Preparation of Thin-Film Composite Nanofiltration Membranes Doped with N- and Cl-Functionalized Graphene Oxide for Water Desalination
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Analytical Techniques
2.3. Preparation and Functionalization of GO
2.4. Fabrication of GO/PA Nanocomposite Membranes
2.5. Performance of NF Membranes
3. Results and Discussion
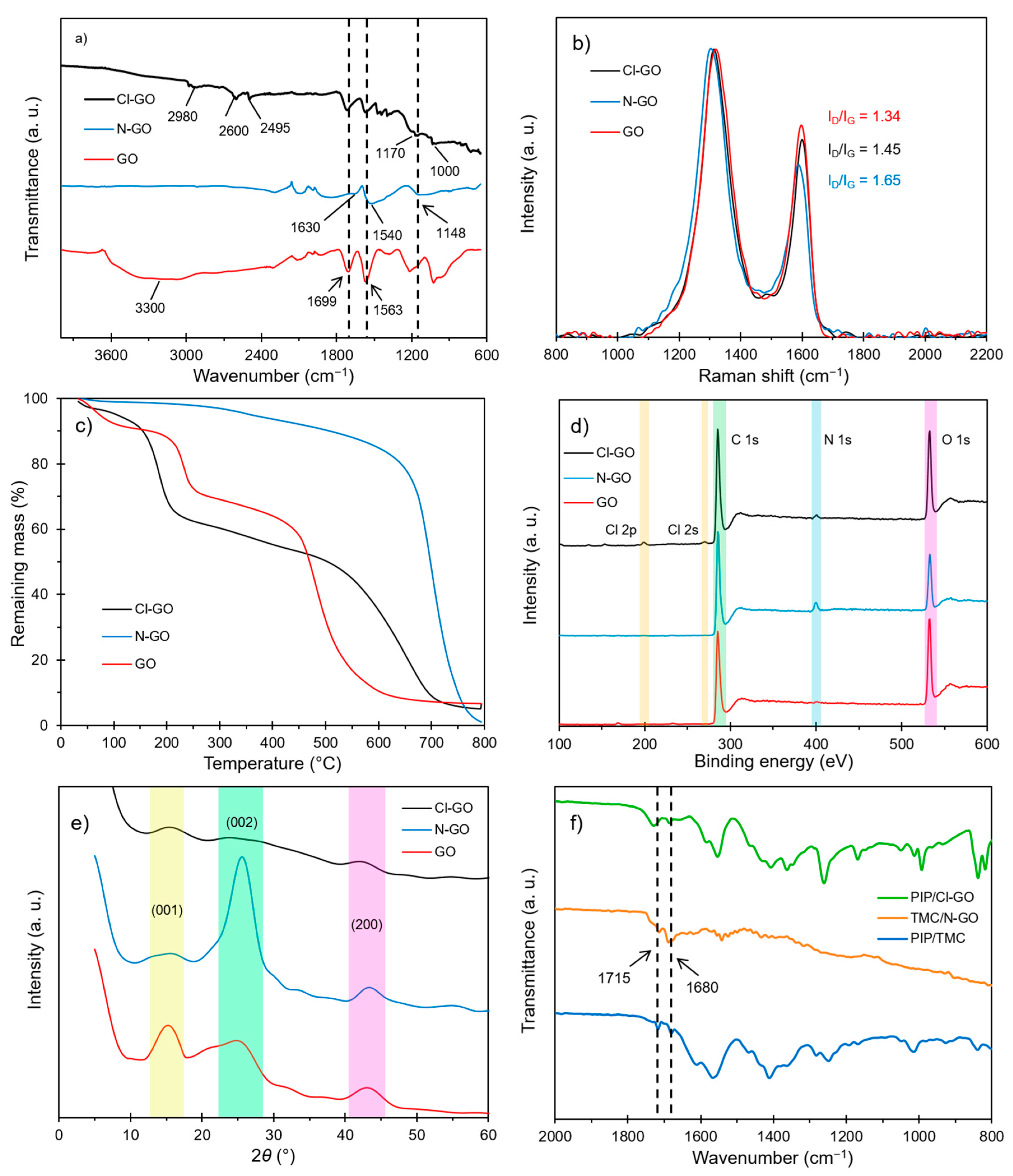
3.1. Characterization of Functionalized GO
3.2. Morphology Imaging and EDS Characteristics of Functionalized GO
