Thermal and Photocatalytic Performance of Unsaturated Polyester Resins Modified with TiO2 Nanoparticles as Panel Bodies for Vehicles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
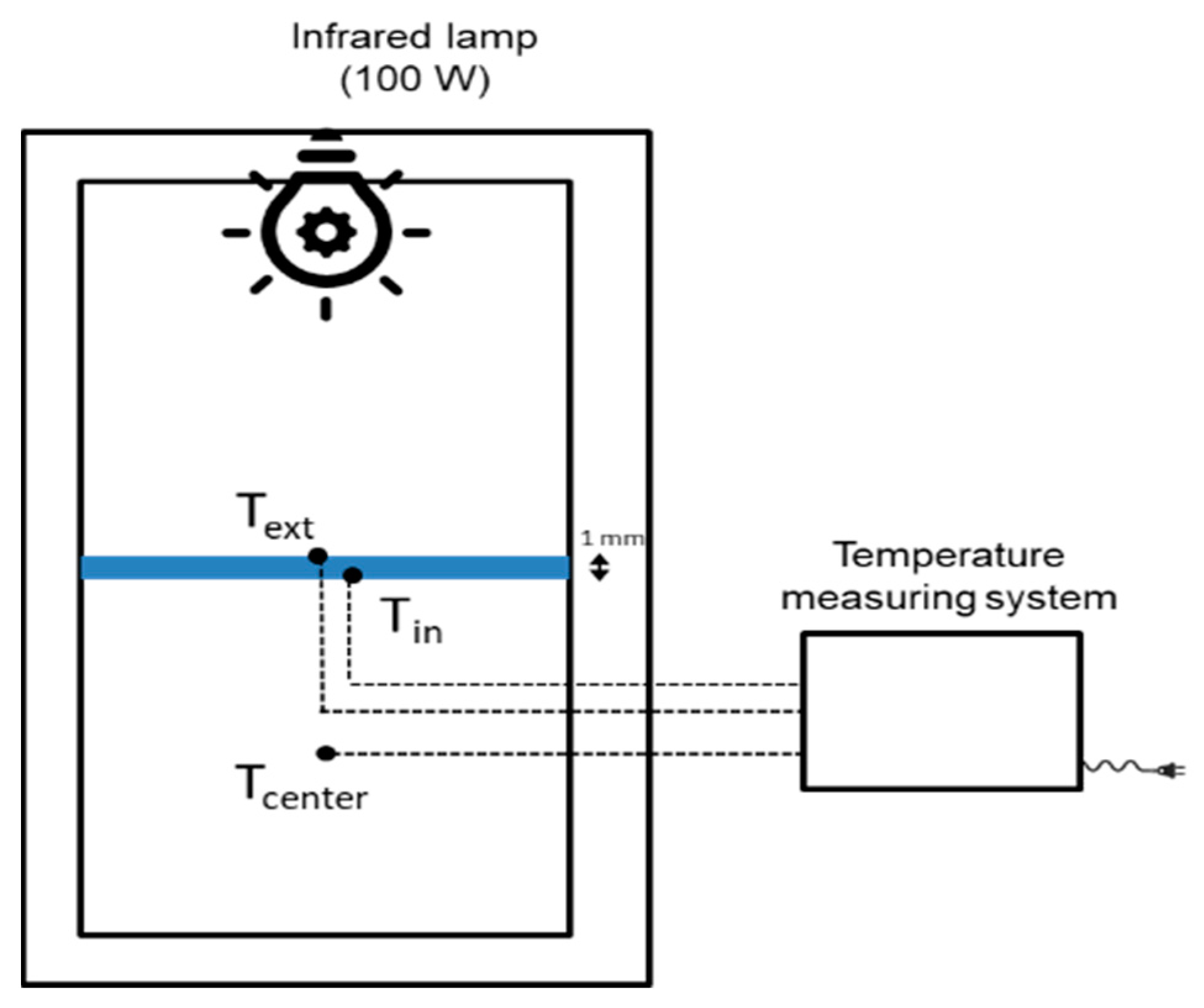
2.3. Characterization Techniques
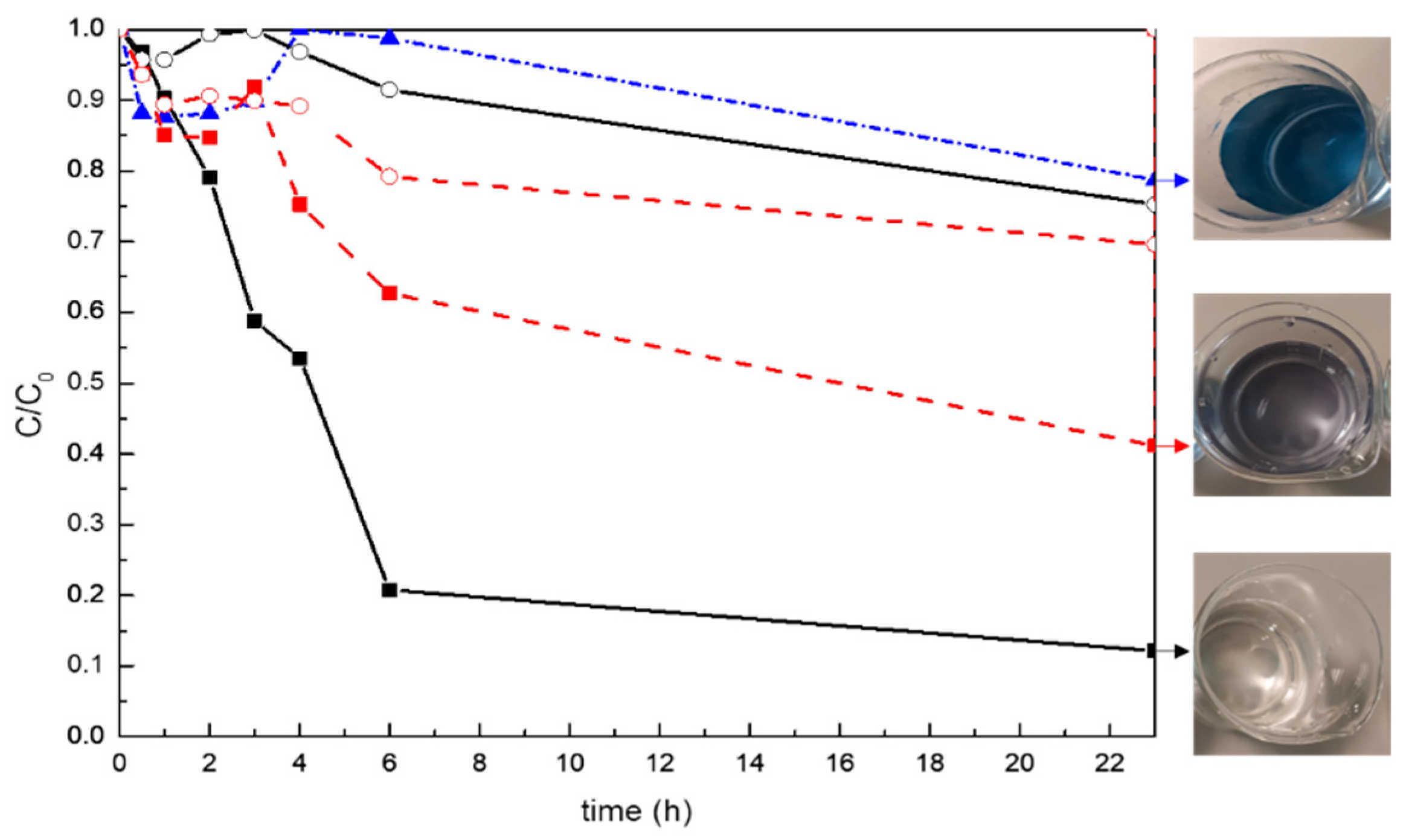
3. Results
