Temperature-Triggered/Switchable Thermal Conductivity of Epoxy Resins
Abstract
1. Introduction
2. Materials and Methods
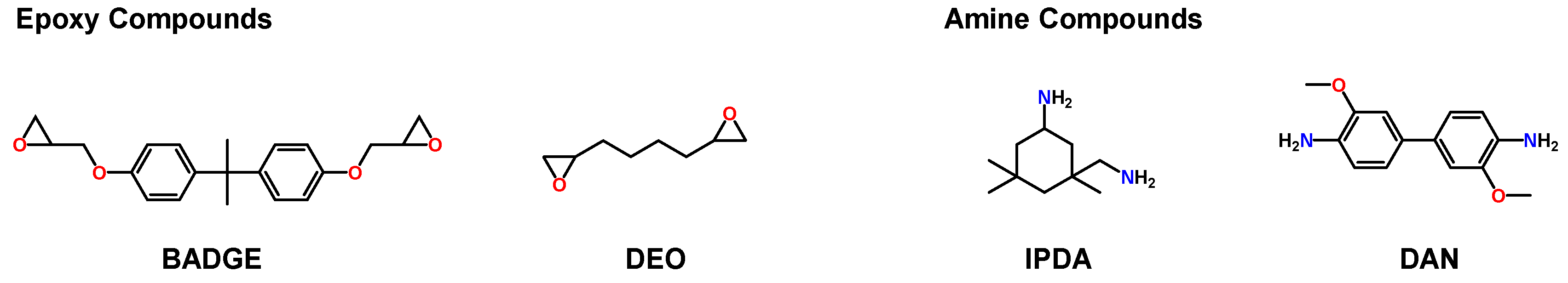
2.1. Materials
2.2. Instrumentation
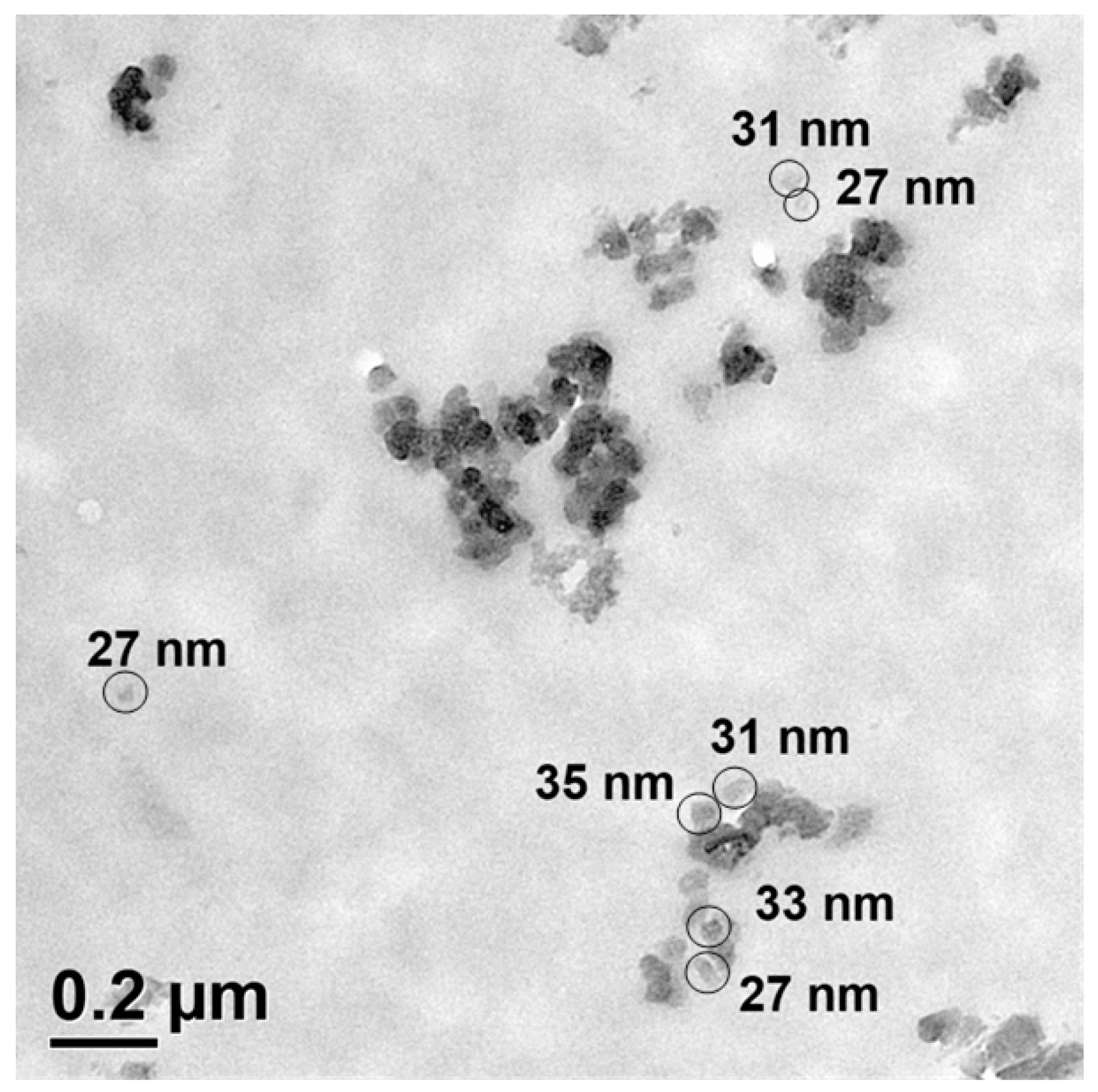
2.3. Analysis of the Size of the Silica Particles
2.4. Preparation of Crosslinked Epoxy Resins and Nanocomposites
3. Results
3.1. Preparation of the Test Specimens/Curing of the Epoxy-Amine Resins
3.2. Physico-Chemical Characterization of the Test Specimens
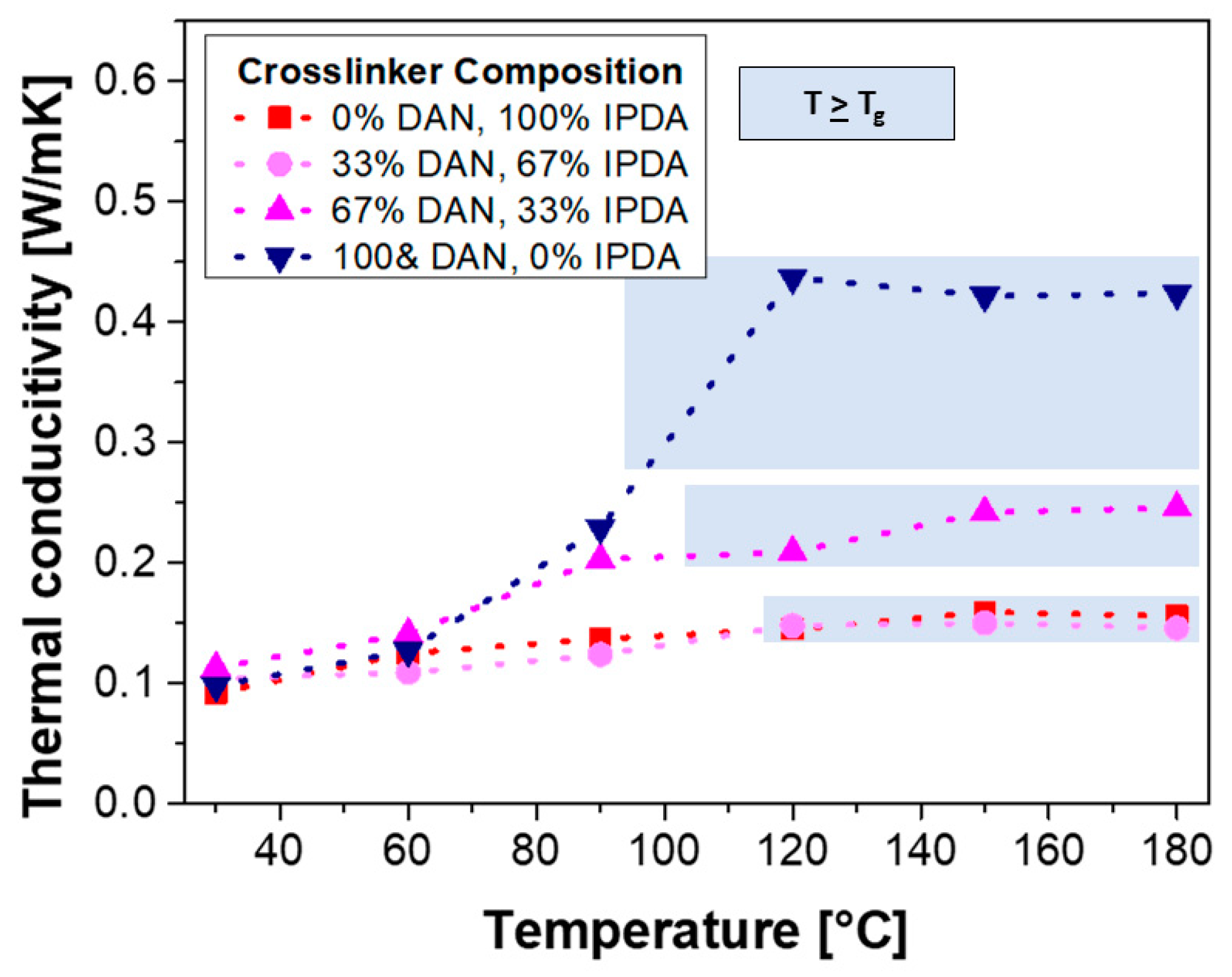
3.3. Thermal Conductivity of the Epoxy-Amine Blends and Composites
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krishnan, S.; Garimella, S.V.; Chrysler, G.M.; Mahajan, R.V. Towards a Thermal Moore’s Law. IEEE Trans. Adv. Packag. 2007, 30, 462–474. [Google Scholar] [CrossRef]
- Kish, L.B. End of Moore’s law: Thermal (noise) death of integration in micro and nano electronics. Phys. Lett. A 2002, 305, 144–149. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Nanoparticle Reinforced Polymers. Polymers 2019, 11, 625. [Google Scholar] [CrossRef]
- Tanaka, T.; Montanari, G.C.; Mulhaupt, R. Polymer nanocomposites as dielectrics and electrical insulation-perspectives for processing technologies, material characterization and future applications. IEEE Trans. Dielect. Electr. Insul. 2004, 11, 763–784. [Google Scholar] [CrossRef]
- Andritsch, T.; Kochetov, R.; Gebrekiros, Y.T.; Lafont, U.; Morshuis, P.H.F.; Smit, J.J. Synthesis and dielectric properties of epoxy based nanocomposites. In Proceedings of the 2009 IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Virginia Beach, VA, USA, 18–21 October 2009; IEEE: Piscataway, NJ, USA, 2009; pp. 523–526, ISBN 978-1-4244-4557-8. [Google Scholar]
- Camargo, P.H.C.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: Synthesis, structure, properties and new application opportunities. Mater. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef]