3.3. Characterization of GO/PA Nanocomposite Membranes
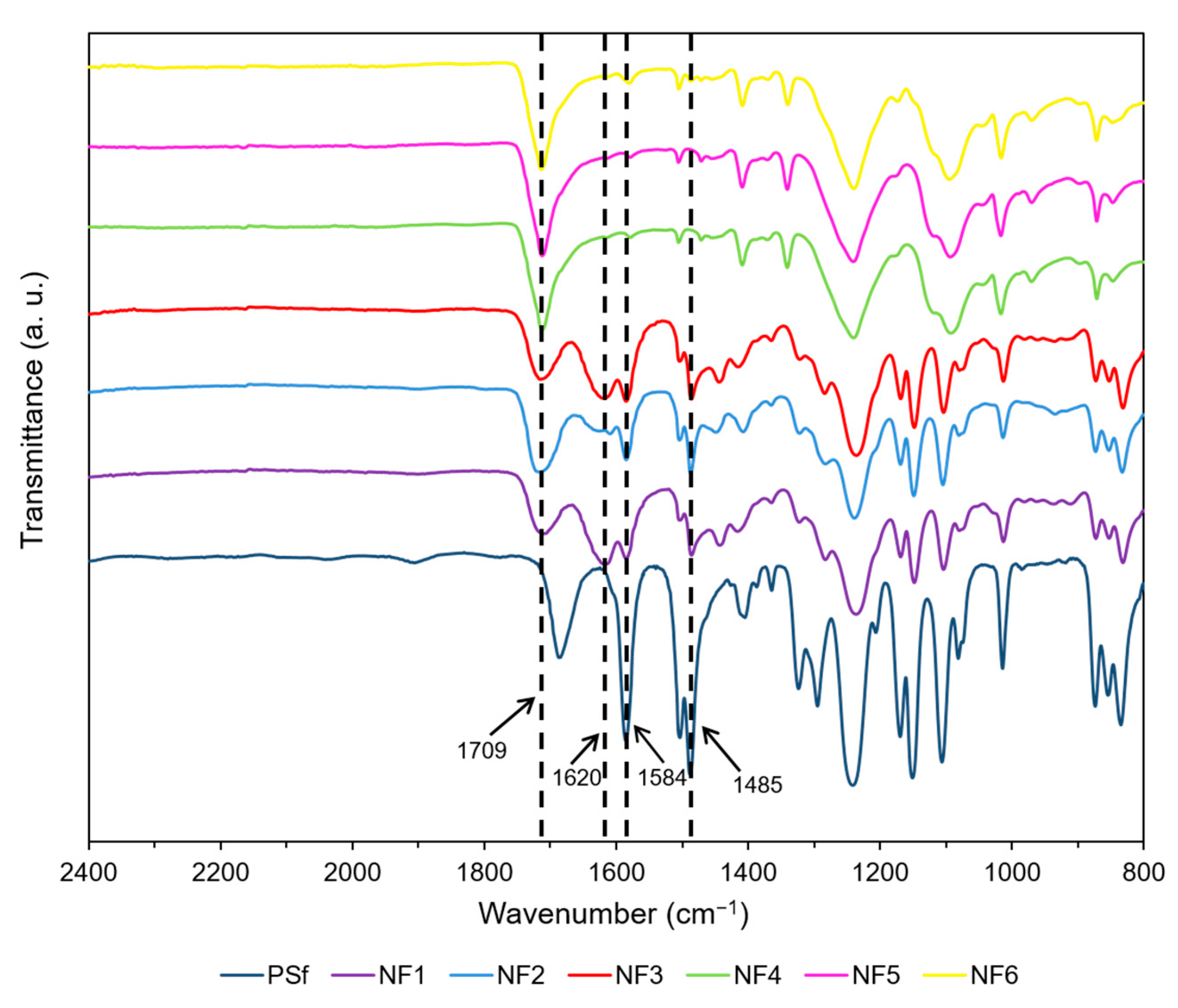
3.3.1. FTIR Spectra of Prepared Membranes
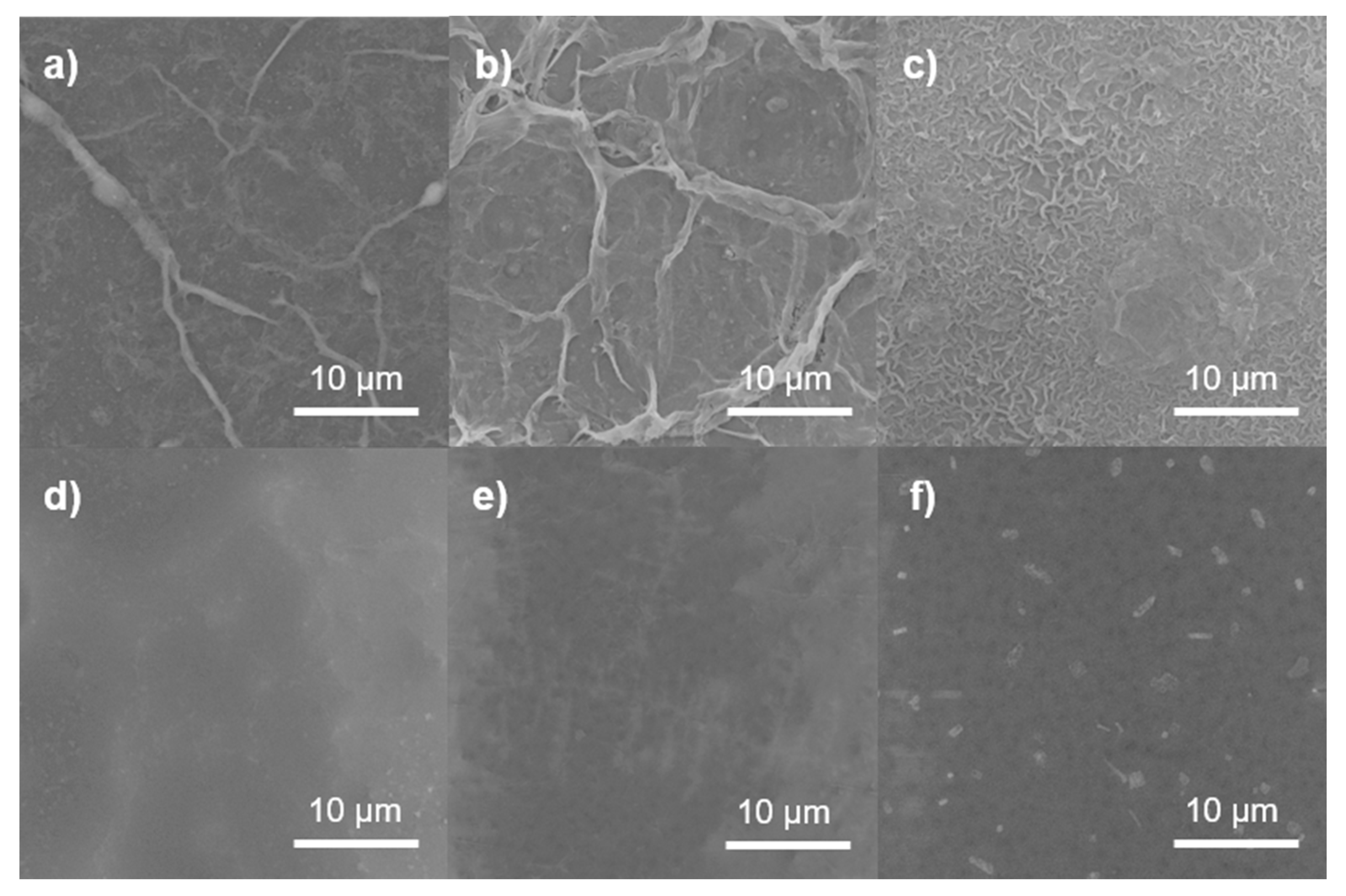
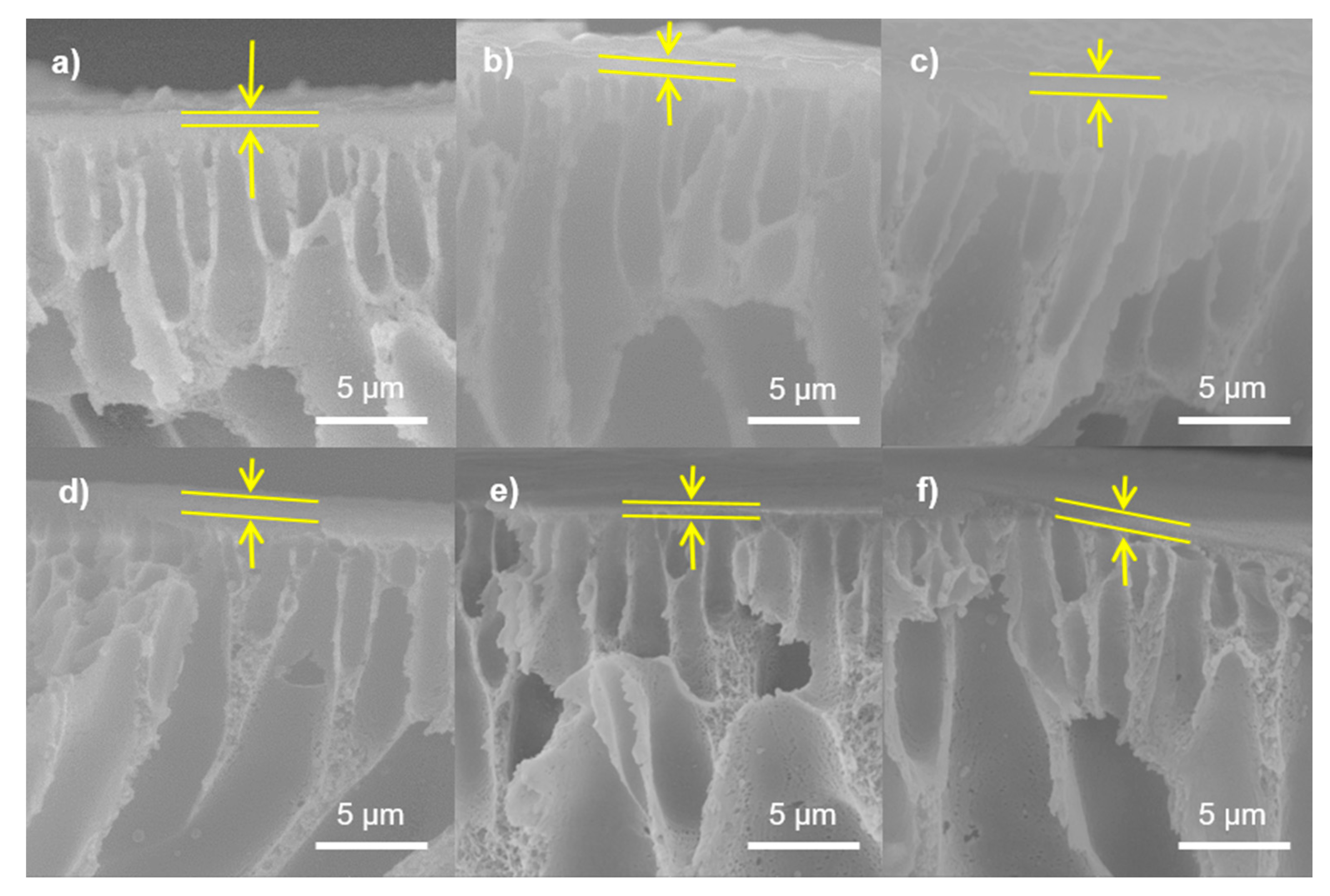
3.3.2. SEM Imaging of GO/PA NF Membranes
