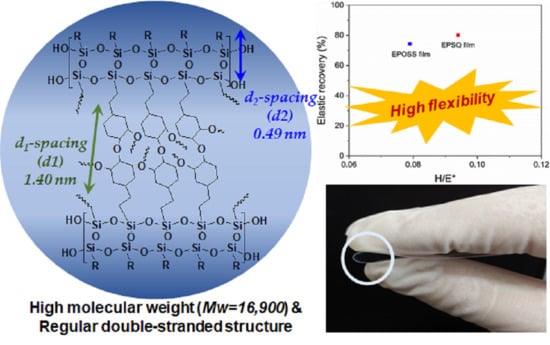
Hard Coating Materials Based on Photo-Reactive Silsesquioxane for Flexible Application: Improvement of Flexible and Hardness Properties by High Molecular Weight
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
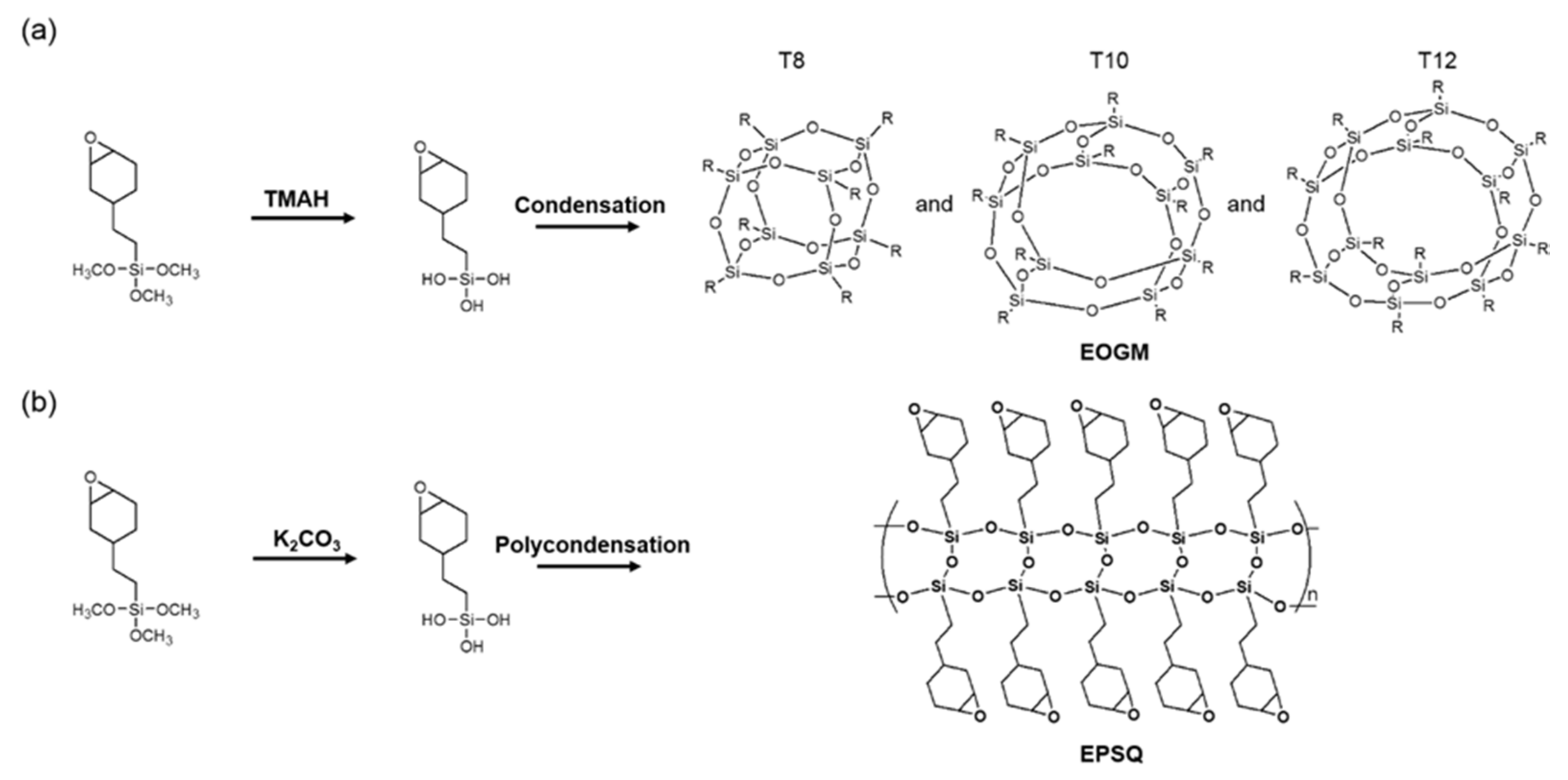
2.2. Synthesis of EPOSS and EPSQ
2.3. Fabrication of EPOSS and EPSQ Films
2.4. Characterization of the EPOSS and EPSQ Films
3. Results
3.1. Preparation and Characterization of Silsesquioxanes
3.2. Thermal Properties of Silsesquioxanes
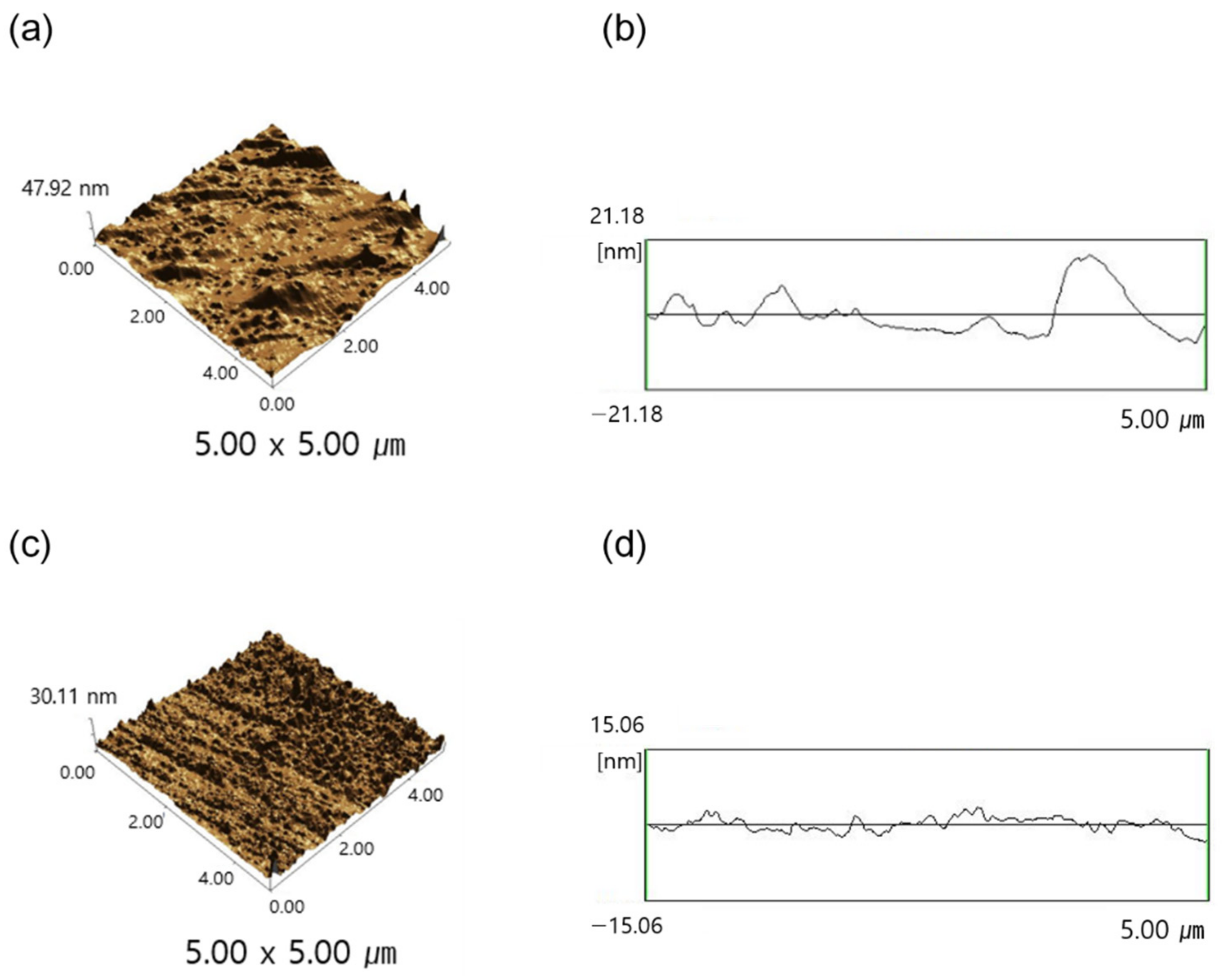
3.3. Characterization of Silsesquioxane Films
3.4. Structural Properties of Silsesquioxane Films
3.5. Optical Properties of Silsesquioxane Films
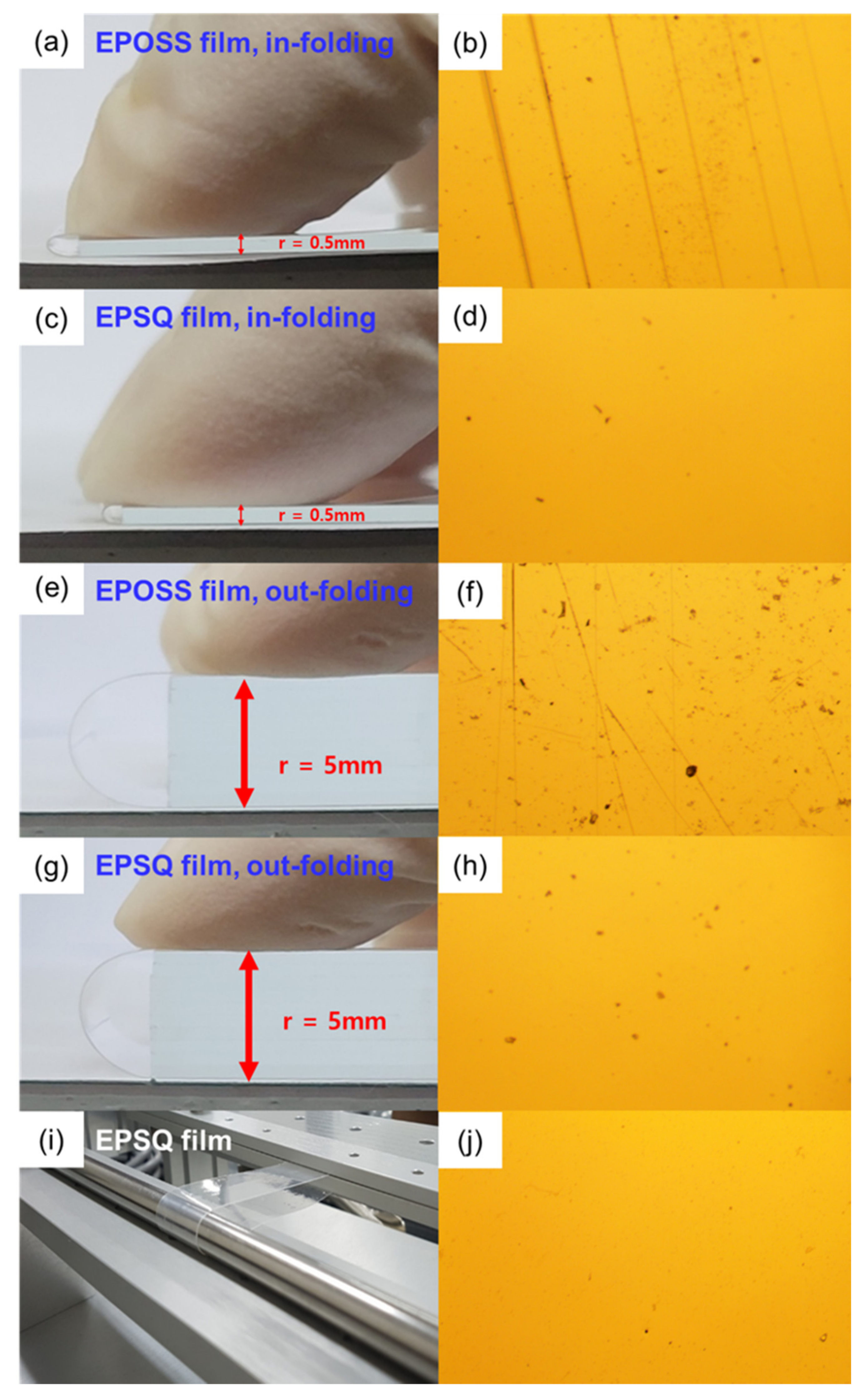
3.6. Mechanical Properties of Silsesquioxane Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, M.; Bae, S.-R.; Hu, L.; Hoang, A.T.; Kim, S.Y.; Ahn, J.-H. Full-color active-matrix organic light-emitting diode display on human skin based on a large-area MoS2 backplane. Sci. Adv. 2020, 6, 5898. [Google Scholar] [CrossRef] [PubMed]
- Han, M.J.; Khang, D.Y. Glass and Plastics Platforms for Foldable Electronics and Displays. Adv. Mater. 2015, 27, 4969–4974. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.J.; Kang, J.H.; Kim, S.J.; Koh, W.-G.; Song, H.J.; Lee, S. Effects of Organic Acids and a Fluoropolymer on the Conductivity and Transparency of Poly(3,4-ethylenedioxythiophene) Films. Macromol. Res. 2018, 26, 410–417. [Google Scholar] [CrossRef]
- Kwon, S.J.; Seok, W.C.; Leem, J.T.; Kang, J.H.; Koh, W.-G.; Song, H.J.; Lee, S. Enhancement of conductivity and transparency for of poly(3,4-ethylenedioxythiophene) films using photo-acid generator as dopant. Polymer 2018, 147, 30–37. [Google Scholar] [CrossRef]
- Yi, C.; Li, W.; Shi, S.; He, K.; Ma, P.; Chen, M.; Yang, C. High-temperature-resistant and colorless polyimide: Preparations, properties, and applications. Sol. Energy 2020, 195, 340–354. [Google Scholar] [CrossRef]
- Seok, W.C.; Leem, J.T.; Kang, J.H.; Kim, Y.J.; Lee, S.; Song, H.J. Change of Characterization and Film Morphology Based on Acrylic Pressure Sensitive Adhesives by Hydrophilic Derivative Ratio. Polymers 2020, 12, 1504. [Google Scholar] [CrossRef]
