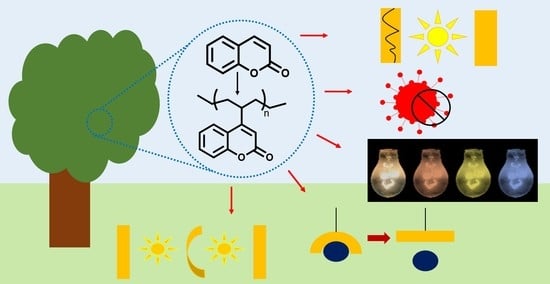
Recent Advances in Functional Polymers Containing Coumarin Chromophores
Abstract
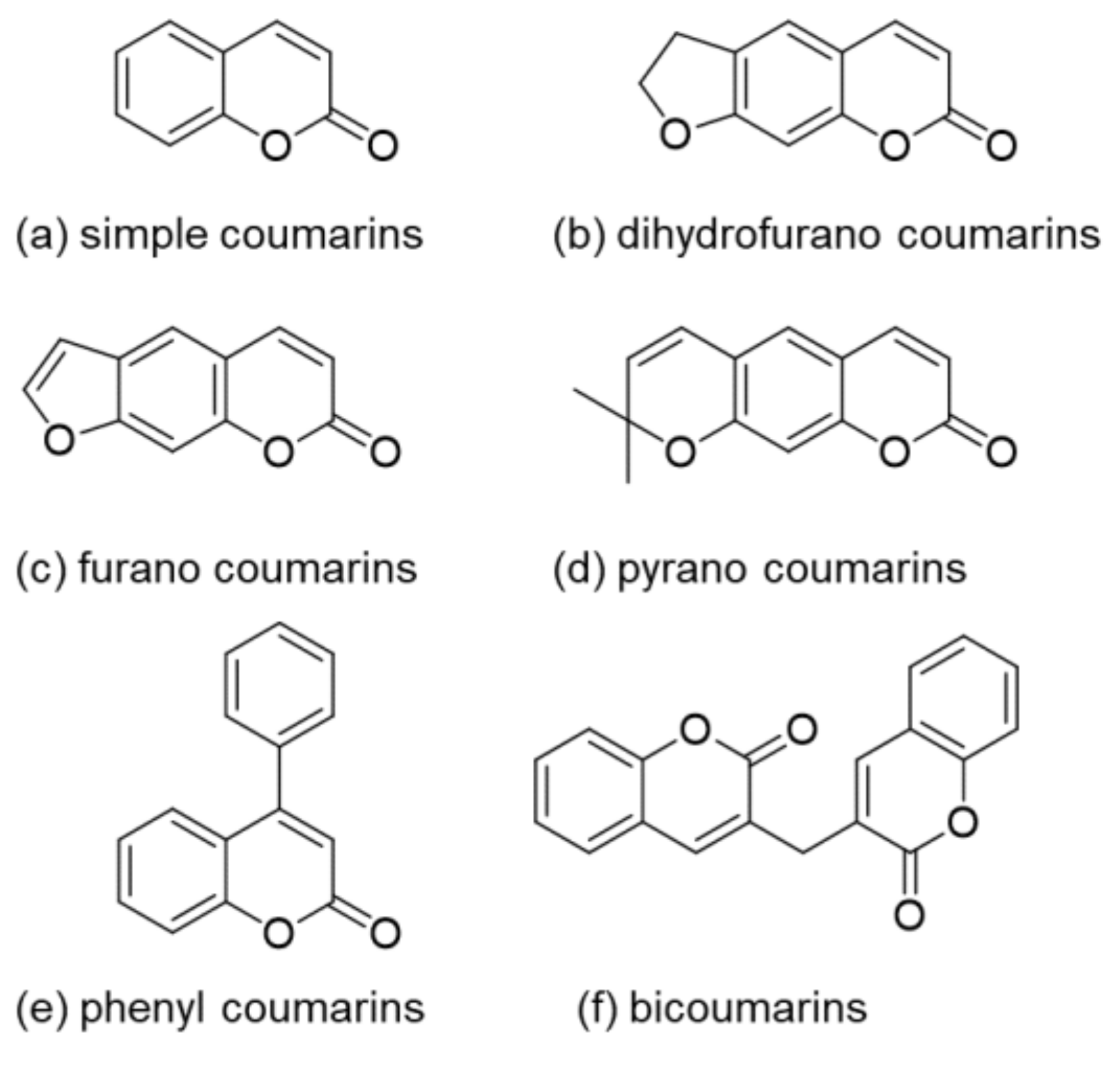
1. Introduction
2. Photoreaction Mechanisms of Coumarin and Its Derivatives
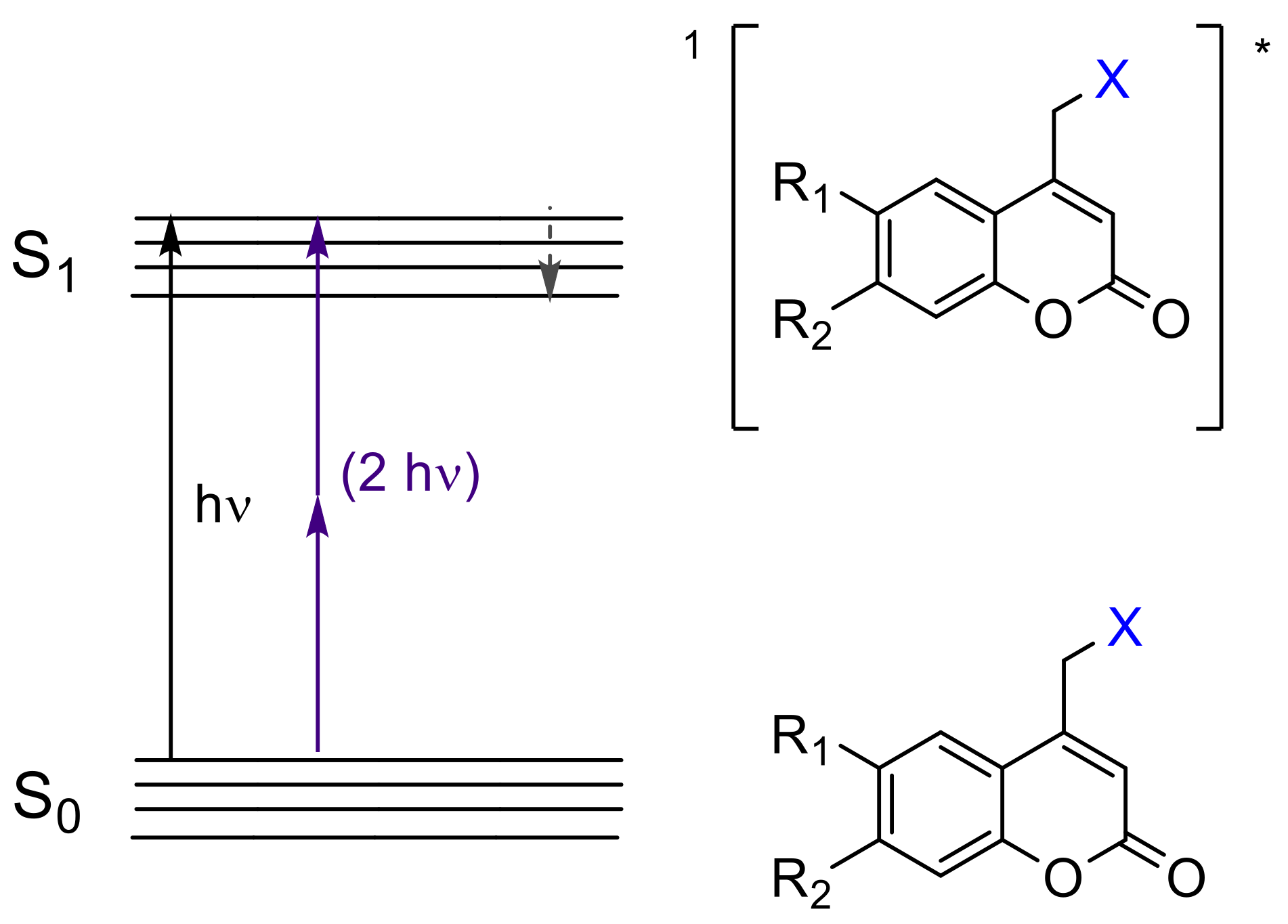
2.1. Photocleavage of Coumarin-Caged Compounds and Photolabile Surfaces Bearing Functional Coumarin Groups (Photofuses)
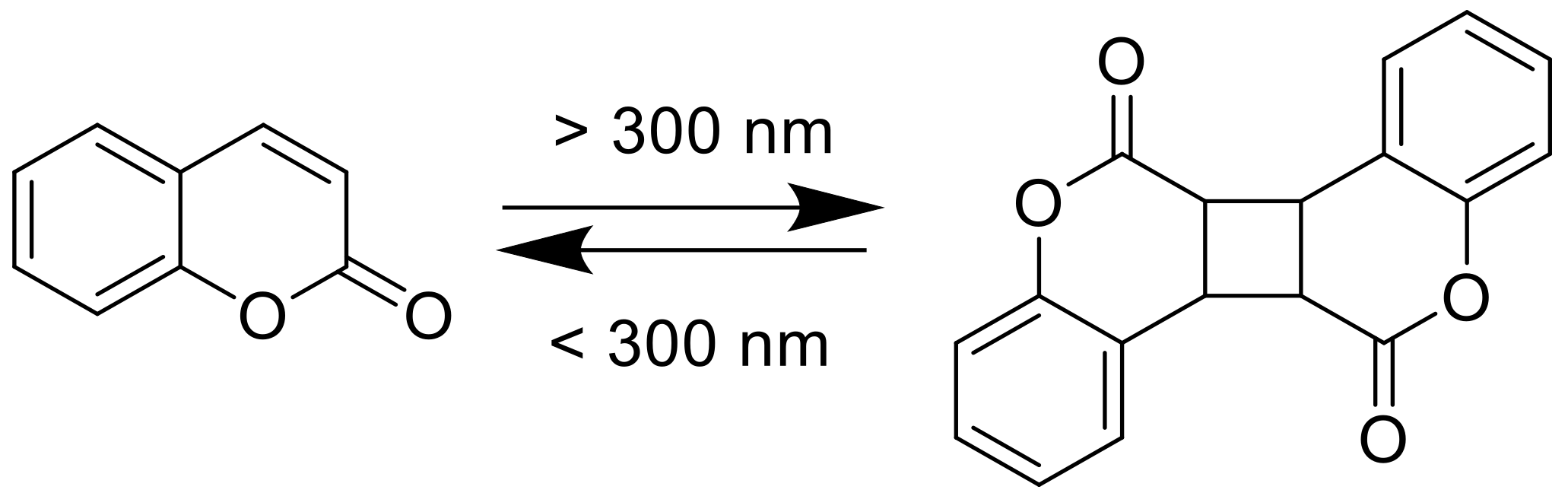
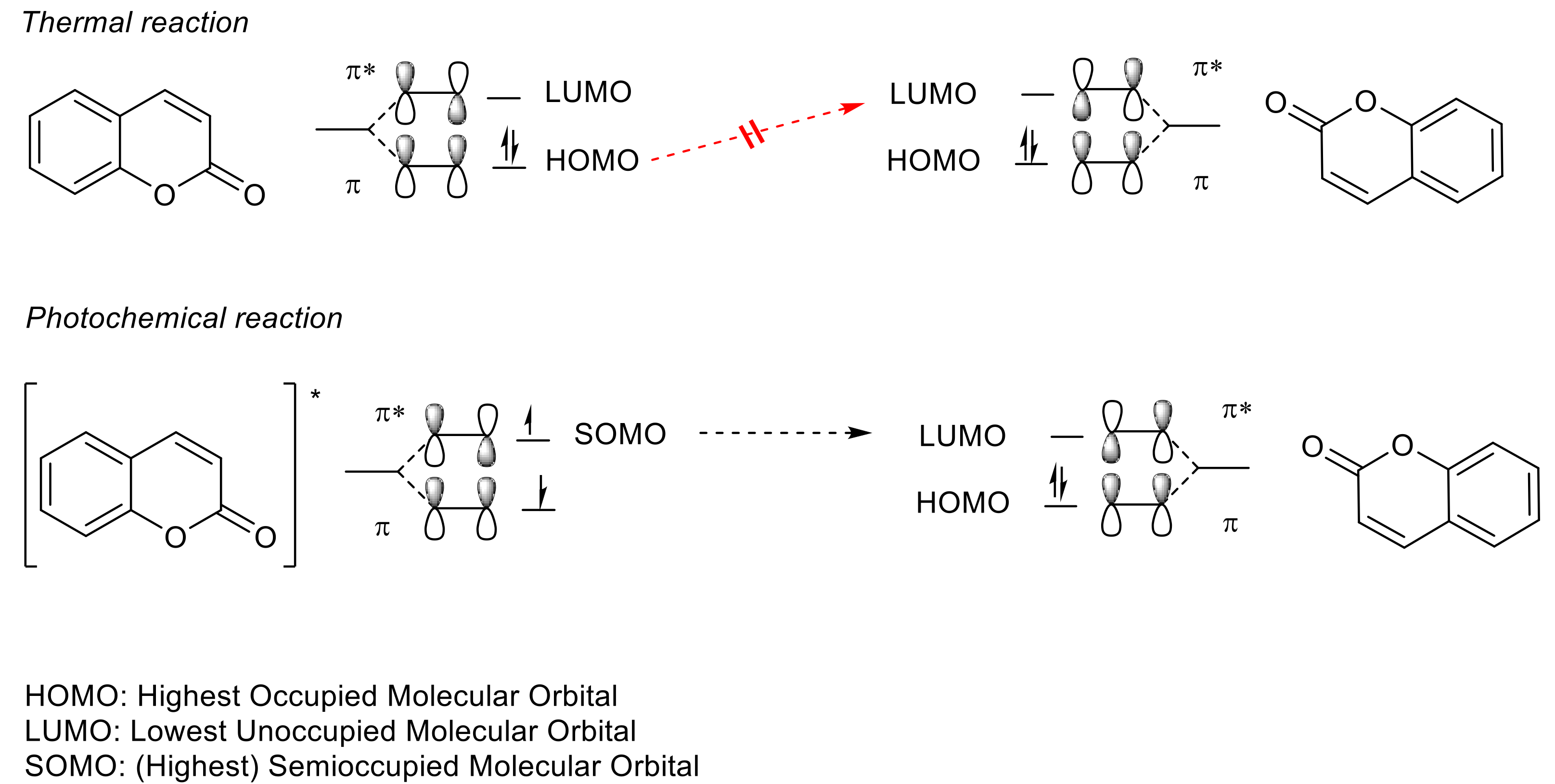
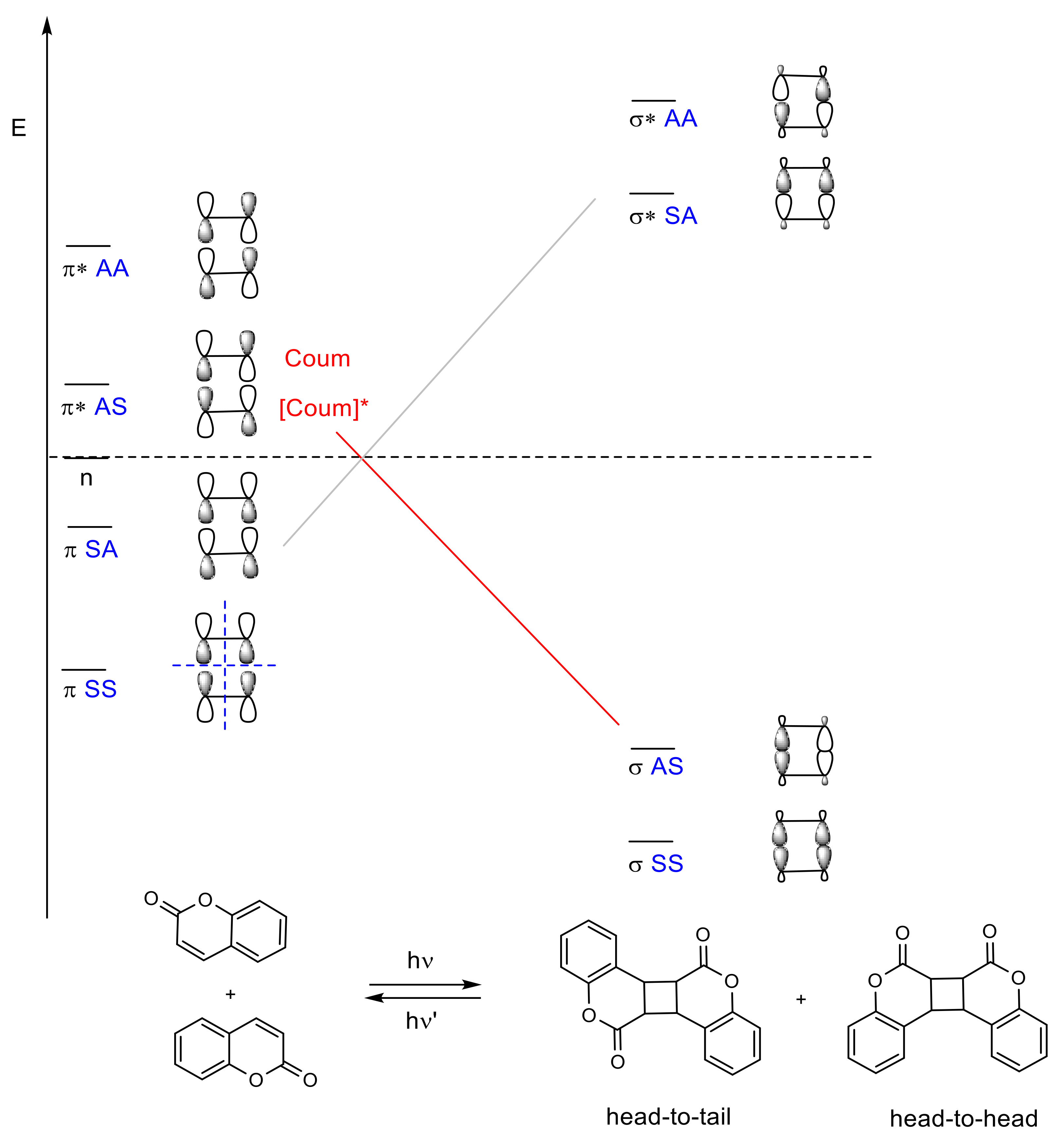
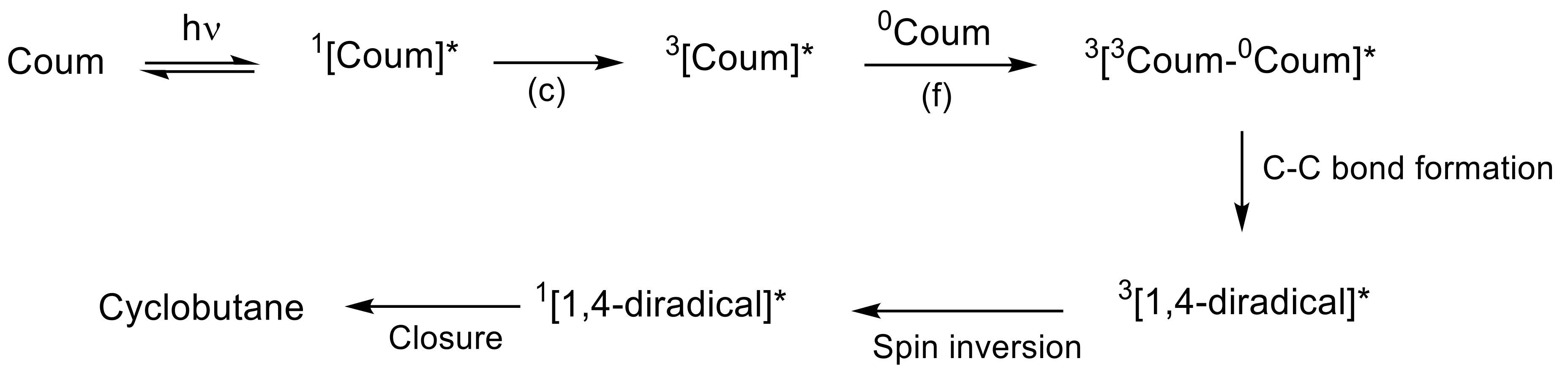
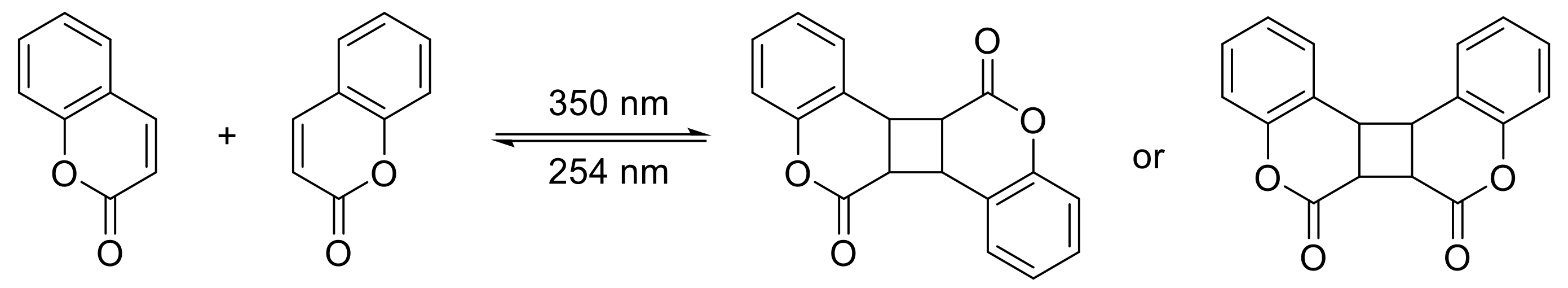
2.2. [2πs + 2πs] Photocycloaddition Reaction of Coumarin Groups
2.3. Coumarin Derivatives Serving as Photoinitiators
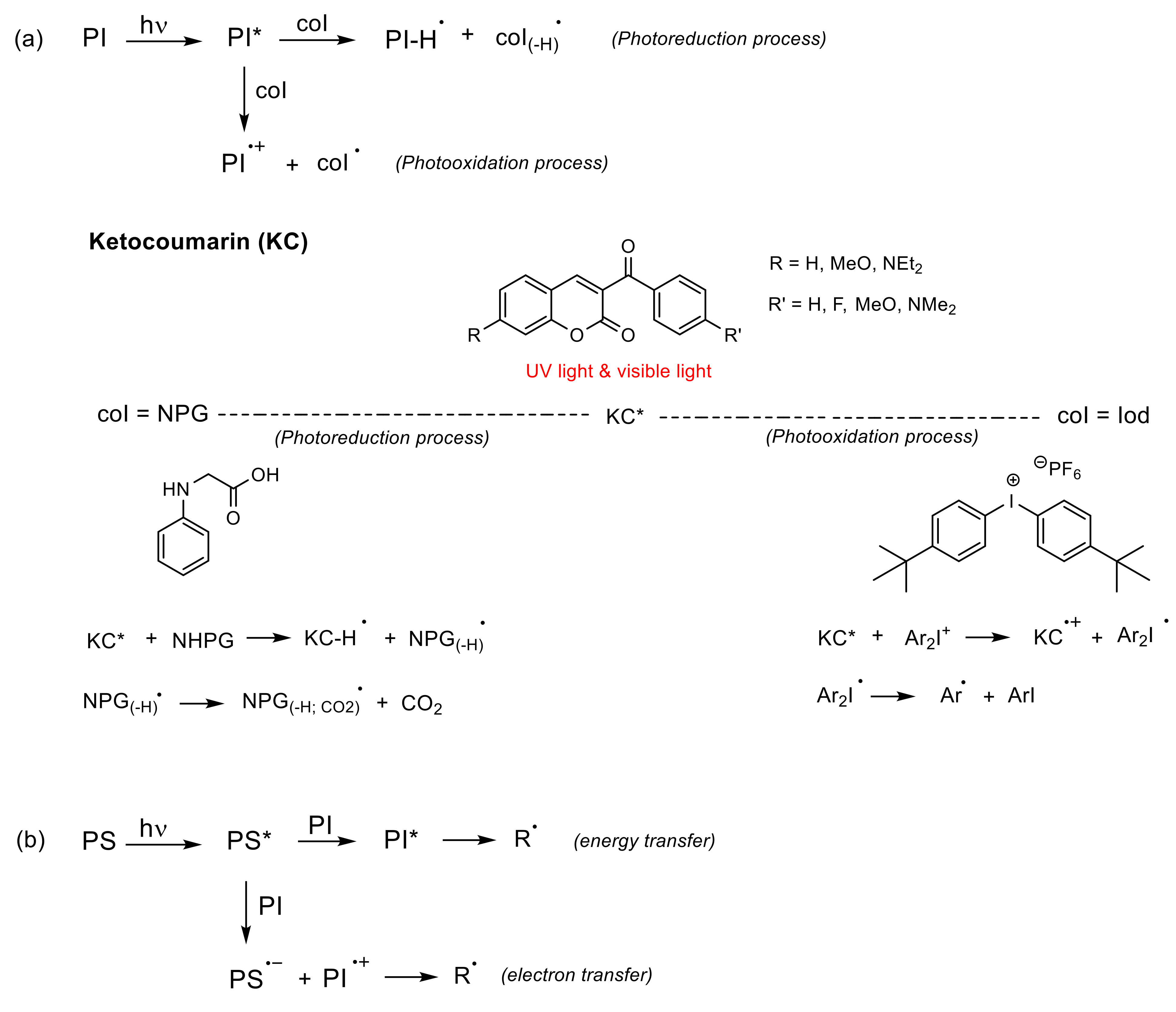
- (a)
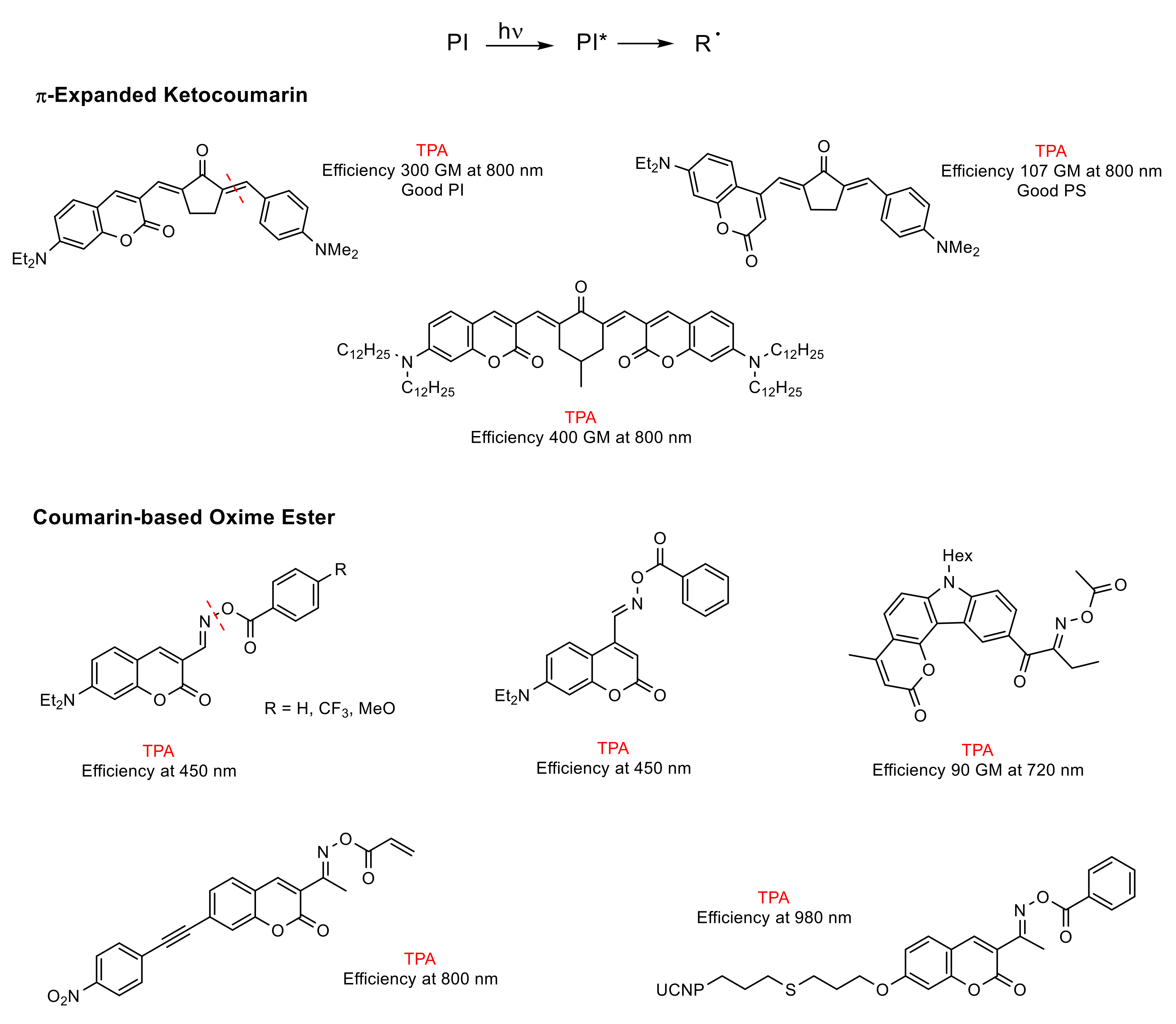
- Unimolecular system (photocleavable PI, Type I): upon absorption, the excited state of the PI undergoes a homolytic cleavage to produce free radicals. Subsequently, an electron transfer from one of these radicals to a monomer generates the radical anion species responsible for the polymerization [86,90]. In contrast to the photocleavage mechanism in (coumarin-4-yl)methyl derivatives, the radical species are generated from the triplet state after the intersystem crossing [87,89,92,94]. This type of coumarin-based 2PIs is generally constituted by conjugated carbonyl groups (photocleavage of a double bond in α-carbonyl position) [92,94] or by oxime-ester (photocleavage of an N-O bond) [87,95,96,97] (Figure 10).
- (b)
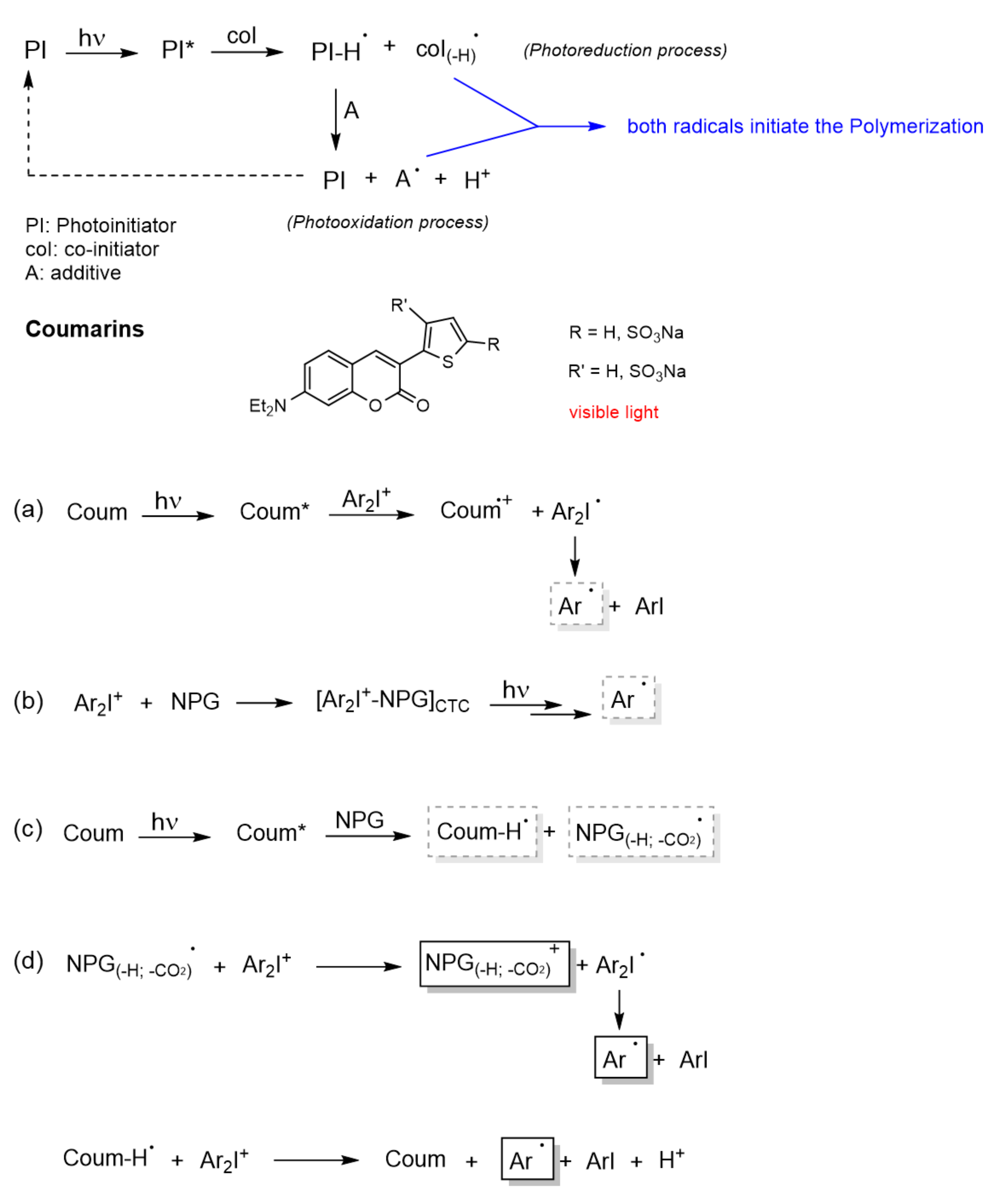
- Bimolecular system (PI/coI (co-initiator) or PI/PS (photosensitizer), Type II) [86]: Once the PI is excited in the PI/coI system, a transfer of an electron/proton takes place between both compounds (see Figure 11a), thus resulting in radicals or ions that initiate the polymerization reaction. Some examples of typical coIs, employed in combination with ketocoumarins as PIs, are bis-(4-tert-butylphenyl)iodonium hexafluorophosphate (Iod or SpeedCure 938), N-phenylglycine (NPG), and ethyl 4-(dimethylamino)benzoate (EDB) [89]. The triplet state pathway is also possible in the mechanism of bimolecular system (KC/Iod), since free energy change for an electron transfer (∆Get) from the aforementioned state is favorable [88,89].
- (c)
- Multicomponent system (three or more compounds): this system involves combinations that allow an improvement in the performance of PIs under the conditions required by the applications [86]. Thus, its mechanism is rather complex.
3. Polymers with Coumarin as Nonreactive Moiety in Electro-Optical Applications
3.1. Fluorescence Studies
3.2. Electroluminescence Studies
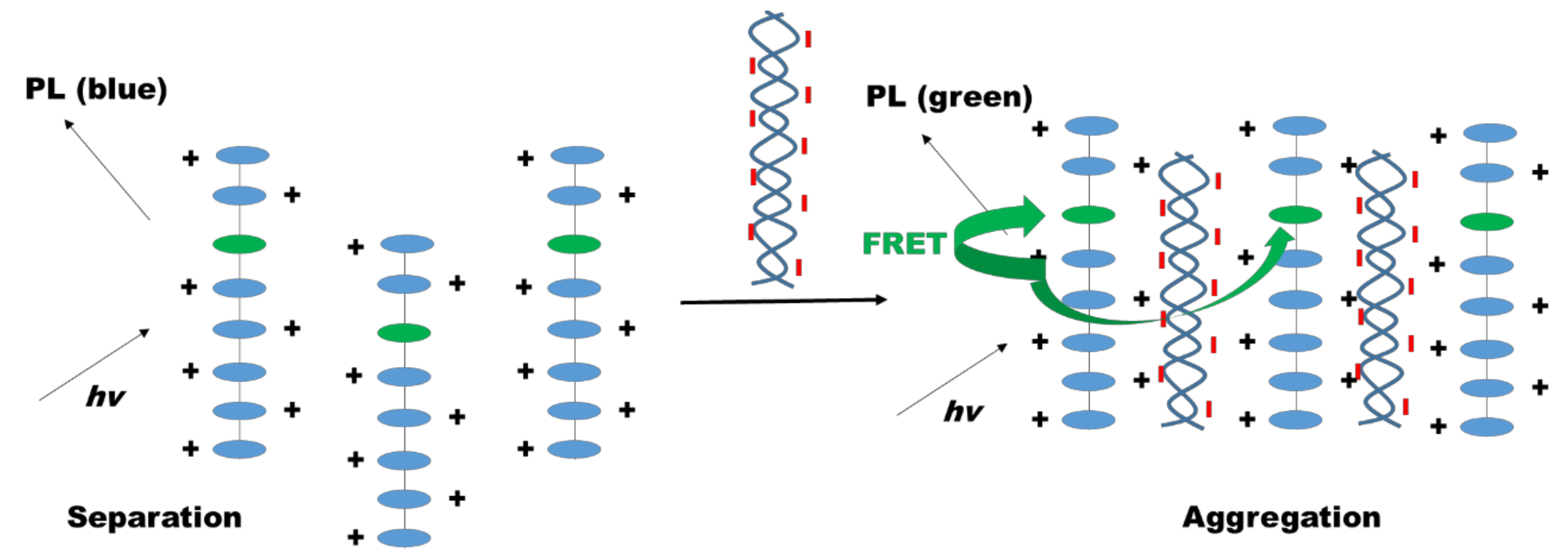
3.3. Light and Energy Harvesting
3.4. Liquid Crystalline Polymers
4. Sustainable Polymers with Coumarin as Nonreactive Moiety
5. Biological and Medical Applications of Polymers with Coumarin as Nonreactive Moiety
5.1. Antimicrobial Coatings
5.2. Biologically Active Polymers
5.3. Gene and Drug Delivery
5.4. Bioimaging
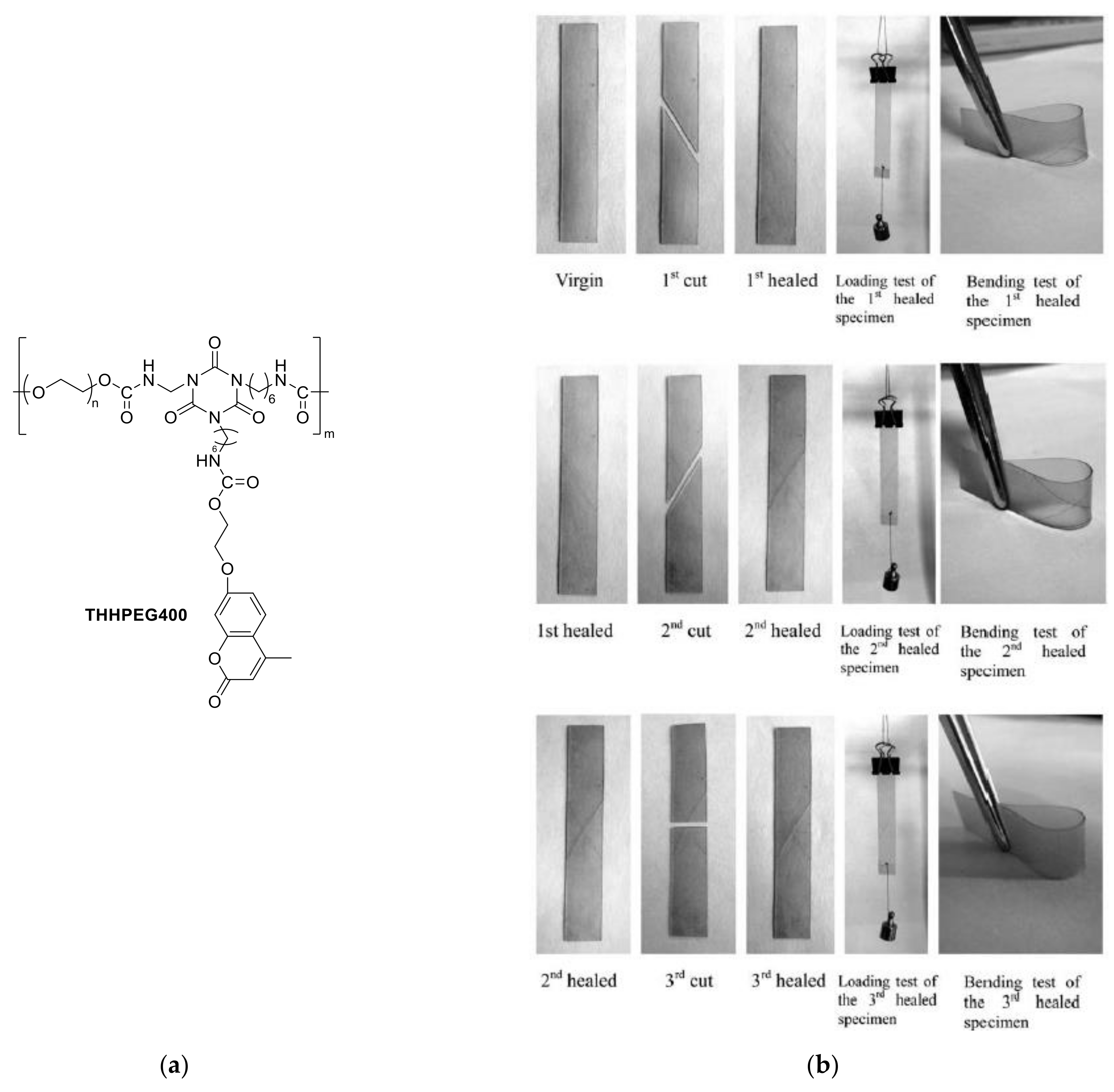
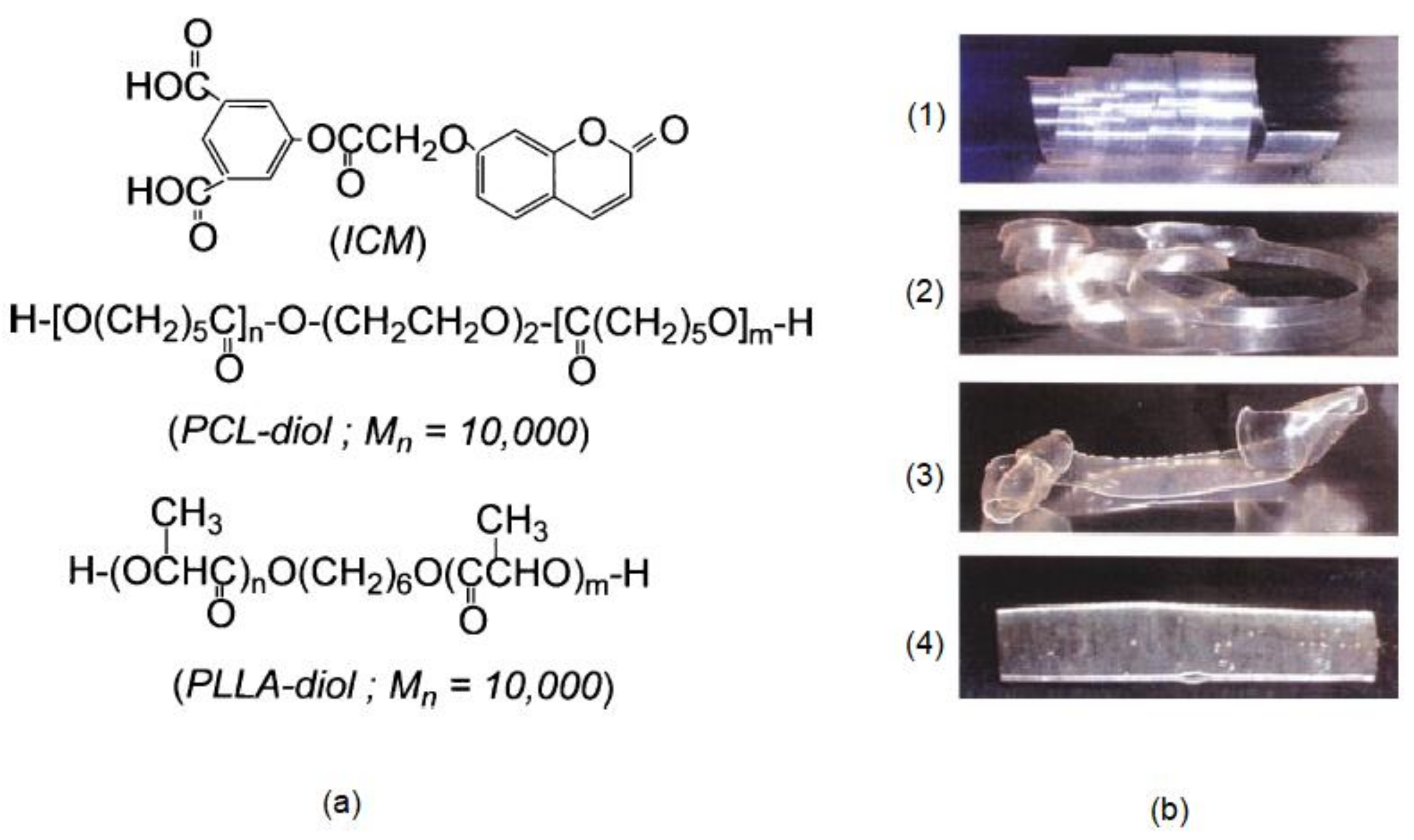
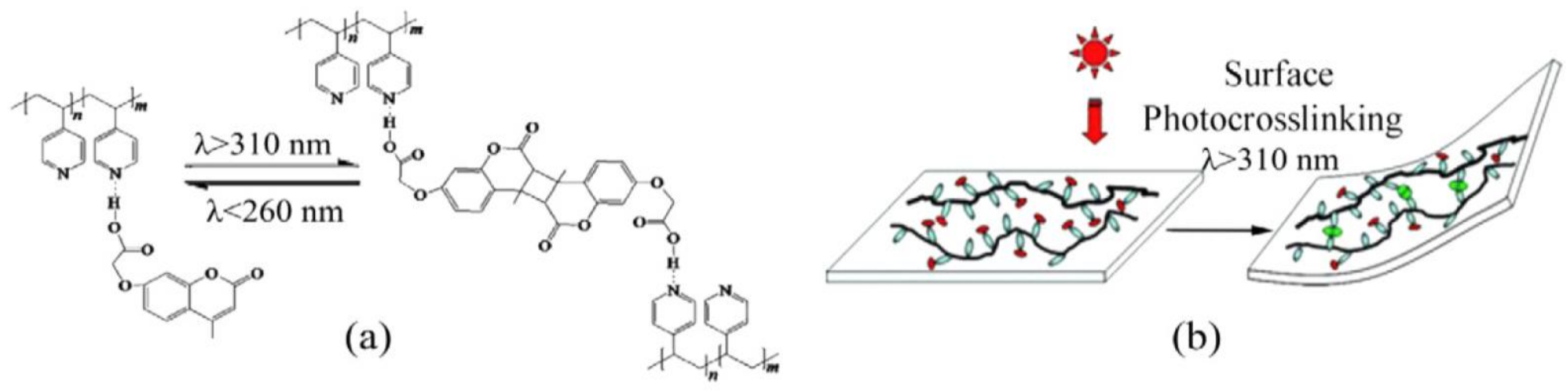
6. Self-Healable Polymers with Coumarin as Reactive Moiety
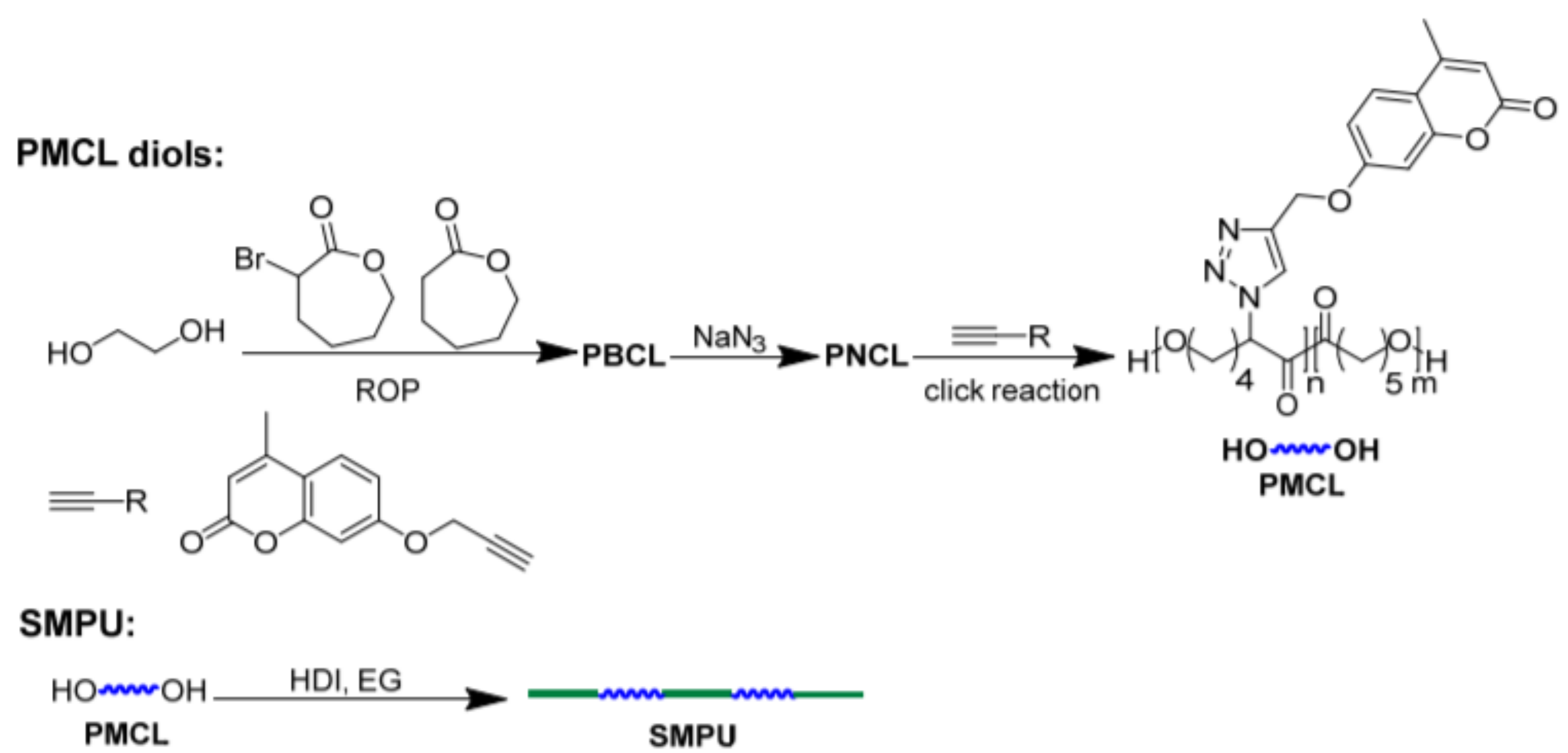
7. Shape-Memory Polymers with Coumarin as Reactive Moiety
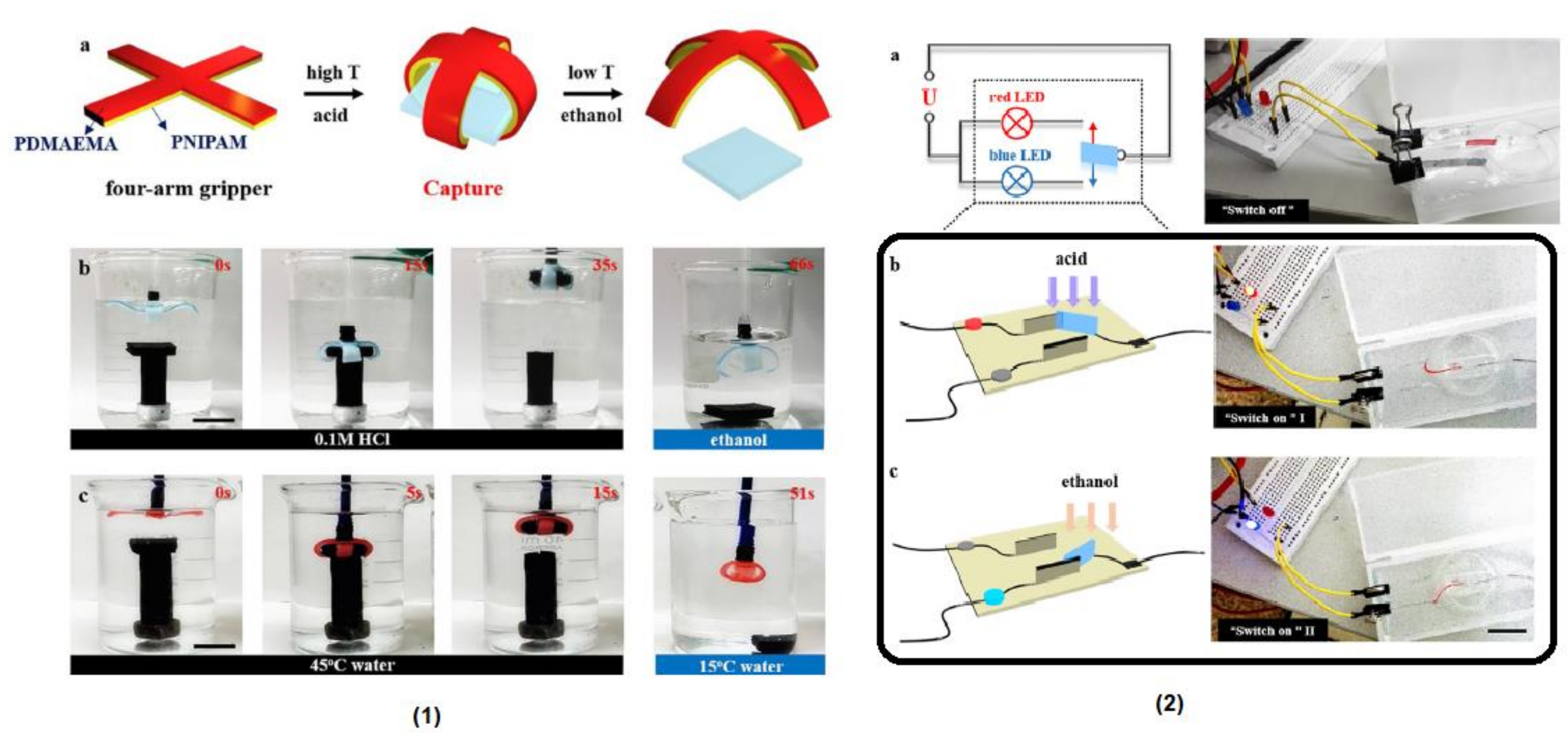
8. Polymers with Coumarin in Soft Robotics Applications
9. Polymers with Coumarin in Tissue Engineering Applications
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple Coumarins and Analogues in Medicinal Chemistry: Occurrence, Synthesis and Biological Activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef] [PubMed]
- Ajay Kumar, K.; Renuka, N.; Pavithra, G.; Vasanth Kumar, G. Comprehensive review on coumarins: Molecules of potential chemical and pharmacological interest. J. Chem. Pharm. Res. 2015, 7, 67–81. [Google Scholar]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef]
- Lei, L.; Xue, Y.-B.; Liu, Z.; Peng, S.-S.; He, Y.; Zhang, Y.; Fang, R.; Wang, J.-P.; Luo, Z.-W.; Yao, G.-M.; et al. Coumarin derivatives from Ainsliaea fragrans and their anticoagulant activity. Sci. Rep. 2015, 5, 13544. [Google Scholar] [CrossRef]
- Lacy, A.; O’Kennedy, R. Studies on Coumarins and Coumarin-Related Compounds to Determine their Therapeutic Role in the Treatment of Cancer. Curr. Pharm. Des. 2004, 10, 3797–3811. [Google Scholar] [CrossRef] [PubMed]
- O’Kennedy, R.; Thornes, R.D. Coumarins: Biology, Applications and Mode of Action; Wiley: Hoboken, NJ, USA, 1997; ISBN 978-0-471-96997-6. [Google Scholar]
- Cooke, D.; Fitzpatrick, B.; O’Kennedy, R.; Mc Cormack, T.; Egan, D. Coumarins—Multifaceted Molecules with Many Analytical and Other Applications. Coumarins: Biology, Applications and Mode of Action; John Wiley & Sons: Hoboken, NJ, USA, 1997. [Google Scholar]
- Cooke, D. Studies on the Mode of Action of Coumarins (Coumarin, 6-hydroxycoumarin, 7-hydroxycoumarin and Esculetin) at a Cellular Level. Ph.D. Thesis, Dublin City University, Dublin, Ireland, 1999. [Google Scholar]
- Huawei, C.; Walsh, C.T. Coumarin formation in novobiocin biosynthesis: V-hydroxylation of the aminoacyl enzyme tyrosyl-S-NovH by a cytochrome P450 NovI. Chem. Biol. 2001, 8, 301–312. [Google Scholar]
- Chamsaz, E.A.; Mankoci, S.; Barton, H.A.; Joy, A. Nontoxic Cationic Coumarin Polyester Coatings Prevent Pseudomonas aeruginosa Biofilm Formation. ACS Appl. Mater. Interfaces 2017, 9, 6704–6711. [Google Scholar] [CrossRef]
- Brahmbhatt, D.I.; Singh, S.; Patel, K.C. Synthesis, characterization and biological activity of some poly(coumarin ethylene)s. Eur. Polym. J. 1999, 35, 317–324. [Google Scholar] [CrossRef]
- Erol, I.; Sanli, G.; Dİlek, M.; Ozcan, L. Synthesis and characterization of novel methacrylate copolymers based on sulfonamide and coumarine: Monomer reactivity ratios, biological activity, thermal stability, and optical properties. J. Polym. Sci. A Polym. Chem. 2010, 48, 4323–4334. [Google Scholar] [CrossRef]
- Chitra, R.; Kayalvizhy, E.; Jeyanthi, P.; Pazhanisamy, P. Synthesis, Characterization and Antimicrobial Screening of Coumarin Copolymers. Chem. Sci. Trans. 2014, 3, 722–730. [Google Scholar] [CrossRef]
- Hamdi, N.; Saoud, M.; Romerosa, A.; Hassen, R.B. Synthesis, spectroscopic and antibacterial investigations of new hydroxy ethers and heterocyclic coumarin derivatives. J. Heterocycl. Chem. 2008, 45, 1835–1842. [Google Scholar] [CrossRef]
- Srivastava, A.; Mishra, V.; Singh, P.; Kumar, R. Coumarin-based polymer and its silver nanocomposite as advanced antibacterial agents: Synthetic path, kinetics of polymerization, and applications. J. Appl. Polym. Sci. 2012, 126, 395–407. [Google Scholar] [CrossRef]
- Venkatesan, S.; Ranjithkumar, B.; Rajeshkumar, S.; Anver Basha, K. Synthesis, characterization, thermal stability and antibacterial activity of coumarin based methacrylate copolymers. Chin. J. Polym. Sci. 2014, 32, 1373–1380. [Google Scholar] [CrossRef]
- Katsori, A.M.; Hadjipavlou-Litina, D. Coumarin derivatives: An updated patent review (2012–2014). Expert Opin. Pat. 2014, 24, 1323–1347. [Google Scholar] [CrossRef] [PubMed]
- Lončarić, M.; Gašo-Sokač, D.; Jokić, S.; Molnar, M. Recent Advances in the Synthesis of Coumarin Derivatives from Different Starting Materials. Biomolecules 2020, 10, 151. [Google Scholar] [CrossRef]
- Patel, H.J.; Patel, M.G.; Patel, R.J.; Patel, K.H.; Patel, R.M. Synthesis, Characterization, Thermal Studies, and Antimicrobial Screening of Poly (acrylate)s Bearing 4-Methyl Coumarin Side Groups. Iran. Polym. J. 2008, 17, 635–644. [Google Scholar]