- Kroeger, F.R.; Swenson, C.A. Absolute linear thermal-expansion measurements on copper and aluminum from 5 to 320 K. J. Appl. Phys. 1977, 48, 853–864. [Google Scholar] [CrossRef]
- Lyon, K.G.; Salinger, G.L.; Swenson, C.A.; White, G.K. Linear thermal expansion measurements on silicon from 6 to 340 K. J. Appl. Phys. 1977, 48, 865–868. [Google Scholar] [CrossRef]
- Adamson, M.J. Thermal expansion and swelling of cured epoxy resin used in graphite/epoxy composite materials. J. Mater. Sci. 1980, 15, 1736–1745. [Google Scholar] [CrossRef]
- McNally, T.; Raymond Murphy, W.; Lew, C.Y.; Turner, R.J.; Brennan, G.P. Polyamide-12 layered silicate nanocomposites by melt blending. Polymer 2003, 44, 2761–2772. [Google Scholar] [CrossRef]
- El-Tonsy, M.M. Automatic measurement of the absolute CTE of thin polymer samples: I—Effect of multiple processing on thermal expansion of polypropylene films. Polym. Test. 2004, 23, 355–360. [Google Scholar] [CrossRef]
- Evans, J.S.O.; Mary, T.A.; Sleight, A.W. Negative Thermal Expansion in a Large Molybdate and Tungstate Family. J. Solid State Chem. 1997, 133, 580–583. [Google Scholar] [CrossRef]
- van Hattum, F.W.J.; Bernardo, C.A.; Finegan, J.C.; Tibbetts, G.G.; Alig, R.L.; Lake, M.L. A study of the thermomechanical properties of carbon fiber-polypropylene composites. Polym. Compos. 1999, 20, 683–688. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Eastman, J.A. (Eds.) Enhancing Thermal Conductivity of Fluids with Nanoparticles. In Proceedings of the 1995 International Mechanical Engineering Congress and Exhibition, San Francisco, CA, USA, 12–17 November 1995. [Google Scholar]
