Natural Polymeric Materials: A Solution to Plastic Pollution from the Agro-Food Sector
Abstract
1. Introduction
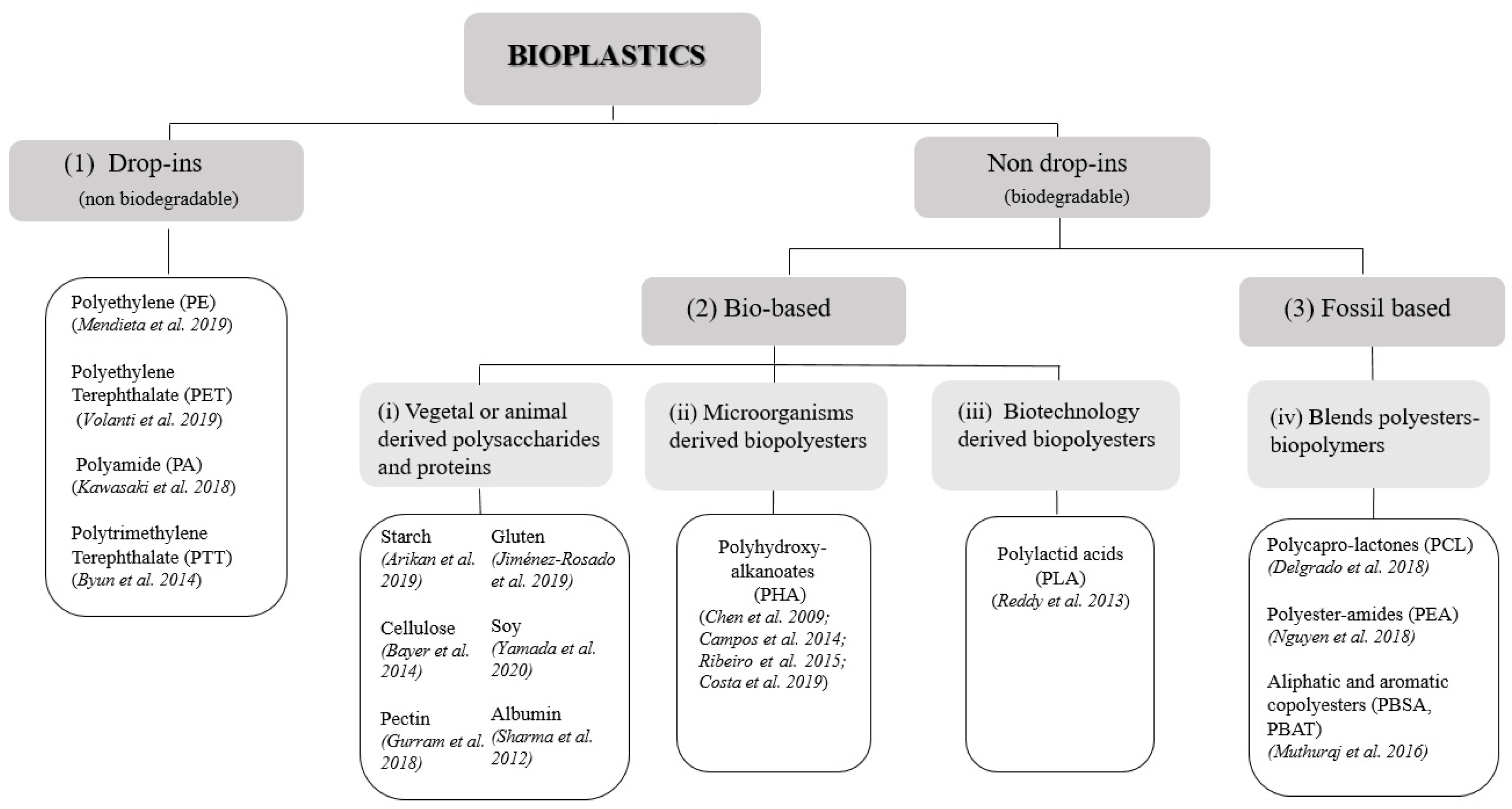
2. Bioplastics: Definition and Classification
2.1. Bioplastics Bio-Based Content and Biodegradability
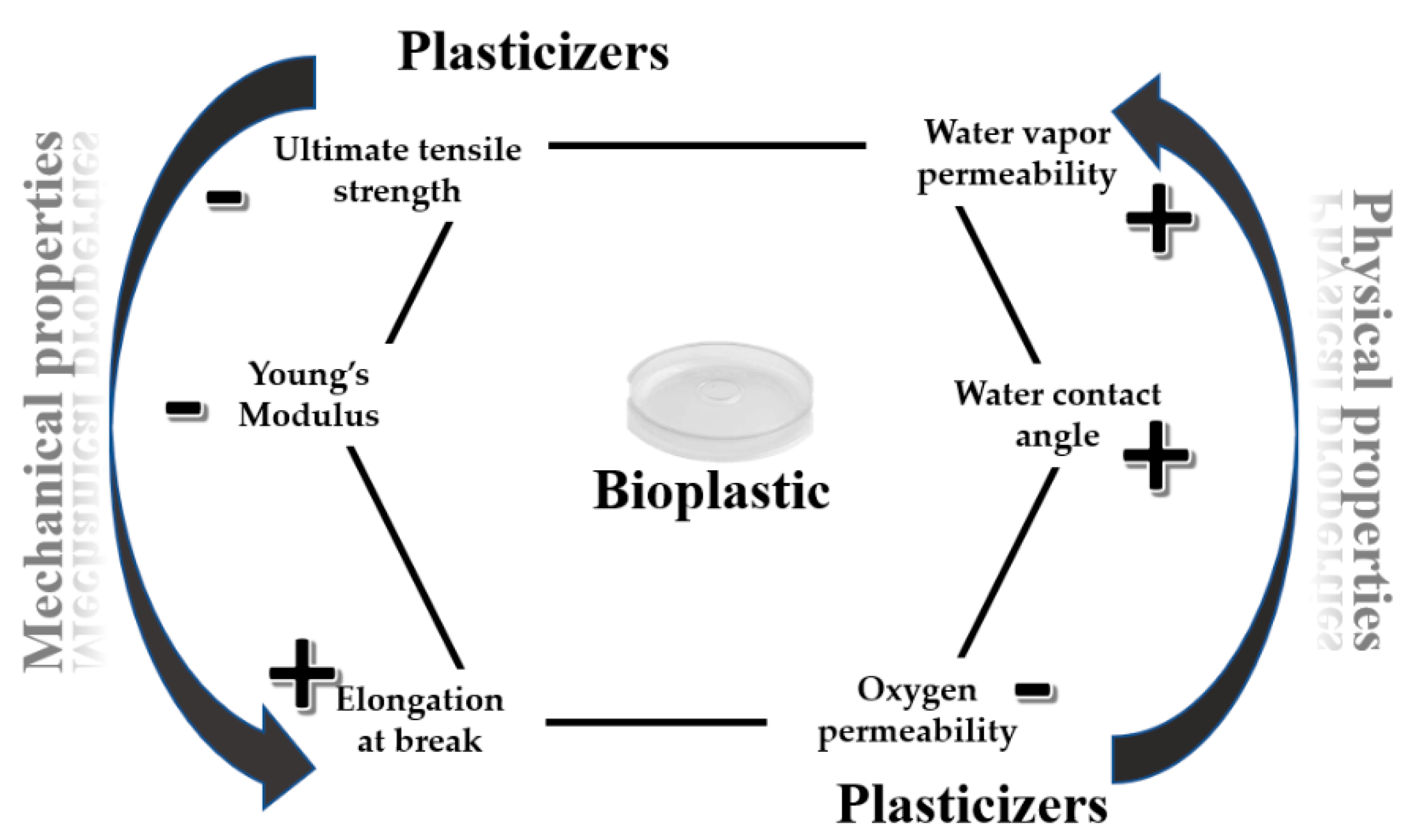
2.2. Bioplastics Mechanical and Physical Properties
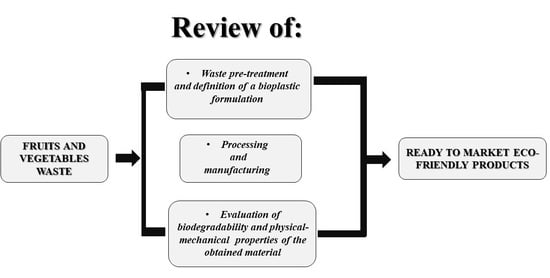
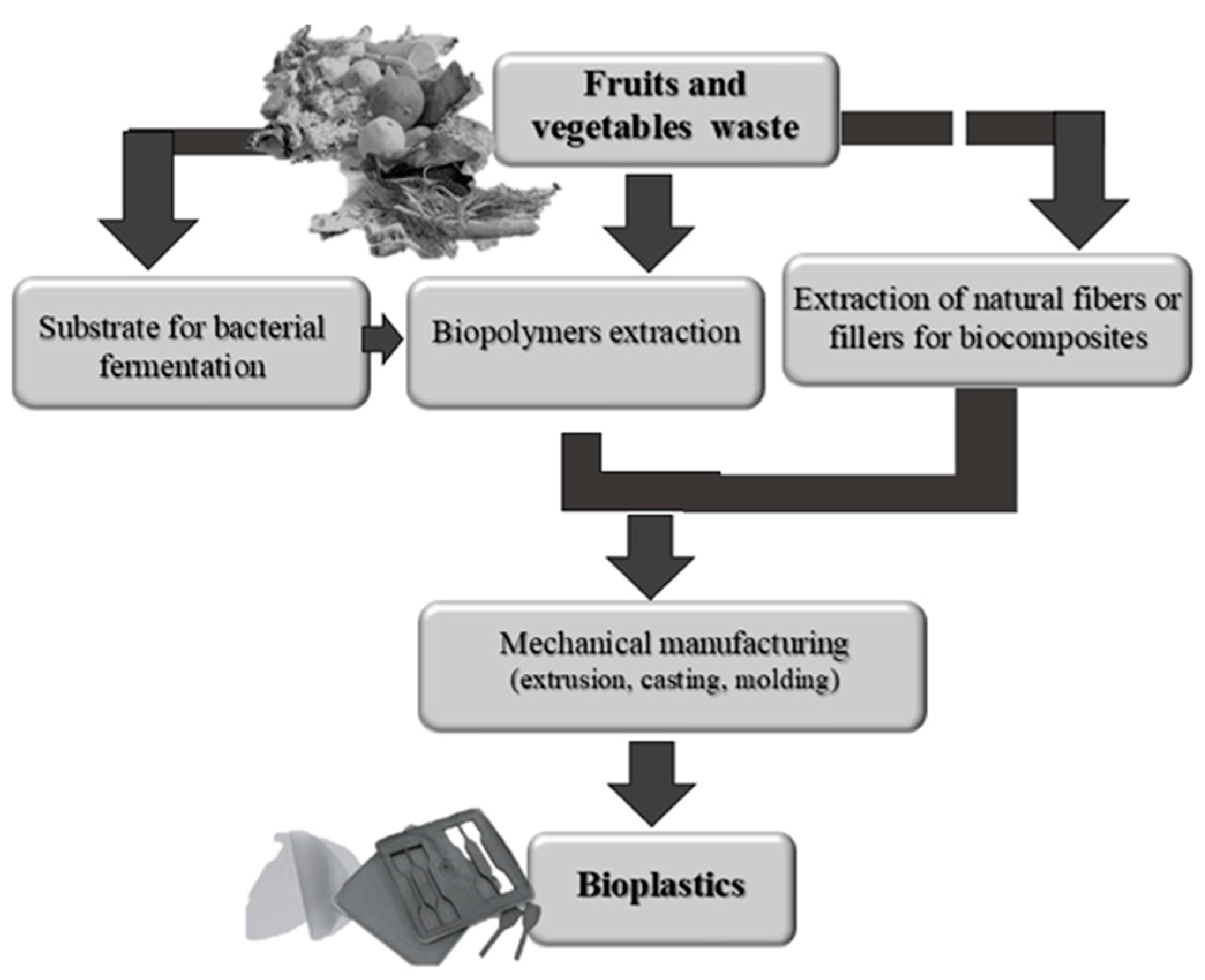
3. Food Waste as Feedstock for Bioplastic Production
3.1. Biopolymers-Based Plastics
3.2. Fruits and Vegetables Waste Usage for Biocomposites Production
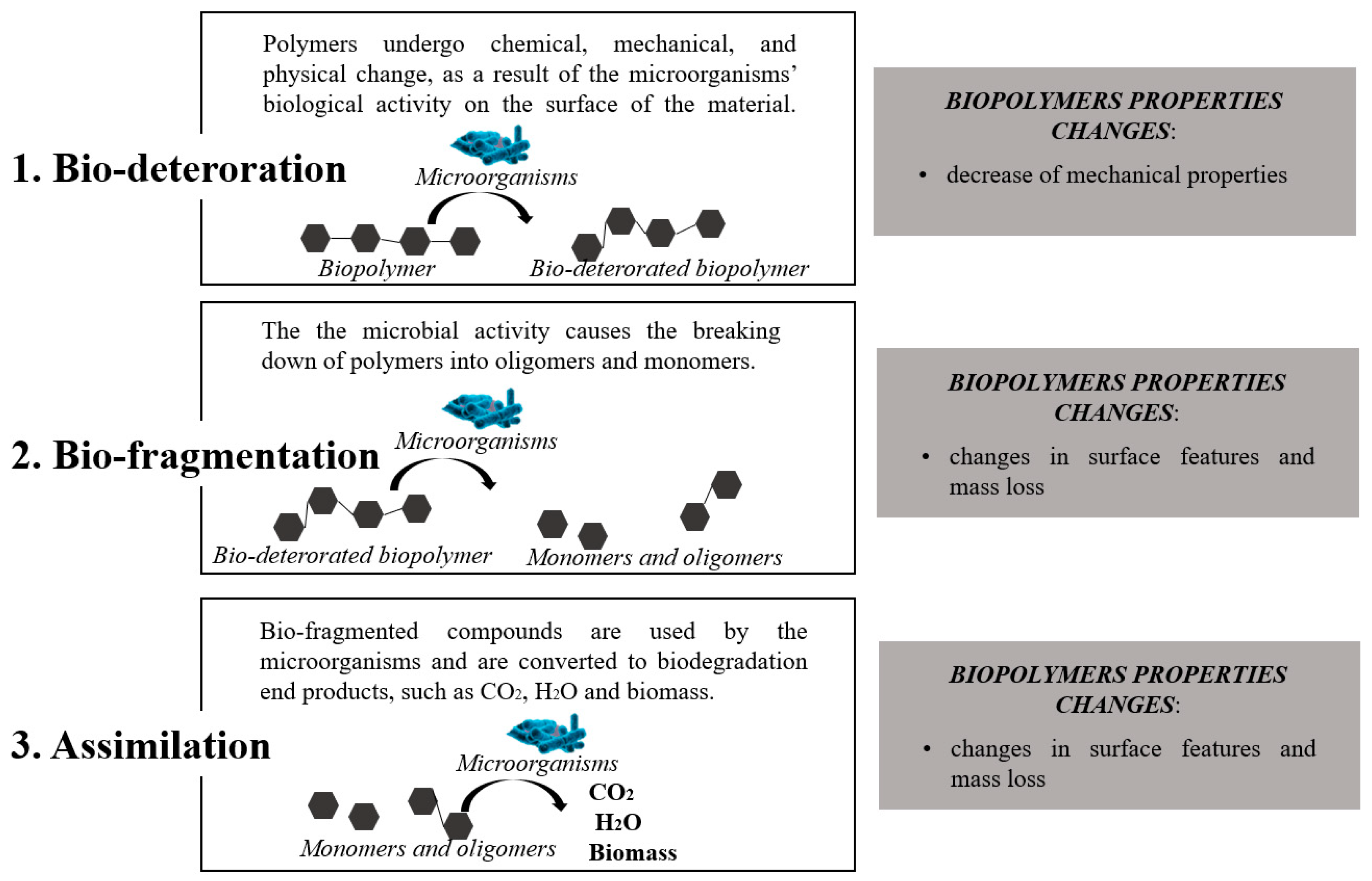
4. Environmental Impacts of Agro-Food Waste Based Bioplastics Production
5. Bioplastics Market
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Food Waste Source | Technology and Pre-Treatment | Processing | Bioplastic Type | Bioplastic Mechanical and Physical Properties | Reference |
|---|---|---|---|---|---|
| Apple pomace | Apple pomace, either washed with water or not, was powdered. A mixture was prepared containing 2% (w/v) of powder and 7% glycerol (w/w of apple pomace powder) and dissolved in 1% (w/v) of citric acid solution under heating (70 °C) and constant magnetic stirring at 560 rpm. Then, the mixture was poured onto a non-sticky plate for casting. A mixture without glycerol was also used in order to prepare a biofilm. | Casting | Pectin based biofilm | Tensile strength (MPa): 3.27–16.49 Elongation at break: 10.77–55.41% | Gustaffson et al. [88] |
| Apple pomace, either washed with water or not, was powdered. A mixture was prepared containing glycerol and apple pomace powder (70:30) was prepared. 40 g of the mixture was placed into a mold. A pressure of 8 MPa was applied for 20 min at 100 °C. | Molding | Pectin based 3D biomaterial | Tensile strength (MPa): 3.02–5.79 Young’s Modulus (MPa): 367.1–633.4 Elongation at break: 0.93–1.56% | Gustaffson et al. [88] | |
| Banana peels and pseudostems | Banana peels were boiled in water for about 30 min; then, they were left to dry and squashed to obtain a paste. 25 g of banana paste were placed in a beaker with 3 mL of HCl (0.1 N) and 2 mL of glycerol and stirred. Subsequently, NaOH (0.1 N) was added in order to neutralize the pH up to 7. | Casting | Cellulose based biofilm | Weight loss after 13 days: 0.04 g Difference of weight after swelling test (g): 0.01–0.10 | Yaradoddi et al. [89] |
| Banana peels were washed, sliced, and blended. Then, they were grounded and ground sample was heated at 150 °C for 2 h at 30 psi. The obtained paste was hydrolyzed (100 mL/50 g sample) by (HCl, 99%). The sample was filtrated and washed with water. 1000 g of sample were mixed with chlorinated paraffin liquid plasticizers (1:8 of sample), 5% acetic acid (5 mL/100 g sample), 5 mL/100 g of polyvinylchloride, cellulose (25%) and 25% starch powder, 5% toluene Phthalates ester and 10%water. Then, 10 mL/100 g of PVC and glycerine were added. The mixture was heated at 150 °C in the oven for 30 min at 30 psi pressure. | Casting | Biocomposite | Tensile strength (MPa/kg·m3): 120.0 Tensile modulus (GPa): 1.1 Water absorption: 0.03% | Sharif Hossain et al. [139] | |
| Banana peels were immersed in a Na2S2O5 solution (1% w/v) for 24 h, oven-dried at 60 °C and milled. 100 g of milled peels were washed three times with ethanol, then washed in 200 mL of acetone. The pectin was extracted with a citric acid solution at pH 2.0 at 87 °C for 160 min and then centrifuged. The supernatant pH was adjusted to 3.5 with KOH, added with ethanol, stirred for 30 min and left to precipitate at 4 °C. The pellet was washed with ethanol 70%, and dried at room temperature. Then, it was stirred and its pH adjusted to 7, and it was again dried and milled. For the extraction of cellulose nanocrystals (CNCs), the alcohol insoluble residue of banana peels was suspended in a mixture of 93 wt% acetic acid and 0.3 wt% HCl in distilled water. Subsequently, the pulp was rinsed and washed in more steps and an acid hydrolysis was conducted with a 30 v% H2SO4 solution at 45 °C, for 150 min. After centrifugation-dilution-sonication cycles, the CNC suspension was dialyzed against deionized water. CNCs (at different wt%), 4.5 g of pectin, 1.35 g glycerol, citric acid, and distilled water were mixed to form biofilms. | Casting | Pectin based biofilm | Tensile strength (MPa) without citric acid: 7.36 ± 1.15 Tensile strength (MPa) with citric acid: 7.92 ± 1.21 Elongation at break (%)without citric acid: 4.69 ± 0.84 Elongation at break (%)with citric acid: 4.26 ± 0.77 Elastic modulus (MPa)without citric acid: 1586 ± 487 Elastic modulus (MPa)with citric acid: 1714 ± 452 Water vapor permeability(g·mm·kPa−1·h−1·m−2) without citric acid: 3.31 ± 0.36 Water vapor permeability(g·mm·kPa−1·h−1·m−2) with citric acid: 3.10 ± 0.33 | Oliveira et al. [75] | |
| 300 g of banana peels were dipped in acetic acid solution and then placed into a beaker containing 800 mL water and boiled for 30 min. The water was decanted off and the peels were left to dry. The banana peels were pureed. To 25 mL of the paste, 3 mL of 0.5 M HCl and 2 mL of 15% glycerol solution were added. The mixture was stirred, 3 mL of 1% corn starch and 3 mL of 0.5 M NaOH were added to the mixture and stirred again. | Casting | Starch based biofilm | Tensile strength (N/m2): 12.22–34.72 Water uptake: 60.65–108.98% | Sultan et al. [90] | |
| Banana pseudostem was sliced, dryer at 50 °C for 5 h and milled. Then, 10 g of pseudo-stem flour were soaked in 300 mL of 5% KOH, centrifuged and bleached with 200 mL of 1% NaClO2 at pH 5 for 1 h in 70 °C. 9 g of the bleached pseudo-stem were mixed with TEMPO solution and 22.5 mL of 12% NaClO. At the end of the reaction, the mixture was homogenized and sonicated. Water containing 0.7% solid nanocellulose was mixed with glycerol, nano-clay or graphene oxide in different proportions. | Casting | Biocomposite | Tensile strength (MPa): ~5–~39 Elongation at break (%): ~1–~9 Oxygen permeability (mL/m2*day*Pa): 2 × 10−6–3.5 × 10−6 Water vapor permeability (g/m2*day*Pa): ~0.007–~0.023 Contact angle at 20 s (°): 21.89–75.03 | Faradilla et al. [91] | |
| Banana peels were boiled for 60 min and then left to dry and blended. In order to obtain a chemical-based material, 100 g of banana paste were mixed with 12 mL of HCl, 8 mL of glycerol and 12 mL of NaOH. The mixture was stirred for 5 min. Alternatively, a natural-based material was obtained by mixing 40 g of banana peels paste with, 1 g of sage, 12 g of glycerol, 12 g of potato starch, 12 g of corn starch and 38 g of water. The mixture was dried using the oven at a temperature of 120 °C for 3–4 h. | Casting | Biocomposite | Tensile strength for chemical-based material (KPa): 228 Tensile strength for natural-based material (KPa): 150 Young’s Modulus for chemical-based material (MPa): 1.53 Young’s Modulus for natural-based material (MPa): 1.88 Elongation at break for chemical-based material: 18.77% Elongation at break for natural-based material: 13.97% | Azieyanti et al. [138] | |
| Banana peels were boiled in water for about 30 min. Water was decanted and the peels were left to dry and then they were squashed to obtain a uniform paste. 25 g of banana paste were mixed with 3 mL of (0.1 N) HCl, 2 mL of glycerol and then 3 mL of 0.1 N NaOH to neutralize the pH up to 7. | Casting | Starch based biofilm | Not reported | Rizwana Beevi et al. [113] | |
| Carrots waste | Carrots waste powder was dispersed in a 5% (w/w) HCl water solution at a concentration of 50 mg/mL under vigorous stirring at 40 °C. After 12 h, the viscous dispersion was dialyzed using a 3500 MWCO membrane against MilliQ water for 72 h and then cast on a petri dish. | Casting | Cellulose based biofilm | Young’s Modulus (MPa): ~1300 Elongation at break: 6% Ultimate strength (MPa): ~37 Water contact angle: >100° Oxygen permeability (cm3μm/m2 day kPa): 96 × 104 | Perotto et al. [111] |