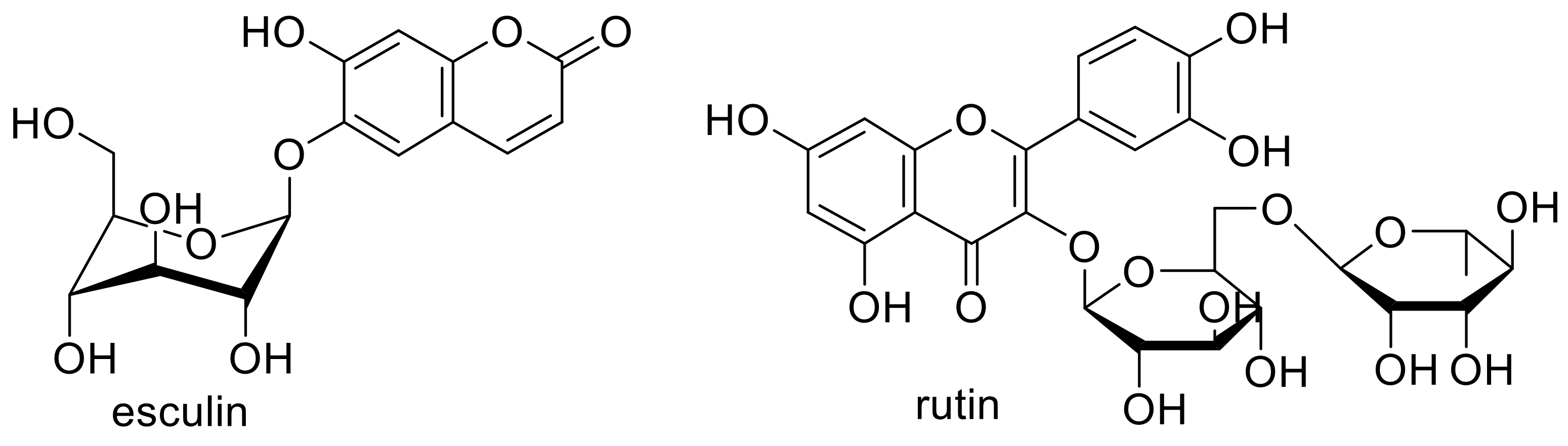
- Chebil, L.; Rhouma, G.B.; Chekir-Ghedira, L.; Ghoul, M. Enzymatic Polymerization of Rutin and Esculin and Evaluation of the Antioxidant Capacity of Polyrutin and Polyesculin. In Biotechnology; Ekinci, D., Ed.; InTech: London, UK, 2015; ISBN 978-953-51-2040-7. [Google Scholar]
- Li, Q.; Wei, L.; Zhang, J.; Gu, G.; Guo, Z. Significantly enhanced antioxidant activity of chitosan through chemical modification with coumarins. Polym. Chem. 2019, 10, 1480–1488. [Google Scholar] [CrossRef]
- Iranshahi, M.; Askari, M.; Sahebkar, A.; Hadjipavlou-Litina, D. Evaluation of antioxidant, anti-inflammatory and lipoxygenase inhibitory activities of the prenylated coumarin umbelliprenin. DARU 2009, 99–104. [Google Scholar]
- Liu, X.; Zhang, R.; Li, T.; Zhu, P.; Zhuang, Q. Novel Fully Biobased Benzoxazines from Rosin: Synthesis and Properties. ACS Sustain. Chem. Eng. 2017, 5, 10682–10692. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A Natural, Privileged and Versatile Scaffold for Bioactive Compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef]
- Olmedo, D.; Sancho, R.; Bedoya, L.M.; López-Pérez, J.L.; Del Olmo, E.; Muñoz, E.; Alcamí, J.; Gupta, M.P.; San Feliciano, A. 3-Phenylcoumarins as inhibitors of HIV-1 replication. Molecules 2012, 17, 9245–9257. [Google Scholar] [CrossRef] [PubMed]
- Bairagi, S.H.; Salaskar, P.P.; Loke, S.D.; Surve, N.N.; Tandel, D.V. Medicinal Significance of Coumarins: A Review. Int. J. Pharm. Res. 2012, 4, 16–19. [Google Scholar]
- Ciamician, G.; Silber, P. Chemische Lichtwirkungen. Chem. Ber 1902, 35, 4128–4131. [Google Scholar] [CrossRef]
- Chen, Y.; Chou, C.-F. Reversible photodimerization of coumarin derivatives dispersed in poly(vinyl acetate). J. Polym. Sci. Part A Polym. Chem. 1995, 33, 2705–2714. [Google Scholar] [CrossRef]
- Trenor, S.R.; Shultz, A.R.; Love, B.J.; Long, T.E. Coumarins in polymers: From light harvesting to photo-cross-linkable tissue scaffolds. Chem. Rev. 2004, 104, 3059–3077. [Google Scholar] [CrossRef]
- Wheelock, C.E. The Fluoreszence of Some Coumarins. J. Chem. Ed. 1950, 81, 1348–1352. [Google Scholar]
- Kotchapradist, P.; Prachumrak, N.; Sunonnam, T.; Tarsang, R.; Namuangruk, S.; Sudyoadsuk, T.; Keawin, T.; Jungsuttiwong, S.; Promarak, V. N-coumarin derivatives as hole-transporting emitters for high efficiency solution-processed pure green electroluminescent devices. Dyes Pigment. 2015, 112, 227–235. [Google Scholar] [CrossRef]
- Prachumrak, N.; Pojanasopa, S.; Tarsang, R.; Namuangruk, S.; Jungsuttiwong, S.; Keawin, T.; Sudyoadsuk, T.; Promarak, V. Synthesis and characterization of carbazole dendronized coumarin derivatives as solution-processed non-doped emitters and hole-transporters for electroluminescent devices. New J. Chem. 2014, 38, 3282. [Google Scholar] [CrossRef]
- Prachumrak, N.; Potjanasopa, S.; Rattanawan, R.; Namuangruk, S.; Jungsuttiwong, S.; Keawin, T.; Sudyoadsuk, T.; Promarak, V. Coumarin-cored carbazole dendrimers as solution-processed non-doped green emitters for electroluminescent devices. Tetrahedron 2014, 70, 6249–6257. [Google Scholar] [CrossRef]
- Teixeira, E.; Lima, J.C.; Parola, A.J.; Branco, P.S. Incorporation of Coumarin-Based Fluorescent Monomers into Co-Oligomeric Molecules. Polymers 2018, 10, 396. [Google Scholar] [CrossRef]
- Gindre, D.; Iliopoulos, K.; Krupka, O.; Evrard, M.; Champigny, E.; Sallé, M. Coumarin-Containing Polymers for High Density Non-Linear Optical Data Storage. Molecules 2016, 21, 147. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, K.; Krupka, O.; Gindre, D.; Sallé, M. Reversible two-photon optical data storage in coumarin-based copolymers. J. Am. Chem. Soc. 2010, 132, 14343–14345. [Google Scholar] [CrossRef] [PubMed]
- Miluski, P.; Kochanowicz, M.; Zmojda, J.; Dorosz, D. Energy conversion in 7-(Diethylamino)coumarin doped PMMA fluorescent fibre. Opt. Quant. Electron. 2017, 49. [Google Scholar] [CrossRef]
- Albertazzi, L.; Storti, B.; Marchetti, L.; Beltram, F. Delivery and subcellular targeting of dendrimer-based fluorescent pH sensors in living cells. J. Am. Chem. Soc. 2010, 132, 18158–18167. [Google Scholar] [CrossRef]
- Chung, J.W.; Lee, K.; Neikirk, C.; Nelson, C.M.; Priestley, R.D. Photoresponsive coumarin-stabilized polymeric nanoparticles as a detectable drug carrier. Small 2012, 8, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Samanta, P.; Kapat, K.; Maiti, S.; Biswas, G.; Dhara, S.; Dhara, D. pH-labile and photochemically cross-linkable polymer vesicles from coumarin based random copolymer for cancer therapy. J. Colloid Interface Sci. 2019, 555, 132–144. [Google Scholar] [CrossRef]
- Rahimi, S.; Khoee, S.; Ghandi, M. Development of photo and pH dual crosslinked coumarin-containing chitosan nanoparticles for controlled drug release. Carbohydr. Polym. 2018, 201, 236–245. [Google Scholar] [CrossRef]
- Lin, H.-M.; Wang, W.-K.; Hsiung, P.-A.; Shyu, S.-G. Light-sensitive intelligent drug delivery systems of coumarin-modified mesoporous bioactive glass. Acta Biomater. 2010, 6, 3256–3263. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, R.; Zhu, X.; Yan, D. Photo-responsive polymeric micelles. Soft Matter 2014, 10, 6121–6138. [Google Scholar] [CrossRef]
- Wang, H.; Miao, W.; Wang, F.; Cheng, Y. A Self-Assembled Coumarin-Anchored Dendrimer for Efficient Gene Delivery and Light-Responsive Drug Delivery. Biomacromolecules 2018, 19, 2194–2201. [Google Scholar] [CrossRef]
- Pelliccioli, A.P.; Wirz, J. Photoremovable protecting groups: Reaction mechanisms and applications. Photochem. Photobiol. Sci. 2002, 1, 441–458. [Google Scholar] [CrossRef] [PubMed]
- Klán, P.; Šolomek, T.; Bochet, C.G.; Blanc, A.; Givens, R.; Rubina, M.; Popik, V.; Kostikov, A.; Wirz, J. Photoremovable protecting groups in chemistry and biology: Reaction mechanisms and efficacy. Chem. Rev. 2013, 113, 119–191. [Google Scholar] [CrossRef] [PubMed]
- Stegmaier, P.; Alonso, J.M.; Campo, A.D. Photoresponsive surfaces with two independent wavelength-selective functional levels. Langmuir 2008, 24, 11872–11879. [Google Scholar] [CrossRef] [PubMed]
- Wylie, R.G.; Shoichet, M.S. Two-photon micropatterning of amines within an agarose hydrogel. J. Mater. Chem. 2008, 18, 2716–2721. [Google Scholar] [CrossRef]
- San Miguel, V.; Bochet, C.G.; del Campo, A. Wavelength-selective caged surfaces: How many functional levels are possible? J. Am. Chem. Soc. 2011, 133, 5380–5388. [Google Scholar] [CrossRef]
- Bowen, A.M.; Ritchey, J.A.; Moore, J.S.; Nuzzo, R.G. Programmable chemical gradient patterns by soft grayscale lithography. Small 2011, 7, 3350–3362. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Miguel, V.S.; del Campo, A. Light-triggered multifunctionality at surfaces mediated by photolabile protecting groups. Macromol. Rapid Commun. 2013, 34, 310–329. [Google Scholar] [CrossRef]
- Schmidt, R.; Geissler, D.; Hagen, V.; Bendig, J. Mechanism of photocleavage of (coumarin-4-yl)methyl esters. J. Phys. Chem. A 2007, 111, 5768–5774. [Google Scholar] [CrossRef]
- Furuta, T.; Wang, S.S.-H.; Dantzker, J.L.; Dore, T.M.; Bybee, W.J.; Callaway, E.M.; Denk, W.; Tsien, R.Y. Brominated 7-hydroxycoumarin-4-ylmethyls: Photolabile protecting groups with biologically useful cross-sections for two photon photolysis. Proc. Natl. Acad. Sci. USA 1999, 96, 1193–1200. [Google Scholar] [CrossRef]
