Optimization of the Electrochemical Performance of a Composite Polymer Electrolyte Based on PVA-K2CO3-SiO2 Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
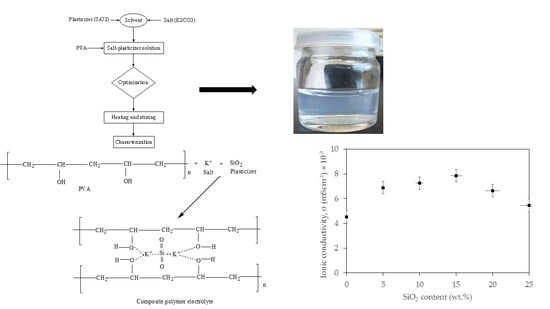
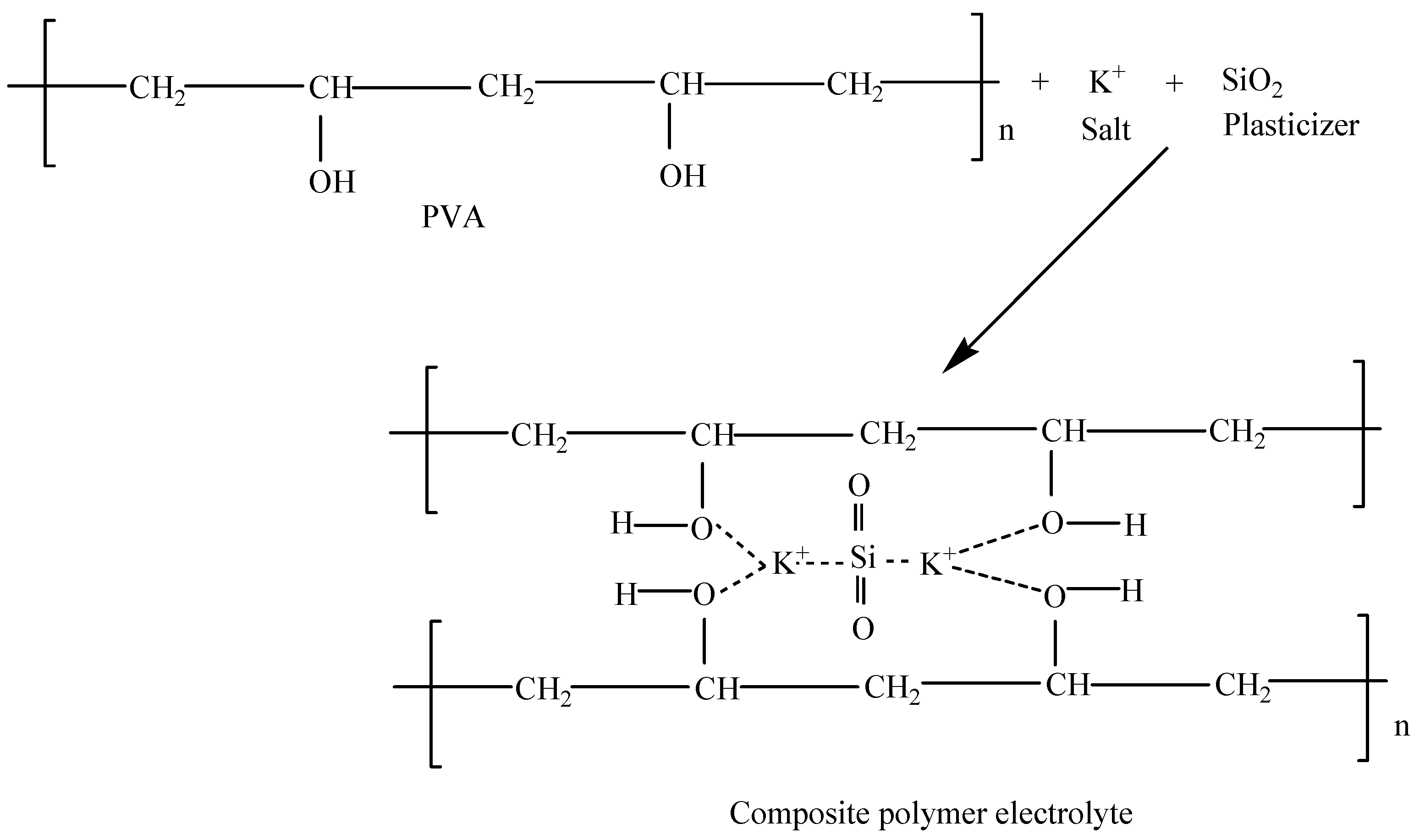
2.2. Preparation of CPE Based on PVA-K2CO3-SiO2 Composite
2.3. Physicochemical Characterization
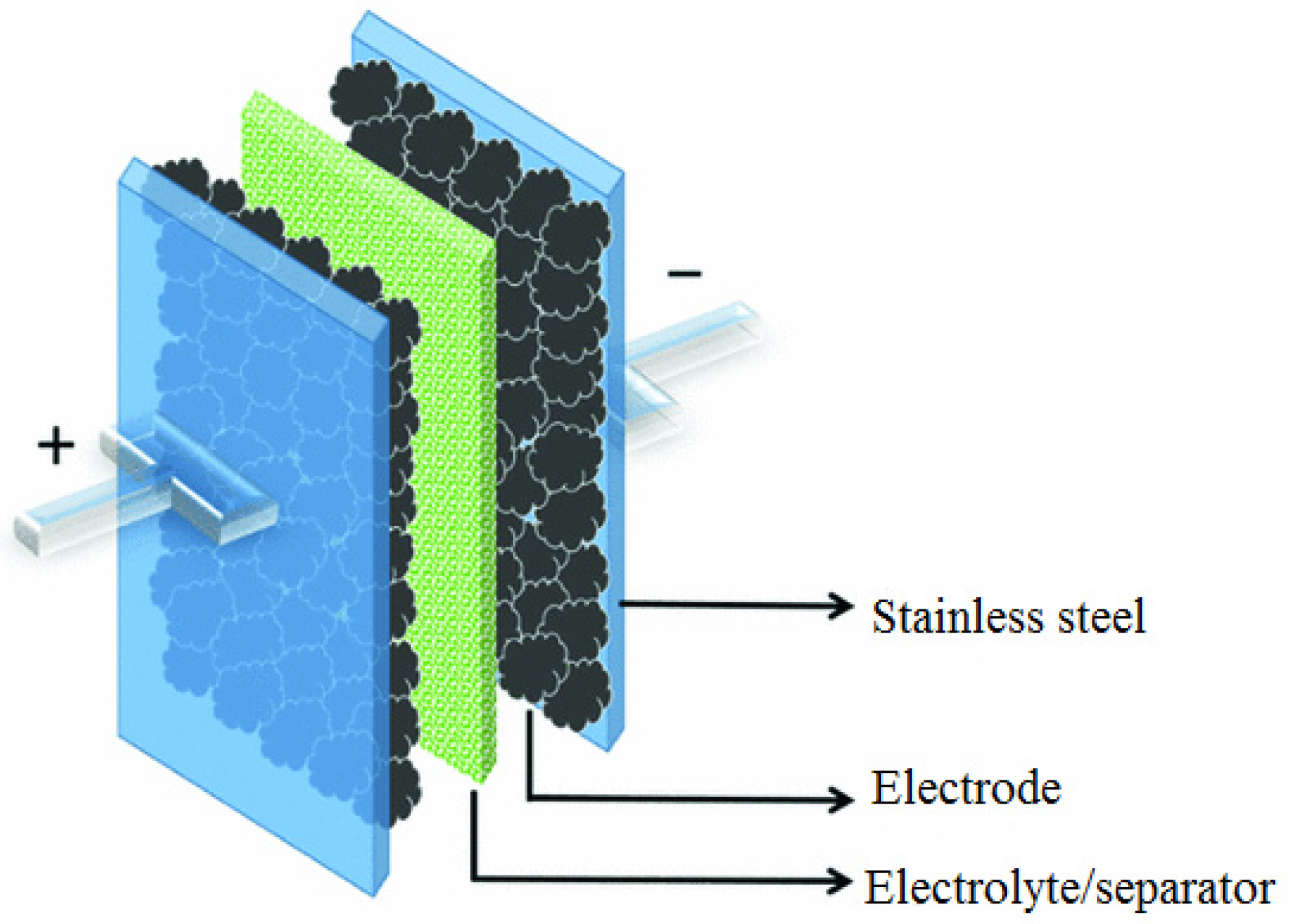
2.4. Electrochemical Characterization
2.4.1. Electrochemical Impedance Spectroscopy (EIS)
2.4.2. Linear Sweep Voltammetry (LSV)
3. Results
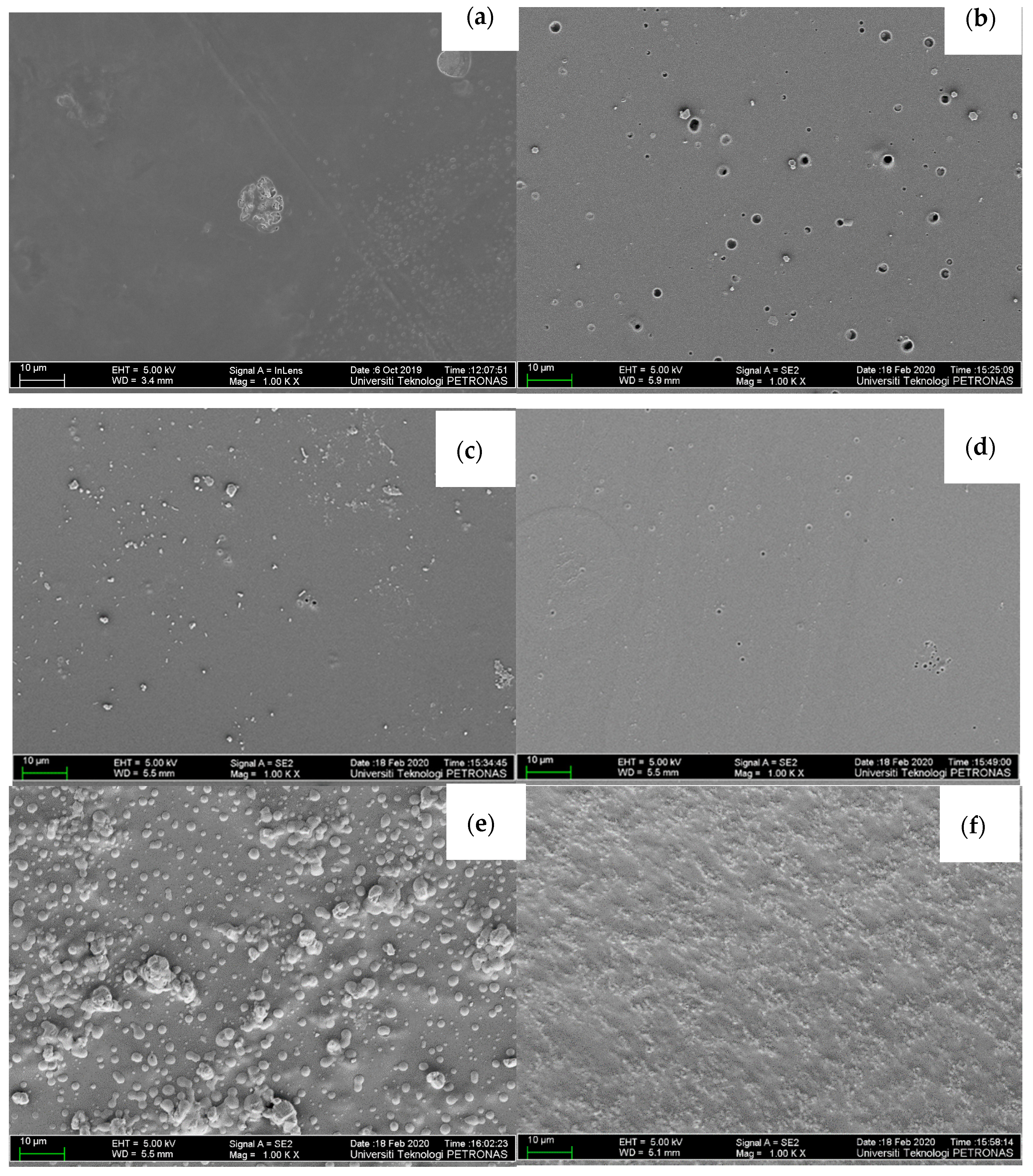
3.1. Structural Analysis of CPE
3.2. Physicochemical Characterization
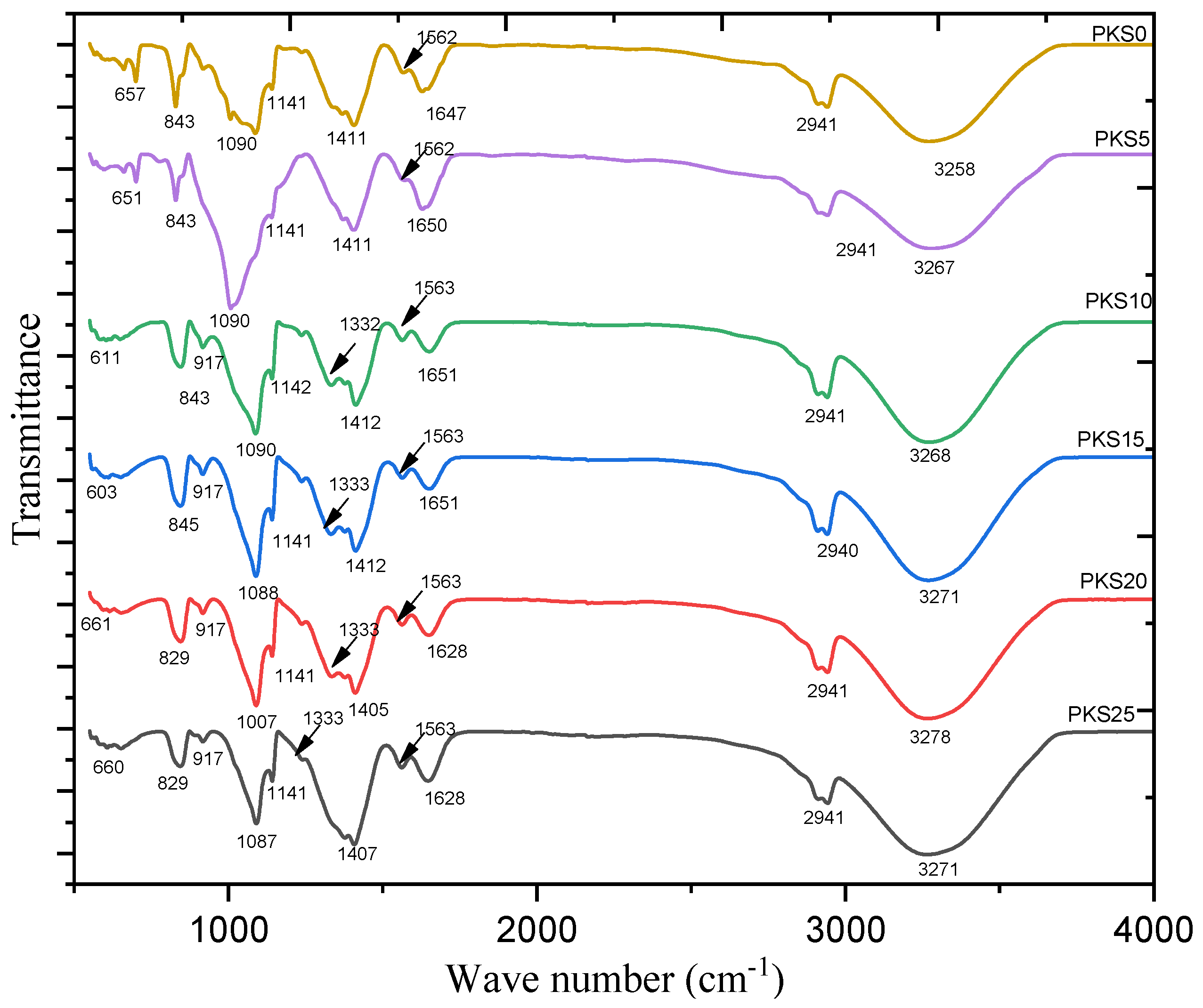
3.2.1. Chemical Structure
3.2.2. Miscibility and SiO2 Presence
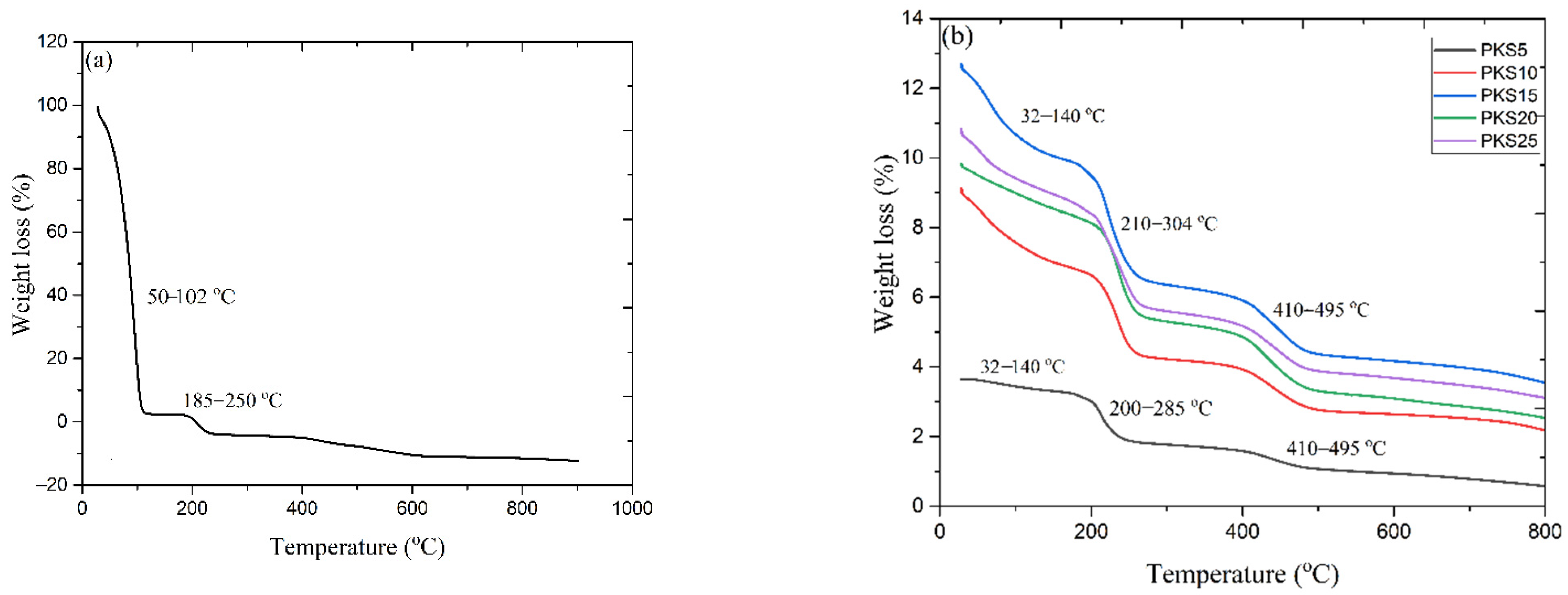
3.2.3. Thermal Stability
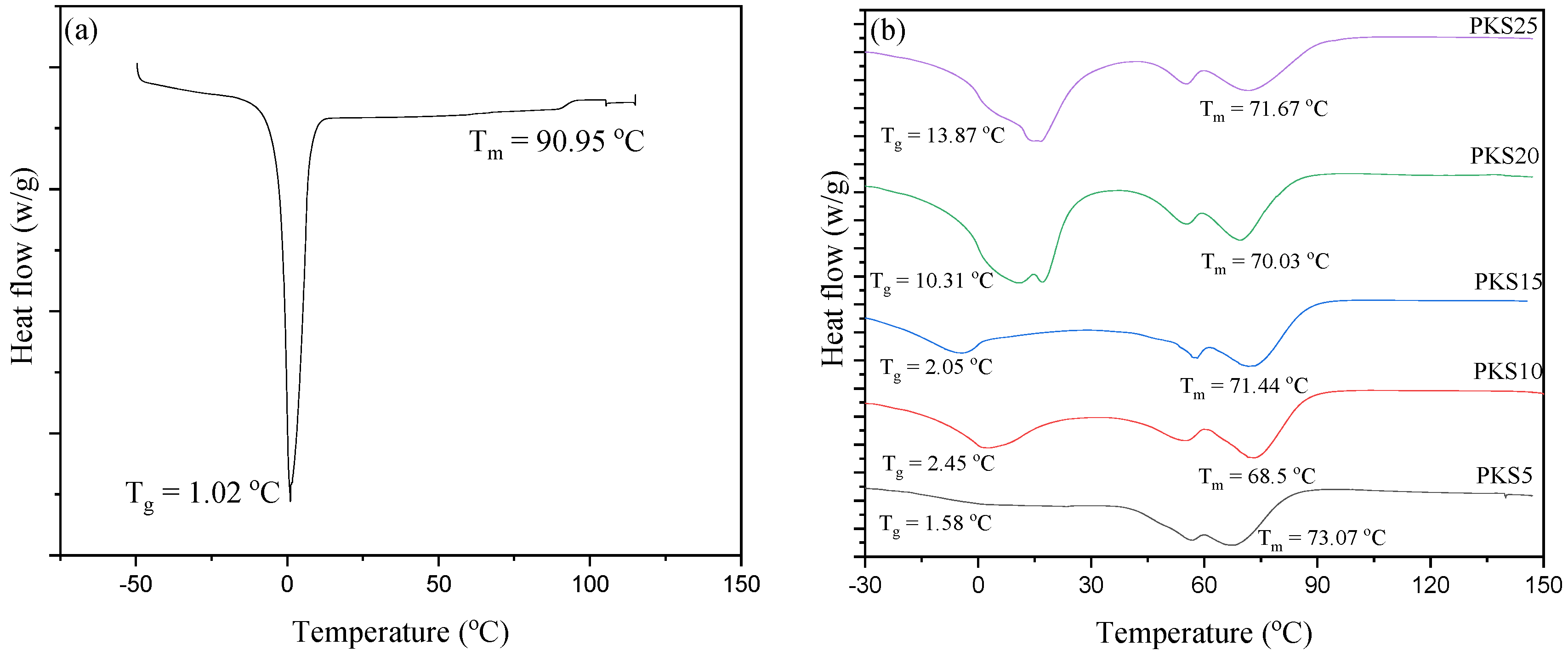
3.2.4. Glass Transition Temperature
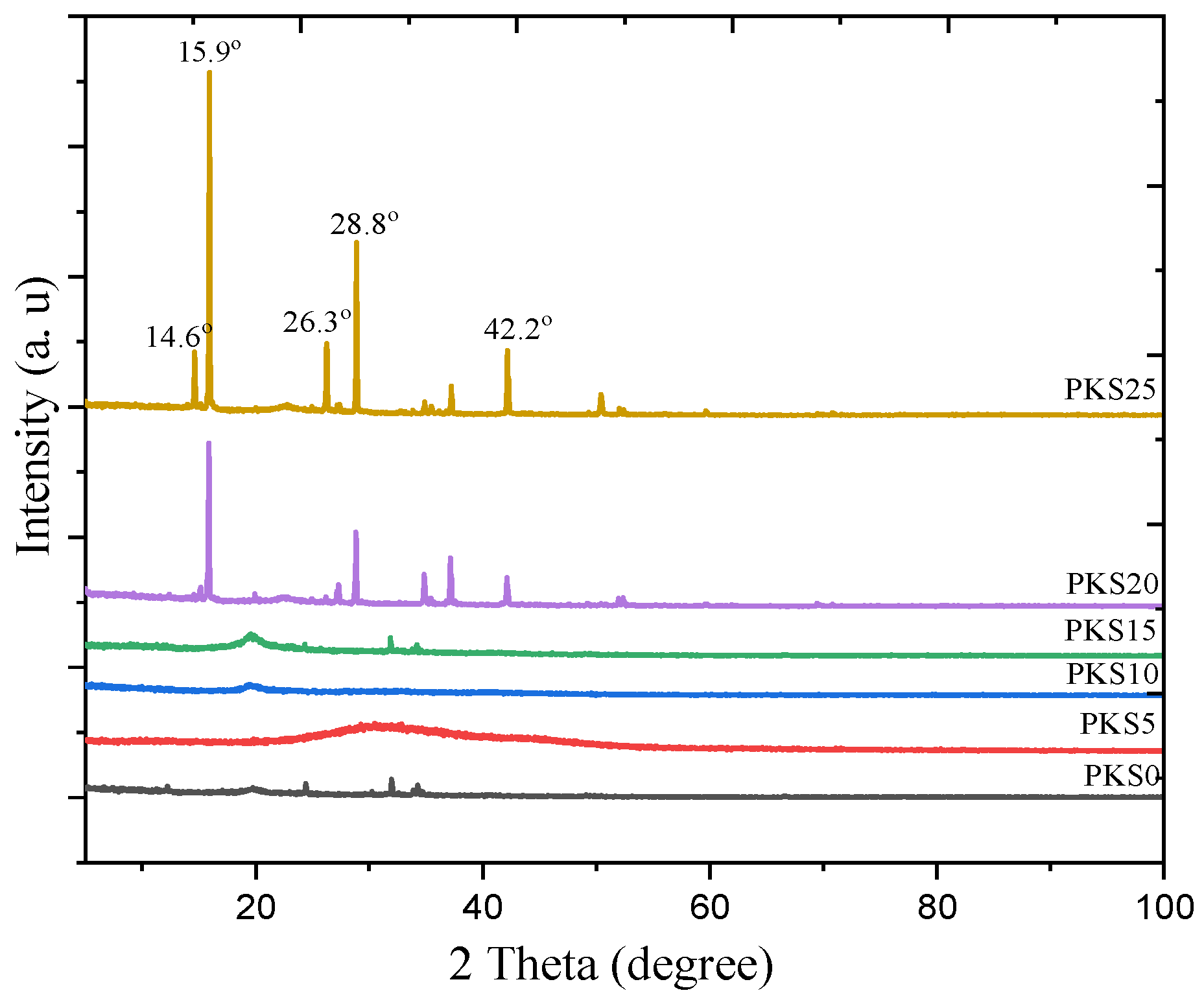
3.2.5. Crystal Phase
3.3. Electrochemical Properties
3.3.1. Resistance
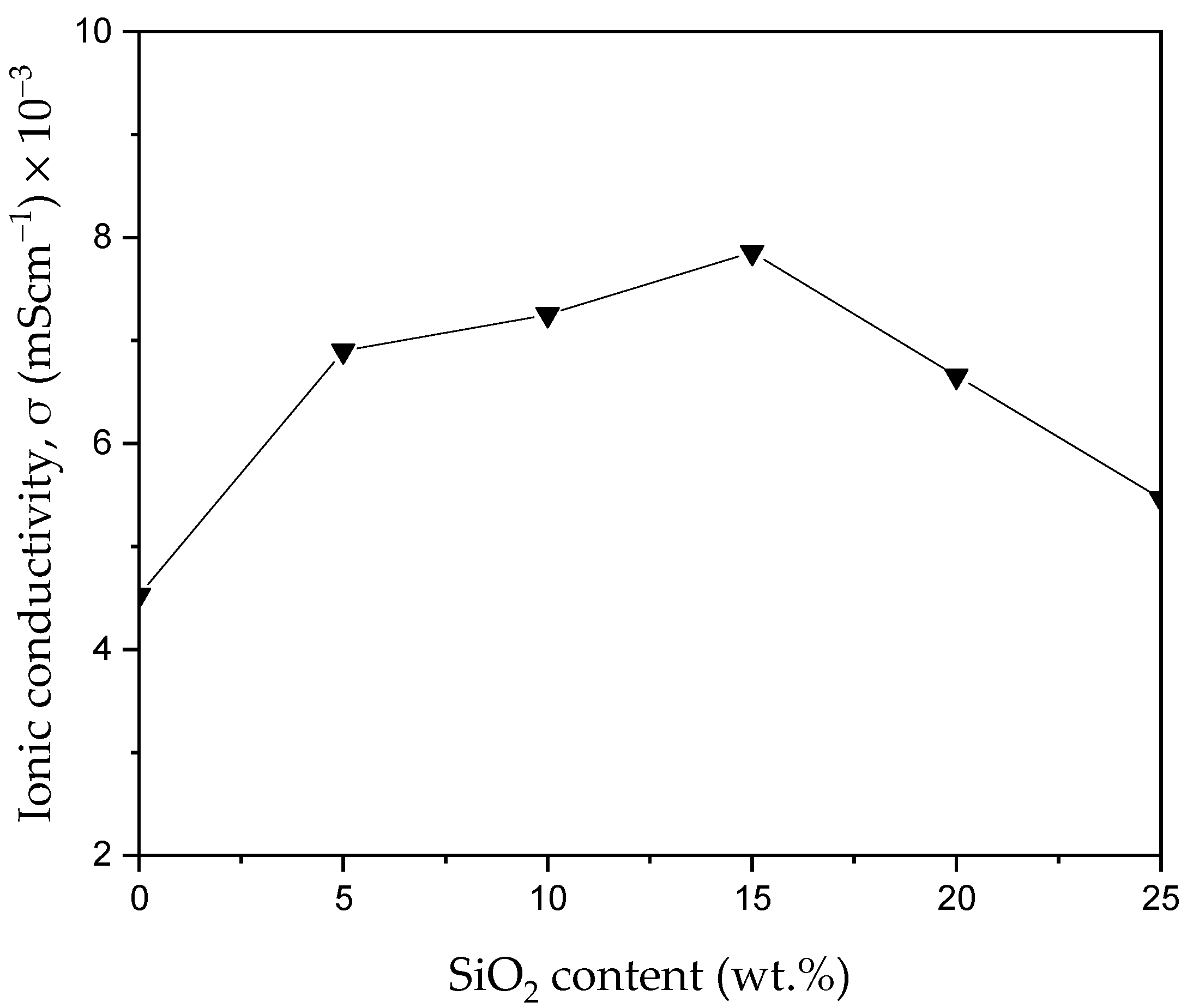
3.3.2. Ionic Conductivity
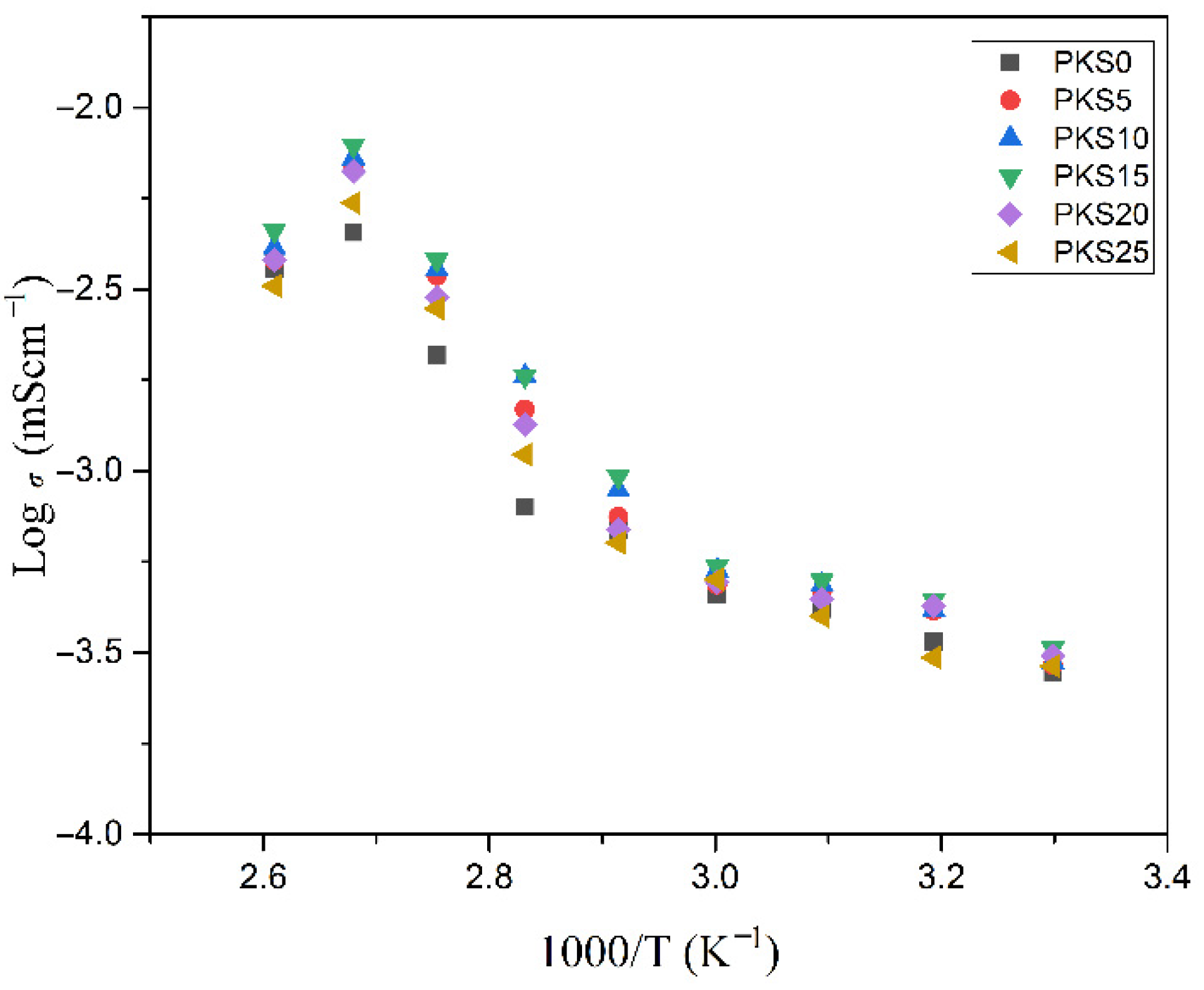
3.3.3. Temperature-Conductivity Relationship
3.3.4. Activation Energy (Ea) of Plasticized CPE
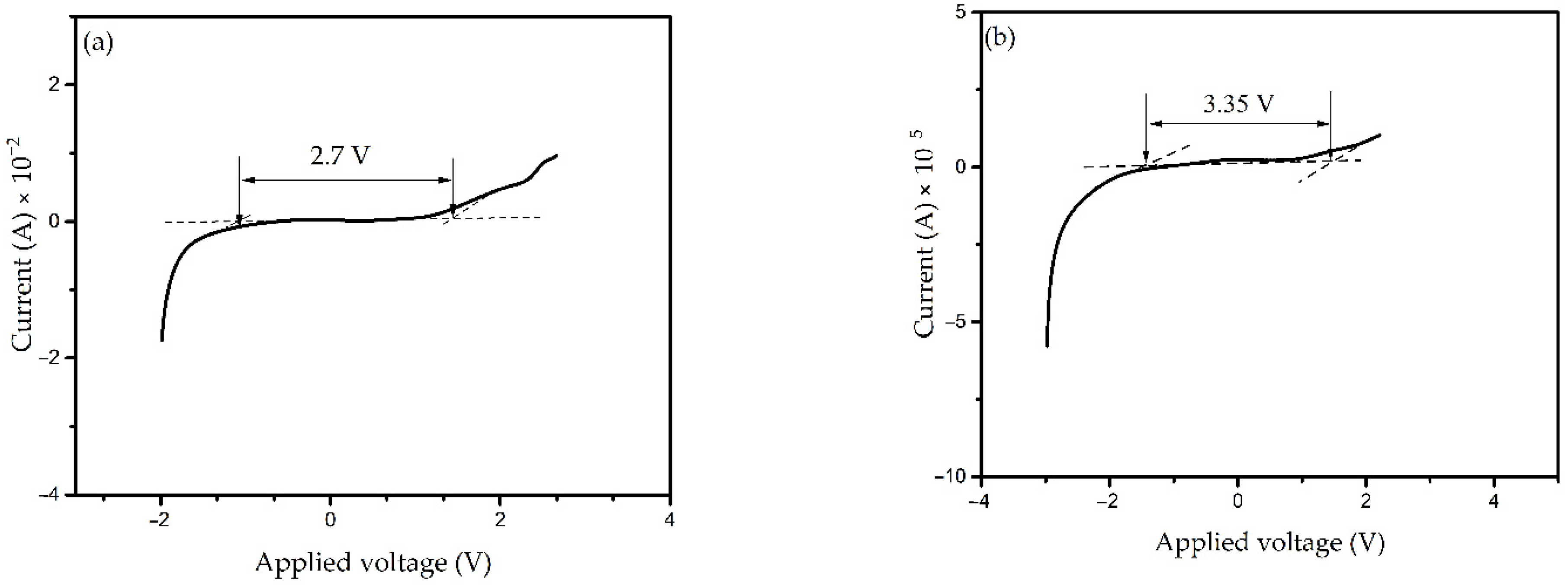
3.3.5. Electrochemical Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiong, G.; Meng, C.; Reifenberger, R.G.; Irazoqui, P.P.; Fisher, T.S. A Review of Graphene-Based Electrochemical Microsupercapacitors. Electroanalysis 2014, 26, 30–51. [Google Scholar] [CrossRef]
- Mun, Y.; Jo, C.; Hyeon, T.; Lee, J.; Ha, K.-S.; Jun, K.-W.; Lee, S.-H.; Hong, S.W.; Lee, H.I.; Yoon, S.; et al. Simple synthesis of hierarchically structured partially graphitized carbon by emulsion/block-copolymer co-template method for high power supercapacitors. Carbon 2013, 64, 391–402. [Google Scholar] [CrossRef]
- Jin, Z.; Yan, X.; Yu, Y.; Zhao, G. Sustainable activated carbon fibers from liquefied wood with controllable porosity for high- performance supercapacitors. J. Mater. Chem. A 2014, 2, 11706–11715. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Naoi, K.; Ishimoto, S.; Miyamoto, J.-I.; Naoi, W. Second generation ‘nanohybrid supercapacitor’: Evolution of capacitive energy storage devices. Energy Environ. Sci. 2012, 5, 9363–9373. [Google Scholar] [CrossRef]
- Li, Y.; Wong, K.W.; Ng, K.M. Ionic liquid decorated mesoporous silica nanoparticles: A new high-performance hybrid electrolyte for lithium batteries. Chem. Commun. 2016, 52, 4369–4372. [Google Scholar] [CrossRef]
- Polu, A.R.; Kumar, R. Preparation and characterization of pva based solid polymer electrolytes for electrochemical cell applications. Chin. J. Polym. Sci. 2013, 31, 641–648. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, X.; Li, C.; Sun, X.; Meng, Q.; Ma, Y.; Wei, Z. Chemically Crosslinked Hydrogel Film Leads to Integrated Flexible Supercapacitors with Superior Performance. Adv. Mater. 2015, 27, 7451–7457. [Google Scholar] [CrossRef]
