Multifactorial Effects of Gelling Conditions on Mechanical Properties of Skin-Like Gelatin Membranes Intended for In Vitro Experimentation and Artificial Skin Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of SLGMs
2.3. Sol-Gel Method Conditions
2.4. Thickness of SLGMs
2.5. Mechanical Properties
2.6. ATR-FTIR Spectroscopy
2.7. Thermal Analysis
2.8. Theoretical Calculations
2.9. Surface Morphology
3. Results and Discussion
3.1. Mechanical Characterization of SLGMs
3.2. Chemical Characterization of SLGMs
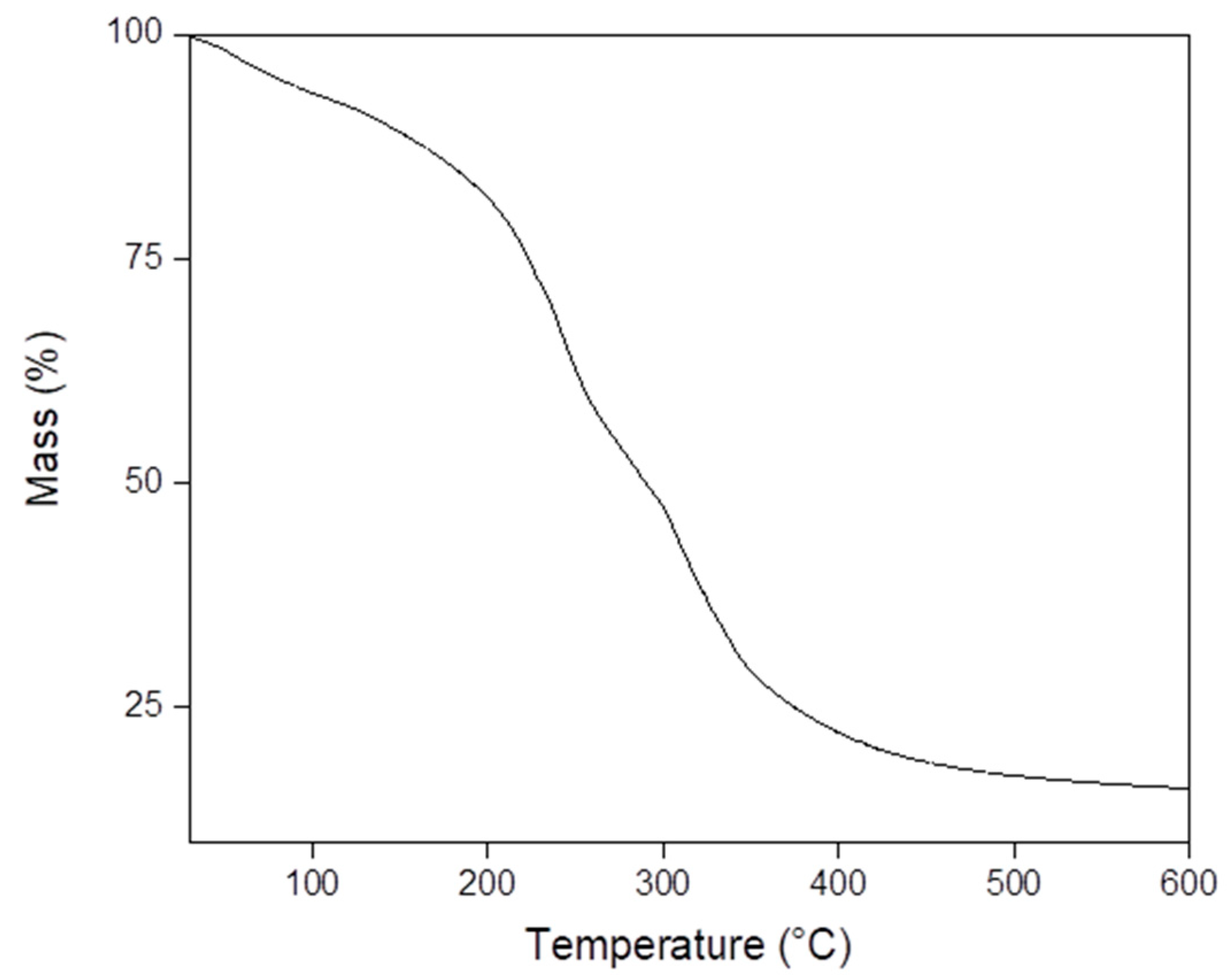
3.3. Thermal Analysis of SLGMs
3.4. Response Surface Analysis
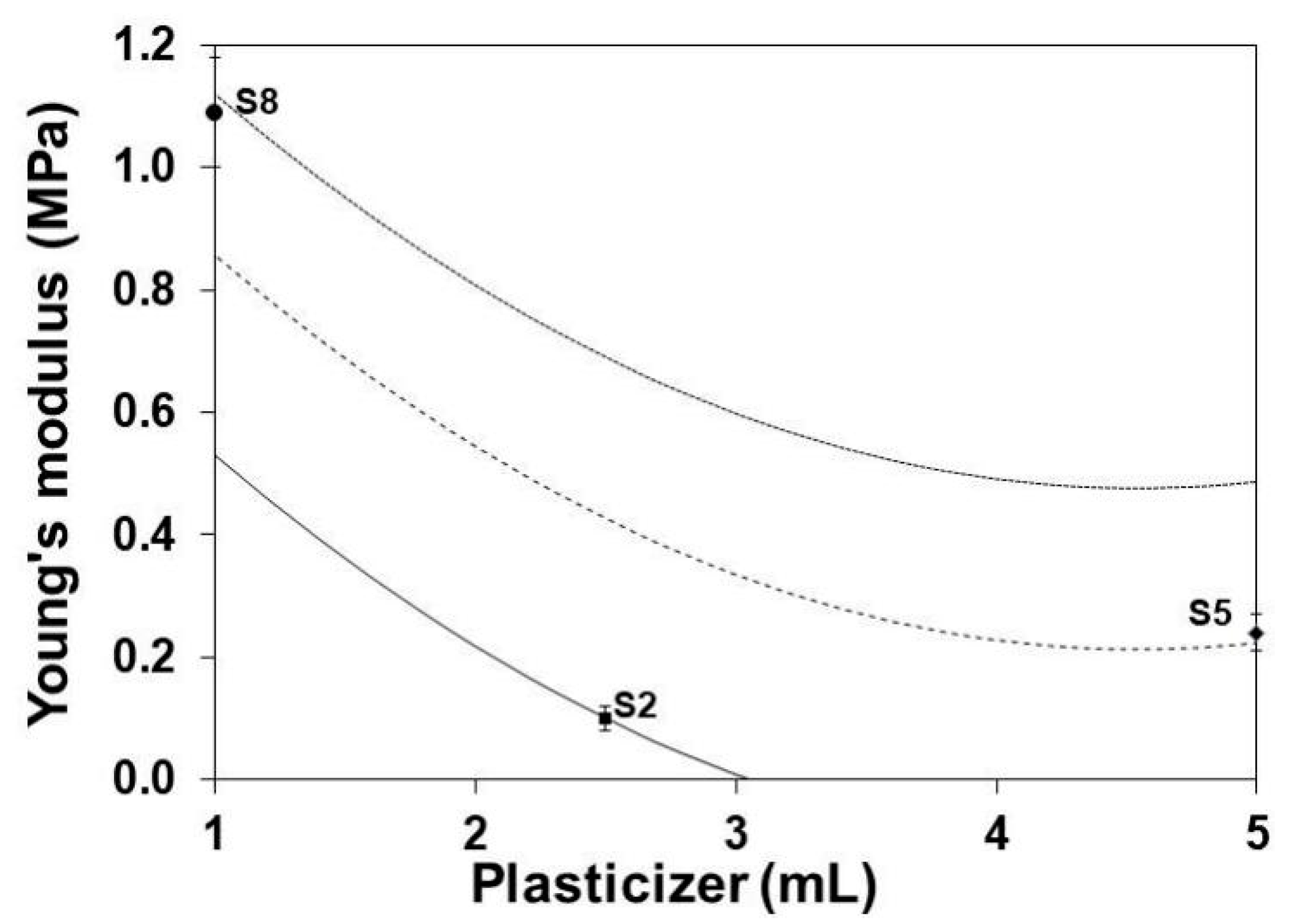
3.5. Young’s Modulus
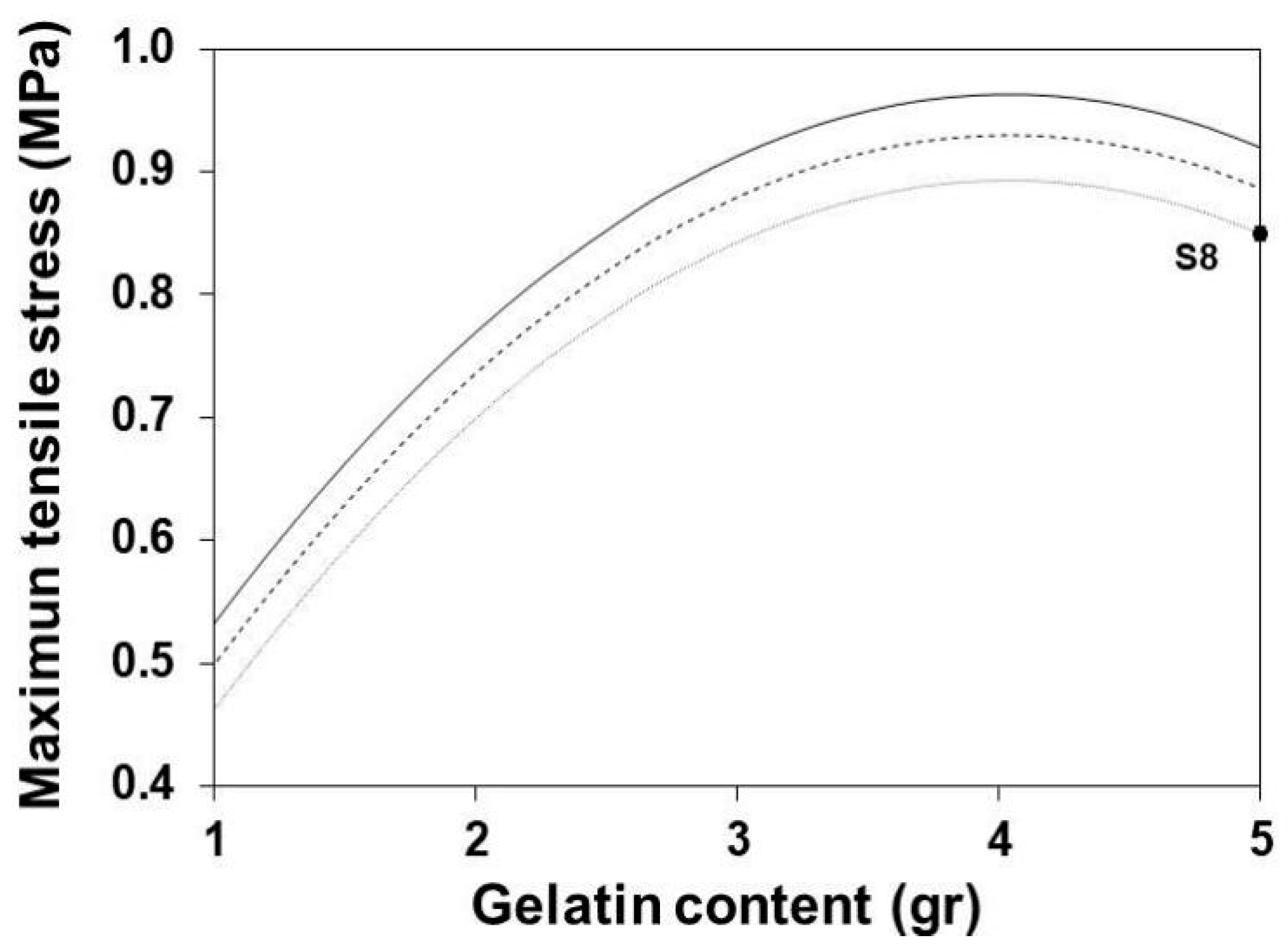
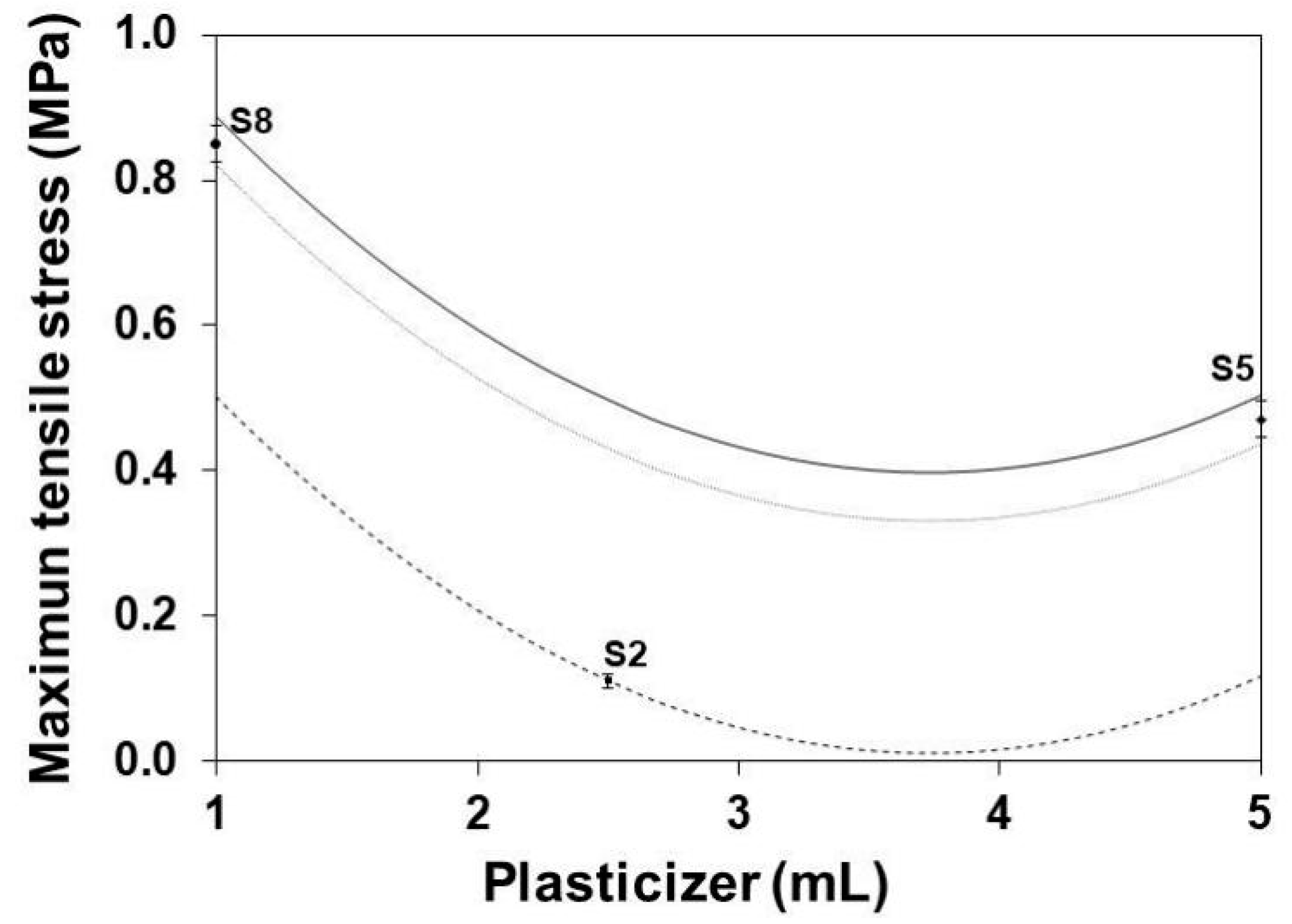
3.6. Maximum Tensile Stress
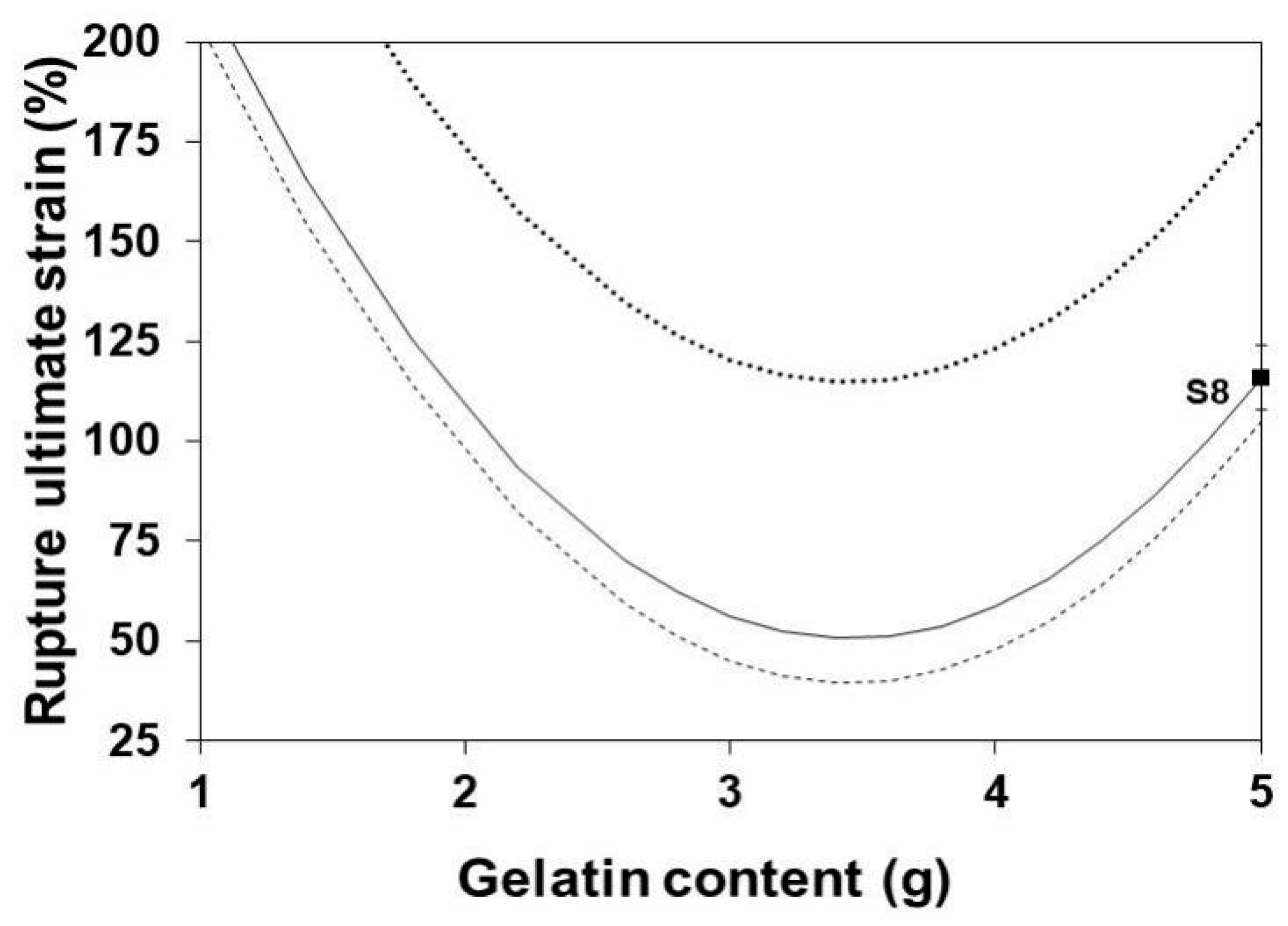
3.7. Rupture Ultimate Strain
3.8. Surface Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kolarsick, P.A.; Kolarsick, M.A.; Goodwin, C. Anatomy and physiology of the skin. J. Dermatol. Nurses Assoc. 2011, 3, 203–213. [Google Scholar] [CrossRef]
- McBride, A.; Bargmann, S.; Pond, D.; Limbert, G. Thermoelastic modelling of the skin at finite deformations. J. Therm. Biol. 2016, 62, 201–209. [Google Scholar] [CrossRef]
- Oomens, C.; Van Vijven, M.; Peters, G. Skin Mechanics. In Biomechanics of Living Organs: Hyperelastic Constitutive Laws for Finite Element Modeling; Payan, Y., Ohayon, J., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 347–357. [Google Scholar]
- Weinstein, G.D.; Boucek, R.J. Collagen and elastin of human dermis. J. Invest. Dermatol. 1960, 35, 227–229. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.; Uitto, J. Anatomy and Organization of Human Skin. In Rook’s Textbook of Dermatology, 9th ed.; Burns, T., Ed.; Wiley: Hoboken, NJ, USA, 2010; Volume 4, pp. 34–86. [Google Scholar]