3.1. Curing Kinetics and Morphology of Developed Composites
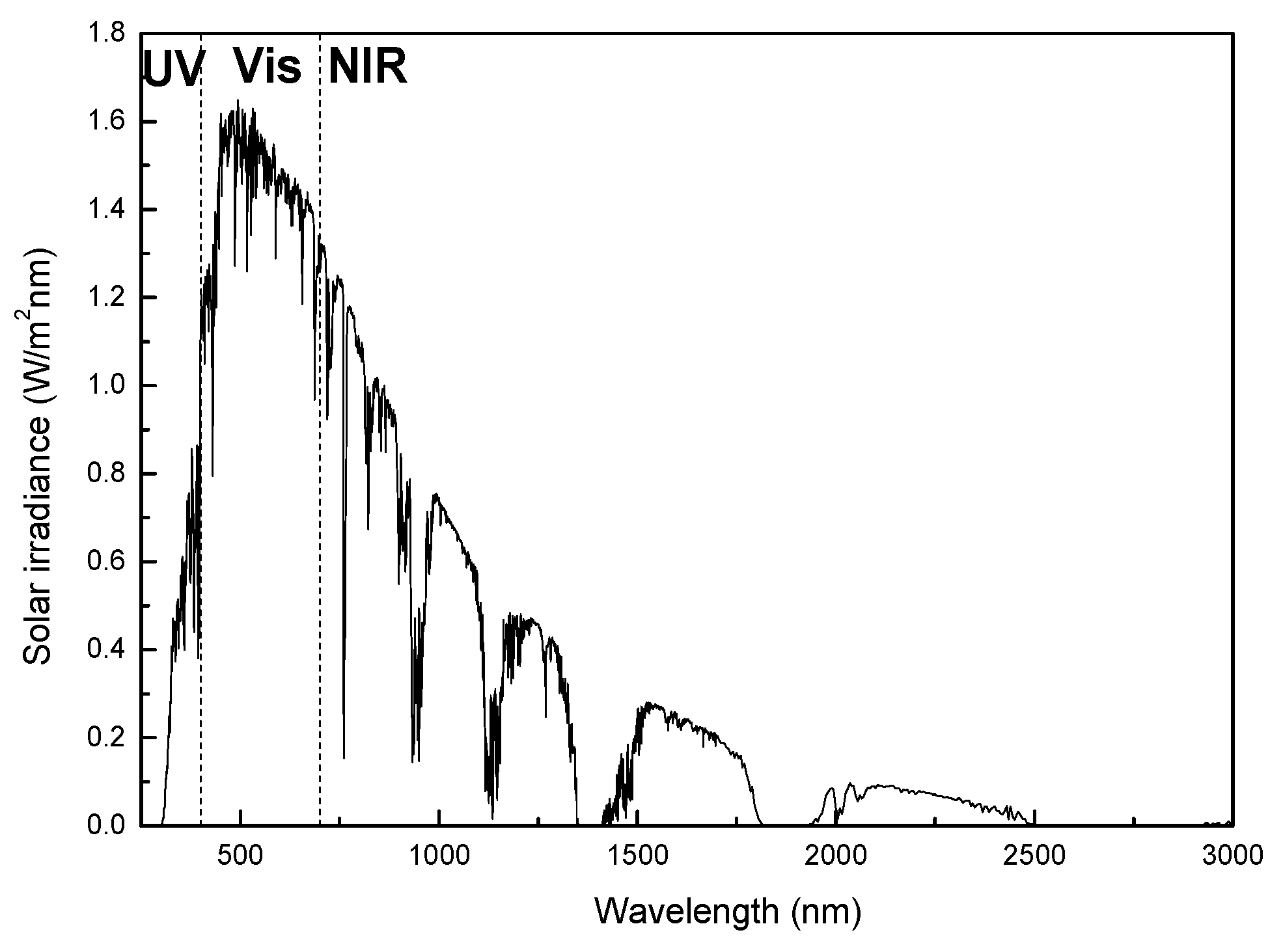
3.2. Thermal Reflectance Properties
3.3. Photocatalytic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maduekwe, M.; Akpan, U.; Isihak, S. Road transport energy consumption and vehicular emissions in Lagos, Nigeria: An application of the LEAP model. Transp. Res. Interdiscip. Perspect. 2020, 6, 100172. [Google Scholar] [CrossRef]
- Reports and Data, New York. 2020. Available online: https://www.globenewswire.com/news-release/2020/04/16/2017606/0/en/Refrigerated-Transport-Market-To-Reach-USD-23-1-Billion-By-2027-Reports-and-Data.html (accessed on 31 May 2021).
