Potential Uses of Musaceae Wastes: Case of Application in the Development of Bio-Based Composites
Abstract
1. Introduction
2. Musaceaes
2.1. Origin and Initial Distribution
2.2. Taxonomic Classification
2.3. Modern Classification of Musaceae
2.4. Morphological Characteristics and Development of Musaceae
2.4.1. Root System
2.4.2. Rhizome and Sprouts
2.4.3. Leaves
2.4.4. Pseudostem
2.4.5. Inflorescence
2.4.6. Bunch
2.4.7. Fruit’s Development and Ripening
- The pre-climateric stage, first stage, or green stage begins after the bunch harvest until some physical change is generated, which is characteristic of climatic breathing. There is a slow metabolic activity, and the commercial objective is to prolong it through storage at a temperature of 13 °C and/or the use of controlled atmospheres. The harvest of a Gros Michel banana, Dominico Hartón, and FHIA 20 plantains requires a time between 8 and 10 weeks after the inflorescence in the department of Caldas (Colombia). Chávez-Salazar et al. [10] reported the respective contents of starch being 5.78, 12.73, and 10.18% in the humid base and 18.73, 32.22, and 31.67% in the dry base, respectively, evidencing a higher content in plantains. On the other hand, its °brix did not exceed 11, being characteristic of a green and physiologically immature plantain during 9 days of storage. In a second study, the increase of starch presented in a Dominico Hartón plantain’s pulp was determined by comparing the harvest between weeks 14 and 18 after inflorescence, reporting 56.5 and 74.8% on a dry basis, respectively [31,32]. In another study conducted in Malaysia, starch from green bananas can be obtained between 70 and 80% on a dry basis [33]. The peel also generates a starch contribution between 16.6 and 48.5% in the dry base [34].
- Maturation stage. Various changes are generated in the fruit such as the peel’s change of colour, going from dark green, followed by light green, and ending in yellow. In addition, there is evidence of softening of the skin and pulp, converting starch into reducing sugars, and generating aroma [7,14]. Barrera et al. [36] reported an increase in total solids and reduction of the rigidity and pH in the fruit as the ripening time increases due to the degradation of the starch, generating an accumulation of reducing sugars, mainly glucose, fructose, and sucrose, until a content of 35–40% is achieved in the fruit when it has an intense yellow peel.
- Final stage. At the end of the breathing process, the fruit’s physiological death is obtained, revealing a brown to black skin, and the pulp changes colour, going from white to brown with a gelatinous texture [7]. The time required for the ripening mentioned in the above stages is between 13 and 20 days [7,14].
2.5. General Climatic Requirements
3. Starchy Products Obtained from Banana and Plantain Bunches
3.1. Native Starch
3.2. Flour
3.3. Starch and Flour from Musaceae
3.4. Methods of Extracting Starch and Flour from Musaceae
4. Lignocellulosic Fibres from Banana Pseudostem
4.1. Lignocellulosic Fibres
4.1.1. Cellulose
4.1.2. Hemicellulose
4.1.3. Lignin
4.2. Pseudostem’s Lignocellulosic Fibres
4.2.1. Chemical Composition
4.2.2. Physical Properties
4.2.3. Tensile Mechanical Properties
4.2.4. Thermal Properties
4.3. Methods of Extraction of Lignocellulosic Fibres from the Musaceae’s Pseudostem
4.3.1. Mobile Blade
4.3.2. Manual Fixed Blade (Hand Stripping)
4.3.3. Spindle Peeling Blade (Spindle Stripping)
4.4. Pre-Treatment Methods for Lignocellulosic Fibres
4.4.1. Physical Methods
- Grinding. This is based on reducing the lignocellulosic fibres’ particle size when desired to produce a biocomposite by implementing a reinforcement with short fibres that achieve lengths of between 0.2 and 2 mm. The use of short fibres increases the specific surface area and reduces the degree of polymerisation and crystallinity of the cellulose. However, this adaptation method must complement a second method to contribute to the adhesion or mechanical anchorage with the matrix. Ball, vibrating balls, discs, or hammers mills can be used in the milling process [67,75,76].
- Corona treatment. From the corona discharge application on the fibre’s surface, a surface oxidative activation is generated, and its polarity increases, allowing a greater degree of compatibility between the hydrophilic fibres with hydrophobic polymeric matrices, especially when using polymers derived from petroleum. By achieving an adequate exposure time and intensity of the corona discharge in the lignocellulosic fibres, increases in the modulus of elasticity and maximum resistance to tension can be obtained. However, if the exposure time is increased to values greater than 15 min, the tenacity is reduced, and the degree of polymeric degradation of the lignocellulosic fibres increases [72].
- Plasma treatment. This procedure is similar to corona treatment based on exposing the lignocellulosic fibres to an electrical discharge, achieving a surface modification. However, for its adequate execution at low temperatures and exposure to atmospheric pressure, it is required to handle a greater number of process variables such as the type of gas to be used (e.g., oxygen, helium), type of frequency (radiofrequency or low frequency), flow, pressure, and concentration or plasma power [50]. Reactive free radicals are produced, as well as variations in surface energy, the generation of surface cross-links, and the development of the hydrophobic character of lignocellulosic fibres [50,72].
- Steam explosion. Lignocellulosic fibres are exposed to saturated water vapour at a temperature of 160 to 290 °C and a pressure of 0.70 to 4.85 MPa for 1 to 60 min in a closed system such as a reactor. Water is the most commonly used solvent; however, changing it to sodium hydroxide (NaOH), sulphuric acid (H2SO4), sulphur oxide (SO2), and sodium hypochlorite (NaClO) solutions increases the intensity of the operation [75,77]. When the fibres are exposed to the solvent at high pressure for short periods and subsequent decompression, the fibre structure explodes [77]. The macromolecule detached with the highest proportion of fibres is hemicellulose, which is hydrolysed and solubilised in water from simple sugars, mainly glucose and xylose. Its structure is altered for lignin, and it is removed in low proportions in lignocellulosic fibres [67,69]. If it is intended to increase the amount of lignin extracted, a temperature higher than the glass transition temperature of lignin (142 °C) should be considered during the unit operation’s execution to obtain a higher fibre surface roughness and increased crystallinity index [77,78].
4.4.2. Chemical Methods
- Silanisation. Silanes are multifunctional molecules that are used as coupling agents to form covalent bonds called siloxane bridges, with phenyltrimethoxysilane being one of the most widely used, due to its high efficiency in generating bonds with lignocellulosic fibres (hydrophilic character) and the matrix (hydrophobic character); however, other coupling agents are found such as epoxy and urethane silanes [72]. Initially, the cellulose presented in the lignocellulosic fibre is modified through a chemical reaction by condensation between the silanol group belonging to the coupling agent and the hydroxyl group found in the cellulose, generating the Si–O–cellulose bond. In contrast, the other end of the coupling agent reacts with the matrix, generating the Si–matrix bond. Subsequently, as it has a modified fibre, its surface’s polarity is reduced, facilitating its mixture with polymeric matrices of a more hydrophobic nature. It also contributes to the reduction of porosities of the fibres from the coating of the coupling agent. This method generates biocomposites with an increase of mechanical resistance greater than that provided by alkalisation and acetylation [74].
- Alkalisation. This is the least complex and least costly treatment, using sodium hydroxide solutions between 2 and 15%, requiring immersion times of between 2 and 24 h and temperatures of between 60 and 120 °C to modify the surface of the lignocellulosic fibres [9,75] by breaking hydrogen bridges between the cellulose and other molecules, facilitating the release of significant portions of lignin, hemicellulose, waxes, pectins, and oils that cover the external cell walls, contributing to surfaces with greater roughness. It has also been shown that the hydroxyl groups (-O-H) in the cellulose are broken or altered, creating more reactive groups (-O-Na) and reducing the hydrophilic nature of the cellulose present in the modified fibre [74]. If the fibres’ immersion time in high sodium hydroxide concentrations is prolonged, damage or cracks may be generated in the fibre. In contrast, with an adequate concentration of alkali, the fibre diameter is reduced, favouring interfacial adhesion with the matrix, since the surface area and the aspect ratio (length/diameter) are increased [50].