- Silver, F.H.; Freeman, J.W.; DeVore, D. Viscoelastic properties of human skin and processed dermis. Skin Res. Technol. 2001, 7, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Healthline. Available online: www.healthline.com/health/stratum-corneum (accessed on 14 November 2019).
- Mazzini, M. Dermatología Práctica, 1st ed.; Lopez Libreros: Buenos Aires, Argentina, 1968; pp. 1–16. [Google Scholar]
- Georges, L. State-of-the-art constitutive models of skin biomechanics. In Computational Biophysics of the Skin; Querleux, B., Ed.; Pan Stanford Publishing Pte. Ltd.: Singapore, 2014; pp. 95–131. [Google Scholar]
- Georges, L. Skin Biophysics: From Experimental Characterization to Advanced Modelling; Georges, L., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; Volume 22, pp. 135–192. [Google Scholar]
- Goldsmith, L.A. My organ is bigger than your organ. Arch. Dermatol. 1990, 126, 301–302. [Google Scholar] [CrossRef]
- Yuan, J.H.; Shi, Y.; Pharr, M.; Feng, X.; Rogers, J.A.; Huang, Y.A. Mechanics Model for Sensors Imperfectly Bonded to the Skin for Determination of the Young’s Moduli of Epidermis and Dermis. J. Appl. Mech. 2016, 83, 084501. [Google Scholar] [CrossRef]
- Bischoff, J.E.; Arruda, E.M.; Grosh, K.J. Finite element modeling of human skin using an isotropic, nonlinear elastic constitutive mode. J. Biomech. 2000, 33, 645–652. [Google Scholar] [CrossRef]
- Dickey, M.D. Stretchable and soft electronics using liquid metals. Adv. Mater. 2017, 29, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Dagdeviren, C.; Shi, Y.; Ma, Y.; Feng, X.; Rogers, J.A.; Huang, Y. Computational models for the determination of depth-dependent mechanical properties of skin with a soft, flexible measurement device. Proc. Math. Phys. Eng. Sci. 2016, 472, 1–19. [Google Scholar] [CrossRef]