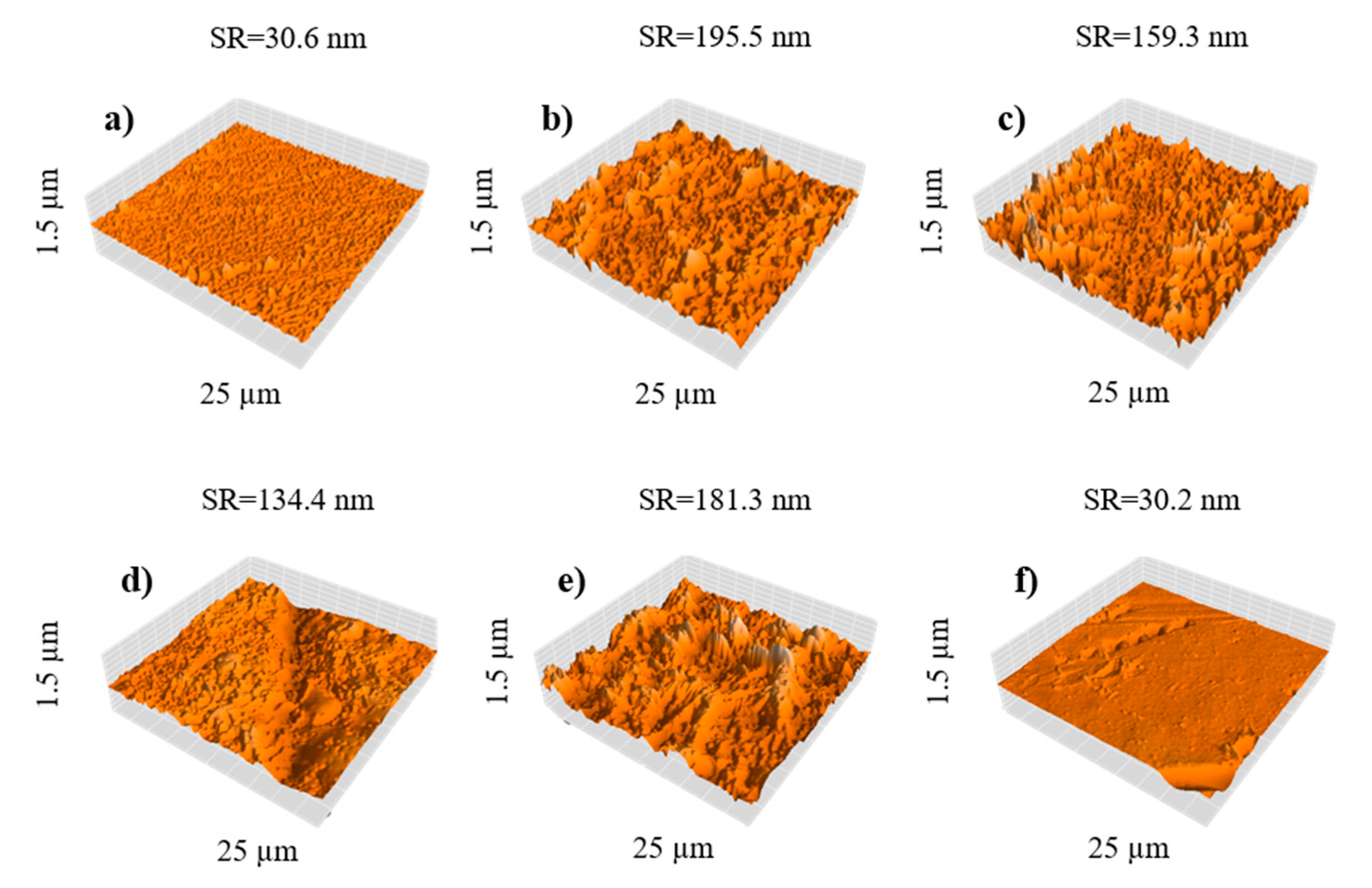
3.3.3. AFM Imaging of GO/PA NF Membranes
3.4. Performance of NF Membranes
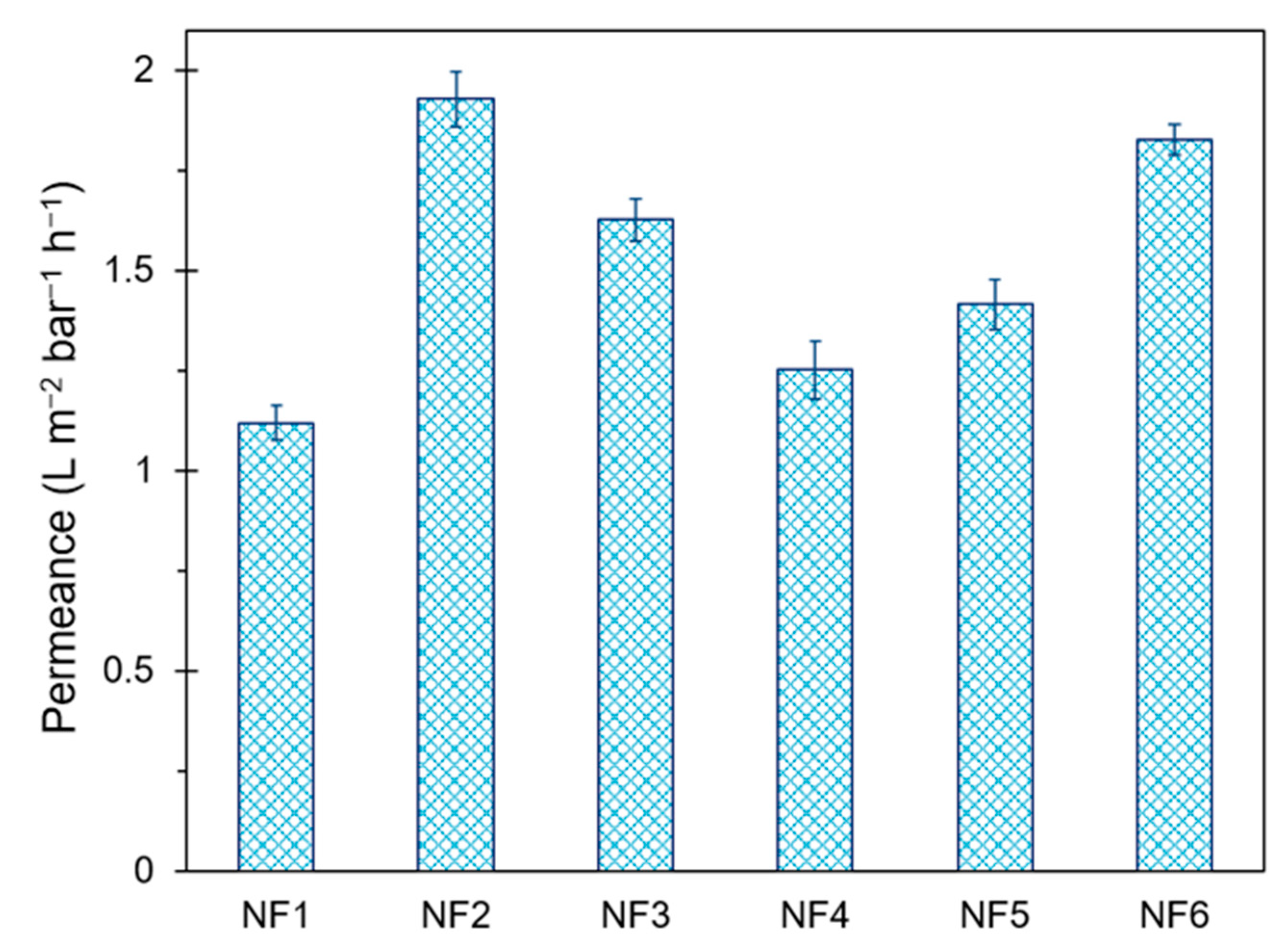
3.4.1. Water Flux Test
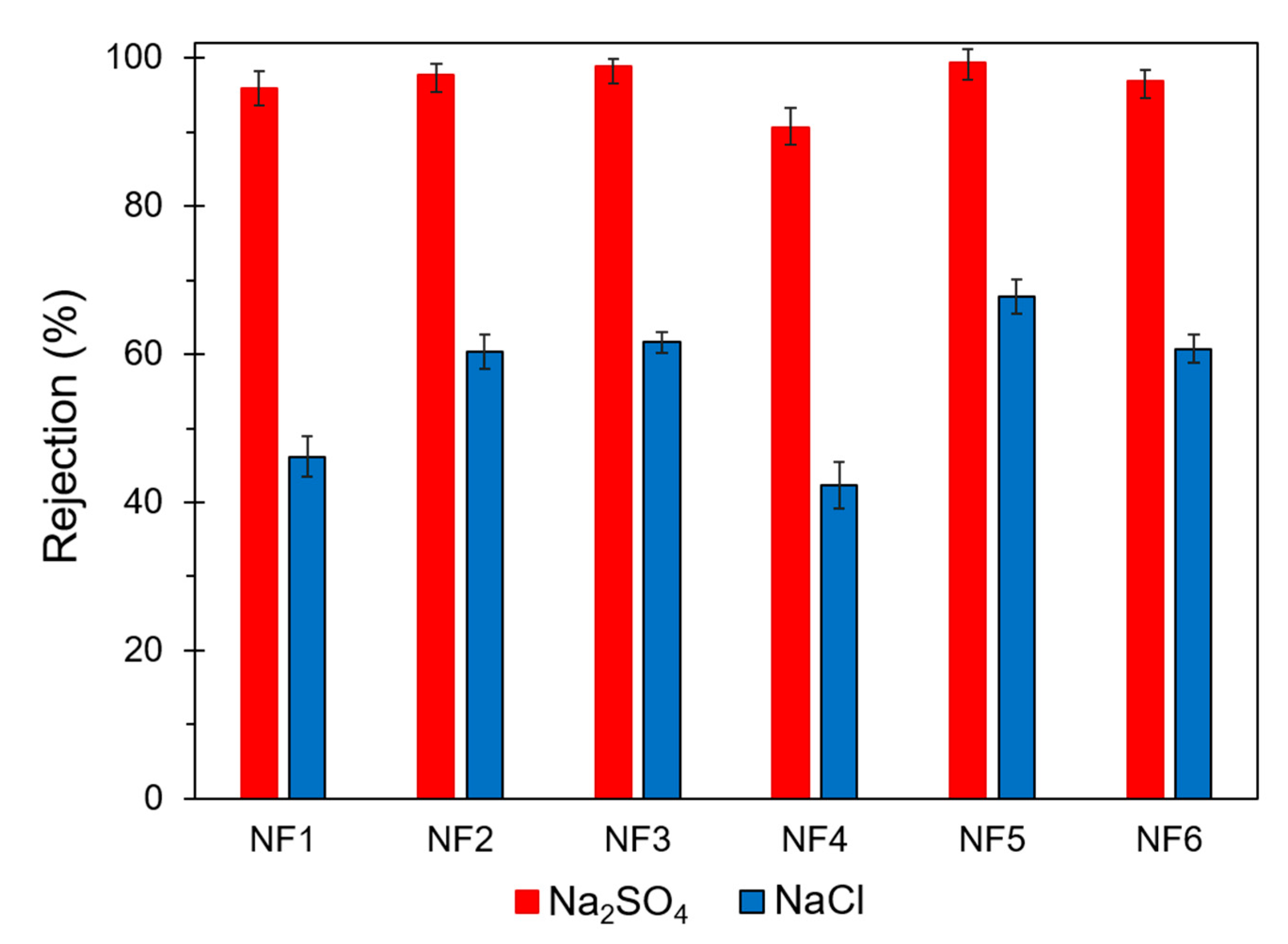
3.4.2. Salt Rejection Capacity