- A European Strategy for low-emission mobility. COM 2016, 501. Available online: https://ec.europa.eu/transport/sites/default/files/themes/strategies/news/doc/2016-07-20-decarbonisation/com%282016%29501_en.pdf (accessed on 31 May 2021).
- The Cost of Air Pollution: Strengthening the Economic Case for Action; World Bank Group: Washington, DC, USA, 2016. [CrossRef] [Green Version]
- Fanizza, C.; De Berardis, B.; Ietto, F.; Soggiu, M.E.; Schirò, R.; Inglessis, M.; Ferdinandi, M.; Incoronato, F. Analysis of major pollutants and physico-chemical characteristics of PM2.5 at an urban site in Rome. Sci. Total Environ. 2018, 616–617, 1457–1468. [Google Scholar] [CrossRef]
- Adekomaya, O.; Jamiru, T. Mitigating Energy Requirement in Transport System via Material Selection. In Proceedings of the IOP Conference Series: Earth and Environmental Science, 4th International Conference on Environmental and Energy Engineering (IC3E 2020), Sanya, China, 12–15 March 2020; Institute of Physics: London, UK, 2020; Volume 495, p. 012010. [Google Scholar] [CrossRef]
- Rizvi, A.; Chu, R.K.M.; Park, C.B. Scalable fabrication of thermally insulating mechanically resilient hierarchically porous polymer foams. ACS Appl. Mat. Int. 2018, 10, 38410–38417. [Google Scholar] [CrossRef]
- An, L.; Wang, J.; Petit, D.; Armstrong, J.N.; Li, C.; Hu, Y.; Huang, Y.; Shao, Z.; Ren, S. A scalable crosslinked fiberglass-aerogel thermal insulation composite. Appl. Mater. Today 2020, 21, 100843. [Google Scholar] [CrossRef]
- Guzel, G.; Deveci, K. Synergistic effects of silica aerogels/xerogels on properties of polymer composites: A review. J. Ind. Eng. Chem. 2020, 89, 13–27. [Google Scholar] [CrossRef]
- Yuan, X.D.; Cheng, W.L. Multi-objective optimization of household refrigerator with novel heat-storage condensers by Genetic algorithm. Energy Convers. Manag. 2014, 84, 550–561. [Google Scholar] [CrossRef]
