Solution-Based Processing for Scaffold Fabrication in Tissue Engineering Applications: A Brief Review
Abstract
:1. Introduction
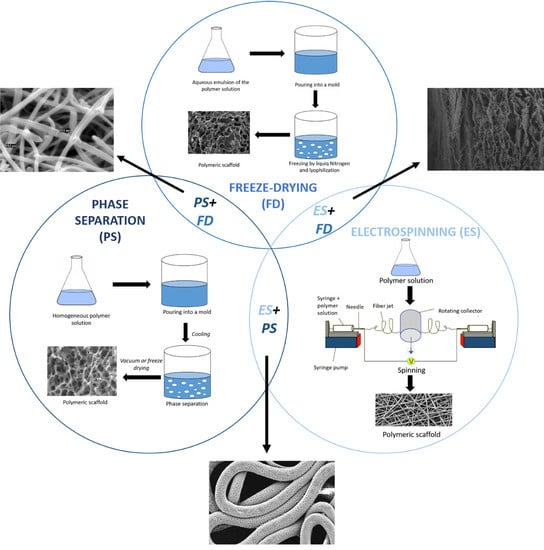
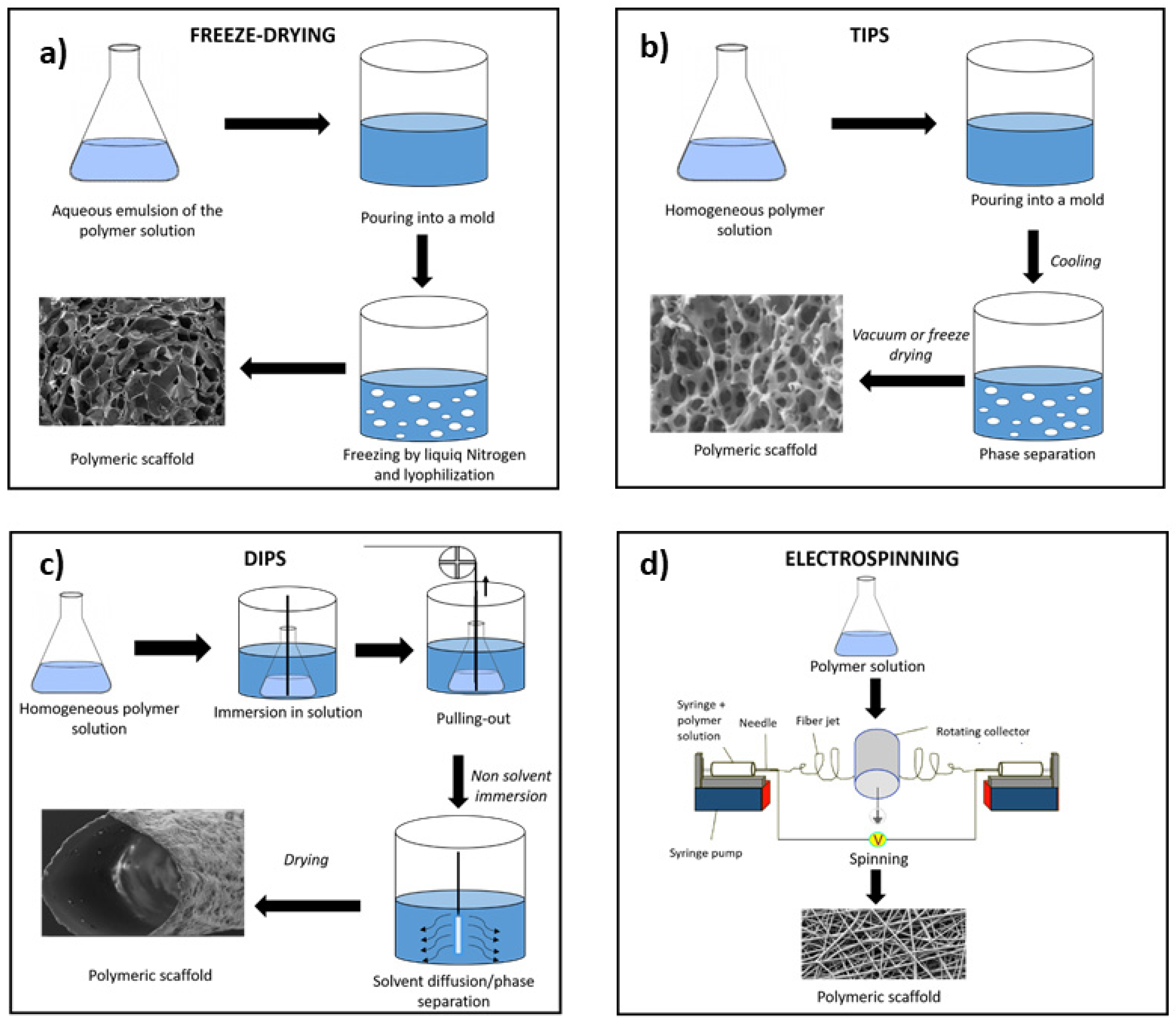
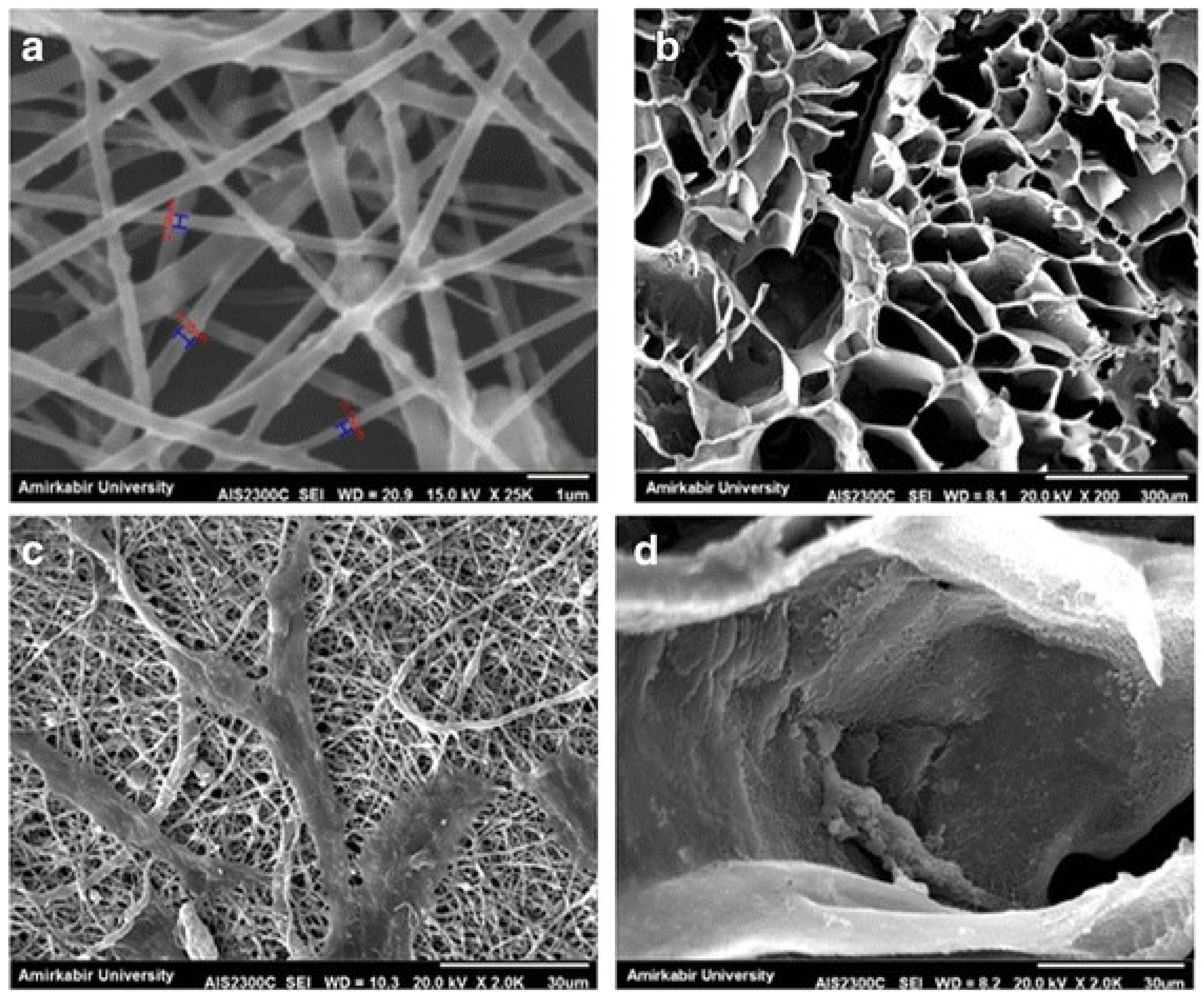
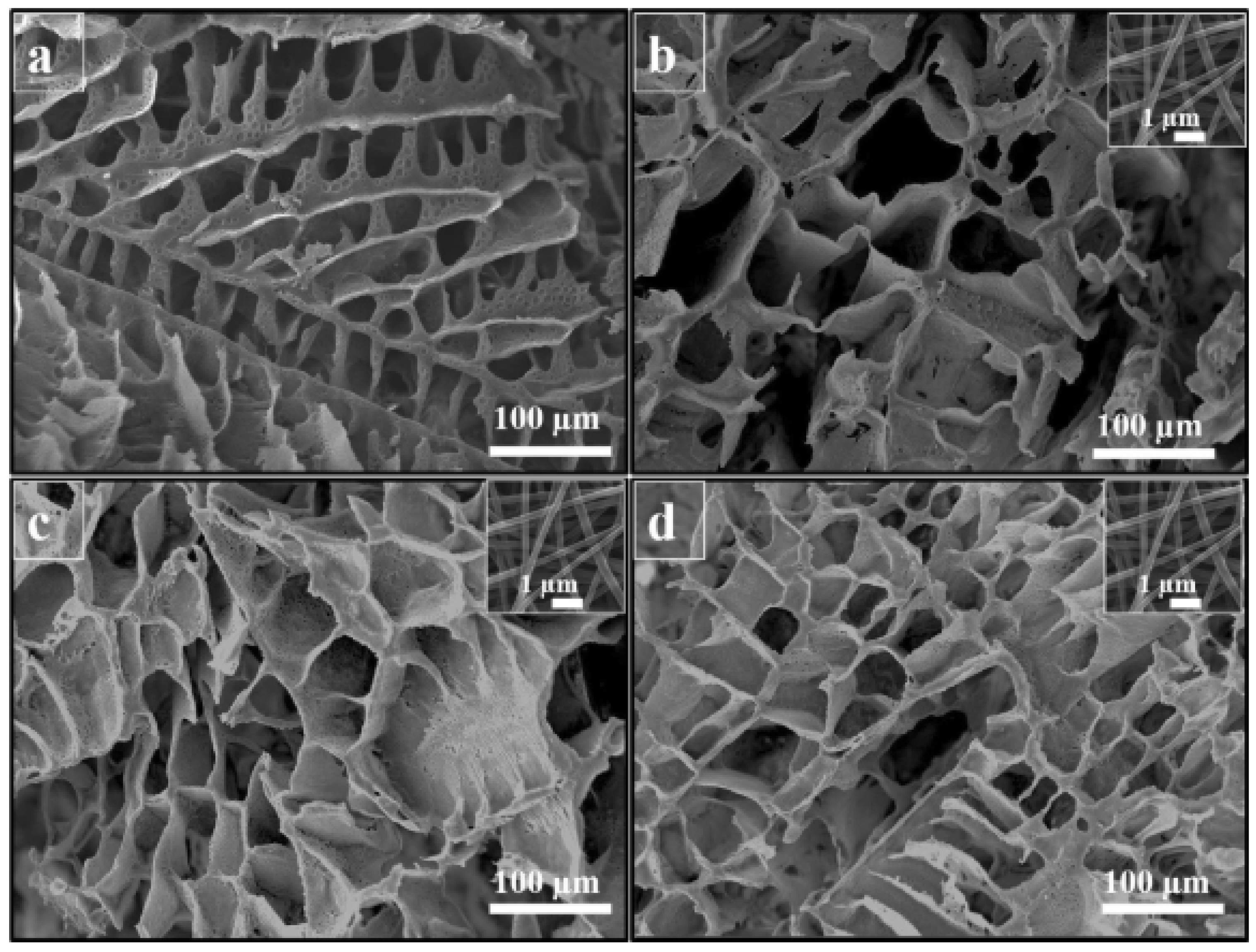
2. Freeze-Drying
3. Phase Separation
3.1. DIPS
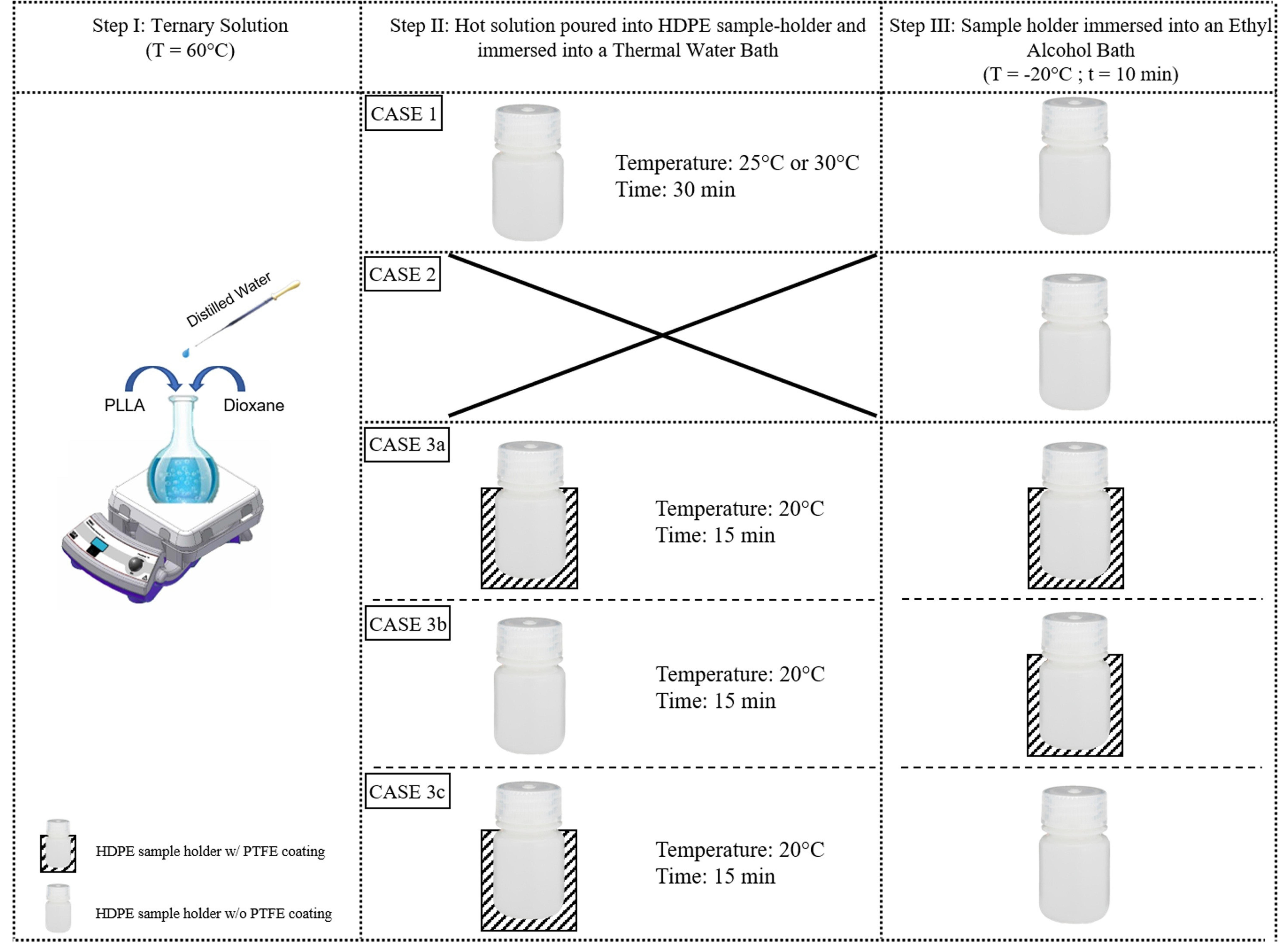
3.2. TIPS
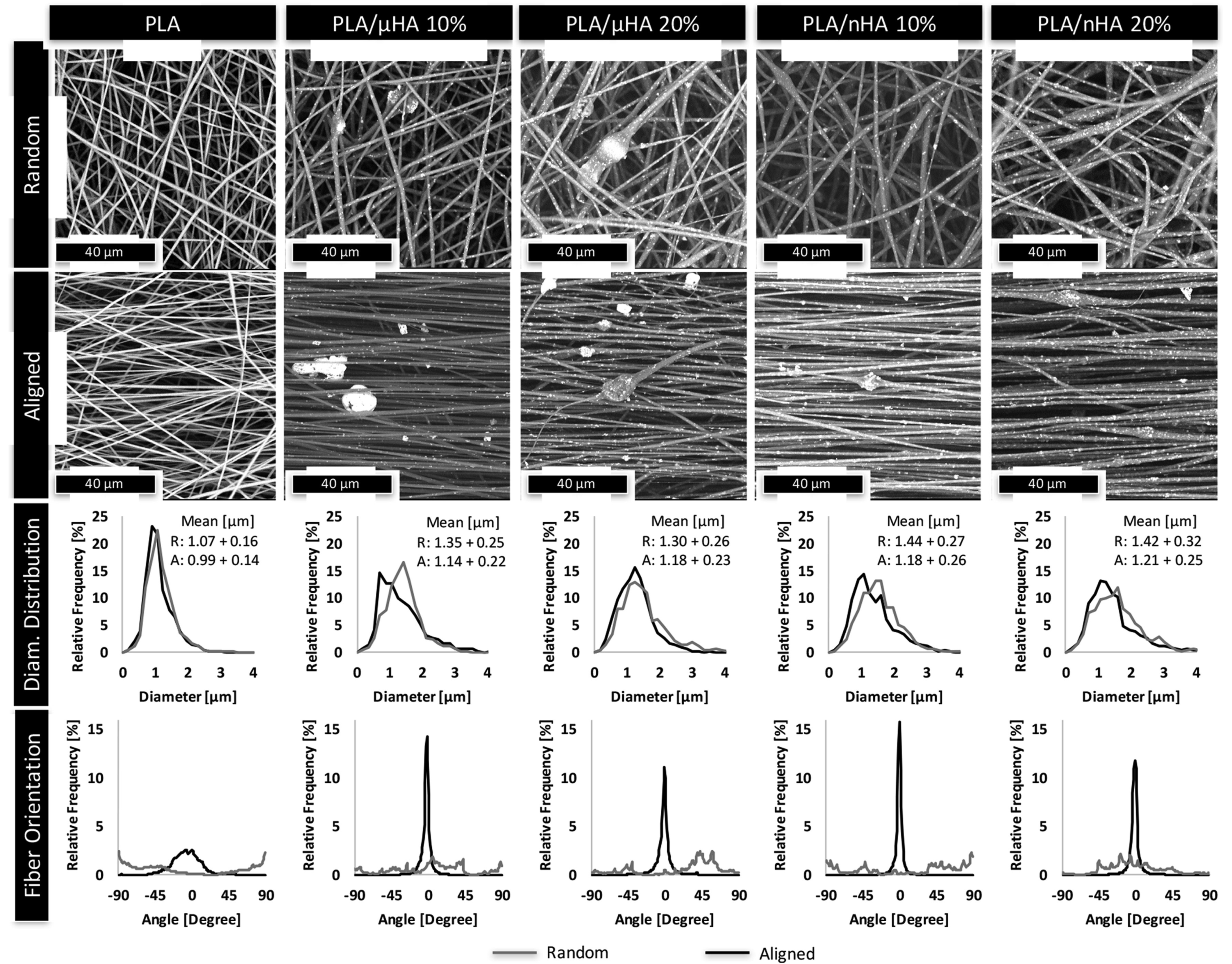
4. Electrospinning
5. Combination of Solution-Based Techniques
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scaffaro, R.; Lopresti, F.; Botta, L.; Rigogliuso, S.; Ghersi, G. Integration of PCL and PLA in a monolithic porous scaffold for interface tissue engineering. J. Mech. Behav. Biomed. Mater. 2016, 63, 303–313. [Google Scholar] [CrossRef]
- Donate, R.; Monzón, M.; Alemán-Domínguez, M.E. Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties. E-Polymers 2020, 20, 571–599. [Google Scholar] [CrossRef]
- Courtenay, J.; Sharma, R.; Scott, J. Recent Advances in Modified Cellulose for Tissue Culture Applications. Molecules 2018, 23, 654. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Leong, K.-F.E.; Du, Z.M.E.; Chua, C.-K. The Design of Scaffolds for Use in Tissue Engineering. Part I. Traditional Factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef] [Green Version]
- Deb, P.; Deoghare, A.B.; Borah, A.; Barua, E.; Das Lala, S. Scaffold Development Using Biomaterials: A Review. Mater. Today Proc. 2018, 5, 12909–12919. [Google Scholar] [CrossRef]
- Gupta, S.; Bissoyi, A.; Bit, A. A Review on 3D Printable Techniques for Tissue Engineering. Bionanoscience 2018, 8, 868–883. [Google Scholar] [CrossRef]
- Bisht, B.; Hope, A.; Mukherjee, A.; Paul, M.K. Advances in the Fabrication of Scaffold and 3D Printing of Biomimetic Bone Graft. Ann. Biomed. Eng. 2021, 49, 1129–1150. [Google Scholar] [CrossRef]
- Norouzi, S.K.; Shamloo, A. Bilayered heparinized vascular graft fabricated by combining electrospinning and freeze drying methods. Mater. Sci. Eng. C 2019, 94, 1067–1076. [Google Scholar] [CrossRef]
- Erickson, A.E.; Sun, J.; Lan Levengood, S.K.; Swanson, S.; Chang, F.C.; Tsao, C.T.; Zhang, M. Chitosan-based composite bilayer scaffold as an in vitro osteochondral defect regeneration model. Biomed. Microdevices 2019, 21, 1–16. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, Y.; Liu, R.; Tian, C. Preparation and characterization of a novel polylactic acid/hydroxyapatite composite scaffold with biomimetic micro-nanofibrous porous structure. J. Mater. Sci. Mater. Med. 2020, 31, 1–11. [Google Scholar] [CrossRef]
- Guo, R.; Chen, S.; Xiao, X. Fabrication and characterization of poly (ethylenimine) modified poly (l-lactic acid) nanofibrous scaffolds. J. Biomater. Sci. Polym. Ed. 2019, 30, 1523–1541. [Google Scholar] [CrossRef]
- Salehi, M.; Bastami, F.; Rezai Rad, M.; Nokhbatolfoghahaei, H.; Paknejad, Z.; Nazeman, P.; Hassani, A.; Khojasteh, A. Investigation of cell-free poly lactic acid/nanoclay scaffolds prepared via thermally induced phase separation technique containing hydroxyapatite nanocarriers of erythropoietin for bone tissue engineering applications. Polym. Adv. Technol. 2021, 32, 670–680. [Google Scholar] [CrossRef]
- McKenna, E.; Klein, T.J.; Doran, M.R.; Futrega, K. Integration of an ultra-strong poly(lactic-co-glycolic acid) (PLGA) knitted mesh into a thermally induced phase separation (TIPS) PLGA porous structure to yield a thin biphasic scaffold suitable for dermal tissue engineering. Biofabrication 2020, 12, 015015. [Google Scholar] [CrossRef] [Green Version]
