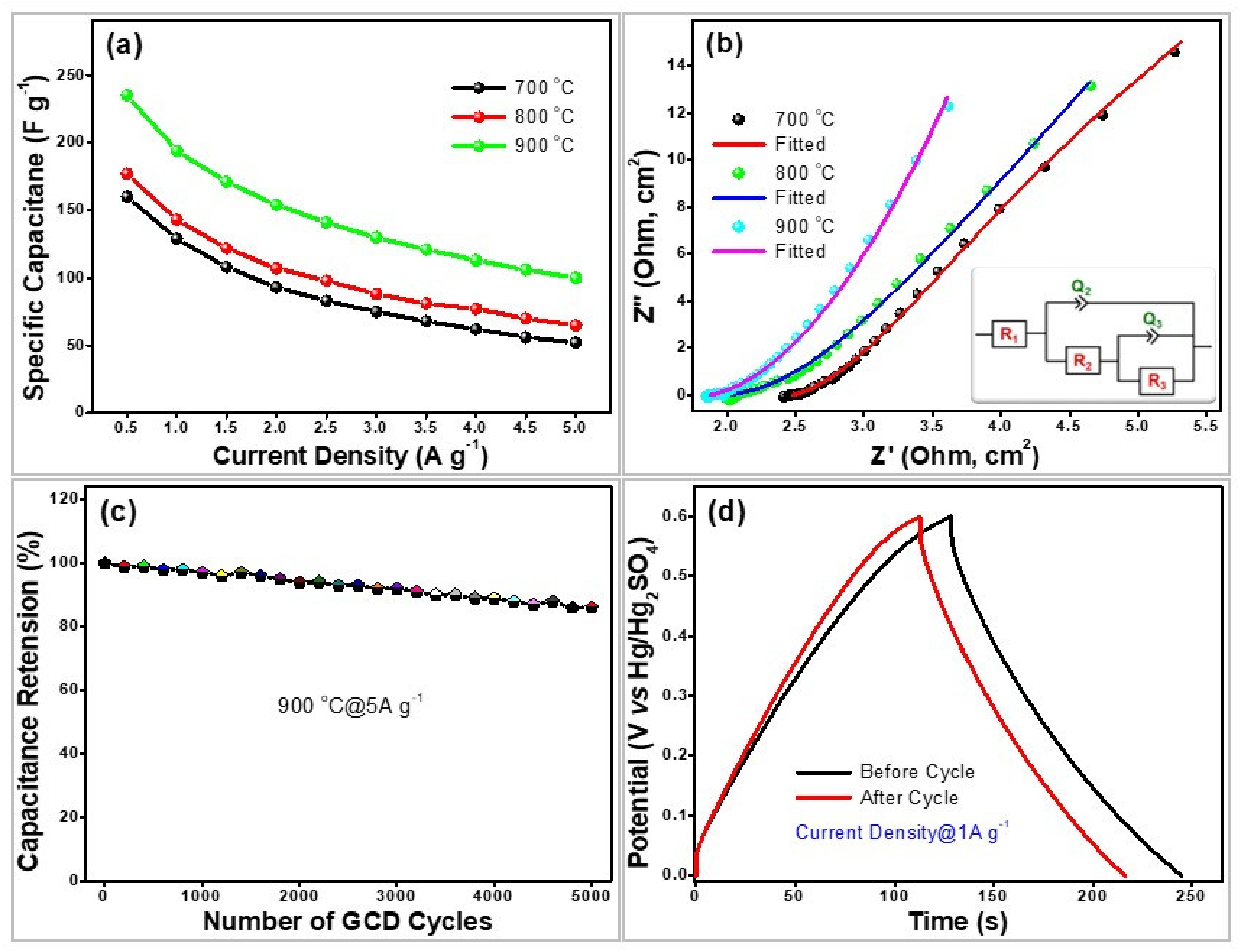
4. Electrochemical Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, C.Z.; Gao, B.; Shen, L.; Yang, S.D.; Hao, L.; Lu, X.J.; Zhang, F.; Zhang, L.J.; Zhang, X.G. Hierarchically structured carbon-based composites: Design, synthesis and their application in electrochemical capacitors. Nanoscale 2010, 3, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Hui, K.S.; Zeng, Z.; Zhang, L.; Mo, M.; Li, M. Hierarchical nitrogen-doped porous carbon with high surface area derived from endothelium corneum gigeriae galli for high-performance supercapacitor. Electrochim. Acta 2014, 130, 464–469. [Google Scholar] [CrossRef]
- Zhai, Y.; Dou, Y.; Zhao, D.; Fulvio, P.F.; Mayes, R.; Dai, S. Carbon Materials for Chemical Capacitive Energy Storage. Adv. Mater. 2011, 23, 4828–4850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Fic, K.; Meller, M.; Frackowiak, E. Strategies for enhancing the performance of carbon/carbon supercapacitors in aqueous electrolytes. Electrochim. Acta 2014, 128, 210–217. [Google Scholar] [CrossRef]
- Inagaki, M.; Konno, H.; Tanaike, O. Carbon materials for electrochemical capacitors. J. Power Sources 2010, 195, 7880–7903. [Google Scholar] [CrossRef]
- Xia, K.; Gao, Q.; Jiang, J.; Hu, J. Hierarchical porous carbons with controlled micropores and mesopores for supercapacitor electrode materials. Carbon 2008, 46, 1718–1726. [Google Scholar] [CrossRef]
- Sawaryn, C.; Landfester, K.; Taden, A. Benzoxazine Miniemulsions Stabilized with Polymerizable Nonionic Benzoxazine Surfactants. Macromolecules 2010, 43, 8933–8941. [Google Scholar] [CrossRef]
- Frackowiak, E.; Beguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Huang, J.; Sumpter, B.; Meunier, V. Theoretical Model for Nanoporous Carbon Supercapacitors. Angew. Chem. Int. Ed. 2008, 47, 520–524. [Google Scholar] [CrossRef]
- Ania, C.; Khomenko, V.; Raymundo-Piñero, E.; Parra, J.B.; Beguin, F. The Large Electrochemical Capacitance of Microporous Doped Carbon Obtained by Using a Zeolite Template. Adv. Funct. Mater. 2007, 17, 1828–1836. [Google Scholar] [CrossRef] [Green Version]
- Hall, P.J.; Mirzaeian, M.; Fletcher, S.I.; Sillars, F.B.; Rennie, A.; Shitta-Bey, G.O.; Wilson, G.; Cruden, A.; Carter, R. Energy storage in electrochemical capacitors: Designing functional materials to improve performance. Energy Environ. Sci. 2010, 3, 1238–1251. [Google Scholar] [CrossRef]
- Hulicova-Jurcakova, D.; Kodama, M.; Shiraishi, S.; Hatori, H.; Zhu, Z.; Lu, G.Q. Nitrogen-Enriched Nonporous Carbon Electrodes with Extraordinary Supercapacitance. Adv. Funct. Mater. 2009, 19, 1800–1809. [Google Scholar] [CrossRef]
- Chmiola, J.; Yushin, G.; Dash, R.; Gogotsi, Y. Effect of pore size and surface area of carbide derived carbons on specific capacitance. J. Power Sources 2006, 158, 765–772. [Google Scholar] [CrossRef]