- Kozako, M.; Okazaki, Y.; Hikita, M.; Tanaka, T. Preparation and evaluation of epoxy composite insulating materials toward high thermal conductivity. In Proceedings of the 2010 10th IEEE International Conference on Solid Dielectrics (ICSD), Potsdam, Germany, 4–9 July 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 1–4, ISBN 978-1-4244-7945-0. [Google Scholar]
- Kochetov, R.; Andritsch, T.; Lafont, U.; Morshuis, P.H.F.; Smit, J.J. Thermal conductivity of nano-filled epoxy systems. In Proceedings of the 2009 IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Virginia Beach, VA, USA, 18–21 October 2009; pp. 658–661, ISBN ISBN 978-1-4244-4557-8. [Google Scholar]
- Moradi, S.; Calventus, Y.; Román, F.; Hutchinson, J.M. Achieving High Thermal Conductivity in Epoxy Composites: Effect of Boron Nitride Particle Size and Matrix-Filler Interface. Polymers 2019, 11, 1156. [Google Scholar] [CrossRef] [PubMed]
- Morak, M.; Marx, P.; Gschwandl, M.; Fuchs, P.F.; Pfost, M.; Wiesbrock, F. Heat Dissipation in Epoxy/Amine-Based Gradient Composites with Alumina Particles: A Critical Evaluation of Thermal Conductivity Measurements. Polymers 2018, 10, 1131. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, S.; Gong, X.; Xue, Q.; Lu, Z. Benzoxazine-epoxy thermosets with smectic phase structures for high thermal conductive materials. Liquid Crystals 2019, 46, 1686–1695. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Q.; Hu, Z.; Wang, S.; Wu, K.; Shi, J.; Liang, L.; Lu, M. Liquid crystalline epoxies bearing biphenyl ether and aromatic ester mesogenic units: Synthesis and thermal properties. J. Macromol. Sci. Part A 2019, 56, 484–495. [Google Scholar] [CrossRef]
- Guo, H.; Zheng, J.; Gan, J.; Liang, L.; Wu, K.; Lu, M. High thermal conductivity epoxies containing substituted biphenyl mesogenic. J. Mater. Sci. Mater. Electron. 2016, 27, 2754–2759. [Google Scholar] [CrossRef]