- de Souza, L.; Alavarse, A.C.; da Vinci, M.A.; Bonvent, J.J. The Synergistic Effect of Polymer Composition, Solvent Volatility, and Collector Distance on Pullulan and PVA Fiber Production by Rotary Jet Spinning. Fibers Polym. 2021, 22, 942–956. [Google Scholar] [CrossRef]
- Campiglio, C.E.; Contessi Negrini, N.; Farè, S.; Draghi, L. Cross-Linking Strategies for Electrospun Gelatin Scaffolds. Materials 2019, 12, 2476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Wu, Z.; Zhou, Y.; Li, L.; Wang, Y.; Wang, Z.; Chen, Y.; Zhang, P. Biomimetic porous collagen/hydroxyapatite scaffold for bone tissue engineering. J. Appl. Polym. Sci. 2017, 134, 45271. [Google Scholar] [CrossRef]
- Sabzi, E.; Abbasi, F.; Ghaleh, H. Interconnected porous nanofibrous gelatin scaffolds prepared via a combined thermally induced phase separation/particulate leaching method. J. Biomater. Sci. Polym. Ed. 2020, 32, 488–503. [Google Scholar] [CrossRef]
- Andrieux, S.; Drenckhan, W.; Stubenrauch, C. Highly ordered biobased scaffolds: From liquid to solid foams. Polymer 2017, 126, 425–431. [Google Scholar] [CrossRef]
- Ghafari, R.; Jonoobi, M.; Amirabad, L.M.; Oksman, K.; Taheri, A.R. Fabrication and characterization of novel bilayer scaffold from nanocellulose based aerogel for skin tissue engineering applications. Int. J. Biol. Macromol. 2019, 136, 796–803. [Google Scholar] [CrossRef]
- Kang, J.; Hwang, J.Y.; Huh, M.; Yun, S.I. Porous Poly(3-hydroxybutyrate) Scaffolds Prepared by Non-Solvent-Induced Phase Separation for Tissue Engineering. Macromol. Res. 2020, 28, 835–843. [Google Scholar] [CrossRef]
- Salerno, A.; Domingo, C. Pore structure properties of scaffolds constituted by aggregated microparticles of PCL and PCL-HA processed by phase separation. J. Porous Mater. 2015, 22, 425–435. [Google Scholar] [CrossRef]
- Nune, S.K.; Rama, K.S.; Dirisala, V.R.; Chavali, M.Y. Electrospinning of collagen nanofiber scaffolds for tissue repair and regeneration. In Nanostructures for Novel Therapy: Synthesis, Characterization and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 281–311. ISBN 9780323461481. [Google Scholar]
- Lopresti, F.; Keraite, I.; Ongaro, A.E.; Howarth, N.M.; Carrubba, V.L.; Kersaudy-Kerhoas, M. Engineered membranes for residual cell trapping on microfluidic blood plasma separation systems: A comparison between porous and nanofibrous membranes. bioRxiv 2020. [Google Scholar] [CrossRef]
- Miszuk, J.M.; Hu, J.; Sun, H. Biomimetic Nanofibrous 3D Materials for Craniofacial Bone Tissue Engineering. ACS Appl. Bio Mater. 2020, 3, 6538–6545. [Google Scholar] [CrossRef]
- Izadyari Aghmiuni, A.; Heidari Keshel, S.; Sefat, F.; Akbarzadeh Khiyavi, A. Fabrication of 3D hybrid scaffold by combination technique of electrospinning-like and freeze-drying to create mechanotransduction signals and mimic extracellular matrix function of skin. Mater. Sci. Eng. C 2021, 120, 111752. [Google Scholar] [CrossRef] [PubMed]
- Sin, D.; Fitzpatrick, J.; Luckman, P.; Wolvetang, E.J.; Cooper-White, J.J. Fabrication of nanopatterned, porous microspheres using a glass capillary microfluidic device. Soft Matter 2012, 8, 10712–10718. [Google Scholar] [CrossRef]
- Gao, J.; Chung, T.S. Membranes made from nonsolvent-thermally induced phase separation (N-TIPS) for decellularization of blood in dry plasma spot (DPS) applications. Chem. Eng. Sci. 2021, 229, 116010. [Google Scholar] [CrossRef]
- Li, Y.; Yang, S.T. Effects of three-dimensional scaffolds on cell organization and tissue development. Biotechnol. Bioprocess Eng. 2001, 6, 311–325. [Google Scholar] [CrossRef]
