Migration Behavior of Lubricants in Polypropylene Composites under Accelerated Thermal Aging
Abstract
1. Introduction
2. Materials and Methods
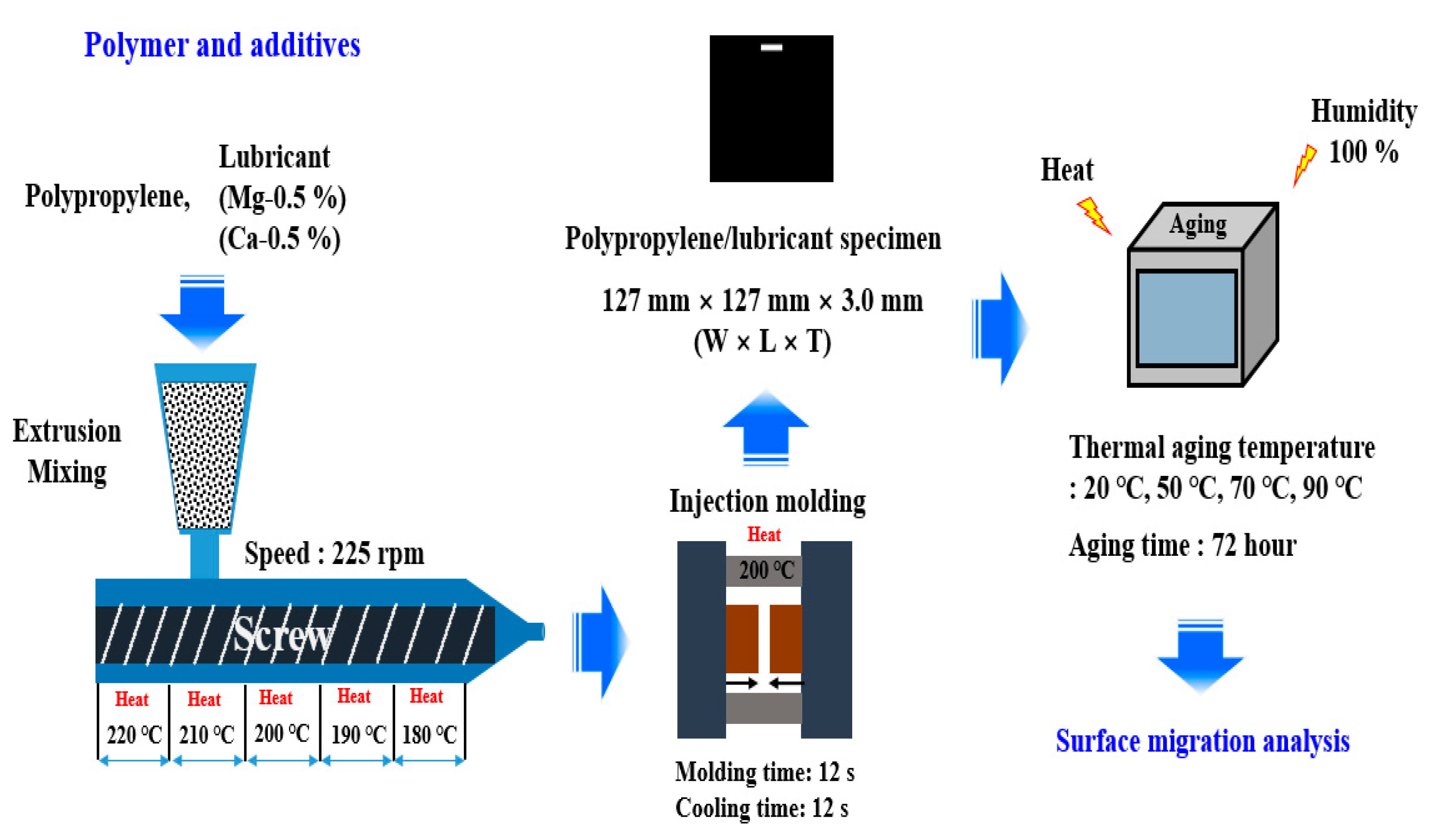
2.1. Materials
2.2. Preparation of PP/Lubricant Composites
2.3. Characterization of PP/Lubricant Composites
2.4. Characterization of PP/Lubricant Composite Surface
2.4.1. Sessile Drop Method
2.4.2. Column Wicking Method
3. Results and Discussion
3.1. Contact Angle and Surface Free Energy of PP/Lubricant Composites
3.2. Surface Free Energies of PP and Lubricants
3.3. Work of Adhesion of PP/Lubricant Composites
3.4. Characterization of PP/Lubricant Composites
3.5. Surface Morphology of PP/Lubricant Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martial, F.; Huguet, J.; Bunel, C. Development of a quantitative analysis method for polypropylene additives using on-line SFE/SFC. Polym. Int. 1999, 48, 299–306. [Google Scholar] [CrossRef]
- Mansouri, H.E.; Yagoubi, N.; Ferrier, D. Extraction of polypropylene additives and their analysis by HPLC. Chromatographia 1998, 48, 491. [Google Scholar] [CrossRef]
- Li, C.; Liang, T.; Lu, W.; Tang, C.; Hu, X.; Cao, M.; Liang, J. Improving the antistatic ability of polypropylene fibers by inner antistatic agent filled with carbon nanotubes. Compos. Sci. Technol. 2004, 64, 2089–2096. [Google Scholar] [CrossRef]
- Zheng, A.; Xu, X.; Xiao, H.; Li, N.; Guan, Y.; Li, S. Antistatic modification of polypropylene by incorporating tween/modified tween. Appl. Surf. Sci. 2012, 258, 8861–8866. [Google Scholar] [CrossRef]
- Chow, W.S.; Tham, W. Effects of antistatic agent on the mechanical, morphological and antistatic properties of polypropylene/organo-montmorillonite nanocomposites. Express Polym. Lett. 2009, 3, 116–125. [Google Scholar] [CrossRef]
- Ding, Y.; Tang, H.; Zhang, X.; Wu, S.; Xiong, R. Antistatic ability of 1-n-tetradecyl-3-methylimidazolium bromide and its effects on the structure and properties of polypropylene. Eur. Polym. J. 2008, 44, 1247–1251. [Google Scholar] [CrossRef]