- Coupling by maleation. Maleation coupling agents such as maleic anhydride generate C-C bonds between the surface of the lignocellulosic fibre and the polymer matrix. Two types of chemical reactions are generated in the maleic anhydrous: (a) between the maleic anhydrous and the hydroxyl groups of the lignocellulosic fibres; (b) between the maleic anhydrous and the polymeric matrix. One of the alternatives for carrying out the chemical reaction consists of melting the polymeric matrix with 0.5% maleic anhydrous and then coating or mixing the maleic matrix with the lignocellulosic fibres, allowing the generation of links with the hydroxyl groups coming from the cellulose and contributing to greater mechanical resistance and a reduction in the absorption of water in the biocomposite [79].
- Acetylation. The use of acetic acid and acetic anhydride is required to modify the lignocellulosic fibre’s surface, generating a hydrophobic character by incorporating acetyl groups (CH3CO) in the hydroxyl groups presented in the cellulose. Initially, the lignocellulosic material must be immersed in acetic acid. The acetic anhydride is added during an immersion time of between 1 and 3 h at a high temperature to accelerate the esterification’s chemical reaction between the hydroxyl group and the anhydrous group. The level of modification can be quantified by the degree of acetylation, with 18% being the maximum value permitted, since there have been considerable reductions in the degree of polymerisation and crystallinity of the cellulose contributing to the reduction of the tensile maximum resistance. However, acetylation values greater than 18% contribute to strengthening the modified fibre’s hydrophobic character [50,72,74]. This type of surface modification provides greater hydrophobic character and tensile strength in banana pseudostem fibres than that generated in plasma treatment [64].
4.4.3. Biological Methods
4.4.4. Influence of Pre-Treatment Methods on Lignocellulosic Fibres from Musaceae Pseudostem
- Steam explosion. This physical technique on fibre from the banana pseudostem generates an increase in the cellulose’s thermal degradation temperature, going from 390 (native fibre) to 400 °C [65]. In a second study of the steam explosion in lignocellulosic fibres from banana pseudostem, an autoclave was used at a temperature of 220 °C, evaluating the structural changes of the fibres when exposed to high-pressure water vapour, using two operating times, 2 and 4 min. When comparing the holocellulose content (cellulose + hemicellulose) of the native fibre with its modified state, reducing its content was evidenced, going from 57.5 to 52.8%, due to the removal of the hemicellulose and amorphous cellulose in the fibres. Simultaneously, the proportion of lignin was increased, starting with a value of 20.3% in its native state until reaching a content of 23.2% when achieving the surface modification of the fibres when exposed for 4 min. By establishing a longer operation time, greater severity of the physical operation on the fibres is established, evidencing greater roughness through Scanning Electronic Microscopy (SEM), a greater index of crystallinity, and the degree of polymerisation of the cellulose is reduced [54,80]. However, replacing water with a 2% NaOH solution in an autoclave for 1 h at a temperature between 110 and 120 °C showed an increase in cellulose content from 64% in its native state to 82.4% in its modified state and a reduction in hemicellulose and lignin from 18.6 to 13.9% and 4.9 to 3.6%, respectively [77].
- Plasma treatment. A surface treatment equipment with plasma technology was used to carry out the surface modification on plantain pseudostem fibres, using the following conditions: ambient temperature, atmospheric pressure, ceramic electrodes with a potential discharge supply of 1 kW, speed of 4 m/min, and variation in dosage of 1, 3, and 6 kW min/m2. The modified fibres were characterised by using the tensile test, FT-IR spectroscopy, thermogravimetric analysis, and contact angle. In the FT-IR analysis, the formations of the 2850 and 2900 cm−1 bands are shown, relating a C-C transformation, contributing to a hydrophobic character in the fibres. The thermal stability of the cellulose shows an increase from 336.3 °C in the native fibre to 337.1 °C in the fibre exposed to a dosage of 1 kW min/m2, 342.1 °C at 3 kW min/m2, and 339.1 °C at 6 kW min/m2, identifying an increase in the mentioned property between 0.2 and 1.7%. The toughness is reduced in the tensile test when the modification is made, going from 0.3 (native fibre) to values between 0.20 and 0.27 N/Tex. At the same time, the contact angle is increased, going from 92.2° in the native fibre to values between 97.5 and 106.8°, being the superior value of the angle belonging to the dosage of 6 kW min/m2, contributing to the increase of the hydrophobic character in the fibre [64].
- The blend of alkalisation with peroxidation. The fibre of the pseudostem from the banana tree (10 g) was exposed to different solutions to remove the non-cellulose components, starting with an immersion of the fibre in a solution of sulphuric acid (H2SO4) at 55 °C for 2 h to remove the external wax, followed by a wash with distilled water to remove residual H2SO4. The second immersion consisted of using a solution composed of 200 mL of hydrogen peroxide (H2O2) with a concentration of 7 g/L, 3% of sodium silicate (Na2SiO3), and 2% of sodium polyphosphate at 95 °C for 1.5 h to remove hemicellulose and lignin. The third immersion consisted of the use of 200 mL of sodium hydroxide (NaOH) solution with a concentration of 9 g/L boiling for 3 h, and then, a wash was carried out using a solution of H2SO4 to neutralise the alkaline residues, and finally, the modified fibres were dried at 105 °C for 24 h. When evaluating the tensile properties of the modified fibres compared to their native state, the removal of lignin and hemicellulose contributed to the increase in the packing of the cellulose, generating an increase in the maximum tensile strength from 210 to 333 MPa, while the modulus of elasticity was reduced from 26.86 to 22.56 GPa, and the deformation at the breakpoint increased from 0.8 to 1.6%. Concerning thermal stability, the cellulose’s thermal degradation temperature increased by 10 °C due to its concentration in the modified fibres. From X-ray diffraction, the crystallographic pattern consisted of two peaks at 16 and 22.5° 2θ. Simultaneously, the crystallinity index of the native fibre presented a value of 56.6%, achieving an increase of 61.2% when performing the chemical modification due to the removal of amorphous structures represented in the hemicellulose [61].
- Acetylation. Two surface modification treatments were used on the plantain pseudostem fibres, the first being a blend of acetic anhydride and acetone at a ratio of 1:10 w/w and the second being a blend of acetic anhydride, epichlorhydrin, and acetone at a ratio of 1:1:20. The native fibres were submitted to immersion in the respective treatments for 24 h at 20 °C. Subsequently, the fibres were washed with acetone and distilled water to remove chemical residues. Then, the fibres were dried in an oven at 105 °C for 24 h. The modified fibres were characterised from the tensile test, FT-IR spectroscopy, thermogravimetric analysis, and contact angle. From the FT-IR analysis and comparison between the native fibres and the modified fibres, a reduction of the absorbance presented in the 3330 and 3600 cm−1 bands was identified in the modified fibres, generating a greater reduction in the fibres exposed to acetic anhydride and epichlorohydrin; it is possible that chemical reactions were generated in a more significant number of hydroxyl groups present in the fibre to establish esterification by the acetic anhydride and alkylation by the epichlorohydrin. In addition, bands of 3700 and 3850 cm−1 are evident in the modified fibres, relating the presence of -CH3 groups due to acetylation and bands of 2850 and 2900 cm−1 due to alkylation to give a greater hydrophobic character to the fibres. The thermal stability of the cellulose presented an increase from 336.3 °C in the native fibre to 359.3 °C in the fibre exposed to acetic anhydride, giving an increase in the mentioned property of 6.8% due to the increase of the cellulose content in the fibre, while the use of epichlorohydrin and the blend of acetic anhydride and epichlorohydrin reduce the degradation temperature to 329.5 and 328.6 °C, respectively. The toughness is reduced in the tensile test when the modification is carried out, going from 0.3 (native fibre) to values between 0.16 and 0.25 N/Tex. Simultaneously, the contact angle is increased, going from 92.2 ° in the native fibre to values between 116.3 and 133.14 °, the higher value given by the acetic anhydride and epichlorohydrin blend, due to the greater hydrophobic character obtained in the fibre [64].