- Olson, J.P.; Kwon, H.-B.; Takasaki, K.T.; Chiu, C.Q.; Higley, M.J.; Sabatini, B.L.; Ellis-Davies, G.C.R. Optically selective two-photon uncaging of glutamate at 900 nm. J. Am. Chem. Soc. 2013, 135, 5954–5957. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Boinapally, S.; Katan, C.; Abe, M. Synthesis and photochemical reactivity of caged glutamates with a π-extended coumarin chromophore as a photolabile protecting group. Tetrahedron Lett. 2013, 54, 7171–7174. [Google Scholar] [CrossRef]
- Chitose, Y.; Abe, M.; Furukawa, K.; Katan, C. Design, Synthesis, and Reaction of π-Extended Coumarin-based New Caged Compounds with Two-photon Absorption Character in the Near-IR Region. Chem. Lett. 2016, 45, 1186–1188. [Google Scholar] [CrossRef]
- Bendig, J.; Helm, S.; Hagen, V. (Coumarin-4-yl)Methyl Ester of cGMP and 8-Br-cGMP: Photochemical Fluorescence Enhancement. J. Fluoresc. 1997, 7, 357–361. [Google Scholar] [CrossRef]
- Schade, B.; Hagen, V.; Schmidt, R.; Herbrich, R.; Krause, E.; Eckardt, T.; Bendig, J. Deactivation Behavior and Excited-State Properties of (Coumarin-4-yl)methyl Derivatives. 1. Photocleavage of (7-Methoxycoumarin-4-yl)methyl-Caged Acids with Fluorescence Enhancement. J. Org. Chem. 1999, 64, 9109–9117. [Google Scholar] [CrossRef]
- Eckardt, T.; Hagen, V.; Schade, B.; Schmidt, R.; Schweitzer, C.; Bendig, J. Deactivation behavior and excited-state properties of (coumarin-4-yl)methyl derivatives. 2. Photocleavage of selected (coumarin-4-yl)methyl-caged adenosine cyclic 3’,5’-monophosphates with fluorescence enhancement. J. Org. Chem. 2002; 67, 703–710. [Google Scholar] [CrossRef]
- Schmidt, R.; Geissler, D.; Hagen, V.; Bendig, J. Kinetics study of the photocleavage of (coumarin-4-yl)methyl esters. J. Phys. Chem. A 2005, 109, 5000–5004. [Google Scholar] [CrossRef]
- Senda, N.; Momotake, A.; Nishimura, Y.; Arai, T. Synthesis and Photochemical Properties of a New Water-Soluble Coumarin, Designed as a Chromophore for Highly Water-Soluble and Photolabile Protecting Group. BCSJ 2006, 79, 1753–1757. [Google Scholar] [CrossRef]
- Sinkel, C.; Greiner, A.; Agarwal, S. A Polymeric Drug Depot Based on 7-(2′-Methacryloyloxyethoxy)-4-methylcoumarin Copolymers for Photoinduced Release of 5-Fluorouracil Designed for the Treatment of Secondary Cataracts. Macromol. Chem. Phys. 2010, 211, 1857–1867. [Google Scholar] [CrossRef]
- López-Vilanova, L.; Martinez, I.; Corrales, T.; Catalina, F. Photoreversible crosslinking of poly-(ethylene-butyl-acrylate) copolymers functionalized with coumarin chromophores using microwave methodology. React. Funct. Polym. 2014, 85, 28–35. [Google Scholar] [CrossRef][Green Version]
- Jellali, R.; Alexandre, M.; Jérôme, C. Photosensitive polydimethylsiloxane networks for adjustable-patterned films. Polym. Chem. 2017, 8, 2499–2508. [Google Scholar] [CrossRef]
- Jellali, R.; Bertrand, V.; Alexandre, M.; Rosière, N.; Grauwels, M.; de Pauw-Gillet, M.-C.; Jérôme, C. Photoreversibility and Biocompatibility of Polydimethylsiloxane-Coumarin as Adjustable Intraocular Lens Material. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef]
- Govindarajan, S.R.; Jain, T.; Choi, J.-W.; Joy, A.; Isayeva, I.; Vorvolakos, K. A hydrophilic coumarin-based polyester for ambient-temperature initiator-free 3D printing: Chemistry, rheology and interface formation. Polymer 2018, 152, 9–17. [Google Scholar] [CrossRef]
- Hughes, T.; Simon, G.P.; Saito, K. Photocuring of 4-arm coumarin-functionalised monomers to form highly photoreversible crosslinked epoxy coatings. Polym. Chem. 2019, 10, 2134–2142. [Google Scholar] [CrossRef]
- Abdollahi, A.; Roghani-Mamaqani, H.; Herizchi, A.; Alidaei-Sharif, H.; Enayati, A.; Sajedi-Amin, S. Light-induced spherical to dumbbell-like morphology transition of coumarin-functionalized latex nanoparticles by a [2π + 2π] cycloaddition reaction: A fast and facile strategy to anisotropic geometry. Polym. Chem. 2020, 11, 2053–2069. [Google Scholar] [CrossRef]
- Ling, J.; Rong, M.Z.; Zhang, M.Q. Coumarin imparts repeated photochemical remendability to polyurethane. J. Mater. Chem. 2011, 21, 18373. [Google Scholar] [CrossRef]
- Ling, J.; Rong, M.Z.; Zhang, M.Q. Photo-stimulated self-healing polyurethane containing dihydroxyl coumarin derivatives. Polymer 2012, 53, 2691–2698. [Google Scholar] [CrossRef]
- Aguirresarobe, R.H.; Martin, L.; Aramburu, N.; Irusta, L.; Fernandez-Berridi, M.J. Coumarin based light responsive healable waterborne polyurethanes. Prog. Org. Coat. 2016, 99, 314–321. [Google Scholar] [CrossRef]
- Wagner, N.; Theato, P. Light-induced wettability changes on polymer surfaces. Polymer 2014, 55, 3436–3453. [Google Scholar] [CrossRef]
- Sato, E.; Nagai, S.; Matsumoto, A. Reversible thickness control of polymer thin films containing photoreactive coumarin derivative units. Prog. Org. Coat. 2013, 76, 1747–1751. [Google Scholar] [CrossRef]
- He, J.; Zhao, Y. Light-responsive polymer micelles, nano- and microgels based on the reversible photodimerization of coumarin. Dye. Pigment. 2011, 89, 278–283. [Google Scholar] [CrossRef]
- Li, J.; Jiang, H.; Hu, W.; Xia, H.; Zou, G.; Zhang, Q. Photo-controlled hierarchical assembly and fusion of coumarin-containing polydiacetylene vesicles. Macromol. Rapid Commun. 2013, 34, 274–279. [Google Scholar] [CrossRef]
- Wen, Y.; Song, Y.; Zhao, D.; Ding, K.; Bian, J.; Zhang, X.; Wang, J.; Liu, Y.; Jiang, L.; Zhu, D. Highly regio- and enantioselective thermal 2 + 2 cycloaddition of coumarin in a crystalline inclusion complex under high vacuum. Chem. Commun. 2005, 2732–2734. [Google Scholar] [CrossRef] [PubMed]
- Goodall, G.W.; Hayes, W. Advances in cycloaddition polymerizations. Chem. Soc. Rev. 2006, 35, 280–312. [Google Scholar] [CrossRef] [PubMed]
- Woodward, R.B.; Hoffmann, R. The Conservation of Orbital Symmetry. Angew. Chem. Int. Ed. 1969, 8, 781–932. [Google Scholar] [CrossRef]
- Crimmins, M.T.; Reinhold, T.L. Organic Reactions: Enone olefin [2 + 2] photochemical cycloadditions. Org. React. 2004, 44, 297–588. [Google Scholar]
- Belfield, K.D.; Bondar, M.V.; Liu, Y.; Przhonska, O.V. Photophysical and photochemical properties of 5,7-dimethoxycoumarin under one- and two-photon excitation. J. Phys. Org. Chem. 2003, 16, 69–78. [Google Scholar] [CrossRef]
- Schuster, D.I.; Lem, G.; Kaprinidis, N.A. New insights into an old mechanism: [2 + 2] photocycloaddition of enones to alkenes. Chem. Rev. 1993, 93, 3–22. [Google Scholar] [CrossRef]
- Brimioulle, R.; Bauer, A.; Bach, T. Enantioselective Lewis Acid Catalysis in Intramolecular 2 + 2 Photocycloaddition Reactions: A Mechanistic Comparison between Representative Coumarin and Enone Substrates. J. Am. Chem. Soc. 2015, 137, 5170–5176. [Google Scholar] [CrossRef]
- Wolff, T.; Görner, H. Photocleavage of dimers of coumarin and 6-alkylcoumarins. J. Photochem. Photobiol. A 2010, 209, 219–223. [Google Scholar] [CrossRef]
- Jiang, M.; Paul, N.; Bieniek, N.; Buckup, T.; Hampp, N.; Motzkus, M. Photocleavage of coumarin dimers studied by femtosecond UV transient absorption spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 4597–4606. [Google Scholar] [CrossRef]
- Maddipatla, M.V.S.N.; Wehrung, D.; Tang, C.; Fan, W.; Oyewumi, M.O.; Miyoshi, T.; Joy, A. Photoresponsive Coumarin Polyesters That Exhibit Cross-Linking and Chain Scission Properties. Macromolecules 2013, 46, 5133–5140. [Google Scholar] [CrossRef]