- Hema, M.; Selvasekarapandian, S.; Arunkumar, D.; Sakunthala, A.; Nithya, H. FTIR, XRD and ac impedance spectroscopic study on PVA based polymer electrolyte doped with NH4X (X=Cl, Br, I). J. Non-Crystalline Solids 2009, 355, 84–90. [Google Scholar] [CrossRef]
- Aziz, S.B.; Faraj, M.G.; Abdullah, O.G. Impedance Spectroscopy as a Novel Approach to Probe the Phase Transition and Microstructures Existing in CS: PEO Based Blend Electrolytes. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Aziz, S.B.; Abdullah, O.G. Effect of PVA Blending on Structural and Ion Transport Properties of CS: AgNt-Based Polymer. Polymers 2017, 9, 622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.; Wang, M.; Zhang, Z.; Qin, G.; Hu, X.; Chen, Q. Preparation and Characterization of PVA Alkaline Solid Polymer Electrolyte with Addition of Bamboo Charcoal. Materials 2018, 11, 679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fic, K.; Lota, G.; Meller, M.; Frackowiak, E. Novel insight into neutral medium as electrolyte for high-voltage supercapacitors. Energy Environ. Sci. 2012, 5, 5842–5850. [Google Scholar] [CrossRef]
- Brandt, A.; Pohlmann, S.; Varzi, A.; Balducci, A.; Passerini, S. Ionic liquids in supercapacitors. MRS Bull. 2013, 38, 554–559. [Google Scholar] [CrossRef]
- Gao, Y. Graphene and Polymer Composites for Supercapacitor Applications: A Review. Nanoscale Res. Lett. 2017, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, Y.; Hu, Z.; Li, Y.; Ling, Z. All-Solid-State Supercapacitors Based on a Carbon-Filled Porous/Dense/Porous Layered Ceramic Electrolyte. J. Electrochem. Soc. 2018, 165, A1269–A1274. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.H.; Lee, Y.G.; Park, J.K.; Seung, D.Y. Effect of silica on the electrochemical characteristics of the plasticized polymer electrolytes based on the P(AN-co-MMA) copolymer. Solid State Ion. 2000, 133, 257–263. [Google Scholar] [CrossRef]
- Pandey, K.; Asthana, N.; Dwivedi, M.M.; Chaturvedi, S.K. Effect of Plasticizers on Structural and Dielectric Behaviour of [PEO + (NH4)2C4H8(COO)2] Polymer Electrolyte. J. Polym. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Shekibi, Y.; Pringle, J.M.; Sun, J.; Pas, S.J.; Rocher, N.M.; Clare, B.R.; Hill, A.J.; MacFarlane, D.R.; Forsyth, M. Lithium-functionalised silica nanoparticles for enhanced ionic conductivity in an organic ionic plastic crystal. J. Mater. Chem. 2010, 20, 338–344. [Google Scholar] [CrossRef]
- Sharma, J.P.; Sekhon, S.S. Effect of plasticizer and fumed silica on ionic conductivity behaviour of proton conducting polymer electrolytes containing HPF6. Bull. Mater. Sci. 2013, 36, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Wang, W.; Yu, R.; Guo, M.; He, C.; Peng, H.; Peng, H.; Xue, Z. Solid polymer electrolyte based on ionic bond or covalent bond functionalized silica nanoparticles. RSC Adv. 2017, 7, 54986–54994. [Google Scholar] [CrossRef] [Green Version]