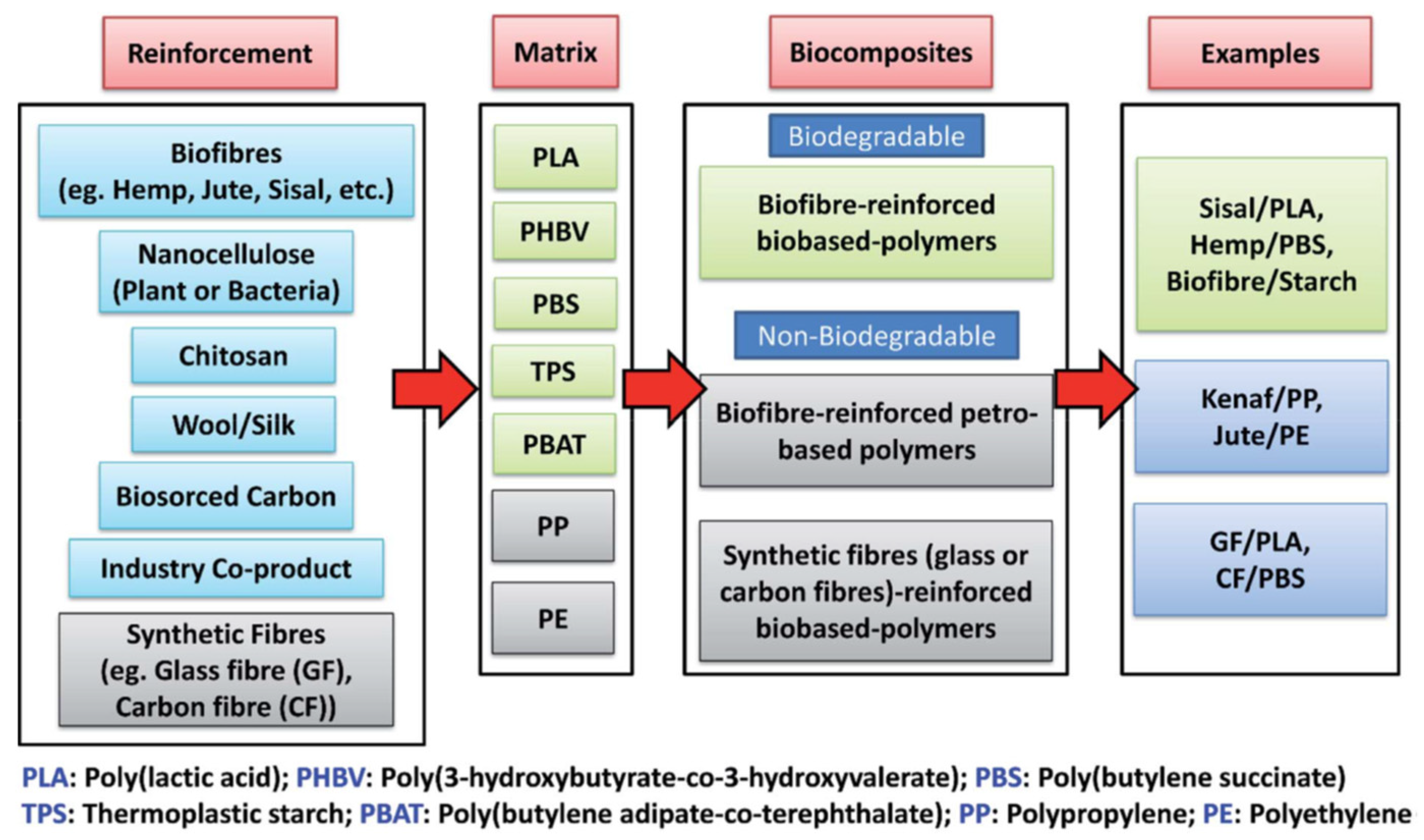
5. Development of Biocomposites Made up of Lignocellulosic Fibres
5.1. Biocomposites
5.1.1. Reinforcements
5.1.2. Matrices
5.1.3. Processing Techniques
5.2. Biocomposites from Starches, Flours, and Fibres from Musaceae
5.2.1. Synthetic Oil-Based Polymers
5.2.2. Agro-Polymers
5.2.3. Polymers of Microbial Origin
5.2.4. Biodegradable Synthetic Polymers
5.3. Applications of Biocomposites
5.4. Containers and Packaging
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics Recycling: Challenges and Opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef]
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Úbeda, B.; Hernández-León, S.; Palma, Á.T.; Navarro, S.; García-de-Lomas, J.; Ruiz, A.; et al. Plastic Debris in the Open Ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.A.; Kalra, B. Biodegradable Polymers for the Environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef]
- Ma, J. Banana Pseudostem: Properties Nutritional Composition and Use as Food. Master’s Thesis, The University of New South Wales, Sidney, Australia, 2015; pp. 1–252. [Google Scholar]
- Bertolini, A. Starches Characterization, Properties and Applications; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2010; ISBN 9781420080230. [Google Scholar]
- Janssen, L.; Moscicki, L. Thermoplastic Starch A Green Material for Various Industries; Wiley-VCH Verlag: Weinheim, Germany, 2009; ISBN 9780470146835. [Google Scholar]
- Robinson, J.; Galán, V. Bananas and Plantains, 2nd ed.; CABI: Oxfordshire, UK, 2010; ISBN 9781845936587. [Google Scholar]
- Hoyos-Leyva, J.D.; Jaramillo-Jiménez, P.A.; Giraldo-Toro, A.; Dufour, D.; Sánchez, T.; Lucas-Aguirre, J.C. Physical, Morphological Characterization and Evaluation of Pasting Curves of Musa spp.|Caracterización Física, Morfológica y Evaluación de Las Curvas de Empastamiento de Musáceas (Musa spp.). Acta Agron. 2012, 61, 214–229. [Google Scholar]
- Adeniyi, A.G.; Ighalo, J.O.; Onifade, D.V. Banana and Plantain Fiber-Reinforced Polymer Composites. J. Polym. Eng. 2019, 39, 597–611. [Google Scholar] [CrossRef]
- Chávez-Salazar, A.; Bello-Pérez, L.A.; Agama-Acevedo, E.; Castellanos-Galeano, F.J.; Álvarez-Barreto, C.I.; Pacheco-Vargas, G. Isolation and Partial Characterization of Starch from Banana Cultivars Grown in Colombia. Int. J. Biol. Macromol. 2017, 98, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.; Jarvis, A.; vanden Bergh, I.; Staver, C.; Turner, D. Changing Climates: Effects on Growing Conditions for Banana and Plantain (Musa spp.) and Possible Responses. In Crop Adaptation to Climate Change; Wiley-Blackwell: Oxford, UK, 2011; pp. 426–438. ISBN 9780813820163. [Google Scholar]
- Manzo-Sánchez, G.; Buenrostro-Nava, M.T.; Guzmán-González, S.; Orozco-Santos, M.; Youssef, M.; Escobedo-Gracia Medrano, R.M. Genetic Diversity in Bananas and Plantains (Musa spp.). Mol. Approaches Genet. Divers. 2015. [Google Scholar] [CrossRef]
- Sales, E.K.; Butardo, N.G.; Paniagua, H.G.; Jansen, H.; Dolezel, J.; Mindanao, S.; Mexico-Veracruz, K.C.; Batan, E. Assessment of Ploidy and Genome Constitution of Some Musa Balbisiana Cultivars Using DArT Markers. Philipp. J. Crop Sci. 2011, 36, 11–18. [Google Scholar]
- Gowen, S. Bananas and Plantains World Crop Series; Springer-S. Chapman & Hall: Dordrecht, The Netherlands, 1995; ISBN 9789401043175. [Google Scholar]
- DANE. El Cultivo Del Plátano (Musa Paradisiaca), Un Importante Alimento Para El Mundo. Boletín Mens. Insumos Factores Asoc. Prod. Agropecu. 2014, 22, 52. [Google Scholar]
- Minagricultura. Anuario Estadístico Del Sector Agropecuario. 2016. Available online: https://repository.agrosavia.co/handle/20.500.12324/34404 (accessed on 28 January 2021).
- Amaya, N. The World’s Leading Plantain Producers–WorldAtlas. Available online: https://www.worldatlas.com/articles/the-world-s-leading-plantain-producers.html (accessed on 28 January 2021).
- Statista. Global Leading Producers of Bananas 2018|Statista. Available online: https://www.statista.com/statistics/811243/leading-banana-producing-countries/ (accessed on 28 January 2021).
- Cedeño, G.; Suarez, C.; Vera, D.; Fadda, C.; Jarvis, D. Early Detection of Resistance to Mycosphaerella Fijiensis in Local Genotypes of Musa in Ecuador. Sci. Agropecu. 2017, 8, 29–42. [Google Scholar] [CrossRef][Green Version]
- UNIBAN Banano. Available online: https://www.uniban.com/index.php/es/productos-1/banano (accessed on 28 January 2021).