- Fouassier, J.-P.; Morlet-Savary, F.; Lalevée, J.; Allonas, X.; Ley, C. Dyes as Photoinitiators or Photosensitizers of Polymerization Reactions. Materials 2010, 3, 5130–5142. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Hu, P.; Zhu, J.; Liu, R.; Li, Z.; Hu, Z.; Chen, Q.; Dietliker, K.; Liska, R. Cleavable Unimolecular Photoinitiators Based on Oxime-Ester Chemistry for Two-Photon Three-Dimensional Printing. ChemPhotoChem 2019, 3, 1090–1094. [Google Scholar] [CrossRef]
- Abdallah, M.; Hijazi, A.; Graff, B.; Fouassier, J.-P.; Rodeghiero, G.; Gualandi, A.; Dumur, F.; Cozzi, P.G.; Lalevée, J. Coumarin derivatives as versatile photoinitiators for 3D printing, polymerization in water and photocomposite synthesis. Polym. Chem. 2019, 10, 872–884. [Google Scholar] [CrossRef]
- Abdallah, M.; Dumur, F.; Hijazi, A.; Rodeghiero, G.; Gualandi, A.; Cozzi, P.G.; Lalevée, J. Keto-coumarin scaffold for photoinitiators for 3D printing and photocomposites. J. Polym. Sci. 2020, 58, 1115–1129. [Google Scholar] [CrossRef]
- Cumpston, B.H.; Ananthavel, S.P.; Barlow, S.; Dyer, D.L.; Ehrlich, J.E.; Erskine, L.L.; Heikal, A.A.; Kuebler, S.M.; Lee, I.Y.S.; McCord-Maughon, D.; et al. Two-photon polymerization initiators for three-dimensional optical data storage and microfabrication. Nature 1999, 398, 51–54. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, Y.; Li, Y. Photoexcitation mechanisms of new D-π-A coumarin derivatives in linear and nonlinear optical processes. J. Mater. Sci. 2016, 27, 7132–7140. [Google Scholar] [CrossRef]
- Xue, J.; Zhao, Y.; Wu, F.; Fang, D.-C. Effect of bridging position on the two-photon polymerization initiating efficiencies of novel coumarin/benzylidene cyclopentanone dyes. J. Phys. Chem. A 2010, 114, 5171–5179. [Google Scholar] [CrossRef]
- Whitby, R.; Kay, A.; Simpson, M.C. Triphenylamine two-photon photoinitiators for 3D laser microfabrication. In Three-Dimensional Microfabrication Using Two-Photon Polymerization; Elsevier: Amsterdam, The Netherlands, 2020; pp. 101–141. ISBN 9780128178270. [Google Scholar]
- Nazir, R.; Danilevicius, P.; Ciuciu, A.I.; Chatzinikolaidou, M.; Gray, D.; Flamigni, L.; Farsari, M.; Gryko, D.T. π-Expanded Ketocoumarins as Efficient, Biocompatible Initiators for Two-Photon-Induced Polymerization. Chem. Mater. 2014, 26, 3175–3184. [Google Scholar] [CrossRef]
- Li, Z.; Zou, X.; Zhu, G.; Liu, X.; Liu, R. Coumarin-Based Oxime Esters: Photobleachable and Versatile Unimolecular Initiators for Acrylate and Thiol-Based Click Photopolymerization under Visible Light-Emitting Diode Light Irradiation. ACS Appl. Mater. Interfaces 2018, 10, 16113–16123. [Google Scholar] [CrossRef]
- Zhou, R.; Malval, J.-P.; Jin, M.; Spangenberg, A.; Pan, H.; Wan, D.; Morlet-Savary, F.; Knopf, S. A two-photon active chevron-shaped type I photoinitiator designed for 3D stereolithography. Chem. Commun. 2019, 55, 6233–6236. [Google Scholar] [CrossRef]
- Li, Z.; Zou, X.; Shi, F.; Liu, R.; Yagci, Y. Highly efficient dandelion-like near-infrared light photoinitiator for free radical and thiol-ene photopolymerizations. Nat. Commun. 2019, 10, 3560. [Google Scholar] [CrossRef] [PubMed]
- Senyurt, A.F.; Hoyle, C.E. Three component ketocoumarin, amine, maleimide photoinitiator II. Eur. Polym. J. 2006, 42, 3133–3139. [Google Scholar] [CrossRef]
- Zivic, N.; Bouzrati-Zerelli, M.; Kermagoret, A.; Dumur, F.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Photocatalysts in Polymerization Reactions. ChemCatChem 2016, 8, 1617–1631. [Google Scholar] [CrossRef]
- Garra, P.; Graff, B.; Morlet-Savary, F.; Dietlin, C.; Becht, J.-M.; Fouassier, J.-P.; Lalevée, J. Charge Transfer Complexes as Pan-Scaled Photoinitiating Systems: From 50 μm 3D Printed Polymers at 405 nm to Extremely Deep Photopolymerization (31 cm). Macromolecules 2018, 51, 57–70. [Google Scholar] [CrossRef]
- Jones, G.; Jackson, W.R.; Choi, C.; Bergmark, W.R. Solvent Effects on Emission Yleld and Llfetime for Coumarin Laser Dyes. Requirements for a Rotatory Decay Mechanism. J. Phys. Chem. 1985, 294–300. [Google Scholar]
- Wagner, B.D. The use of coumarins as environmentally-sensitive fluorescent probes of heterogeneous inclusion systems. Molecules 2009, 14, 210–237. [Google Scholar] [CrossRef]
- Song, P.S.; Gordon, W.H. A Spectroscopic Study of the Excited States of Coumarin. J. Phys. Chem. 1970, 72, 4234–4240. [Google Scholar] [CrossRef]
- Hoshiyama, M.; Kubob, K.; Igarashi, T.; Sakurai, T. Complexation and proton dissociation behavior of 7-hydroxy-4-methylcoumarin and related compounds in the presence of β-cyclodextrin. J. Photochem. Photobiol. A 2001, 138, 227–233. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, F.; Shen, K.; Ren, Y.; Li, Y. Dendritic effects on photophysical and fluorescence properties of coumarin functionalized dendrigraft polybutadiene. Polymer 2014, 55, 1202–1208. [Google Scholar] [CrossRef]
- Tocco, G.; Carbonaro, C.M.; Meli, G.; Podda, G. Evaluation of photoluminescence properties of some poly(ethylene glycol)-supported coumarin derivatives. Molecules 2009, 14, 1044–1055. [Google Scholar] [CrossRef]
- Goonewardena, S.N.; Leroueil, P.R.; Gemborys, C.; Tahiliani, P.; Emery, S.; Baker, J.R.; Zong, H. Fluorogenic ‘click-on’ dendrimer reporter for rapid profiling of cell proliferation. Bioorg. Med. Chem. Lett. 2013, 23, 2230–2233. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Wang, L.; Li, H.; Zhou, J.; Cao, D. Synthesis of coumarin-containing conjugated polymer for naked-eye detection of DNA and cellular imaging. Sens. Actuators B 2013, 181, 234–243. [Google Scholar] [CrossRef]
- Behl, G.; Sikka, M.; Chhikara, A.; Chopra, M. PEG-coumarin based biocompatible self-assembled fluorescent nanoaggregates synthesized via click reactions and studies of aggregation behavior. J. Colloid Interface Sci. 2014, 416, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.-J.; Huong, D.T.; Han, J.-H.; Kim, J.-Y.; Lee, W.-J.; Shin, H.-J.; Han, E.-T.; Park, H. Performance of coumarin-derived dendrimer-based fluorescence-linked immunosorbent assay (FLISA) to detect malaria antigen. Malar. J. 2014, 13, 266. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.-J.; Huong, D.T.; Hong, N.N.; Li, C.-Y.; Choi, K.; Yu, K.; Choi, D.-Y.; Chong, C.-K.; Choi, H.S.; Mallik, S.K.; et al. Rapid and quantitative detection of zoonotic influenza A virus infection utilizing coumarin-derived dendrimer-based fluorescent immunochromatographic strip test (FICT). Theranostics 2014, 4, 1239–1249. [Google Scholar] [CrossRef][Green Version]
- Lalitha, K.; Nagarajan, S. Strongly fluorescent organogels and self-assembled nanostructures from pyrene coupled coumarin derivatives: Application in cell imaging. J. Mater. Chem. B 2015, 3, 5690–5701. [Google Scholar] [CrossRef]
- Hande, P.E.; Samui, A.B.; Kulkarni, P.S. Selective nanomolar detection of mercury using coumarin based fluorescent Hg(II)—Ion imprinted polymer. Sens. Actuators B 2017, 246, 597–605. [Google Scholar] [CrossRef]
- McFadden, P.D.; Frederick, K.; Argüello, L.A.; Zhang, Y.; Vandiver, P.; Odegaard, N.; Loy, D.A. UV fluorescent epoxy adhesives from non-covalent and covalent incorporation of coumarin dyes. ACS Appl. Mater. Interfaces 2017, 9, 10061–10068. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, H.; Guo, M.; Du, L.; Liu, G.; Wang, P. Synthesis of polymeric fluorescent brightener based on coumarin and its performances on paper as light stabilizer, fluorescent brightener and surface sizing agent. Appl. Surf. Sci. 2016, 367, 167–173. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, H.; Liu, G.; Wang, P.; Xiang, R. Synthesis and Application of a Multifunctional Fluorescent Polymer Based on Coumarin. BioResources 2016, 11, 373–385. [Google Scholar] [CrossRef][Green Version]