- Ketabi, S.; Lian, K. Effect of SiO2 on conductivity and structural properties of PEO–EMIHSO4 polymer electrolyte and enabled solid electrochemical capacitors. Electrochim. Acta 2013, 103, 174–178. [Google Scholar] [CrossRef]
- Abdulkadir, B.A.; Dennis, J.O.; Shukur, M.F.B.A.; Nasef, M.M.E.; Usman, F. Preparation and characterization of gel polymer electrolyte based on PVA-K2CO3. Polym. Technol. Mater. 2020, 59, 1679–1697. [Google Scholar] [CrossRef]
- Shukur, M.F.; Ithnin, R.; Sonsudin, F.; Yahya, R.; Ahmad, Z.; Kadir, M.F.Z. Conduction Mechanism and Dielectric Properties of Solid Biopolymer Electrolyte Incorporated with Silver Nitrate. Adv. Mater. Res. 2013, 701, 115–119. [Google Scholar] [CrossRef]
- Yusof, Y.M.; Illias, H.A.; Shukur, M.F.; Kadir, M.F.Z. Characterization of starch-chitosan blend-based electrolyte doped with ammonium iodide for application in proton batteries. Ionics 2016, 23, 681–697. [Google Scholar] [CrossRef]
- Garaga, M.N.; Aguilera, L.; Yaghini, N.; Matic, A.; Persson, M.; Martinelli, A. Achieving enhanced ionic mobility in nanoporous silica by controlled surface interactions. Phys. Chem. Chem. Phys. 2017, 19, 5727–5736. [Google Scholar] [CrossRef] [Green Version]
- Dodda, J.M.; Bělský, P.; Chmelař, J.; Remiš, T.; Smolná, K.; Tomáš, M.; Kullová, L.; Kadlec, J. Comparative study of PVA/SiO2 and PVA/SiO2/glutaraldehyde (GA) nanocomposite membranes prepared by single-step solution casting method. J. Mater. Sci. 2015, 50, 6477–6490. [Google Scholar] [CrossRef]
- Liu, Y.; Lee, J.Y.; Hong, L. In situ preparation of poly (ethylene oxide)–SiO2 composite polymer electrolytes. J. Power Sources 2004, 129, 303–311. [Google Scholar] [CrossRef]
- Liew, C.-W.; Ramesh, S.; Arof, A. A novel approach on ionic liquid-based poly(vinyl alcohol) proton conductive polymer electrolytes for fuel cell applications. Int. J. Hydrogen Energy 2014, 39, 2917–2928. [Google Scholar] [CrossRef]
- Gouda, M.; Gouveia, W.; Afonso, M.; Šljukić, B.; El Essawy, N.; Nassr, A.; Santos, J.L.M. Poly(vinyl alcohol)-based crosslinked ternary polymer blend doped with sulfonated graphene oxide as a sustainable composite membrane for direct borohydride fuel cells. J. Power Sources 2019, 432, 92–101. [Google Scholar] [CrossRef]
- Aziz, S.B.; Woo, T.J.; Kadir, M.; Ahmed, H.M. A conceptual review on polymer electrolytes and ion transport models. J. Sci. Adv. Mater. Devices 2018, 3, 1–17. [Google Scholar] [CrossRef]
- Long, L.; Wang, S.; Xiao, M.; Meng, Y. Polymer electrolytes for lithium polymer batteries. J. Mater. Chem. A 2016, 4, 10038–10069. [Google Scholar] [CrossRef]
- An, Q.; Li, F.; Ji, Y.; Chen, H. Influence of polyvinyl alcohol on the surface morphology, separation and anti-fouling performance of the composite polyamide nanofiltration membranes. J. Membr. Sci. 2011, 367, 158–165. [Google Scholar] [CrossRef]