- Fu, N.; Zhang, X.; Sui, L.; Liu, M.; Lin, Y. Application of Scaffold Materials in Cartilage Tissue Engineering. In Cartilage Regeneration, Stem Cell Biology and Regenerative Medicine; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2017; pp. 21–39. [Google Scholar]
- Keeney, M.; Pandit, A. The osteochondral junction and its repair via bi-phasic tissue engineering scaffolds. Tissue Eng. Part B Rev. 2009, 15, 55–73. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, T.H.; Im, G., II; Lee, J.H. Investigation of pore size effect on chondrogenic differentiation of adipose stem cells using a pore size gradient scaffold. Biomacromolecules 2010, 11, 1948–1955. [Google Scholar] [CrossRef]
- Eberli, D. Tissue Engineering for Tissue and Organ Regeneration; InTech: Rijeka, Croatia, 2011; ISBN 9789533076881. [Google Scholar]
- Vacanti, J.P.; Langer, R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 1999, 354, S32–S34. [Google Scholar] [CrossRef]
- Serbo, J.V.; Gerecht, S. Vascular tissue engineering: Biodegradable scaffold platforms to promote angiogenesis. Stem Cell Res. Ther. 2013, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ou, S.-F.; Tsao, Y.-L.; Lin, W.-C.; Wang, Y.-T.; Wang, L.; Fan, F.-Y. Novel Epigallocatechin-3-Gallate (EGCG)-Loaded Mesoporous Bioglass Scaffolds for Bone Recruitment Applications. Appl. Sci. 2020, 11, 243. [Google Scholar] [CrossRef]
- Liang, C.; Luo, Y.; Yang, G.; Xia, D.; Liu, L.; Zhang, X.; Wang, H. Graphene Oxide Hybridized nHAC/PLGA Scaffolds Facilitate the Proliferation of MC3T3-E1 Cells. Nanoscale Res. Lett. 2018, 13, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-Based Composite Scaffold Matrices for Tissue Engineering Applications. Mol. Biotechnol. 2018, 60, 506–532. [Google Scholar] [CrossRef]
- Fereshteh, Z. Freeze-drying technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds: Materials, Technologies, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 151–174. ISBN 9780081009802. [Google Scholar]
- Ranganathan, N.; Mugeshwaran, A.; Bensingh, R.J.; Kader, M.A.; Nayak, S.K. Biopolymeric scaffolds for tissue engineering application. In Biomedical Engineering and Its Applications in Healthcare; Springer: Singapore, 2019; pp. 249–274. ISBN 9789811337055. [Google Scholar]
- Brougham, C.M.; Levingstone, T.J.; Shen, N.; Cooney, G.M.; Jockenhoevel, S.; Flanagan, T.C.; O’Brien, F.J. Freeze-Drying as a Novel Biofabrication Method for Achieving a Controlled Microarchitecture within Large, Complex Natural Biomaterial Scaffolds. Adv. Healthc. Mater. 2017, 6, 1700598. [Google Scholar] [CrossRef] [PubMed]
- Grenier, J.; Duval, H.; Barou, F.; Lv, P.; David, B.; Letourneur, D. Mechanisms of pore formation in hydrogel scaffolds textured by freeze-drying. Acta Biomater. 2019, 94, 195–203. [Google Scholar] [CrossRef]
- Valencia, C.; Valencia, C.; Zuluaga, F.; Valencia, M.; Mina, J.; Grande-Tovar, C. Synthesis and Application of Scaffolds of Chitosan-Graphene Oxide by the Freeze-Drying Method for Tissue Regeneration. Molecules 2018, 23, 2651. [Google Scholar] [CrossRef] [Green Version]
- Mesgar, A.S.; Mohammadi, Z.; Khosrovan, S. Improvement of mechanical properties and in vitro bioactivity of freeze-dried gelatin/chitosan scaffolds by functionalized carbon nanotubes. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 267–276. [Google Scholar] [CrossRef]