- Chen, J.; Ling, Z.; Fang, X.; Zhang, Z. Experimental and numerical investigation of form-stable dodecane/hydrophobic fumed silica composite phase change materials for cold energy storage. Energy Convers. Manag. 2015, 105, 817–825. [Google Scholar] [CrossRef]
- Gin, B.; Farid, M.M.; Bansal, P.K. Effect of door opening and defrost cycle on a freezer with phase change panels. Energy Convers. Manag. 2010, 51, 2698–2706. [Google Scholar] [CrossRef]
- Cavalier, G.; From, A. 26 challenges facing refrigerated trucks for sustainable development. In Proceedings of the 23rd IIR International Congress of Refrigeration, Prague, Czech Republic, 21–26 August 2011. [Google Scholar]
- Cozza, E.S.; Alloisio, M.; Comite, A.; Di Tanna, G.; Vicini, S. NIR-reflecting properties of new paints for energy-efficient buildings. Sol. Energy 2015, 116, 108–116. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Ângelo, J.; Andrade, L.; Madeira, L.M.; Mendes, A. An overview of photocatalysis phenomena applied to NOx abatement. J. Environ. Manag. 2013, 129, 522–539. [Google Scholar] [CrossRef]
- Wood, D.; Shaw, S.; Cawte, T.; Shanen, E.; Van Heyst, B. An overview of photocatalyst immobilization methods for air pollution remediation. Chem. Eng. J. 2020, 391, 123490. [Google Scholar] [CrossRef]
- Yu, D.; Wang, L.; Yang, T.; Yang, G.; Wang, D.; Ni, H.; Wu, M. Tuning Lewis acidity of iron-based metal-organic frameworks for enhanced catalytic ozonation. Chem. Eng. J. 2021, 404, 127075. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Gu, P.; Ma, R.; Wen, T.; Zhao, G.; Li, L.; Ai, Y.; Hu, C.; Wang, X. Enhanced photodegradation of toxic organic pollutants using dual-oxygen-doped porous g-C3N4: Mechanism exploration from both experimental and DFT studies. Appl. Catal. B Environ. 2019, 248, 1–10. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, N.K.; Singla, M.L. Size dependent reflective properties of TiO2 nanoparticles and reflectors made thereof. Dig. J. Nanomater. Biostruct. 2012, 7, 607–619. [Google Scholar]