| Cassava peels | 100 g of cassava peels were washed and soaked in sodium metabisulphyte 1%. Then, they were crushed with 100 mL of water. Slurry resulted was extracted with water (ratio of 1:1) two times. The extracts were precipitated for 3 h. The precipitate was dried; then 5 g were mixed with glacial acetic acid 1%, chitosan (20–50%), glycerol (30%), liquid smoke (0–2 mL) and stirred at 70 °C. | Casting | Biocomposite | Tensile strength (MPa): 35.28–96.04 Elongation at break (%): 14.87–52.27 Water resistance (%): 22.68–78.40 | Fathanah et al. [117] |
| 5.0 g of dried cassava peels waste were mixed with 1.5 mL of glycerol, 0.5 mL of kaffir lime essential oil and 0.7 g of citric acid. The mixture was stirred for 45 min, and heated to 80 °C. Two other samples were prepared without essential oil and without citric acid, respectively. | Casting | Starch based biofilm | Tensile strength (N/cm): 0.3–2.5 | Masruri et al. [115] | |
| Cassava peels were mashed into a pulp. Then, 10 g were extracted with 50 mL of water. The extract was washed with water. Then, the juice precipitated and dried under direct sunlight to form a flour or starch. 3 g of starch were mixed with glycerol (25% wt)) and chitosan (2 and 3% wt). The mixture was heated at a 80–90 °C and stirred. | Casting | Biocomposite | Tensile strength (Kgf/cm2): 4.16–27.41 Elongation at break (%): 30.37–94.25 | Dasumiati et al. [116] | |
| Cauliflower waste | Cauliflower waste powder was dispersed in a 5% (w/w) HCl water solution at a concentration of 50 mg/mL under vigorous stirring at 40 °C. After 12 h, the viscous dispersion was dialyzed using a 3500 MWCO membrane against MilliQ water for 72 h and then cast on a petri dish. | Casting | Cellulose based biofilm | Young’s Modulus (MPa): ~500 Elongation at break: 4% Ultimate strength (MPa): ~7 Water contact angle: ~80° | Perotto et al. [111] |
| Citrus waste | Citrus peel derived pectin powder was mixed with glycerol (30% (w/w)) for 2 min to get a uniform dough which was then formed into ball shape (total weight of 2.5 g). The obtained blend was placed in molding press. The compression molding process was performed for 10 min under operation conditions of 1.33 MPa and 120 °C. Pectin-based biomass films were also produced by incorporation of lyophilized and milled fungal biomass. Biomass concentrations varied in the range of 0–35% of the total mixture. Glycerol content was kept at 30%. | Molding | Pectin based biofilm | Tensile strength (MPa) without fungal biomass: 15.7 ± 0.5 Elongation at break without fungal biomass (%): 5.5 ± 1.7 Young’s Modulus (MPa) without fungal biomass: 298 ± 58 Tensile strength (MPa) with fungal biomass: 5.2–19.3 Elongation at break with fungal biomass (%): 1.4–4.5 Young’s Modulus (MPa) with fungal biomass: 187–1350 Water Vapor Permeability Coefficient ((kg·s−1·m−1·Pa−1) × 1013) without fungal biomass: 7 Water Vapor Permeability Coefficient ((kg·s−1·m−1·Pa−1) × 1013) with fungal biomass: ~2–4 | Gurram et al. [102] |
| Cocoa pod husks | Cocoa pod husks were washed to remove residual sugars and alcohols and then dried in an oven at 40 °C overnight. Solutions of 3% by weight of solids in TFA were prepared in glass vials. Vials were sealed with Parafilm and were placed in a benchtop lab shaker for 29 days. The obtained solution was centrifuged in order to remove any residuals. | Casting | Cellulose based biofilm | Tensile stress at break (MPa): 70 Water adsorption %: <10 (at a relative humidity <50%) Water adsorption %: >10 (at a relative humidity >80%) Initial decomposition temperature under 44% of relative humidity (°C): ~200 | Bayer et al. [37] |
| 100 g of milled cocoa by-products were placed in 400 g of diethyl ether under stirring at room temperature for 24 h. After evaporation, the supernatant gave a first residue. In addition, the solid residue was separated by filtration and, subsequently, underwent the second extraction with 400 g of ethanol under stirring at room temperature for 24 h. Once again, the supernatant was evaporated and the second residue was collected. The third residue was used as obtained after filtration. | Extrusion | Biocomposite | Oxygen permeability [cc·mm/(m2·bar·24 h)]: 844–1104 for PLA and 982-5784 for PP Wettability (contact angle): 88 ± 2° | Battegazzore et al. [76] | |
| Cocoa shell waste (CSW) was grinded. The micronized CSW powder was added to 10 mL of heptane and stirred for 2 min. Then, the silicone (Elastosil E43) was added to the CSW dispersion and vigorously stirred for further 5 min to obtain a homogenous blend. | Casting | Biocomposite | Tensile strain at break %: 15–250 Young Modulus (MPa): 1.96 ± 0.13–10.67 ± 0.19 Water vapor permeability (g (m d Pa)−1): 1.51·10−5–14.93·10−5 BOD saturation level (mg O2/L): 36.1–38.2 | Tran et al. [135] | |
| Hazelnut skin | 50 g of hazelnut skin were placed in 400 g of diethyl ether under stirring at room temperature for 24 h. After evaporation, the supernatant gave a first residue. In addition, the solid residue was separated by filtration and, subsequently, underwent the second extraction with 400 g of ethanol under stirring at room temperature for 24 h. Once again, the supernatant was evaporated and the second residue was collected. The third residue was used as obtained after filtration. | Melt-blending extrusion | Biocomposite | Oxygen permeability [cc·mm/(m2·bar·24 h)]: 11.7–1875 for PLA and 122-338 for PP Wettability (contact angle): 80 ± 2° | Battegazzore et al. [76] |
| Hemp fibers | Dried hemp fibers were aggregated with tomato fibers in different percentages (100% tomato fibers, 90% tomato fibers, 70% tomato fibers). 50.0 g of the aggregated were soaked in 100 mL of a 2% (w/v) sodium alginate water solution. | Molding | Biocomposite | Young Modulus (MPa): 62.51–97.08 Tensile stress at break (MPa): 0.46–1.20 Maximum load (N) *: 8.7–14.8 Displacement (mm) *: 3.22–4.75 Water up-take percentage Wst%: 128–186 Time for complete biodegradation: 16 days after the transplanting | Schettini et al. [105] |
| Jackfruit seeds | Jackfruit seeds were removed from the skin of the arrows and then washed and powdered. The obtained powder was mixed with sorbitol (from 0 to 6 mL) and poly vinyl alcohol (from 0 to 3 g). | Not specified | Not specified | Tensile strength (MPa): 0–~2.2 Elongation at break: 0–7% | Lestari et al. [93] |
| Mangosteen peels | Mangosteen peels were sun dried for about 48 h at room temperature. Then, they were grinded and sieved. To prepare cellulose fibers, about 50 g of the Mangosteen peels powder were treated with 700 mL of 0.1 M NaOH, under heating and stirring. Then the insoluble pulp was bleached with 500 mL of NaOCl buffered to a pH 5 and washed with distilled water. The cellulose fibers were air dried. Then 10 g of fibers were hydrolyzed in 100 mL of 95% H2SO4 at 500 °C, then diluted with distilled water, centrifuged and sonicated. The resulting suspension cellulose nanocrystals (CNCs) were dried in a freeze drier at 3 °C. CNCs were mixed in different wt% (0–19%) with 10 g of cassava starch, 60 mL of distilled water, 5 mL of vinegar and 7 mL of glycerol. The mixture was stirred at 105 °C up to 200 °C. | Casting | Biocomposite | Tensile strength (MPa): ~1.3–~2 Young’s Modulus (GPa): ~15–~26 Elongation at break (%): ~15–~23 | Muhammad et al. [103] |
| Miscanthus | Chopped 2 mm miscanthus were dried at 80 °C for at least 24 h. They were mixed with oat hull and blends of PBS/PBAT (80/20) in the presence of peroxide (0.02 phr) with feeder at 100 rpm and 180 °C. | Extrusion | Biocomposite | Tensile strength with 20% Mischatus fiber (MPa): 1.0 Tensile strength with 40% Mischatus fiber (MPa): 1.0 Young’s Modulus with 20% Mischatus fiber (MPa): 64 Young’s Modulus with 40% Mischatus fiber (MPa): 404 Water absorption with 20% Mischatus fiber: 1–3% Water absorption with 40% Mischatus fibre: 1–7% | Wu et al. [133] |
