1. Introduction
The production of chemicals generally involves multiple catalytic reactions with a corresponding product separation/purification and catalyst separation between each reaction. The ability to perform multiple reactions in “one pot” decreases the number of workups and purifications, as well as the volume of solvent required. These cascade or domino reactions simplify the process of organic synthesis, lower cost, and reduce waste and product purification steps [
1,
2].
Such cascade reactions can be performed with multifunctional heterogeneous catalysts in order to ease the separation and reuse of the catalyst [
1]. Polymer-based systems have been a versatile approach to achieving such multifunctional catalysts [
3,
4,
5,
6,
7]. One approach has been to precisely control position of the active sites of multiple catalysts when incorporated into a polymer support to ensure that incompatible catalysts are isolated. For example, monomers with acidic and basic catalytic sites were copolymerized to achieve acidic and basic porous polymers. The multifunctional acid-base catalyst was used for the cascade reaction involving two steps of an acid-catalyzed deacetalization followed by a base-catalyzed Knoevanagel condensation reaction [
1]. Similar studies have been performed with bottlebrush copolymers with acidic and basic catalytic sites [
2]. Acid-base bifunctional crosslinked polymers have also been incorporated into the pores of mesoporous silica and combined with Ni or Ni–Pd alloy nanoparticles. The multifunctional catalyst system was used for the three-step, one-pot reaction of a deacetalization-Knoevenagel condensation reduction reaction [
8]. An alternative approach has been incorporating multiple catalysts into a polymer-based nanocarrier. For example, pluoronic nanocarriers were used to coencapsulate two enzyme biocatalysts, formate dehydrogenase and mannitol dehydrogenase, for the conversion of D-fructose to D-mannitol [
9].
Micellar systems are particularly promising for performing one-pot reactions because they facilitate the use of water as a bulk solvent, and the micellar structure can provide site isolation [
10,
11]. For example, shell cross-linked micelles have also been used for the site isolation of incompatible catalysts to facilitate a three-step tandem one-pot reaction performed in water. Upon self-assembly in water, the concentric structure of the micelle is comprised of carboxylic acids in the hydrophilic outermost corona, rhodium-based catalysts in the intermediate crosslinked layer, and basic catalytic sites in the hydrophobic core. The structure of the nanoreactor was designed to produce an ester product through a tandem reaction. [
12]. Similar micellar systems encapsulating coporphyrin catalysts in the micelle core with Rh-based catalysts in the shell have also been reported to produce chiral alcohols via a two-step tandem reaction [
13]. Similarly, the organocatalytst TEMPO has been incorporated into the shell of a polymer micelle with Rh-based catalysts in the hydrophobic core to facilitate redox catalysis in aqueous media [
10].
While one-pot reactions can be performed with two micelle populations [
10] or micelles with enzymes [
14], it is important to note that engineering two catalysts into a single nanoreactor reduces the reaction time fourfold compared to a one-pot cascade reaction performed using two different nanoreactors [
10]. This improvement in performance was attributed to reduced diffusion limitations [
10]. However, engineering these micelle systems with multiple catalysts can be challenging and typically requires a new polymer synthesis for each micelle system.
Alternative systems that use a single metal nanoparticle catalyst have also been considered. For example, gold nanoparticles stabilized with polyionic liquids were used for a one-pot cascade reaction of ethyl cyanoacetate and p-nitrobenzaldehyde. Sodium borohydride was then added to reduce the NO
2 groups. The tandem reaction was performed in one pot [
15]. Tandem reactions have also been performed with silver nanoparticles within polymer reactors made from hydrogel bilayers. The encapsulated silver nanoparticles were used for the reduction of the intermediate, p-nitrophenol, to p-aminophenol. The reactor was temperature responsive. No reaction was observed at 30 °C due to the collapsed state of the first layer and low resulting conversion of p-nitrophenyl acetate. No product was formed at 45 °C due to the collapsed state of the second layer resulting in low conversion of the intermediate. At 60 °C, both reactions occurred in tandem.
Further integrating reactions and separation processes could minimize solvent usage. Such an ability would be impactful because traditionally, downstream separation processes account for as much as two thirds of total manufacturing costs [
16]. Green process engineering approaches to combine reaction and separation have included reactive membranes as well as combining continuous flow synthesis with nanofiltration. These promising approaches have recently been demonstrated with single-step reactions [
16,
17,
18].