- Aranzabe, E.; Villasante, P.M.; March, R.; Arriortua, M.I.; Vadillo, J.; Larrañaga, A.; Aranzabe, A. Preparation and characterization of high NIR reflective pigments based in ultramarine blue. Energy Build. 2016, 126, 170–176. [Google Scholar] [CrossRef]
- Blanco, M.; Monteserín, C.; Angulo, A.; Pérez-Márquez, A.; Maudes, J.; Murillo, N.; Aranzabe, E.; Ruiz-Rubio, L.; Vilas, J.L. TiO2 doped electrospun nanofibrous membrane for photocatalytic water treatment. Polymers 2019, 11, 747. [Google Scholar] [CrossRef] [Green Version]
- Martín, J.L. Kinetic Analysis of two DSC peaks in the curing of an unsaturated polyester resin catalyzed with methylethylketone peroxide and cobalt octoate. Polym. Eng. Sci. 2007, 47, 62–70. [Google Scholar] [CrossRef]
- Vargas, M.A.; Vazquez, H.; Guthausen, G. Non-isothermal curing kinetics and physical properties of MMT-reinforced unsaturated polyester (UP) resins. Thermochim. Acta 2015, 611, 10–19. [Google Scholar] [CrossRef]
- Monti, M.; Puglia, D.; Natali, M.; Torre, L.; Kenny, J.M. Effect of carbon nanofibers on the cure kinetics of unsaturated polyester resin: Thermal and chemorheological modelling. Compos. Sci. Technol. 2011, 71, 1507–1516. [Google Scholar] [CrossRef] [Green Version]
- Moreira dos Santos, L.; Pandolfo Carone, C.L.; Oliveira Einloft, S.M.; Ligabue, R.A. Preparation and properties of aromatic polyester/TiO2 nanocomposites from polyethylene terephthalate. Mater. Res. 2016, 19, 158–166. [Google Scholar] [CrossRef] [Green Version]
- Ghanem, A.F.; Badawy, A.A.; Ismail, N.; Rayn Tian, Z.; Abdel Rehim, M.H.; Rabia, A. Photocatalytic activity of hyperbranched polyester/TiO2 nanocomposites. Appl. Catal. A Gen. 2014, 472, 191–197. [Google Scholar] [CrossRef]
- Vargas, M.A.; Montiel, R.; Vázquez, H. Effect of ammonium and aminosilane montmorillonites organo-clays on the curing kinetics of unsaturated polyester (UP) resin nanocomposites. Thermochim. Acta 2016, 630, 21–30. [Google Scholar] [CrossRef]
