Electrospun Fibers of Polybutylene Succinate/Graphene Oxide Composite for Syringe-Push Protein Absorption Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Solution Preparation for Electrospun Fiber Composite Membrane
2.2.2. Fabrication of Electrospun Fiber Composite Membrane by Electrospinning
2.2.3. Mechanical Properties Test and Uniformity Measurement of Membranes
2.2.4. Examination of Morphology by Scanning Electron Microscopy
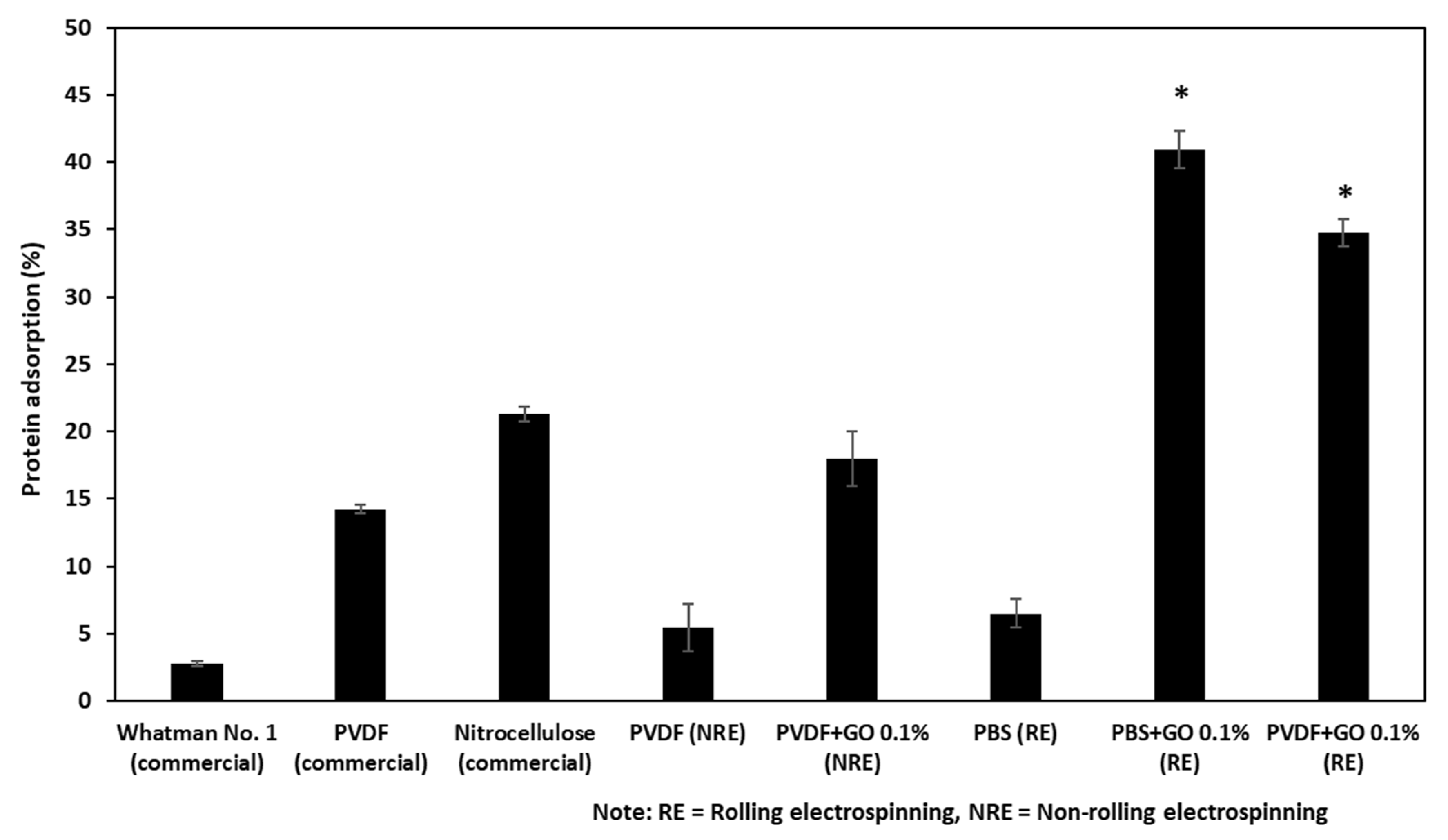
2.2.5. Comparison of Membrane Protein Binding Capacity
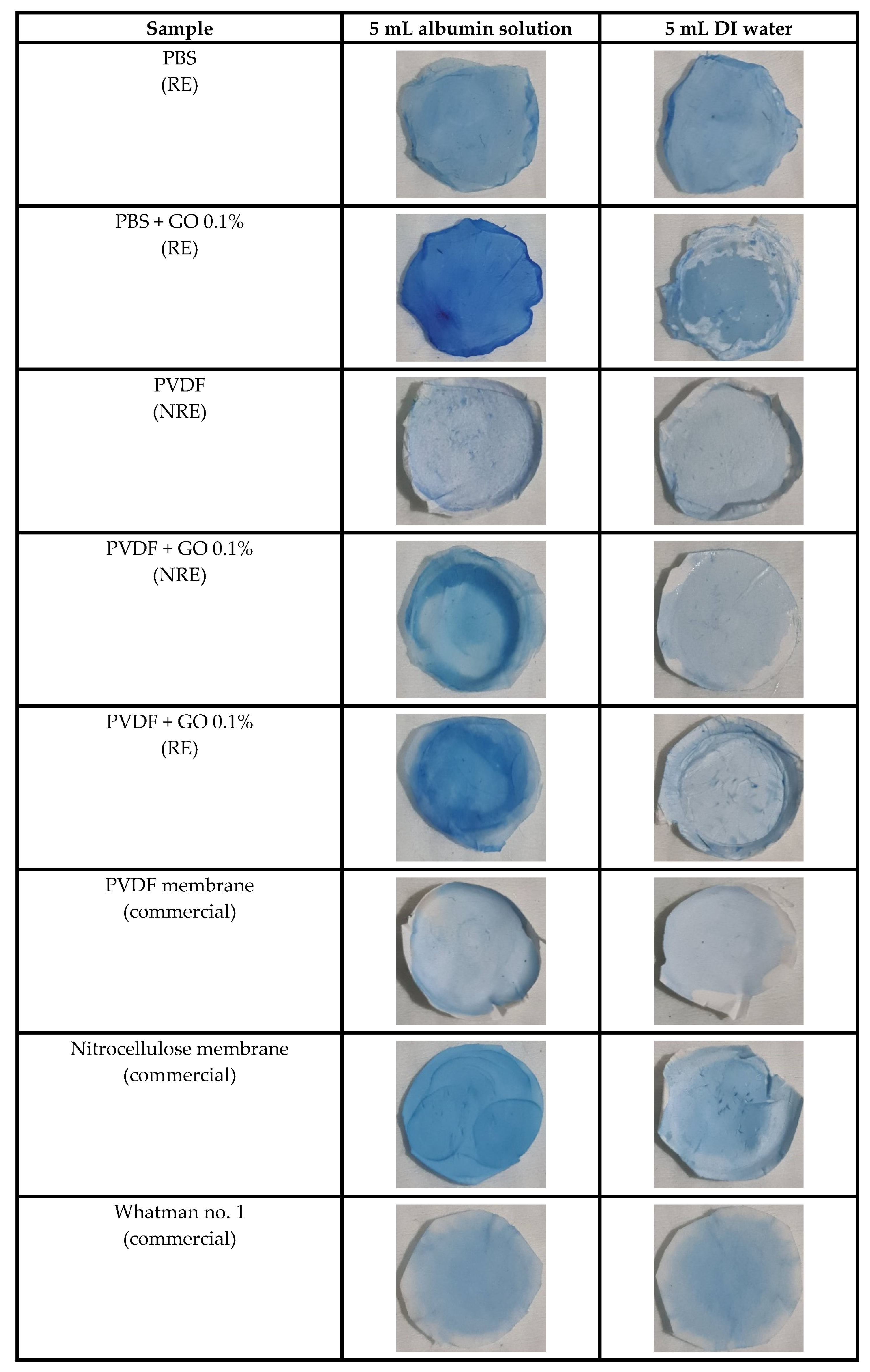
2.2.6. Staining of Protein on the Filtered Specimens
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Mechanical Properties and Membrane Thickness Uniformity
3.2. Morphology of the Fabricated Membranes
3.3. The Protein Binding Capacity of the Fabricated Filter Membranes and Commercially Available Filter Membranes
3.4. Qualitative Investigation of Absorbed Protein on the Membrane by Protein Staining
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Senturk, A.; Sahin, A.T.; Armutlu, A.; Kiremit, M.C.; Acar, O.; Erdem, S.; Bagbudar, S.; Esen, T.; Tuncbag, N.; Ozlu, N. Quantitative proteomics identifies secreted diagnostic biomarkers as well as tumor-dependent prognostic targets for clear cell Renal Cell Carcinoma. Mol. Cancer Res. MCR 2021. [Google Scholar] [CrossRef] [PubMed]