- Lee, A.S.; Jo, Y.Y.; Jeon, H.; Choi, S.-S.; Baek, K.-Y.; Hwang, S.S. Mechanical properties of thiol-ene UV-curable thermoplastic polysilsesquioxanes. Polymer 2015, 68, 140–146. [Google Scholar] [CrossRef]
- Seubert, C.; Nietering, K.; Nichols, M.; Wykoff, R.; Bollin, S. An Overview of the Scratch Resistance of Automotive Coatings: Exterior Clearcoats and Polycarbonate Hardcoats. Coatings 2012, 2, 221–234. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, G.M.; Bae, J.G.; Kim, Y.H.; Bae, B.S. High-Performance and Simply-Synthesized Ladder-Like Structured Methacrylate Siloxane Hybrid Material for Flexible Hard Coating. Polymers 2018, 10, 449. [Google Scholar] [CrossRef]
- Musil, J.; Sklenka, J.; Prochazka, J. Protective over-layer coating preventing cracking of thin films deposited on flexible substrates. Surf. Coat. Technol. 2014, 240, 275–280. [Google Scholar] [CrossRef]
- Kim, D.; Kim, S.H.; Kim, J.H.; Lee, J.C.; Ahn, J.P.; Kim, S.W. Failure criterion of silver nanowire electrodes on a polymer substrate for highly flexible devices. Sci. Rep. 2017, 7, 45903. [Google Scholar] [CrossRef]
- Chansomwong, K.; Kim, Y.H.; Lee, H.; Bae, B.-S. Facile preparation of wear-resistant and anti-fingerprint hard coating with chemisorption of fluorosilane by simple wet coating. J. Sol-Gel Sci. Technol. 2020, 95, 447–455. [Google Scholar] [CrossRef]
- Seo, J.; Moon, S.W.; Kang, H.; Choi, B.H.; Seo, J.H. Foldable and Extremely Scratch-Resistant Hard Coating Materials from Molecular Necklace-like Cross-Linkers. ACS Appl. Mater. Interfaces 2019, 11, 27306–27317. [Google Scholar] [CrossRef]
- Parola, S.; Julián-López, B.; Carlos, L.D.; Sanchez, C. Optical Properties of Hybrid Organic-Inorganic Materials and their Applications. Adv. Funct. Mater. 2016, 26, 6506–6544. [Google Scholar] [CrossRef]
- Diaz, U.; Corma, A. Organic-Inorganic Hybrid Materials: Multi-Functional Solids for Multi-Step Reaction Processes. Chemistry 2018, 24, 3944–3958. [Google Scholar] [CrossRef]
- Yilgör, E.; Yilgör, I. Silicone containing copolymers: Synthesis, properties and applications. Prog. Polym. Sci. 2014, 39, 1165–1195. [Google Scholar] [CrossRef]
- Zolper, T.J.; Seyam, A.; Li, Z.; Chen, C.; Jungk, M.; Stammer, A.; Marks, T.J.; Chung, Y.-W.; Wang, Q. Friction and Wear Protection Performance of Synthetic Siloxane Lubricants. Tribol. Lett. 2013, 51, 365–376. [Google Scholar] [CrossRef]
- Peng, S. Silicon, Silica and Silicone in New York. Silicon 2015, 7, 307–308. [Google Scholar] [CrossRef]