- Liu, X.; Gu, Y. Study on the volumetric expansion of benzoxazine curing with different catalysts. J. Appl. Polym. Sci. 2002, 84, 1107–1113. [Google Scholar] [CrossRef]
- Agag, T.; Arza, C.R.; Maurer, F.H.J.; Ishida, H. Primary Amine-Functional Benzoxazine Monomers and Their Use for Amide-Containing Monomeric Benzoxazines. Macromolecules 2010, 43, 2748–2758. [Google Scholar] [CrossRef]
- Sawaryn, C.; Landfester, K.; Taden, A. Benzoxazine Miniemulsions Stabilized with Multifunctional Main-chain Benzoxazine Protective Colloids. Macromolecules 2011, 44, 5650–5658. [Google Scholar] [CrossRef]
- Thubsuang, U.; Chotirut, S.; Thongnok, A.; Promraksa, A.; Nisoa, M.; Manmuanpom, N.; Wongkasemjit, S.; Chaisuwan, T. Facile preparation of polybenzoxazine-based carbon microspheres with nitrogen functionalities: Effects of mixed solvents on pore structure and supercapacitive performance. Front. Chem. Sci. Eng. 2020, 14, 1072–1086. [Google Scholar] [CrossRef]
- Yagci, Y.; Kiskan, B.; Ghosh, N.N. Recent advancement on polybenzoxazine-A newly developed high performance thermoset. J. Polym. Sci. Part. A Polym. Chem. 2009, 47, 5565–5576. [Google Scholar] [CrossRef]
- Wan, L.; Wang, J.; Xie, L.; Sun, Y.; Li, K. Nitrogen-Enriched Hierarchically Porous Carbons Prepared from Polybenzoxazine for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 15583–15596. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef] [Green Version]
- Nishihara, H.; Kyotani, T. Templated Nanocarbons for Energy Storage. Adv. Mater. 2012, 24, 4473–4498. [Google Scholar] [CrossRef]
- Xu, B.; Hou, S.; Cao, G.; Wu, F.; Yang, Y. Sustainable nitrogen-doped porous carbon with high surface areas prepared from gelatin for supercapacitors. J. Mater. Chem. 2012, 22, 19088–19093. [Google Scholar] [CrossRef]
- Li, L.; Liu, E.; Li, J.; Yang, Y.; Shen, H.; Huang, Z.; Xiang, X.; Li, W. A doped activated carbon prepared from polyaniline for high performance supercapacitors. J. Power Sources 2010, 195, 1516–1521. [Google Scholar] [CrossRef]
- Kim, C.; Ngoc, B.T.N.; Yang, K.S.; Kojima, M.; Kim, Y.A.; Endo, M.; Yang, S.C. Self-Sustained Thin Webs Consisting of Porous Carbon Nanofibers for Supercapacitors via the Electrospinning of Polyacrylonitrile Solutions Containing Zinc Chloride. Adv. Mater. 2007, 19, 2341–2346. [Google Scholar] [CrossRef]
- Dutta, S.; Bhaumik, A.; Wu, K.C.-W. Hierarchically porous carbon derived from polymers and biomass: Effect of interconnected pores on energy applications. Energy Environ. Sci. 2014, 7, 3574–3592. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, C.; Yu, T.; Liu, F.; Zhang, F.; Wan, Y.; Zhang, L.; Wang, C.; Tu, B.; Webley, P.A.; et al. Facile Synthesis of Hierarchically Porous Carbons from Dual Colloidal Crystal/Block Copolymer Template Approach. Chem. Mater. 2007, 19, 3271–3277. [Google Scholar] [CrossRef]