| Oat Hull | Chopped 2 mm oat hull were dried at 80 °C for at least 24 h. They were mixed with miscanthus and blends of PBS/PBAT (80/20) in the presence of peroxide (0.02 phr) with feeder at 100 rpm and 180 °C. | Extrusion | Biocomposite | Tensile strength with 20% oat hull (MPa): 0.4 Tensile strength with 40% oat hull (MPa): 0.29 Young’s Modulus with 20% oat hull (MPa): 54 Young’s Modulus with 40% oat hull (MPa): 257 Water absorption with 20% oat hull: 1–5% Water absorption with 40% oat hull: 2–9% | Wu et al. [133] |
| Orange peels | Dried orange peels were washed with a HCl (0.03 N) at 60 °C and agitated for 30 min. The residue was hydrolyzed with HCl (0.04 N) at 90 °C for 20 min. Then, pectin extraction was performed with hot water at 90 °C and agitated for 30 min. The obtained pectin jelly was mixed with corn starch, layered silicates, glycerol and water; the mixture was left overnight in an oven at 70 °C. | Extrusion followed by casting | Biocomposite | Equilibrium recoverable compliance (m2/N): 3.71 × 10−9 Water vapor transmission rate (g/m2h): 9.87 Oxygen gas transmission rate (mL/m2day): 1366 ± 194 | Cokajgil et al. [100] |
| Orange waste (OW) was washed with water then dried for 16 h at 40 °C and milled to a fine powder. A mixture of 2% (w/v) of OW powder was prepared in 1% (w/v) citric acid solution under constant magnetic stirring at 70 °C. The acid solution also contained 7% (w/w) glycerol and 1 drop of organic antifoam/100 mL solution. The suspension was sieved before it was poured onto PTFE plates and dried at 40 °C. | Casting | Pectin and cellulose based biofilm | Tensile strengths (MPa): 28–36 Time for 90% degradation: 15 days | Batori et al. [92] | |
| Palm empty fruit bunch | Palm empty fruit bunch was dried, powdered and cooked for 8 h at 80 °C. 10% NaOH solution was added and the mixture was autoclaved for 15 min at 121 °C. The obtained mixture was added with 10% sodium hypochlorite processed using ultrafine grinder in wet milling method. 2% cellulose was mixed with 2 L of water and passed through the grinder for up to 30 cycles until a nanocellulose gel was formed. 5% of the produced nanocellulose was introduced in the Enviplast® formula. | Extrusion | Biocomposite | Tensile strength (kgf/cm2): 191.30 Elongation at break (%): 197.12 Water Vapour Transmission Rate (g/m2/24 h): 299.42 | Iriani et al. [140] |
| Parsley stems | Parsley stems were washed to remove residual sugars and alcohols and then dried in an oven at 40 °C overnight. Solutions of 3% by weight of solids in TFA were prepared in glass vials. Vials were sealed with Parafilm and were placed in a benchtop lab shaker for 29 days. The obtained solution was centrifuged in order to remove any residuals. | Casting | Cellulose based biofilm | Tensile stress at break (MPa): 5 Water adsorption %: <10 (at a relative humidity <50%) Water adsorption %: >10 (at a relative humidity >80%) Initial decomposition temperature under 44% of relative humidity (°C): ~200 | Bayer et al. [37] |
| Parsley stems powder was dispersed in a 5% (w/w) HCl water solution at a concentration of 50 mg/mL under vigorous stirring at 40 °C. After 12 h, the viscous dispersion was dialyzed using a 3500 MWCO membrane against MilliQ water for 72 h and then cast on a petri dish. | Casting | Cellulose based biofilm | Young’s Modulus (MPa): ~200 Elongation at break: 10% Ultimate strength (MPa): ~7 Water contact angle: ~60 | Perotto et al. [111] | |
| Passion fruit peels | Passion fruits peels were dried and milled to fine flour. A mixture of corn and cassava starches, 45 and 55%, respectively, was placed in a homogenizer and blended for 10 min with the passion fruit peel. 2000 g of the mix was processed by the extruder. For the plasticizer solutions (1 L for each treatment), different amounts of glycerol and water were prepared (60, 64, 70, 76, and 80% glycerol content). The extrudates were cut into 5-g pieces, placed between Teflon sheets, compressed, and molded at 5 ton and 90 °C for 30 s. | Extrusion followed by molding | Biocomposite | Tensile strength (MPa): 1.6–9.0 Elongation at break: 24.7–54.5% Young’s Modulus (MPa): 2.4–29.9 Water vapor permeability (g·mm/m2·h·kPa): 0.256–0.436 Water solubility index: 50.4–68.3% Contact angle (°): 5.3–72.2 | Moro et al. [132] |
| Peanut hulls | Peanut hulls, were stored at 4 °C, and then reduce to powder. The mixture including 17 g of peanut hulls mashed to a size of around 100 microns was blended minutes, gradually adding potato flour from skins (30 g), whole milk (48 mL) and glycerol (5 mL). The mixed ingredients formed a compound that was cooked in a fan-assisted oven at 180 °C for 13 min. | Casting | Biocomposite | Weight loss %: 6.5 ± 0.5 | Troiano et al. [134] |
| Pine flower waste | Pine flowers were soaked water at 100 °C for 2 h, washed and dried for 24 h at 50 °C. Then, they were grounded and soaked in hot water for 4 h and roasted for 24 h at 60 °C. For cellulose isolation, 50 g of grounded pine flower were mixed with 500 mL of 6% NaOH at 70 °C for 4 h. After serial washing and filtration steps, 5 g of obtained cellulose were mixed with 10%, 30%, and 60% citric acid in 100 mL respectively and ultrasonicated. Then, a polyvinyl alcohol (PVA) solution was prepared by mixing 4% PVA, 25% glycerol, and 71% distilled water (w/w), and a starch solution by mixing 3% starch, 12% glycerol and 85% distilled water (w/w). Both solutions were mixed together (PVA: starch ratio of 80:20 (w/w)) and finally 1% nanocellulose, 10% turmeric extract and 10% natural dragon fruit extract were added. | Casting | Biocomposite | Not reported | Nasihin et al. [137] |
| Pineapple leaf | Pineapple leaf were scaffed and then the fibers were cut into small sizes and grinded. The grinded fibers were then sieved to get the highest amount of fiber length available. Then they were placed in oven for 24 h and subsequently mixed with tapioca-based bioplastic resin (70–90% w/w). The mixture was first extruded into pellet at 160 °C. The pellets were then placed in the mold and hot pressed at 160 °C for 5 min at 8 MPa, and then cold pressed at room temperature for 5 min at 8 MPa. | Extrusion followed by molding | Biocomposite | Tensile Modulus (GPa): 1.029–1.145 Tensile strain (mm/mm): 0.007–0.011 | Mathivanan et al. [130] |
| Potato peels | Potato peels were granulated and centrifuged at 15000 rpm for 20 min. The supernatant was filtered and the starch was obtained. 13.5 g of dried starch was extracted from 330 g wet potato peels. After filtration, starch was dried at 50 °C for 2 h. 13.5 g of starch were mixed with 135 mL of tap water, 16.2 mL of vinegar, and 10.8 mL of glycerin. The mixture was heated (100 °C) and kept waiting at that temperature for 20 min. | Casting | Starch based biofilm | Water absorption: 48.46% within two hours and 83.57% within 24 h Time for complete biodegradation: 28 days in moist soil. | Arikan et al. [114] |
| Potato peels were boiled with water and then the starch was extracted from the water by filtration. The starch was mixed with glycerine, vinegar and water in different ratio, at 105 °C. | Casting | Starch based biofilm | Resistence to compressive stress (MPa): 0.5–1.1 | Samer et al. [118] | |