- Duong, H.D.; Shin, Y.; Rhee, J.I. Development of novel optical pH sensors based on coumarin 6 and nile blue A encapsulated in resin particles and specific support materials. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110323. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Sun, T.; Grattan, K.T.V. Novel coumarin-based pH sensitive fluorescent probes for the highly alkaline pH region. Dye. Pigment. 2020, 177, 108312. [Google Scholar] [CrossRef]
- Salbeck, J. Electroluminescence with Organic Compounds. Ber. Bunsenges. Phys. Chem. 1996, 100, 1667–1677. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, Z.-y.; Peng, Q.; Jiang, Q.; Xie, R.-G.; Han, S.-H.; Dong, L.-g.; Peng, J.-B.; Cao, Y.; Xie, M.-G. Luminescent properties of coumarin-doped MEH-PPV and novel coumarin-terminated MEH-PPV. Mater. Chem. Phys. 2005, 93, 95–99. [Google Scholar] [CrossRef]
- Yiğit, D.; Hacioglu, S.O.; Güllü, M.; Toppare, L. Synthesis and spectroelectrochemical characterization of multi-colored novel poly(3,6-dithienylcarbazole) derivatives containing azobenzene and coumarin chromophore units. Electrochim. Acta 2016, 196, 140–152. [Google Scholar] [CrossRef]
- Webber, S.E. Photon-Harvesting Polymers. Chem. Rev. 1990, 90, 1469–1483. [Google Scholar] [CrossRef]
- Lang, J.M.; Drickamer, H.G. High-pressure Study of Energy Transfer between Coumarin 138 and Rhodamine B in a Solid Polymeric Matrix. J. Phys. Chem. 1993, 97, 5058–5064. [Google Scholar] [CrossRef]
- Chen, M.; Ghiggino, K.P.; Mau, A.W.H.; Rizzardo, E.; Sasse, W.H.F.; Thang, S.H.; Wilson, G.J. Synthesis of Functionalized RAFT Agents for Light Harvesting Macromolecules. Macromolecules 2004, 37, 5479–5481. [Google Scholar] [CrossRef]
- Hania, P.R.; Heijs, D.J.; Bowden, T.; Pugžlys, A.; van Esch, J.; Knoester, J.; Duppen, K. Ultrafast Energy Transport in a First-Generation Coumarin−Tetraphenylporphyrin Dendrimer. J. Phys. Chem. B 2004, 108, 71–81. [Google Scholar] [CrossRef]
- Augulis, R.; Pugzlys, A.; Hurenkamp, J.H.; Feringa, B.L.; van Esch, J.H.; van Loosdrecht, P.H.M. Optical energy transport and interactions between the excitations in a coumarin-perylene bisimide dendrimer. J. Phys. Chem. A 2007, 111, 12944–12953. [Google Scholar] [CrossRef]
- Aydinli, M.; Tutaş, M.; Atasoy, B.; Bozdemir, Ö.A. Synthesis and characterization of poly(aryl ether) dendritic structures functionalized with coumarin derivatives. React. Funct. Polym. 2005, 65, 317–327. [Google Scholar] [CrossRef]
- Mao, M.; Song, Q.-H. Non-conjugated dendrimers with a porphyrin core and coumarin chromophores as peripheral units: Synthesis and photophysical properties. Dye. Pigment. 2012, 92, 975–981. [Google Scholar] [CrossRef]
- Cavero, E.; Serrano, J.L.; Giménez, R.; Piñol, M. Liquid crystalline dendrimers based on cinnamates and coumarins. Liq. Cryst. 2016, 43, 1408–1421. [Google Scholar] [CrossRef]
- Bucoş, M.; Sierra, T.; Golemme, A.; Termine, R.; Barberá, J.; Giménez, R.; Serrano, J.L.; Romero, P.; Marcos, M. Multifunctional supramolecular dendrimers with an s-triazine ring as the central core. Liquid crystalline, fluorescence and photoconductive properties. Chem. A Eur. J. 2014, 10027–10037. [Google Scholar] [CrossRef]
- Concellón, A.; Bucoş, M.; Serrano, J.L.; Romero, P.; Marcos, M. Supramolecular liquid crystalline dendrimers with a porphyrin core and functional carboxylic acid dendrons. RSC Adv. 2016, 6, 65179–65185. [Google Scholar] [CrossRef]
- Concellón, A.; Marcos, M.; Romero, P.; Serrano, J.L.; Termine, R.; Golemme, A. Not Only Columns: High Hole Mobility in a Discotic Nematic Mesophase Formed by Metal-Containing Porphyrin-Core Dendrimers. Angew. Chem. 2017, 129, 1279–1283. [Google Scholar] [CrossRef]
- Concellón, A.; Termine, R.; Golemme, A.; Romero, P.; Marcos, M.; Serrano, J.L. High hole mobility and light-harvesting in discotic nematic dendrimers prepared via ‘click’ chemistry. J. Mater. Chem. C 2019, 7, 2911–2918. [Google Scholar] [CrossRef]
- Parker, L. The World’s Plastic Pollution Crisis Explained. Available online: https://www.nationalgeographic.com/environment/habitats/plastic-pollution/ (accessed on 26 November 2020).
- Bozell, J.J.; Patel, M.K. Feedstocks for the Future: Renewables for the Production of Chemicals and Materials; ACS Symposium Series; American Chemical Societ: Washington, DC, USA, 2006. [Google Scholar]
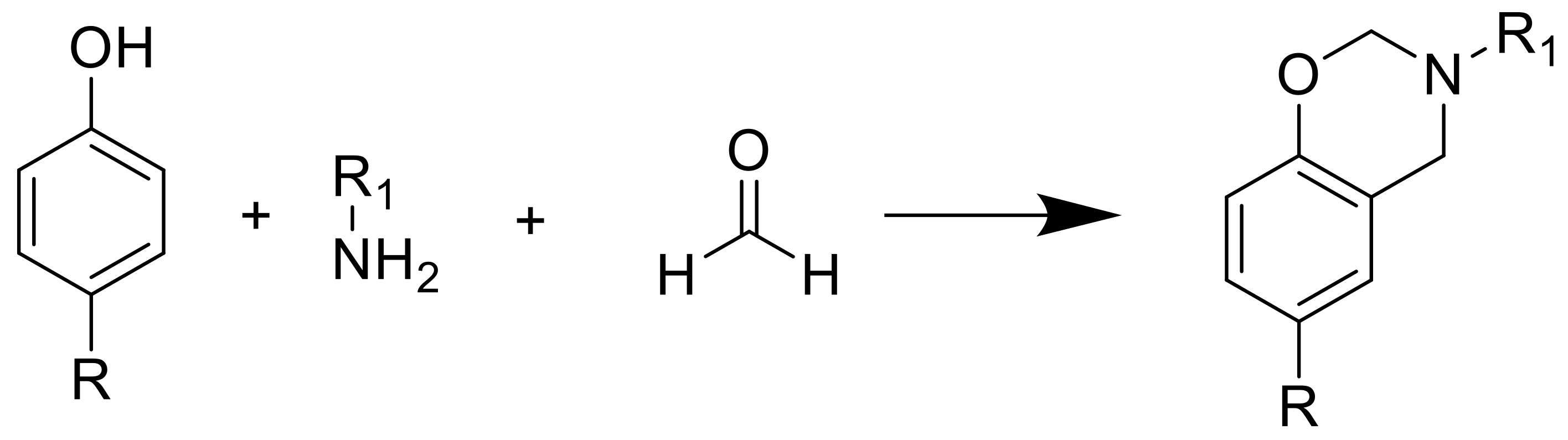
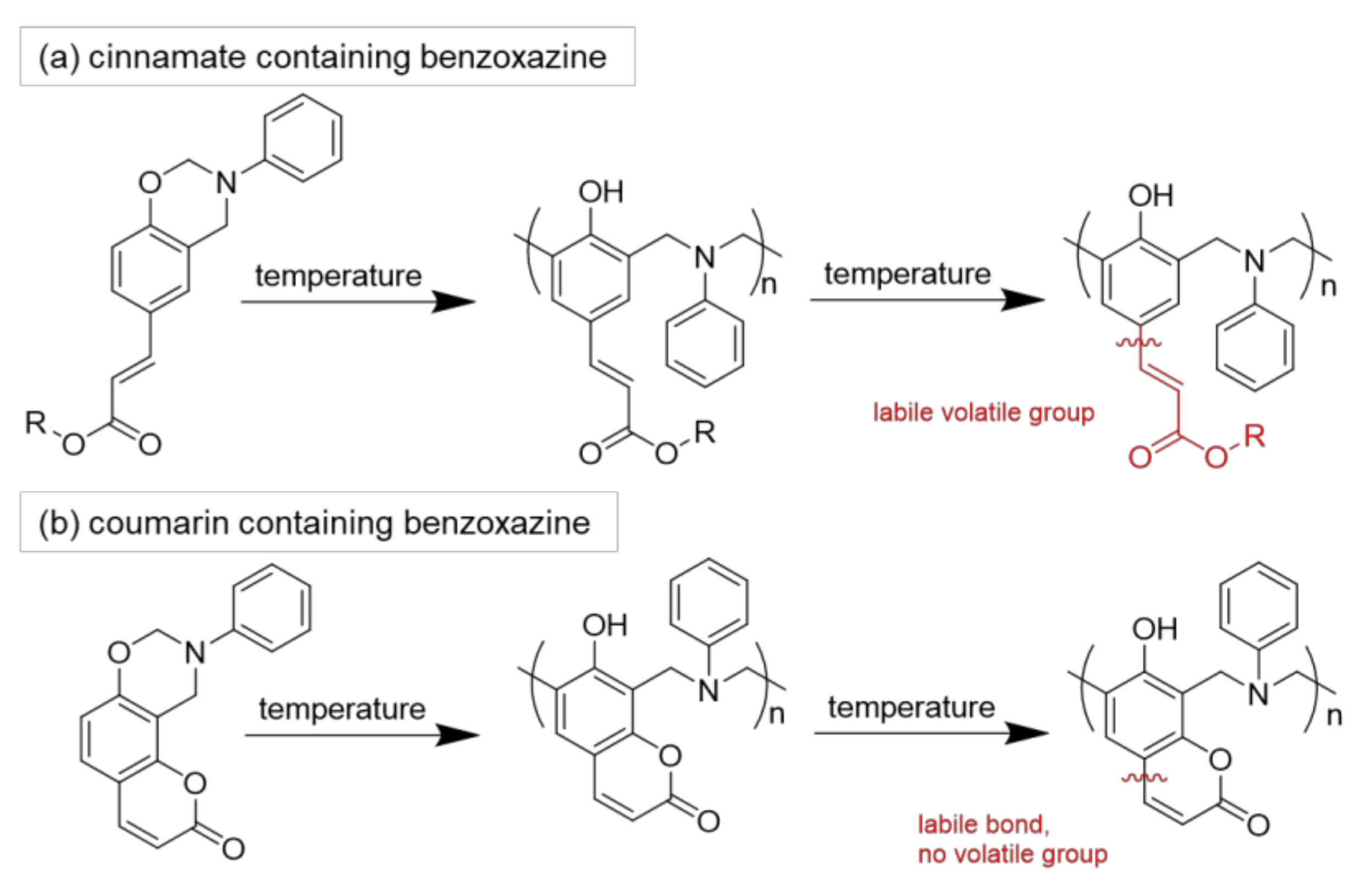
- Chiou, K.; Ishida, H. Incorporation of Natural Renewable Components and Waste Byproducts to Benzoxazine Based High Performance Materials. COC 2013, 17, 913–925. [Google Scholar] [CrossRef]
- Comí, M.; Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Renewable benzoxazine monomers from “lignin-like” naturally occurring phenolic derivatives. J. Polym. Sci. Part A 2013, 51, 4894–4903. [Google Scholar] [CrossRef]
- Arza, R.C.; Froimowicz, P.; Ishida, H. Smart chemical design incorporating umbelliferone as natural renewable resource toward the preparation of thermally stable thermosets materials based on benzoxazine chemistry. RSC Adv. 2015, 5, 97855–97861. [Google Scholar] [CrossRef]
- Froimowicz, P.; Arza, R.C.; Ohashi, S.; Ishida, H. Tailor-made and chemically designed synthesis of coumarin-containing benzoxazines and their reactivity study toward their thermosets. J. Polym. Sci. Part A 2016, 54, 1428–1435. [Google Scholar] [CrossRef]
- Froimowicz, P.; Arza, R.C.; Han, L.; Ishida, H. Smart, Sustainable, and Ecofriendly Chemical Design of Fully Bio-Based Thermally Stable Thermosets Based on Benzoxazine Chemistry. ChemSusChem 2016, 9, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, L.; Zhang, H.; Tang, Z. Synthesis and curing performance of a novel bio-based epoxy monomer from soybean oil. Eur. J. Lipid Sci. Technol. 2017, 119, 1600429. [Google Scholar] [CrossRef]
- Cai, X.; Li, C.; Qiao, C.; Peng, D. Renewable Coumarin-Derived Network as a Toughening Structure for Petroleum-Based Epoxy Resins. ACS Omega 2019, 4, 16080–16087. [Google Scholar] [CrossRef] [PubMed]
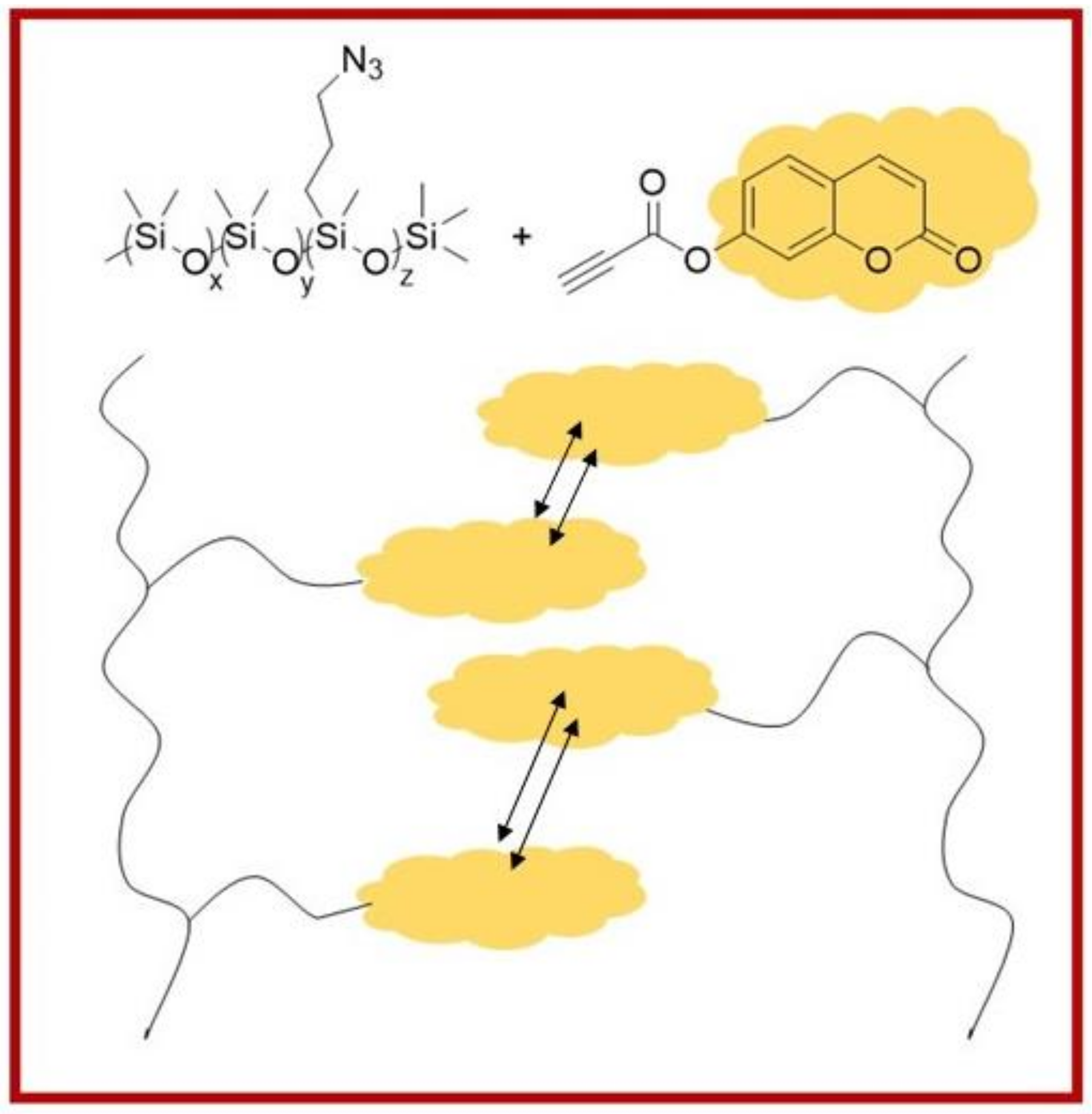
- Fawcett, A.S.; Brook, M.A. Thermoplastic Silicone Elastomers through Self-Association of Pendant Coumarin Groups. Macromolecules 2014, 47, 1656–1663. [Google Scholar] [CrossRef]
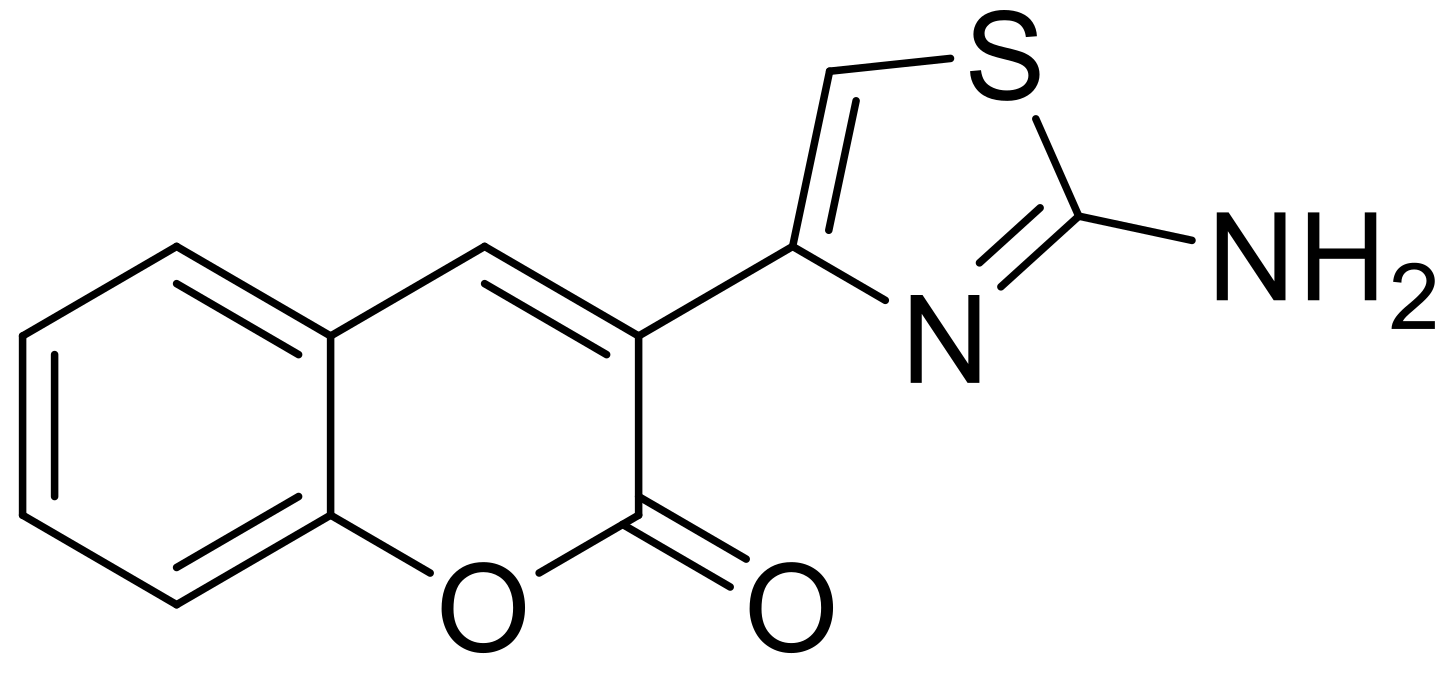
- El-Wahab, H.A.; El-Fattah, M.A.; El-Khalik, N.A.; Nassar, H.S.; Abdelall, M.M. Synthesis and characterization of coumarin thiazole derivative 2-(2-amino-1,3-thiazol-4-yl)-3H-benzo[f]chromen-3-one with anti-microbial activity and its potential application in antimicrobial polyurethane coating. Prog. Org. Coat. 2014, 77, 1506–1511. [Google Scholar] [CrossRef]
- Abd El-Fattah, M.; Abd El-Wahab, H.; Bashandy, M.S.; El-Eisawy, R.A.; Abd El-hai, F.; Saeed, M. Potential application of some coumarin derivatives incorporated thiazole ring as ecofriendly antimicrobial, flame retardant and corrosion inhibitor additives for polyurethane coating. Prog. Org. Coat. 2017, 111, 57–66. [Google Scholar] [CrossRef]
- Singh, L.R.; Avula, S.R.; Raj, S.; Srivastava, A.; Palnati, G.R.; Tripathi, C.K.M.; Pasupuleti, M.; Sashidhara, K.V. Coumarin-benzimidazole hybrids as a potent antimicrobial agent: Synthesis and biological elevation. J. Antibiot. 2017, 70, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Bhattacharya, K.; Sullivan, M.; Walsh, M.; Creaven, B.S.; Laffir, F.; Duffy, B.; McHale, P. Non-cytotoxic antibacterial silver-coumarin complex doped sol-gel coatings. Colloids Surf. B Biointerfaces 2013, 102, 412–419. [Google Scholar] [CrossRef]