- Dureja, H.; Tiwary, A.K.; Gupta, S. Simulation of skin permeability in chitosan membranes. Int. J. Pharm. 2001, 213, 193–198. [Google Scholar] [CrossRef]
- Dąbrowska, A.; Rotaru, G.M.; Spano, F.; Affolter, C.; Fortunato, G.; Lehmann, S.; Derler, S.; Spencer, N.D.; Rossi, R.M. A water-responsive, gelatine-based human skin model. Tribol. Int. 2017, 113, 316–322. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; Scognamiglio, M.; Reverchon, E. A new tool to produce alginate-based aerogels for medical applications, by supercritical gel drying. J. Supercrit. Fluids 2019, 146, 152–158. [Google Scholar] [CrossRef]
- Marchal, C.; Nadi, M.; Tosser, A.J.; Roussey, C.; Gaulard, M.L. Dielectric properties of gelatine phantoms used for simulations of biological tissues between 10 and 50 MHz. Int. J. Hyperth. 1989, 5, 725–732. [Google Scholar] [CrossRef]
- Navarro, L.; Ceaglio, N.; Rintoul, I. Structure and properties of biocompatible poly (glycerol adipate) elastomers modified with ethylene glycol. Polym. J. 2017, 49, 625–632. [Google Scholar] [CrossRef]
- Navarro, L.; Mogosanu, D.E.; Ceaglio, N.; Luna, J.; Dubruel, P.; Rintoul, I. Novel poly (diol sebacate) s as additives to modify paclitaxel release from poly (lactic-co-glycolic acid) thin films. J. Pharm. Sci. 2017, 106, 2106–2114. [Google Scholar] [CrossRef]
- Imeson, A. Food Stabilisers, Thickeners and Gelling Agents; Wiley-Blackwell: Chichester, UK, 2010; pp. 116–143. [Google Scholar]
- Król, Ż.; Malik, M.; Marycz, K.; Jarmoluk, A. Physicochemical properties of biopolymer hydrogels treated by direct electric current. Polymers 2016, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Basha, M.A. Optical properties and colorimetry of gelatine gels prepared in different saline solutions. J. Adv. Res. 2018, 16, 55–65. [Google Scholar] [CrossRef]