- Zhai, C.; Fei, H.; Hu, J.; Wang, Z.; Xu, S.; Zuo, Q.; Li, Z.; Wang, Z.; Liang, W.; Fan, W. Repair of Articular Osteochondral Defects Using an Integrated and Biomimetic Trilayered Scaffold. Tissue Eng. Part A 2018, 24, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Bonde, M.; Srinivasan, G. Biodegradable polymer scaffold for tissue engineering. Trends Biomater. Artif. Organs 2011, 25, 20–29. [Google Scholar]
- Dattola, E.; Parrotta, E.I.; Scalise, S.; Perozziello, G.; Limongi, T.; Candeloro, P.; Coluccio, M.L.; Maletta, C.; Bruno, L.; De Angelis, M.T.; et al. Development of 3D PVA scaffolds for cardiac tissue engineering and cell screening applications. RSC Adv. 2019, 9, 4246–4257. [Google Scholar] [CrossRef] [Green Version]
- Mozafari, M.; Kargozar, S.; de Santiago, G.T.; Mohammadi, M.R.; Milan, P.B.; Foroutan Koudehi, M.; Aghabarari, B.; Nourani, M.R. Synthesis and characterisation of highly interconnected porous poly(ε-caprolactone)-collagen scaffolds: A therapeutic design to facilitate tendon regeneration. Mater. Technol. 2018, 33, 29–37. [Google Scholar] [CrossRef]
- Namini, M.S.; Bayat, N.; Tajerian, R.; Ebrahimi-Barough, S.; Azami, M.; Irani, S.; Jangjoo, S.; Shirian, S.; Ai, J. A comparison study on the behavior of human endometrial stem cell-derived osteoblast cells on PLGA/HA nanocomposite scaffolds fabricated by electrospinning and freeze-drying methods. J. Orthop. Surg. Res. 2018, 13, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haugh, M.G.; Murphy, C.M.; O’Brien, F.J. Novel freeze-drying methods to produce a range of collagen- glycosaminoglycan scaffolds with tailored mean pore sizes. Tissue Eng. Part C Methods 2010, 16, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Barba, I. Scaffold Designing. In Bio-Ceramics with Clinical Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2014; Volume 9781118406, pp. 291–313. [Google Scholar]
- Liu, S.; Zheng, Y.; Hu, J.; Wu, Z.; Chen, H. Fabrication and characterization of polylactic acid/polycaprolactone composite macroporous micro-nanofiber scaffolds by phase separation. New J. Chem. 2020, 44, 17382–17390. [Google Scholar] [CrossRef]
- Zeng, S.; Cui, Z.; Yang, Z.; Si, J.; Wang, Q.; Wang, X.; Peng, K.; Chen, W. Characterization of highly interconnected porous poly(lactic acid) and chitosan-coated poly(lactic acid) scaffold fabricated by vacuum-assisted resin transfer molding and particle leaching. J. Mater. Sci. 2016, 51, 9958–9970. [Google Scholar] [CrossRef]
- Lu, T.; Li, Y.; Chen, T. Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. Int. J. Nanomed. 2013, 8, 337–350. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Abedalwafa, M.; Wang, F.; Li, C. Biodegradable Poly-Epsilon-Caprolactone (PCL) for tissue Engineering Applications: A Review. Rev. Adv. Mater. Sci 2013, 34, 123–140. [Google Scholar]
- la Carrubba, V.; Pavia, F.C.; Brucato, V. Tubular scaffold for vascular tissue engineering application. Int. J. Mater. Form. 2010, 3, 567–570. [Google Scholar] [CrossRef]
- Geven, M.A.; Lapomarda, A.; Guillaume, O.; Sprecher, C.M.; Eglin, D.; Vozzi, G.; Grijpma, D.W. Osteogenic differentiation of hBMSCs on porous photo-crosslinked poly(trimethylene carbonate) and nano-hydroxyapatite composites. Eur. Polym. J. 2021, 147, 110335. [Google Scholar] [CrossRef]