Building on these examples, we aim to establish an approach for performing integrated multiple step one-pot reactions and separation using polymer micelles as an alternative to systems that require the unique synthesis of amphiphilic molecules. In our previous work, we established that flash nanoprecipitation is a useful tool for producing self-assembled polymer nanoreactors with tunable properties, e.g., size [
19], catalyst loading [
19], and hydrophobic core material [
20]. In this work, we aim to demonstrate that the self-assembled amphiphilic nanoreactors containing a single metal nanoparticle catalyst in the hydrophobic core can facilitate multiple step synthesis in one pot and separation of the product. Given that the self-assembly occurs by noncovalent interactions, we are further interested in comparing the performance of the polymer nanoreactor with alternative stabilizers of the catalyst. Specifically, we aim to investigate what advantages the self-assembled polymer structure provides compared to small molecule and hydrophilic ligands. Overall, the novel aspects of this work are demonstrating that the amphiphilic self-assembled nanoreactors containing a single catalyst can facilitate multiple reactions without requiring the unique synthesis of amphiphilic molecules and that the amphiphilic polymer structures perform better than small molecule and hydrophilic ligands.
Specifically, in this proof-of-concept study, we use imine synthesis via a one-pot condensation reaction of benzaldehyde and 4-nitrophenol as a model reaction since imines are an important class of molecules [
21,
22] that are commonly used for the preparation of heterocycles, anti-inflammatory agents, and anticancer agents, and the reduction of 4-nitrophenol is well studied [
23,
24]. As a catalyst, we incorporate gold nanoparticles into polystyrene nanoreactors stabilized by polyethylene glycol. The effect of pH on the reaction is discussed. The amphiphilic polymer nanoreactors are compared to gold nanoparticles stabilized by hydrophilic stabilizers such as citrate and PEG.
3. Results and Discussion
Gold nanoparticles were incorporated into amphiphilic polymer nanoreactors using block-copolymer directed self-assembly via flash nanoprecipitation [
19,
20]. To perform polymer directed self-assembly, hydrophobic gold nanoparticles were dispersed with a dissolved amphiphilic block copolymer (polystyrene-b-polyethylene glycol (PS-b-PEG)) and polystyrene coprecipitant in a water miscible organic solvent (THF) and rapidly mixed with water in a confined impinging jet mixer. Upon mixing with water, the gold nanoparticles aggregated and polystyrene precipitated. Simultaneously, the block copolymer micellized, directing the self-assembly of the nanoreactors. The hydrophobic block of the amphiphilic block copolymer adsorbed to the precipitating nanoreactor components via hydrophobic interactions; the hydrophilic PEG block sterically stabilized the nanoreactor. In previous work, we established the formulation parameters to achieve polystyrene nanoreactors with tunable size and gold loading [