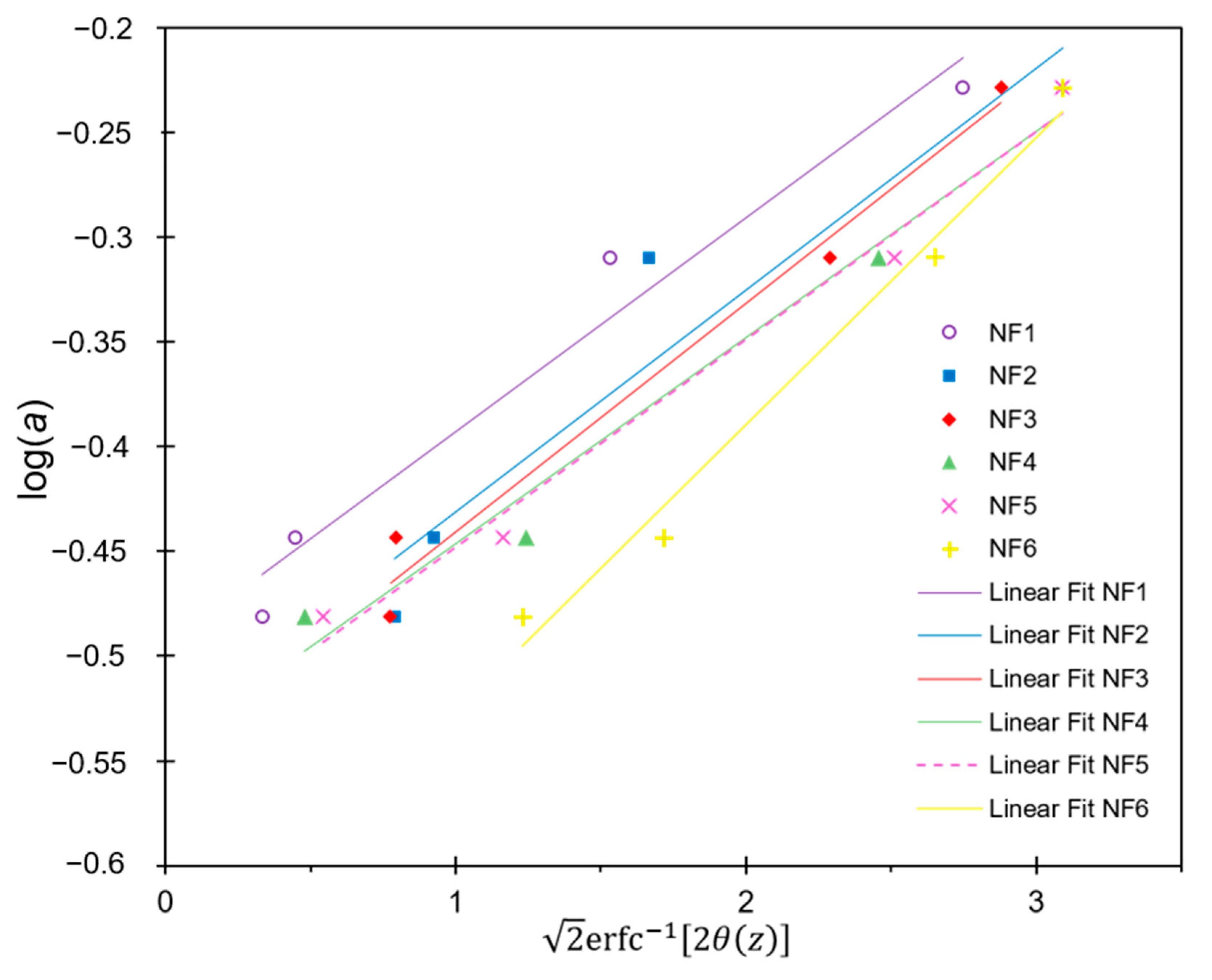
3.4.3. Molecular Weight Cut-Off Determination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Health Topics: Water Fact Sheet. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 6 March 2020).
