Shear Stress-Triggered Deformation of Microparticles in a Tapered Microchannel
Abstract
1. Introduction
2. Materials and Methods
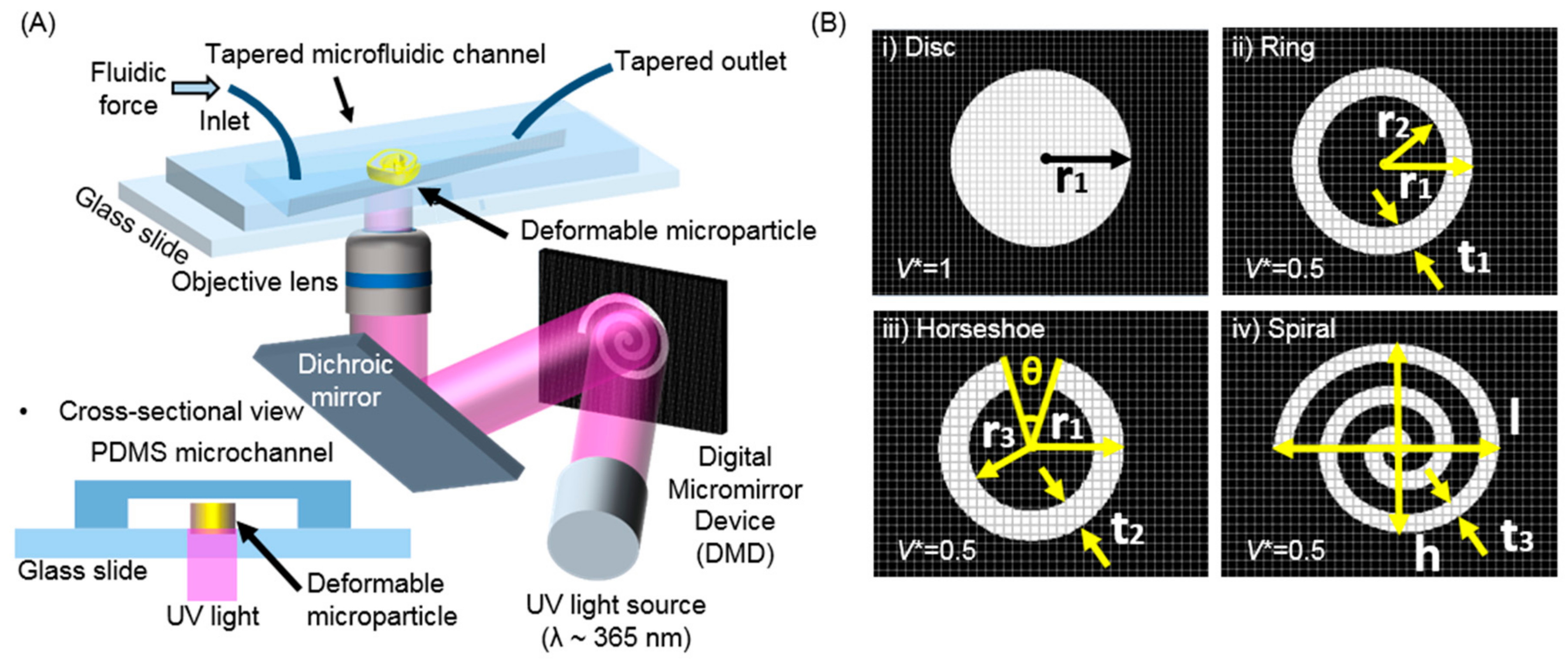
2.1. Microfluidic Platforms for the Generation of Microparticles and the In-Situ Measurement of Deformability
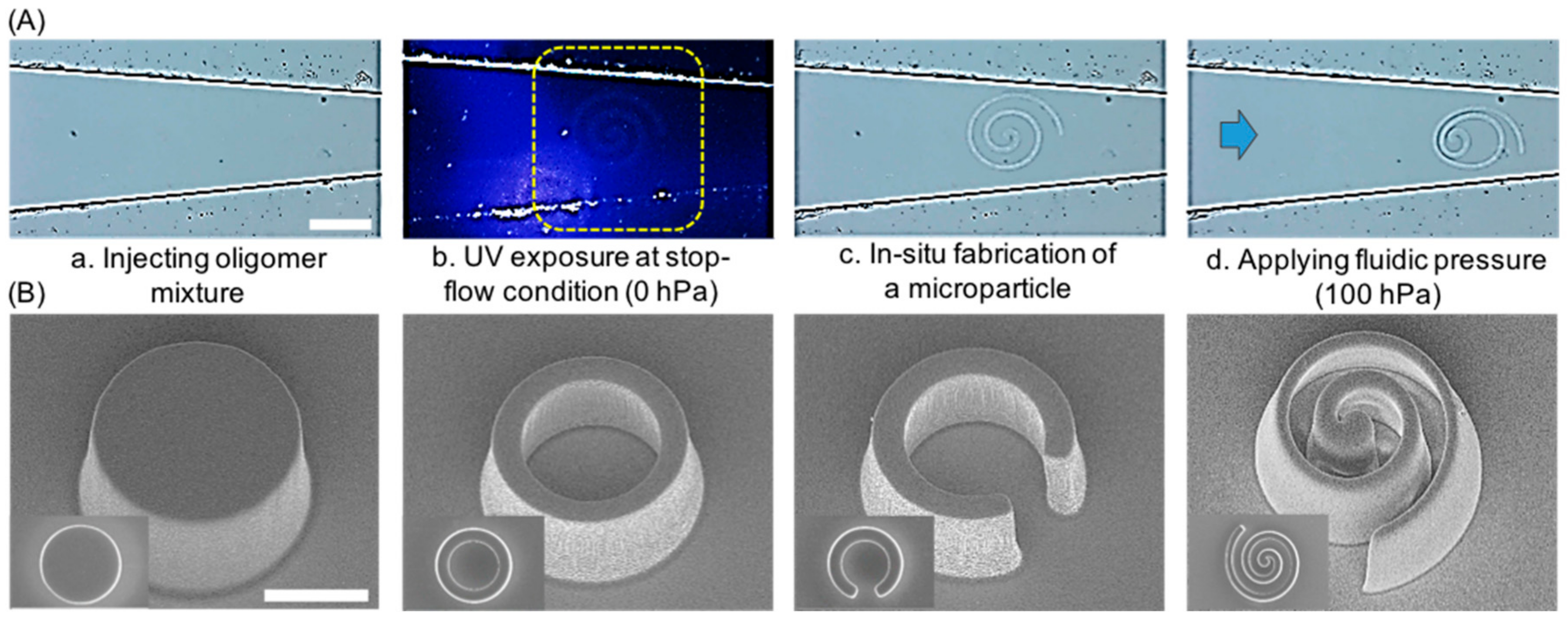
2.2. Generation and Observation of the Deformable Polymeric Particles
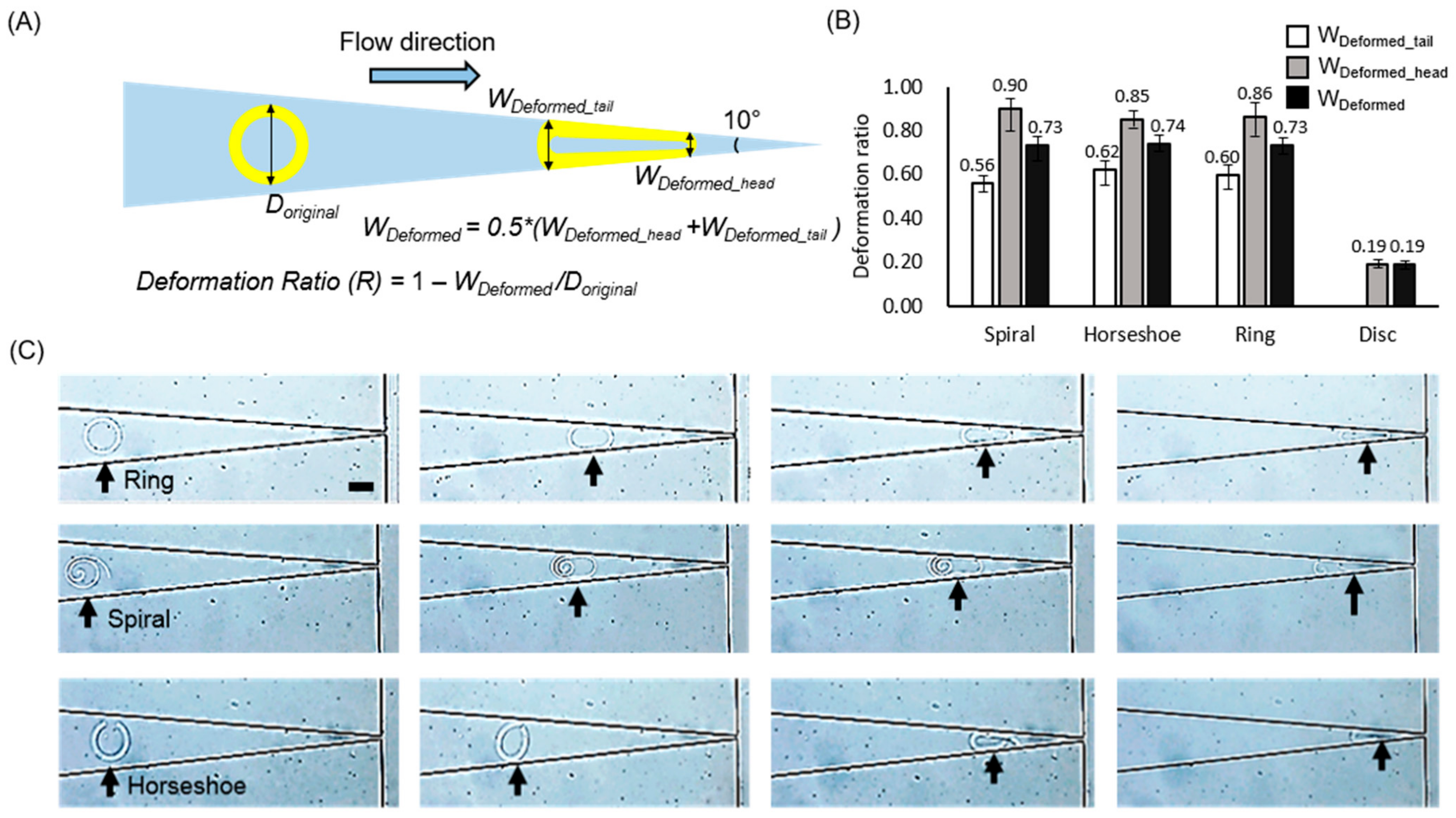
2.3. Deformation Measurements and Modeled the Drug-Releasing Ability of Microparticles
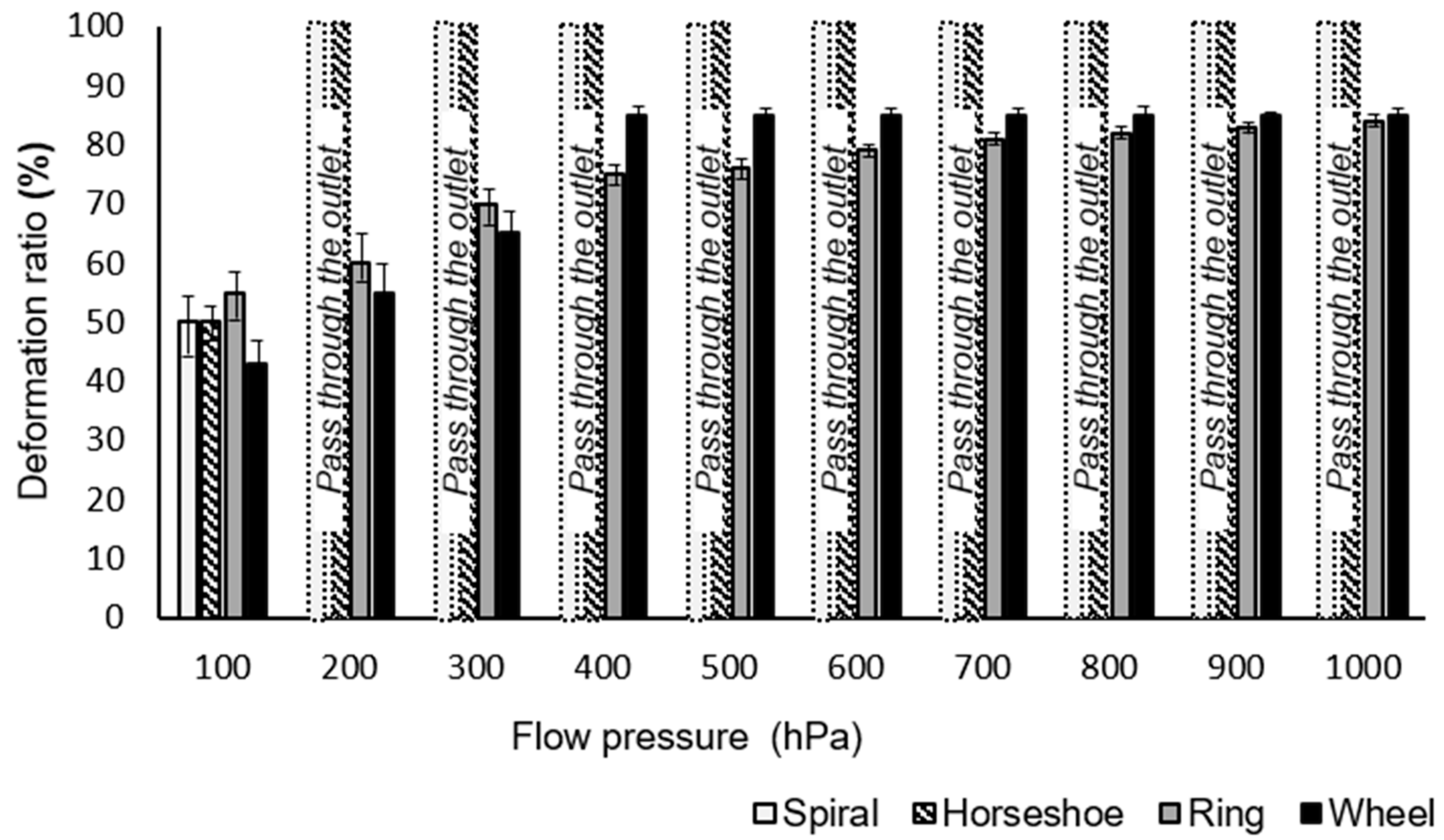
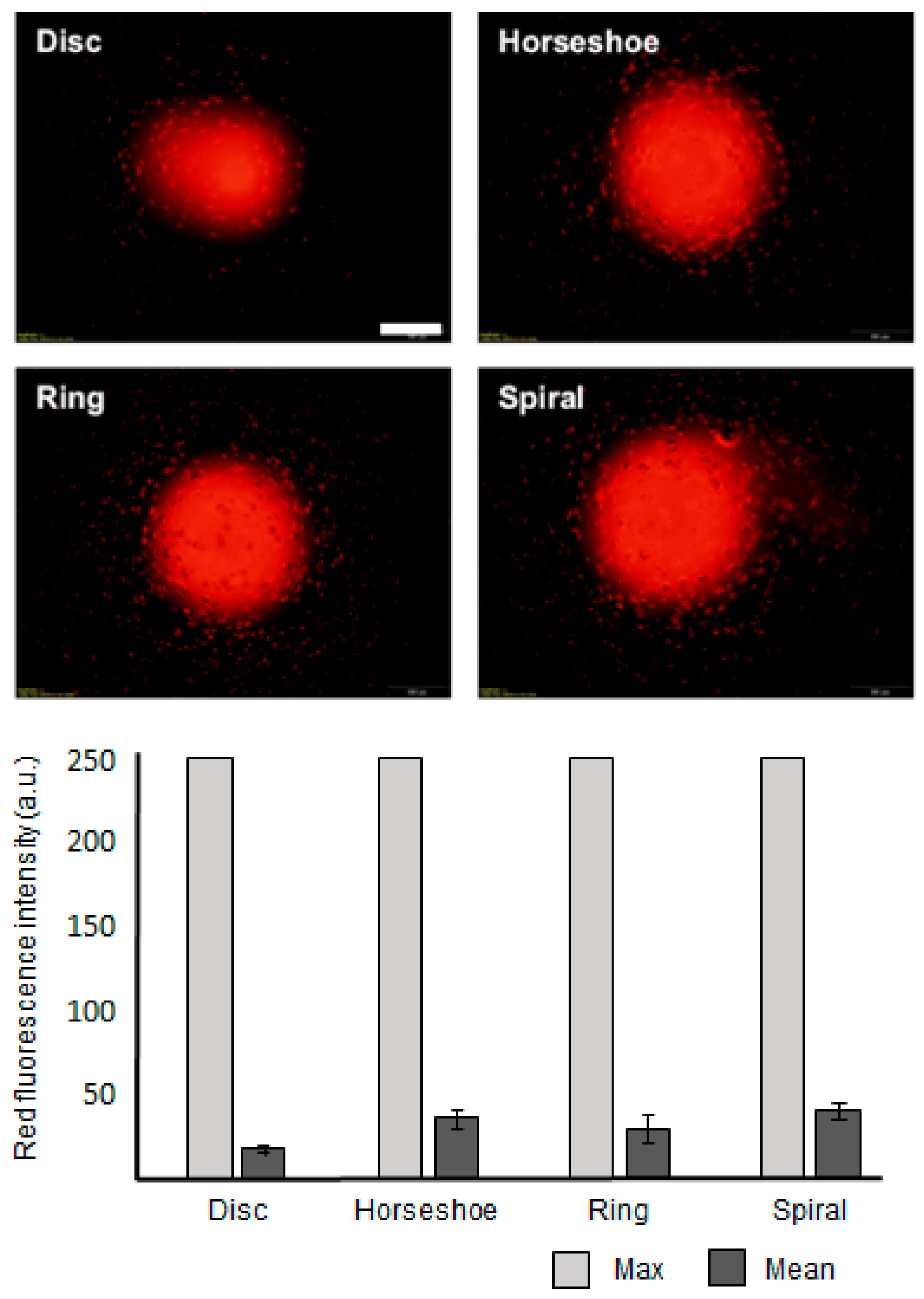
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dao, M.; Lim, C.T.; Suresh, S. Mechanics of the human red blood cell deformed by optical tweezers. J. Mech. Phys. Solids 2003, 51, 2259–2280. [Google Scholar] [CrossRef]
- Doshi, N.; Zahr, A.S.; Bhaskar, S.; Lahann, J.; Mitragotri, S. Red blood cell-mimicking synthetic biomaterial particles. Proc. Natl. Acad. Sci. USA 2009, 106, 21495–21499. [Google Scholar] [CrossRef] [PubMed]
- Secomb, T.W. Mechanics of Blood Flow in Capillaries. In Proceedings of the 2nd Micro and Nano Flows Conference, West London, UK, 1–2 September 2009. [Google Scholar]