- Vogl, O.; Albertsso, A.C.; Janovic, Z. New developments in speciality polymers: Polymeric stabilizers. Polymer 1985, 1288–1296. [Google Scholar] [CrossRef]
- Yen, T.F.; Davar, M.; Rembaum, A. Potentialities of a New Class of Anticlotting and Antihemorrhagic Polymers. J. Macromol. Sci. 1970, 4, 693–714. [Google Scholar] [CrossRef]
- Pandey, M.K.; Balwani, S.; Sharma, P.K.; Parmar, V.S.; Ghosh, B.; Watterson, A.C. Design, synthesis and anti-inflammatory evaluation of PEGylated 4-methyl and 4,8-dimethylcoumarins. Eur. J. Pharm. Sci. 2010, 39, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Kancheva, V.D.; Saso, L.; Boranova, P.V.; Khan, A.; Saroj, M.K.; Pandey, M.K.; Malhotra, S.; Nechev, J.Z.; Sharma, S.K.; Prasad, A.K.; et al. Structure-activity relationship of dihydroxy-4-methylcoumarins as powerful antioxidants: Correlation between experimental & theoretical data and synergistic effect. Biochimie 2010, 92, 1089–1100. [Google Scholar] [CrossRef]
- Pandey, M.K.; Tyagi, R.; Tomar, S.; Kumar, J.; Parmar, V.S.; Watterson, A.C. Design and Synthesis of Novel Pegylated 4-Methylcoumarins. J. Macromol. Sci. Part A 2007, 44, 1293–1298. [Google Scholar] [CrossRef]
- van Dijk, M.; Rijkers, D.T.S.; Liskamp, R.M.J.; van Nostrum, C.F.; Hennink, W.E. Synthesis and applications of biomedical and pharmaceutical polymers via click chemistry methodologies. Bioconjug. Chem. 2009, 20, 2001–2016. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer 2006, 6, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. Engl. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Wu, P.; Malkoch, M.; Hunt, J.N.; Vestberg, R.; Kaltgrad, E.; Finn, M.G.; Fokin, V.V.; Sharpless, K.B.; Hawker, C.J. Multivalent, bifunctional dendrimers prepared by click chemistry. Chem. Commun. 2005, 46, 5775–5777. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, K.; Prasad, Y.S.; Maheswari, C.U.; Sridharan, V.; John, G.; Nagarajan, S. Stimuli responsive hydrogels derived from a renewable resource: Synthesis, self-assembly in water and application in drug delivery. J. Mater. Chem. B 2015, 3, 5560–5568. [Google Scholar] [CrossRef]
- Danilovtseva, E.N.; Pal’shin, V.A.; Krishnan, U.M.; Annenkov, V.V.; Zelinskiy, S.N. Tagging synthetic polymers with coumarin group for study nucleic acid interaction with gene delivery agents. MethodsX 2019, 6, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Yang, M.; Duan, Y. Chemistry, biology, and medicine of fluorescent nanomaterials and related systems: New insights into biosensing, bioimaging, genomics, diagnostics, and therapy. Chem. Rev. 2014, 114, 6130–6178. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Mahmood, U. Molecular Imaging. Radiology 2001, 219, 316–333. [Google Scholar] [CrossRef] [PubMed]
- Ballou, B.; Ernst, L.A.; Waggoner, A.S. Fluorescence Imaging of Tumors In Vivo. Curr. Med. Chem. 2005, 12, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Naylor, A.M.; Goddard, W.A. Starburst Dendrimers: Molecular-Level Control of Size, Shape, Surface Chemistry, Topology, and Flexibility from Atoms to Macroscopic Matter. Angew. Chem. Int. Ed. Engl. 1990, 29, 138–175. [Google Scholar] [CrossRef]
- Wool, R.P. Self-healing materials: A review. Soft Matter 2008, 4, 400–418. [Google Scholar] [CrossRef]
- Sitnikov, N.N.; Khabibullina, I.A.; Mashchenko, V.I.; Rizakhanov, R.N. Prospects of Application of Self-Healing Materials and Technologies Based on Them. Inorg. Mater. Appl. Res. 2018, 9, 785–793. [Google Scholar] [CrossRef]
- Dhanasekaran, R.; Sreenatha Reddy, S.; Sai Kumar, A. Application of Self-Healing Polymers to Overcome Impact, Fatigue and Erosion Damages. Mater. Today 2018, 5, 21373–21377. [Google Scholar] [CrossRef]
- Aïssa, B.; Therriault, D.; Haddad, E.; Jamroz, W. Self-Healing Materials Systems: Overview of Major Approaches and Recent Developed Technologies. Adv. Mater. Sci. Eng. 2012, 2012, 1–17. [Google Scholar] [CrossRef]
- Cordier, P.; Tournilhac, F.; Soulié-Ziakovic, C.; Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 2008, 451, 977–980. [Google Scholar] [CrossRef]
- Kupfer, S.; Zedler, L.; Guthmuller, J.; Bode, S.; Hager, M.D.; Schubert, U.S.; Popp, J.; Gräfe, S.; Dietzek, B. Self-healing mechanism of metallopolymers investigated by QM/MM simulations and Raman spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 12422–12432. [Google Scholar] [CrossRef]
- Blaiszik, B.J.; Kramer, S.L.B.; Olugebefola, S.C.; Moore, J.S.; Sottos, N.R.; White, S.R. Self-Healing Polymers and Composites. Annu. Rev. Mater. Res. 2010, 40, 179–211. [Google Scholar] [CrossRef]
- Bergman, S.D.; Wudl, F. Mendable polymers. J. Mater. Chem. 2008, 18, 41–62. [Google Scholar] [CrossRef]
- Cardenas-Daw, C.; Kroeger, A.; Schaertl, W.; Froimowicz, P.; Landfester, K. Reversible Photocycloadditions, a Powerful Tool for Tailoring (Nano)Materials. Macromol. Chem. Phys. 2012, 213, 144–156. [Google Scholar] [CrossRef]
- Habault, D.; Zhang, H.; Zhao, Y. Light-triggered self-healing and shape-memory polymers. Chem. Soc. Rev. 2013, 42, 7244–7256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Q.; Rong, M.Z. Intrinsic self-healing of covalent polymers through bond reconnection towards strength restoration. Polym. Chem. 2013, 4, 4878. [Google Scholar] [CrossRef]
- Tanaka, K. Supramolecular photodimerization of coumarins. Molecules 2012, 17, 1408–1418. [Google Scholar] [CrossRef]
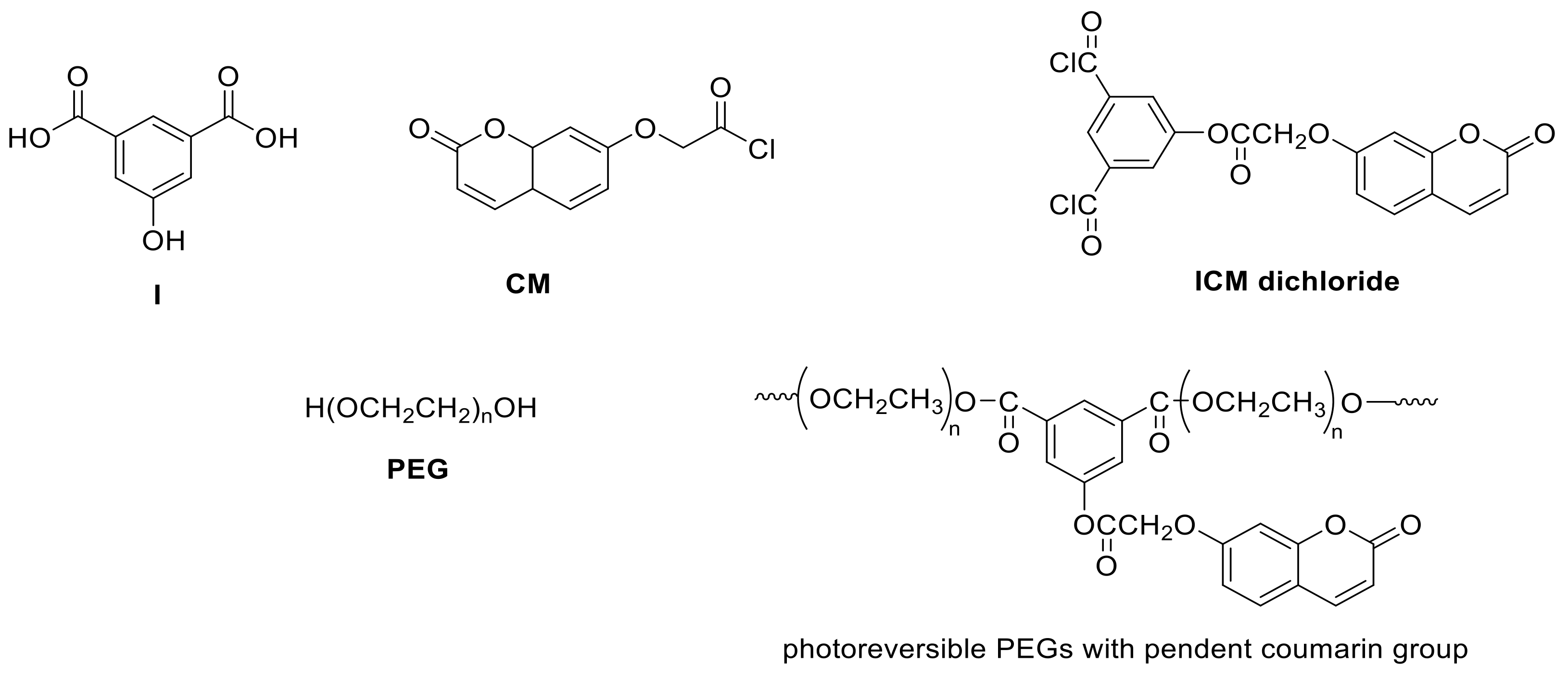
- Nagata, M.; Yamamoto, Y. Photoreversible poly(ethylene glycol)s with pendent coumarin group and their hydrogels. React. Funct. Polym. 2008, 68, 915–921. [Google Scholar] [CrossRef]
- Fawcett, A.S.; Hughes, T.C.; Zepeda-Velazquez, L.; Brook, M.A. Phototunable Cross-Linked Polysiloxanes. Macromolecules 2015, 48, 6499–6507. [Google Scholar] [CrossRef]
- Cuevas, J.M.; Seoane-Rivero, R.; Navarro, R.; Marcos-Fernández, Á. Coumarins into Polyurethanes for Smart and Functional Materials. Polymers 2020, 12, 630. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Li, J.; Ling, L.; Zhang, G.; Sun, R.; Wong, C.P. UV-triggered self-healing polyurethane with enhanced stretchability and elasticity. Polymer 2019, 172, 187–195. [Google Scholar] [CrossRef]
- Abdallh, M.; Hearn, M.T.W.; Simon, G.P.; Saito, K. Light triggered self-healing of polyacrylate polymers crosslinked with 7-methacryloyoxycoumarin crosslinker. Polym. Chem. 2017, 8, 5875–5883. [Google Scholar] [CrossRef]
- Huang, W.M.; Ding, Z.; Wang, C.C.; Wei, J.; Zhao, Y.; Purnawali, H. Shape Memory Materials; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Rose, A.; Zhu, Z.; Madigan, C.F.; Swager, T.M.; Bulović, V. Sensitivity gains in chemosensing by lasing action in organic polymers. Nature 2005, 434, 876–879. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Qi, H.J.; Xie, T. Recent progress in shape memory polymer: New behavior, enabling materials, and mechanistic understanding. Prog. Polym. Sci. 2015, 49-50, 79–120. [Google Scholar] [CrossRef]
- Cho, J.W.; Kim, J.W.; Jung, Y.C.; Goo, N.S. Electroactive Shape-Memory Polyurethane Composites Incorporating Carbon Nanotubes. Macromol. Rapid Commun. 2005, 26, 412–416. [Google Scholar] [CrossRef]
- Schmidt, A.M. Electromagnetic Activation of Shape Memory Polymer Networks Containing Magnetic Nanoparticles. Macromol. Rapid Commun. 2006, 27, 1168–1172. [Google Scholar] [CrossRef]
- Li, G.; Fei, G.; Xia, H.; Han, J.; Zhao, Y. Spatial and temporal control of shape memory polymers and simultaneous drug release using high intensity focused ultrasound. J. Mater. Chem. 2012, 22, 7692. [Google Scholar] [CrossRef]
