Study of the Influence of Magnetite Nanoparticles Supported on Thermally Reduced Graphene Oxide as Filler on the Mechanical and Magnetic Properties of Polypropylene and Polylactic Acid Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TrGO/Fe3O4 Nanoparticles
2.2.1. Graphene Oxide Synthesis and Thermally Reduction Process
2.2.2. TrGO/Magnetite Nanomaterial Synthesis
2.3. Characterization of Nanoparticles
2.4. Preparation of Nanocomposites
2.5. Characterization of Nanocomposites
2.6. Theoretical Models
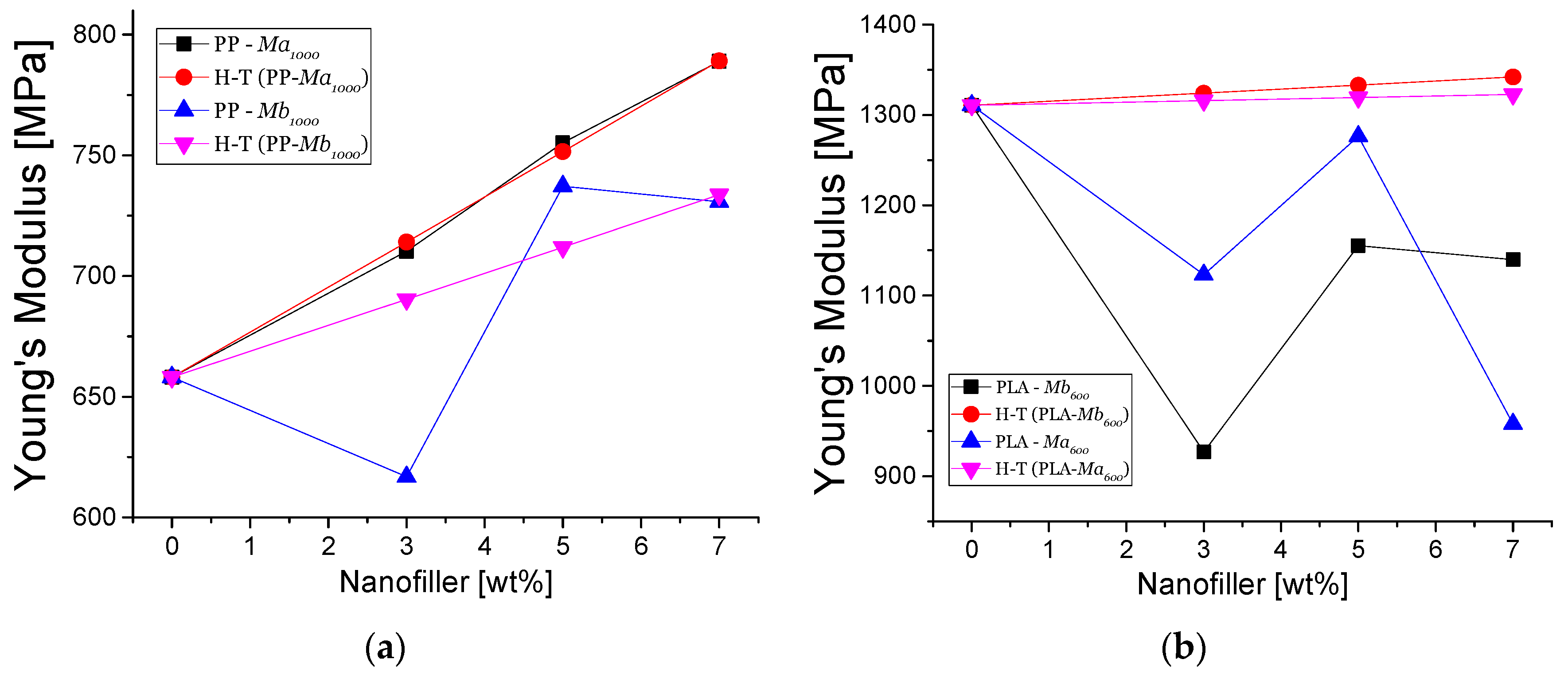
2.6.1. Halpin-Tsai Model
2.6.2. Langevin Model
3. Results and Discussion
3.1. Characterization of Thermally Reduced Graphene Oxide
3.2. Characterization of TrGO/Fe3O4 Nanomaterials
3.3. Magnetic Properties of Nanomaterials
3.4. Properties of PP and PLA of Nanocomposites
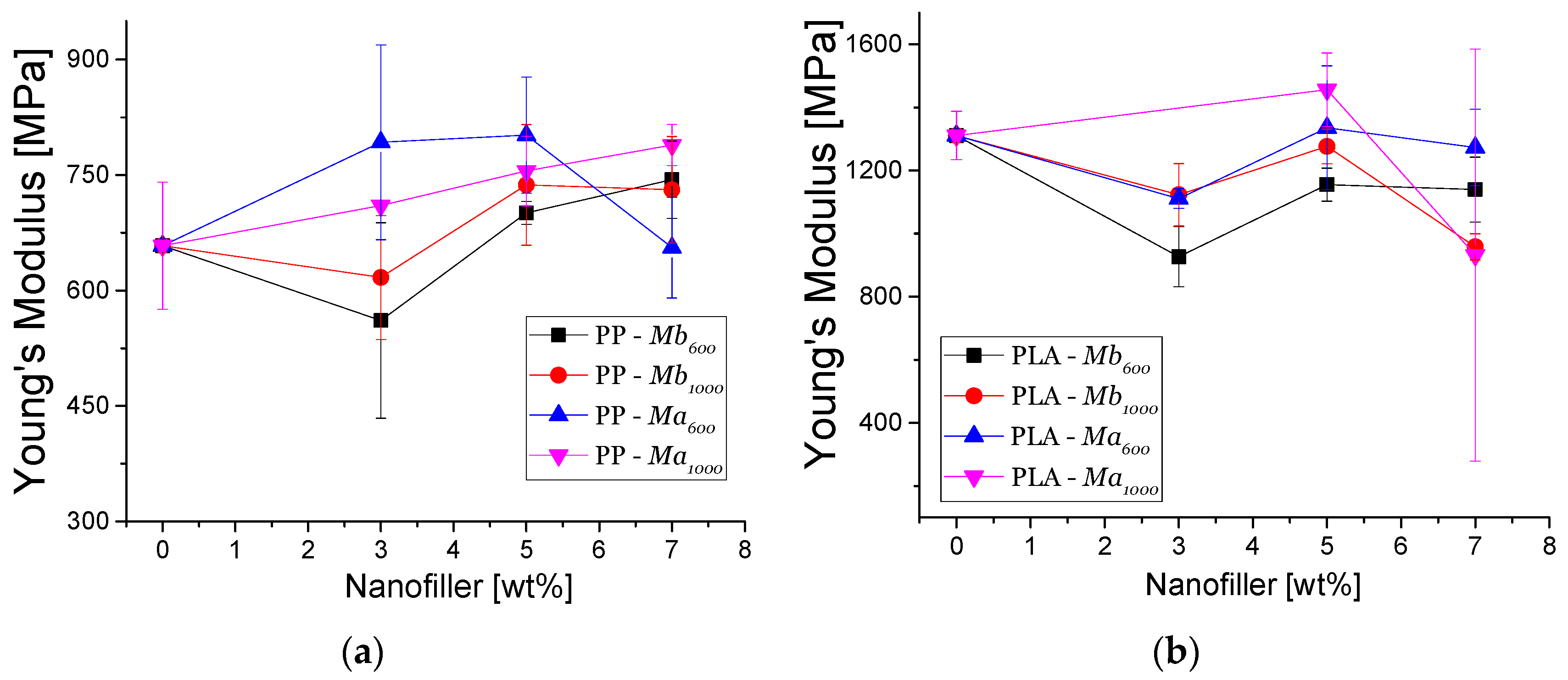
3.4.1. Mechanical Properties
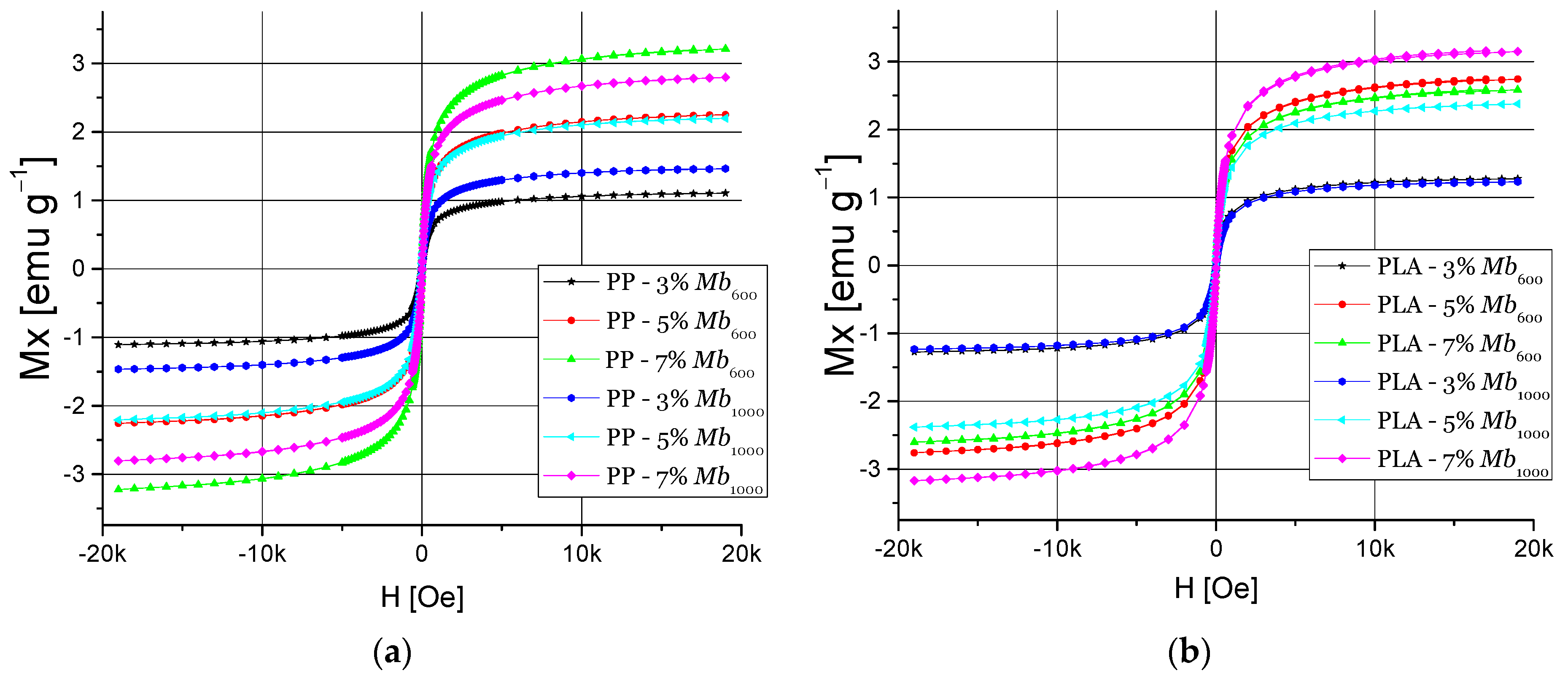
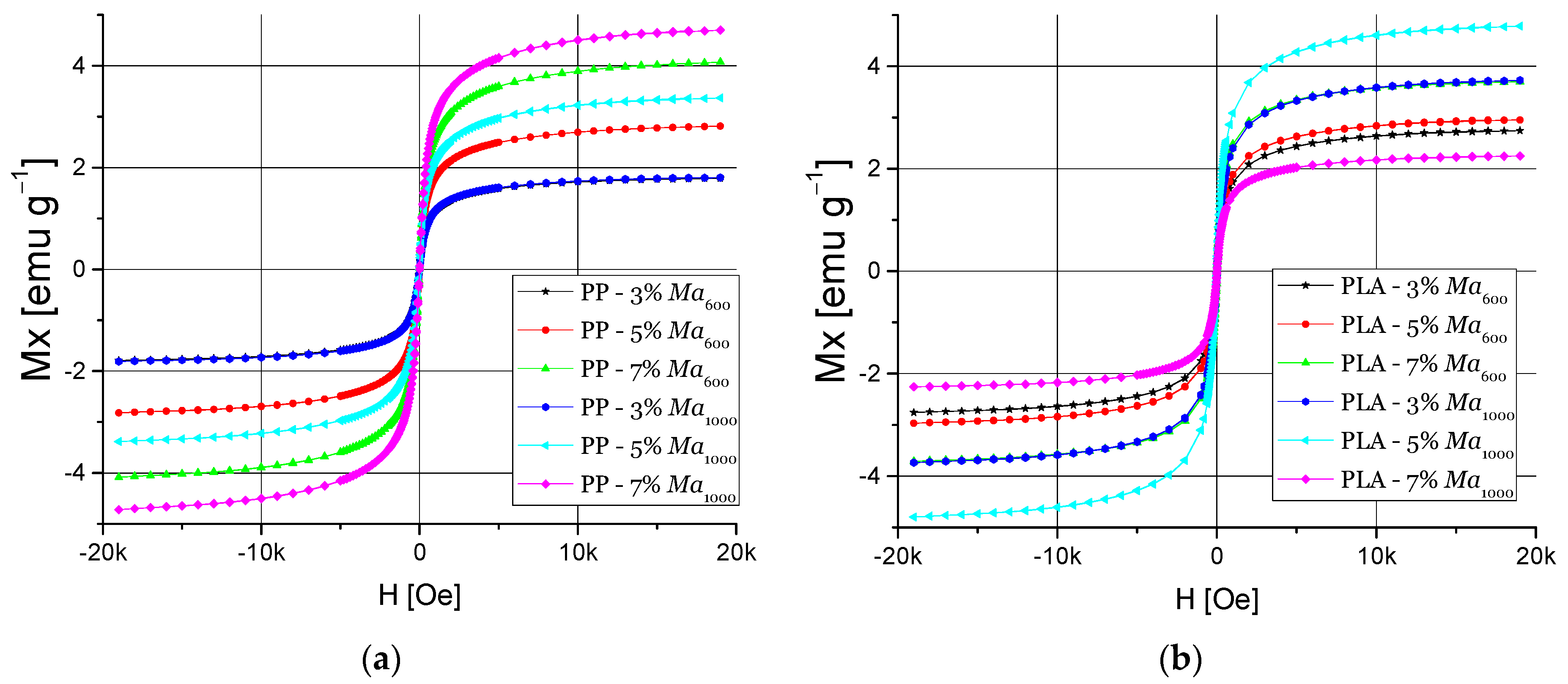
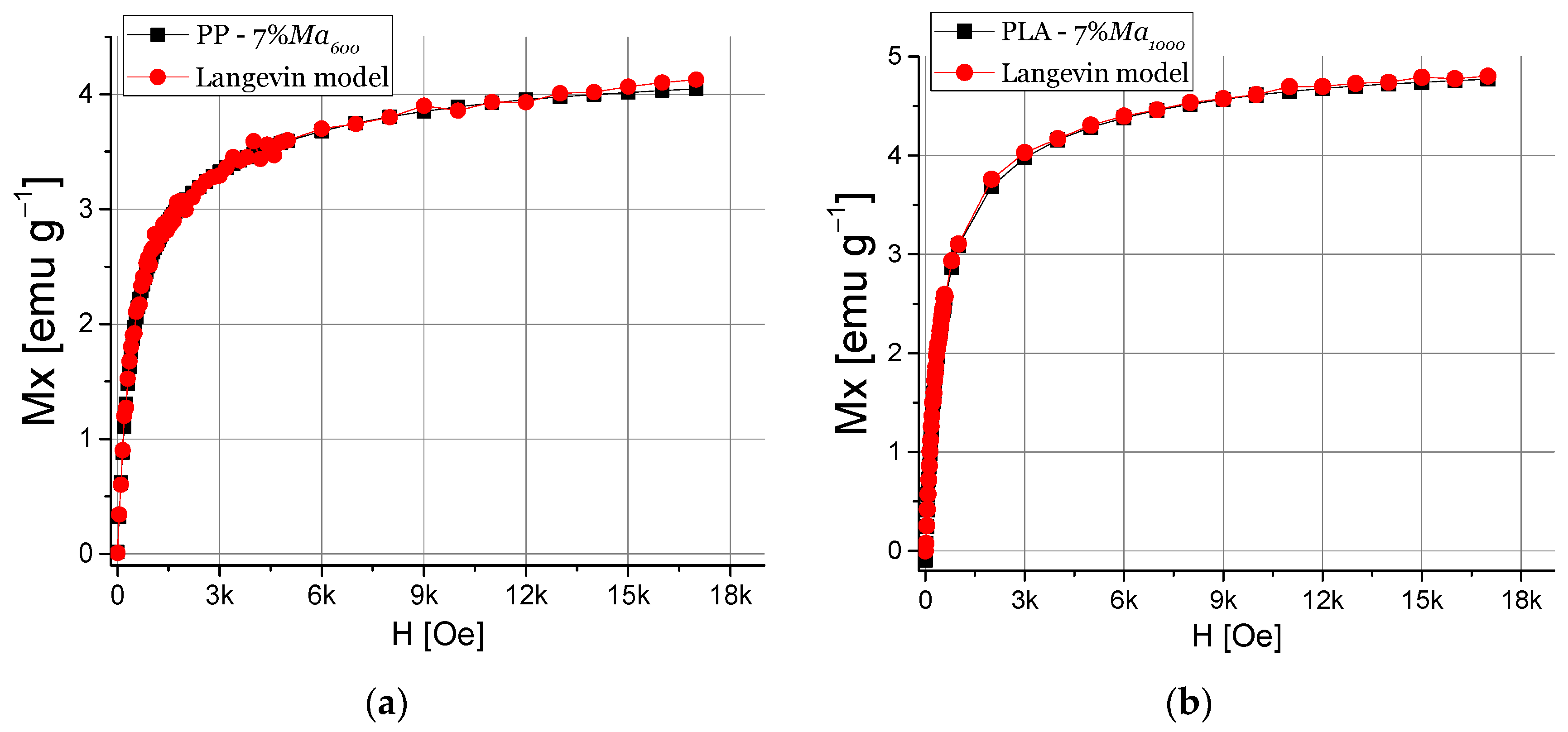
3.4.2. Magnetic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garzón, C.; Palza, H. Electrical behavior of polypropylene composites melt mixed with carbon-based particles: Effect of the kind of particle and annealing process. Compos. Sci. Technol. 2014, 99, 117–123. [Google Scholar] [CrossRef]
- Méndez, R.; Constant, B.; Garzon, C.; Nisar, M.; Nachtigall, S.M.B.; Quijada, R. Barrier, mechanical and conductive properties of polycaprolactam nanocomposites containing carbon-based particles: Effect of the kind of particle. Polymer (Guildford) 2017, 130, 10–16. [Google Scholar] [CrossRef]
- Aguilar-Bolados, H.; Lopez-Manchado, M.A.; Brasero, J.; Avilés, F.; Yazdani-Pedram, M. Effect of the morphology of thermally reduced graphite oxide on the mechanical and electrical properties of natural rubber nanocomposites. Compos. Part B Eng. 2016, 87, 350–356. [Google Scholar] [CrossRef]