- Espitia, P.; Pardo, Y.; Montalvo, A. Características Del Análisis Proximal de Harinas Obtenidas de Frutos de Plátanos Variedades Papocho y Pelipita (Musa ABB Simmonds) Proximate Analysis Characteristics of Flours Obtained from Papocho and Pelipita Plantains (Musa ABB Simmonds). Acta Agronómica 2013, 62, 189–195. [Google Scholar]
- Giraldo, M.C.; Ligarreto, G.A. Análisis de La Variabilidad Genética de La Colección Colombiana de Musáceas Usando Marcadores Isoenzimáticos. Acta Agronómica 2011, 60, 108–119. [Google Scholar]
- Guapacha, S.E.; Salazar, M.S.; Aguillón, J.; Landázuri, P. Similaridad cariotípica entre diversas variedades de musa spp del Quindío-Colombia. Cultiv. Trop. 2017, 38, 119–126. [Google Scholar]
- Nadal-Medina, R.; Manzo-Sánchez, G.; Orozco-Romero, J.; Orozco-Santos, M.; Guzmán-González, S. Diversidad Genética de Bananos y Plátanos. Rev. Fitotec. Mex. 2009, 32, 1–7. [Google Scholar]
- Cuellar, A.; Álvarez, E.; Castaño, J. Evaluación de Resistencia de Genotipos de Plátano y Banano a La Sigatoka Negra (Mycosphaerella Fijiensis Morelet.). Rev. Fac. Nac. Agron. Medellín 2011, 64, 5853–5865. [Google Scholar]
- Pillay, M.; Ude, G.; Kole, C. Genetics, Genomics and Breeding of Bananas; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2012; ISBN 9781466505162. [Google Scholar]
- Pereira, A.L.S.; do Nascimento, D.M.; Souza, M.S.M.; Cassales, A.R.; Saraiva Morais, J.P.; de Paula, R.C.M.; Rosa, M.F.; Feitosa, J.P.A. Banana (Musa sp. cv. Pacovan) Pseudostem Fibers Are Composed of Varying Lignocellulosic Composition throughout the Diameter. BioResources 2014, 9, 7749–7763. [Google Scholar] [CrossRef]
- Mamun, A.A.; Heim, H.P.; Faruk, O.; Bledzki, A.K. The use of banana and abaca fibres as reinforcements in composites. In Biofiber Reinforcements in Composite Materials; Elsevier Inc.: Cambridge, UK, 2015; pp. 236–272. ISBN 9781782421276. [Google Scholar]
- Aziz, N.A.A.; Ho, L.H.; Azahari, B.; Bhat, R.; Cheng, L.H.; Ibrahim, M.N.M. Chemical and Functional Properties of the Native Banana (Musa Acuminata x Balbisiana Colla cv. Awak) Pseudo-Stem and Pseudo-Stem Tender Core Flours. Food Chem. 2011, 128, 748–753. [Google Scholar] [CrossRef]
- Subagyo, A.; Chafidz, A. Banana Pseudo-Stem Fiber: Preparation, Characteristics, and Applications. In Banana Nutrition-Function and Processing Kinetics; IntechOpen: London, UK, 2018; pp. 1–19. [Google Scholar]
- Mejía Gutierrez, L.F. Evaluación Del Comportamiento Físico y Químico Poscosecha Del Plátano Dominico Harton (Musa AAB Simmonds) Cultivado En El Municipio de Belalcazar (Caldas). Master’s Thesis, Universidad Nacional de Colombia, Belalcázar, Colombia, 2013. [Google Scholar]
- Mejía-Gutiérrez, L.F.; Giraldo-Gómez, G.I.; Ramírez-Ramírez, D.D.J. Incidence of the Harvesting Age on Postharvest Characteristics Behavior of Dominico Hartón Plantain (Musa AAB Simmonds) | Efecto de La Edad de Cosecha En Las Características Poscosecha Dei Plátano Dominico-Hartón (Musa AAB Simmonds). Acta Agron. 2012, 61, 345–352. [Google Scholar]
- Shittu, R.; Lasekan, O.; Karim, R.; Sulaiman, R. Plantain-Starch: Microstructural, Physicochemical, and Morphological Characteristics of Two Cultivars Grown in Malaysia. Starch Stärke 2016, 68, 1187–1195. [Google Scholar] [CrossRef]
- Hernández-Carmona, F.; Morales-Matos, Y.; Lambis-Miranda, H.; Pasqualino, J. Starch Extraction Potential from Plantain Peel Wastes. J. Environ. Chem. Eng. 2017, 5, 4980–4985. [Google Scholar] [CrossRef]
- Guiné, R.; Costa, D. Chemichal composition and bioactive compounds in bananas and postharvest alterations. In Bananas Cultivation, Consumption and Crop Diseases; Pearson, V., Ed.; Nova Science Publishers: New York, NY, USA, 2016; pp. 1–145. ISBN 9781634854184. [Google Scholar]
- Barrera, J.; Arrazola, G.; Cayón, D. Caracterización Fisicoquímica y Fisiológica Del Proceso de Maduración de Plátano Hartón (Musa AAB Simmonds) En Dos Sistemas de Producción. Acta Agronómica 2010, 59, 20–29. [Google Scholar]
- Palencia, G.E.; Gómez, S.R.; Martín, J.E. Manejo Sostenible Del Cultivo Del Plátano. Corpoica; Produmedios: Bucaramanga, Colombia, 2006; p. 28. [Google Scholar]
- Lindeboom, N.; Chang, P.R.; Tyler, R.T. Analytical, Biochemical and Physicochemical Aspects of Starch Granule Size, with Emphasis on Small Granule Starches: A Review. Starch Stärke 2004, 56, 89–99. [Google Scholar] [CrossRef]
- Singh, N.; Singh, J.; Kaur, L.; Singh, N.; Singh, B. Morphological, Thermal and Rheological Properties of Starches from Different Botanical Sources. Food Chem. 2003, 81, 219–231. [Google Scholar] [CrossRef]
- Gunaratne, A.; Hoover, R. Effect of Heat-Moisture Treatment on the Structure and Physicochemical Properties of Tuber and Root Starches. Carbohydr. Polym. 2002, 49, 425–437. [Google Scholar] [CrossRef]
- Ceballos, H.; de La Cruz, C. Taxonomía y Morfología de La Yuca. In La Yuca en el Tercer Milenio; Centro Internacional de Agricultura Tropical: Cali, Colombia, 2002; pp. 17–33. ISBN 958-694-043-8. [Google Scholar]
- Giraldo Toro, A.; Gibert, O.; Briffaz, A.; Ricci, J.; Dufour, D.; Tran, T.; Bohuon, P. Starch Gelatinization and in Vitro Digestibility Behaviour after Heat Treatment: Comparison between Plantain Paste and Piece of Pulp. Carbohydr. Polym. 2016, 147, 426–435. [Google Scholar] [CrossRef]
- Giraldo Toro, A.; Gibert, O.; Ricci, J.; Dufour, D.; Mestres, C.; Bohuon, P. Digestibility Prediction of Cooked Plantain Flour as a Function of Water Content and Temperature. Carbohydr. Polym. 2015, 118, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Bohrer, B.M. The Effect of Tropical Flours (Breadfruit and Banana) on Structural and Technological Properties of Beef Emulsion Modeling Systems. Meat Sci. 2020, 163, 108082. [Google Scholar] [CrossRef] [PubMed]