- Wang, Z.; Stein, A. Morphology Control of Carbon, Silica, and Carbon/Silica Nanocomposites: From 3D Ordered Macro-/Mesoporous Monoliths to Shaped Mesoporous Particles. Chem. Mater. 2008, 20, 1029–1040. [Google Scholar] [CrossRef]
- Han, S.; Hyeon, T. Simple silica-particle template synthesis of mesoporous carbons. Chem. Commun. 1999, 1955–1956. [Google Scholar] [CrossRef]
- Wang, S.; Han, C.; Wang, J.; Deng, J.; Zhu, M.; Yao, J.; Li, H.; Wang, Y. Controlled Synthesis of Ordered Mesoporous Carbohydrate-Derived Carbons with Flower-like Structure and N-Doping by Self-Transformation. Chem. Mater. 2014, 26, 6872–6877. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Shakila, A.; Muthusamy, S. Synthesis and characterization of novel bio-based benzoxazines from eugenol. RSC Adv. 2014, 4, 7959–7966. [Google Scholar] [CrossRef]
- Periyasamy, T.; Asrafali, S.P.; Muthusamy, S. New benzoxazines containing polyhedral oligomeric silsesquioxane from eugenol, guaiacol and vanillin. New J. Chem. 2014, 39, 1691–1702. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Parveen, A.S.; Sarojadevi, M. Synthesis and Copolymerization of Fully Biobased Benzoxazines from Renewable Resources. ACS Sustain. Chem. Eng. 2014, 2, 2790–2801. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Manoharan, R.K.; Parveen, A.S.; Atchudan, R.; Kim, S.-C. Sustainability and antimicrobial assessments of apigenin based polybenzoxazine film. Polymers 2019, 172, 100–109. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Atchudan, R.; Balasubramanian, R.; Parveen, A.S.; Kim, S.-C. Direct synthesis of nitrogen-rich carbon sheets via polybenzoxazine as highly active electrocatalyst for water splitting. Int. J. Hydrogen Energy 2018, 43, 13266–13275. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Atchudan, R.; Parveen, A.S.; Lee, Y.R.; Kim, S.-C. Polybenzoxazine originated N-doped mesoporous carbon ropes as an electrode material for high-performance supercapacitors. J. Alloy. Compd. 2018, 750, 384–391. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, M.; Reddeppa, N.; Xu, D.; Jing, Q.-S.; Zha, R. Nitrogen self-doped carbon aerogels derived from trifunctional benzoxazine monomers as ultralight supercapacitor electrodes. Nanoscale 2018, 10, 6549–6557. [Google Scholar] [CrossRef]
- Jin, H.; Wang, X.; Gu, Z.; Polin, J. Carbon materials from high ash biochar for supercapacitor and improvement of capacitance with HNO3 surface oxidation. J. Power Sources 2013, 236, 285–292. [Google Scholar] [CrossRef]