| Pumpkin peels | 1 g of dry pumpkin peel residue was suspended in 50 mL of 2 wt% NaOH solution and stirred for 4 h at 100 °C. Then 80 mL of 0.5 M NaOH containing 2% (v/v) of H2O2 per each gram of material were added. The solution was stirred during 1 h. Then, 20 mL of 2 M NaOH solution was added and the suspension stirred during 5 h at 55 °C. Subsequently, the residue was washed with distilled water until achieve neutral pH. Finally, the residue was filtered and dried. 12 mL of [(BMIM)Cl] was used per each 0.25 g of material to dissolute cellulose. The isolated cellulose was acetylated and 0.02 g were added to a solution of PLA (0.18 g) in DCM (25 mL). The mixture was stirred for 1 h, at 50 °C and then it was casted. | Casting | Biocomposite | Storage Moduls (GPa): 1.85 at 40 °C | Coto et al. [136] |
| Radicchio waste | Radicchio waste powder was dispersed in a 5% (w/w) HCl water solution at a concentration of 50 mg/mL under vigorous stirring at 40 °C. After 12 h, the viscous dispersion was dialyzed using a 3500 MWCO membrane against MilliQ water for 72 h and then cast on a petri dish. | Casting | Cellulose based biofilm | Young’s Modulus (MPa): ~200 Elongation at break: 5% Ultimate strength (MPa): ~5 Water contact angle: ~80° | Perotto et al. [111] |
| Rice straw and hulls | Rice hulls were washed to remove residual sugars and alcohols and then dried in an oven at 40 °C overnight. Solutions of 3% by weight of solids in TFA were prepared in glass vials. Vials were sealed with Parafilm and were placed in a benchtop lab shaker for 29 days. The obtained solution was centrifuged in order to remove any residuals. | Casting | Cellulose based biofilm | Tensile stress at break (MPa): 7 Water adsorption %: <10 (at a relative humidity <50%) Water adsorption %: >10 (at a relative humidity >80%) Initial decomposition temperature under 44% of relative humidity (°C): ~225 | Bayer et al. [37] |
| 200 g of dried rice straw samples were placed in a solid-liquid extractor with 1 L of Milli-Q water. Total extraction was performed for approximately 3 h and with 30 cycles and 12 strikes per cycle and the static phase for 10 min. Then, 10 g of the powdered and dried rice straw, previously washed and dried, was mixed with 200 mL of TFA and maintained under magnetic stirring (800 rpm) at room temperature for 3 days and, poured into a low edge crystallizing container maintained under laminar hood. | Casting | Cellulose based biofilm | Tensile strength at break (MPa): 45 (dried dumbbells) Elongation at break (%): 6.1 (dried dumbbells) Tensile strength at break (MPa): 10 (wet dumbbells) Elongation at break (%): 63 (wet dumbbells) Water adsorption %: 40.7–42.6 Time for complete biodegradation: 105 days in soil. | Bilo et al. [110] | |
| Rice straw was dried, powdered and cooked for 8 h at 80 °C. 10% NaOH solution was added and the mixture was autoclaved for 15 min at 121 °C. The obtained mixture was added with 10% sodium hypochlorite processed using ultrafine grinder in wet milling method. 2% cellulose was mixed with 2 L of water and passed through the grinder for up to 30 cycles until a nanocellulose gel was formed. 5% of the produced nanocellulose was introduced in the Enviplast® formula. | Extrusion | Biocomposite | Tensile strength (kgf/cm2): 168.36 Elongation at break (%): 156.36 Water Vapour Transmission Rate (g/m2/24 h): 301.06 | Iriani et al. [140] | |
| Rice straw were chopped to prepare the fibres with lengths ranging from 0.5 to 2 mm. The fibers were dehydrated for 24 h within a vacuum oven at 105 °C. Three chosen polymers (PLA, Lignin, PP) were separately compounded with 20% of rice straw fiber. | Extrusion | Biocomposite | Not reported | Dahy et al. [142] | |
| Rice waste powdered. The powder of rice waste was mixed with chitosan (from 30 to 60%) and glycerol (from 0 to 3 mL), heated at 50–60 °C for 30 min. | Molding | Cellulose based biofilm | Tensile strength (MPa): ~0–60 Elongation at break: 2–4% Water resistance: 0.1–0.6% | Lestari et al. [93] | |
| Sago waste | Sago waste was soaked in hot water at 40 °C for 2 h and air dried. The sago waste was then treated with 2% NaOH aqueous solution at 60 °C for 1 h, filtered and washed. The samples were dried at 40 °C. The obtained fibers were bleached with NaClO2/glacial CH3COOH mixture at 80 °C. Then, they were washed with distilled water and dried at 60 °C. The cellulose fibers were dissolved in 10 mL of distilled water, stirred, ultrasonicated for 30 min and, subsequently, mixed with a sago starch 4% (w/w) solution. | Casting | Biocomposite | Tensile strength (MPa):86.66–123.03 Young’s Modulus (MPa): 1710–2958 Elongation at break (%): 3.85–4.62 Water absorption (%): ~100–~200 | Yacob et al. [141] |
| Soy waste | Soy waste was bleached with a solution of distilled water:sodium hypochlorite (70:30). Then, it was separated from the solvent, rinsed with distilled water and dried in the oven for one hour at 100 °C. Subsequently, it was powdered and 3.0 g were mixed with corn starch (9.5 g), glycerol (5 mL), vinegar (5 mL) and water (60 mL). The mixing process was carried out at 25 °C and 50 rpm for 10 min. | Casting | Biocomposite | Maximum value of force before fracture (N): 6.71 Water absorption (%): 114.17 | Muhammad et al. [119] |
| Spinach steams | Spinach steams were washed to remove residual sugars and alcohols and then dried in an oven at 40 °C overnight. Solutions of 3% by weight of solids in TFA were prepared in glass vials. Vials were sealed with Parafilm and were placed in a benchtop lab shaker for 29 days. The obtained solution was centrifuged in order to remove any residuals. | Casting | Cellulose based biofilm | Tensile stress at break (MPa): 1 Water adsorption %: <10 (at a relative humidity <50%) Water adsorption %: >10 (at a relative humidity >80%) Initial decomposition temperature under 44% of relative humidity (°C): ~130 | Bayer et al. [37] |
| Tea leaves waste | Tea leaves waste were dried under vacuum at 70 °C, grounded, and sifted. Tea waste powder (TW) was dried under vacuum at 70 °C. TW bioplastics were synthesized with 1 g of TW powder in 20 mL of 3% citric acid solution (TW-CA) or only with water (TW-H2O). Carboxymethylcellulose sodium salt at 5% was investigated also as an additive to TW bioplastics (TW-CMC). All samples were magnetically stirred in an oil bath at 60 °C for 12 h, then casted. | Casting | Cellulose based biofilm | Ultimate tensile strength (MPa): 2–~6 Elongation at break: 0.5%–~13% Water contact angles (°): ~40–~120 | Liu et al. [112] |
| Tomato waste | After the extraction of polysaccharides, carotenoids and polyphenols from peels and seeds, the residual dried fibers were combined with hemp fibers in different percentages (0% hemp fibers, 10% hemp fibers, 30% hemp fibers). 50.0 g of the aggregated fibers were soaked in 100 mL of a 2% (w/v) sodium alginate water solution. | Molding | Biocomposite | Young Modulus (MPa): 62.51–97.08 Tensile stress at break (MPa): 0.46–1.20 Maximum load (N) *: 8.7–14.8 Displacement (mm) *: 3.22–4.75 Water up-take percentage Wst%:128–186 Time for complete biodegradation: 16 days after the transplanting | Schettini et al. [105] |
| Tomato pomace was subjected to alkaline hydrolysis (100 °C for 6 h with a NaOH 0.5 M solution in water) to obtain cutin monomers. The supernatant was discarded and the resulting solution was acidified with HCl 3 M up to final pH 3. 80.0 mg of tomato pomace monomers were blended with tin (II) 2-ethylhexanoate; the mixtures were placed on open carbon-doped Teflon molds and heated in air inside a convention oven. | Melt polycondensation | Aliphatic polyester | Young Modulus (MPa): 14–214 Hardness (MPa): 1.8–26.3 Water contact angle: 81°–109° Water up-take percentage: 2.1–6.1% | Heredia-Guarreiro et al. [124] |
References
- Plastics Europe An Analysis of European Plastics Production, Demand and Waste Data. Available online: https://www.plasticseurope.org/application/files/9715/7129/9584/FINAL_web_version_Plastics_the_facts2019_14102019.pdf (accessed on 6 April 2020).