- Gelatina Elástica. Available online: https://education.mrsec.wisc.edu/gelatina-elastica/ (accessed on 14 November 2019).
- Díaz, O.; Ferreiro, T.; Rodríguez-Otero, J.L.; Cobos, Á. Characterization of chickpea (Cicer arietinum L.) flour films: Effects of pH and plasticizer concentration. Int. J. Mol. Sci. 2019, 20, 1246. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Shi, H.; Cao, A.; Jia, J. Characterization of gelatin/chitosan polymer films integrated with docosahexaenoic acids fabricated by different methods. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.C.; Shangguan, X.; Sha, X.; Wang, H.; Zhang, L.; Bansal, N. Fish gelatin modifications: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 260–269. [Google Scholar] [CrossRef]
- Suderman, N.; Isa, M.I.N.; Sarbon, N.M. The effect of plasticizers on the functional properties of biodegradable gelatin-based film: A review. Food Biosci. 2018, 24, 111–119. [Google Scholar] [CrossRef]
- Mao, J.; Zhao, L.; De Yao, K.; Shang, Q.; Yang, G.; Cao, Y. Study of novel chitosan-gelatin artificial skin in vitro. J. Biomed. Mater. Res. A 2003, 64, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Velasquillo-Martínez, C.; García-Carvajal, Z.Y. Composite hydrogels based on gelatin, chitosan and polyvinyl alcohol to biomedical applications: A review. Int. J. Polym. Mater. 2020, 69, 1–20. [Google Scholar] [CrossRef]
- Owda, A.Y.; Casson, A.J. Electrical properties, accuracy, and multi-day performance of gelatine phantoms for electrophysiology. BioRxiv 2020, 1–29. [Google Scholar] [CrossRef]
- Velcescu, A.; Lindley, A.; Cursio, C.; Krachunov, S.; Beach, C.; Brown, C.A.; Jones, A.K.; Casson, A.J. Flexible 3D-printed EEG electrodes. Sensors 2019, 19, 1650. [Google Scholar] [CrossRef] [PubMed]