- Byczyński, Ł.; Dutkiewicz, M.; Maciejewski, H. Synthesis and properties of high-solids hybrid materials obtained from epoxy functional urethanes and siloxanes. Prog. Org. Coat. 2015, 84, 59–69. [Google Scholar] [CrossRef]
- Fugel, M.; Hesse, M.F.; Pal, R.; Beckmann, J.; Jayatilaka, D.; Turner, M.J.; Karton, A.; Bultinck, P.; Chandler, G.S.; Grabowsky, S. Covalency and Ionicity Do Not Oppose Each Other-Relationship Between Si-O Bond Character and Basicity of Siloxanes. Chemistry 2018, 24, 15275–15286. [Google Scholar] [CrossRef]
- Abe, Y.; Gunji, T. Oligo- and polysiloxanes. Prog. Polym. Sci. 2004, 29, 149–182. [Google Scholar] [CrossRef]
- Bae, J.-y.; Kim, Y.; Kim, H.-Y.; Lim, Y.-w.; Bae, B.-S. Sol–gel synthesized linear oligosiloxane-based hybrid material for a thermally-resistant light emitting diode (LED) encapsulant. RSC Adv. 2013, 3, 8871–8877. [Google Scholar] [CrossRef]
- Ghermezcheshme, H.; Mohseni, M.; Yahyaei, H. Use of nanoindentaion and nanoscratch experiments to reveal the mechanical behavior of POSS containing polyurethane nanocomposite coatings: The role of functionality. Tribol. Int. 2015, 88, 66–75. [Google Scholar] [CrossRef]
- Kaneko, Y. Ionic silsesquioxanes: Preparation, structure control, characterization, and applications. Polymer 2018, 144, 205–224. [Google Scholar] [CrossRef]
- Zhang, W.; Camino, G.; Yang, R. Polymer/polyhedral oligomeric silsesquioxane (POSS) nanocomposites: An overview of fire retardance. Prog. Polym. Sci. 2017, 67, 77–125. [Google Scholar] [CrossRef]
- Li, Z.; Kong, J.; Wang, F.; He, C. Polyhedral oligomeric silsesquioxanes (POSSs): An important building block for organic optoelectronic materials. J. Mater. Chem. C 2017, 5, 5283–5298. [Google Scholar] [CrossRef]
- Pohl, S.; Janka, O.; Füglein, E.; Kickelbick, G. Thermoplastic Silsesquioxane Hybrid Polymers with a Local Ladder-Type Structure. Macromolecules 2021, 54, 3873–3885. [Google Scholar] [CrossRef]
- Du, Y.; Liu, H. Cage-like silsesquioxanes-based hybrid materials. Dalton. Trans. 2020, 49, 5396–5405. [Google Scholar] [CrossRef]
- Fan, L.; Wang, X.; Wu, D. Polyhedral Oligomeric Silsesquioxanes (POSS)-based Hybrid Materials: Molecular Design, Solution Self-Assembly and Biomedical Applications. Chin. J. Chem. 2021, 39, 757–774. [Google Scholar] [CrossRef]