- Otegbayo, B.; Lana, O.; Ibitoye, W. Isolation And Physicochemical Characterization Of Starches Isolated From Plantain (Musa paradisiaca) And Cooking Banana (Musa sapientum). J. Food Biochem. 2010, 34, 1303–1318. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Andrade-Mahecha, M.M.; Sobral, P.J.D.A.; Menegalli, F.C. Isolation and Characterization of the Flour and Starch of Plantain Bananas (Musa paradisiaca). Starch Stärke 2012, 64, 382–391. [Google Scholar] [CrossRef]
- Montoya, J.; Quintero, V.D.; Lucas, J.C. Evaluación Fisicotermica y Reológica de Harina y Almidón de Plátano Dominico Hartón (Musa Paradisiaca Abb). Temas Agrar. 2014, 19, 214. [Google Scholar] [CrossRef]
- Rodriguez-Ambriz, S.L.; Mendez-Montealvo, G.; Velazquez, G.; Bello-Perez, L.A. Thermal, Rheological, and Structural Characteristics of Banana Starches Isolated Using Ethanol. Starch Stärke 2017, 69. [Google Scholar] [CrossRef]
- Bismarck, A.; Mishra, S.; Lampke, T. Plant Fibers as Reinforcement for Green Composites. In Natural Fibers, Biopolymers, and Biocomposites; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2005; ISBN 9780203508206. [Google Scholar]
- Gholampour, A.; Ozbakkaloglu, T. A Review of Natural Fiber Composites: Properties, Modification and Processing Techniques, Characterization, Applications; Springer: New York, NY, USA, 2020; Volume 55, ISBN 1085301903990. [Google Scholar]
- Li, X.; Tabil, L.G.; Panigrahi, S. Chemical Treatments of Natural Fiber for Use in Natural Fiber-Reinforced Composites: A Review. J. Polym. Environ. 2007, 15, 25–33. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Natural Fibers, Biopolymers, and Biocomposites; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2005; ISBN 9780203508206. [Google Scholar]
- Mukhopadhyay, S.; Fangueiro, R. Physical Modification of Natural Fibers and Thermoplastic Films for Composites-A Review. J. Thermoplast. Compos. Mater. 2009, 22, 135–162. [Google Scholar] [CrossRef]
- Müssig, J. Industrial Applications of Natural Fibres: Structure, Properties and Technical Applications; John Wiley & Sons, Ltd.: West Sussex, UK, 2010; ISBN 9780470695081. [Google Scholar]
- Mohit, H.; Arul Mozhi Selvan, V. A Comprehensive Review on Surface Modification, Structure Interface and Bonding Mechanism of Plant Cellulose Fiber Reinforced Polymer Based Composites. Compos. Interfaces 2018, 25, 629–667. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Fangueiro, R.; Shivankar, V. Variability of Tensile Properties of Fibers from Pseudostem of Banana Plant. Text. Res. J. 2009, 79, 387–393. [Google Scholar] [CrossRef]
- Azwa, Z.N.; Yousif, B.F.; Manalo, A.C.; Karunasena, W. A Review on the Degradability of Polymeric Composites Based on Natural Fibres. Mater. Des. 2013, 47, 424–442. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Characterizing Natural Cellulose Fibers from Velvet Leaf (Abutilon Theophrasti) Stems. Bioresour. Technol. 2008, 99, 2449–2454. [Google Scholar] [CrossRef]
- Manilal, V.; Sony, J. Banana Pseudostem Characterization and Its Fiber Property Evaluation on Physical and Bioextraction. J. Nat. Fibers 2011, 8, 149–160. [Google Scholar] [CrossRef]
- Assis, F.S.; Margem, F.M.; Cordeiro, T.C.; Figueiredo, A.B.H.; Braga, F.O.; Monteiro, S.N. Photoacoustic Thermal Characterization of Banana Fibers. Mater. Res. 2015, 18, 240–245. [Google Scholar] [CrossRef][Green Version]
- Xu, S.; Xiong, C.; Tan, W.; Zhang, Y. Microstructural, Thermal, and Tensile Characterization of Banana Pseudo-Stem Fibers Obtained with Mechanical, Chemical, and Enzyme Extraction. BioResources 2015, 10, 3724–3735. [Google Scholar] [CrossRef]
- Mumthas, A.C.S.I.; Wickramasinghe, G.L.D.; Gunasekera, U.S.W. Effect of Physical, Chemical and Biological Extraction Methods on the Physical Behaviour of Banana Pseudo-Stem Fibres: Based on Fibres Extracted from Five Common Sri Lankan Cultivars. J. Eng. Fibers Fabr. 2019, 14. [Google Scholar] [CrossRef]
- Gañán, P.; Zuluaga, R.; Restrepo, A.; Labidi, J.; Mondragon, I. Plantain Fibre Bundles Isolated from Colombian Agro-Industrial Residues. Bioresour. Technol. 2008, 99, 486–491. [Google Scholar] [CrossRef]
- Rodríguez, L.; Fangueiro, R.; Orrego, C. Effect of Chemical and Plasma DBD Treatments on Pseudostem Plantain Fiber Properties. Rev. Latinoam. Metal. Mater. 2015, 35, 295–304. [Google Scholar]
- Sheng, Z.; Shen, Y.; Dai, H.; Pan, S.; Ai, B.; Zheng, L.; Zheng, X.; Xu, Z. Physicochemical Characterization of Raw and Modified Banana Pseudostem Fibers and Their Adsorption Capacities for Heavy Metal Pb2+ and Cd2+ in Water. Polym. Compos. 2016, 39, 1869–1877. [Google Scholar] [CrossRef]
- Cadavid, Y.; Cadena, E.; Velez, J.; Santa, J. Degradation of dyes using plantain fibers modified with nanoparticules. In Natural Fibres: Advances in Science and Technology Towards Industrial Applications; Springer: Dordrecht, The Netherlands, 2016; pp. 99–111. ISBN 9789401775151. [Google Scholar]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass Pretreatment: Fundamentals toward Application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Galbe, M.; Zacchi, G. Pretreatment: The Key to Efficient Utilization of Lignocellulosic Materials. Biomass Bioenergy 2012, 46, 70–78. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of Promising Technologies for Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The Path Forward for Biofuels and Biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef]
- Belgacem, M.N.; Bataille, P.; Sapieha, S. Effect of Corona Modification on the Mechanical Properties of Polypropylene/Cellulose Composites. J. Appl. Polym. Sci. 1994, 53, 379–385. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Fink, H.P.; Sain, M. Biocomposites Reinforced with Natural Fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- Pizzi, A.; Kueny, R.; Lecoanet, F.; Massetau, B.; Carpentier, D.; Krebs, A.; Loiseau, F.; Molina, S.; Ragoubi, M. High Resin Content Natural Matrix-Natural Fibre Biocomposites. Ind. Crop. Prod. 2009, 30, 235–240. [Google Scholar] [CrossRef]