- Dolui, S.K. Synthesis and Characterisation of Polyvinyl alcohol (PVA)/Silica Hybrid Composites Derived through the Sol-Gel Method in Aqueous Medium: Effect of Acid Content, Silica Content and Viscosity of PVA on the Dispersion. J. Sol-Gel Sci. Technol. 2004, 29, 107–114. [Google Scholar]
- Rosi, M.; Iskandar, F.; Abdullah, M. Hydrogel-Polymer Electrolytes Based on Polyvinyl Alcohol and Hydroxyethylcellulose for Supercapacitor Applications. Int. J. Electrochem. Sci. 2014, 9, 4251–4256. [Google Scholar]
- Chen, L.; Fu, J.; Lu, Q.; Shi, L.; Li, M.; Dong, L.; Xu, Y.; Jia, R. Cross-linked polymeric ionic liquids ion gel electrolytes by in situ radical polymerization. Chem. Eng. J. 2019, 378, 122245. [Google Scholar] [CrossRef]
- Yup, J.; Kang, D.A.; Un, N.; Min, J.; Hak, J. Bicontinuously crosslinked polymer electrolyte membranes with high ion conductivity and mechanical strength. J. Memb. Sci. 2019, 589, 117250. [Google Scholar]
- Na, R.; Lu, N.; Zhang, S.; Huo, G.; Yang, Y.; Zhang, C.; Mu, Y.; Luo, Y.; Wang, G. Facile synthesis of a high-performance, fire-retardant organic gel polymer electrolyte for flexible solid-state supercapacitors. Electrochim. Acta 2018, 290, 262–272. [Google Scholar] [CrossRef]
- Nishihara, M.; Terayama, Y.; Haji, T.; Lyth, S.M.; Satokawa, S.; Matsumoto, H. Proton-conductive nano zeolite-PVA composite film as a new water-absorbing electrolyte for water electrolysis. Express Polym. Lett. 2018, 12, 256–264. [Google Scholar] [CrossRef]
- Chang, J.; Lai, H.; Samarahan, K. Polyvinyl alcohol/silica/clay composites: Effect of clay on surface morphology and thermo-mechanical properties. J. Teknol. Sci. Eng. 2016, 78, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Sik, D.; Bum, H.; Won, J.; Lee, Y.M. Preparation and characterization of crosslinked PVA/SiO2 hybrid membranes containing sulfonic acid groups for direct methanol fuel cell applications. J. Membr. Sci. 2004, 240, 37–48. [Google Scholar]
- Choo, K.; Ching, Y.C.; Chuah, C.H.; Julai, S.; Liou, N.-S. Preparation and Characterization of Polyvinyl Alcohol-Chitosan Composite Films Reinforced with Cellulose Nanofiber. Materials 2016, 9, 644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ming, J.; Yuan, C.; Zen, H.; Chen, C. Two step modification of poly (vinyl alcohol) by UV radiation with 2-hydroxy ethyl methacrylate and sol–gel process for the application of polymer electrolyte membrane. J. Membr. Sci. 2009, 341, 186–194. [Google Scholar]
- Çavuş, S.; Durgun, E. Poly(vinyl alcohol) Based Polymer Gel Electrolytes: Investigation on Their Conductivity and Characterization. Acta Phys. Pol. A 2016, 129, 621–624. [Google Scholar] [CrossRef]