- Xu, Y.; Long, J.; Zhang, R.; Du, Y.; Guan, S.; Wang, Y.; Huang, L.; Wei, H.; Liu, L.; Huang, Y. Greatly improving thermal stability of silicone resins by modification with POSS. Polym. Degrad. Stab. 2020, 174, 109082. [Google Scholar] [CrossRef]
- Shen, R.; Du, Y.; Yang, X.; Liu, H. Silsesquioxanes-based porous functional polymers for water purification. J. Mater. Sci. 2020, 55, 7518–7529. [Google Scholar] [CrossRef]
- Jo, Y.Y.; Lee, A.S.; Baek, K.-Y.; Lee, H.; Hwang, S.S. Thermally reversible self-healing polysilsesquioxane structure-property relationships based on Diels-Alder chemistry. Polymer 2017, 108, 58–65. [Google Scholar] [CrossRef]
- Baatti, A.; Erchiqui, F.; Bébin, P.; Godard, F.; Bussières, D. A two-step Sol-Gel method to synthesize a ladder polymethylsilsesquioxane nanoparticles. Adv. Powder Technol. 2017, 28, 1038–1046. [Google Scholar] [CrossRef]
- Wang, R.; Rong, T.; Cao, G.S.; Jia, X.W.; Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Synthesis and synergetic effects of ladder-like silsesquioxane/epoxy compositional gradient hybrid coating. Prog. Org. Coat. 2019, 130, 58–65. [Google Scholar] [CrossRef]
- Serra, A.; Ramis, X.; Fernández-Francos, X. Epoxy Sol-Gel Hybrid Thermosets. Coatings 2016, 6, 8. [Google Scholar] [CrossRef]
- Hoffmann, F.; Froba, M. Vitalising porous inorganic silica networks with organic functions--PMOs and related hybrid materials. Chem. Soc. Rev. 2011, 40, 608–620. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Raju, K.V.S.N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Asemani, H.R.; Luo, L.; Mannari, V. Corrosion-resistant organic-inorganic hybrid pretreatments obtained by UV-initiated process suitable for primer-less coating systems. Prog. Org. Coat. 2020, 147, 105878. [Google Scholar] [CrossRef]
- Lee, A.S.; Choi, S.S.; Song, S.J.; Baek, K.Y.; Hwang, S.S. Cationically photopolymerizable epoxy-functionalized thermoplastic polysilsesquioxanes: Synthesis and properties. RSC Adv. 2014, 4, 56532–56538. [Google Scholar] [CrossRef]
- Choi, G.M.; Jin, J.; Shin, D.; Kim, Y.H.; Ko, J.H.; Im, H.G.; Jang, J.; Jang, D.; Bae, B.S. Flexible Hard Coating: Glass-Like Wear Resistant, Yet Plastic-Like Compliant, Transparent Protective Coating for Foldable Displays. Adv. Mater. 2017, 29, 1700205. [Google Scholar] [CrossRef]