19]. Building on this work, our focus was to demonstrate that the polymer nanoreactors dispersed in water could facilitate a multistep reaction in one pot with spontaneous precipitation of the product from the reaction mixture to reduce the solvent waste associated with liquid phase chemical processing.
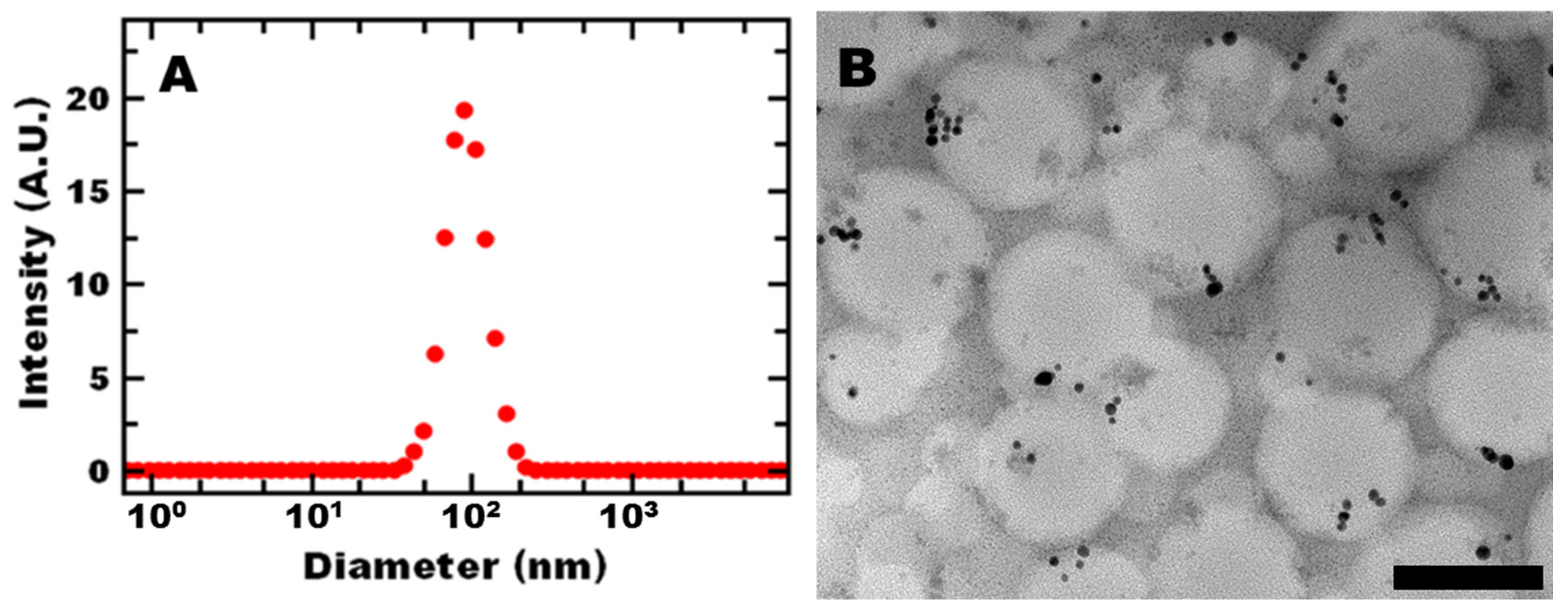
Based on our previous work, we aimed to produce sub-100-nm nanoreactors (through selection of the block copolymer, polystyrene, and gold concentration). At this size, no internal mass transfer limitations were observed [
19]. After formulation, the polymer nanoreactors had an average hydrodynamic diameter of 91 ± 5 nm and PDI of 0.163 ± 0.018 by DLS (
Figure 1A). Transmission electron microscopy (TEM) (
Figure 1B) confirmed that the nanoreactors were spherical and contained gold. The overall nanoreactor size was consistent with DLS measurements. Gold nanoparticles appear to be incorporated in the nanoparticle core. Some gold nanoparticles may also be associated with the PEG shell. Gold unassociated with the nanoreactors would be expected to precipitate out of the dispersion as well as affect the size distribution measured by DLS, which was not observed. These results are consistent with previous reports [
19,
20].
To further analyze the gold content in the nanoreactors, the gold loading in the nanoreactors was determined by measuring the gold content in the nanoreactor dispersion using inductively coupled plasma optical emission spectrometry (ICP-OES) analysis and thermogravimetric analysis (TGA) to determine the nanoreactor concentration (mg/mL). We note that based on TGA analysis, the nanoreactor concentration was over 80% of the nominal concentration, which was comparable to previous reports of recovery using centrifugal processing [
29]. The gold loading was determined to be 1.1 ± 0.1%. (wt. gold/wt. nanoreactors).
Initially, we confirmed that the gold nanoparticles incorporated in the nanoreactors were catalytically active using the reduction of 4-nitrophenol with sodium borohydride. The 4-nitrophenol reduction is a well-known model reaction for studying the activity of gold nanoparticle catalysts [
23,
24]. We compared the activity of the nanoreactors to citrate-stabilized gold nanoparticles and PEGylated gold nanoparticles under the same gold, 4-nitrophenol, and sodium borohydride conditions. The results are reported in
Table 1. At a sodium borohydride concentration of 0.1 M, the polymer nanoreactors demonstrated twofold greater activity than citrate AuNP. The activity of the nanoreactors is slightly lower (0.7-fold) than the PEG AuNP. This demonstrates that incorporating gold nanoparticle catalysts in an amphiphilic polymer nanoreactor is not detrimental to catalytic performance. The enhanced apparent catalytic activity compared to citrate AuNPs could be attributed to reagent solubility differences in the localized reaction environments [
6].