- Yuan, D.; Chen, J.; Zeng, J.; Tan, S. Preparation of monodisperse carbon nanospheres for electrochemical capacitors. Electrochem. Commun. 2008, 10, 1067–1070. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, J.; Wang, Y.; Ning, G.; Fan, Z.; Wei, T.; Cheng, J.; Zhang, M.; Jing, X. Template synthesis of hollow carbon spheres anchored on carbon nanotubes for high rate performance supercapacitors. Carbon 2013, 52, 209–218. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, X.; Wu, C.; Liu, J.; Wang, H.; Gao, J.; Zhang, Y.; Shu, H. Supercapacitive performance of hierarchical porous carbon microspheres prepared by simple one-pot method. J. Power Sources 2014, 254, 10–17. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, J.; Nagaraju, D.H.; Jiang, L.; Marinov, V.R.; Lubineau, G.; Alshareef, H.N.; Oh, M. Flexible, Highly Graphitized Carbon Aerogels Based on Bacterial Cellulose/Lignin: Catalyst-Free Synthesis and its Application in Energy Storage Devices. Adv. Funct. Mater. 2015, 25, 3193–3202. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, D.; Wang, Y.; Hou, B. Electrocatalytic activity of nitrogen-doped graphene synthesized via a one-pot hydrothermal process towards oxygen reduction reaction. J. Power Sources 2013, 227, 185–190. [Google Scholar] [CrossRef]
- Andreas, H.A.; Conway, B.E. Examination of the double-layer capacitance of an high specific-area C-cloth electrode as titrated from acidic to alkaline pHs. Electrochim. Acta 2006, 51, 6510–6520. [Google Scholar] [CrossRef]
- Fujisawa, K.; Cruz-Silva, R.; Yang, K.-S.; Kim, Y.A.; Hayashi, T.; Endo, M.; Terrones, M.; Dresselhaus, M.S. Importance of open, heteroatom-decorated edges in chemically doped-graphene for supercapacitor applications. J. Mater. Chem. A 2014, 2, 9532–9540. [Google Scholar] [CrossRef]
- Kerisit, S.; Schwenzer, B.; Vijayakumar, M. Effects of Oxygen-Containing Functional Groups on Supercapacitor Performance. J. Phys. Chem. Lett. 2014, 5, 2330–2334. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Lee, Y.R. Green synthesis of nitrogen-doped graphitic carbon sheets with use of Prunus persica for supercapacitor applications. Appl. Surf. Sci. 2017, 393, 276–286. [Google Scholar] [CrossRef]
- Candelaria, S.L.; Garcia, B.B.; Liu, D.; Cao, G.; Candelaria, S.L.; Garcia, B.B.; Liu, D.; Cao, G. Nitrogen modification of highly porous carbon for improved supercapacitor performance. J. Mater. Chem. 2012, 22, 9884–9889. [Google Scholar] [CrossRef]
- Wang, D.-W.; Li, F.; Chen, Z.-G.; Lu, G.Q.; Cheng, H.-M. Synthesis and Electrochemical Property of Boron-Doped Mesoporous Carbon in Supercapacitor. Chem. Mater. 2008, 20, 7195–7200. [Google Scholar] [CrossRef]
- Guo, Y.; Shi, Z.-Q.; Chen, M.-M.; Wang, C.-Y. Hierarchical porous carbon derived from sulfonated pitch for electrical double layer capacitors. J. Power Sources 2014, 252, 235–243. [Google Scholar] [CrossRef]
- Atchudan, R.; Perumal, S.; Karthikeyan, D.; Pandurangan, A.; Lee, Y.R. Synthesis and characterization of graphitic mesoporous carbon using metal–metal oxide by chemical vapor deposition method. Microporous Mesoporous Mater. 2015, 215, 123–132. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Chang, K.-H.; Hu, C.-C. Differentiate the pseudocapacitance and double-layer capacitance contributions for nitrogen-doped reduced graphene oxide in acidic and alkaline electrolytes. J. Power Sources 2013, 227, 300–308. [Google Scholar] [CrossRef]