- Kargar, F.; Barani, Z.; Salgado, R.; Debnath, B.; Lewis, J.S.; Aytan, E.; Lake, R.K.; Balandin, A.A. Thermal Percolation Threshold and Thermal Properties of Composites with High Loading of Graphene and Boron Nitride Fillers. ACS Appl. Mater. Interfaces 2018, 10, 37555–37565. [Google Scholar] [CrossRef]
- Yung, K.C.; Liem, H. Enhanced thermal conductivity of boron nitride epoxy-matrix composite through multi-modal particle size mixing. J. Appl. Polym. Sci. 2007, 106, 3587–3591. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Negi, A.; He, J.; Hu, G.; Tian, S.; Liu, J. Synergistic Effects of Boron Nitride (BN) Nanosheets and Silver (Ag) Nanoparticles on Thermal Conductivity and Electrical Properties of Epoxy Nanocomposites. Polymers 2020, 12, 426. [Google Scholar] [CrossRef]
- Pizzi, A.; Papadopoulos, A.N.; Policardi, F. Wood Composites and Their Polymer Binders. Polymers 2020, 12, 1115. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Estrada, O.; Chavez, B.; Stewart, C.; D’Auria, J.C. Tropane and Granatane Alkaloid Biosynthesis: A Systematic Analysis. Molecules 2016, 21, 1510. [Google Scholar] [CrossRef] [PubMed]
- Anjou, K.; Erik, S. The Aroma or Cranberries II. Vaccinium macrocarpon Aid. Acta Chemmica Scand. 1967, 2076–2082. [Google Scholar] [CrossRef] [PubMed]
- Rossegger, E.; Schenk, V.; Wiesbrock, F. Design Strategies for Functionalized Poly(2-oxazoline)s and Derived Materials. Polymers 2013, 5, 956–1011. [Google Scholar] [CrossRef]
- Fimberger, M.; Tsekmes, I.-A.; Kochetov, R.; Smit, J.J.; Wiesbrock, F. Crosslinked Poly(2-oxazoline)s as “Green” Materials for Electronic Applications. Polymers 2016, 8, 6. [Google Scholar] [CrossRef]
- Schenk, V.; Ellmaier, L.; Rossegger, E.; Edler, M.; Griesser, T.; Weidinger, G.; Wiesbrock, F. Water-developable poly(2-oxazoline)-based negative photoresists. Macromol. Rapid Commun. 2012, 33, 396–400. [Google Scholar] [CrossRef]