- Swensen, A.C.; He, J.; Fang, A.C.; Ye, Y.; Nicora, C.D.; Shi, T.; Liu, A.Y.; Sigdel, T.K.; Sarwal, M.M.; Qian, W.J. A Comprehensive Urine Proteome Database Generated From Patients With Various Renal Conditions and Prostate Cancer. Front. Med. 2021, 8, 548212. [Google Scholar] [CrossRef]
- Vanarsa, K.; Enan, S.; Patel, P.; Strachan, B.; Sam Titus, A.; Dennis, A.; Lotan, Y.; Mohan, C. Urine protein biomarkers of bladder cancer arising from 16-plex antibody-based screens. Oncotarget 2021, 12, 783–790. [Google Scholar] [CrossRef]
- Verbeke, F.; Siwy, J.; Van Biesen, W.; Mischak, H.; Pletinck, A.; Schepers, E.; Neirynck, N.; Magalhães, P.; Pejchinovski, M.; Pontillo, C.; et al. The urinary proteomics classifier chronic kidney disease 273 predicts cardiovascular outcome in patients with chronic kidney disease. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2021, 36, 811–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, J.; Song, W.; Tian, X.; Liu, Y.; Wang, Y.; Ma, J.; Wang, C.; Yan, G. A proteomic analysis of urine biomarkers in autism spectrum disorder. J. Proteom. 2021, 242, 104259. [Google Scholar] [CrossRef] [PubMed]