- Hassanjani, A.; Abdollah, R.; Safura, O. Determination of degradation kinetics of polyvinyl alcohol/X-zeolite nanocomposite. J. Therm. Anal. Calorim. 2016, 10, 1–10. [Google Scholar]
- Kadir, M.F.Z.; Hamsan, M.H. Green electrolytes based on dextran-chitosan blend and the effect of NH4SCN as proton provider on the electrical response studies. Ionics 2018, 24, 2379–2398. [Google Scholar] [CrossRef]
- Liew, C.-W.; Ramesh, S.; Arof, A. Good prospect of ionic liquid based-poly(vinyl alcohol) polymer electrolytes for supercapacitors with excellent electrical, electrochemical and thermal properties. Int. J. Hydrogen Energy 2014, 39, 2953–2963. [Google Scholar] [CrossRef]
- Liew, C.-W.; Ramesh, S. Comparing Triflate and Hexafluorophosphate Anions of Ionic Liquids in Polymer Electrolytes for Supercapacitor Applications. Materials 2014, 7, 4019–4033. [Google Scholar] [CrossRef] [Green Version]
- Muchakayala, R.; Song, S.; Wang, J.; Fan, Y.; Bengeppagari, M.; Chen, J.; Tan, M. Development and supercapacitor application of ionic liquid-incorporated gel polymer electrolyte films. J. Ind. Eng. Chem. 2018, 59, 79–89. [Google Scholar] [CrossRef]
- Chodankar, N.R.; Dubal, D.P.; Lokhande, A.C.; Lokhande, C.D. Ionically conducting PVA–LiClO4 gel electrolyte for high performance flexible solid state supercapacitors. J. Colloid Interface Sci. 2015, 460, 370–376. [Google Scholar] [CrossRef]
- Mishra, K.; Garg, A.; Sharma, R.; Gautam, R.; Pundir, S.S. Effect of blending of PMMA on PVdF-HFP + NaCF3SO3-EC-PC gel polymer electrolyte. Mater. Today Proc. 2019, 12, 621–627. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, J.-M.; Zhuang, Q.-C.; Jin, L.-Y.; Sun, S.-G. A novel PEO-based composite solid-state polymer electrolyte with methyl group-functionalized SBA-15 filler for rechargeable lithium batteries. J. Solid State Electrochem. 2008, 12, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Yusof, Y.M.; Majid, N.A.; Kasmani, R.M.; Illias, H.A.; Kadir, M.F.Z. The Effect of Plasticization on Conductivity and Other Properties of Starch/Chitosan Blend Biopolymer Electrolyte Incorporated with Ammonium Iodide. Mol. Cryst. Liq. Cryst. 2014, 603, 73–88. [Google Scholar] [CrossRef]
- Scrosati, B.; Croce, F.; Persi, L. Impedance Spectroscopy Study of PEO-Based Nanocomposite Polymer Electrolytes. J. Electrochem. Soc. 2000, 147, 1718–1721. [Google Scholar] [CrossRef]
- Wang, X.; Hao, X.; Xia, Y.; Liang, Y.; Xia, X.; Tu, J. A polyacrylonitrile (PAN)-based double-layer multifunctional gel polymer electrolyte for lithium-sulfur batteries. J. Membr. Sci. 2019, 582, 37–47. [Google Scholar] [CrossRef]
- Ramesh, S.; Wen, L.C. Investigation on the effects of addition of SiO2 nanoparticles on ionic conductivity, FTIR, and thermal properties of nanocomposite PMMA–LiCF3SO3–SiO2. Ionics 2010, 16, 255–262. [Google Scholar] [CrossRef]
- Tron, A.; Nosenko, A.; Park, Y.D.; Mun, J. Enhanced ionic conductivity of the solid electrolyte for lithium-ion batteries. J. Solid State Chem. 2018, 258, 467–470. [Google Scholar] [CrossRef]