- Schmid, S.H.; Wells, R.; Schildkraut, R. Microscopy and viscometry of blood flowing under uniform shear rate (rheoscopy). J. Appl. Physiol. 1969, 26, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Isogai, Y.; Mochizuki, K.; Ashikaga, M. A new method of measuring red cell deformability and the effects of pentoxifylline. Curr. Med. Res. Opin. 1981, 7, 352–358. [Google Scholar] [PubMed]
- Chesla, S.E.; Selvaraj, P.; Zhu, C. Measuring two-dimensional receptor-ligand binding kinetics by micropipette. Biophys. J. 1998, 75, 1553–1572. [Google Scholar] [CrossRef]
- Kato, G.J.; McGowan, V.; Machado, R.F.; Little, J.A.; Taylor VI, J.; Morris, C.R.; Nichols, J.S.; Wang, X.; Poljakovic, M.; Morris, S.M., Jr.; et al. Lactate dehydrogenase as a biomarker of hemolysis associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertrnsion and death in patients with sickle cell disease. Blood 2006, 107, 2279–2285. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Kim, J.; Suga, K.; Ishigami, T.; Park, H.; Bang, J.W.; Seo, S.; Choi, M.; Chang, P.-S.; Umakoshi, H.; et al. Microfluidic platforms with monolithically integrated hierarchical apertures for the facile and rapid formation of cargo-carrying vesicles. Lab Chip 2014, 15, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Kim, J.; Park, H.; Won, B.J.; Seop, H.M.; Bae, Y.; Ha, L.; Yoon, K.D.; Min, K.S.; Jung, P.T.; et al. Replication of flexible polymer membranes with geometry-controllable nano-apertures via a hierarchical mould-based dewetting. Nat. Commun. 2014, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.E.; Park, W.; Park, H.; Yu, K.; Park, N.; Kwon, S. Optofluidic maskless lithography system for real-time synthesis of photopolymerized microstructures in microfluidic channels. Appl. Phys. Lett. 2007, 91, 041106. [Google Scholar] [CrossRef]
- Merkel, T.J.; Jones, S.W.; Herlihy, K.P.; Kersey, F.R.; Shields, A.R.; Napier, M.; Luft, J.C.; Wu, H.; Zamboni, W.C.; Wang, A.Z.; et al. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 586–591. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.; Kim, H.; Kim, J.; Kwon, S. Colour-barcoded magnetic microparticles for multiplexed bioassays. Nat. Mater. 2010, 9, 745–749. [Google Scholar] [CrossRef]
- Bae, H.J.; Bae, S.; Park, C.; Han, S.; Kim, J.; Kim, L.N.; Kim, K.; Song, S.H.; Park, W.; Kwon, S. Biomimetic microfingerprints for anti-counterfeiting strategies. Adv. Mater. 2015, 27, 2083–2089. [Google Scholar] [CrossRef] [PubMed]