- Le, C.; Kunacheva, C.; Stuckey, D.C. “Protein” Measurement in Biological Wastewater Treatment Systems: A Critical Evaluation. Environ. Sci. Technol. 2016, 50, 3074–3081. [Google Scholar] [CrossRef]
- Westgate, P.J.; Park, C. Evaluation of Proteins and Organic Nitrogen in Wastewater Treatment Effluents. Environ. Sci. Technol. 2010, 44, 5352–5357. [Google Scholar] [CrossRef]
- Chutipongtanate, S.; Changtong, C.; Weeraphan, C.; Hongeng, S.; Srisomsap, C.; Svasti, J. Syringe-push membrane absorption as a simple rapid method of urine preparation for clinical proteomics. Clin. Proteom. 2015, 12, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, L.; Liu, X.; Liu, L.; Li, M.; Gao, Y. Urimem, a membrane that can store urinary proteins simply and economically, makes the large-scale storage of clinical samples possible. Sci. China Life Sci. 2014, 57, 336–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial bio-nanocomposites and their potential applications in food packaging. Food Control. 2020, 112, 107086. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.H.; Yu, J.Y. Preparation and morphology of poly(butylene succinate) nanofibers via electrospinning. Fibres Text. East. Eur. 2007, 15, 30–33. [Google Scholar]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Ko, F.K.; Wan, Y. Introduction to Nanofiber Materials; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Zhang, Y.; Wu, C.; Guo, S.; Zhang, J. Interactions of graphene and graphene oxide with proteins and peptides. Nanotechnol. Rev. 2013, 2, 27–45. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Platnieks, O.; Gaidukovs, S.; Neibolts, N.; Barkane, A.; Gaidukova, G.; Thakur, V.K. Poly(butylene succinate) and graphene nanoplatelet–based sustainable functional nanocomposite materials: Structure-properties relationship. Mater. Today Chem. 2020, 18, 100351. [Google Scholar] [CrossRef]
- Huang, L.; Arena, J.T.; McCutcheon, J.R. Surface modified PVDF nanofiber supported thin film composite membranes for forward osmosis. J. Membr. Sci. 2016, 499, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Grafe, T.; Graham, K. Polymeric Nanofibers and Nanofiber Webs: A New Class of Nonwovens. Int. Nonwovens J. 2003, os-12, 1558925003os-1551200113. [Google Scholar] [CrossRef] [Green Version]