- Gao, Y.; Picot, O.T.; Bilotti, E.; Peijs, T. Influence of filler size on the properties of poly(lactic acid) (PLA)/graphene nanoplatelet (GNP) nanocomposites. Eur. Polym. J. 2017, 86, 117–131. [Google Scholar] [CrossRef]
- Gómez, M.; Díaz, A.; Reyes, P.; Yazdani-Pedram, M.; Bohrz Nachtigall, S.M.; Palza, H.; Quijada, R. Effect of thermally reduced graphene oxides obtained at different temperatures on the barrier and mechanical properties of polypropylene/TRGO and polyamide-6/TRGO nanocomposites. Polym. Compos. 2019, 40, E1746–E1756. [Google Scholar] [CrossRef]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/Polymer Nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Aguilar-Bolados, H.; Brasero, J.; Lopez-Manchado, M.A.; Yazdani-Pedram, M. High performance natural rubber/thermally reduced graphite oxide nanocomposites by latex technology. Compos. Part B Eng. 2014, 67, 449–454. [Google Scholar] [CrossRef]
- Maldonado-Magnere, S.; Yazdani-Pedram, M.; Aguilar-Bolados, H.; Quijada, R. Thermally Reduced Graphene Oxide/Thermoplastic Polyurethane Nanocomposites: Mechanical and Barrier Properties. Polymers 2021, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Cuenca-Bracamonte, Q.; Yazdani-Pedram, M.; Hernández Santana, M.; Aguilar-Bolados, H. Electrical Properties of Poly(Monomethyl Itaconate)/Few-Layer Functionalized Graphene Oxide/Lithium Ion Nanocomposites. Polymers 2020, 12, 2673. [Google Scholar] [CrossRef] [PubMed]
- Alammar, A.; Park, S.-H.; Ibrahim, I.; Arun, D.; Holtzl, T.; Dumée, L.F.; Lim, H.N.; Szekely, G. Architecting neonicotinoid-scavenging nanocomposite hydrogels for environmental remediation. Appl. Mater. Today 2020, 21, 100878. [Google Scholar] [CrossRef]
- Yap, P.L.; Auyoong, Y.L.; Hassan, K.; Farivar, F.; Tran, D.N.H.; Ma, J.; Losic, D. Multithiol functionalized graphene bio-sponge via photoinitiated thiol-ene click chemistry for efficient heavy metal ions adsorption. Chem. Eng. J. 2020, 395, 124965. [Google Scholar] [CrossRef]