- Lee, A.; Liew, M.S. Ecologically derived waste management of conventional plastics. J. Mater. Cycles Waste Manag. 2020, 22, 1–10. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef] [PubMed]
- Amodeo, C.; Sofo, A.; Tito, M.T.; Scopa, A.; Masi, S.; Pascale, R.; Mancini, I.M.; Caniani, D. Environmental factors influencing landfill gas biofiltration: Lab scale study on methanotrophic bacteria growth. J. Environ. Sci. Health Part A 2018, 53, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Caniani, D.; Caivano, M.; Pascale, R.; Bianco, G.; Mancini, I.M.; Masi, S.; Mazzone, G.; Firouzian, M.; Rosso, D. CO2 and N2O from water resource recovery facilities: Evaluation of emissions from biological treatment, settling, disinfection, and receiving water body. Sci. Total Environ. 2019, 648, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Caivano, M.; Pascale, R.; Mazzone, G.; Buchicchio, A.; Masi, S.; Bianco, G.; Caniani, D. N2O and CO2 Emissions from secondary settlers in WWTPs: Experimental results on full and pilot scale plants. In Lecture Notes in Civil Engineering; Springer: Cham, Switzerland, 2017; pp. 412–418. [Google Scholar]
- Caivano, M.; Pascale, R.; Mazzone, G.; Masi, S.; Panariello, S.; Caniani, D. Disinfection unit of water resource recovery facilities: Critical issue for N2O Emission. In Lecture Notes in Civil Engineering; Springer: Cham, Switzerland, 2017; pp. 444–450. [Google Scholar]
- Pascale, R.; Caivano, M.; Buchicchio, A.; Mancini, I.M.; Bianco, G.; Caniani, D. Validation of an analytical method for simultaneous high-precision measurements of greenhouse gas emissions from wastewater treatment plants using a gas chromatography-barrier discharge detector system. J. Chromatogr. A 2017, 1480, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Bradney, L.; Wijesekara, H.; Palansooriya, K.N.; Obadamudalige, N.; Bolan, N.S.; Ok, Y.S.; Rinklebe, J.; Kim, K.H.; Kirkham, M.B. Particulate plastics as a vector for toxic trace-element uptake by aquatic and terrestrial organisms and human health risk. Environ. Int. 2019, 131, 104937. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Lots, F.A.E.; Behrens, P.; Vijver, M.G.; Horton, A.A.; Bosker, T. A large-scale investigation of microplastic contamination: Abundance and characteristics of microplastics in European beach sediment. Mar. Pollut. Bull. 2017, 123, 219–226. [Google Scholar] [CrossRef]
- Okunola A, A.; Kehinde I, O.; Oluwaseun, A.; Olufiropo E, A. Public and Environmental Health Effects of Plastic Wastes Disposal: A Review. J. Toxicol. Risk Assess. 2019, 5, 1–13. [Google Scholar] [CrossRef]
- Gu, F.; Guo, J.; Zhang, W.; Summers, P.A.; Hall, P. From waste plastics to industrial raw materials: A life cycle assessment of mechanical plastic recycling practice based on a real-world case study. Sci. Total Environ. 2017, 601–602, 1192–1207. [Google Scholar] [CrossRef]
- Yates, M.R.; Barlow, C.Y. Resources, Conservation and Recycling Life cycle assessments of biodegradable, commercial biopolymers—A critical review. Resour. Conserv. Recycl. 2013, 78, 54–66. [Google Scholar] [CrossRef]
- Kumar, S.; Thakur, K. Bioplastics-classification, production and their potential food applications. J. Hill Agric. 2017, 8, 118. [Google Scholar] [CrossRef]
- Polman, E.M.N.; Gruter, G.J.M.; Parsons, J.R.; Tietema, A. Comparison of the aerobic biodegradation of biopolymers and the corresponding bioplastics: A review. Sci. Total Environ. 2021, 753, 141953. [Google Scholar] [CrossRef] [PubMed]
- Brodin, M.; Vallejos, M.; Opedal, M.T.; Area, M.C.; Chinga-Carrasco, G. Lignocellulosics as sustainable resources for production of bioplastics—A review. J. Clean. Prod. 2017, 162, 646–664. [Google Scholar] [CrossRef]
- Karan, H.; Funk, C.; Grabert, M.; Oey, M.; Hankamer, B. Green Bioplastics as Part of a Circular Bioeconomy. Trends Plant Sci. 2019, 24, 237–249. [Google Scholar] [CrossRef]
- Tsang, Y.F.; Kumar, V.; Samadar, P.; Yang, Y.; Lee, J.; Ok, Y.S.; Song, H.; Kim, K.H.; Kwon, E.E.; Jeon, Y.J. Production of bioplastic through food waste valorization. Environ. Int. 2019, 127, 625–644. [Google Scholar] [CrossRef]
- Gowman, A.C.; Picard, M.C.; Lim, L.T.; Misra, M.; Mohanty, A.K. Fruit waste valorization for biodegradable biocomposite applications: A review. BioResources 2019, 14, 10047–10092. [Google Scholar] [CrossRef]
- Maraveas, C. Production of sustainable and biodegradable polymers from agricultural waste. Polymers 2020, 12, 1127. [Google Scholar] [CrossRef]
- Matheus, J.R.V.; Miyahira, R.F.; Fai, A.E.C. Biodegradable films based on fruit puree: A brief review. Crit. Rev. Food Sci. Nutr. 2020, 1–8. [Google Scholar] [CrossRef]
- European Bioplastics Bioplastics Facts and Figures. Available online: https://docs.european-bioplastics.org/publications/EUBP_Facts_and_figures.pdf (accessed on 6 April 2020).