- Lir, I.; Haber, M.; Dodiuk-Kenig, H. Skin surface model material as a substrate for adhesion-to-skin testing. J. Adhes. Sci. Technol. 2007, 21, 1497–1512. [Google Scholar] [CrossRef]
- Keshavarzi, F.; Zajforoushan Moghaddam, S.; Barré Pedersen, M.; Østergaard Knudsen, N.; Jafarzadeh, S.; Thormann, E. Water vapor permeation through topical films on a moisture-releasing skin Model. Ski. Res. Technol. 2021, 27, 153–162. [Google Scholar] [CrossRef] [PubMed]
- da Silva, F.R.; Silva, R.O.; de Castro Oliveira, H.M.; Dourado, L.F.N.; da Costa, B.L.; Lima, B.S.; Nunes, P.S. Gelatin-based membrane containing usnic acid-loaded liposomes: A new treatment strategy for corneal healing. Biomed. Pharmacother. 2020, 130, 1–12. [Google Scholar] [CrossRef]
- Shirazaki, P.; Varshosaz, J.; Kharazi, A.Z. Electrospun gelatin/poly (glycerol sebacate) membrane with controlled release of antibiotics for wound dressing. Adv. Biomed. Res. 2017, 6, 1–7. [Google Scholar]
- Schottner, G. Hybrid sol—gel-derived polymers: Applications of multifunctional materials. Chem. Mater. 2001, 13, 3422–3435. [Google Scholar] [CrossRef]
- Bellini, F.; Alberini, I.; Ferreyra, M.G.; Rintoul, I. Absolute Determination of the Gelling Point of Gelatin under Quasi-thermodynamic Equilibrium. J. Food Sci. 2015, 80, C935–C941. [Google Scholar] [CrossRef]
- Dieter, G.E.; Schmidt, L.C. Engineering Design, 5th ed.; McGraw-Hill Higher Education: Boston, NY, USA, 2011; pp. 700–712. [Google Scholar]
- Alarcon-Segovia, L.C.; Daza-Agudelo, J.I.; Glisoni, R.J.; Acha, C.; De Zan, M.M.; Rintoul, I. A multiparametric model for the industrialization of co-precipitation synthesis of nano-commodities. Nanotechnology 2020, 31, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Daza-Agudelo, J.I.; Badano, J.M.; Rintoul, I. Kinetics and thermodynamics of swelling and dissolution of PVA gels obtained by freeze-thaw technique. Chem. Phys. 2018, 216, 14–21. [Google Scholar] [CrossRef]