- Das, L.; Das, P.; Bhowal, A.; Bhattachariee, C. Synthesis of hybrid hydrogel nano-polymer composite using Graphene oxide, Chitosan and PVA and its application in waste water treatment. Environ. Technol. Innov. 2020, 18, 100664. [Google Scholar] [CrossRef]
- Sharif, F.; Arjmand, M.; Moud, A.A.; Sundararaj, U.; Roberts, E.P.L. Segregated Hybrid Poly(methyl methacrylate)/Graphene/Magnetite Nanocomposites for Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2017, 9, 14171–14179. [Google Scholar] [CrossRef] [PubMed]
- Blanco, C.; Santamarı, R.; Botas, C.; Patricia, A.; Rodrı, F.; Granda, M.; Gutie, M.D.; Mene, R. Critical temperatures in the synthesis of graphene-like materials by thermal exfoliation—Reduction of graphite oxide. Carbon 2013, 52, 476–485. [Google Scholar] [CrossRef]
- Botas, C.; Álvarez, P.; Blanco, C.; Gutiérrez, M.D.; Ares, P.; Zamani, R.; Arbiol, J.; Morante, J.R.; Menéndez, R. Tailored graphene materials by chemical reduction of graphene oxides of different atomic structure. RSC Adv. 2012, 2, 9643–9650. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Koinuma, M.; Kim, S.Y.; Watanabe, Y.; Taniguchi, T.; Hatakeyama, K.; Tateishi, H.; Ida, S. Simple photoreduction of graphene oxide nanosheet under mild conditions. ACS Appl. Mater. Interfaces 2010, 2, 3461–3466. [Google Scholar] [CrossRef]
- Aguilar-Bolados, H.; Vargas-Astudillo, D.; Yazdani-Pedram, M.; Acosta-Villavicencio, G.; Fuentealba, P.; Contreras-Cid, A.; Verdejo, R.; López-Manchado, M.A. Facile and Scalable One-Step Method for Amination of Graphene Using Leuckart Reaction. Chem. Mater. 2017, 29, 6698–6705. [Google Scholar] [CrossRef]
- Aguilar-Bolados, H.; Contreras-Cid, A.; Yazdani-Pedram, M.; Acosta-Villavicencio, G.; Flores, M.; Fuentealba, P.; Neira-Carrillo, A.; Verdejo, R.; López-Manchado, M.A. Synthesis of fluorinated graphene oxide by using an easy one-pot deoxyfluorination reaction. J. Colloid Interface Sci. 2018, 524, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Bolados, H.; Yazdani-Pedram, M.; Quinteros-Jara, E.; Cuenca-Bracamonte, Q.; Quijada, R.; Carretero-González, J.; Avilés, F.; Lopez-Manchado, M.A.; Verdejo, R. Synthesis of sustainable, lightweight and electrically conductive polymer brushes grafted multi-layer graphene oxide. Polym. Test. 2021, 93, 106986. [Google Scholar] [CrossRef]