- Kim, D.G.; Jung, B.; Kim, K.H.; Cho, K.Y.; Jeong, Y.C. Highly robust and transparent flexible cover window films based on UV-curable polysilsesquioxane nano sol. J. Appl. Polym. Sci. 2020, 137, 49012. [Google Scholar] [CrossRef]
- Kim, H.; Bae, J.-y.; Kim, Y.H.; Kim, Y.B.; Bae, B.-S. Low temperature curable epoxy siloxane hybrid materials for LED encapsulant. J. Appl. Polym. Sci. 2014, 131, 33968. [Google Scholar] [CrossRef]
- Ahn, Y.; Kim, S.; Kim, M.S.; Youk, J.H.; Kim, B.-G. Ladder-Structured Polysilsesquioxane/Al2O3 Nanocomposites for Transparent Wear-Resistant Windows. Fibers Polym. 2018, 19, 1295–1302. [Google Scholar] [CrossRef]
- Gao, J.; Du, Y.; Dong, C. Rheological behavior and mechanical properties of PVC/MAP-POSS nanocomposites. Polym. Compos. 2010, 31, 1822–1827. [Google Scholar] [CrossRef]
- Tokunaga, T.; Koge, S.; Mizumo, T.; Ohshita, J.; Kaneko, Y. Facile preparation of a soluble polymer containing polyhedral oligomeric silsesquioxane units in its main chain. Polym. Chem. 2015, 6, 3039–3045. [Google Scholar] [CrossRef]
- Ervithayasuporn, V.; Chimjarn, S. Synthesis and isolation of methacrylate- and acrylate-functionalized polyhedral oligomeric silsesquioxanes (T8, T10, and T12) and characterization of the relationship between their chemical structures and physical properties. Inorg. Chem. 2013, 52, 13108–13112. [Google Scholar] [CrossRef] [PubMed]
- Kozuma, T.; Kaneko, Y. Preparation of carboxyl-functionalized polyhedral oligomeric silsesquioxane by a structural transformation reaction from soluble rod-like polysilsesquioxane. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 2511–2518. [Google Scholar] [CrossRef]
- Hwang, S.O.; Lee, A.S.; Lee, J.Y.; Park, S.-H.; Jung, K.I.; Jung, H.W.; Lee, J.-H. Mechanical properties of ladder-like polysilsesquioxane-based hard coating films containing different organic functional groups. Prog. Org. Coat. 2018, 121, 105–111. [Google Scholar] [CrossRef]
- Choi, S.-S.; Lee, A.S.; Hwang, S.S.; Baek, K.-Y. Structural Control of Fully Condensed Polysilsesquioxanes: Ladderlike vs Cage Structured Polyphenylsilsesquioxanes. Macromolecules 2015, 48, 6063–6070. [Google Scholar] [CrossRef]
- Gorkem Sencevik, R.; Atilla Tasdelen, M. Poly(methyl methacrylate)/POSS hybrid networks by type II photoinitiated free radical polymerization. Polym. Compos. 2014, 35, 1614–1620. [Google Scholar] [CrossRef]