When we attempted a one-pot cascade reaction adding all the reactants (4-nitrophenol, sodium borohydride, and benzaldehyde) at the same time, analysis by GC-MS indicated that benzaldehyde reacted with the sodium borohydride, producing benzyl alcohol rather than the desired product. This result is consistent with previous studies [
30]. Since the benzaldehyde was converted to benzyl alcohol, the desired cascade reaction of reduction of 4-nitrophenol to 4-aminophenol followed by reaction with benzaldehyde was not achieved.
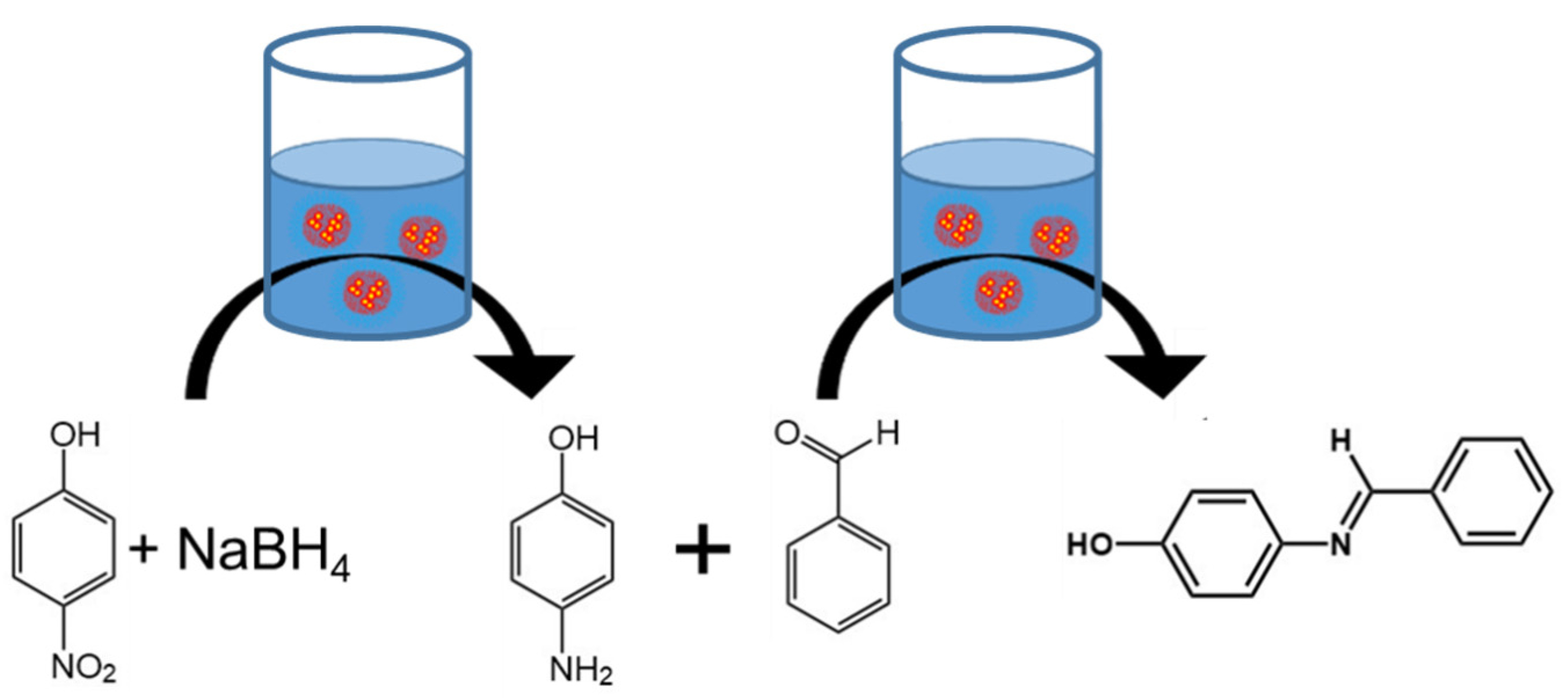
From these results, we determined that the sodium borohydride used for the reduction of 4-nitrophenol caused an unwanted side reaction in the presence of benzaldehyde. Therefore, to perform the two-step reaction in one pot, the reactions needed to be performed sequentially. Specifically, the sodium borohydride needed to be consumed prior to the addition of benzaldehyde. Thus, our approach to this “one pot” reaction was to first add the 4-nitrophenol and sodium borohydride to produce 4-aminophenol, neutralize the nanoreactor dispersion with formic acid, and then add benzaldehyde. An overview of this one-pot approach is provided in
Figure 2.