- Reddy, M.J.; Kumar, J.S.; Rao, U.S.; Chu, P.P. Structural and ionic conductivity of PEO blend PEG solid polymer electrolyte. Solid State Ion. 2006, 177, 253–256. [Google Scholar] [CrossRef]
- Yusof, Y.M.; Shukur, M.F.; Illias, H.A.; Kadir, M.F.Z. Conductivity and electrical properties of corn starch–chitosan blend biopolymer electrolyte incorporated with ammonium iodide. Phys. Scr. 2014, 89, 1–10. [Google Scholar] [CrossRef]
- Shukur, M.F.; Yusof, Y.M.; Zawawi, S.M.M.; Illias, H.A.; Kadir, M.F.Z. Conductivity and transport studies of plasticized chitosan-based proton conducting biopolymer electrolytes. Phys. Scr. 2013, 157. [Google Scholar] [CrossRef]
- Zhao, B.; Lu, X.; Wang, Q.; Yang, J.; Zhao, J.; Zhou, H. Enhancing the ionic conductivity in a composite polymer electrolyte with ceramic nanoparticles anchored to charged polymer brushes. Chin. Chem. Lett. 2020, 31, 831–835. [Google Scholar] [CrossRef]
- Shukur, M.F.; Azmi, M.S.; Zawawi, S.M.M.; Majid, N.A.; Illias, H.A.; Kadir, M.F.Z. Conductivity studies of biopolymer electrolytes based on chitosan incorporated with NH4Br. Phys. Scr. 2013, 157. [Google Scholar] [CrossRef]
- Shukur, M.; Ithnin, R.; Illias, H.; Kadir, M. Proton conducting polymer electrolyte based on plasticized chitosan–PEO blend and application in electrochemical devices. Opt. Mater. 2013, 35, 1834–1841. [Google Scholar] [CrossRef]
- Agrawal, R.C.; Mahipal, Y.K.; Ashrafi, R. Materials and ion transport property studies on hot-press casted solid polymer electrolyte membranes: [(1−x) PEO: X KIO3]. Solid State Ion. 2011, 192, 6–8. [Google Scholar] [CrossRef]
- Raj, C.J.; Varma, K.B.R. Synthesis and electrical properties of the (PVA)0.7(KI)0.3·xH2SO4 (0 ≤ x ≤ 5) polymer electrolytes and their performance in a primary Zn/MnO2 battery. Electrochim. Acta 2010, 56, 649–656. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abidin, Z.; Arof, A. Effect of silver nanoparticles on the DC conductivity in chitosan–silver triflate polymer electrolyte. Phys. B: Condens. Matter 2010, 405, 4429–4433. [Google Scholar] [CrossRef]
- Kou, Z.; Miao, C.; Mei, P.; Zhang, Y.; Yan, X.; Jiang, Y.; Xiao, W. Enhancing the cycling stability of all-solid-state lithium-ion batteries assembled with Li1.3Al0.3Ti1.7(PO4)3 solid electrolytes prepared from precursor solutions with appropriate pH values. Ceram. Int. 2020, 46, 9629–9636. [Google Scholar] [CrossRef]
- Wang, Z.; Miao, C.; Xiao, W.; Zhang, Y.; Mei, P.; Yan, X.; Jiang, Y.; Tian, M. Effect of different contents of organic-inorganic hybrid particles poly(methyl methacrylate)eZrO2 on the properties of poly(vinylidene fluoride-hexafluoroprolene)-based composite gel polymer electrolytes. Electrochim. Acta 2018, 272, 127–134. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, Z.; Zhang, Y.; Fang, R.; Yuan, Z.; Miao, C.; Yan, X.; Jiang, Y. Enhanced performance of P(VDF-HFP)-based composite polymer electrolytes doped with organic-inorganic hybrid particles PMMA-ZrO2 for lithium ion batteries. J. Power Sources 2018, 382, 128–134. [Google Scholar] [CrossRef]