- Recursos Educativos de Química Orgánica. Available online: http://www.ugr.es/~quiored/lab/tablas_espec/ir.htm (accessed on 14 November 2019).
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Physico-chemical properties, morphology and antioxidant activity of film from fish skin gelatin incorporated with root essential oils. J. Food Eng. 2013, 117, 350–360. [Google Scholar] [CrossRef]
- Chao, S.C.; Wang, M.J.; Pai, N.S.; Yen, S.K. Preparation and characterization of gelatin–hydroxyapatite composite microspheres for hard tissue repair. Mater. Sci. Eng. C 2015, 57, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Brazel, C.S. The plasticizer market: An assessment of traditional plasticizers and research trends to meet new challenges. Prog. Polym. Sci. 2004, 9, 1223–1248. [Google Scholar] [CrossRef]
- Gontard, N.; Guilbert, S.; Cuq, J.L. Water and glycerol as plasticizers affect mechanical and water vapor barrier properties of an edible wheat gluten film. J. Food Sci. 1993, 58, 206–211. [Google Scholar] [CrossRef]
- Jacobsen, S.; Fritz, H.G. Plasticizing polylactide the effect of different plasticizers on the mechanical properties. Polym. Eng. Sci. 1999, 39, 1303–1310. [Google Scholar] [CrossRef]
- Jia, P.; Xia, H.; Tang, K.; Zhou, Y. Plasticizers derived from biomass resources: A short review. Polymers 2018, 10, 1303. [Google Scholar] [CrossRef]
- Vanin, F.M.; Sobral, P.J.A.; Menegalli, F.C.; Carvalho, R.A.; Habitante, A.M.Q.B. Effects of plasticizers and their concentrations on thermal and functional properties of gelatin-based films. Food Hydrocoll. 2005, 19, 899–907. [Google Scholar] [CrossRef]
- Cao, N.; Yang, X.; Fu, Y. Effects of various plasticizers on mechanical and water vapor barrier properties of gelatin films. Food Hydrocoll. 2009, 23, 729–735. [Google Scholar] [CrossRef]