- Conoscenti, G.; Schneider, T.; Stoelzel, K.; Carfì Pavia, F.; Brucato, V.; Goegele, C.; La Carrubba, V.; Schulze-Tanzil, G. PLLA scaffolds produced by thermally induced phase separation (TIPS) allow human chondrocyte growth and extracellular matrix formation dependent on pore size. Mater. Sci. Eng. C 2017, 80, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Thavornyutikarn, B.; Chantarapanich, N.; Sitthiseripratip, K.; Thouas, G.A.; Chen, Q. Bone tissue engineering scaffolding: Computer-aided scaffolding techniques. Prog. Biomater. 2014, 3, 61–102. [Google Scholar] [CrossRef] [Green Version]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M.V. Preparation and characterization of membranes formed by nonsolvent induced phase separation: A review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Montesanto, S.; Mannella, G.A.; Carfì Pavia, F.; La Carrubba, V.; Brucato, V. Coagulation bath composition and desiccation environment as tuning parameters to prepare skinless membranes via diffusion induced phase separation. J. Appl. Polym. Sci. 2015, 132, 42151. [Google Scholar] [CrossRef]
- Gangolli, R.A.; Devlin, S.M.; Gerstenhaber, J.A.; Lelkes, P.I.; Yang, M. A Bilayered Poly (Lactic-Co-Glycolic Acid) Scaffold Provides Differential Cues for the Differentiation of Dental Pulp Stem Cells. Tissue Eng. Part A 2019, 25, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Rezabeigi, E.; Wood-Adams, P.M.; Drew, R.A.L. Morphological examination of highly porous polylactic acid/Bioglass scaffolds produced via nonsolvent induced phase separation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2433–2442. [Google Scholar] [CrossRef]
- Montesanto, S.; Smithers, N.P.; Bucchieri, F.; Brucato, V.; Carrubba, V.L.; Davies, D.E.; Conforti, F. Establishment of a pulmonary epithelial barrier on biodegradable poly-L-lactic-acid membranes. PLoS ONE 2019, 14, e0210830. [Google Scholar] [CrossRef]
- Kasoju, N.; Hawkins, N.; Pop-Georgievski, O.; Kubies, D.; Vollrath, F. Silk fibroin gelation via non-solvent induced phase separation. Biomater. Sci. 2016, 4, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Wittmar, A.S.M.; Koch, D.; Prymak, O.; Ulbricht, M. Factors Affecting the Nonsolvent-Induced Phase Separation of Cellulose from Ionic Liquid-Based Solutions. ACS Omega 2020, 5, 27314–27322. [Google Scholar] [CrossRef]
- Figoli, A.; De Luca, G.; Longavita, E.; Drioli, E. PEEKWC Capsules Prepared by Phase Inversion Technique: A Morphological and Dimensional Study. Sep. Sci. Technol. 2007, 42, 2809–2827. [Google Scholar] [CrossRef]
- Jung, J.T.; Kim, J.F.; Wang, H.H.; Di Nicolo, E.; Drioli, E.; Lee, Y.M. Understanding the non-solvent induced phase separation (NIPS) effect during the fabrication of microporous PVDF membranes via thermally induced phase separation (TIPS). J. Memb. Sci. 2016, 514, 250–263. [Google Scholar] [CrossRef]
- Lombardo, M.E.; Carfì Pavia, F.; Vitrano, I.; Ghersi, G.; Brucato, V.; Rosei, F.; La Carrubba, V. PLLA scaffolds with controlled architecture as potential microenvironment for in vitro tumor model. Tissue Cell 2019, 58, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yang, F.; Li, C.; Wang, F.; Zhu, H.; Guo, Y. Effect of the molecular weight of polymer and diluent on the performance of hydrophilic poly(vinyl butyral) porous heddle via thermally induced phase separation. Mater. Chem. Phys. 2021, 261, 124227. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Y.; Liu, Y.; Pan, Y.; Yao, Q. Novel three-dimensional bioglass functionalized gelatin nanofibrous scaffolds for bone regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Farzamfar, S.; Naseri-Nosar, M.; Sahrapeyma, H.; Ehterami, A.; Goodarzi, A.; Rahmati, M.; Ahmadi Lakalayeh, G.; Ghorbani, S.; Vaez, A.; Salehi, M. Tetracycline hydrochloride-containing poly (ε-caprolactone)/poly lactic acid scaffold for bone tissue engineering application: In vitro and in vivo study. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 472–479. [Google Scholar] [CrossRef]