- Baker, R.W. Membrane Technology and Applications, 2nd ed.; John Wiley & Sons: Chichester, UK, 2012; p. 575. [Google Scholar] [CrossRef]
- Homayoonfal, M.; Akbari, A.; Mehrnia, M.R. Preparation of polysulfone nanofiltration membranes by UV-assisted grafting polymerization for water softening. Desalination 2010, 263, 217–225. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, K. The high flux poly (m-phenylene isophthalamide) nanofiltration membrane for dye purification and desalination. Desalination 2011, 282, 19–26. [Google Scholar] [CrossRef]
- Zhu, Y.; Dou, P.; He, H.; Lan, H.; Xu, S.; Zhang, Y.; He, T.; Niu, J. Improvement of permeability and rejection of an acid resistant polysulfonamide thin-film composite nanofiltration membrane by a sulfonated poly (ether ether ketone) interlayer. Sep. Purif. Technol. 2020, 239, 116528. [Google Scholar] [CrossRef]
- Guo, Y.-S.; Ji, Y.-L.; Wu, B.; Wang, N.-X.; Yin, M.-J.; An, Q.-F.; Gao, C.-J. High-flux zwitterionic nanofiltration membrane constructed by in-situ introduction method for monovalent salt/antibiotics separation. J. Membr. Sci. 2020, 593, 117441. [Google Scholar] [CrossRef]
- Wang, J.; He, R.; Han, X.; Jiao, D.; Zhu, J.; Lai, F.; Liu, X.; Liu, J.; Zhang, Y.; Van Der Bruggen, B. High performance loose nanofiltration membranes obtained by a catechol-based route for efficient dye/salt separation. Chem. Eng. J. 2019, 375, 121982. [Google Scholar] [CrossRef]
- Gu, J.-E.; Jun, B.-M.; Kwon, Y.-N. Effect of chlorination condition and permeability of chlorine species on the chlorination of a polyamide membrane. Water Res. 2012, 46, 5389–5400. [Google Scholar] [CrossRef]
- Haghighat, N.; Vatanpour, V. Fouling decline and retention increase of polyvinyl chloride nanofiltration membranes blended by polypyrrole functionalized multiwalled carbon nanotubes. Mater. Today Commun. 2020, 23, 100851. [Google Scholar] [CrossRef]
- Jye, L.W.; Ismail, A.F. Nanofiltration Membranes: Synthesis, Characterization, and Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—Development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef]
- Hai, Y.; Zhang, J.; Shi, C.; Zhou, A.; Bian, C.; Li, W. Thin film composite nanofiltration membrane prepared by the interfacial polymerization of 1,2,4,5-benzene tetracarbonyl chloride on the mixed amines cross-linked poly (ether imide) support. J. Membr. Sci. 2016, 520, 19–28. [Google Scholar] [CrossRef]
- Morales-Cuevas, J.B.; Pérez-Sicairos, S.; Lin, S.W.; Salazar-Gastélum, M.I. Evaluation of a modified spray-applied interfacial polymerization method for preparation of nanofiltration membranes. J. Appl. Polym. Sci. 2019, 136, 1–10. [Google Scholar] [CrossRef]
- Esfandian, F.; Peyravi, M.; Ghoreyshi, A.A.; Jahanshahi, M.; Rad, A.S. Fabrication of TFC nanofiltration membranes via co-solvent assisted interfacial polymerization for lactose recovery. Arab. J. Chem. 2019, 12, 5325–5338. [Google Scholar] [CrossRef]
- Echaide-Górriz, C.; Zapata, J.A.; Etxeberría-Benavides, M.; Téllez, C.; Coronas, J. Polyamide/MOF bilayered thin film composite hollow fiber membranes with tuned MOF thickness for water nanofiltration. Sep. Purif. Technol. 2020, 236, 116265. [Google Scholar] [CrossRef]
- Arumugham, T.; Kaleekkal, N.J.; Rana, D. Fabrication of novel aromatic amine functionalized nanofiltration (NF) membranes and testing its dye removal and desalting ability. Polym. Test. 2018, 72, 1–10. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, J.; Wu, D.; Feng, X. Layer-by-layer self-assembled chitosan/PAA nanofiltration membranes. Sep. Purif. Technol. 2018, 207, 142–150. [Google Scholar] [CrossRef]
- Guo, D.; Xiao, Y.; Li, T.; Zhou, Q.; Shen, L.; Li, R.; Xu, Y.; Lin, H. Fabrication of high-performance composite nanofiltration membranes for dye wastewater treatment: Mussel-inspired layer-by-layer self-assembly. J. Colloid Interface Sci. 2020, 560, 273–283. [Google Scholar] [CrossRef]
- Ben-Sasson, M.; Lu, X.; Bar-Zeev, E.; Zodrow, K.R.; Nejati, S.; Qi, G.; Giannelis, E.P.; Elimelech, M. In situ formation of silver nanoparticles on thin-film composite reverse osmosis membranes for biofouling mitigation. Water Res. 2014, 62, 260–270. [Google Scholar] [CrossRef]
- Abadikhah, H.; Kalali, E.N.; Behzadi, S.; Khan, S.A.; Xu, X.; Shabestari, M.E.; Agathopoulos, S. High flux thin film nanocomposite membrane incorporated with functionalizedTiO2@reduced graphene oxide nanohybrids for organic solvent nanofiltration. Chem. Eng. Sci. 2019, 204, 99–109. [Google Scholar] [CrossRef]
- Li, Y.-X.; Cao, Y.; Wang, M.; Xu, Z.-L.; Zhang, H.-Z.; Liu, X.-W.; Li, Z. Novel high-flux polyamide/TiO2 composite nanofiltration membranes on ceramic hollow fibre substrates. J. Membr. Sci. 2018, 565, 322–330. [Google Scholar] [CrossRef]
- Low, Z.-X.; Wang, Z.; Leong, S.; Razmjou, A.; Dumée, L.F.; Zhang, X.; Wang, H. Enhancement of the Antifouling Properties and Filtration Performance of Poly(ethersulfone) Ultrafiltration Membranes by Incorporation of Nanoporous Titania Nanoparticles. Ind. Eng. Chem. Res. 2015, 54, 11188–11198. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Trilles, C.A.; De Guzman, M.R.; Pereira, J.M.; Aquino, R.R.; Huang, S.-H.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Improved performance of thin-film nanocomposite nanofiltration membranes as induced by embedded polydopamine-coated silica nanoparticles. Sep. Purif. Technol. 2019, 224, 113–120. [Google Scholar] [CrossRef]
- Beisl, S.; Monteiro, S.; Santos, R.; Figueiredo, A.S.; Sánchez-Loredo, M.G.; Lemos, M.A.; Lemos, F.; Minhalma, M.; de Pinho, M.N. Synthesis and bactericide activity of nanofiltration composite membranes—Cellulose acetate/silver nanoparticles and cellulose acetate/silver ion exchanged zeolites. Water Res. 2019, 149, 225–231. [Google Scholar] [CrossRef]