- Hamzeh, S.; Motamedzadegan, A.; Shahidi, S.A.; Ahmadi, M.; Regenstein, J.M. Effects of drying condition on physico-chemical properties of foam-mat dried shrimp powder. J. Aquat. Food Prod. Tech. 2019, 28, 794–805. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Gómez-Guillén, M.C.; Fernández-Martín, F.; Montero, P. Effects of gelatin origin, bovine-hide and tuna-skin, on the properties of compound gelatin—chitosan films. Food Hydrocoll. 2011, 25, 1461–1469. [Google Scholar] [CrossRef]





| Sample | Parameters | |||
|---|---|---|---|---|
| A (g) | B (mL) | C (mL) | D (min) | |
| S1 | 1.0 | 25 | 1.0 | 1.0 |
| S2 | 1.0 | 50 | 2.5 | 7.5 |
| S3 | 1.0 | 75 | 5.0 | 15.0 |
| S4 | 2.5 | 25 | 2.5 | 1.0 |
| S5 | 2.5 | 50 | 5.0 | 7.5 |
| S6 | 2.5 | 75 | 1.0 | 15.0 |
| S7 | 5.0 | 25 | 5.0 | 1.0 |
| S8 | 5.0 | 50 | 1.0 | 7.5 |
| S9 | 5.0 | 75 | 2.5 | 15.0 |
| Sample | Thickness (mm) | YM (MPa) | MTS (MPa) | RUS (%) |
|---|---|---|---|---|
| S1 | 0.40 ± 0.03 | 0.32 ± 0.03 | 0.30 ± 0.02 | 0.97 ± 0.13 |
| S2 | 0.67 ± 0.05 | 0.10 ± 0.02 | 0.11 ± 0.01 | 0.34 ± 0.01 |
| S3 | 0.58 ± 0.02 | 0.08 ± 0.01 | 0.03 ± 0.01 | 0.34 ± 0.06 |
| S4 | 0.69 ± 0.01 | 0.26 ± 0.04 | 0.17 ± 0.01 | 0.88 ± 0.18 |
| S5 | 0.96 ± 0.05 | 0.24 ± 0.03 | 0.47 ± 0.03 | 1.34 ± 0.49 |
| S6 | 0.40 ± 0.02 | 0.90 ± 0.13 | 0.77 ± 0.09 | 1.74 ± 0.17 |
| S7 | 1.21 ± 0.04 | 0.28 ± 0.05 | 0.28 ± 0.09 | 1.69 ± 0.02 |
| S8 | 0.94 ± 0.03 | 1.04 ± 0.09 | 0.85 ± 0.03 | 1.51 ± 0.10 |
| S9 | 0.86 ± 0.03 | 0.98 ± 0.15 | 0.48 ± 0.12 | 2.26 ± 0.15 |
| Peak Wavenumber (cm−1) | |||||
|---|---|---|---|---|---|
| Sample | OH | AI | AII | AIII | C-O |
| S1 | 3033 | 1654 | 1541 | 1249 | 1026 |
| S2 | 3299 | 1643 | 1552 | 1259 | 1041 |
| S3 | -- | 1654 | 1544 | 1259 | 1002 |