- Miura, K. Method of packaging and deployment of large membranes in space. Inst. Sp. Astronaut. Sci. Rep. 1985, 618, 1–9. [Google Scholar]
- Eun, C.S.; Kim, J.; Yoon, O.D.; Song, Y.; Hoon, L.S.; Min, S.; Kwon, S. One-step pipetting and assembly of encoded chemical-laden microparticles for high-throughput multiplexed bioassays. Nat. Commun. 2014, 5, 3468. [Google Scholar]
- Choi, Y.; Bae, H.J.; Lee, A.C.; Choi, H.; Lee, D.; Ryu, T.; Hyun, J.; Kim, S.; Kim, H.; Song, S.H.; et al. DNA Micro-Disks for the Management of DNA-Based Data Storage with Index and Write-Once–Read-Many (WORM) Memory Features. Adv. Mater. 2020, 32, 1–8. [Google Scholar] [CrossRef]
- Kim, J.; Wang, Y.; Park, H.; Park, M.C.; Moon, S.E.; Hong, S.M.; Koo, C.M.; Suh, K.Y.; Yang, S.; Cho, H. Nonlinear Frameworks for Reversible and Pluripotent Wetting on Topographic Surfaces. Adv. Mater. 2017, 29, 1605078. [Google Scholar] [CrossRef]
- Chen, L.; An, H.Z.; Doyle, P.S. Synthesis of Nonspherical Microcapsules through Controlled Polyelectrolyte Coating of Hydrogel Templates. Langmuir 2015, 31, 9228–9235. [Google Scholar] [CrossRef]