- Topolniak, I.; Hodoroaba, V.D.; Pfeifer, D.; Braun, U.; Sturm, H. Boehmite Nanofillers in Epoxy Oligosiloxane Resins: Influencing the Curing Process by Complex Physical and Chemical Interactions. Materials 2019, 12, 1513. [Google Scholar] [CrossRef]
- Mei, H.; Wang, R.; Ren, W.; Zhang, Y. The grafting reaction of epoxycyclohexyl polyhedral oligomeric silsesquioxanes with carboxylic methoxypolyethylene glycols and the properties of composite solid polymer electrolytes with the graftomer. J. Appl. Polym. Sci. 2017, 134, 44460. [Google Scholar] [CrossRef]
- Song, H.-J.; Kim, D.-H.; Lee, E.-J.; Moon, D.-K. Conjugated polymers consisting of quinacridone and quinoxaline as donor materials for organic photovoltaics: Orientation and charge transfer properties of polymers formed by phenyl structures with a quinoxaline derivative. J. Mater. Chem. A 2013, 1, 6010–6020. [Google Scholar] [CrossRef]
- Lee, E.J.; Song, H.J. Donor-Acceptor Polymer Based on Planar Structure of Alkylidene-Fluorene Derivative: Correlation of Power Conversion Efficiency among Polymer and Various Acceptor Units. Polymers 2020, 12, 2859. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, P.; Sun, L.; Wang, L. Hydrophobic Epoxy Caged Silsesquioxane Film (EP-POSS): Synthesis and Performance Characterization. Nanomaterials 2021, 11, 472. [Google Scholar] [CrossRef]
- Hui, B.; Ye, L. Polyurethane–imide–polyhedral oligomeric silsesquioxane hybrid nano-composites. J. Therm. Anal. Calorim. 2018, 136, 2383–2396. [Google Scholar] [CrossRef]
- Cobos, M.; Ramos, J.R.; Guzman, D.J.; Fernandez, M.D.; Fernandez, M.J. PCL/POSS Nanocomposites: Effect of POSS Derivative and Preparation Method on Morphology and Properties. Polymers 2018, 11, 33. [Google Scholar] [CrossRef]
- Hwang, S.O.; Lee, J.Y.; Lee, J.-H. Effect of the silsesquioxane structure on the mechanical properties of the silsesquioxane-reinforced polymer composite films. Prog. Org. Coat. 2019, 137, 105316. [Google Scholar] [CrossRef]
- Florea, N.M.; Lungu, A.; Badica, P.; Craciun, L.; Enculescu, M.; Ghita, D.G.; Ionescu, C.; Zgirian, R.G.; Iovu, H. Novel nanocomposites based on epoxy resin/epoxy-functionalized polydimethylsiloxane reinforced with POSS. Compos. Part B Eng. 2015, 75, 226–234. [Google Scholar] [CrossRef]