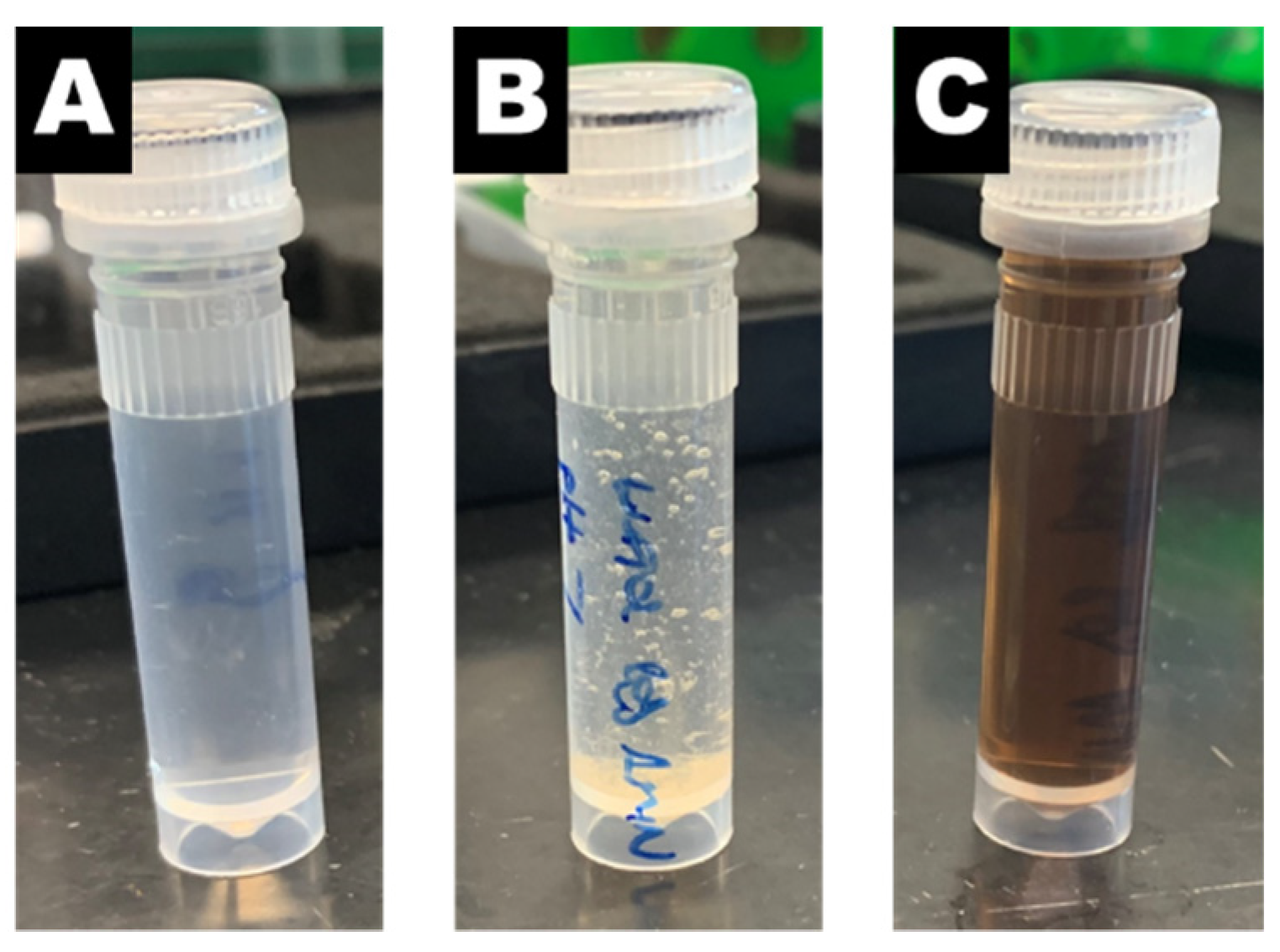
To determine the appropriate pH for the second reaction, we examined the effect of pH on the desired reaction between 4-aminophenol and benzaldehyde. Experiments in the condensation of benzaldehyde with 4-aminophenol without nanoreactors (i.e., no gold) showed that the pH of the reaction solution strongly influences the outcome of the reaction. At pH 4, the reaction solution remained colorless with no precipitate formed (
Figure 3A). No desired product was detected by GC-MS or NMR. In contrast, increasing the pH to neutral resulted in precipitate formation (
Figure 3B). NMR analysis confirmed that the precipitate formed was the desired 4-benzylideneaminophenol product (
Figure S1). Further increasing the pH to 10 resulted in a dark brown reaction solution (
Figure 3C). The desired product was not detected by either GC-MS or NMR. The color change may be attributed to the oxidation-induced polymerization of 4-aminophenol [
31]. From these experiments, the condensation of benzaldehyde and 4-aminopheno does not require gold but does require neutral pH. Therefore, after the reaction between 4-nitrophenol and sodium borohydride, we aimed to ensure that the sodium borohydride was completely consumed and to adjust the pH of the reaction solution to 7 when performing the one-pot reaction with nanoreactors.