- Kabir, M.M.; Wang, H.; Lau, K.T.; Cardona, F. Chemical Treatments on Plant-Based Natural Fibre Reinforced Polymer Composites: An Overview. Compos. Part B Eng. 2012, 43, 2883–2892. [Google Scholar] [CrossRef]
- Cardona, C.; Moncada, J.; Aristizabal, V. Biorefineries: Design and Analysis; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2019; ISBN 9781138080027. [Google Scholar]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef]
- Deepa, B.; Abraham, E.; Cherian, B.M.; Bismarck, A.; Blaker, J.J.; Pothan, L.A.; Leao, A.L.; de Souza, S.F.; Kottaisamy, M. Structure, Morphology and Thermal Characteristics of Banana Nano Fibers Obtained by Steam Explosion. Bioresour. Technol. 2011, 102, 1988–1997. [Google Scholar] [CrossRef]
- Ibrahim, M.; Agblevor, F.; El-Zawawy, W. Isolation and Characterization of Cellulose and Lignin from Steam-Exploded Lignocellulosic Biomass. BioResources 2010, 5, 397–418. [Google Scholar]
- Huneault, M.A.; Li, H. Morphology and Properties of Compatibilized Polylactide/Thermoplastic Starch Blends. Polymer 2007, 48, 270–280. [Google Scholar] [CrossRef]
- Ibrahim, M.; Dufresne, A.; El-Zawawy, W.; Agblevor, F. Banana Fibers and Microfibrils as Lignocellulosic Reinforcements in Polymer Composites. Carbohydr. Polym. 2010, 81, 811–819. [Google Scholar] [CrossRef]
- Nagalakshmaiah, M.; Afrin, S.; Malladi, R.P.; Elkoun, S.; Robert, M.; Ansari, M.A.; Svedberg, A.; Karim, Z. Biocomposites: Present Trends and Challenges for the Future. Green Compos. Automot. Appl. 2018, 197–215. [Google Scholar] [CrossRef]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. A Review of the Recent Developments in Biocomposites Based on Natural Fibres and Their Application Perspectives. Compos. Part A Appl. Sci. Manuf. 2015, 77, 1–25. [Google Scholar] [CrossRef]
- Chang, B.P.; Misra, M. Studies on Durability of Sustainable Biobased Composites: A Review. RSC Adv. 2020, 10, 17955–17999. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated Cellulose and New Nanocomposite Materials: A Review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Callister, W.D., Jr. Material Science and Engineering—An Introduction, 7th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2007; ISBN-13: 978-0-471-73696-7. [Google Scholar]
- Hulleman, S.H.D.; Janssen, F.H.P.; Feil, H. The Role of Water during Plasticization of Native Starches. Polymer 1998, 39, 2043–2048. [Google Scholar] [CrossRef]
- Fishman, M.L.; Coffin, D.R.; Konstance, R.P.; Onwulata, C.I. Extrusion of Pectin/Starch Blends Plasticized with Glycerol. Carbohydr. Polym. 2000, 41, 317–325. [Google Scholar] [CrossRef]
- Van Soest, J.J.G.; Knooren, N. Influence of Glycerol and Water Content on the Structure and Properties of Extruded Starch Plastic Sheets during Aging. J. Appl. Polym. Sci. 1997, 64, 1411–1422. [Google Scholar] [CrossRef]
- Laohakunjit, N.; Noomhorm, A. Effect of Plasticizers on Mechanical and Barrier Properties of Rice Starch Film. Starch Stärke 2004, 56, 348–356. [Google Scholar] [CrossRef]
- Khan, B.; Khan, M.; Samin, G.; Jahan, Z. Thermoplastic Starch: A Posible Biodegradable Food Packaging Material-A Review. J. Food Process Eng. 2017, 40, 1–17. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, M.; Chen, L. Interface and Bonding Mechanisms of Plant Fibre Composites: An Overview. Compos. Part B Eng. 2016, 101, 31–45. [Google Scholar] [CrossRef]
- Müller, C.M.O.; Yamashita, F.; Laurindo, J.B. Evaluation of the Effects of Glycerol and Sorbitol Concentration and Water Activity on the Water Barrier Properties of Cassava Starch Films through a Solubility Approach. Carbohydr. Polym. 2008, 72, 82–87. [Google Scholar] [CrossRef]
- Liu, H.; Yu, L.; Chen, L.; Li, L. Retrogradation of Corn Starch after Thermal Treatment at Different Temperatures. Carbohydr. Polym. 2007, 69, 756–762. [Google Scholar] [CrossRef]
- van Soest, J.J.G.; Hulleman, S.H.D.; de Wit, D.; Vliegenthart, J.F.G. Changes in the Mechanical Properties of Thermoplastic Potato Starch in Relation with Changes in B-Type Crystallinity. Carbohydr. Polym. 1996, 29, 225–232. [Google Scholar] [CrossRef]
- Liu, Q.; Thompson, D.B. Effects of Moisture Content and Different Gelatinization Heating Temperatures on Retrogradation of Waxy-Type Maize Starches. Carbohydr. Res. 1998, 314, 221–235. [Google Scholar] [CrossRef]
- Alanís-López, P.; Pérez-González, J.; Rendón-Villalobos, R.; Jiménez-Pérez, A.; Solorza-Feria, J. Extrusion and Characterization of Thermoplastic Starch Sheets from “Macho” Banana. J. Food Sci. 2011, 76. [Google Scholar] [CrossRef]
- Versino, F.; López, O.V.; García, M.A. Sustainable Use of Cassava (Manihot Esculenta) Roots as Raw Material for Biocomposites Development. Ind. Crop. Prod. 2015, 65, 79–89. [Google Scholar] [CrossRef]
- Florencia, V.; López, O.V.; García, M.A. Exploitation of By-Products from Cassava and Ahipa Starch Extraction as Filler of Thermoplastic Corn Starch. Compos. Part B Eng. 2020, 182, 107653. [Google Scholar] [CrossRef]
- Moriana, R.; Vilaplana, F.; Karlsson, S.; Ribes-Greus, A. Improved Thermo-Mechanical Properties by the Addition of Natural Fibres in Starch-Based Sustainable Biocomposites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 30–40. [Google Scholar] [CrossRef]
- Sisti, L.; Totaro, G.; Marchese, P. PBS Makes Its Entrance into the Family of Biobased Plastics. In Biodegradable and Biobased Polymer Environmental and Biomedical Applications; Scrivener Publishing LLC: Salem, MA, USA, 2016; ISBN 978-1-119-11733-9. [Google Scholar]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.C. Poly (Lactic Acid) Blends: Processing, Properties and Applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Dean, K.; Li, L. Polymer Blends and Composites from Renewable Resources. Prog. Polym. Sci. 2006, 31, 576–602. [Google Scholar] [CrossRef]
- Zaman, H.U.; Beg, M.D.H. Banana Fiber Strands-Reinforced Polymer Matrix Composites. Compos. Interfaces 2016, 23, 281–295. [Google Scholar] [CrossRef]