- Huang, W. Graphene Oxide Nanopapers. In Nanopapers: From Nanochemistry and Nanomanufacturing to Advanced Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 1–26. ISBN 9780323480208. [Google Scholar]
- McAllister, M.J.; Li, J.L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K.; et al. Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Schniepp, H.C.; Li, J.L.; McAllister, M.J.; Sai, H.; Herrera-Alonson, M.; Adamson, D.H.; Prud’homme, R.K.; Car, R.; Seville, D.A.; Aksay, I.A. Functionalized single graphene sheets derived from splitting graphite oxide. J. Phys. Chem. B 2006, 110, 8535–8539. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, J.; Hou, Y. Fe3O4 nanostructures: Synthesis, growth mechanism, properties and applications. Chem. Commun. 2011, 47, 5130–5141. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, L.; Gutierrez, S.; Menendez, N.; Morales, M.P.; Herrasti, P. Magnetite nanoparticles: Electrochemical synthesis and characterization. Electrochim. Acta 2008, 53, 3436–3441. [Google Scholar] [CrossRef]
- Xu, J.; Yang, H.; Fu, W.; Du, K.; Sui, Y.; Chen, J.; Zeng, Y.; Li, M.; Zou, G. Preparation and magnetic properties of magnetite nanoparticles by sol-gel method. J. Magn. Magn. Mater. 2007, 309, 307–311. [Google Scholar] [CrossRef]
- Valenzuela, R.; Fuentes, M.C.; Parra, C.; Baeza, J.; Duran, N.; Sharma, S.K.; Knobel, M.; Freer, J. Influence of stirring velocity on the synthesis of magnetite nanoparticles (Fe3O4) by the co-precipitation method. J. Alloys Compd. 2009, 488, 227–231. [Google Scholar] [CrossRef]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Hatel, R.; Goumri, M.; Ratier, B.; Baitoul, M. Graphene derivatives/Fe3O4/polymer nanocomposite films: Optical and electrical properties. Mater. Chem. Phys. 2017, 193, 156–163. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Flynn, A.; Chiou, B.S.; Imam, S.; Orts, W.; Chiellini, E. Thermal, mechanical and morphological characterization of plasticized PLA-PHB blends. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Lamkin-Kennard, K.A.; Popovic, M.B. Molecular and Cellular Level—Applications in Biotechnology and Medicine Addressing Molecular and Cellular Level. In Biomechatronics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 201–233. [Google Scholar]
- Hu, C.; Li, Z.; Wang, Y.; Gao, J.; Dai, K.; Zheng, G.; Liu, C.; Shen, C.; Song, H.; Guo, Z. Comparative assessment of the strain-sensing behaviors of polylactic acid nanocomposites: Reduced graphene oxide or carbon nanotubes. J. Mater. Chem. C 2017, 5, 2318–2328. [Google Scholar] [CrossRef]
- Maier, C.; Calafut, T. 11—Applications. In Plastics Design Library; Maier, C., Calafut, T.B.T.-P., Eds.; William Andrew Publishing: Norwich, NY, USA, 1998; pp. 87–107. ISBN 978-1-884207-58-7. [Google Scholar]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable polymers and green-based antimicrobial packaging materials: A mini-review. Adv. Ind. Eng. Polym. Res. 2020, 3, 27–35. [Google Scholar] [CrossRef]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.W.; Voon, C.H. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Moztahida, M.; Nawaz, M.; Kim, J.; Shahzad, A.; Kim, S.; Jang, J.; Lee, D.S. Reduced graphene oxide-loaded-magnetite: A Fenton-like heterogeneous catalyst for photocatalytic degradation of 2-methylisoborneol. Chem. Eng. J. 2019, 370, 855–865. [Google Scholar] [CrossRef]
- Ahn, T.; Kim, J.H.; Yang, H.M.; Lee, J.W.; Kim, J.D. Formation pathways of magnetite nanoparticles by coprecipitation method. J. Phys. Chem. C 2012, 116, 6069–6076. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Walton, K.S.; Snurr, R.Q. Applicability of the BET Method for Determining Surface Areas of Microporous Metal–Organic Frameworks. J. Am. Chem. Soc. 2007, 129, 8552–8556. [Google Scholar] [CrossRef] [PubMed]