- Martín, J.L. Kinetic analysis of an asymmetrical DSC peak in the curing of an unsaturated polyester resin catalysed with MEKP and cobalt octoate. Polymer 1999, 40, 3451–3462. [Google Scholar] [CrossRef]
- Calza, P.; Rigo, L.; Sangermano, M. Investigations of photocatalytic activities of photosensitive semiconductors dispersed into epoxy matrix. Appl. Catal. B Environ. 2011, 106, 657–663. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Krishnan, P.; Yu, L.E.; Zhang, M.H. Using lightweight cement composite and photocatalytic coating to reduce cooling energy consumption of buildings. Constr. Build. Mater. 2017, 145, 555–564. [Google Scholar] [CrossRef]
- Synnefa, A.; Santamouris, M. White or light colored cool roofing materials. Adv. Dev. Cool Mater. Built Environ. 2013, 33–71. [Google Scholar] [CrossRef]
- Song, Z.; Qin, J.; Qu, J.; Song, J.; Zhang, W.; Shi, Y.; Zhang, T.; Xue, X.; Zhang, R.; Zhang, H.; et al. A systematic investigation of the factors affecting the optical properties of near infrared transmitting cool non-white coatings. Sol. Energy Mater. Sol. Cells 2014, 125, 206–214. [Google Scholar] [CrossRef]
- Aranzabe, E.; Arriortua, M.I.; Larranaga, A.; Aranzabe, A.; Villasante, P.M.; March, R. Designing multifunctional pigments for an improved energy efficiency in buildings. Energy Build. 2017, 147, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Ahn, K.; Kim, K.; Kim, J. Thermal conductivity and electric properties of epoxy composites filled with TiO2-coated copper nanowire. Polymer 2015, 76, 313–320. [Google Scholar] [CrossRef]
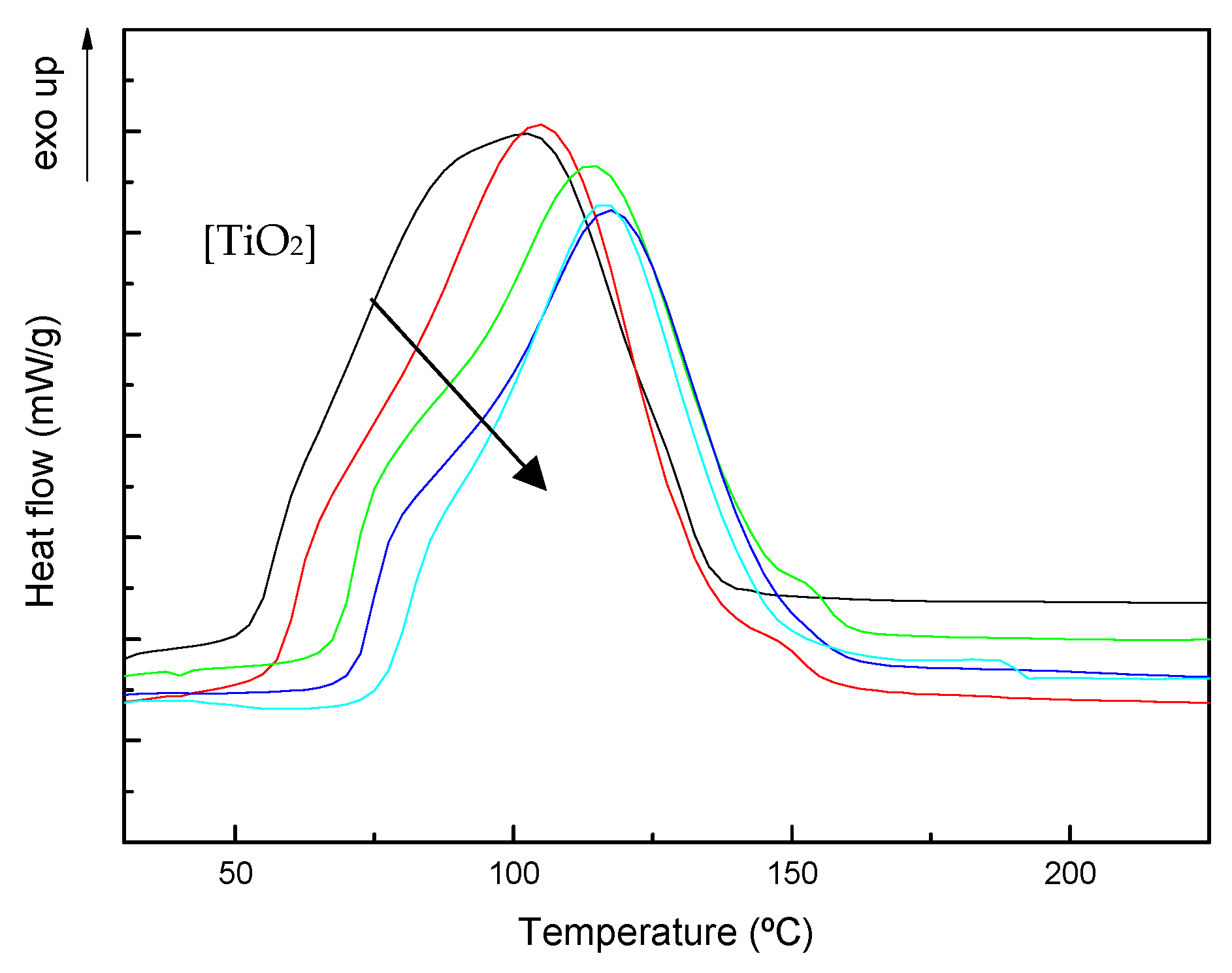
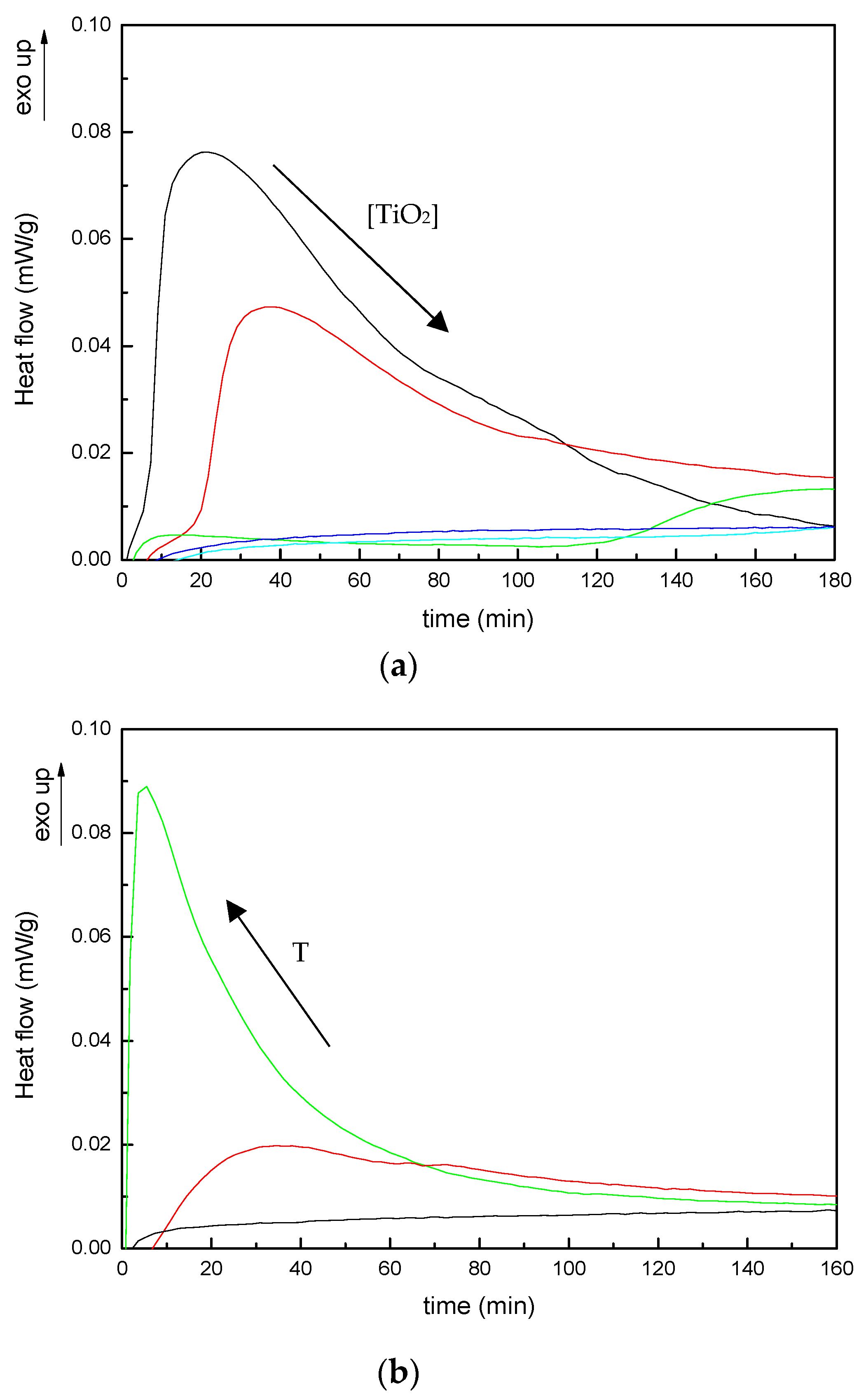




| TiO2 wt% | Tonset (°C) | Tp1 (°C) | Tp2 (°C) | ΔHT (J/g) | Tg∞ (°C) |
|---|---|---|---|---|---|
| 0 | 50.0 | 60.0 | 100.0 | 291.0 | 66.8 |
| 5 | 56.0 | 64.5 | 105.0 | 328.0 | 67.2 |