- Nematollahzadeh, A.; Shojaei, A.; Abdekhodaie, M.J.; Sellergren, B. Molecularly imprinted polydopamine nano-layer on the pore surface of porous particles for protein capture in HPLC column. J. Colloid Interface Sci. 2013, 404, 117–126. [Google Scholar] [CrossRef] [PubMed]
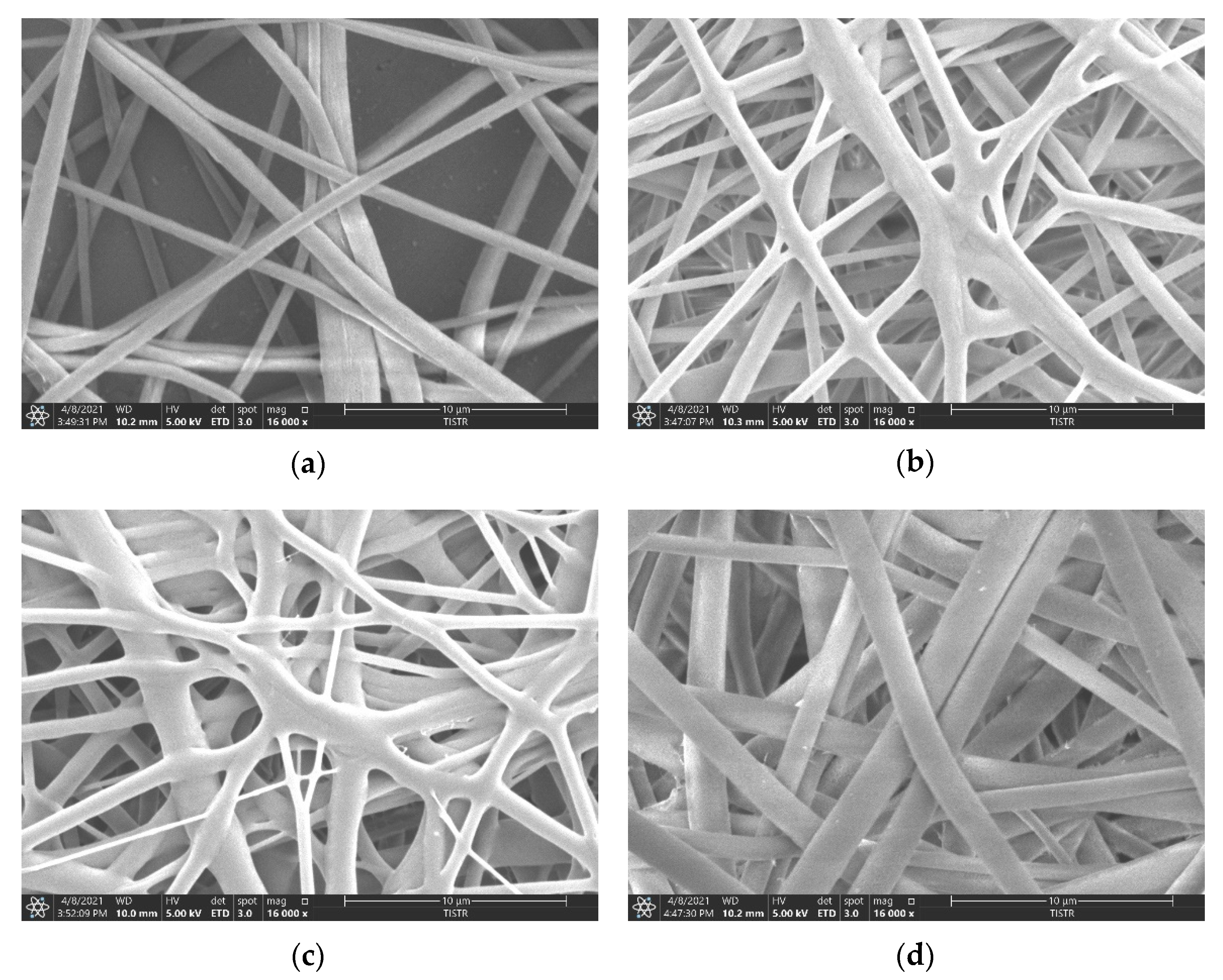
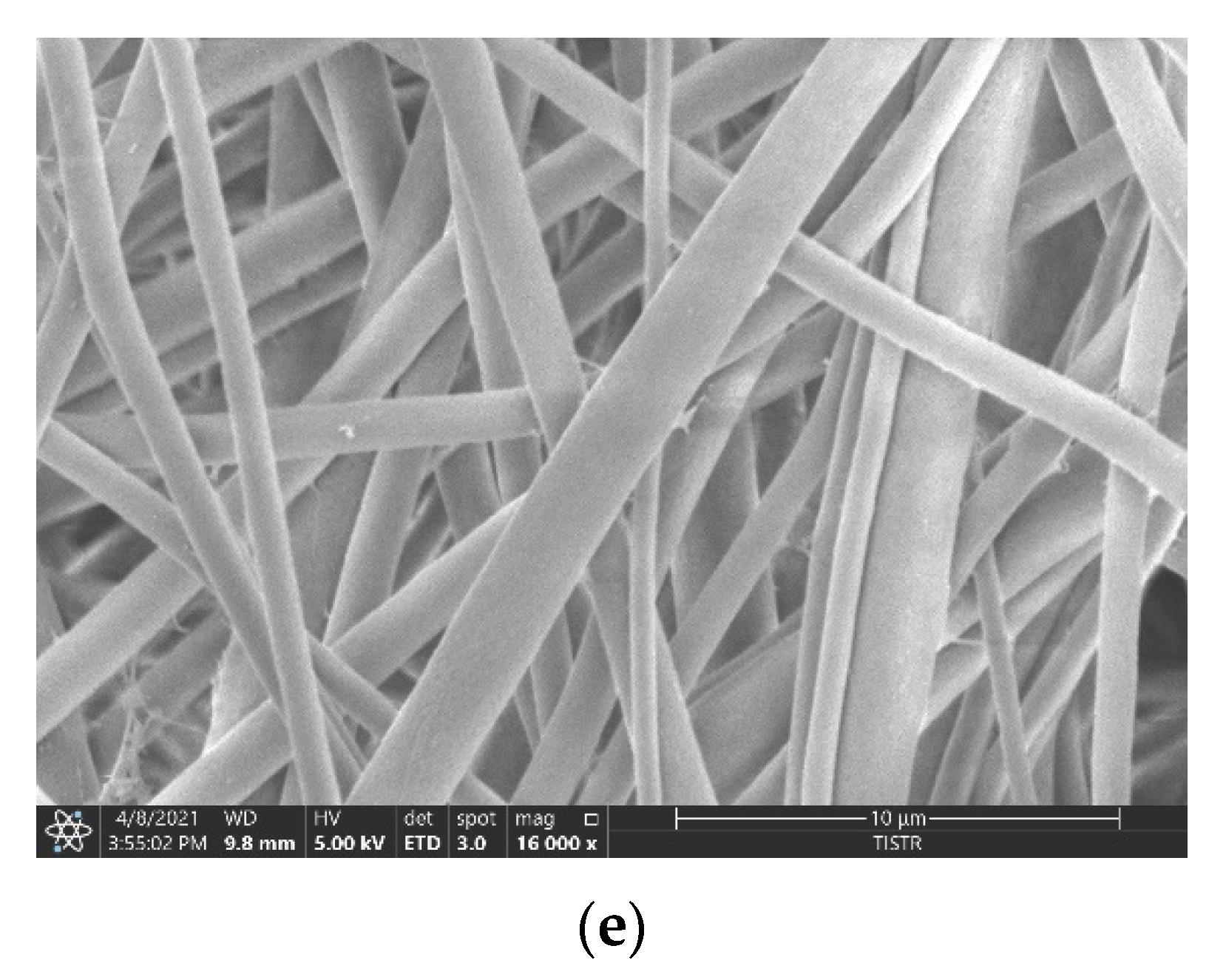
| Sample | Thickness (μm) | Young’s Modulus (MPa) | Max. Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|
| PBS (RE) | 30.8 ± 7.9 | 193 ± 3.0 | 24 ± 3.6 | 58 ± 6.5 |
| PBS + GO 0.1% (RE) | 45.2 ± 6.9 | 170 ± 2.0 | 31 ± 4.6 | 50 ± 2.6 |
| PVDF (NRE) | 116.8 ± 47.3 | 143 ± 5.6 | 18 ± 3.6 | 61 ± 7.2 |
| PVDF + GO 0.1% (NRE) | 87.6 ± 47.3 | 101 ± 2.6 | 24 ± 2.0 | 53 ± 5.2 |
| PVDF + GO 0.1% (RE) | 82.4 ± 3.2 | 181 ± 2.0 | 32 ± 4.0 | 57 ± 5.2 |
| Nitrocellulose (commercial) | 140.4 ± 1.0 | 4.8 ± 0.6 | 0.18 ± 0.1 | 9 ± 2.6 |
| PVDF (commercial) | 120.2 ± 1.0 | 5.3 ± 1.2 | 0.12 ± 0.1 | 9 ± 3.5 |
| Sample | Average Diameter of Fibers (μm) | Average Pore Area (μm2) |
|---|---|---|
| PBS (RE) | 1.00 ± 0.27 | 27.01 ± 4.48 |
| PBS + GO 0.1% (RE) | 0.87 ± 0.16 | 23.32 ± 2.98 |
| PVDF (NRE) | 0.90 ± 0.29 | 36.26 ± 5.86 |
| PVDF + GO 0.1% (NRE) | 1.17 ± 0.22 | 37.48 ± 6.25 |