- Bai, T.; Han, Y.; Zhang, P.; Wang, W.; Liu, W. Zinc ion-triggered two-way macro-/microscopic shape changing and memory effects in high strength hydrogels with pre-programmed unilateral patterned surfaces. Soft Matter 2012, 8, 6846. [Google Scholar] [CrossRef]
- Zheng, D.; Arima, H.; Sato, S.; Gasparrini, A.; Heeley, E.; Delcourt, C.; Lo, S.; Huang, Y.; Wang, J.; Stapf, C.; et al. Low Ambient Temperature and Intracerebral Hemorrhage: The INTERACT2 Study. PLoS ONE 2016, 11, e0149040. [Google Scholar] [CrossRef]
- Miyata, T.; Asami, N.; Uragami, T. A reversibly antigen-responsive hydrogel. Nature 1999, 399, 766–769. [Google Scholar] [CrossRef]
- Lendlein, A.; Langer, R. Biodegradable, elastic shape-memory polymers for potential biomedical applications. Science 2002, 296, 1673–1676. [Google Scholar] [CrossRef]
- Sokolowski, W.; Metcalfe, A.; Hayashi, S.; Yahia, L.; Raymond, J. Medical applications of shape memory polymers. Biomed. Mater. 2007, 2, S23–S27. [Google Scholar] [CrossRef] [PubMed]
- Maitland, D.J.; Metzger, M.F.; Schumann, D.; Lee, A.; Wilson, T.S. Photothermal properties of shape memory polymer micro-actuators for treating stroke. Lasers Surg. Med. 2002, 30, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Snyder, E.A.; Tong, T.H. Towards Novel Light Activated Shape Memory Polymer. MRS Online Proc. Libr. Arch. 2005, 872. [Google Scholar] [CrossRef]
- Jin Yoo, H.; Chae Jung, Y.; Gopal Sahoo, N.; Whan Cho, J. Polyurethane-Carbon Nanotube Nanocomposites Prepared by In-Situ Polymerization with Electroactive Shape Memory. J. Macromol. Sci. Part B 2006, 45, 441–451. [Google Scholar] [CrossRef]
- Gök, M.O.; Bilir, M.Z.; Gürcüm, B.H. Shape-Memory Applications in Textile Design. Procedia Soc. Behav. Sci. 2015, 195, 2160–2169. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Kelch, S.; Lendlein, A. Polymers Move in Response to Light. Adv. Mater. 2006, 18, 1471–1475. [Google Scholar] [CrossRef]
- Defize, T.; Thomassin, J.-M.; Ottevaere, H.; Malherbe, C.; Eppe, G.; Jellali, R.; Alexandre, M.; Jérôme, C.; Riva, R. Photo-Cross-Linkable Coumarin-Based Poly(ε-caprolactone) for Light-Controlled Design and Reconfiguration of Shape-Memory Polymer Networks. Macromolecules 2019, 52, 444–456. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, Q.; Gao, P.; Chi, B.; Nie, J.; He, Y. Photopolymerization of Coumarin-Containing Reversible Photoresponsive Materials Based on Wavelength Selectivity. Ind. Eng. Chem. Res. 2019, 58, 2970–2975. [Google Scholar] [CrossRef]
- Nagata, M.; Yamamoto, Y. Photocurable Shape-Memory Copolymers of ε-Caprolactone and L-Lactide. Macromol. Chem. Phys. 2010, 211, 1826–1835. [Google Scholar] [CrossRef]
- Nagata, M.; Yamamoto, Y. Synthesis and characterization of photocrosslinked poly(ε-caprolactone)s showing shape-memory properties. J. Polym. Sci. A Polym. Chem. 2009, 47, 2422–2433. [Google Scholar] [CrossRef]
- Zhao, X.; Dang, Y.; Deng, J.; Zhang, J. Photoinduced shape fixity and thermal-induced shape recovery properties based on polyvinyl alcohol bearing coumarin. Colloid Polym. Sci. 2014, 292, 85–95. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, Z.; Huang, H.; Chen, Y. The design of triple shape memory polymers with stable yet tunable temporary shapes by introducing photo-responsive units into a crystalline domain. Polym. Chem. 2019, 10, 1537–1543. [Google Scholar] [CrossRef]
- Yongwei, W.; Huahua, H.; Yongming, C. Synthesis of triple shape memory polyurethanes by introducing photo-responsive coumarin units into the crystalline soft segment. Mater. Today Proc. 2019, 16, 1507–1511. [Google Scholar]
- He, J.; Zhao, Y.; Zhao, Y. Photoinduced bending of a coumarin-containing supramolecular polymer. Soft Matter 2009, 5, 308–310. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Longenecker, R.; Mu, T.; Hanna, M.; Burke, N.A.D.; Stöver, H.D.H. Thermally Responsive 2-Hydroxyethyl Methacrylate Polymers: Soluble–Insoluble and Soluble–Insoluble–Soluble Transitions. Macromolecules 2011, 44, 8962–8971. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Zhang, X.-Z.; Jo Lewis, P.; Chu, C.-C. Fabrication and characterization of a smart drug delivery system: Microsphere in hydrogel. Biomaterials 2005, 26, 3299–3309. [Google Scholar] [CrossRef]
- Pan, Y.; Gao, Y.; Shi, J.; Wang, L.; Xu, B. A versatile supramolecular hydrogel of nitrilotriacetic acid (NTA) for binding metal ions and magnetorheological response. J. Mater. Chem. 2011, 21, 6804. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, N.; Fu, C.; Li, K.; Tao, L.; Feng, L.; Wei, Y. Thermo and pH dual-responsive materials for controllable oil/water separation. ACS Appl. Mater. Interfaces 2014, 6, 2026–2030. [Google Scholar] [CrossRef]
- Xu, B.; Jiang, H.; Li, H.; Zhang, G.; Zhang, Q. High strength nanocomposite hydrogel bilayer with bidirectional bending and shape switching behaviors for soft actuators. RSC Adv. 2015, 5, 13167–13170. [Google Scholar] [CrossRef]
- Shi, K.; Liu, Z.; Wei, Y.-Y.; Wang, W.; Ju, X.-J.; Xie, R.; Chu, L.-Y. Near-Infrared Light-Responsive Poly(N-isopropylacrylamide)/Graphene Oxide Nanocomposite Hydrogels with Ultrahigh Tensibility. ACS Appl. Mater. Interfaces 2015, 7, 27289–27298. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Wu, J.; Dinh, N.-D.; Chen, C.-H. Gradient Porous Elastic Hydrogels with Shape-Memory Property and Anisotropic Responses for Programmable Locomotion. Adv. Funct. Mater. 2015, 25, 7272–7279. [Google Scholar] [CrossRef]
- Yu, Z.; Cai, Z.; Chen, Q.; Liu, M.; Ye, L.; Ren, J.; Liao, W.; Liu, S. Engineering β-sheet peptide assemblies for biomedical applications. Biomater. Sci. 2016, 4, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.P.; Konst, S. Novel hydrogel actuator inspired by reversible mussel adhesive protein chemistry. Adv. Mater. Weinh. 2014, 26, 3415–3419. [Google Scholar] [CrossRef]
- Francis, W.; Dunne, A.; Delaney, C.; Florea, L.; Diamond, D. Spiropyran based hydrogels actuators—Walking in the light. Sens. Actuators B 2017, 250, 608–616. [Google Scholar] [CrossRef]
- Li, Y.; Huang, G.; Zhang, X.; Li, B.; Chen, Y.; Lu, T.; Lu, T.J.; Xu, F. Magnetic Hydrogels and Their Potential Biomedical Applications. Adv. Funct. Mater. 2013, 23, 660–672. [Google Scholar] [CrossRef]
- Ionov, L. Biomimetic Hydrogel-Based Actuating Systems. Adv. Funct. Mater. 2013, 23, 4555–4570. [Google Scholar] [CrossRef]
- Cheng, Y.; Ren, K.; Yang, D.; Wei, J. Bilayer-type fluorescence hydrogels with intelligent response serve as temperature/pH driven soft actuators. Sens. Actuators B 2018, 255, 3117–3126. [Google Scholar] [CrossRef]
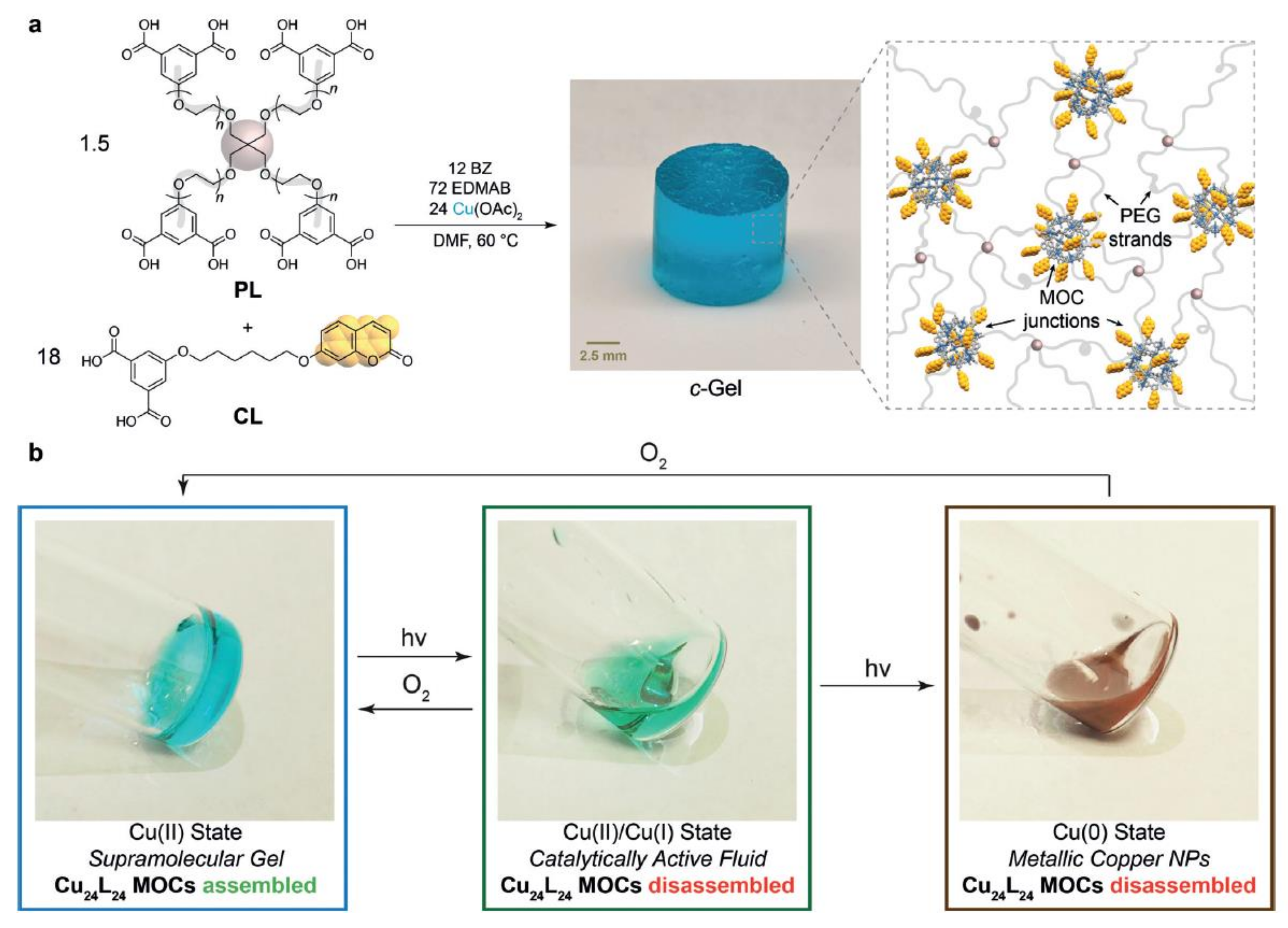
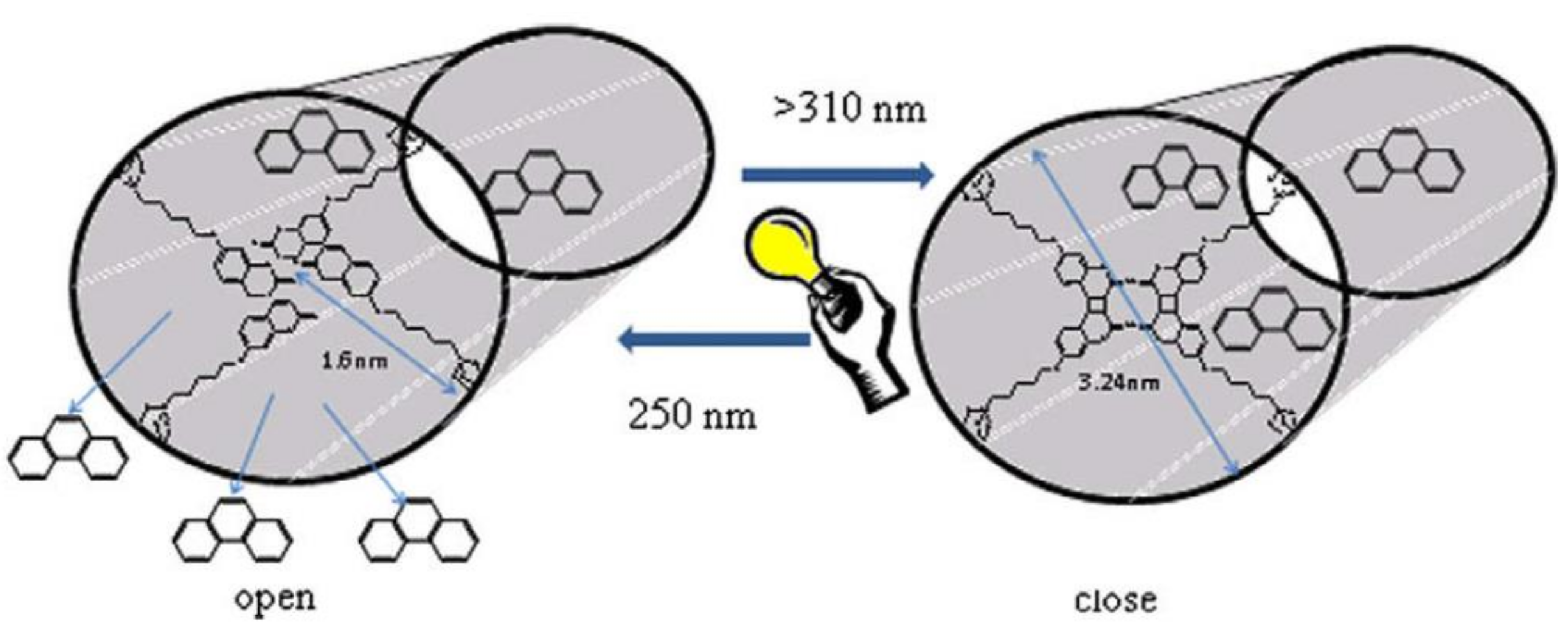
- Oldenhuis, N.J.; Qin, K.P.; Wang, S.; Ye, H.-Z.; Alt, E.A.; Willard, A.P.; van Voorhis, T.; Craig, S.L.; Johnson, J.A. Photoswitchable Sol-Gel Transitions and Catalysis Mediated by Polymer Networks with Coumarin-Decorated Cu24 L24 Metal-Organic Cages as Junctions. Angew. Chem. Int. Ed. Engl. 2020, 59, 2784–2792. [Google Scholar] [CrossRef]
- Kamyshny, A.; Magdassi, S. Conductive nanomaterials for 2D and 3D printed flexible electronics. Chem. Soc. Rev. 2019, 48, 1712–1740. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Han, S.I.; Kim, D.; Hyeon, T.; Kim, D.-H. High-performance stretchable conductive nanocomposites: Materials, processes, and device applications. Chem. Soc. Rev. 2019, 48, 1566–1595. [Google Scholar] [CrossRef] [PubMed]