To perform the two-step sequential one-pot reaction, first 4-nitrophenol and sodium borohydride were added to the nanoreactor dispersion. Following the 4-nitrophneol reduction to 4-aminophenol, indicated by the color change from yellow to colorless (i.e., 100% conversion of 4-nitrophenol by UV–vis spectroscopy), formic acid was added to neutralize the reaction mixture to ensure decomposition of sodium borohydride via hydrolysis [
32]. Then, benzaldehyde was added with small amounts of sodium hydroxide (20 μL of 10 M NaOH) to achieve pH 7 (
Figure 2). In this case, there was a color change to light brown associated with the desired reaction as well as a precipitate. The precipitate was characteristic of the condensation of benzaldehyde with 4-aminophenol at neutral pH. Since the desired product is hydrophobic, it spontaneously phase separated from the reaction mixture and could be easily separated from the nanoreactors. We note that at the same conditions we confirmed that no product was formed without nanoreactors because the multistep reaction could not proceed since gold is required for the first reaction.
Following the cascade reaction, the nanoreactor dispersion was centrifuged, the nanoreactor dispersion was decanted, and the resulting pellet was analyzed. The resulting pellet was partially solubilized using deuterated acetone. The components following extraction were analyzed by NMR. NMR analysis confirmed that the pellet contained the desired 4-benzylideneaminophenol product (
Figure S1). The mass of product extracted from the pellet was measured (
Table 2), and the yield (based on the molar amounts of 4-nitrophenol and benzaldehyde) was 65%. The turnover frequency based on the mass of product, mass of gold, and reaction time was 9 h
−1. The yield and turnover frequency achieved are comparable to previous reports [
33]. Importantly, these results demonstrate that multiple reactions could be achieved in “one pot” using water as the bulk solvent at ambient temperature and pressure. Furthermore, the product spontaneously precipitated from the reaction mixture.
Overall, these results demonstrate that the self-assembled polymer micelle nanoreactors incorporating gold nanoparticle catalysts dispersed in water facilitate integrated reaction and separation. Such integrated methods are important as up to two thirds of total manufacturing costs can be attributed to traditional downstream separation processes [
16]. This proof-of-concept work demonstrates that polymer nanoreactors self-assembled by noncovalent interactions are a possible technology for enabling green processing engineering to complement alternative approaches, including membrane reactors [
17,
18], as well as continuous flow synthesis and nanofiltration [
16]. Building on this proof-of-concept study, further future effort is needed to address methods to achieve precipitate with improved purity. Products with higher purity may be possible with a higher temperature and pressure [
21] or platinum-based catalysts [
34].
Evaluating green chemistry metrics, we examined the E factor. Since we used water as the bulk solvent, the E factor for the reaction itself is relatively low: ~25. The E factor is approximately half of a one-pot synthesis method using a nickel-based catalyst, ethanol as the solvent, and an elevated temperature (105 °C) and pressure (1.4 MPa) [
21]. The E factor for the reaction was also comparable to results reported using a bifunctional metal/solid acid catalyst on mesoporous carbon [
33].
Depending on the purity of the desired product, the E factor will increase. For example, the extraction of the desired product from the pellet increases the E factor to ~400, although we note that the process was not designed to minimize solvent usage. This result is comparable to previous studies reporting product purification by extraction [
33]. Further purification by column chromatography is solvent intensive with E factors of ~10,000–25,000 [
35,
36]. Clearly, the isolation of the desired reaction product with solvent contributes significantly to the E factor. Therefore, performing multiple reaction steps in “one pot” to avoid the product isolation of intermediate steps would be beneficial for reducing the waste associated with liquid phase chemical processing.