- Janssen, L.P.B.M.; Moscicki, L. Termoplastic Starch: A Green Material for Various Industries, 1st ed.; Janssen, P.B.M., Moscicki, L., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; ISBN 9780470146835. [Google Scholar]
- Reddy, R.L.; Reddy, V.S.; Gupta, G.A. Study of Bio-plastics As Green & Sustainable Alternative to Plastics. Int. J. Emerg. Technol. Adv. Eng. 2013, 3, 76–81. [Google Scholar]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef]
- Ross, G.; Ross, S.; Tighe, B.J. Bioplastics: New Routes, New Products. In Brydson’s Plastics Materials, 8th ed.; Elsevier: Oxford, UK, 2017; pp. 631–652. ISBN 9780323358248. [Google Scholar]
- De Almeida Oroski, F.; Chaves Alves, F.; Bomtempo, V. Bioplastics Tipping Point: Drop-in or non-drop-in? J. Bus. Chem. 2014, 11, 42–50. [Google Scholar]
- Xiao, B.; Zheng, M.; Pang, J.; Jiang, Y.; Wang, H.; Sun, R.; Wang, A.; Wang, X.; Zhang, T. Synthesis and Characterization of Poly(ethylene terephthalate) from Biomass-Based Ethylene Glycol: Effects of Miscellaneous Diols. Ind. Eng. Chem. Res. 2015, 54, 5862–5869. [Google Scholar] [CrossRef]
- Chen, L.; Pelton, R.E.O.; Smith, T.M. Comparative life cycle assessment of fossil and bio-based polyethylene terephthalate (PET) bottles. J. Clean. Prod. 2016, 137, 667–676. [Google Scholar] [CrossRef]
- Volanti, M.; Cespi, D.; Passarini, F.; Neri, E.; Cavani, F.; Mizsey, P.; Fozer, D. Terephthalic acid from renewable sources: Early-stage sustainability analysis of a bio-PET precursor. Green Chem. 2019, 21, 885–896. [Google Scholar] [CrossRef]
- Soták, T.; Schmidt, T.; Hronec, M. Hydrogenolysis of polyalcohols in the presence of metal phosphide catalysts. Appl. Catal. A Gen. 2013, 459, 26–33. [Google Scholar] [CrossRef]
- Yang, L.; Yan, X.; Wang, Q.; Wang, Q.; Xia, H. One-pot catalytic conversion of cellulose into polyols with Pt/CNTs catalysts. Carbohydr. Res. 2015, 404, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Zheng, M.; Sun, R.; Wang, A.; Wang, X.; Zhang, T. Synthesis of ethylene glycol and terephthalic acid from biomass for producing PET. Green Chem. 2016, 18, 342–359. [Google Scholar] [CrossRef]
- Jain, R.; Kosta, S.; Tiwari, A. Polyhydroxyalkanoates: A way to sustainable development of bioplastics. Chron. Young Sci. 2010, 1, 10–15. [Google Scholar] [CrossRef]
- Koller, M. Poly(hydroxyalkanoates) for Food Packaging: Application and Attempts towards Implementation. Appl. Food Biotechnol. 2014, 1, 3–15. [Google Scholar]
- Bayer, I.S.; Guzman-Puyol, S.; Heredia-Guerrero, J.A.; Ceseracciu, L.; Pignatelli, F.; Ruffilli, R.; Cingolani, R.; Athanassiou, A. Direct transformation of edible vegetable waste into bioplastics. Macromolecules 2014, 47, 5135–5143. [Google Scholar] [CrossRef]
- Sharma, S.; Luzinov, I. Water Aided Fabrication of Whey and Albumin Plastics. J. Polym. Environ. 2012, 20, 681–689. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Zarate-Ramírez, L.S.; Romero, A.; Bengoechea, C.; Partal, P.; Guerrero, A. Bioplastics based on wheat gluten processed by extrusion. J. Clean. Prod. 2019, 239, 1–8. [Google Scholar] [CrossRef]
- Zárate-Ramírez, L.S.; Romero, A.; Bengoechea, C.; Partal, P.; Guerrero, A. Thermo-mechanical and hydrophilic properties of polysaccharide/gluten-based bioplastics. Carbohydr. Polym. 2014, 112, 16–23. [Google Scholar] [CrossRef]
- Luengo, J.M.; García, B.; Sandoval, A.; Naharro, G.; Olivera, E.R. Bioplastics from microorganisms. Curr. Opin. Microbiol. 2003, 6, 251–260. [Google Scholar] [CrossRef]
- Ribeiro, P.L.L.; da Silva, A.C.M.S.; Filho, J.A.M.; Druzian, J.I. Impact of different by-products from the biodiesel industry and bacterial strains on the production, composition, and properties of novel polyhydroxyalkanoates containing achiral building blocks. Ind. Crops Prod. 2015, 69, 212–223. [Google Scholar] [CrossRef]
- Campos, M.I.; Figueiredo, T.V.B.; Sousa, L.S.; Druzian, J.I. The influence of crude glycerin and nitrogen concentrations on the production of PHA by Cupriavidus necator using a response surface methodology and its characterizations. Ind. Crops Prod. 2014, 52, 338–346. [Google Scholar] [CrossRef]
- Chen, G.Q. A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem. Soc. Rev. 2009, 38, 2434–2446. [Google Scholar] [CrossRef]
- Costa, S.S.; Miranda, A.L.; de Morais, M.G.; Costa, J.A.V.; Druzian, J.I. Microalgae as source of polyhydroxyalkanoates (PHAs) —A review. Int. J. Biol. Macromol. 2019, 131, 536–547. [Google Scholar] [CrossRef]
- Delgado, M.; Felix, M.; Bengoechea, C. Development of bioplastic materials: From rapeseed oil industry by products to added-value biodegradable biocomposite materials. Ind. Crops Prod. 2018, 125, 401–407. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Defersha, F.; Mohanty, A.K. Influence of processing parameters on the impact strength of biocomposites: A statistical approach. Compos. Part A Appl. Sci. Manuf. 2016, 83, 120–129. [Google Scholar] [CrossRef]
- Nguyen, H.T.H.; Qi, P.; Rostagno, M.; Feteha, A.; Mille, S.A. The quest for high glass transition temperature bioplastics. J. Mater. Chem. A 2013, 1, 1–38. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef] [PubMed]
- Averous, L.; Moro, L.; Dole, P.; Fringant, C. Properties of thermoplastic blends: Starch-polycaprolactone. Polymer 2000, 41, 4157–4167. [Google Scholar] [CrossRef]
- Bianco, G.; Agerbirk, N.; Losito, I.; Cataldi, T.R.I. Acylated glucosinolates with diverse acyl groups investigated by high resolution mass spectrometry and infrared multiphoton dissociation. Phytochemistry 2014, 100, 92–102. [Google Scholar] [CrossRef]
- Cataldi, T.R.I.; Orlando, D.; Nardiello, D.; Rubino, A.; Bianco, G.; Abate, S.; Ciriello, R.; Guerrieri, A. A three-factor Doehlert matrix design in optimising the determination of octadecyltrimethylammonium bromide by cation-exchange chromatography with suppressed conductivity detection. Anal. Chim. Acta 2007, 597, 129–136. [Google Scholar] [CrossRef]
- Zianni, R.; Bianco, G.; Lelario, F.; Losito, I.; Palmisano, F.; Cataldi, T.R.I. Fatty acid neutral losses observed in tandem mass spectrometry with collision-induced dissociation allows regiochemical assignment of sulfoquinovosyl-diacylglycerols. J. Mass Spectrom. 2013, 48, 205–215. [Google Scholar] [CrossRef]
- Bianco, G.; Zianni, R.; Anzillotta, G.; Palma, A.; Vitacco, V.; Scrano, L.; Cataldi, T.R.I. Dibenzo-p-dioxins and dibenzofurans in human breast milk collected in the area of Taranto (Southern Italy): First case study. Anal. Bioanal. Chem. 2013, 405, 2405–2410. [Google Scholar] [CrossRef]
- Pascale, R.; Onzo, A.; Ciriello, R.; Scrano, L.; Bufo, S.A.; Bianco, G. LC/MS Based Food Metabolomics; Elsevier: Oxford, UK, 2020; ISBN 9780081005965. [Google Scholar]
- Cataldi, T.R.I.; Bianco, G.; Abate, S.; Losito, I. Identification of unsaturated N-acylhomoserine lactones in bacterial isolates of Rhodobacter sphaeroides by liquid chromatography coupled to electrospray ionization-hybrid linear ion trap-Fourier transform ion cyclotron resonance mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 1817–1826. [Google Scholar] [CrossRef]
- Pascale, R.; Bianco, G.; Cataldi, T.R.I.; Kopplin, P.S.; Bosco, F.; Vignola, L.; Uhl, J.; Lucio, M.; Milella, L. Mass spectrometry-based phytochemical screening for hypoglycemic activity of Fagioli di Sarconi beans (Phaseolus vulgaris L.). Food Chem. 2018, 242, 497–504. [Google Scholar] [CrossRef]
- Bianco, G.; Pascale, R.; Carbone, C.F.; Acquavia, M.A.; Cataldi, T.R.I.; Schmitt-Kopplin, P.; Buchicchio, A.; Russo, D.; Milella, L. Determination of soyasaponins in Fagioli di Sarconi beans (Phaseolus vulgaris L.) by LC-ESI-FTICR-MS and evaluation of their hypoglycemic activity. Anal. Bioanal. Chem. 2018, 410, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Pascale, R.; Bianco, G.; Cataldi, T.R.I.; Buchicchio, A.; Losito, I.; Altieri, G.; Genovese, F.; Tauriello, A.; Di Renzo, G.C.; Lafiosca, M.C. Investigation of the Effects of Virgin Olive Oil Cleaning Systems on the Secoiridoid Aglycone Content Using High Performance Liquid Chromatography–Mass Spectrometry. JAOCS J. Am. Oil Chem. Soc. 2018, 95, 665–671. [Google Scholar] [CrossRef]
- Pascale, R.; Acquavia, M.A.; Cataldi, T.R.I.; Onzo, A.; Coviello, D.; Bufo, S.A.; Scrano, L.; Ciriello, R.; Guerrieri, A.; Bianco, G. Profiling of quercetin glycosides and acyl glycosides in sun-dried peperoni di Senise peppers (Capsicum annuum L.) by a combination of LC-ESI (-) -MS/MS and polarity prediction in reversed-phase separations. Anal. Bioanal. Chem. 2020, 412, 3005–3015. [Google Scholar] [CrossRef] [PubMed]
- Pascale, R.; Bianco, G.; Coviello, D.; Cristina Lafiosca, M.; Masi, S.; Mancini, I.M.; Bufo, S.A.; Scrano, L.; Caniani, D. Validation of a liquid chromatography coupled with tandem mass spectrometry method for the determination of drugs in wastewater using a three-phase solvent system. J. Sep. Sci. 2020, 43, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Onzo, A.; Acquavia, M.A.; Cataldi, T.R.I.; Ligonzo, M.; Coviello, D.; Pascale, R.; Martelli, G.; Bondoni, M.; Scrano, L.; Bianco, G. Coceth sulfate characterization by electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2020, 34, 1–10. [Google Scholar] [CrossRef]
- Thakur, S.; Chaudhary, J.; Sharma, B.; Tamulevicius, S.; Thakur, V.K. ScienceDirect Sustainability of bioplastics: Opportunities and challenges. Curr. Opin. Green Sustain. Chem. 2018, 13, 68–75. [Google Scholar] [CrossRef]
- Quarta, G. Determination of the Biobased Content in Plastics by Radiocarbon. Radiocarbon 2013, 55, 1834–1844. [Google Scholar] [CrossRef]
- Sherwood, J.; Clark, J.H.; Farmer, T.J.; Herrero-Davila, L.; Moity, L. Recirculation: A new concept to drive innovation in sustainable product design for bio-based products. Molecules 2017, 22, 48. [Google Scholar] [CrossRef]
- Iwata, T. Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew. Chemie-Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef]
- Lucas, N.; Bienaime, C.; Belloy, C.; Queneudec, M.; Silvestre, F.; Nava-saucedo, J. Polymer biodegradation: Mechanisms and estimation techniques. Chemosphere 2008, 73, 429–442. [Google Scholar] [CrossRef]
- Ruggero, F.; Gori, R.; Lubello, C. Methodologies to assess biodegradation of bioplastics during aerobic composting and anaerobic digestion: A review. Waste Manag. Res. 2019, 37, 959–975. [Google Scholar] [CrossRef] [PubMed]