- Affdl, J.C.H.; Kardos, J.L. The Halpin-Tsai equations: A review. Polym. Eng. Sci. 1976, 16, 344–352. [Google Scholar] [CrossRef]
- Zare, Y. Development of Halpin-Tsai model for polymer nanocomposites assuming interphase properties and nanofiller size. Polym. Test. 2016, 51, 69–73. [Google Scholar] [CrossRef]
- García-Palacios, J.L.; Lázaro, F.J. Langevin-dynamics study of the dynamical properties of small magnetic particles. Phys. Rev. B Condens. Matter Mater. Phys. 1998, 58, 14937–14958. [Google Scholar] [CrossRef]
- Baumgartner, J.; Dey, A.; Bomans, P.H.H.; Le Coadou, C.; Fratzl, P.; Sommerdijk, N.A.J.M.; Faivre, D. Nucleation and growth of magnetite from solution. Nat. Mater. 2013, 12, 310–314. [Google Scholar] [CrossRef]
- Zubir, N.A.; Yacou, C.; Motuzas, J.; Zhang, X.; Diniz da Costa, J.C. Structural and functional investigation of graphene oxide–Fe3O4 nanocomposites for the heterogeneous Fenton-like reaction. Sci. Rep. 2014, 4, 4594. [Google Scholar] [CrossRef]
- Beams, R.; Gustavo Cançado, L.; Novotny, L. Raman characterization of defects and dopants in graphene. J. Phys. Condens. Matter 2015, 27, 083002. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, W.; Wang, G.; Yu, Y.; Qin, S.; Fang, J.; Wang, F.; Zhang, X.A. The Raman redshift of graphene impacted by gold nanoparticles. AIP Adv. 2015, 5, 057133. [Google Scholar] [CrossRef]
- Chicot, D.; Roudet, F.; Lepingle, V.; Louis, G. Strain gradient plasticity to study hardness behavior of magnetite (Fe3O4) under multicyclic indentation. J. Mater. Res. 2009, 24, 749–759. [Google Scholar] [CrossRef]
- Weidenfeller, B.; Riehemann, W.; Lei, Q. Mechanical spectroscopy of polymer-magnetite composites. Mater. Sci. Eng. A 2004, 370, 278–283. [Google Scholar] [CrossRef]