- Kim, D.H.; Seok, W.C.; Leem, J.T.; Han, Y.W.; Kang, J.H.; Song, H.J. Ordered orientation and compact molecule packing due to coplanar backbone structure of interlayer: Improvement in fill factor for photovoltaic device. Eur. Polym. J. 2019, 116, 330–335. [Google Scholar] [CrossRef]
- Jo, Y.Y.; Lee, A.S.; Baek, K.-Y.; Lee, H.; Hwang, S.S. Multi-crosslinkable self-healing polysilsesquioxanes for the smart recovery of anti-scratch properties. Polymer 2017, 124, 78–87. [Google Scholar] [CrossRef]
- Loka, C.; Lee, K.; Moon, S.W.; Choi, Y.; Lee, K.-S. Enhanced transmittance of sapphire by silicon oxynitride thin films annealed at high temperatures. Mater. Lett. 2018, 213, 354–357. [Google Scholar] [CrossRef]
- Amini, M.; Keshavarzi, R.; Mirkhani, V.; Moghadam, M.; Tangestaninejad, S.; Mohammadpoor-Baltork, I.; Sadegh, F. From dense blocking layers to different templated films in dye sensitized and perovskite solar cells: Toward light transmittance management and efficiency enhancement. J. Mater. Chem. A 2018, 6, 2632–2642. [Google Scholar] [CrossRef]
- Leem, J.W.; Choi, M.; Dudem, B.; Yu, J.S. Hierarchical structured polymers for light-absorption enhancement of silicon-based solar power systems. RSC Adv. 2016, 6, 55159–55166. [Google Scholar] [CrossRef]
- Tkachenko, I.M.; Kobzar, Y.L.; Korolovych, V.F.; Stryutsky, A.V.; Matkovska, L.K.; Shevchenko, V.V.; Tsukruk, V.V. Novel branched nanostructures based on polyhedral oligomeric silsesquioxanes and azobenzene dyes containing different spacers and isolation groups. J. Mater. Chem. C 2018, 6, 4065–4076. [Google Scholar] [CrossRef]
- Gao, N.; Liu, W.; Yan, Z.; Wang, Z. Synthesis and properties of transparent cycloaliphatic epoxy–silicone resins for opto-electronic devices packaging. Opt. Mater. 2013, 35, 567–575. [Google Scholar] [CrossRef]
- Kasimuthumaniyan, S.; Reddy, A.A.; Krishnan, N.M.A.; Gosvami, N.N. Understanding the role of post-indentation recovery on the hardness of glasses: Case of silica, borate, and borosilicate glasses. J. Non-Cryst. Solids 2020, 534, 119955. [Google Scholar] [CrossRef]
- Kim, K.-H.; Jung, B.; Jeong, Y.-C. Foldable hard coating materials based on reaction-controlled polysilsesquioxane resin for flexible electronic devices. Prog. Org. Coat. 2020, 143, 105639. [Google Scholar] [CrossRef]
- Musil, J. Flexible hard nanocomposite coatings. RSC Adv. 2015, 5, 60482–60495. [Google Scholar] [CrossRef]