- Gupte, M.J.; Swanson, W.B.; Hu, J.; Jin, X.; Ma, H.; Zhang, Z.; Liu, Z.; Feng, K.; Feng, G.; Xiao, G.; et al. Pore Size Directs Bone Marrow Stromal Cell Fate and Tissue Regeneration in Nanofibrous Macroporous Scaffolds by Mediating Vascularization. Acta Biomater. 2018, 82, 1–11. [Google Scholar] [CrossRef]
- Díaz, E.; Aresti, J.; León, J. Evaluation of physicochemical and mechanical properties with the in vitro degradation of PCL/nHA/MWCNT composite scaffolds. J. Reinf. Plast. Compos. 2020, 40, 134–142. [Google Scholar] [CrossRef]
- Heo, S.-J.; Kim, S.-E.; Wei, J.; Hyun, Y.-T.; Yun, H.-S.; Kim, D.-H.; Shin, J.W.; Shin, J.-W. Fabrication and characterization of novel nano- and micro-HA/PCL composite scaffolds using a modified rapid prototyping process. J. Biomed. Mater. Res. Part A 2008, 9999A. [Google Scholar] [CrossRef]
- Carfì Pavia, F.; Conoscenti, G.; Greco, S.; La Carrubba, V.; Ghersi, G.; Brucato, V. Preparation, characterization and in vitro test of composites poly-lactic acid/hydroxyapatite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2018, 119, 945–953. [Google Scholar] [CrossRef]
- Carfì Pavia, F.; Palumbo, F.S.; La Carrubba, V.; Bongiovì, F.; Brucato, V.; Pitarresi, G.; Giammona, G. Modulation of physical and biological properties of a composite PLLA and polyaspartamide derivative obtained via thermally induced phase separation (TIPS) technique. Mater. Sci. Eng. C 2016, 67, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Zeinali, R.; Del Valle, L.J.; Torras, J.; Puiggalí, J. Recent progress on biodegradable tissue engineering scaffolds prepared by thermally-induced phase separation (Tips). Int. J. Mol. Sci. 2021, 22, 3504. [Google Scholar] [CrossRef]
- Lou, T.; Wang, X.; Yan, X.; Miao, Y.; Long, Y.Z.; Yin, H.L.; Sun, B.; Song, G. Fabrication and biocompatibility of poly(l-lactic acid) and chitosan composite scaffolds with hierarchical microstructures. Mater. Sci. Eng. C 2016, 64, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Balakrishnan, N.T.M.; Joyner, J.D.; Medhavi, N.; Manaf, O.; Jabeen Fatima, M.J.; Ahn, J.-H.; Ali, W.; Prasanth, R. Electrospinning: The State of Art Technique for the Production of Nanofibers and Nanofibrous Membranes for Advanced Engineering Applications; Springer: Singapore, 2021; pp. 23–71. [Google Scholar]
- Lopresti, F.; Pavia, F.C.; Ceraulo, M.; Capuana, E.; Brucato, V.; Ghersi, G.; Botta, L.; La Carrubba, V. Physical and biological properties of electrospun poly(d,l-lactide)/nanoclay and poly(d,l-lactide)/nanosilica nanofibrous scaffold for bone tissue engineering. J. Biomed. Mater. Res. Part A 2021. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M. Electrospun multifunctional tissue engineering scaffolds. Front. Mater. Sci. 2014, 8, 3–19. [Google Scholar] [CrossRef]
- Koyyada, A.; Orsu, P. Recent Advancements and Associated Challenges of Scaffold Fabrication Techniques in Tissue Engineering Applications. Regen. Eng. Transl. Med. 2020, 1–13. [Google Scholar] [CrossRef]
- Du, G.Y.; He, S.W.; Sun, C.X.; Mi, L.D. Bone Morphogenic Protein-2 (rhBMP2)-Loaded Silk Fibroin Scaffolds to Enhance the Osteoinductivity in Bone Tissue Engineering. Nanoscale Res. Lett. 2017, 12, 573. [Google Scholar] [CrossRef] [Green Version]
- Smoak, M.M.; Han, A.; Watson, E.; Kishan, A.; Grande-Allen, K.J.; Cosgriff-Hernandez, E.; Mikos, A.G. Fabrication and characterization of electrospun decellularized muscle-derived scaffolds. Tissue Eng. Part C Methods 2019, 25, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; He, L.; Yang, R.; Chen, B.; Xie, X.; Jiang, B.; Weidong, T.; Ding, Y. Enhanced effects of electrospun collagen-chitosan nanofiber membranes on guided bone regeneration. J. Biomater. Sci. Polym. Ed. 2020, 31, 155–168. [Google Scholar] [CrossRef]