| PVDF + GO 0.1% (RE) | 1.05 ± 0.22 | 33.29 ± 8.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sathirapongsasuti, N.; Panaksri, A.; Boonyagul, S.; Chutipongtanate, S.; Tanadchangsaeng, N. Electrospun Fibers of Polybutylene Succinate/Graphene Oxide Composite for Syringe-Push Protein Absorption Membrane. Polymers 2021, 13, 2042. https://doi.org/10.3390/polym13132042
Sathirapongsasuti N, Panaksri A, Boonyagul S, Chutipongtanate S, Tanadchangsaeng N. Electrospun Fibers of Polybutylene Succinate/Graphene Oxide Composite for Syringe-Push Protein Absorption Membrane. Polymers. 2021; 13(13):2042. https://doi.org/10.3390/polym13132042
Chicago/Turabian StyleSathirapongsasuti, Nuankanya, Anuchan Panaksri, Sani Boonyagul, Somchai Chutipongtanate, and Nuttapol Tanadchangsaeng. 2021. "Electrospun Fibers of Polybutylene Succinate/Graphene Oxide Composite for Syringe-Push Protein Absorption Membrane" Polymers 13, no. 13: 2042. https://doi.org/10.3390/polym13132042
APA StyleSathirapongsasuti, N., Panaksri, A., Boonyagul, S., Chutipongtanate, S., & Tanadchangsaeng, N. (2021). Electrospun Fibers of Polybutylene Succinate/Graphene Oxide Composite for Syringe-Push Protein Absorption Membrane. Polymers, 13(13), 2042. https://doi.org/10.3390/polym13132042