- Muthupandi, G.; Lim, K.R.; Na, Y.-S.; Park, J.; Lee, D.; Kim, H.; Park, S.; Choi, Y.S. Pile-up and sink-in nanoindentation behaviors in AlCoCrFeNi multi-phase high entropy alloy. Mater. Sci. Eng. A 2017, 696, 146–154. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, G.M.; Shin, D.; Kim, Y.H.; Jang, D.; Bae, B.S. Transparent Urethane-Siloxane Hybrid Materials for Flexible Cover Windows with Ceramic-Like Strength, yet Polymer-Like Modulus. ACS Appl. Mater. Interfaces 2018, 10, 43122–43130. [Google Scholar] [CrossRef] [PubMed]
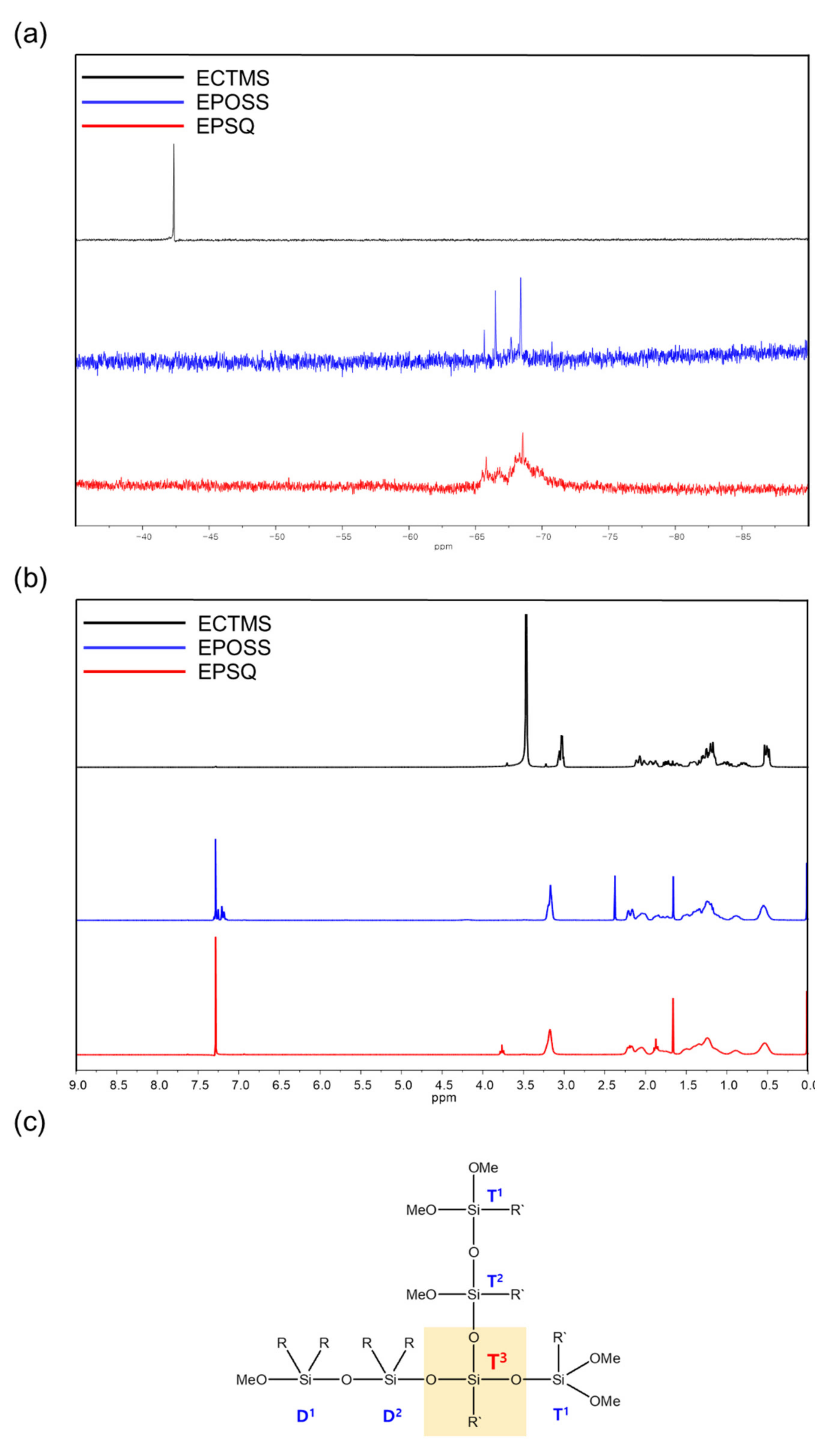
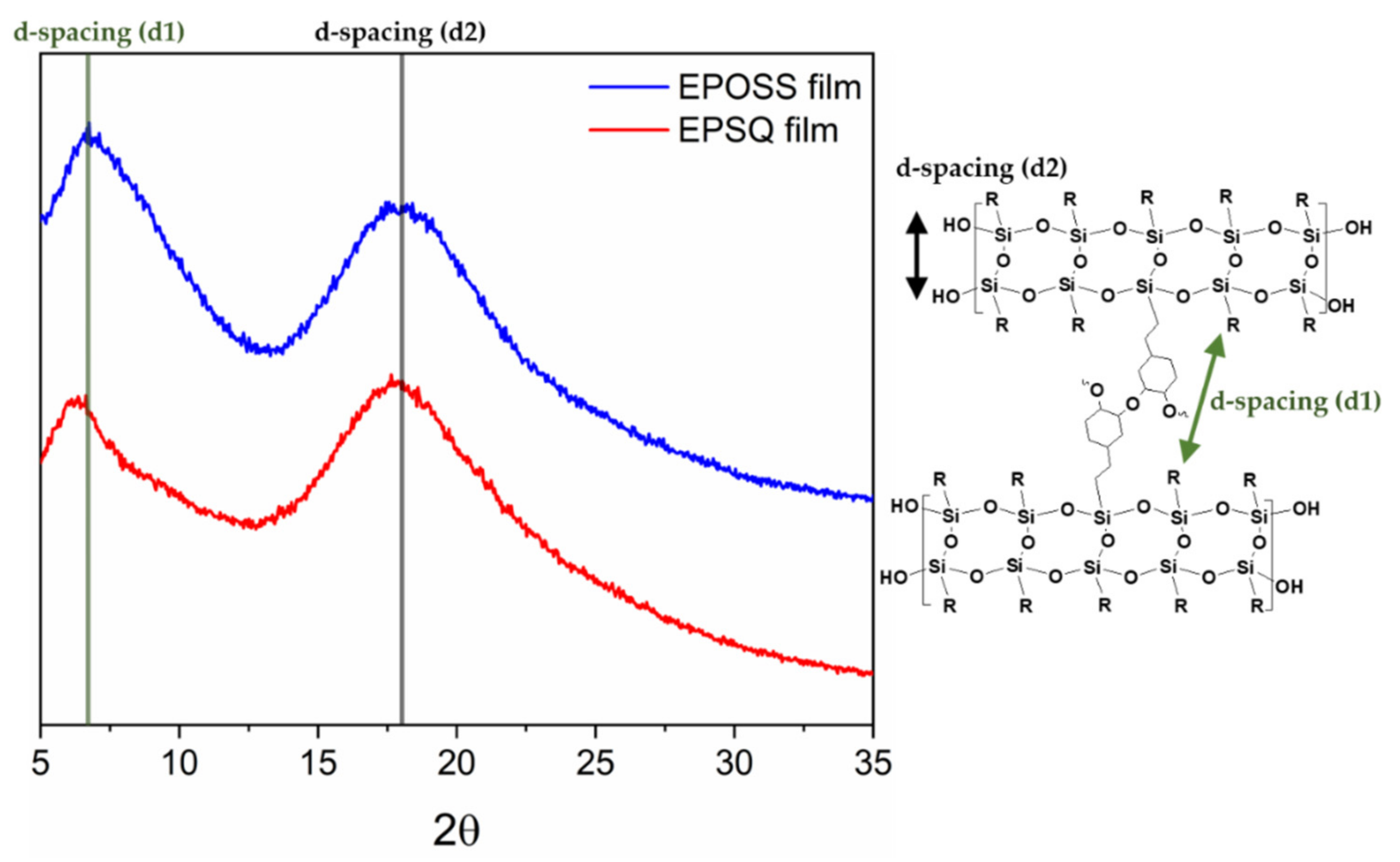
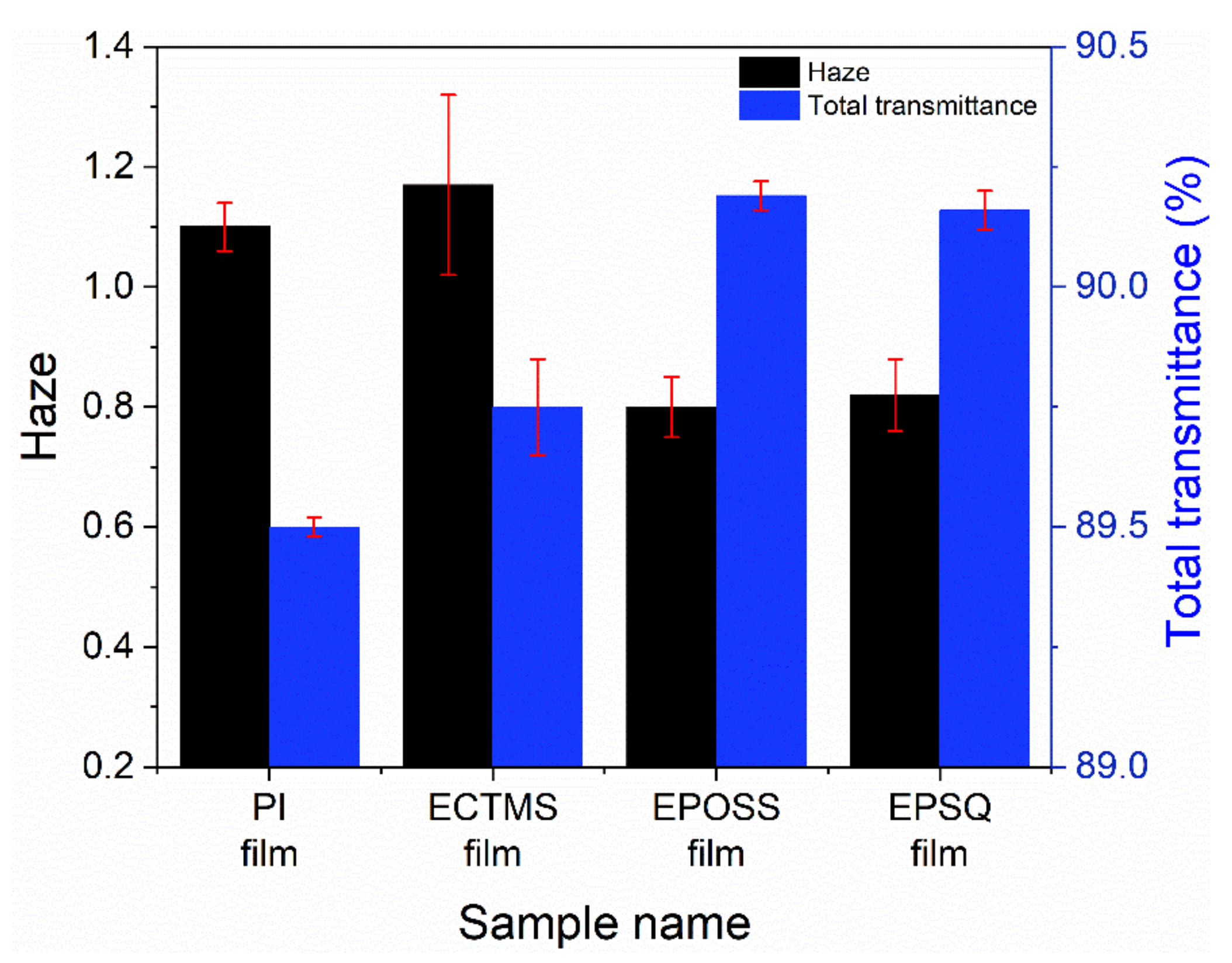

| MW a | PDI | Td10 b (°C) | Weight of Residue at 700 °C c (%) | |
|---|---|---|---|---|
| ECTMS | 246 | - | 152 | 0 |
| EPOSS | 1700 | 1.16 | 468 | 41 |
| EPSQ | 16,900 | 4.15 | 459 | 39 |
| Pencil hardness, 1 kg (H) | Hardness (GPa) a | E* (GPa) b | H/E* c | Elastic Recovery (%) | In-Folding Test (r = 0.5 mm) | Out-Folding Test (r = 5 mm) | |
|---|---|---|---|---|---|---|---|
| EPOSS film | 5 | 0.36 | 4.83 | 0.075 | 74.4 | Crack | Crack |
| EPSQ film | 8 | 0.49 | 5.15 | 0.094 | 80.1 | No crack | No crack |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leem, J.T.; Seok, W.C.; Yoo, J.B.; Lee, S.; Song, H.J. Hard Coating Materials Based on Photo-Reactive Silsesquioxane for Flexible Application: Improvement of Flexible and Hardness Properties by High Molecular Weight. Polymers 2021, 13, 1564. https://doi.org/10.3390/polym13101564
Leem JT, Seok WC, Yoo JB, Lee S, Song HJ. Hard Coating Materials Based on Photo-Reactive Silsesquioxane for Flexible Application: Improvement of Flexible and Hardness Properties by High Molecular Weight. Polymers. 2021; 13(10):1564. https://doi.org/10.3390/polym13101564
Chicago/Turabian StyleLeem, Jong Tae, Woong Cheol Seok, Ji Beom Yoo, Sangkug Lee, and Ho Jun Song. 2021. "Hard Coating Materials Based on Photo-Reactive Silsesquioxane for Flexible Application: Improvement of Flexible and Hardness Properties by High Molecular Weight" Polymers 13, no. 10: 1564. https://doi.org/10.3390/polym13101564
APA StyleLeem, J. T., Seok, W. C., Yoo, J. B., Lee, S., & Song, H. J. (2021). Hard Coating Materials Based on Photo-Reactive Silsesquioxane for Flexible Application: Improvement of Flexible and Hardness Properties by High Molecular Weight. Polymers, 13(10), 1564. https://doi.org/10.3390/polym13101564