- Caneparo, C.; Baratange, C.; Chabaud, S.; Bolduc, S. Conditioned medium produced by fibroblasts cultured in low oxygen pressure allows the formation of highly structured capillary-like networks in fibrin gels. Sci. Rep. 2020, 10, 9291. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.; Ramakrishna, S. Design strategies of tissue engineering scaffolds with controlled fiber orientation. Tissue Eng. 2007, 13, 1845–1866. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef]
- Shelke, N.B.; James, R.; Laurencin, C.T.; Kumbar, S.G. Polysaccharide biomaterials for drug delivery and regenerative engineering. Polym. Adv. Technol. 2014, 25, 448–460. [Google Scholar] [CrossRef]
- Bressan, E.; Favero, V.; Gardin, C.; Ferroni, L.; Iacobellis, L.; Favero, L.; Vindigni, V.; Berengo, M.; Sivolella, S.; Zavan, B. Biopolymers for Hard and Soft Engineered Tissues: Application in Odontoiatric and Plastic Surgery Field. Polymers 2011, 3, 509–526. [Google Scholar] [CrossRef]
- Mano, J.F.; Silva, G.A.; Azevedo, H.S.; Malafaya, P.B.; Sousa, R.A.; Silva, S.S.; Boesel, L.F.; Oliveira, J.M.; Santos, T.C.; Marques, A.P.; et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 1–19. [Google Scholar] [CrossRef]
- Trenor, S.R.; Long, T.E.; Love, B.J. Photoreversible Chain Extension of Poly(ethylene glycol). Macromol. Chem. Phys. 2004, 205, 715–723. [Google Scholar] [CrossRef]
- Habraken, W.J.E.M.; Wolke, J.G.C.; Jansen, J.A. Ceramic composites as matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Polini, A.; Bai, H.; Tomsia, A.P. Dental applications of nanostructured bioactive glass and its composites. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Yan, X.; Yu, C.; Zhou, X.; Tang, J.; Zhao, D. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew. Chem. Int. Ed. Engl. 2004, 43, 5980–5984. [Google Scholar] [CrossRef]
- Luo, L.-J.; Huang, C.-C.; Chen, H.-C.; Lai, J.-Y.; Matsusaki, M. Effect of deacetylation degree on controlled pilocarpine release from injectable chitosan-g-poly(N-isopropylacrylamide) carriers. Carbohydr. Polym. 2018, 197, 375–384. [Google Scholar] [CrossRef]
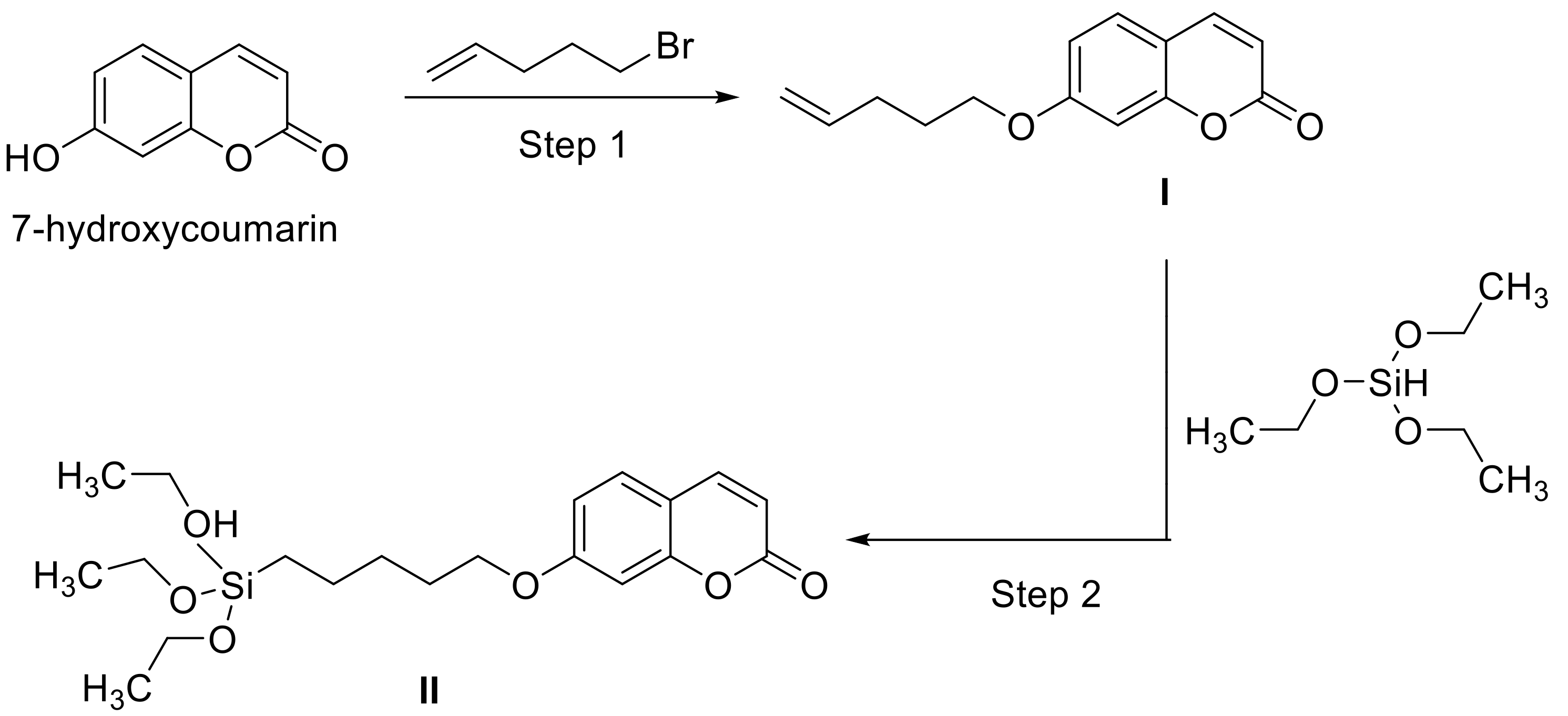
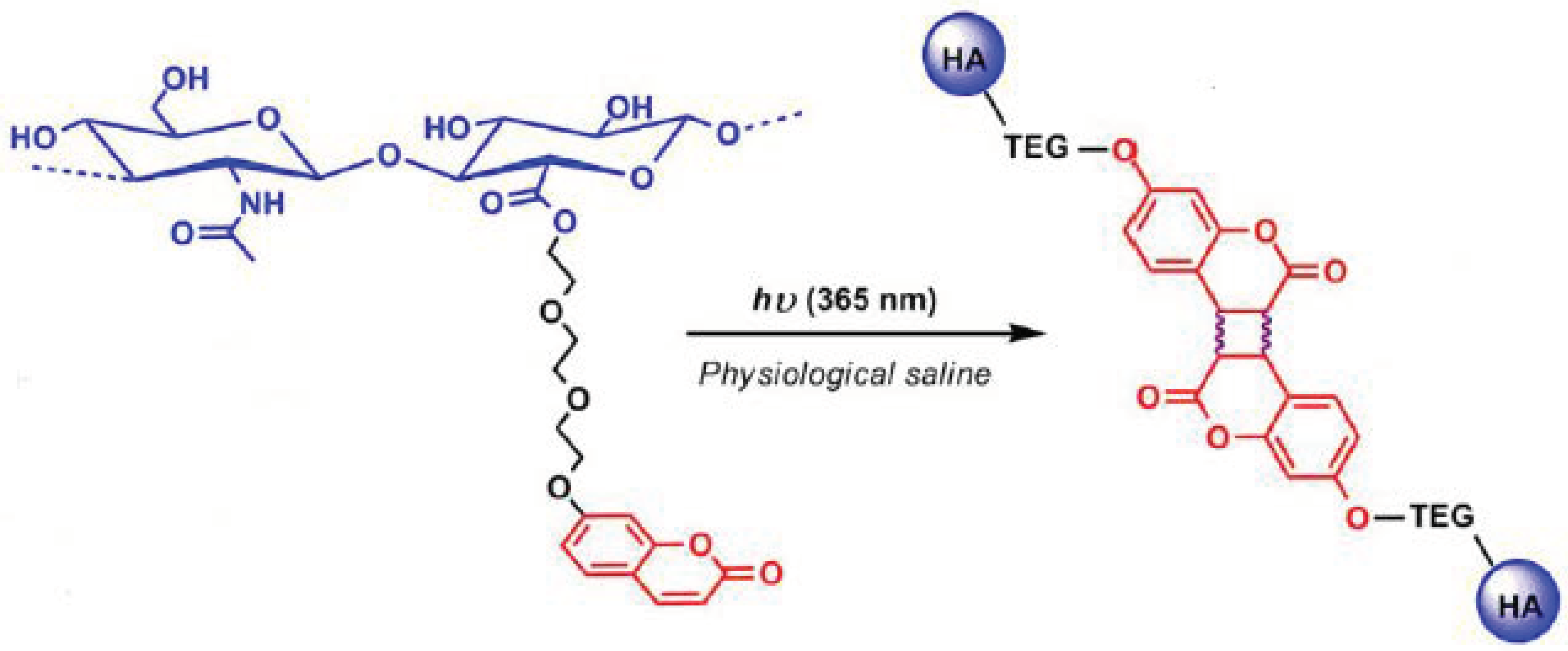
- Beninatto, R.; Barbera, C.; de Lucchi, O.; Borsato, G.; Serena, E.; Guarise, C.; Pavan, M.; Luni, C.; Martewicz, S.; Galesso, D.; et al. Photocrosslinked hydrogels from coumarin derivatives of hyaluronic acid for tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 625–634. [Google Scholar] [CrossRef]
- Tabet, A.; Forster, R.A.; Parkins, C.C.; Wu, G.; Scherman, O.A. Modulating stiffness with photo-switchable supramolecular hydrogels. Polym. Chem. 2019, 10, 467–472. [Google Scholar] [CrossRef]
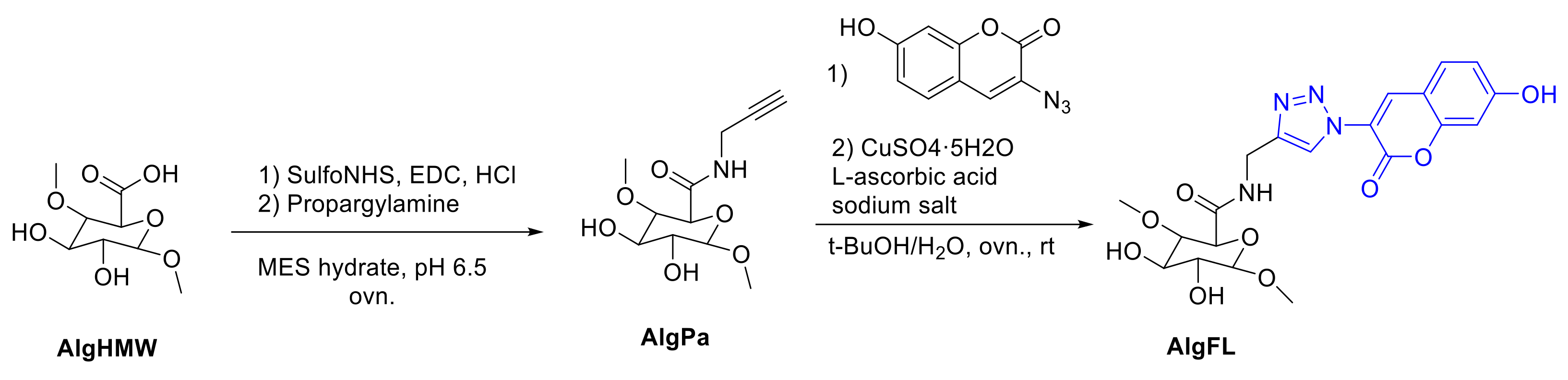
- Araújo, M.; Bidarra, S.J.; Alves, P.M.; Valcarcel, J.; Vázquez, J.A.; Barrias, C.C. Coumarin-grafted blue-emitting fluorescent alginate as a potentially valuable tool for biomedical applications. J. Mater. Chem. B 2020, 8, 813–825. [Google Scholar] [CrossRef]
- Borzacchiello, A.; Russo, L.; Malle, B.M.; Schwach-Abdellaoui, K.; Ambrosio, L. Hyaluronic Acid Based Hydrogels for Regenerative Medicine Applications. BioMed Res. Int. 2015, 2015, 871218. [Google Scholar] [CrossRef]
- Rossi, C.A.; Flaibani, M.; Blaauw, B.; Pozzobon, M.; Figallo, E.; Reggiani, C.; Vitiello, L.; Elvassore, N.; de Coppi, P. In vivo tissue engineering of functional skeletal muscle by freshly isolated satellite cells embedded in a photopolymerizable hydrogel. FASEB J. 2011, 25, 2296–2304. [Google Scholar] [CrossRef] [PubMed]
- Seidlits, S.K.; Khaing, Z.Z.; Petersen, R.R.; Nickels, J.D.; Vanscoy, J.E.; Shear, J.B.; Schmidt, C.E. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials 2010, 31, 3930–3940. [Google Scholar] [CrossRef]
- Campisi, M.; de Lucchi, O.; Beninatto, R.; Borsato, G. Photocrosslinked Hyaluronic Acid Derivatives, and the Preparation Process and Use Thereof. U.S. Patent 9,889,226, 24 December 2015. [Google Scholar]
- Wang, R.; Bardelang, D.; Waite, M.; Udachin, K.A.; Leek, D.M.; Yu, K.; Ratcliffe, C.I.; Ripmeester, J.A. Inclusion complexes of coumarin in cucurbiturils. Org. Biomol. Chem. 2009, 7, 2435–2439. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Han, D.; Yan, W.; Yuan, Z.; Wang, Q.; Zou, L. Multi-mode supermolecular polymerization driven by host–guest interactions. RSC Adv. 2018, 8, 13722–13727. [Google Scholar] [CrossRef]
- Aliaga, M.E.; García-Río, L.; Pessêgo, M.; Montecinos, R.; Fuentealba, D.; Uribe, I.; Martín-Pastor, M.; García-Beltrán, O. Host–guest interaction of coumarin-derivative dyes and cucurbit[7]uril: Leading to the formation of supramolecular ternary complexes with mercuric ions. New J. Chem. 2015, 39, 3084–3092. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Zhang, X.; Zhou, W.; Huang, L. One-pot fluorescent labeling of saccharides with fluorescein-5-thiosemicarbazide for imaging polysaccharides transported in living cells. Carbohydr. Res. 2011, 346, 2156–2164. [Google Scholar] [CrossRef] [PubMed]








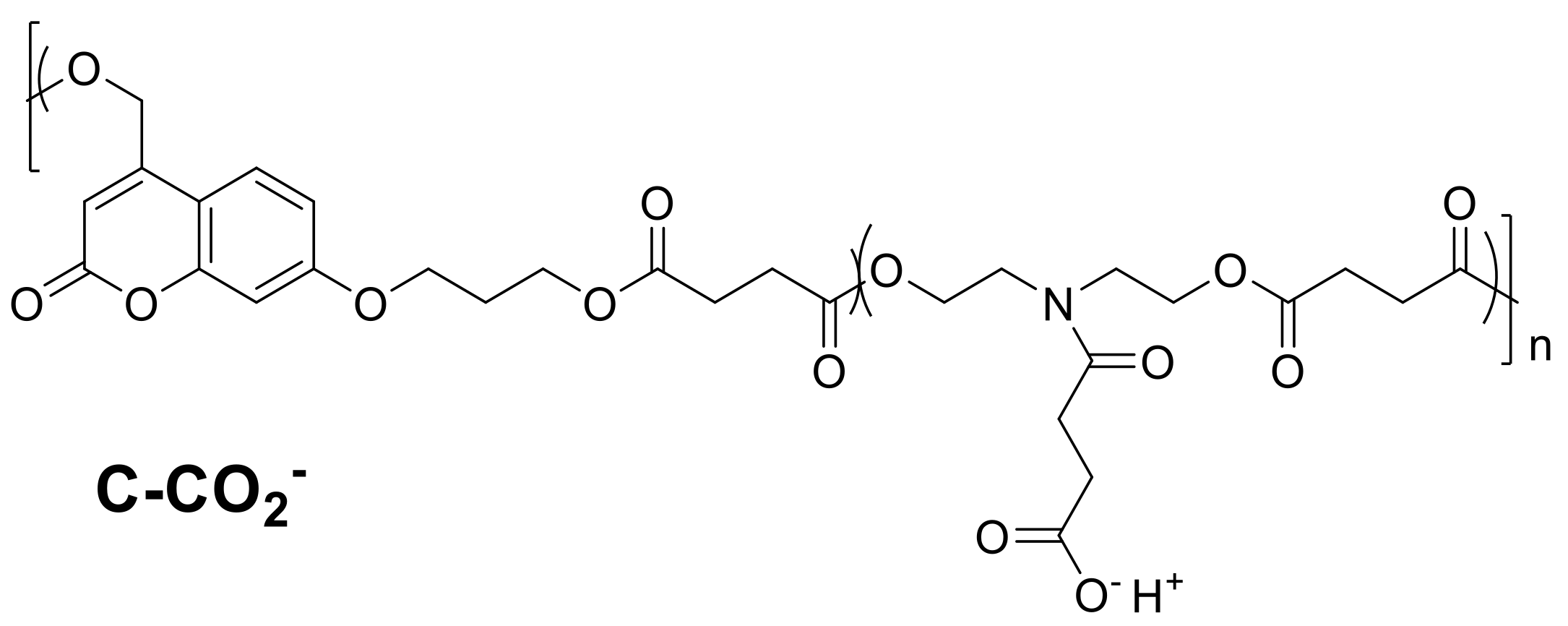
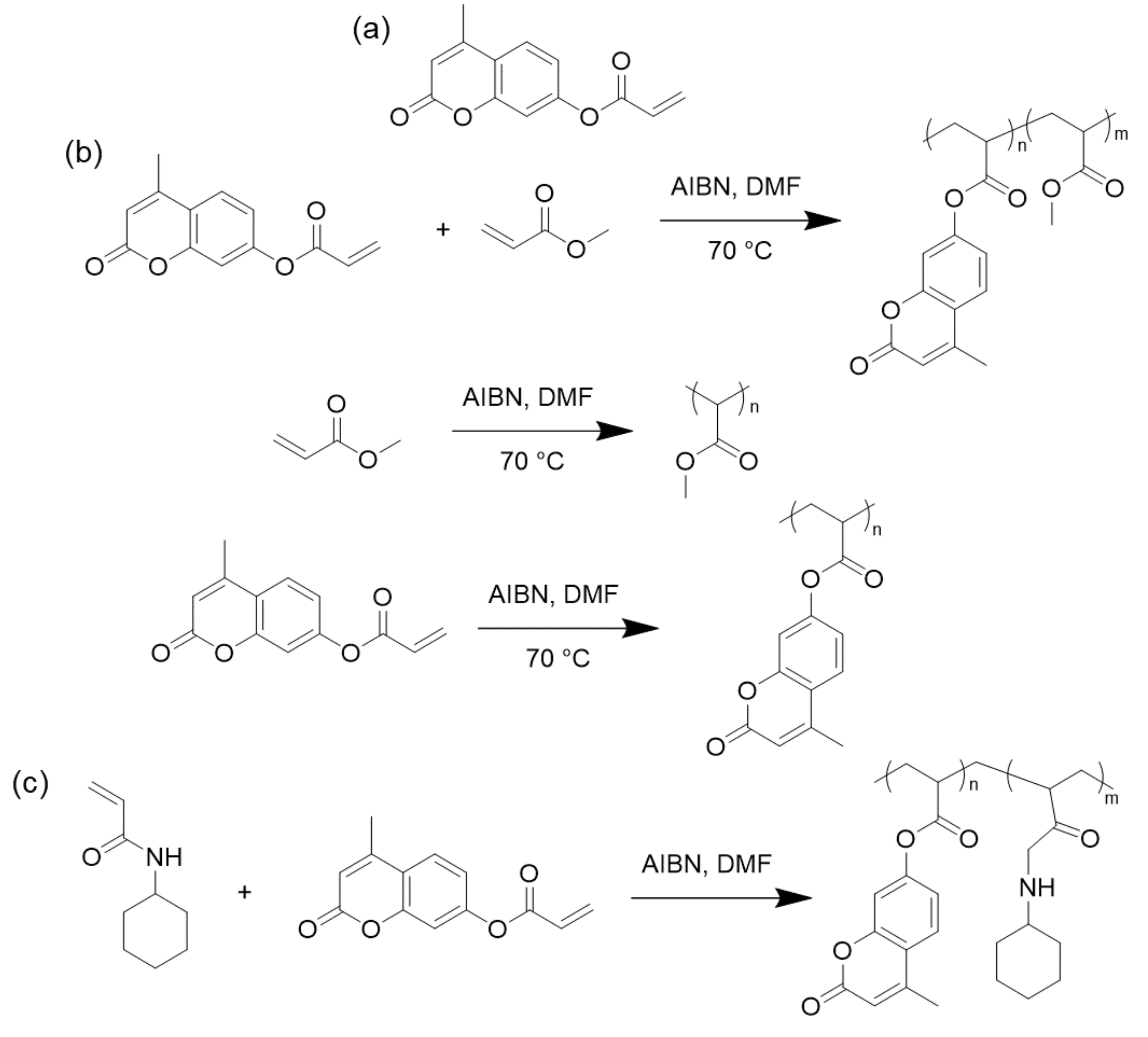
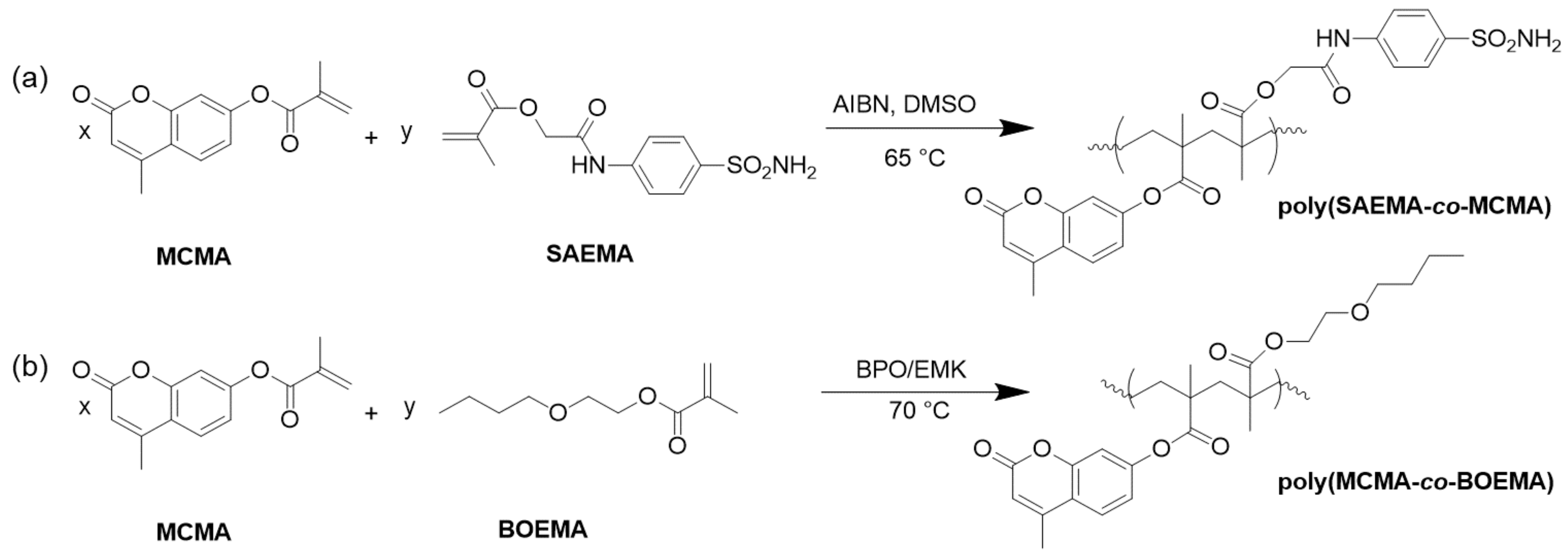
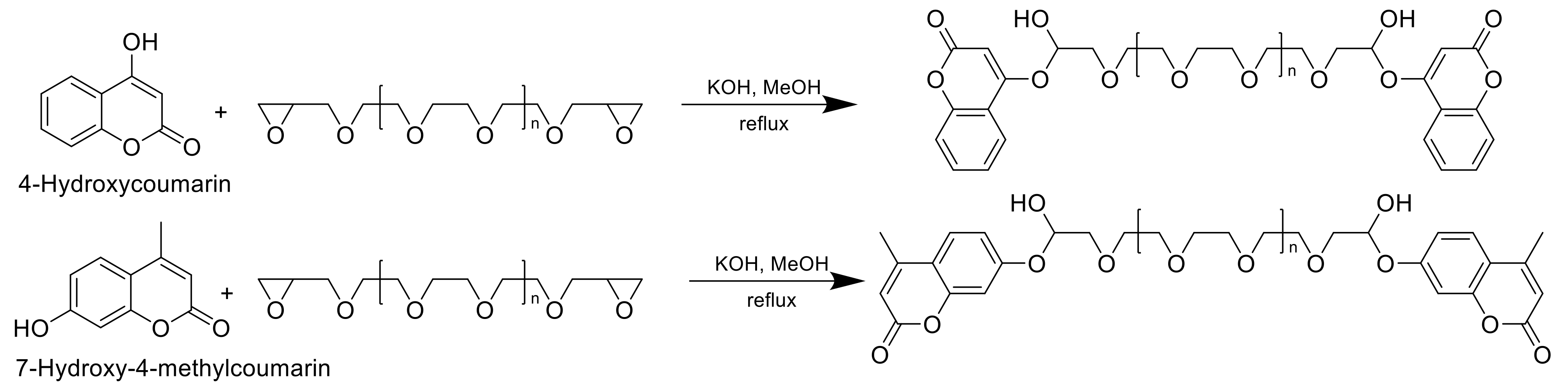





| Resin | Melting Temperature (°C) | Polymerization Temperature (°C) | |
|---|---|---|---|
| Onset | Max | ||
| PH-a | 54 | 255 | 261 |
| U-a | 147 | 215 | 220 |
| MU-a | 153 | 229 | 232 |
| Polymers | Tg (°C) | ||
|---|---|---|---|
| Virgin Sample | Irradiation with 254 nm | Irradiation with 365 nm | |
| BMA | 70 | 52 | 67 |
| MA | 65 | 50 | 62 |
| HMA | 46 | 43 | 44 |
| EA | – | 32 | 35 |
| Sample | Photocross-Linking Conditions | Rf(0 to 1) (%) a | Rf(1 to 0) (%) b | ∆Ɛrel (1 to 2) (%) c | ΔƐrel (2 to 0) (%) d | |
|---|---|---|---|---|---|---|
| Time (min) | Light Intensity (mW/cm2) | |||||
| SMPU | 5 | 34.0 | 99 | 90.1 | 85.1 | 14.9 |
| SMPU | 10 | 34.0 | 99 | 92.1 | 79.1 | 20.9 |
| SMPU | 15 | 34.0 | 99 | 91 | 77.6 | 22.4 |
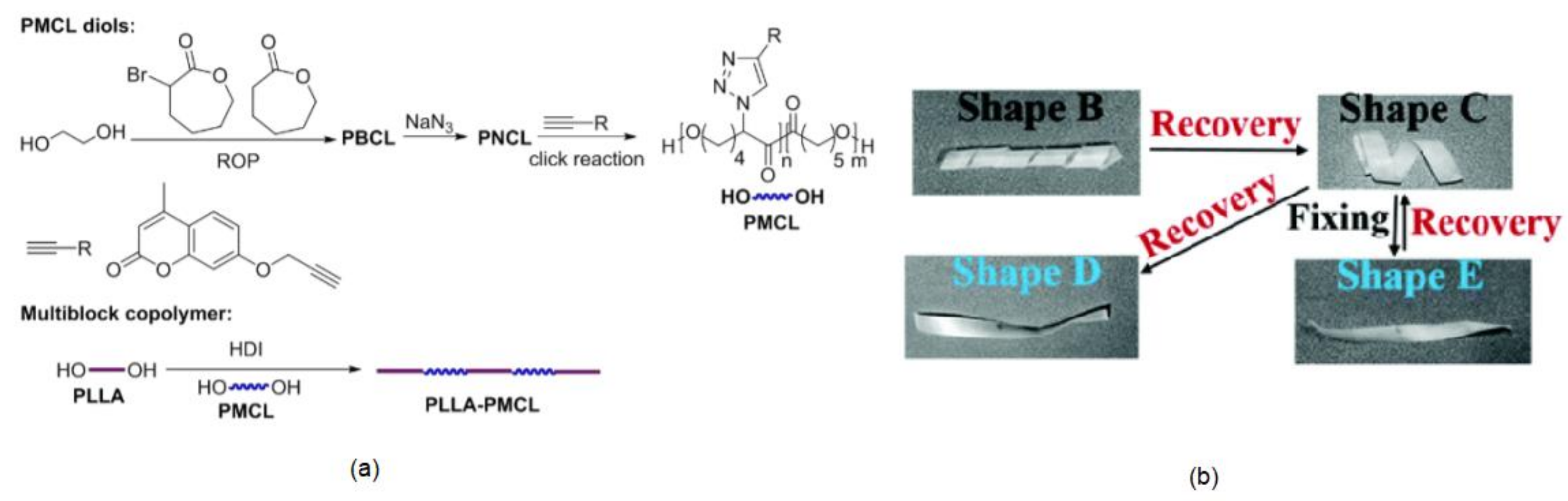
| Entry | Sample | Photocross-linking Conditions | Rf(A to B) (%) | Rr(B to A) (%) | ∆Ɛrel (B to C) (%) | ΔƐrel (C to A) (%) | |
|---|---|---|---|---|---|---|---|
| Time (min) | Light Intensity (mW/cm2) | ||||||
| 1 | PLLA-PMCL1 | 1 | 27.8 | 99.0 ± 1.0 | 80.7 ± 1.2 | 86.2 ± 1.2 | 13.8 ± 1.2 |
| 2 | PLLA-PMCL2 | 1 | 27.8 | 99.0 ± 1.0 | 72.2 ± 1.3 | 88.1 ± 1.3 | 11.9 ± 1.3 |
| 3 | PLLA-PMCL2 | 0.5 | 27.8 | 99.0 ± 1.0 | 85.9 ± 1.2 | 88.0 ± 1.2 | 12.0 ± 1.2 |
| 4 | PLLA-PMCL2 | 8 | 7.0 | 99.0 ± 1.0 | 89.4 ± 1.2 | 67.8 ± 1.2 | 21.5 ± 1.2 |
| 5 | PLLA-PMCL1 | 10 | 34.0 | 99.0 ± 1.0 | 67.9 ± 1.3 | 47.5 ± 1.3 | 52.5 ± 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazin, I.; Rossegger, E.; Guedes de la Cruz, G.; Griesser, T.; Schlögl, S. Recent Advances in Functional Polymers Containing Coumarin Chromophores. Polymers 2021, 13, 56. https://doi.org/10.3390/polym13010056
Cazin I, Rossegger E, Guedes de la Cruz G, Griesser T, Schlögl S. Recent Advances in Functional Polymers Containing Coumarin Chromophores. Polymers. 2021; 13(1):56. https://doi.org/10.3390/polym13010056
Chicago/Turabian StyleCazin, Ines, Elisabeth Rossegger, Gema Guedes de la Cruz, Thomas Griesser, and Sandra Schlögl. 2021. "Recent Advances in Functional Polymers Containing Coumarin Chromophores" Polymers 13, no. 1: 56. https://doi.org/10.3390/polym13010056
APA StyleCazin, I., Rossegger, E., Guedes de la Cruz, G., Griesser, T., & Schlögl, S. (2021). Recent Advances in Functional Polymers Containing Coumarin Chromophores. Polymers, 13(1), 56. https://doi.org/10.3390/polym13010056