- Ciriello, R.; Lo Magro, S.; Guerrieri, A. Assay of serum cholinesterase activity by an amperometric biosensor based on a co-crosslinked choline oxidase/overoxidized polypyrrole bilayer. Analyst 2018, 143, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Ciriello, R.; De Gennaro, F.; Frascaro, S.; Guerrieri, A. A novel approach for the selective analysis of L-lysine in untreated human serum by a co-crosslinked L-lysine–α-oxidase/overoxidized polypyrrole bilayer based amperometric biosensor. Bioelectrochemistry 2018, 124, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Massardier-Nageotte, V.; Pestre, C.; Cruard-Pradet, T.; Bayard, R. Aerobic and anaerobic biodegradability of polymer films and physico-chemical characterization. Polym. Degrad. Stab. 2006, 91, 620–627. [Google Scholar] [CrossRef]
- Kale, G.; Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.E.; Singh, S.P. Compostability of bioplastic packaging materials: An overview. Macromol. Biosci. 2007, 7, 255–277. [Google Scholar] [CrossRef]
- Folino, A.; Karageorgiou, A.; Calabrò, P.S.; Komilis, D. Biodegradation of wasted bioplastics in natural and industrial environments: A review. Sustainability 2020, 12, 6030. [Google Scholar] [CrossRef]
- Wang, K.; Wang, W.; Ye, R.; Liu, A.; Xiao, J.; Liu, Y.; Zhao, Y. Mechanical properties and solubility in water of corn starch-collagen composite films: Effect of starch type and concentrations. Food Chem. 2017, 216, 209–216. [Google Scholar] [CrossRef]
- Oliveira, T.Í.S.; Rosa, M.F.; Ridout, M.J.; Cross, K.; Brito, E.S.; Silva, L.M.A.; Mazzetto, S.E.; Waldron, K.W.; Azeredo, H.M.C. Bionanocomposite films based on polysaccharides from banana peels. Int. J. Biol. Macromol. 2017, 101, 1–8. [Google Scholar] [CrossRef]
- Battegazzore, D.; Bocchini, S.; Alongi, J.; Frache, A. Plasticizers, antioxidants and reinforcement fillers from hazelnut skin and cocoa by-products: Extraction and use in PLA and PP. Polym. Degrad. Stab. 2014, 108, 297–306. [Google Scholar] [CrossRef]
- Granda, L.A.; Espinach, X.; Méndez, J.A.; Tresserras, J.; Delgado-Aguilar, M.; Mutjé, P. Semichemical fibres of Leucaena collinsii reinforced polypropylene composites: Young’s Modulus analysis and fibre diameter effect on the stiffness. Compos. Part B Eng. 2016, 92, 332–337. [Google Scholar] [CrossRef]
- Palomba, D.; Vazquez, G.E.; Díaz, M.F. Chemometrics and Intelligent Laboratory Systems Prediction of elongation at break for linear polymers. Chemom. Intell. Lab. Syst. 2014, 139, 121–131. [Google Scholar] [CrossRef]
- Sanjay, M.R.; Arpitha, G.R.; Yogesha, B. Study on Mechanical Properties of Natural-Glass Fibre Reinforced Polymer Hybrid Composites: A Review. Mater. Today Proc. 2015, 2, 2959–2967. [Google Scholar] [CrossRef]
- Suderman, N.; Isa, M.I.N.; Sarbon, N.M. Food Bioscience The effect of plasticizers on the functional properties of biodegradable gelatin-based film: A review. Food Biosci. 2018, 24, 111–119. [Google Scholar] [CrossRef]
- Vieira, G.M.A.; da Silva, A.M.; dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Suyatma, N.E.; Tighzert, L.; Copinet, A.; Coma, V. Effects of hydrophilic plasticizers on mechanical, thermal, and surface properties of chitosan films. J. Agric. Food Chem. 2005, 53, 3950–3957. [Google Scholar] [CrossRef]
- Di Gioia, L.; Guilbert, S. Corn protein-based thermoplastic resins: Effect of some polar and amphiphilic plasticizers. J. Agric. Food Chem. 1999, 47, 1254–1261. [Google Scholar] [CrossRef]
- Santosa, F.X.B.; Padua, G.W. Tensile properties and water absorption of zein sheets plasticized with oleic and linoleic acids. J. Agric. Food Chem. 1999, 47, 2070–2074. [Google Scholar] [CrossRef]
- Saberi, B.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Physical and mechanical properties of a new edible film made of pea starch and guar gum as affected by glycols, sugars and polyols. Int. J. Biol. Macromol. 2017, 104, 345–359. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A. Physical, mechanical, barrier, and thermal properties of polyol-plasticized biodegradable edible film made from kefiran. Carbohydr. Polym. 2011, 84, 477–483. [Google Scholar] [CrossRef]
- Tihminlioglu, F.; Atik, I.D.; Ozen, B. Water vapor and oxygen-barrier performance of corn—Zein coated polypropylene films. J. Food Eng. 2010, 96, 342–347. [Google Scholar] [CrossRef]
- Gustafsson, J.; Landberg, M.; Bátori, V.; Åkesson, D.; Taherzadeh, M.J.; Zamani, A. Development of bio-based films and 3D objects from apple pomace. Polymers 2019, 11, 289. [Google Scholar] [CrossRef] [PubMed]
- Yaradoddi, J.; Patil, V.; Ganachari, S.; Banapurmath, N.; Hunashyal, A.; Shettar, A. Biodegradable plastic production from fruit waste material and its sustainable use for green apllications. Int. J. Pharm. Res. Allied Sci. 2016, 5, 56–65. [Google Scholar]
- Sultan, N.F.K.; Johari, W.L.W. The Development of Banana Peel/Corn Starch Bioplastic Film: A Preliminary Study. Bioremediat. Sci. Technol. Res. 2017, 5, 12–17. [Google Scholar]
- Faradilla, R.H.F.; Lee, G.; Roberts, J.; Martens, P.; Stenzel, M.; Arcot, J. Effect of glycerol, nanoclay and graphene oxide on physicochemical properties of biodegradable nanocellulose plastic sourced from banana pseudo-stem. Cellulose 2018, 25, 399–416. [Google Scholar] [CrossRef]
- Bátori, V.; Jabbari, M.; Åkesson, D.; Lennartsson, P.R.; Taherzadeh, M.J.; Zamani, A. Production of Pectin-Cellulose Biofilms: A New Approach for Citrus Waste Recycling. Int. J. Polym. Sci. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Lestari, R.A.S.; Kasmiyatun, M.; Dermawan, K.; Aini, A.N.; Riyati, N.; Putri, F.R. Bioplastic from Jackfruit Seeds and Rice. IOP Conf. Ser. Mater. Sci. Eng. 2020, 835, 1–10. [Google Scholar] [CrossRef]
- Sun, S.; Song, Y.; Zheng, Q. Morphology and mechanical properties of thermo-molded bioplastics based on glycerol-plasticized wheat gliadins. J. Cereal Sci. 2008, 48, 613–618. [Google Scholar] [CrossRef]
- Caner, C.; Vergano, P.J.; Wiles, J.L. Chitosan film mechanical and permeation properties as affected by acid, plasticizer, and storage. J. Food Sci. 1998, 63, 1049–1053. [Google Scholar] [CrossRef]
- Aguilar, J.M.; Bengoechea, C.; Pérez, E.; Guerrero, A. Effect of different polyols as plasticizers in soy based bioplastics. Ind. Crops Prod. 2020, 153, 1–9. [Google Scholar] [CrossRef]
- Alonso-González, M.; Ramos, M.; Bengoechea, C.; Romero, A.; Guerrero, A. Evaluation of Composition on Processability and Water Absorption of Wheat Gluten-Based Bioplastics. J. Polym. Environ. 2020, 1–10. [Google Scholar] [CrossRef]
- Bashir, A.S.M.; Manusamy, Y. Recent Developments in Biocomposites Reinforced with Natural Biofillers from Food Waste. Polym. Plast. Technol. Eng. 2015, 54, 87–99. [Google Scholar] [CrossRef]
- Cinar, S.O.; Chong, Z.K.; Kucuker, M.A.; Wieczorek, N.; Cengiz, U.; Kuchta, K. Bioplastic Production from Microalgae: A Review. Int. J. Environ. Res. Public Health 2020, 17, 3842. [Google Scholar] [CrossRef]
- Çokaygil, Z.; Banar, M.; Seyhan, A.T. Orange peel-derived pectin jelly and corn starch-based biocomposite film with layered silicates. J. Appl. Polym. Sci. 2014, 131, 1–12. [Google Scholar] [CrossRef]
- González-Gutiérrez, J.; Partal, P.; García-Morales, M.; Gallegos, C. Effect of processing on the viscoelastic, tensile and optical properties of albumen/starch-based bioplastics. Carbohydr. Polym. 2011, 84, 308–315. [Google Scholar] [CrossRef]
- Gurram, R.; Souza Filho, P.F.; Taherzadeh, M.J.; Zamani, A. A Solvent-Free Approach for Production of Films from Pectin and Fungal Biomass. J. Polym. Environ. 2018, 26, 4282–4292. [Google Scholar] [CrossRef]
- Muhammad, A.; Roslan, A.; Sanusi, S.N.A.; Shahimi, M.Q.; Nazari, N.Z. Mechanical properties of bioplastic form cellulose nanocrystal (CNC) mangosteen peel using glycerol as plasticizer. J. Phys. Conf. Ser. 2019, 1349, 1–8. [Google Scholar] [CrossRef]
- Oliveira, T.Í.S.; Rosa, M.F.; Cavalcante, F.L.; Pereira, P.H.F.; Moates, G.K.; Wellner, N.; Mazzetto, S.E.; Waldron, K.W.; Azeredo, H.M.C. Optimization of pectin extraction from banana peels with citric acid by using response surface methodology. Food Chem. 2016, 198, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Schettini, E.; Santagata, G.; Malinconico, M.; Immirzi, B.; Scarascia Mugnozza, G.; Vox, G. Recycled wastes of tomato and hemp fibres for biodegradable pots: Physico-chemical characterization and field performance. Resour. Conserv. Recycl. 2013, 70, 9–19. [Google Scholar] [CrossRef]
- Jha, A.; Kumar, A. Biobased technologies for the efficient extraction of biopolymers from waste biomass. Bioprocess Biosyst. Eng. 2019, 42, 1893–1901. [Google Scholar] [CrossRef]
- Pingali, S.V.; Urban, V.S.; Heller, W.T.; McGaughey, J.; O’Neill, H.; Foston, M.B.; Li, H.; Wyman, C.E.; Myles, D.A.; Langan, P.; et al. Understanding Multiscale Structural Changes during Dilute Acid Pretreatment of Switchgrass and Poplar. ACS Sustain. Chem. Eng. 2017, 5, 426–435. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Zhao, H.; Holladay, J.E.; Kwak, J.H.; Zhang, Z.C. Inverse Temperature-Dependent Pathway of Cellulose Decrystallization in Trifluoroacetic Acid. J. Phys. Chem. B 2007, 111, 5295–5300. [Google Scholar] [CrossRef] [PubMed]
- Bilo, F.; Pandini, S.; Sartore, L.; Depero, L.E.; Gargiulo, G.; Bonassi, A.; Federici, S.; Bontempi, E. A sustainable bioplastic obtained from rice straw. J. Clean. Prod. 2018, 200, 357–368. [Google Scholar] [CrossRef]
- Perotto, G.; Ceseracciu, L.; Simonutti, R.; Paul, U.C.; Guzman-Puyol, S.; Tran, T.N.; Bayer, I.S.; Athanassiou, A. Bioplastics from vegetable waste: Via an eco-friendly water-based process. Green Chem. 2018, 20, 894–902. [Google Scholar] [CrossRef]
- Liu, M.; Arshadi, M.; Javi, F.; Lawrence, P.; Davachi, S.M.; Abbaspourrad, A. Green and facile preparation of hydrophobic bioplastics from tea waste. J. Clean. Prod. 2020, 276, 1–10. [Google Scholar] [CrossRef]
- Rizwana Beevi, K.; Sameera Fathima, A.R.; Thahira Fathima, A.I.; Thameemunisa, N.; Noorjahan, C.M.; Deepika, T. Bioplastic Synthesis Using Banana Peels And Potato Starch And Characterization. Int. J. Sci. Technol. Res. 2020, 9, 1809–1814. [Google Scholar]
- Arikan, E.B.; Bilgen, H.D. Production of bioplastic from potato peel waste and investigation of its biodegradability. Int. Adv. Res. Eng. J. 2019, 03, 093–097. [Google Scholar] [CrossRef]
- Masruri, M.; Azhar, A.Z.; Rosyada, I.; Febrianto, A. The effect of kaffir lime (Citrus hystrix DC) essential oil on bioplastic derived from cassava peel waste. J. Phys. Conf. Ser. 2019, 1374, 1–6. [Google Scholar] [CrossRef]