- Al-Hinai, M.H.; Sathe, P.; Al-Abri, M.Z.; Dobretsov, S.; Al-Hinai, A.T.; Dutta, J. Antimicrobial Activity Enhancement of Poly (ether sulfone) Membranes by in Situ Growth of ZnO Nanorods. ACS Omega 2017, 2, 3157–3167. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.-M.; Xu, Z.-L.; Tang, Y.-J.; Ji, C.-H. Polypiperazine-amide Nanofiltration Membrane Modified by Different Functionalized Multiwalled Carbon Nanotubes (MWCNTs). ACS Appl. Mater. Interfaces 2016, 8, 19135–19144. [Google Scholar] [CrossRef]
- Wu, M.-B.; Lv, Y.; Yang, H.-C.; Liu, L.-F.; Zhang, X.; Xu, Z.-K. Thin film composite membranes combining carbon nanotube intermediate layer and microfiltration support for high nanofiltration performances. J. Membr. Sci. 2016, 515, 238–244. [Google Scholar] [CrossRef]
- Zhao, H.; Qiu, S.; Wu, L.; Zhang, L.; Chen, H.; Gao, C. Improving the performance of polyamide reverse osmosis membrane by incorporation of modified multi-walled carbon nanotubes. J. Membr. Sci. 2014, 450, 249–256. [Google Scholar] [CrossRef]
- Wang, L.; Song, X.; Wang, T.; Wang, S.; Wang, Z.; Gao, C. Fabrication and characterization of polyethersulfone/carbon nanotubes (PES/CNTs) based mixed matrix membranes (MMMs) for nanofiltration application. Appl. Surf. Sci. 2015, 330, 118–125. [Google Scholar] [CrossRef]
- Li, X.; Zhao, C.; Yang, M.; Yang, B.; Hou, D.; Wang, T. Reduced graphene oxide-NH2 modified low pressure nanofiltration composite hollow fiber membranes with improved water flux and antifouling capabilities. Appl. Surf. Sci. 2017, 419, 418–428. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, R.; He, Y.; Zhao, G.; Zhu, H. Graphene oxide-in-polymer nanofiltration membranes with enhanced permeability by interfacial polymerization. J. Membr. Sci. 2018, 564, 813–819. [Google Scholar] [CrossRef]
- Kang, Y.; Obaid, M.; Jang, J.; Kim, I.S. Sulfonated graphene oxide incorporated thin film nanocomposite nanofiltration membrane to enhance permeation and antifouling properties. Desalination 2019, 470, 114125. [Google Scholar] [CrossRef]
- Nam, Y.T.; Kim, S.J.; Kang, K.M.; Jung, W.-B.; Kim, D.W.; Jung, H.-T. Enhanced nanofiltration performance of graphene-based membranes on wrinkled polymer supports. Carbon N. Y. 2019, 148, 370–377. [Google Scholar] [CrossRef]
- Hua, D.; Chung, T.-S. Polyelectrolyte functionalized lamellar graphene oxide membranes on polypropylene support for organic solvent nanofiltration. Carbon N. Y. 2017, 122, 604–613. [Google Scholar] [CrossRef]
- Anand, A.; Unnikrishnan, B.; Mao, J.-Y.; Lin, H.-J.; Huang, C.-C. Graphene-based nanofiltration membranes for improving salt rejection, water flux and antifouling–A review. Desalination 2018, 429, 119–133. [Google Scholar] [CrossRef]
- Muzyka, R.; Kwoka, M.; Smędowski, Ł.; Díez, N.; Gryglewicz, G. Oxidation of graphite by different modified Hummers methods. New Carbon Mater. 2017, 32, 15–20. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, H.; Meng, N.; Jian, M.; Wang, H.; Zhang, X. Graphene oxide incorporated thin film nanocomposite membrane at low concentration monomers. J. Membr. Sci. 2018, 565, 380–389. [Google Scholar] [CrossRef]
- Lai, G.; Lau, W.; Goh, P.; Tan, Y.; Ng, B.; Ismail, A. A novel interfacial polymerization approach towards synthesis of graphene oxide-incorporated thin film nanocomposite membrane with improved surface properties. Arab. J. Chem. 2019, 12, 75–87. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, S.-T.; Liu, J.-H.; Dong, E.; Wang, Y.; Cao, A.; Liu, Y.; Wang, H. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol. Lett. 2011, 200, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Liu, C.; Ma, H.; Hong, Z.; Xie, Q.; Lu, Y. Fabrication of pH-sensitive thin-film nanocomposite nanofiltration membranes with enhanced performance by incorporating amine-functionalized graphene oxide. Appl. Surface Sci. 2019, 487, 1209–1221. [Google Scholar] [CrossRef]
- Wen, P.; Chen, Y.; Hu, X.; Cheng, B.; Liu, D.; Zhang, Y.; Nair, S. Polyamide thin film composite nanofiltration membrane modified with acyl chlorided graphene oxide. J. Membr. Sci. 2017, 535, 208–220. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Irani, V.; Tavasoli, A.; Vahidi, M. Preparation of amine functionalized reduced graphene oxide/methyl diethanolamine nanofluid and its application for improving the CO2 and H2S absorption. J. Colloid Interface Sci. 2018, 527, 57–67. [Google Scholar] [CrossRef]
- Ayala, C.E.; Villalpando, A.; Nguyen, A.L.; McCandless, G.T.; Kartika, R. Chlorination of Aliphatic Primary Alcohols via Triphosgene–Triethylamine Activation. Org. Lett. 2012, 14, 3676–3679. [Google Scholar] [CrossRef]
- Lin, S.W.; Martínez-Ayala, A.V.; Pérez-Sicairos, S.; Félix-Navarro, R.M. Preparation and characterization of low-pressure and high MgSO4 rejection thin-film composite NF membranes via interfacial polymerization process. Polym. Bull. 2019, 76, 5619–5632. [Google Scholar] [CrossRef]
- Ambre, J.P.; Dhopte, K.B.; Nemade, P.R.; Dalvi, V.H. High flux hyperbranched starch-graphene oxide piperazinamide composite nanofiltration membrane. J. Environ. Chem. Eng. 2019, 7, 103300. [Google Scholar] [CrossRef]
- Otero, J.; Mazarrasa, O.; Villasante, J.; Silva, V.; Prádanos, P.; Calvo, J.; Hernández, A. Three independent ways to obtain information on pore size distributions of nanofiltration membranes. J. Membr. Sci. 2008, 309, 17–27. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Shao, L.; Lau, C.H. High flux polyethylene glycol based nanofiltration membranes for water environmental remediation. J. Membr. Sci. 2015, 476, 95–104. [Google Scholar] [CrossRef]
- Larkin, P.J. Illustrated and Raman Spectra Demonstrating Important Functional Groups; Elsevier: New York, NY, USA, 2018; pp. 153–210. [Google Scholar]