| 10 | 68.0 | 75.0 | 113.0 | 279.5 | 66.0 |
| 15 | 71.0 | 79.5 | 117.0 | 256.5 | 64.2 |
| 20 | 75.0 | 86.0 | 116.5 | 231.0 | 64.7 |
| (wt%). | TSR (%) |
|---|---|
| 0 | 8.8 |
| 5 | 61.1 |
| 10 | 64.1 |
| 15 | 69.9 |
| 20 | 72.6 |
| System | Process | Cycle | Color Removal (%) | k (h−1) | R |
|---|---|---|---|---|---|
| neat UP | adsorption | 1st | 30.4 | 0.01364 | 0.82 |
| 5rd | 2.7 | 0.01240 | 0.72 | ||
| neat UP | photocatalysis | 1st | 58.9 | 0.03673 | 0.90 |
| 5rd | 9.9 | 0.00257 | 0.76 | ||
| UP + 20 wt%TiO2 | adsorption | 1st | 24.7 | 0.01203 | 0.92 |
| 5rd | 4.1 | 0.00189 | 0.71 | ||
| UP + 20 wt%TiO2 | photocatalysis | 1st | 87.9 | 0.09223 | 0.76 |
| 5rd | 100 | 0.26496 | 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, M.; Monteserín, C.; Uranga, N.; Gómez, E.; Aranzabe, E.; García, J.I. Thermal and Photocatalytic Performance of Unsaturated Polyester Resins Modified with TiO2 Nanoparticles as Panel Bodies for Vehicles. Polymers 2021, 13, 2036. https://doi.org/10.3390/polym13132036
Blanco M, Monteserín C, Uranga N, Gómez E, Aranzabe E, García JI. Thermal and Photocatalytic Performance of Unsaturated Polyester Resins Modified with TiO2 Nanoparticles as Panel Bodies for Vehicles. Polymers. 2021; 13(13):2036. https://doi.org/10.3390/polym13132036
Chicago/Turabian StyleBlanco, Miren, Cristina Monteserín, Nerea Uranga, Estíbaliz Gómez, Estíbaliz Aranzabe, and Jose Ignacio García. 2021. "Thermal and Photocatalytic Performance of Unsaturated Polyester Resins Modified with TiO2 Nanoparticles as Panel Bodies for Vehicles" Polymers 13, no. 13: 2036. https://doi.org/10.3390/polym13132036
APA StyleBlanco, M., Monteserín, C., Uranga, N., Gómez, E., Aranzabe, E., & García, J. I. (2021). Thermal and Photocatalytic Performance of Unsaturated Polyester Resins Modified with TiO2 Nanoparticles as Panel Bodies for Vehicles. Polymers, 13(13), 2036. https://doi.org/10.3390/polym13132036