- Yang, X.; Wu, D.; Chen, X.; Fu, R. Nitrogen-Enriched Nanocarbons with a 3-D Continuous Mesopore Structure from Polyacrylonitrile for Supercapacitor Application. J. Phys. Chem. C 2010, 114, 8581–8586. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Xu, J.; Wang, M.; Zhu, L.; Dai, L.; Jaroniec, M. Nitrogen Enriched Porous Carbon Spheres: Attractive Materials for Supercapacitor Electrodes and CO2 Adsorption. Chem. Mater. 2014, 26, 2820–2828. [Google Scholar] [CrossRef]
- Nasini, U.B.; Bairi, V.G.; Ramasahayam, S.K.; Bourdo, S.E.; Viswanathan, T.; Shaikh, A.U. Phosphorous and nitrogen dual heteroatom doped mesoporous carbon synthesized via microwave method for supercapacitor application. J. Power Sources 2014, 250, 257–265. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Sethuraman, M.G.; Lee, Y.R. Supercapacitor performance of carbon supported Co 3 O 4 nanoparticles synthesized using Terminalia chebula fruit. J. Taiwan Inst. Chem. Eng. 2016, 68, 489–495. [Google Scholar] [CrossRef]
- Yu, P.; Li, Y.; Zhao, X.; Wu, L.; Zhang, Q. Graphene-Wrapped Polyaniline Nanowire Arrays on Nitrogen-Doped Carbon Fabric as Novel Flexible Hybrid Electrode Materials for High-Performance Supercapacitor. Langmuir 2014, 30, 5306–5313. [Google Scholar] [CrossRef]
- Xu, B.; Hou, S.; Zhang, F.; Cao, G.; Chu, M.; Yang, Y. Nitrogen-doped mesoporous carbon derived from biopolymer as electrode material for supercapacitors. J. Electroanal. Chem. 2014, 712, 146–150. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thirukumaran, P.; Atchudan, R.; Shakila Parveen, A.; Santhamoorthy, M.; Ramkumar, V.; Kim, S.-C. N-Doped Mesoporous Carbon Prepared from a Polybenzoxazine Precursor for High Performance Supercapacitors. Polymers 2021, 13, 2048. https://doi.org/10.3390/polym13132048
Thirukumaran P, Atchudan R, Shakila Parveen A, Santhamoorthy M, Ramkumar V, Kim S-C. N-Doped Mesoporous Carbon Prepared from a Polybenzoxazine Precursor for High Performance Supercapacitors. Polymers. 2021; 13(13):2048. https://doi.org/10.3390/polym13132048
Chicago/Turabian StyleThirukumaran, Periyasamy, Raji Atchudan, Asrafali Shakila Parveen, Madhappan Santhamoorthy, Vanaraj Ramkumar, and Seong-Cheol Kim. 2021. "N-Doped Mesoporous Carbon Prepared from a Polybenzoxazine Precursor for High Performance Supercapacitors" Polymers 13, no. 13: 2048. https://doi.org/10.3390/polym13132048
APA StyleThirukumaran, P., Atchudan, R., Shakila Parveen, A., Santhamoorthy, M., Ramkumar, V., & Kim, S.-C. (2021). N-Doped Mesoporous Carbon Prepared from a Polybenzoxazine Precursor for High Performance Supercapacitors. Polymers, 13(13), 2048. https://doi.org/10.3390/polym13132048