- Ramesh, S.; Liew, C.-W.; Ramesh, K. Evaluation and investigation on the effect of ionic liquid onto PMMA-PVC gel polymer blend electrolytes. J. Non-Cryst. Solids 2011, 357, 2132–2138. [Google Scholar] [CrossRef]
- Wang, Z.; Kou, Z.; Miao, C.; Xiao, W. Improved performance all-solid-state electrolytes with high compacted density of monodispersed spherical Li1.3Al0.3Ti1.7(PO4)3 particles. Ceram. Int. 2019, 45, 14469–14473. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Abdullah, R.M.; Kadir, M.F.Z. A Promising Polymer Blend Electrolytes Based on Chitosan: Methyl Cellulose for EDLC Application with High Specific Capacitance and Energy Density. Mollecules 2019, 24, 2503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noor, N.A.M.; Isa, M.I.N. Investigation on transport and thermal studies of solid polymer electrolyte based on carboxymethyl cellulose doped ammonium thiocyanate for potential application in electrochemical devices. Int. J. Hydrogen Energy 2019, 44, 8298–8306. [Google Scholar] [CrossRef]
- Shukur, M.F.; Kadir, M.F.Z. Hydrogen ion conducting starch-chitosan blend based electrolyte for application in electrochemical devices. Electrochim. Acta 2015, 158, 152–165. [Google Scholar] [CrossRef]
- Tan, X.; Wu, Y.; Tang, W.; Song, S.; Yao, J.; Wen, Z.; Lu, L.; Savilov, S.V.; Hu, N.; Molenda, J. Preparation of Nanocomposite Polymer Electrolyte via In Situ Synthesis of SiO2 Nanoparticles in PEO. Nanomaterials 2020, 10, 157. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Yang, J.; Li, H.; Nuli, Y.; Wang, J. Electrolytes for advanced lithium ion batteries using silicon-based anodes. J. Mater. Chem. A 2019, 7, 9432–9446. [Google Scholar] [CrossRef]
- Arof, A.K.; Kufian, M.Z.; Syukur, M.F.; Aziz, M.F.; Abdelrahman, A.E.; Majid, S.R. Electrical double layer capacitor using poly(methyl methacrylate)—C4BO8Li gel polymer electrolyte and carbonaceous material from shells of mata kucing (Dimocarpus longan) fruit. Electrochim. Acta 2012, 74, 39–45. [Google Scholar] [CrossRef]
- Shuhaimi, N.E.A.; Majid, S.R.; Arof, A.K.; Shuhaimi, N.E.A.; Majid, S.R.; Arof, A.K. On complexation between methyl cellulose and ammonium nitrate on complexation between methyl cellulose and ammonium nitrate. Mater. Res. Innov. 2016, 13, 239–242. [Google Scholar] [CrossRef]
- Samsudin, A.S.; Lai, H.M.; Isa, M.I.N. Biopolymer Materials Based Carboxymethyl Cellulose as a Proton Conducting Biopolymer Electrolyte for Application in Rechargeable Proton Battery. Electrochim. Acta 2014, 129, 1–13. [Google Scholar] [CrossRef]



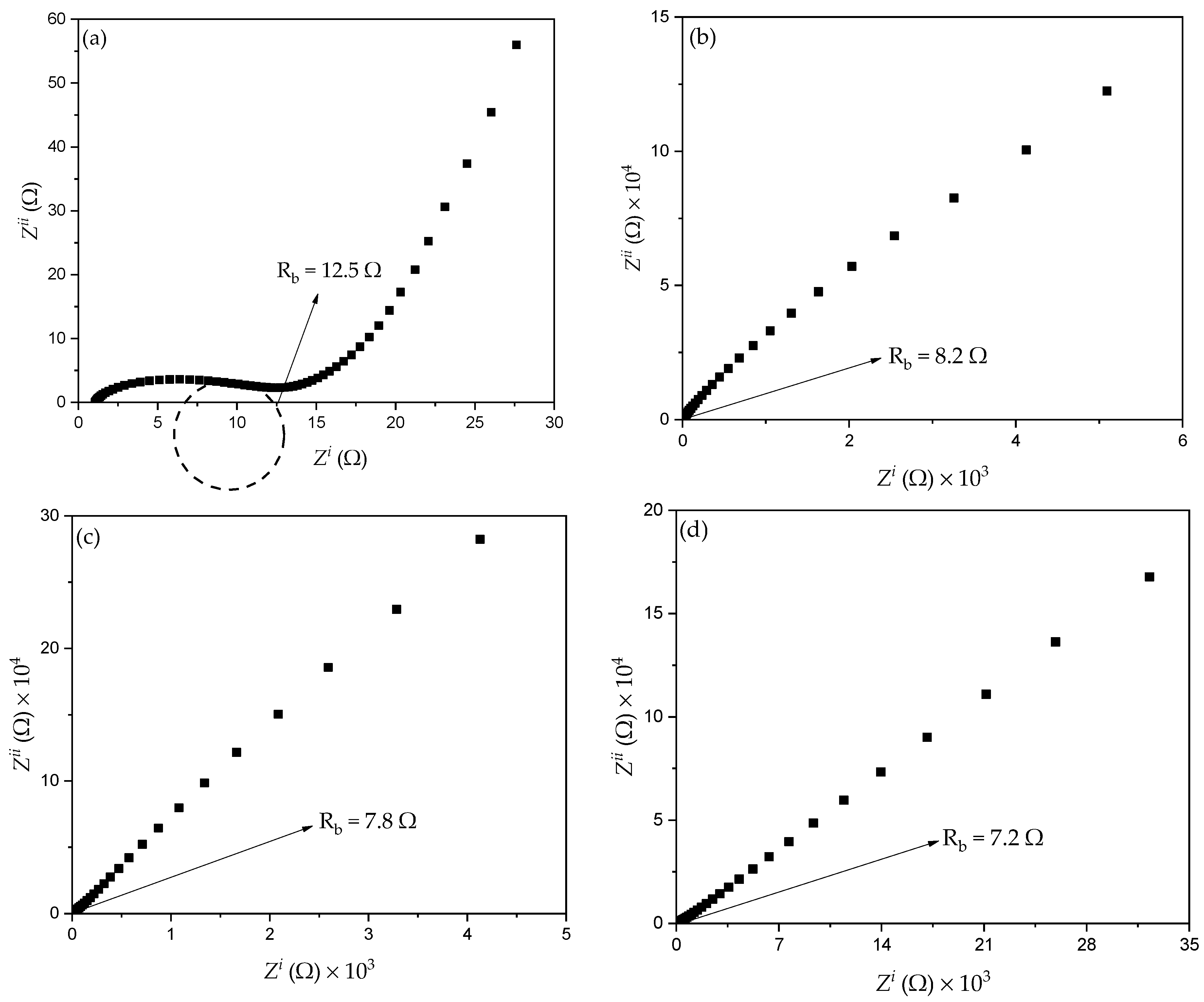