- Ward, I.M.; Coates, P.D.; Dumoulin, M.M. Solid Phase Processing of Polymers; Hanser Publishers: Munich, Germany, 2000; pp. I–XIX. [Google Scholar] [CrossRef]
- Raquez, J.M.; Narayan, R.; Dubois, P. Recent Advances in Reactive Extrusion Processing of Biodegradable Polymer-Based Compositions. Macromol. Mater. Eng. 2008, 293, 447–470. [Google Scholar] [CrossRef]
- Agüero, Á.; Garcia-Sanoguera, D.; Lascano, D.; Rojas-Lema, S.; Ivorra-Martinez, J.; Fenollar, O.; Torres-Giner, S. Evaluation of Different Compatibilization Strategies to Improve the Performance of Injection-Molded Green Composite Pieces Made of Polylactide Reinforced with Short Flaxseed Fibers. Polymers 2020, 12, 821. [Google Scholar] [CrossRef]
- Singh, R.; Heldman, D. Introduction to Food Engineering, 5th ed.; Elsevier: Burlington, NJ, USA, 2014; ISBN 9780123985309. [Google Scholar]
- Rodríguez, L.J.; Orrego, C.E.; Ribeiro, I.; Peças, P. Life-Cycle Assessment and Life-Cycle Cost Study of Banana (Musa sapientum) Fiber Biocomposite Materials. Proc. Procedia CIRP 2018, 69, 585–590. [Google Scholar] [CrossRef]
- Venkateshwaran, N.; Elaya Perumal, A.; Arunsundaranayagam, D. Fiber Surface Treatment and Its Effect on Mechanical and Visco-Elastic Behaviour of Banana/Epoxy Composite. Mater. Des. 2013, 47, 151–159. [Google Scholar] [CrossRef]
- Cadena, C.E.M.; Vélez, R.J.M.; Santa, J.F.; Otálvaro, G.V. Natural Fibers from Plantain Pseudostem (Musa paradisiaca) for Use in Fiber-Reinforced Composites. J. Nat. Fibers 2017, 14, 678–690. [Google Scholar] [CrossRef]
- Ibrahim, H.; Mehanny, S.; Darwish, L.; Farag, M. A Comparative Study on the Mechanical and Biodegradation Characteristics of Starch-Based Composites Reinforced with Different Lignocellulosic Fibers. J. Polym. Environ. 2018, 26, 2434–2447. [Google Scholar] [CrossRef]
- Guimarães, J.L.; Wypych, F.; Saul, C.K.; Ramos, L.P.; Satyanarayana, K.G. Studies of the Processing and Characterization of Corn Starch and Its Composites with Banana and Sugarcane Fibers from Brazil. Carbohydr. Polym. 2010, 80, 130–138. [Google Scholar] [CrossRef]
- Mo, X.; Qi, X.; Zhong, Y.; Li, R.; Mo, C. Preparation and Properties of Tapioca Starch-Banana Fiber Composites Modified with Magnesium Hydroxide. Adv. Mater. Res. 2011, 194, 1707–1710. [Google Scholar] [CrossRef]
- Mo, X.; Zhong, Y.; Liang, C.; Yu, S. Studies on the Properties of Banana Fibers-Reinforced Thermoplastic Cassava Starch Composites: Preliminary Results. Adv. Mater. Res. 2010, 87–88, 439–444. [Google Scholar] [CrossRef]
- Hermansyah, H.; Carissa, R.; Faiz, M.B.; Deni, P. Food Grade Bioplastic Based on Corn Starch with Banana Pseudostem Fibre/Bacterial Cellulose Hybrid Filler. Adv. Mater. Res. 2014, 997, 158–168. [Google Scholar] [CrossRef]
- Ma, X.; Yu, J.; Kennedy, J.F. Studies on the Properties of Natural Fibers-Reinforced Thermoplastic Starch Composites. Carbohydr. Polym. 2005, 62, 19–24. [Google Scholar] [CrossRef]
- Nery, T.B.R.; dos Santos, Z.I.G.; José, N.M. Development and Characterization of Polyhydroxybutyrate and Banana Fiber Biocomposites|Desenvolvimento e Caracterização de Biocompósitos de Polihidroxibutirato e Fibra de Bananeira. Rev. Mater. 2018, 23. [Google Scholar] [CrossRef]
- Jandas, P.J.; Mohanty, S.; Nayak, S.K. Surface Treated Banana Fiber Reinforced Poly (Lactic Acid) Nanocomposites for Disposable Applications. J. Clean. Prod. 2013, 52, 392–401. [Google Scholar] [CrossRef]
- Shih, Y.F.; Huang, C.C. Polylactic Acid (PLA)/Banana Fiber (BF) Biodegradable Green Composites. J. Polym. Res. 2011, 18, 2335–2340. [Google Scholar] [CrossRef]
- Rodríguez-Soto, K.; Piñeros-Castro, N.; Ortega-Toro, R. Laminated Composites Reinforced with Chemically Modified Sheets-Stalk of Musa Cavendish. Rev. Mex. Ing. Química 2019, 18, 749–758. [Google Scholar] [CrossRef]
- Jandas, P.J.; Mohanty, S.; Nayak, S.K. Renewable Resource-Based Biocomposites of Various Surface Treated Banana Fiber and Poly Lactic Acid: Characterization and Biodegradability. J. Polym. Environ. 2012, 20, 583–595. [Google Scholar] [CrossRef]
- Wang, H.; Memon, H.; Hassan, E.A.M.; Elagib, T.H.H.; Hassan, F.E.A.A.; Yu, M. Rheological and Dynamic Mechanical Properties of Abutilon Natural Straw and Polylactic Acid Biocomposites. Int. J. Polym. Sci. 2019, 2019. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Recent Developments in Sugar Palm (Arenga Pinnata) Based Biocomposites and Their Potential Industrial Applications: A Review. Renew. Sustain. Energy Rev. 2016, 54, 533–549. [Google Scholar] [CrossRef]
- Ramasubbu, R.; Madasamy, S. Fabrication of Automobile Component Using Hybrid Natural Fiber Reinforced Polymer Composite. J. Nat. Fibers 2020, 1–11. [Google Scholar] [CrossRef]
- Kumar, S.; Zindani, D.; Bhowmik, S. Investigation of Mechanical and Viscoelastic Properties of Flax- and Ramie-Reinforced Green Composites for Orthopedic Implants. J. Mater. Eng. Perform. 2020, 29, 3161–3171. [Google Scholar] [CrossRef]
- Durr, A.; Rayapudi, R.; Peesapaty, N. Eco-Friendly and Biodegradable Edible Utensils Including Cutlery and Chopsticks and Methods of Making Them. WIPO PCT No. WO 2012/098448Al, 26 July 2012. [Google Scholar]
- Villada, H.; Navia, D.; Castañeda, J. Biodegradable Packaging Obtained from Cassava Flour and Fique Fiber and Their Manufacture Process. U.S. Patent US9109116B2, 18 August 2015. [Google Scholar]
- Wagner, J.; Kirsch, W. Recyclable and Compostable Eating Utensils and Other Products Made from Crop-Based Resin & Method of Manufacture. U.S. Patent Application No. 12/157, 181, 21 May 2009. [Google Scholar]
- Reay, G. Biodegradable Article with Embedded Seed. U.S. Patent WO2009156855A2, 30 December 2009. [Google Scholar]
- Rodríguez, L.J.; Fabbri, S.; Orrego, C.E.; Owsianiak, M. Life Cycle Inventory Data for Banana-Fiber-Based Biocomposite Lids. Data Brief 2020, 30, 105605. [Google Scholar] [CrossRef] [PubMed]