- Carrasco, F.; Pagès, P.; Gámez-Pérez, J.; Santana, O.O.; Maspoch, M.L. Processing of poly(lactic acid): Characterization of chemical structure, thermal stability and mechanical properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Marin, P.; Tschan, M.J.-L.; Isnard, F.; Robert, C.; Haquette, P.; Trivelli, X.; Chamoreau, L.; Guérineau, V.; del Rosal, I.; Maron, L.; et al. Polymerization of rac-Lactide Using Achiral Iron Complexes: Access to Thermally Stable Stereocomplexes. Angew. Chem. Int. Ed. 2019, 58, 12585–12589. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Liang, F.; Gou, J. Nanopaper enabled shape-memory nanocomposite with vertically aligned nickel nanostrand: Controlled synthesis and electrical actuation. Soft Matter 2011, 7, 7416–7423. [Google Scholar] [CrossRef]
- Lu, H.; Min Huang, W. Synergistic effect of self-assembled carboxylic acid-functionalized carbon nanotubes and carbon fiber for improved electro-activated polymeric shape-memory nanocomposite. Appl. Phys. Lett. 2013, 102, 231910. [Google Scholar] [CrossRef]
- Aguilar, H.; Yazdani-Pedram, M.; Toro, P.; Quijada, R.; López-Manchado, M.Á. Synergic effect of two inorganic fillers on the mechanical and thermal properties of hybrid polypropylene composites. J. Chil. Chem. Soc. 2014, 59, 2468–2473. [Google Scholar] [CrossRef]
- Carosio, F.; Kochumalayil, J.; Cuttica, F.; Camino, G.; Berglund, L. Oriented Clay Nanopaper from Biobased Components—Mechanisms for Superior Fire Protection Properties. ACS Appl. Mater. Interfaces 2015, 7, 5847–5856. [Google Scholar] [CrossRef]
- Aguilar-Bolados, H.; Quijada, R.; Yazdani-Pedram, M.; Maldonado-Magnere, S.; Verdejo, R.; Lopez-Manchado, M.A. SEBS-Grafted Itaconic Acid as Compatibilizer for Elastomer Nanocomposites Based on BaTiO3 Particles. Polymers 2020, 12, 643. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzidou, K.; Fukushima, H.; Miyagawa, H.; Drzal, L.T. Flexural and tensile moduli of polypropylene nanocomposites and comparison of experimental data to Halpin-Tsai and Tandon-Weng models. Polym. Eng. Sci. 2007, 47, 1796–1803. [Google Scholar] [CrossRef]