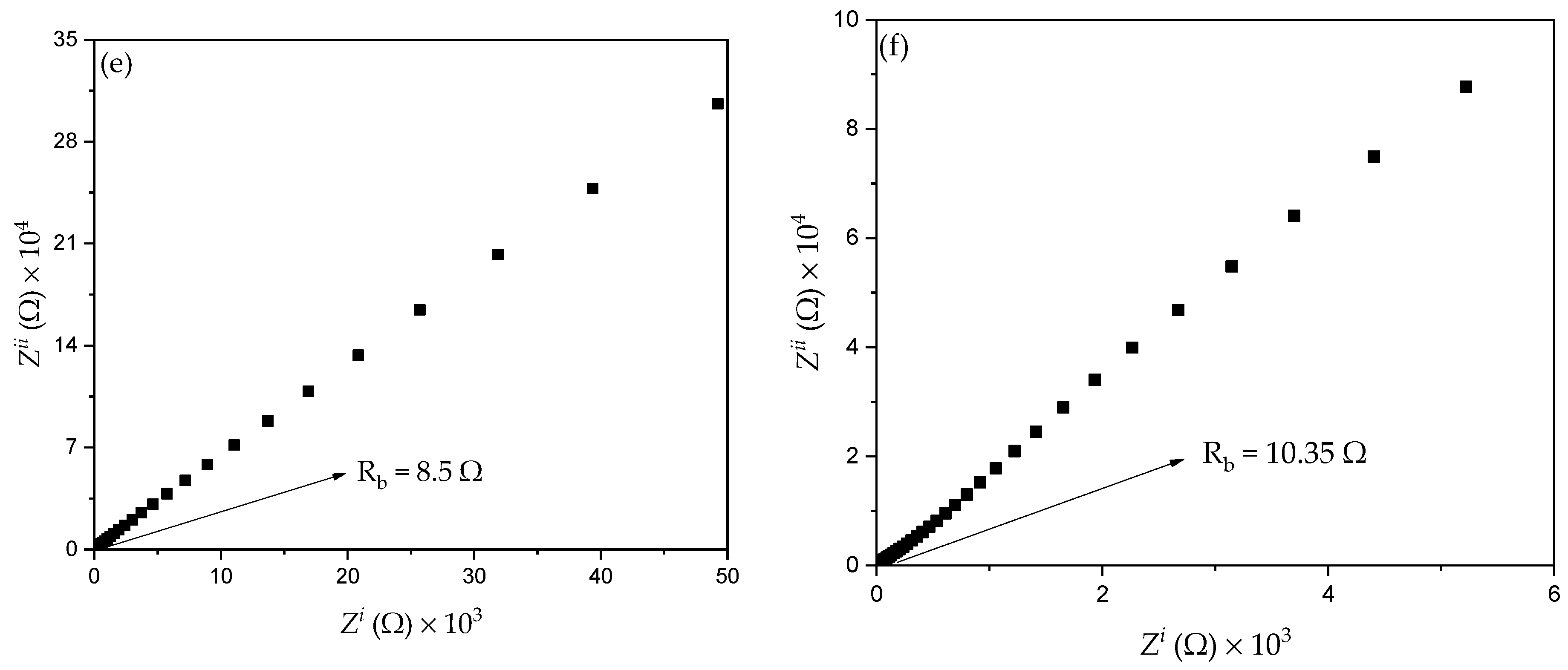

| Designation | SiO2 Composition (wt.%) |
|---|---|
| PKS0 | 0 |
| PKS5 | 15 |
| PKS10 | 10 |
| PKS15 | 15 |
| PKS20 | 20 |
| PKS25 | 25 |
| Description Vibration Modes | Wavenumber (cm−1) |
|---|---|
| Stretching of O–H of PVA | 3258 |
| Asymmetric C–H stretching of pure PVA | 2941 |
| C=O broadening | 1647 |
| Strong C=C broadening | 1562 |
| Winding vibration of CH2 | 1411 |
| CH wagging vibrations | 1141 |
| C–O stretching of acetyl group | 1090 |
| C–H rocking | 843 |
| Free- anions | 657 |
| Symmetric Si–O–Si stretch | 917 |
| Asymmetric Si–O–Si stretch | 1332 |
| Electrolytes | Plasticizers | σ, (mScm–1) | Ref |
|---|---|---|---|
| PVA-K2CO3 | SiO2 | 3.25 × 10–4 | This study |
| Li2O−LiF−P2O5 | MoO3 | 4.40 × 10–7 | [61] |
| Lithium polystyrene sulfonate brushes (LiPSS) | SiO2 | 7.20 × 10–4 | [61] |
| PVC | SiO2 | 5.0 × 10–4 | [61] |
| PEO/PEG | KI | 5.27 × 10–5 | [58] |
| Starch-chitosan doped with NH4I | Glycerol | 1.28 × 10–3 | [53] |
| Starch–chitosan | NH4I | 3.04 × 10–4 | [59] |
| Chitosan-based solid biopolymer | NH4SCN/glycerol | 1.51 × 10–3 | [62] |
| Chitosan | NH4Br | 2.15 × 10–4 | [60] |
| Chitosan-PEO NH4NO3 | Ethylene carbonate (EC) | 2.06 × 10–3 | [63] |
| Samples | Activation Energy (Ea) (eV) |
|---|---|
| PKS0 | 5.51 × 10−4 |
| PKS5 | 1.43 × 10−4 |
| PKS10 | 1.30 × 10−4 |
| PKS15 | 1.09 × 10−4 |
| PKS20 | 1.55 × 10−4 |
| PKS25 | 1.85 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulkadir, B.A.; Ojur Dennis, J.; Al-Hadeethi, Y.; Shukur, M.F.B.A.; Mkawi, E.M.; Al-Harbi, N.; Ibnaouf, K.H.; Aldaghri, O.; Usman, F.; Abbas Adam, A. Optimization of the Electrochemical Performance of a Composite Polymer Electrolyte Based on PVA-K2CO3-SiO2 Composite. Polymers 2021, 13, 92. https://doi.org/10.3390/polym13010092
Abdulkadir BA, Ojur Dennis J, Al-Hadeethi Y, Shukur MFBA, Mkawi EM, Al-Harbi N, Ibnaouf KH, Aldaghri O, Usman F, Abbas Adam A. Optimization of the Electrochemical Performance of a Composite Polymer Electrolyte Based on PVA-K2CO3-SiO2 Composite. Polymers. 2021; 13(1):92. https://doi.org/10.3390/polym13010092
Chicago/Turabian StyleAbdulkadir, Bashir Abubakar, John Ojur Dennis, Yas Al-Hadeethi, Muhammad Fadhlullah Bin Abd. Shukur, E. M. Mkawi, Nuha Al-Harbi, K. H. Ibnaouf, O. Aldaghri, Fahad Usman, and Abdullahi Abbas Adam. 2021. "Optimization of the Electrochemical Performance of a Composite Polymer Electrolyte Based on PVA-K2CO3-SiO2 Composite" Polymers 13, no. 1: 92. https://doi.org/10.3390/polym13010092
APA StyleAbdulkadir, B. A., Ojur Dennis, J., Al-Hadeethi, Y., Shukur, M. F. B. A., Mkawi, E. M., Al-Harbi, N., Ibnaouf, K. H., Aldaghri, O., Usman, F., & Abbas Adam, A. (2021). Optimization of the Electrochemical Performance of a Composite Polymer Electrolyte Based on PVA-K2CO3-SiO2 Composite. Polymers, 13(1), 92. https://doi.org/10.3390/polym13010092