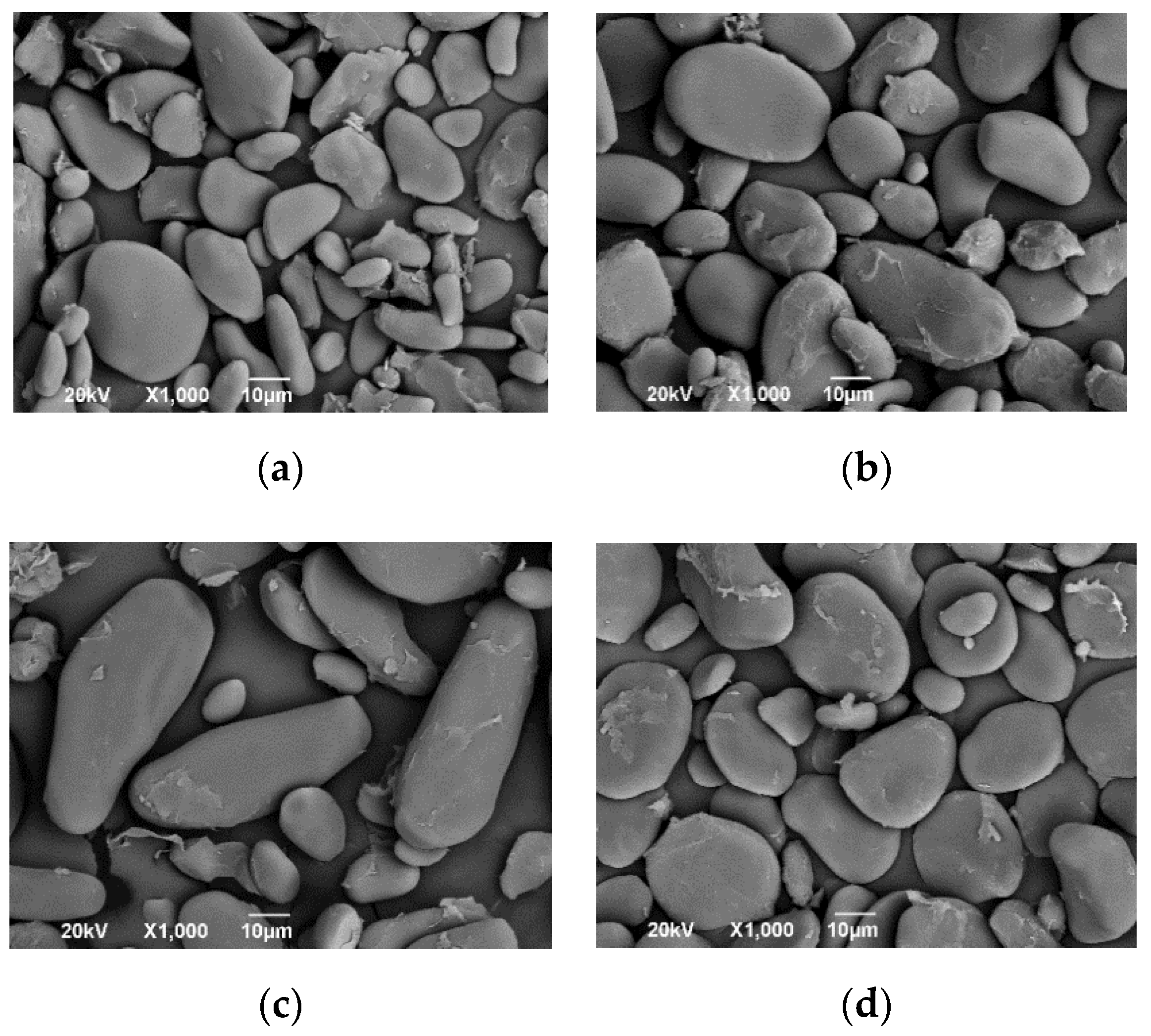

| Genomic Group | Score | Species |
|---|---|---|
| AA Diploide | 15–25 | Bocadillo 1 |
| AAA Triploide | 15–25 | Gros Michel 1, Cavendish Enano 1, Cavendish Valery 1, Dwarf Cavendish 1 Indio 1, Guineo 1, Cachaco 1, Morado 1 |
| AAB Triploide | 26–46 | Hartón 2, Dominico Hartón 2, Maqueño 2, Pompo comino 2, Prata Ana 1 |
| ABB Triploide | 59–63 | Topoco o Bluggoe 3, Pisang Awak 1,3, Pelipita 2, Popocho 2 |
| ABBB Tetraploide | 67–69 | Klue Teparod 3 |
| AAAB Tetraploide | 67–69 | Atan 3, Goldfinger o FHIA 01 1, Prata Graúda 1 |
| AABB Tetraploide | 67–69 | Kalamagol, FHIA 26 1 |
| BB Diploide | 70–75 | Abuhon 2, Pisang Wulung 2 |
| BBB Triploide | 70–75 | Saba 3 |
| Specie | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Moisture (%) | Others (%) | Author |
|---|---|---|---|---|---|---|
| Banana | 31.3 | 14.9 | 15.1 | 9.7 | 4.46 (extractives) 8.65 (Ash) | [56] |
| Banana | 62.5 | 12.5 | 7.5 | N.R. | 4.0 (Pectin) | [55] |
| Banana | 64.0 | 19.0 | 5.0 | 10–11 | N.R. | [9] |
| Plantain | 56.8 | 11.8 | 19.1 | 10–11 | 1.3 (Extractives) | [9] |
| Nendran Plantain | 59.3 | 10.2 | 17.5 | 9.1 | 1.0 (Ash) | [59] |
| Abaca | 56–63 | 20–25 | 7–9 | 15 | 3 (Wax) | [50] |
| Cotton | 85–90 | 5.7 | 0.7–1.6 | 1.0 | 0.6 (Wax) 0–1 (Pectin) | [52] |
| Coconut | 32–43 | 0.15 a 0.25 | 40–45 | 3–4 | N.R. | [52] |
| Abutilon | 67–71 | N.R. | 17 | N.R. | 3.2 (Ash) | [58] |
| Specie | Diameter (µm) | Length (cm) | Author |
|---|---|---|---|
| Banana | 56–143 | N.R. | [61] |
| Banana Ambul | 355 | 100–200 | [62] |
| Plantain | 80–250 | N.R. | [30] |
| Nendran Plantain | 50–250 | N.R. | [59] |
| Abaca | 114–400 | 250–350 | [55] |
| Cotton | 11–22 | 10.3–65 | [50] |
| Coconut | 100–460 | 35–62 | [50,55] |
| Abutilon | 11.4 | 8.5 | [58] |
| Specie | Tensile Strength (MPa) | Modulus of Elasticy (GPa) | Tensile Strain (%) | Tenacity (N/Tex) | Author |
|---|---|---|---|---|---|
| Banana | 210 | 26.86 | 0.8 | N.R. | [61] |
| Banana | 54–754 | 7.7–20 | 10.35 | N.R. | [30] |
| Banana | 800 | 32 | 3.7 | N.R. | [60] |
| Nendran Plantain | 182.33–631.74 | N.R. | 1.24–2.1 | N.R. | [59] |
| Dominico Hartón Plantain | 200–300 | N.R. | 1.9 | 0.47 | [63] |
| Plantain | N.R. | N.R. | N.R. | 0.30 | [64] |
| Cotton | 287–800 | 5.5–12.6 | 3–10 | N.R. | [52] |
| Coconut | 108–252 | 4–6 | 15–40 | N.R. | [51,52] |
| Abutilon | N.R. | N.R. | 2.5 | N.R. | [58] |
| Especie | TD Hemicellulose (°C) | TD Cellulose (°C) | TD Lignin (°C) | Author |
|---|---|---|---|---|
| Banana | N.R. | 250–370 (65–71%) | 200–500 (20–30%) | [61] |
| Banana | N.R. | 260–390 | 400–500 | [65] |
| Banana | 178 | 296 | 501 | [30] |
| Plantain | N.R. | 336.3 | >400 | [64] |
| TPS | Tensile Strength (MPa) | Modulus Of Elasticity (MPa) | Tensile Strain At Break (%) | Author |
|---|---|---|---|---|
| Rice (30% glycerol) | 1.8 | N.R. | 8.0 | [89] |
| Rice (40% sorbitol) | 3.2 | N.R. | 23.0 | |
| Cassava (30% glycerol) | 1.7 | 38.8 | 11.0 | [97] |
| Corn | 1.2 | 22.7 | 62.6 | [98] |
| Biocomposite | Tensile Strength (Mpa) | Modulus of Elasticity (Mpa) | Tensile Strain (%) | Impact Resistance (Kj/M2) | Author |
|---|---|---|---|---|---|
| LDPE/NBF | 18.6 | 645.0 | 22.3–40.3 | 12.3 | [103] |
| LDPE/MBF1 | 26.9 | 889.3 | N.R. | 16.7 | |
| LDPE/MBF2 | 29.2 | 912.6 | N.R. | 19.5 | |
| Polyester/NPF | 27.7 | 1038–1042 | 3.4–3.9 | N.R. | [108] |
| Polyester/MPF | 30.7 | 1229–1231 | 3.0–4.6 | N.R. | |
| Epoxy/NBF | 14.5 | 725 | N.R. | 2.2 | [109] |
| Epoxy/MBF | 33.6 | 1680 | N.R. | 12.2 |
| Biocomposite | Tensile Strength (Mpa) | Modulus Of Elasticity (Mpa) | Tensile Strain (%) | Author |
|---|---|---|---|---|
| TPS corn/MPF | 21.6–29.2 | 3410–4010 | 1.7–2.3 | [111] |
| TPS corn/NPF | 3.87–4.23 | 88.2–106.1 | 12.06–12.4 | [112] |
| TPS cassava/NBF | 14.6 | 700 | 4.8 | [113] |
| TPS cassava/MBF | 24.8 | 3100 | 1.2 | [113] |
| Biocomposite | Tensile Strength (Mpa) | Flexure Strength (Mpa) | Modulus of Elasticity At Flexure (Mpa) | Impact Resistance (Kj/M2) | Author |
|---|---|---|---|---|---|
| PHB | 23.8–24.2 | 28.2–28.6 | 2655.2 | 8.1–8.5 | [117] |
| PHB/MBF5% | 25.9–27.1 | 34.9–35.3 | 2870.5 | 10.2–11 | |
| PHB/MBF10% | 19.9–21.1 | 30.2–30.6 | 2450.2 | 9.2–9.6 |
| Biocomposite | Tensile Strength (MPa) | Modulus of Elasticity (Mpa) | Flexure Strength (MPa) | Impact Resistance (J/m) | Author |
|---|---|---|---|---|---|
| PLA/MBF1 | 78.6 | 7200 | 65.4 | 17.1 | [119] |
| APLA/NPF | 47–49 | 2575–2815 | N.R. | N.R. | [120] |
| APLA/MPF2 | 50.3–52.7 | 2600–2820 | N.R. | N.R. | |
| SPLA/NPF | 46–51 | 2584–2796 | N.R. | N.R. | |
| SPLA/MPF2 | 48.9–51.3 | 2600–2840 | N.R. | N.R. | |
| PLA/NBF | 13.2–16 | 4593–4669 | N.R. | 17.7–20.5 | [118] |
| PLA/MBF3 | 14.5–17.5 | 4580–4692 | N.R. | 17.3–22.1 | |
| PLA/NBF + C30B | 42.3–57.7 | 4639–4827 | N.R. | 27.7–40.5 | |
| PLA/MBF3 + C30B | 44.7–57.3 | 5033–5141 | N.R. | 29.7–39.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castañeda Niño, J.P.; Mina Hernandez, J.H.; Valadez González, A. Potential Uses of Musaceae Wastes: Case of Application in the Development of Bio-Based Composites. Polymers 2021, 13, 1844. https://doi.org/10.3390/polym13111844
Castañeda Niño JP, Mina Hernandez JH, Valadez González A. Potential Uses of Musaceae Wastes: Case of Application in the Development of Bio-Based Composites. Polymers. 2021; 13(11):1844. https://doi.org/10.3390/polym13111844
Chicago/Turabian StyleCastañeda Niño, Juan Pablo, José Herminsul Mina Hernandez, and Alex Valadez González. 2021. "Potential Uses of Musaceae Wastes: Case of Application in the Development of Bio-Based Composites" Polymers 13, no. 11: 1844. https://doi.org/10.3390/polym13111844
APA StyleCastañeda Niño, J. P., Mina Hernandez, J. H., & Valadez González, A. (2021). Potential Uses of Musaceae Wastes: Case of Application in the Development of Bio-Based Composites. Polymers, 13(11), 1844. https://doi.org/10.3390/polym13111844