- Dasumiati; Saridewi, N.; Malik, M. Food packaging development of bioplastic from basic waste of cassava peel (manihot uttilisima) and shrimp shell. IOP Conf. Ser. Mater. Sci. Eng. 2019, 602, 1–9. [Google Scholar] [CrossRef]
- Fathanah, U.; Lubis, M.R.; Nasution, F.; Masyawi, M.S. Characterization of bioplastic based from cassava crisp home industrial waste incorporated with chitosan and liquid smoke. IOP Conf. Ser. Mater. Sci. Eng. 2018, 334, 1–8. [Google Scholar] [CrossRef]
- Samer, M.; Khalefa, Z.; Abdelall, T.; Moawya, W.; Farouk, A.; Abdelaziz, S.; Soliman, N.; Salah, A.; Gomaa, M.; Mohamed, M. Bioplastics production from agricultural crop residues. Agric. Eng. Int. CIGR J. 2019, 21, 190–194. [Google Scholar]
- Muhammad, A.; Rashidi, A.R.; Roslan, A.; Idris, S.A. Development of bio based plastic materials for packaging from soybeans waste. AIP Conf. Proc. 2017, 1885, 1–8. [Google Scholar] [CrossRef]
- Emaga, T.H.; Robert, C.; Sebastien, N.R.; Wathelet, B.; Paquot, M. Dietary fibre components and pectin chemical features of peels during ripening in banana and plantain varieties. Bioresour. Technol. 2008, 99, 4346–4354. [Google Scholar] [CrossRef] [PubMed]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Khan, A.; Khan, R.A.; Salmieri, S.; Le Tien, C.; Riedl, B.; Bouchard, J.; Chauve, G.; Tan, V.; Kamal, M.R.; Lacroix, M. Mechanical and barrier properties of nanocrystalline cellulose reinforced chitosan based nanocomposite films. Carbohydr. Polym. 2012, 90, 1601–1608. [Google Scholar] [CrossRef]
- Olsson, E.; Hedenqvist, M.S.; Johansson, C.; Järnström, L. Influence of citric acid and curing on moisture sorption, diffusion and permeability of starch films. Carbohydr. Polym. 2013, 94, 765–772. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Caputo, G.; Guzman-Puyol, S.; Tedeschi, G.; Heredia, A.; Ceseracciu, L.; Benitez, J.J.; Athanassiou, A. Sustainable polycondensation of multifunctional fatty acids from tomato pomace agro-waste catalyzed by tin (II) 2-ethylhexanoate. Mater. Today Sustain. 2019, 3, 1–10. [Google Scholar] [CrossRef]
- Domínguez, E.; Heredia-Guerrero, J.A.; Heredia, A. Plant cutin genesis: Unanswered questions. Trends Plant Sci. 2015, 20, 551–558. [Google Scholar] [CrossRef]
- Deng, M.; Zhang, G.; Zeng, Y.; Pei, X.; Huang, R.; Lin, J. Simple process for synthesis of layered sodium silicates using rice husk ash as silica source. J. Alloys Compd. 2016, 683, 412–417. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Hinrichsen, G. Biofibres, biodegradable polymers and biocomposites: An overview. Macromol. Mater. Eng. 2000, 276–277, 1–24. [Google Scholar] [CrossRef]
- Ashori, A.; Nourbakhsh, A. Bio-based composites from waste agricultural residues. Waste Manag. 2010, 30, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Malinconico, M.; Santagata, G. Effect of cross-linking with calcium ions on the physical properties of alginate films. Biomacromolecules 2007, 8, 3193–3197. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, D.; Norfazilah, H.; Siregar, J.P.; Rejab, M.R.M.; Bachtiar, D.; Cionita, T. The study of mechanical properties of pineapple leaf fibre reinforced tapioca based bioplastic resin composite. MATEC Web Conf. 2016, 74, 1–4. [Google Scholar] [CrossRef]
- Yapo, B.M.; Koffi, K.L. Dietary fiber components in yellow passion fruit rind—A potential fiber source. J. Agric. Food Chem. 2008, 56, 5880–5883. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.M.A.; Ascheri, J.L.R.; Ortiz, J.A.R.; Carvalho, C.W.P.; Meléndez-Arévalo, A. Bioplastics of Native Starches Reinforced with Passion Fruit Peel. Food Bioprocess Technol. 2017, 10, 1798–1808. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Sustainable green composites from biodegradable plastics blend and natural fibre with balanced performance: Synergy of nano-structured blend and reactive extrusion. Compos. Sci. Technol. 2020, 200, 1–8. [Google Scholar] [CrossRef]
- Troiano, M.; Santulli, C.; Roselli, G.; Di Girolami, G.; Cinaglia, P.; Gkrilla, A. DIY Bioplastics from Peanut Hulls Waste in a Starch-Milk Based Matrix. FME Trans. 2018, 46, 503–512. [Google Scholar] [CrossRef]
- Tran, T.N.; Heredia-Guerrero, J.A.; Mai, B.T.; Ceseracciu, L.; Marini, L.; Athanassiou, A.; Bayer, I.S. Bioelastomers Based on Cocoa Shell Waste with Antioxidant Ability. Adv. Sustain. Syst. 2017, 1, 1700002. [Google Scholar] [CrossRef]
- Côto, T.; Moura, I.; de Sá, A.; Vilarinho, C.; Machado, A.V. Sustainable materials based on cellulose from food sector agro-wastes. J. Renew. Mater. 2018, 6, 688–696. [Google Scholar] [CrossRef]
- Nasihin, Z.D.; Masruri, M.; Warsito, W.; Srihardyastutie, A. Preparation of Nanocellulose Bioplastic with a Gradation Color of Red and Yellow. IOP Conf. Ser. Mater. Sci. Eng. 2020, 833, 1–6. [Google Scholar] [CrossRef]
- Azieyanti, N.A.; Amirul, A.; Othman, S.Z.; Misran, H. Mechanical and Morphology Studies of Bioplastic-Based Banana Peels. J. Phys. Conf. Ser. 2020, 1529, 1–6. [Google Scholar] [CrossRef]
- Sharif Hossain, A.B.M.; Ibrahim, N.A.; AlEissa, M.S. Nano-cellulose derived bioplastic biomaterial data for vehicle bio-bumper from banana peel waste biomass. Data Brief 2016, 8, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Iriani, E.S.; Permana, A.W.; Yuliani, S.; Kailaku, S.I.; Sulaiman, A.A. The effect of agricultural waste nanocellulose on the properties of bioplastic for fresh fruit packaging. IOP Conf. Ser. Earth Environ. Sci. 2019, 309, 1–7. [Google Scholar] [CrossRef]
- Yacob, N.; Yusof, M.R.; Mohamed, A.Z.; Badri, K.H. Effect of cellulose fiber loading on the properties of starch-based films. AIP Conf. Proc. 2019, 2111, 1–7. [Google Scholar] [CrossRef]
- Dahy, H. Efficient Fabrication of Sustainable Building Products from Annually Generated Non-wood Cellulosic Fibres and Bioplastics with Improved Flammability Resistance. Waste Biomass Valoriz. 2019, 10, 1167–1175. [Google Scholar] [CrossRef]
- Lu, S.-T.; Hamerton, I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Shruti, V.C.; Kutralam-Muniasamy, G. Bioplastics: Missing link in the era of Microplastics. Sci. Total Environ. 2019, 697, 134139. [Google Scholar] [CrossRef]
- Bhagwat, G.; Gray, K.; Wilson, S.P.; Muniyasamy, S.; Vincent, S.G.T.; Bush, R.; Palanisami, T. Benchmarking Bioplastics: A Natural Step Towards a Sustainable Future. J. Polym. Environ. 2020, 28, 3055–3075. [Google Scholar] [CrossRef]
- Hottle, T.A.; Bilec, M.M.; Landis, A.E. Sustainability assessments of bio-based polymers. Polym. Degrad. Stab. 2013, 98, 1898–1907. [Google Scholar] [CrossRef]
- Kakadellis, S.; Harris, Z.M. Don’t scrap the waste: The need for broader system boundaries in bioplastic food packaging life-cycle assessment—A critical review. J. Clean. Prod. 2020, 274, 122831. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Attinà, E.; Basile, C.; Mallamaci, C.; Muscolo, A. Use of Recalcitrant Agriculture Wastes to Produce Biogas and Feasible Biofertilizer. Waste Biomass Valoriz. 2016, 7, 267–280. [Google Scholar] [CrossRef]
- Heckman, J.H. Food packaging regulation in the United States and the European Union. Regul. Toxicol. Pharmacol. 2005, 42, 96–122. [Google Scholar] [CrossRef] [PubMed]
- Peelman, N.; Ragaert, P.; De Meulenaer, B.; Adons, D.; Peeters, R.; Cardon, L.; Van Impe, F.; Devlieghere, F. Application of bioplastics for food packaging. Trends Food Sci. Technol. 2013, 32, 128–141. [Google Scholar] [CrossRef]
- Malinconico, M.; Immirzi, B.; Santagata, G.; Schettini, E.; Vox, G.; Mugnozza, G.S. An overview on innovative biodegradable materials for agricultural applications. In Progress in Polymer Degradation and Stability Research; Moeller, H.W., Ed.; Nova Science Publishers: New York, NY, USA, 2008; pp. 69–114. ISBN 9781600218286. [Google Scholar]
| Biopolymer Name | Biopolymer Type | Properties | Fruits and Vegetable Wastes Used as Biopolymer Source |
|---|---|---|---|
| Cellulose | Polysaccharide | Highly structured intermolecular hydrogen bonding network; impossibility of melting or dissolution by standard processes such as thermoforming. | Banana peels, carrots waste, cauliflower waste, cocoa pod husks, orange peels, parsley steams, radicchio waste, rice hulls, spinach steams, tea leaves waste. |
| Starch | Polysaccharide | Strong inter- and intra-molecular hydrogen bonding; water sensitivity and poor fowability; brittleness. | Banana peels, cassava peels, potato peels. |
| Pectin | Polysaccharide | Gelling ability but poor tensile and barrier properties; water sensitivity. | Apple pomace, banana peels, citrus waste, orange peels |
| Cutin | Polyester of hydroxy fatty acids | Amorphous and flexible three-dimensional polymer; hydrophobic, low water sensitivity. | Tomato waste |
| Advantages | Drawbacks | |
|---|---|---|
| Production | Reduction of greenhouse gas emission; saving fossil fuels, possibility of using a local resource, less energy during the manufacturing cycle. | Use of croplands to produce items, not cost-competitive compared to conventional plastics |
| Use | No toxic, no release of chemicals into food if used as packaging | Often characterized by thermal instability, brittleness, low melt strength, high water vapor and oxygen permeability; when hydrophilic polymers are used, they possess low water vapor barrier and vulnerability to degradation. |
| Disposal | Biodegradable; broken down by naturally occurring bacteria; do not persist for many years in the environment. | Controlled fate in order to kickstart the expected biodegradation process; a specific disposal procedure must be followed to avoid they fragment into microplastics which accumulate in the environment. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acquavia, M.A.; Pascale, R.; Martelli, G.; Bondoni, M.; Bianco, G. Natural Polymeric Materials: A Solution to Plastic Pollution from the Agro-Food Sector. Polymers 2021, 13, 158. https://doi.org/10.3390/polym13010158
Acquavia MA, Pascale R, Martelli G, Bondoni M, Bianco G. Natural Polymeric Materials: A Solution to Plastic Pollution from the Agro-Food Sector. Polymers. 2021; 13(1):158. https://doi.org/10.3390/polym13010158
Chicago/Turabian StyleAcquavia, Maria Assunta, Raffaella Pascale, Giuseppe Martelli, Marcella Bondoni, and Giuliana Bianco. 2021. "Natural Polymeric Materials: A Solution to Plastic Pollution from the Agro-Food Sector" Polymers 13, no. 1: 158. https://doi.org/10.3390/polym13010158
APA StyleAcquavia, M. A., Pascale, R., Martelli, G., Bondoni, M., & Bianco, G. (2021). Natural Polymeric Materials: A Solution to Plastic Pollution from the Agro-Food Sector. Polymers, 13(1), 158. https://doi.org/10.3390/polym13010158