- Lai, S.M.; Wu, S.H.; Lin, G.G.; Don, T.M. Unusual mechanical properties of melt-blended poly(lactic acid) (PLA)/clay nanocomposites. Eur. Polym. J. 2014, 52, 193–206. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]





| Sample | XRD | Elemental Analysis | BET Surface Area | ||||
|---|---|---|---|---|---|---|---|
| d002 nm | Lc nm | %C | %H | %N | %O | m2·g−1 | |
| GO | 0.702 | 4.08 | 54.4 | 1.9 | 0.1 | 43.6 | 69.7 |
| TrGO600 | 0.350 | 3.42 | 83.9 | 0.3 | 0.1 | 15.7 | 304 |
| TrGO1000 | 0.340 | 4.01 | 90.8 | 0.4 | 0.1 | 8.70 | 267 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constant-Mandiola, B.; Aguilar-Bolados, H.; Geshev, J.; Quíjada, R. Study of the Influence of Magnetite Nanoparticles Supported on Thermally Reduced Graphene Oxide as Filler on the Mechanical and Magnetic Properties of Polypropylene and Polylactic Acid Nanocomposites. Polymers 2021, 13, 1635. https://doi.org/10.3390/polym13101635
Constant-Mandiola B, Aguilar-Bolados H, Geshev J, Quíjada R. Study of the Influence of Magnetite Nanoparticles Supported on Thermally Reduced Graphene Oxide as Filler on the Mechanical and Magnetic Properties of Polypropylene and Polylactic Acid Nanocomposites. Polymers. 2021; 13(10):1635. https://doi.org/10.3390/polym13101635
Chicago/Turabian StyleConstant-Mandiola, Benjamin, Héctor Aguilar-Bolados, Julian Geshev, and Raul Quíjada. 2021. "Study of the Influence of Magnetite Nanoparticles Supported on Thermally Reduced Graphene Oxide as Filler on the Mechanical and Magnetic Properties of Polypropylene and Polylactic Acid Nanocomposites" Polymers 13, no. 10: 1635. https://doi.org/10.3390/polym13101635
APA StyleConstant-Mandiola, B., Aguilar-Bolados, H., Geshev, J., & Quíjada, R. (2021). Study of the Influence of Magnetite Nanoparticles Supported on Thermally Reduced Graphene Oxide as Filler on the Mechanical and Magnetic Properties of Polypropylene and Polylactic Acid Nanocomposites. Polymers, 13(10), 1635. https://doi.org/10.3390/polym13101635