- Nouman, M.; Saunier, J.; Jubeli, E.; Yagoubi, N. Additive blooming in polymer materials: Consequences in the pharmaceutical and medical field. Polym. Degrad. Stab. 2017, 143, 239–252. [Google Scholar] [CrossRef]
- Chougule, R.; Khare, V.R.; Pattada, K. A fuzzy logic based approach for modeling quality and reliability related customer satisfaction in the automotive domain. Expert Syst. Appl. 2013, 40, 800–810. [Google Scholar] [CrossRef]
- Médard, N.; Poleunis, C.; Eynde, X.V.; Bertrand, P. Characterization of additives at polymer surfaces by ToF-SIMS. Surf. Interface Anal. 2002, 34, 565–569. [Google Scholar] [CrossRef]
- Marcato, B.; Guerra, S.; Vianello, M.; Scalia, S. Migration of antioxidant additives from various polyolefinic plastics into oleaginous vehicles. Int. J. Pharm. 2003, 257, 217–225. [Google Scholar] [CrossRef]
- Li, X.-M.; Yang, R.-J. Study on blooming of tetrabromobisphenol a bis(2,3-dibromopropyl ether) in blends with polypropylene. J. Appl. Polym. Sci. 2006, 101, 20–24. [Google Scholar] [CrossRef]
- Bang, D.Y.; Kyung, M.; Kim, M.J.; Jung, B.Y.; Cho, M.C.; Choi, S.M.; Kim, Y.W.; Lim, S.K.; Lim, D.S.; Won, A.J.; et al. Human risk assessment of endocrine-disrupting chemicals derived from plastic food containers. Compr. Rev. Food Sci. Food Saf. 2012, 11, 453–470. [Google Scholar] [CrossRef]
- Vitrac, O.; Hayert, M. Risk assessment of migration from packaging materials into foods. AIChE J. 2005, 51, 1080–1095. [Google Scholar] [CrossRef]
- Wang, Z.-W.; Li, B.; Lin, Q.-B.; Hu, C.-Y. Two-phase molecular dynamics model to simulate the migration of additives from polypropylene material to food. Int. J. Heat Mass Transf. 2018, 122, 694–706. [Google Scholar] [CrossRef]
- Tan, Y.; Guo, M. Using surface free energy method to study the cohesion and adhesion of asphalt mastic. Constr. Build. Mater. 2013, 47, 254–260. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Holec, D.; Dumitraschkewitz, P.; Vollath, D.; Fischer, F.D. Surface energy of Au nanoparticles depending on their size and shape. Nanomaterials 2020, 10, 484. [Google Scholar] [CrossRef] [PubMed]