| S4 | 3350 | 1672 | 1531 | 1257 | 1004 |
| S5 | 3298 | 1637 | 1541 | 1265 | 1014 |
| S6 | 3296 | 1650 | 1550 | 1257 | 1060 |
| S7 | 3298 | 1637 | 1541 | 1249 | 1045 |
| S8 | 3298 | 1647 | 1546 | 1259 | 1026 |
| S9 | -- | -- | -- | 1263 | 1017 |
| Coef. | YM | MTS | RUS |
|---|---|---|---|
| a | 278,080 | −87,244 | 532 |
| A | 316,167 | 376,667 | −189 |
| B | 9467 | 25,333 | 0.3 |
| C | −466,333 | −489,833 | −57 |
| D | −1560 | −5274 | −20 |
| A2 | −28,111 | −46,667 | 27 |
| B2 | -42.67 | −218.7 | −0.01 |
| C2 | 51,333 | 65,667 | 7 |
| D2 | 424.9 | 17.09 | 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alarcón-Segovia, L.C.; Daza-Agudelo, J.I.; Rintoul, I. Multifactorial Effects of Gelling Conditions on Mechanical Properties of Skin-Like Gelatin Membranes Intended for In Vitro Experimentation and Artificial Skin Models. Polymers 2021, 13, 1991. https://doi.org/10.3390/polym13121991
Alarcón-Segovia LC, Daza-Agudelo JI, Rintoul I. Multifactorial Effects of Gelling Conditions on Mechanical Properties of Skin-Like Gelatin Membranes Intended for In Vitro Experimentation and Artificial Skin Models. Polymers. 2021; 13(12):1991. https://doi.org/10.3390/polym13121991
Chicago/Turabian StyleAlarcón-Segovia, Lilian C., Jorge I. Daza-Agudelo, and Ignacio Rintoul. 2021. "Multifactorial Effects of Gelling Conditions on Mechanical Properties of Skin-Like Gelatin Membranes Intended for In Vitro Experimentation and Artificial Skin Models" Polymers 13, no. 12: 1991. https://doi.org/10.3390/polym13121991
APA StyleAlarcón-Segovia, L. C., Daza-Agudelo, J. I., & Rintoul, I. (2021). Multifactorial Effects of Gelling Conditions on Mechanical Properties of Skin-Like Gelatin Membranes Intended for In Vitro Experimentation and Artificial Skin Models. Polymers, 13(12), 1991. https://doi.org/10.3390/polym13121991