We further analyzed the effect of the cascade reaction steps on the stability of the polymer nanoreactors. Stability was defined as maintaining a hydrodynamic diameter and PDI within 25% of the initial DLS measurement, as long as the PDI remained below a maximum value of 0.400. As can be seen in
Table 3, the nanoreactor size and polydispersity was not affected by the first reaction dispersion or pH adjustment. Following the second reaction (imine formation), there were multiple peaks apparent by dynamic light scattering and a significant increase in PDI. This increase in PDI suggests a broad distribution of large aggregates [
37]. We attributed this increase in PDI to precipitation of the desired reaction product. To confirm that the nanoreactors were stable following the reaction, we centrifuged the dispersion following the second reaction to separate the precipitated product from the nanoreactor dispersion. We analyzed the resulting supernatant by DLS. After centrifugation to remove the precipitated product, the original size and PDI of the nanoreactor were recovered. Specifically, there was only one peak by DLS that corresponds to a diameter around the initial size of the nanoreactors, and the PDI again falls below 0.400. This result suggests that the nanoreactors are stable throughout both steps of the reaction. To support this analysis, following centrifuging, we analyzed the pellet for gold using ICP-OES analysis. Minimal gold was detected in the pellet, suggesting that the nanoreactors were stable during both steps of the reaction (
Table 2). Since the product spontaneously phase separates from the nanoreactors, this is a promising approach to ease reuse of the catalyst.
Finally, we were interested in comparing the performance of the polymer nanoreactors with PEG-coated gold nanoparticles and citrate-stabilized gold nanoparticles in the one-pot reaction. Performing the one-pot reaction with either PEG AuNP and citrate AuNP yielded ~1.5 mg of product which is comparable to the polymer nanoreactors (
Table 2). However, both yielded more of the undesired product than the polymer nanoreactors (statistically significant with 90% confidence). Thus, the fraction of product that made up the precipitate was approximately twofold higher for the amphiphilic, self-assembled nanoreactors than the citrate-stabilized gold nanoparticles or PEG-stabilized gold nanoparticles. This result demonstrates that the amphiphilic structure of the self-assembled polymer nanoreactor enhances the performance compared to small molecule or hydrophilic polymer stabilizers. This enhancement is consistent with studies and has been attributed to reactant solubility in the hydrophobic core of the nanoreactor [
20].
Interestingly, ICP-OES analysis of the precipitate resulting from the one-pot reactions using PEG AuNP and citrate AuNP reactions indicated that a significant amount of gold precipitated out of the reaction solution (3.6 ± 0.2 and 4.3 ± 0.4 μg, respectively) (
Table 2). In contrast, minimal precipitation (<0.9 μg) was observed in the one-pot reaction when using the nanoreactors. These results suggest that the ligand-stabilized (PEG and citrate) gold nanoparticles were less stable than the polymer nanoreactors. Specifically, the amount of gold retained in the dispersion using the self-assembled polymer structures was improved approximately fourfold compared to alternative ligand-stabilized gold nanoparticles. This lack of stability of ligand-stabilized gold nanoparticles in the two-step reaction could be due to removal of the stabilizing ligands by sodium borohydride [
24], resulting in colloidal instability.
Notably, while PEG AuNP and citrate AuNP yielded comparable amounts of product as the nanoreactors, there was significant gold in the precipitate as measured by ICP-OES, indicating that these systems are not stable during the cascade reaction and precipitate with the product. This would complicate both isolation of the product as well as reuse of the catalyst. Therefore, the block-copolymer-stabilized nanoreactors are a promising approach for reuse of the catalyst since the product spontaneously phase separates from the nanoreactors without contamination by the catalyst. Taken together, these results indicated that the product spontaneously separated from the reaction mixture, and the nanoreactors were stable in the dispersion. Therefore, the nanoreactor dispersions could be recycled. Micelle-based nanoreactors have been recycled using this approach, which has been demonstrated [
38,
39]. Future work to understand the recyclability of the nanoreactors, i.e., the performance with multiple reaction cycles, is a key future direction for understanding the functional limitations of the nanoreactors. Further studies to understand the material selection with respect to reactants, products, and polymer nanoreactor components that facilitate the reaction and phase separation would also be valuable.