- Mullin, T.; Deschanel, S.; Bertoldi, K.; Boyce, M.C. Pattern Transformation Triggered by Deformation. Phys. Rev. Lett. 2007, 99, 084301. [Google Scholar] [CrossRef]
- Park, A.; Jeong, H.H.; Lee, J.; Kim, K.P.; Lee, C.S. Effect of shear stress on the formation of bacterial biofilm in a microfluidic channel. Biochip J. 2011, 5, 236–241. [Google Scholar] [CrossRef]
- Cho, Y.; Shin, J.-H.; Costa, A.; Kim, T.A.; Kunin, V.; Li, J.; Lee, S.Y.; Yang, S.; Han, H.N.; Choi, I.-S.; et al. Engineering the shape and structure of materials by fractal cut. Proc. Natl. Acad. Sci. USA 2014, 111, 17390–17395. [Google Scholar] [CrossRef]
- Caldorera, M.M.; Kang, M.K.; Moore, Z.; Singh, V.; Sreenivasan, S.V.; Shi, L.; Huang, R.; Roy, K. Swelling behavior of nanoscale, shape- and size-specific, hydrogel particles fabricated using imprint lithography. Soft Matter 2011, 7, 2879–2887. [Google Scholar] [CrossRef]
- Chan, V.; Jeong, J.H.; Bajaj, P.; Collens, M.; Saif, T.; Kong, H.; Bashir, R. Multi-material bio-fabrication of hydrogel cantilevers and actuators with stereolithography. Lab Chip 2012, 12, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Coclite, A.; Pascazio, G.; Tullio, M.D.; Decuzzi, P. Predicting the vascular adhesion of deformable drug carriers in narrow capillaries traversed by blood cells. J. Fluids Struct. 2018, 82, 638–650. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, K.; Park, Y. Measurement Techniques for Red Blood Cell Deformability: Recent Advances. Blood Cell-An Overv. Stud. Hematol. 2012, 10, 167–194. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.; Bae, J.; Choi, Y.; Park, W. Shear Stress-Triggered Deformation of Microparticles in a Tapered Microchannel. Polymers 2021, 13, 55. https://doi.org/10.3390/polym13010055
Park C, Bae J, Choi Y, Park W. Shear Stress-Triggered Deformation of Microparticles in a Tapered Microchannel. Polymers. 2021; 13(1):55. https://doi.org/10.3390/polym13010055
Chicago/Turabian StylePark, Cheolheon, Junghyun Bae, Yeongjae Choi, and Wook Park. 2021. "Shear Stress-Triggered Deformation of Microparticles in a Tapered Microchannel" Polymers 13, no. 1: 55. https://doi.org/10.3390/polym13010055
APA StylePark, C., Bae, J., Choi, Y., & Park, W. (2021). Shear Stress-Triggered Deformation of Microparticles in a Tapered Microchannel. Polymers, 13(1), 55. https://doi.org/10.3390/polym13010055