- Sandri, G.; Rossi, S.; Bonferoni, M.C.; Miele, D.; Faccendini, A.; Del Favero, E.; Di Cola, E.; Icaro Cornaglia, A.; Boselli, C.; Luxbacher, T.; et al. Chitosan/glycosaminoglycan scaffolds for skin reparation. Carbohydr. Polym. 2019, 220, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Wongkanya, R.; Chuysinuan, P.; Pengsuk, C.; Techasakul, S.; Lirdprapamongkol, K.; Svasti, J.; Nooeaid, P. Electrospinning of alginate/soy protein isolated nanofibers and their release characteristics for biomedical applications. J. Sci. Adv. Mater. Devices 2017, 2, 309–316. [Google Scholar] [CrossRef]
- Salah, N.A. In Vitro Bronchial Mucosa Model Using Air-Liquid Interface Culture on PLLA Electrospun Membrane; European Society for Biomaterials (ESB): Athens, Greece, 2017. [Google Scholar]
- Shabannejad, M.; Nourbakhsh, M.S.; Salati, A.; Bahrami, Z. Fabrication and Characterization of Electrospun Scaffold Based on Polycaprolactone-Aloe vera and Polyvinyl Alcohol for Skin Tissue Engineering. Fibers Polym. 2020, 21, 1694–1703. [Google Scholar] [CrossRef]
- Lopresti, F.; Carfì Pavia, F.; Vitrano, I.; Kersaudy-Kerhoas, M.; Brucato, V.; La Carrubba, V. Effect of hydroxyapatite concentration and size on morpho-mechanical properties of PLA-based randomly oriented and aligned electrospun nanofibrous mats. J. Mech. Behav. Biomed. Mater. 2020, 101, 103449. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Botta, L. Preparation, characterization and hydrolytic degradation of PLA/PCL co-mingled nanofibrous mats prepared via dual-jet electrospinning. Eur. Polym. J. 2017, 96, 266–277. [Google Scholar] [CrossRef]
- Han, J.H.; Ko, U.H.; Kim, H.J.; Kim, S.; Jeon, J.S.; Shin, J.H. Electrospun Microvasculature for Rapid Vascular Network Restoration. Tissue Eng. Regen. Med. 2021, 18, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, A.; Markatos, D.N.; Mitropoulou, A.; Panagiotopoulos, I.; Koletsis, E.; Mavrilas, D. A novel polymeric fibrous microstructured biodegradable small-caliber tubular scaffold for cardiovascular tissue engineering. J. Mater. Sci. Mater. Med. 2021, 32, 21. [Google Scholar] [CrossRef]
- Christy, P.N.; Basha, S.K.; Kumari, V.S.; Bashir, A.K.H.; Maaza, M.; Kaviyarasu, K.; Arasu, M.V.; Al-Dhabi, N.A.; Ignacimuthu, S. Biopolymeric nanocomposite scaffolds for bone tissue engineering applications—A review. J. Drug Deliv. Sci. Technol. 2020, 55, 101452. [Google Scholar] [CrossRef]
- Tan, G.Z.; Zhou, Y. Electrospinning of biomimetic fibrous scaffolds for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2019, 1–14. [Google Scholar] [CrossRef]
- Calore, A.R.; Sinha, R.; Harings, J.; Bernaerts, K.V.; Mota, C.; Moroni, L. Additive Manufacturing Using Melt Extruded Thermoplastics for Tissue Engineering. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2021; Volume 2147, pp. 75–99. [Google Scholar]
- Gorth, D.; Webster, T.J. Matrices for tissue engineering and regenerative medicine. In Biomaterials for Artificial Organs; Elsevier Inc.: Amsterdam, The Netherlands, 2010; pp. 270–286. ISBN 9781845696535. [Google Scholar]
- Dabouian, A.; Bakhshi, H.; Irani, S.; Pezeshki-Modaress, M. β-Carotene: A natural osteogen to fabricate osteoinductive electrospun scaffolds. RSC Adv. 2018, 8, 9941–9945. [Google Scholar] [CrossRef] [Green Version]