- Michel, M.; Ferrier, E. Effect of curing temperature conditions on glass transition temperature values of epoxy polymer used for wet lay-up applications. Constr. Build. Mater. 2020, 231, 117206. [Google Scholar] [CrossRef]
- Ehlers, J.-E.; Rondan, N.G.; Huynh, L.K.; Pham, H.; Marks, M.; Truong, T.N. Theoretical Study on Mechanisms of the Epoxy−Amine Curing Reaction. Macromolecules 2007, 40, 4370–4377. [Google Scholar] [CrossRef]
- Riccardi, C.C.; Adabbo, H.E.; Williams, R.J.J. Curing reaction of epoxy resins with diamines. J. Appl. Polym. Sci. 1984, 29, 2481–2492. [Google Scholar] [CrossRef]
- Moroni, A.; Mijovic, J.; Pearce, E.M.; Foun, C.C. Cure kinetics of epoxy resins and aromatic diamines. J. Appl. Polym. Sci. 1986, 32, 3761–3773. [Google Scholar] [CrossRef]
- Horie, K.; Hiura, H.; Sawada, M.; Mita, I.; Kambe, H. Calorimetric investigation of polymerization reactions. III. Curing reaction of epoxides with amines. J. Polym. Sci. A-1 Polym. Chem. 1970, 8, 1357–1372. [Google Scholar] [CrossRef]
- Niedermann, P.; Szebényi, G.; Toldy, A. Effect of Epoxidized Soybean Oil on Curing, Rheological, Mechanical and Thermal Properties of Aromatic and Aliphatic Epoxy Resins. J. Polym. Environ. 2014, 22, 525–536. [Google Scholar] [CrossRef]
- Yamamoto, O. Thermal Conductivity of Cross-linked Polymers. Polym. J. 1971, 2, 509–517. [Google Scholar] [CrossRef][Green Version]
- Pleşa, I.; Noţingher, P.V.; Stancu, C.; Wiesbrock, F.; Schlögl, S. Polyethylene Nanocomposites for Power Cable Insulations. Polymers 2018, 11, 24. [Google Scholar] [CrossRef]
- Coasne, B.; Alba-Simionesco, C.; Audonnet, F.; Dosseh, G.; Gubbins, K.E. Adsorption and structure of benzene on silica surfaces and in nanopores. Langmuir 2009, 25, 10648–10659. [Google Scholar] [CrossRef]



| Epoxy Type | Ratio DAN/IPDA (wt.-%/wt.-%) | Content SiO2 (wt.-%) | Tg (°C) |
|---|---|---|---|
| BADGE | 0:100 | 0 | 154 |