| Liquids | Surface Free Energy Components (mJ/m2) | ||
|---|---|---|---|
| γl | γld | γlp | |
| Distilled water | 72.8 | 21.8 | 51.0 |
| Diiodomethane | 50.8 | 50.4 | 0.38 |
| Liquids | γL (mJ/m2) | γLLW (mJ/m2) | γL+ (mJ/m2) | γL− (mJ/m2) | η (mPa⋅s) |
|---|---|---|---|---|---|
| Distilled water | 72.8 | 21.8 | 25.5 | 25.5 | 1.002 |
| Diiodomethane | 50.8 | 50.8 | 0 | 0 | 2.762 |
| Formamide | 58.0 | 39.0 | 2.28 | 39.6 | 4.550 |
| Toluene | 28.3 | 28.3 | 0 | 2.7 | 0.59 |
| Chloroform | 27.3 | 27.3 | 3.8 | 0 | 0.786 |
| Surface Free Energy γa (mJ/m2) | Dispersion Force γad (mJ/m2) | Polarity Force γap (mJ/m2) | |
|---|---|---|---|
| Polypropylene | 31.88 ± 2.1 | 31.76 ± 3.2 | 0.12 ± 2.7 |
| Lubricant | γs (mJ/m2) | γsLW (mJ/m2) | γsAB (mJ/m2) | γs+ (mJ/m2) | γs− (mJ/m2) |
|---|---|---|---|---|---|
| Mg | 7.60 ± 0.4 | 5.34 ± 0.1 | 2.26 ± 0.3 | 0.25 ± 0.2 | 2.01 ± 0.1 |
| Ca | 6.49 ± 0.6 | 4.84 ± 0.1 | 1.65 ± 0.5 | 0.31 ± 0.3 | 1.34 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bak, M.-G.; Won, J.-S.; Koo, S.-W.; Oh, A.; Lee, H.-K.; Kim, D.-S.; Lee, S.-G. Migration Behavior of Lubricants in Polypropylene Composites under Accelerated Thermal Aging. Polymers 2021, 13, 1723. https://doi.org/10.3390/polym13111723
Bak M-G, Won J-S, Koo S-W, Oh A, Lee H-K, Kim D-S, Lee S-G. Migration Behavior of Lubricants in Polypropylene Composites under Accelerated Thermal Aging. Polymers. 2021; 13(11):1723. https://doi.org/10.3390/polym13111723
Chicago/Turabian StyleBak, Mun-Gyu, Jong-Sung Won, Seon-Woong Koo, Arom Oh, Han-Ki Lee, Dae-Sik Kim, and Seung-Goo Lee. 2021. "Migration Behavior of Lubricants in Polypropylene Composites under Accelerated Thermal Aging" Polymers 13, no. 11: 1723. https://doi.org/10.3390/polym13111723
APA StyleBak, M.-G., Won, J.-S., Koo, S.-W., Oh, A., Lee, H.-K., Kim, D.-S., & Lee, S.-G. (2021). Migration Behavior of Lubricants in Polypropylene Composites under Accelerated Thermal Aging. Polymers, 13(11), 1723. https://doi.org/10.3390/polym13111723