- Carfì Pavia, F.; La Carrubba, V.; Ghersi, G.; Greco, S.; Brucato, V. Double flow bioreactor for in vitro test of drug delivery. Curr. Drug Deliv. 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Montanheiro, T.L.d.A.; Montagna, L.S.; Patrulea, V.; Jordan, O.; Borchard, G.; Lobato, G.M.M.; Catalani, L.H.; Lemes, A.P. Evaluation of cellulose nanocrystal addition on morphology, compression modulus and cytotoxicity of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) scaffolds. J. Mater. Sci. 2019, 54, 7198–7210. [Google Scholar] [CrossRef]
- Matsuzaki, Y.; Iwaki, R.; Reinhardt, J.W.; Chang, Y.C.; Miyamoto, S.; Kelly, J.; Zbinden, J.; Blum, K.; Mirhaidari, G.; Ulziibayar, A.; et al. The effect of pore diameter on neo-tissue formation in electrospun biodegradable tissue-engineered arterial grafts in a large animal model. Acta Biomater. 2020, 115, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Ngadiman, N.; Yusof, N.; Idris, A.; Fallahiarezoudar, E.; Kurniawan, D. Novel Processing Technique to Produce Three Dimensional Polyvinyl Alcohol/Maghemite Nanofiber Scaffold Suitable for Hard Tissues. Polymers 2018, 10, 353. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Zhu, J.; Zhang, H.; You, Z.; Morsi, Y.; Mo, X.; Zhu, T. Facile preparation of a controlled-release tubular scaffold for blood vessel implantation. J. Colloid Interface Sci. 2019, 539, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Samadian, H.; Farzamfar, S.; Vaez, A.; Ehterami, A.; Bit, A.; Alam, M.; Goodarzi, A.; Darya, G.; Salehi, M. A tailored polylactic acid/polycaprolactone biodegradable and bioactive 3D porous scaffold containing gelatin nanofibers and Taurine for bone regeneration. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Geng, M.; Zhang, Q.; Gu, J.; Yang, J.; Du, H.; Jia, Y.; Zhou, X.; He, C. Construction of a nanofiber network within 3D printed scaffolds for vascularized bone regeneration. Biomater. Sci. 2021, 9, 2631–2646. [Google Scholar] [CrossRef]
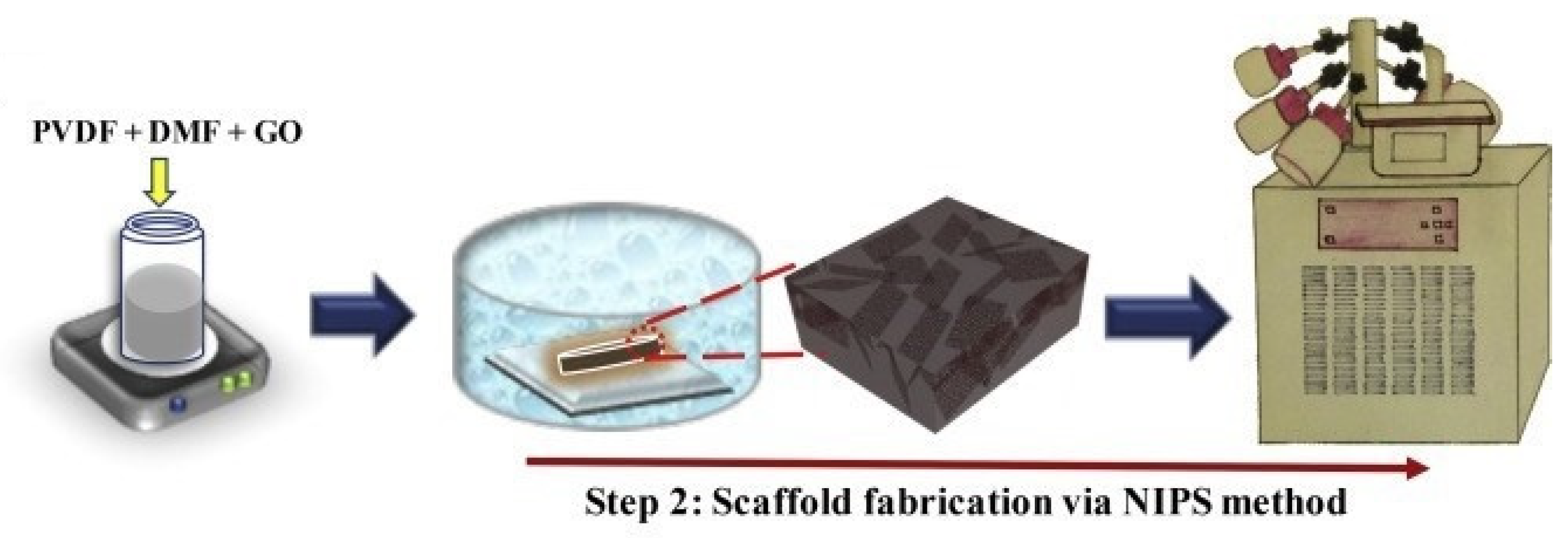
- Abzan, N.; Kharaziha, M.; Labbaf, S. Development of three-dimensional piezoelectric polyvinylidene fluoride-graphene oxide scaffold by non-solvent induced phase separation method for nerve tissue engineering. Mater. Des. 2019, 167, 107636. [Google Scholar] [CrossRef]
- Holland, T.A.; Mikos, A.G. Review: Biodegradable polymeric scaffolds. Improvements in bone tissue engineering through controlled drug delivery. Adv. Biochem. Eng. Biotechnol. 2006, 102, 161–185. [Google Scholar] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capuana, E.; Lopresti, F.; Carfì Pavia, F.; Brucato, V.; La Carrubba, V. Solution-Based Processing for Scaffold Fabrication in Tissue Engineering Applications: A Brief Review. Polymers 2021, 13, 2041. https://doi.org/10.3390/polym13132041
Capuana E, Lopresti F, Carfì Pavia F, Brucato V, La Carrubba V. Solution-Based Processing for Scaffold Fabrication in Tissue Engineering Applications: A Brief Review. Polymers. 2021; 13(13):2041. https://doi.org/10.3390/polym13132041
Chicago/Turabian StyleCapuana, Elisa, Francesco Lopresti, Francesco Carfì Pavia, Valerio Brucato, and Vincenzo La Carrubba. 2021. "Solution-Based Processing for Scaffold Fabrication in Tissue Engineering Applications: A Brief Review" Polymers 13, no. 13: 2041. https://doi.org/10.3390/polym13132041
APA StyleCapuana, E., Lopresti, F., Carfì Pavia, F., Brucato, V., & La Carrubba, V. (2021). Solution-Based Processing for Scaffold Fabrication in Tissue Engineering Applications: A Brief Review. Polymers, 13(13), 2041. https://doi.org/10.3390/polym13132041