| BADGE | 33:67 | 0 | 152 |
| BADGE | 67:33 | 0 | 149 |
| BADGE | 100:0 | 0 | 148 |
| BADGE | 0:100 | 5 | 152 |
| BADGE | 33:67 | 5 | 151 |
| BADGE | 67:33 | 5 | 146 |
| BADGE | 100:0 | 5 | 146 |
| DEO | 0:100 | 0 | 118 |
| DEO | 33:67 | 0 | 118 |
| DEO | 67:33 | 0 | 103 |
| DEO | 100:0 | 0 | 92 |
| DEO | 0:100 | 5 | 100 |
| DEO | 33:67 | 5 | 92 |
| DEO | 67:33 | 5 | 88 |
| DEO | 100:0 | 5 | 85 |
| Epoxy Type | Ratio DAN/IPDA | Content SiO2 | Free Surface Energy | Polar Fraction | Disperse Fraction |
|---|---|---|---|---|---|
| (wt.-%/wt.-%) | (wt.-%) | (mN·m−1) | (mN·m−1) | (mN·m−1) | |
| BADGE | 0:100 | 0 | 22.3 | 4.7 | 17.5 |
| BADGE | 33:67 | 0 | 36.3 | 4.2 | 32.0 |
| BADGE | 67:33 | 0 | 38.4 | 3.4 | 35.1 |
| BADGE | 100:0 | 0 | 39.5 | 1.1 | 38.3 |
| DEO | 0:100 | 0 | 25.0 | 5.0 | 19.5 |
| DEO | 33:67 | 0 | 32.7 | 4.3 | 28.4 |
| DEO | 67:33 | 0 | 34.8 | 3.2 | 31.6 |
| DEO | 100:0 | 0 | 41.3 | 0.1 | 41.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Windberger, M.S.; Dimitriou, E.; Rendl, S.; Wewerka, K.; Wiesbrock, F. Temperature-Triggered/Switchable Thermal Conductivity of Epoxy Resins. Polymers 2021, 13, 65. https://doi.org/10.3390/polym13010065
Windberger MS, Dimitriou E, Rendl S, Wewerka K, Wiesbrock F. Temperature-Triggered/Switchable Thermal Conductivity of Epoxy Resins. Polymers. 2021; 13(1):65. https://doi.org/10.3390/polym13010065
Chicago/Turabian StyleWindberger, Matthias Sebastian, Evgenia Dimitriou, Sarah Rendl, Karin Wewerka, and Frank Wiesbrock. 2021. "Temperature-Triggered/Switchable Thermal Conductivity of Epoxy Resins" Polymers 13, no. 1: 65. https://doi.org/10.3390/polym13010065
APA StyleWindberger, M. S., Dimitriou, E., Rendl, S., Wewerka, K., & Wiesbrock, F. (2021). Temperature-Triggered/Switchable Thermal Conductivity of Epoxy Resins. Polymers, 13(1), 65. https://doi.org/10.3390/polym13010065