- Long, D.A. Infrared and Raman characteristic group frequencies. Tables and chartsGeorge Socrates John Wiley and Sons, Ltd, Chichester, Third Edition, 2001. Price £135. J. Raman Spectrosc. 2004, 35, 905. [Google Scholar] [CrossRef]
- Briggs, D. Handbook of X-ray Photoelectron Spectroscopy C. D. Wanger, W.M. Riggs, L.E. Davis, J.F. Moulder and G. E. Muilenberg Perkin-Elmer Corp., Physical Electronics Division, Eden Prairie, Minnesota, USA, 1979. 190 pp. $195. Surf. Interface Anal. 1981, 3. [Google Scholar] [CrossRef]
- Lai, L.; Chen, L.; Zhan, D.; Sun, L.; Liu, J.; Lim, S.H.; Poh, C.K.; Shen, Z.; Lin, J. One-step synthesis of NH2-graphene from in situ graphene-oxide reduction and its improved electrochemical properties. Carbon N. Y. 2011, 49, 3250–3257. [Google Scholar] [CrossRef]
- Latiff, N.M.; Mayorga-Martinez, C.C.; Wang, L.; Sofer, Z.; Fisher, A.C.; Pumera, M. Microwave irradiated N- and B,Cl-doped graphene: Oxidation method has strong influence on capacitive behavior. Appl. Mater. Today 2017, 9, 204–211. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Chen, J.; Yin, Y.; Wu, H. Structurally stable graphene oxide-based nanofiltration membranes with bioadhesive polydopamine coating. Appl. Surf. Sci. 2018, 427, 1092–1098. [Google Scholar] [CrossRef]
- Ghaemi, N.; Madaeni, S.S.; Alizadeh, A.; Daraei, P.; Badieh, M.M.S.; Falsafi, M.; Vatanpour, V. Fabrication and modification of polysulfone nanofiltration membrane using organic acids: Morphology, characterization and performance in removal of xenobiotics. Sep. Purif. Technol. 2012, 96, 214–228. [Google Scholar] [CrossRef]
- Soroush, A.; Barzin, J.; Barikani, M.; Fathizadeh, M. Interfacially polymerized polyamide thin film composite membranes: Preparation, characterization and performance evaluation. Desalination 2012, 287, 310–316. [Google Scholar] [CrossRef]
- Sirinupong, T.; Youravong, W.; Tirawat, D.; Lau, W.; Lai, G.; Ismail, A. Synthesis and characterization of thin film composite membranes made of PSF-TiO2/GO nanocomposite substrate for forward osmosis applications. Arab. J. Chem. 2018, 11, 1144–1153. [Google Scholar] [CrossRef]
- Geise, G.M.; Park, H.B.; Sagle, A.C.; Freeman, B.D.; McGrath, J.E. Water permeability and water/salt selectivity tradeoff in polymers for desalination. J. Membr. Sci. 2011, 369, 130–138. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Fang, L.; Lin, H.; Liu, F.; Tang, C.Y. Electrosprayed polyamide nanofiltration membrane with intercalated structure for controllable structure manipulation and enhanced separation performance. J. Membr. Sci. 2020, 602, 117971. [Google Scholar] [CrossRef]
- Ganesh, B.M.; Isloor, A.M.; Ismail, A.F. Enhanced hydrophilicity and salt rejection study of graphene oxide-polysulfone mixed matrix membrane. Desalination 2013, 313, 199–207. [Google Scholar] [CrossRef]


| Reagent | NF1 | NF2 | NF3 | NF4 | NF5 | NF6 | |
|---|---|---|---|---|---|---|---|
| PIP | 0.25% | 0.25% | 0.25% | 0.25% | 0.25% | 0.25% | |
| NaOH | 0.50% | 0.50% | 0.50% | 0.50% | 0.50% | 0.50% | |
| Aqueous phase | PVA | 0.25% | 0.25% | 0.25% | 0.25% | 0.25% | 0.25% |
| GO | - | 0.004% | - | - | - | - | |
| N-GO | - | - | 0.004% | - | - | 0.002% | |
| TMC | 1.00% | 1.00% | 1.00% | 1.00% | 1.00% | 1.00% | |
| Organic phase | GO | - | - | - | 0.004% | - | - |
| Cl-GO | - | - | - | - | 0.004% | 0.002% |
| Sample | C1 | C2 | C3 | N1 | N2 |
|---|---|---|---|---|---|
| GO | 69.7 | 5.2 | 25.1 | N/D | N/D |
| N-GO | 62.4 | 16.8 | 20.8 | 78.0 | 20.0 |
| Cl-GO | 68.7 | 9.8 | 21.5 | 24.5 | 75.5 |
| Sample | C | O | N | Cl |
|---|---|---|---|---|
| GO | 54% | 43% | 4% | 0% |
| N-GO | 57% | 19% | 23% | 1% |
| Cl-GO | 70% | 6% | 8% | 16% |
| Membrane | Permeance (L m−2 bar−1 h−1) | Rejection for Na2SO4 (%) | Rejection for NaCl (%) | MWCO (g mol−1) | Surface Roughness (nm) |
|---|---|---|---|---|---|
| NF1 | 1.12 ± 0.04 | 95.9 ± 2.3 | 46.2 ± 2.7 | 264 ± 5 | 30.6 ± 0.6 |
| NF2 | 1.93 ± 0.07 | 97.7 ± 1.6 | 60.3 ± 2.3 | 221 ± 8 | 195.5 ± 3.9 |
| NF3 | 1.63 ± 0.05 | 98.9 ± 1.1 | 61.6 ± 1.4 | 212 ± 7 | 159.3 ± 3.2 |
| NF4 | 1.25 ± 0.07 | 90.7 ± 2.6 | 42.3 ± 3.1 | 203 ± 6 | 134.4 ± 2.7 |
| NF5 | 1.42 ± 0.06 | 99.4 ± 1.8 | 67.8 ± 2.3 | 202 ± 8 | 181.3 ± 3.6 |
| NF6 | 1.83 ± 0.04 | 96.9 ± 1.5 | 60.7 ± 1.9 | 145 ± 7 | 30.2 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Picazo, F.J.; Pérez-Sicairos, S.; Fimbres-Weihs, G.A.; Lin, S.W.; Salazar-Gastélum, M.I.; Trujillo-Navarrete, B. Preparation of Thin-Film Composite Nanofiltration Membranes Doped with N- and Cl-Functionalized Graphene Oxide for Water Desalination. Polymers 2021, 13, 1637. https://doi.org/10.3390/polym13101637
García-Picazo FJ, Pérez-Sicairos S, Fimbres-Weihs GA, Lin SW, Salazar-Gastélum MI, Trujillo-Navarrete B. Preparation of Thin-Film Composite Nanofiltration Membranes Doped with N- and Cl-Functionalized Graphene Oxide for Water Desalination. Polymers. 2021; 13(10):1637. https://doi.org/10.3390/polym13101637
Chicago/Turabian StyleGarcía-Picazo, Francisco J., Sergio Pérez-Sicairos, Gustavo A. Fimbres-Weihs, Shui W. Lin, Moisés I. Salazar-Gastélum, and Balter Trujillo-Navarrete. 2021. "Preparation of Thin-Film Composite Nanofiltration Membranes Doped with N- and Cl-Functionalized Graphene Oxide for Water Desalination" Polymers 13, no. 10: 1637. https://doi.org/10.3390/polym13101637
APA StyleGarcía-Picazo, F. J., Pérez-Sicairos, S., Fimbres-Weihs, G. A., Lin, S. W., Salazar-Gastélum, M. I., & Trujillo-Navarrete, B. (2021). Preparation of Thin-Film Composite Nanofiltration Membranes Doped with N- and Cl-Functionalized Graphene Oxide for Water Desalination. Polymers, 13(10), 1637. https://doi.org/10.3390/polym13101637









